Abstract
Background
Chronic kidney disease (CKD)-mineral and bone disorder (MBD) and fracture risk are both closely related to declining renal function. Controlling hyperphosphatemia with phosphate binders is a basic principle of CKD-MBD treatment. The aim of this study was to identify differences in fracture risk between pre-dialysis CKD patients and end-stage renal disease (ESRD) on dialysis, and to evaluate the effects of phosphate binders on fracture risk in ESRD patients.
Methods
Data from a total of 89,533 CKD patients comprising CKD diagnosis, dialysis, fracture history, and phosphate binder prescription history from 2012 to 2016 were retrieved from the Health Insurance Review and Assessment Service Database. Multivariate Cox regression analyses were performed to identify whether dialysis or phosphate binders were associated with an increased fracture risk.
Results
Overall, the rate of fractures in pre-dialysis CKD patients was 74 per 1,000 patient-years, while that in dialysis patients was 84 per 1,000 patient-years. The risk of fracture in ESRD patients was higher than pre-dialysis CKD patients (hazard ratio, 1.16; 95% confidence interval, 1.12–1.21; P < 0.001) after adjusting for confounding variables. In addition, the fracture risk in patients who were not taking phosphate binders was 20.0% higher compared to ESRD patients taking phosphate binders.
Conclusion
Fractures were more prevalent in ESRD patients on dialysis than pre-dialysis CKD patients. Use of phosphate binders was associated with a lower fracture risk in ESRD patients.
Keywords: Chronic renal insufficiency, Dialysis, Fracture, Phosphate binder
Introduction
Chronic kidney disease (CKD) is a momentous issue both for individuals and society as a whole. The prevalence of CKD has been steadily increasing along with the size of the elderly population and the increasing prevalence of other chronic diseases such as diabetes mellitus and hypertension, and is associated with a tremendous increase in medical expenditures [1]. Individually, the various co-morbidities and complications associated with CKD significantly lower lifespan and quality of life.
CKD patients are susceptible to fractures, especially hip fractures that can lead to prolonged immobilization and subsequent fatal complications such as thromboembolism, pneumonia, and pressure sores [2,3]. Osteoporosis is the typical etiology of fractures in the general population. However, CKD-mineral and bone disorder (CKD-MBD), which refers to abnormalities of calcium and phosphorus metabolism, bone formation/turnover dysregulation, and vascular calcification is also associated with an increased risk of fracture. Importantly, patients with CKD-MBD can sustain fractures in cases with weak forces [4].
At the center of CKD-MBD is phosphorus, which has an important role as an essential mineral to control our body. Eighty to ninety percent of the body’s phosphorus content is stored in the bones and teeth, while the remainder resides in cells, body fluids, and the blood to control cell metabolism and energy generation [5]. As renal function declines, phosphorus excretion through the kidneys decreases, resulting in hyperphosphatemia and hypocalcemia. This imbalance of phosphate and calcium stimulates excretion of parathyroid hormone, which acts to release calcium from bones into the blood, leading to weakening of bones. Phosphate binders are widely used to treat patients with hyperphosphatemia, as they promote excretion of phosphorus into the feces. However, previous research has focused primarily on the role of phosphate binders in improving vascular calcifications and reducing cardiovascular mortality [6–8].
Here, we studied the impact of renal function decline on fracture risk in non-dialysis and dialysis-dependent CKD patients. In addition, we investigated the difference in fracture risk according to use of phosphate binders in dialysis-dependent CKD patients using data obtained from the Health Insurance Review and Assessment Service Database.
Methods
Ethics
Our study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Ewha Womans University College of Medicine (IRB number: EUMC 2017-08-028-001). Data was obtained from the Health Insurance Review and Assessment Service Database after anonymization. The IRB committee waived the requirement for informed consent due to the nature of the data and study design.
Study design and population
We analyzed data from the Health Insurance Review & Assessment Service Database from 2012 to 2016. First, patients diagnosed with CKD or end-stage renal disease (ESRD) (coded as N18.1 to N18.5, and N18.9) were enrolled. Next, patients were classified into pre-dialysis CKD patient or dialysis patient groups. Specifically, if a patient’s record included a hemodialysis treatment code (O7010 or O7020), peritoneal dialysis operation code (O7070), or peritoneal dialysate prescription code, they were classified into the dialysis patient group. Conversely, patients who did not fit the criteria for an ESRD patient on dialysis were classified into the pre-dialysis CKD group. To include incident pre-dialysis CKD or ESRD on dialysis patients only during the study period (2013–2016), we selected and excluded patients who already had CKD diagnosis codes or dialysis codes in 2012 (wash-out period). We were not focused on renal allograft patients in this study, so patients who underwent a renal allograft in 2012 were excluded.
Data on major and minor fracture diagnoses were obtained using fracture diagnosis codes for the spine (S32-), hip (S72-), upper extremities (S42-, S42.2, S42.4, S52-, S62-), lower extremities (S92-, S82-, S72.3, S72.4), skull (S02), thorax (S22), or other (S82-). Fracture data was collected from the time of CKD or ESRD diagnosis up to 2016 for all patients. We attempted to identify differences in fracture risk between age groups under 65 years and 65 years and over. We also performed analyses to identify the risk of fracture between the pre-dialysis CKD and ESRD patients on dialysis groups. Lastly, we analyzed the association between fracture risk and use of phosphate binders in patients on dialysis.
Statistical analysis
Categorical variables including dialysis status, age, sex, and fracture occurrence were expressed as frequencies (percentages). The number of fractures were counted, and the person-years of the study population were calculated by multiplying the number of patients and the follow-up period (years) or the period until the time of fracture. The total number of fractures was first divided by total person-years in the respective CKD and ESRD groups, and logistic regression analysis was conducted to find the statistical significance of fracture risk. Kaplan–Meier plot analyses were performed to evaluate differences in fracture risk according to dialysis status, female sex, and age over 65 years. A multivariate Cox proportional hazard regression analysis was then conducted to identify the fracture risk of dialysis patients.
Lastly, Kaplan–Meier analysis was carried out to verify whether there was any difference in the time from dialysis initiation to fracture diagnosis between those who were and were not taking phosphate binders. In addition, multivariate Cox proportional hazard regression analysis was performed to determine the effect of phosphate binders on fracture risk.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, USA).
Results
Baseline characteristics of the study population
Of the 89,533 CKD patients, 76,106 (85.0%) were pre-dialysis CKD patients. Among the 13,427 dialysis patients, 12,098 patients (90.1%) were receiving hemodialysis and 1,329 patients (9.9%) were undergoing peritoneal dialysis treatment. A total of 37,610 (42.0%) of the patients were female, and 51,723 (57.8%) of the study patients were over the age of 65 years. A total of 25,771 (28.8%) patients had between 0 and 5 points on the Charlson Comorbidity Index (CCI) scoring system, 35,840 (40.0%) had between 6 and 8 points, and 27,922 (31.2%) had between 9 and 27 points (Table 1).
Table 1.
Baseline characteristics of the study population
| Variable | Total (n = 89,533) | Pre-dialysis (n = 76,106) | Dialysis (n = 13,427) | P value |
|---|---|---|---|---|
| Age group (yr) | < 0.001 | |||
| < 65 | 37,810 (42.2) | 30,594 (40.2) | 7,216 (53.7) | |
| ≥ 65 | 51,723 (57.8) | 45,512 (59.8) | 6,211 (46.3) | |
| Sex, male | 51,923 (58.0) | 43,925 (57.7) | 7,998 (59.6) | < 0.001 |
| Charlson Comorbidity Index | < 0.001 | |||
| 0–5 | 25,771 (28.8) | 23,140 (30.4) | 2,631 (19.6) | |
| 6–8 | 35,840 (40.0) | 30,382 (39.9) | 5,458 (40.6) | |
| 9–27 | 27,922 (31.2) | 22,584 (29.7) | 5,338 (39.8) | |
| Phosphate binder use | ||||
| Calcium-based | NA | NA | 7,456 (55.5) | |
| Non-calcium-based | NA | NA | 2,462 (18.3) | |
Data are presented as number (%).
NA, not available.
Fractures in patients with CKD according to dialysis, sex, and age
In Supplementary Table 1 (available online), we present the number of fractures per total patient-years in CKD patients divided into dialysis and pre-dialysis groups. Overall, the rate of fractures in pre-dialysis CKD patients was 74 per 1,000 patient-years, while that of dialysis-dependent CKD patients was 84 per 1,000 patient-years. Based on multivariate logistic regression analysis (Table 2), the odds ratio (OR) for total fracture events in dialysis patients was 1.10 (95% confidence interval [CI] 1.05–1.16, P < 0.001) compared to pre-dialysis patients.
Table 2.
Fracture risks in the dialysis group according to the fracture site
| Total | Spine | Hip | Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Pre-dialysis | Reference | Reference | Reference | Reference | ||||||||
| Dialysis | 1.10 | 1.05–1.16 | < 0.001 | 0.98 | 0.90–1.08 | 0.694 | 1.66 | 1.54–1.82 | < 0.001 | 1.04 | 0.99–1.10 | 0.133 |
Odds ratio (OR) and 95% confidence interval (CI) were attained by multivariate logistic regression analysis adjusted for age group and sex.
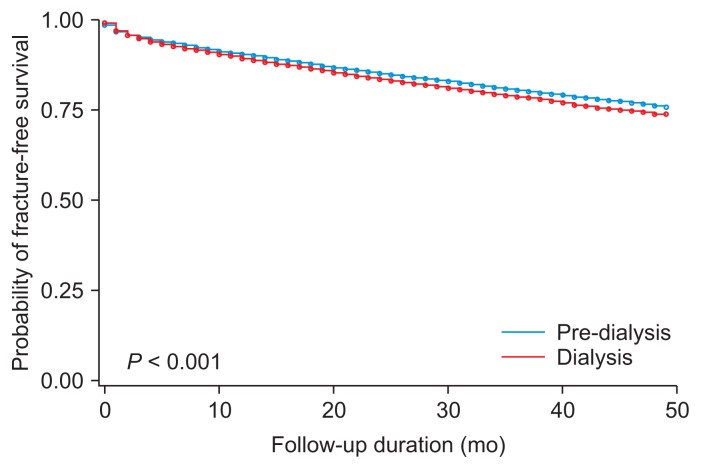
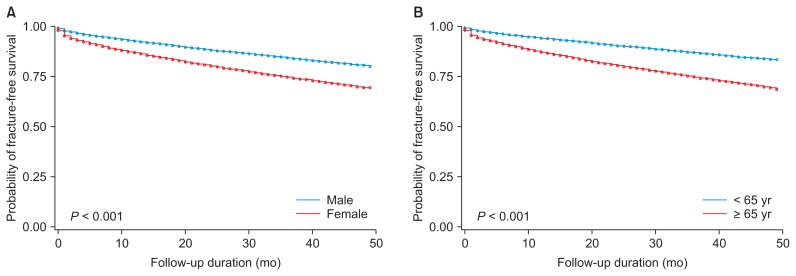
We next compared the risk of fractures using Kaplan–Meier curves depending on dialysis status, gender, and age (cutoff 65 years). Fig. 1 shows that the incidence of fracture was significantly higher in dialysis patients than in pre-dialysis patients. Furthermore, when pre-dialysis CKD and dialysis-dependent ESRD patients were analyzed by gender and age group, the incidence of fractures was significantly higher among females compared to males, and individuals 65 years and older compared to those less than 65 (Fig. 2).
Figure 1.
Kaplan–Meier plot of fracture incidence in pre-dialysis chronic kidney disease and end-stage renal disease patients.
Figure 2.
Kaplan–Meier plot of fracture incidence in patients with pre-dialysis and dialysis chronic kidney disease according to (A) sex and (B) age group.
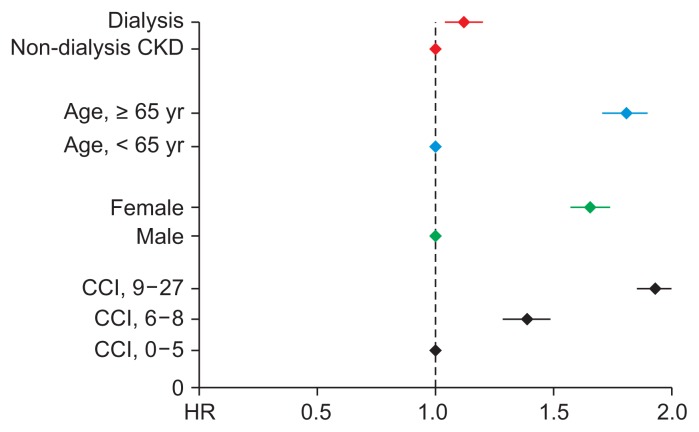
Cox proportional hazard regression analysis was performed to determine the risk of fracture in dialysis patients compared to pre-dialysis patients after adjusting for confounding variables. Univariate analysis showed the hazard ratio (HR) for fracture risk in dialysis patients was 1.11 (95% CI 1.07–1.16, P < 0.001). After adjusting for age, gender, and CCI, the fracture risk in dialysis patients was higher than in pre-dialysis dependent CKD patients (HR 1.16, 95% CI 1.12–1.21, P < 0.001) (Table 3 and Fig. 3).
Table 3.
Cox regression analysis for fracture risk of ESRD patients compared to pre-dialysis CKD patients
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Dialysis | ||||||
| Pre-dialysis CKD | Reference | Reference | ||||
| ESRD | 1.11 | 1.07–1.16 | < 0.001 | 1.16 | 1.12–1.21 | < 0.001 |
| Age (yr) | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 2.07 | 2.01–2.14 | < 0.001 | 1.80 | 1.74–1.86 | < 0.001 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.71 | 1.66–1.76 | < 0.001 | 1.69 | 1.64–1.74 | < 0.001 |
| Charlson Comorbidity Index | ||||||
| 0–5 | Reference | Reference | ||||
| 6–8 | 1.61 | 1.55–1.68 | < 0.001 | 1.41 | 1.35–1.47 | < 0.001 |
| 9–27 | 2.35 | 2.25–2.45 | < 0.001 | 1.92 | 1.84–2.01 | < 0.001 |
CI, confidence interval; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR, hazard ratio.
Figure 3. Fracture risk of end-stage renal disease patients compared to pre-dialysis chronic kidney disease (CKD) patients.
CCI, Charlson Comorbidity Index; HR, hazard ratio.
Fracture risk in dialysis patients according to the use of phosphate binders
We next investigated the incidence of fracture according to the use of phosphate binders in dialysis patients. Of the 13,427 dialysis patients, 9,918 were taking phosphate binders. Among those taking phosphate binders, 1,935 patients (19.5%) experienced fractures. Among the patients who were not taking phosphate binders (n = 3,509), 671 (19.1%) experienced a fracture (Table 4).
Table 4.
Baseline demographic and fracture incidence data according to phosphate binder prescription in end-stage renal disease patients
| Variable | Taking phosphate binders (n = 9,918) | Not taking phosphate binders (n = 3,509) |
|---|---|---|
| Fracture | ||
| Yes | 1,935 (19.5) | 671 (19.1) |
| No | 7,983 (80.5) | 2,838 (80.9) |
| Age (yr) | ||
| < 65 | 5,878 (59.3) | 1,338 (38.1) |
| ≥ 65 | 4,040 (40.7) | 2,171 (61.9) |
| Sex | ||
| Male | 5,945 (59.9) | 2,053 (58.5) |
| Female | 3,973 (40.1) | 1,456 (41.5) |
| Charlson Comorbidity Index | ||
| 0–5 | 1,972 (19.9) | 659 (18.8) |
| 6–8 | 4,090 (41.2) | 1,368 (39.0) |
| 9–27 | 3,856 (38.9) | 1,482 (42.2) |
Data are presented as number (%).
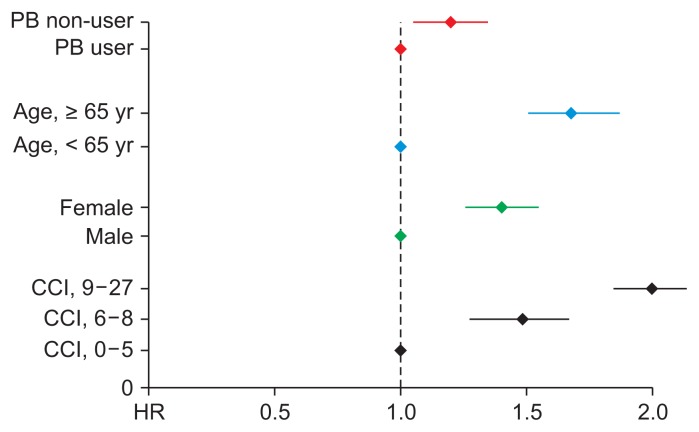
We also investigated fracture risk according to the use of phosphate binders in dialysis patients using Cox proportional hazard regression analysis. The crude HR for sustaining a fracture in the group not taking phosphate binders was 1.33 (95% CI 1.22–1.45, P < 0.001) compared to the group taking phosphate binders. Multivariate analyses to adjust for confounding factors were performed using two models. Model 1 adjusted for age and sex, while model 2 adjusted for the factors used in model 1 as well as CCI. In both models, no use of phosphate binders was significantly associated with increased fracture risk (model 1: HR 1.19, 95% CI 1.09–1.30, P < 0.001; model 2: HR 1.20, 95% CI 1.09–1.31, P < 0.001). The HRs for each factor (dialysis, age, gender, and CCI scores) are described in Fig. 3. In dialysis patients, the risk of fracture in dialysis patients not taking phosphate binders was 20.0% higher compared to patients taking phosphate binders (Table 5 and Fig. 4).
Table 5.
Cox regression analysis for fracture risk in patients who did and did not take phosphate binders in end-stage renal disease
| Variable | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| HR | 95% CI | P value | Model 1 | Model 2 | |||||
|
|
|
||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Phosphate binders | |||||||||
| Users | Reference | Reference | Reference | ||||||
| Non-users | 1.33 | 1.22–1.45 | < 0.001 | 1.19 | 1.09–1.30 | < 0.001 | 1.20 | 1.09–1.31 | < 0.001 |
| Age (yr) | |||||||||
| < 65 | Reference | Reference | Reference | ||||||
| ≥ 65 | 2.04 | 1.89–2.21 | < 0.001 | 1.95 | 1.80–2.11 | < 0.001 | 1.70 | 1.57–1.84 | < 0.001 |
| Sex | |||||||||
| Male | Reference | Reference | Reference | ||||||
| Female | 1.47 | 1.36–1.59 | < 0.001 | 1.39 | 1.29–1.50 | < 0.001 | 1.45 | 1.34–1.56 | < 0.001 |
| Charlson Comorbidity Index | |||||||||
| 0–5 | Reference | Reference | |||||||
| 6–8 | 1.63 | 1.43–1.85 | < 0.001 | 1.51 | 1.33–1.72 | < 0.001 | |||
| 9–27 | 2.43 | 2.15–2.75 | < 0.001 | 2.05 | 1.81–2.33 | < 0.001 | |||
Model 1: phosphate binder prescription, age, sex; model 2: model 1 + Charlson Comorbidity Index.
CI, confidence interval; HR, hazard ratio.
Figure 4. Fracture risk of patients with end-stage renal disease according to use of phosphate binders (PB).
CCI, Charlson Comorbidity Index; HR, hazard ratio.
Discussion
This large-scaled, nationwide, observational study showed that the fracture risk of dialysis patients was 16.0% higher than that of pre-dialysis CKD patients. In addition, dialysis patients who were not taking phosphate binders had a 20.0% higher risk of fracture compared to those who were taking phosphate binders.
Fractures can be a fatal complication in patients with CKD, and these patients have a higher fracture risk for a number of reasons. First, bones of CKD patients are fragile. Increasing age over 65 years as well as increased steroid exposure, alterations in vitamin D metabolism, and parathyroid hormone abnormalities all have an impact on bone quantity and strength in CKD patients. To date, most of the therapeutic modalities aimed at reducing fracture risk have targeted the general population, and not CKD patients. Bisphosphonates, which are representative osteoporosis medications, cannot be prescribed to advanced CKD patients with a creatinine clearance under 30 mL/min/1.73 m2. Therefore, despite a higher prevalence of osteoporosis in patients with advanced CKD and ESRD, they cannot be treated appropriately. Next, ESRD patients are more likely to experience dizziness, and thus have a higher risk of falling. Multiple other factors including anemia, intradialytic hypotension, and autonomic neuropathy due to diabetes, all common manifestations in ESRD patients, can also influence dizziness and falls [9]. Hip fracture, which frequently occurs after a falling accident, is strongly associated with poor clinical outcomes. According to the additional analysis based on fracture site, the dialysis group hip fracture risk was 66% higher than that of the pre-dialysis group (OR 1.66, 95% CI 1.54–1.82, P < 0.001) (Table 2). Therefore, nephrologists need to recognize the higher risk for critical fractures, including hip fracture, among dialysis patients.
Controversy persists as to whether decreased renal function is associated with osteoporosis and increased fracture risk, and the statistical significance of the relationship between renal function and fracture risk varies according to fracture site and study population, especially in patients with mild to moderate renal dysfunction [10–12]. However, the majority of studies that have studied ESRD populations have identified a higher risk of fracture compared to the general population [3,13–15]. The significance of this study was the delineation of a higher risk of fracture in patients with ESRD compared to those with pre-dialysis CKD. This study is also significant for presenting statistics regarding the risk of fracture in a Korean population.
In this study, dialysis patients who were not taking phosphate binders had a higher risk of fracture compared to patients taking phosphate binders. There are several explanations for this finding. First, control of secondary hyperparathyroidism by treating hyperphosphatemia using phosphate binders may, at least in part, contribute to a decreased fracture risk. A reduction in fracture risk in patients taking phosphate binders has not been identified in randomized controlled trials; however, phosphate binders may nevertheless impact fracture risk. For example, one study showed that treatment of secondary hyperparathyroidism with cinacalcet was associated with a reduced risk of fracture (relative risk 0.46, 95% CI 0.22–0.95) [16].
A second explanation for the difference in fracture risk according to phosphate binder treatment is that calcium-based phosphate binders may specifically lead to reduced fracture risk. One study showed a clear association between use of calcium-based phosphate binders and reduced fracture risk in child patients (HR 0.37, 95% CI 0.15–0.91, P = 0.03) [17], which the authors attributed to calcium supplementation by the binding agent. Importantly, calcium is the most deficient nutrient in the Korean diet, with only 78% of males and 67% of females meeting the recommended daily dietary allowance [18]. According to the 2017 Korean ESRD registry report, 60% of hemodialysis patients and 53% of peritoneal dialysis patients are being treated with calcium-based phosphate binders [19]. In this way, calcium supplementation secondary to the use of calcium-based phosphate binders may also contribute to the reduced fracture risk seen in these patients.
Lastly, protein-energy wasting (PEW) in dialysis patients might be associated with differences in fracture risk. PEW refers to decreased protein and energy in the body and is common among ESRD patients [20]. Inadequate intake as well as nonspecific inflammation is a widely known as component of PEW, which leads to muscle wasting in advanced cases. Patients who take small amounts of protein and energy because of PEW may not necessarily take phosphate binders. In other words, patients who do not take phosphate binders because of PEW may have existing fragile bone and muscle, leading to increased risk of fracture.
There were some limitations to our study. First, we did not have data regarding the specific diagnosis of osteoporosis and CKD stages among study patients. In particular, we could not verify the estimated glomerular filtration rate for individual patients because we only used CKD diagnostic codes to extract CKD patients from the Health Insurance Review and Assessment Service Database. As a result, we tended to recruit advanced CKD patients rather than early stage CKD patients. This bias may have underestimated the incidence of CKD and caused sampling error. In addition, the proportion of patients over 65 years was statistically higher in the pre-dialysis CKD patient group than in the dialysis-dependent ESRD patient group (Table 1). However, the elderly generally tend to experience more fractures than the young. This sampling bias may slightly influence our main results inversely, that is, by underestimating fracture risks in ESRD patients. Next, we did not have information about the nutritional status of patients due to the nature of the dataset. However, as discussed above, PEW may be associated with use of phosphate binders. Thus, further research will be necessary to determine whether use of phosphate binders is associated with reduced nutritional status. Fourthly, a phosphate binder user was defined only as a patient prescribed phosphate binders for over 30 days. However, phosphate binders are prescribed off and on based on current phosphate levels. A more detailed definition of phosphate binder user is needed to investigate the accurate impact of phosphate binders on fracture risks, but we did not use such a definition. Lastly, we could not obtain data on phosphate binder type, such as calcium-based phosphate binders versus non-calcium-based phosphate binders. This might have a different impact on bone and fracture risks, but we could not verify the effect according to the type of phosphate binders in this study.
Taken together, the results of this study demonstrated that fracture was more prevalent in dialysis patients compared to pre-dialysis CKD patients, and ESRD patients on dialysis taking phosphate binders experienced less fractures compared to patients not taking phosphate binders. This study confirmed that decreased renal function might be associated with a higher fracture risk, and suggests the possibility of an association between phosphate binders and fracture risk. Additional studies, including randomized controlled trials, will be needed to verify our results.
Supplementary Information
Acknowledgments
This work was supported by Kyowa Hakko Kirin Korea Co., Ltd.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Young Eun Kwon wrote the manuscript. Hyung Yun Choi participated in the study design and performed the statistical analysis. Sol Kim helped to draft the manuscript and provided technical support. Dong-Ryeol Ryu provided intellectual content of critical importance to the work. Hyung Jung Oh participated in the conception, analysis, and interpretation of data. All authors read and approved the final manuscript.
References
- 1.Lee H, Oh K. Prevalence of chronic kidney disease in Korea, 2013. PHWR. 2015;8:242–244. [Google Scholar]
- 2.Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 5.Bringhurst FR, Demay MB, Krane SM, Kronenberg HM. Bone and mineral metabolism in health and disease. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison’s principles of internal medicine. 19th ed. New York: McGraw-Hill Education; 2015. [Google Scholar]
- 6.Cozzolino M, Staniforth ME, Liapis H, et al. Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int. 2003;64:1653–1661. doi: 10.1046/j.1523-1755.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhai CJ, Yu XS, Sun QL, et al. Effect of lanthanum carbonate versus calcium-based phosphate binders in dialysis patients: a meta-analysis. Clin Nephrol. 2014;82:372–378. doi: 10.5414/CN108361. [DOI] [PubMed] [Google Scholar]
- 8.Raggi P, Bommer J, Chertow GM. Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis. 2004;13:134141. [PubMed] [Google Scholar]
- 9.Abdel-Rahman EM, Turgut F, Turkmen K, Balogun RA. Falls in elderly hemodialysis patients. QJM. 2011;104:829–838. doi: 10.1093/qjmed/hcr108. [DOI] [PubMed] [Google Scholar]
- 10.Kaji H, Yamauchi M, Yamaguchi T, Shigematsu T, Sugimoto T. Mild renal dysfunction is a risk factor for a decrease in bone mineral density and vertebral fractures in Japanese postmenopausal women. J Clin Endocrinol Metab. 2010;95:4635–4642. doi: 10.1210/jc.2010-0099. [DOI] [PubMed] [Google Scholar]
- 11.Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22:203–210. doi: 10.1359/jbmr.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167:133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 13.Lin ZZ, Wang JJ, Chung CR, et al. Epidemiology and mortality of hip fracture among patients on dialysis: Taiwan National Cohort Study. Bone. 2014;64:235–239. doi: 10.1016/j.bone.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25:159–165. doi: 10.1007/s00198-013-2435-1. [DOI] [PubMed] [Google Scholar]
- 15.Wakasugi M, Kazama JJ, Taniguchi M, et al. Increased risk of hip fracture among Japanese hemodialysis patients. J Bone Miner Metab. 2013;31:315–321. doi: 10.1007/s00774-012-0411-z. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 17.Denburg MR, Kumar J, Jemielita T, et al. Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J Am Soc Nephrol. 2016;27:543–550. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon S. Intakes of calcium and dairy products in Korea National Health and Nutrition Examination Survey. PHWR. 2013;6:821–827. [Google Scholar]
- 19.ESRD Registry Committee, Korean Society of Nephrology (KSN) Current renal replacement therapy in Korea; Insan Memorial Dialysis Registry, 2017 [Internet] Seoul: KSN; 2018. [cited 2019 Aug 1]. Available from: http://www.ksn.or.kr/rang_board/list.html?code=sinchart_eng. [Google Scholar]
- 20.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.