Abstract
Background
Chronic kidney disease (CKD) is a growing public health concern, and available treatments are insufficient in limiting disease progression. New strategies, including regenerative cell-based therapies, have emerged as therapeutic alternatives. Results from several groups, including our own, have reported evidence of a supportive role for mesenchymal stromal cells (MSCs) in functional recovery and prevention of tissue damage in murine models of CKD. Prompted by these data, an open pilot study was conducted to assess the safety and efficacy of a single injection of autologous adipose tissue-derived MSCs (AT-MSCs) for treatment of CKD.
Methods
AT-MSCs were infused intravenously into six CKD patients at a dose of 1 million cells/kg. Patients were stabilized and followed for one year prior to MSC infusion and one year following infusion.
Results
No patients presented with adverse effects. Statistically significant improvement in urinary protein excretion was observed in AT-MSCs transplanted patients, from a median of 0.75 g/day (range, 0.15–9.57) at baseline to 0.54 g/day (range, 0.01v2.66) at month 12 (P = 0.046). The glomerular filtration rate was not significantly decreased post-infusion of AT-MSCs.
Conclusion
Findings from this pilot study demonstrate that intravenous infusion of autologous expanded AT-MSCs into CKD patients was not associated with adverse effects and could benefit patients already undergoing standard medical treatment.
Keywords: Adipose tissue-derived mesenchymal stromal cells, Chronic kidney disease, Mesenchymal stromal cell transplantation, Proteinuria, Stem cells
Introduction
Chronic kidney disease (CKD) is characterized by a progressive and permanent loss of kidney function. CKD represents a major public health problem due to its growing incidence and prevalence, the high cost of renal substitutive therapies and a marked increase of morbidity and mortality [1,2]. Available pharmacologic agents do not halt disease progression and have significant risks [3], thus prompting the search for new therapeutic strategies [4]. Mesenchymal stromal cells (MSCs) are multipotent stromal cells that reside within the vascular network of vascularized organs and have broad tissue-supporting roles throughout the body [5]. The ability of MSCs to preserve renal structure and function in experimental acute renal injury [6] and also in CKD has been demonstrated in rodent models of diabetic nephropathy [7], partial nephrectomy [8], and allograft nephropathy [9]. Beneficial effects of MSCs have included restriction of the inflammatory response [6], decreases in proteinuria, and preservation of the tubular and interstitial architecture [7]. Of note, whole bone marrow (BM) transplant does not appear to exert similar beneficial effects [10]. CKD has been linked to a loss of stem cell quantity and function, particularly as patients progress to end stage disease [11]. Evidence also suggests that the pro-inflammatory state associated with CKD and uremic toxins, such as homocysteine [12] or p-cresol [13], can hasten stem cell senescence and apoptosis [14]. Hyperparathyroidism and insufficient erythropoietin activity can also contribute to progenitor cell dysfunction [15].
A recent meta-analysis demonstrated that cell-based therapies are effective in preclinical models of CKD [16], and systemic administration of MSCs is considered safe to treat renal and cardiovascular diseases in humans [17,18]. However, clinical translation of MSC-based therapeutics for human CKD is still in an early phase, and the optimal cell source, mode of administration and expected clinical effects are still not well characterized.
MSCs derived from adipose tissue (AT-MSCs) exhibit differentiation potential, kinetics, cell senescence, gene transduction efficiency and multi-lineage differentiation capacity similar to BM-derived MSCs (BM-MSCs) [19]. Since BM samples require invasive procedures and yield fewer MSCs, AT-MSCs have been tested in different experimental and clinical scenarios. Indeed, we previously evaluated the use of AT-MSCs in a murine model of CKD and reported marked recovery in renal function and disease biomarkers [20]. We thus hypothesized that the injection of autologous ex vivo expanded AT-MSCs could exert positive functional effects in CKD patients with moderately advanced disease. Although the inclusion of a parallel control group is the desirable approach for a clinical trial, the purpose of this study was to acquire preliminary data that could inform the design of a future randomized, placebo-controlled, prospective trial.
Methods
The National Health System Review Board located at the Servicio de Salud Metropolitano Oriente in Santiago, Chile, approved the protocol and the informed consent form, which was signed by each patient prior to any intervention. The study was carried out in accordance with good clinical practice (GCP) guidelines, the Declaration of Helsinki and the rules of the International Society for Stem Cell Research (ISSCR) contained in the Guidelines for the Clinical Translation of Stem Cells, published in December 2008.
Patients, inclusion/exclusion criteria
Given the scarcity of data on the effect of AT-MSCs in CKD, we collected clinical data that might inform the design of a future trial. Therefore, CKD patients (n = 7) were enrolled for treatment with MSCs, using the following inclusion criteria.
CKD with an estimated glomerular filtration rate (eGFR) between 20 and 40 mL/min/1.73 m2 using the Modification of Diet in Kidney Disease (MDRD) formula, daily proteinuria > 150 mg, and blood pressure < 140/90 mmHg with or without antihypertensive medications, at the recruitment visit. Diabetic patients were required to have a glycated hemoglobin ≤ 7.5%.
Clinical and laboratory evidence of progressive disease in the twelve months prior to the recruitment date.
No other significant co-morbidity or condition that could affect the clinical disease course. These exclusion criteria included: active cancer or immunosuppressive treatments; women intending to be pregnant and/or not on effective contraception; and breast-feeding women. Additionally, patients could not have planned elective surgical procedures or significant allergies reported.
All were receiving evidence based optimized stable pharmacological treatment for at least 12 months prior to recruitment, including dietary restricted salt (≤ 2 g/day of sodium) and protein (0.8 g/day) and renin angiotensin axis blockade (enalapril ≤ 40 mg/day or losartan ≤ 100 mg/day) with the addition of furosemide, nitrendipine, atenolol or doxazosin as needed to achieve blood pressure control (< 140/90 mmHg). Interventions were not changed (drugs and dosage) during the follow-up period.
Primary end point
Change in CKD functional parameters, including the GFR and quantitative 24-hour urinary protein excretion rate in the 12-month period following MSC infusion. Because of the pilot nature of this study and the small sample size with no control group, variables were measured during the 12 months prior to treatment (control period) and compared to measurements taken during the 12 months following MSC administration (intervention period).
Secondary endpoints
Clinical or biochemical changes suggestive of treatment-associated adverse events or warnings as described below.
Clinical procedures
Adipose tissue harvest: Adipose tissue (20–25 g) was aspirated from the abdominal subcutaneous fat pad from all patients by a single plastic surgeon, using a 19-G bore needle attached to a standard plastic syringe under local anesthesia.
MSC isolation and in vitro expansion
Each autologous adipose tissue sample was suspended in sterile phosphate-buffered saline (PBS), passed through a 70-μm Falcon cell strainer (BD Biosciences, San Jose, USA) and centrifuged at 350 × g for 10 minutes. The MSC isolation and in vitro expansion were performed as described previously [19]. After three passages, MSCs were characterized for adipogenic, chondrogenic and osteogenic tri-differentiation.
MSC characterization
Immuno-phenotyping of MSCs
MSCs were immune-phenotyped by flow cytometry using a FACSCanto II cytometer (BD Biosciences) after staining with the following anti-human monoclonal antibodies: CD105, CD90, CD73, HLA-ABC, HLA-DR, CD34 and CD45 (all from BD Pharmingen, San Jose, USA). In brief, cells were harvested, washed with cytometer buffer (PBS + 0.2% bovine serum albumin + 0.01% sodium azide; all from Sigma-Aldrich, St. Louis, USA) and incubated with the fluorescently-labeled antibodies in cytometer buffer for 20 minutes at 4°C. Matched isotype antibodies were used as negative controls.
Differentiation
To induce adipogenic differentiation, confluent cells were cultured in medium supplemented with 1 × 10−6 M dexamethasone, 0.02 mg/mL indomethacin and 10 μg/ mL insulin (Sigma-Aldrich). After 12 days, cell differentiation into lipid-laden adipocytes was confirmed by oil red O staining (Sigma-Aldrich). For chondrogenic differentiation, cells were incubated for 1 hour at 5 × 103 cells/ μL in 10 μL of culture medium to achieve conditions for micromass formation. Cells were cultured in medium supplemented with 107 M dexamethasone, 50 μg/mL ascorbic acid, and 10 ng/mL of transforming growth factor-β3 (Sigma-Aldrich) for 7 days, and chondrogenic differentiation was assessed by Safranin O staining (Merck, Darmstadt, Germany). To induce osteogenic differentiation, adherent cells were grown at 3 × 104 cells/cm2 in culture medium with 10−7 M dexamethasone, 50 g/mL ascorbic acid, and 10 mM β-glycerophosphate (Sigma-Aldrich). After 21 days of culture, calcium deposits were detected by Alizarin Red staining (Sigma-Aldrich).
Release criteria
The criteria for MSC batch release included: > 90% of cells expressing CD105, CD73, and CD90, and < 3% of cells expressing CD34, CD45, and CD14; negative microbiology testing for mycoplasma, aerobic bacteria, anaerobic bacteria and fungi; and viability > 85% by trypan blue. Freshly harvested cells were resuspended in a 1:1 mixture of Ringer’s lactate and autologous patient serum and transported in a cooled container to the administration site.
Cell infusion
Patients were transiently hospitalized to receive 1 million AT-MSCs per kg of body weight suspended in 120 mL Ringer’s lactate solution. Infusions were done into a peripheral vein over 30 to 40 minutes. Asymptomatic patients were discharged after 2 hours of observation. Given the ample evidence from controlled trials of the safety of MSCs administered intravenously (i.v.) at much higher doses [18], we did not screen regularly for ischemic events.
Follow-up
Patients were instructed to communicate with the clinician in charge in the case of an adverse event. Vital signs were measured and physical examinations were performed at monthly follow-up appointments. Additional follow-up evaluations included the assessment of serum creatinine, 24 hour urinary quantitative protein excretion rate, additional blood and urinary testing, and screening for solid organ or coagulation abnormalities, including transaminases and prothrombin or active thromboplastin times. Collection of adverse events was done in accordance with US Food and Drug Administration 21 312.32 Code of Federal regulations and International Conference of Harmonization ICH E-6 Good Clinical Practices. Given the two-yearly periods of follow-up, the adverse event type and frequency were retrieved as follows; All adverse events occurring between MSC infusion and the monthly follow-up visit (month 1). All further events that were not attributable to CKD were judged by treating physicians and were reported as adverse effects either related to the MSC treatment or not related, according to GCP rules.
Statistical analysis
Based on the limited sample size and unknown distribution, we employed non-parametric analyses and Wilcoxon or Mann–Whitney tests. We also computed the slopes of the linear regression equations for the change in eGFRs of each individual patient, spanning the four quarterly measurements from the 12 months prior to treatment and the 12 monthly measurements taken after the MSC infusion. The percent change was calculated as difference for each variable from pre- to post-treatment divided by pre-treatment level multiplied by 100. The P values less than 0.05 were considered statistically significant.
Results
Patients
Seven patients with stage III–IV CKD and no prior dialysis therapy were recruited between January 2013 and August 2014. Specific diagnoses were 1) focal and segmental glomerulosclerosis (FSGS), 2) immunoglobulin A (IgA) nephropathy, 3) diabetic nephropathy, 4) post-acute kidney (AKI) injury RIFLE stage L, 5) Tubulo-interstitial chronic nephritis due to Sjögren’s disease, 6) renal dysplasia, and 7) hypertensive nephrosclerosis. Patient clinical characteristics are shown in Table 1. One patient with diabetic nephropathy was excluded from the study due to poor proliferation and a resulting low number of AT-MSCs in culture.
Table 1.
Baseline clinical characteristics of patients and the number mesenchymal cells infused
| Patient No. | Age (yr) | Sex | CKD diagnosis | HTN | Serum creatinine (mg/dL) | Patient weight (kg) | Number of cells infused (× 106) |
|---|---|---|---|---|---|---|---|
| 1 | 22 | Male | FSGS | Yes | 3.30 | 68 | 40.0 |
| 2 | 58 | Male | CKD post-AKI | Yes | 2.18 | 98 | 98.5 |
| 3 | 36 | Female | Hypertensive nephrosclerosis | Yes | 1.83 | 75 | 72.5 |
| 4 | 59 | Female | Renal dysplasia | Yes | 1.49 | 72 | 72.0 |
| 5 | 42 | Male | IgA nephropathy | No | 2.42 | 65 | 65.0 |
| 6 | 36 | Female | Sjögren-associated chronic tubulointerstitial nephritis | No | 2.07 | 63 | 65.0 |
AKI, acute kidney injury; CKD, chronic kidney disease; FSGS, focal segmental glomerulosclerosis; HTN, hypertension; IgA, immunoglobulin A.
All patients were on stable renin angiotensin blockade during the study period, as described in the inclusion criteria. While this achieved blood pressures < 140/90 mmHg in four cases, two patients required the addition of 40 mg/day nitrendipine to reach goal blood pressure. Patients reported not fully adhering to dietary protein restriction; nevertheless, four patients decreased (1–4 kg) and two increased in body weight (2–4 kg) in the year following AT-MSC infusion. Only the nephrotic patient with FSGS had low serum albumin (< 3.5 g/dL).
Cell characterization
The AT-MSCs isolated from 6 patients that completed study procedures proliferated normally and exhibited typical tri-lineage differentiation into adipocyte, chondrocyte and osteoblast lineages (Fig. 1A–F). As described, AT-MSCs expressed CD105, CD73, and CD90 (Fig. 1G) and were negative for CD45, CD34, CD14, and HLA-DR (data not shown). There was no difference in the expression of these markers between CKD and control AT-MSCs derived from a healthy individual (Fig. 1G).
Figure 1. AT-MSCs from CKD patients exhibit normal expression of stem cell phenotypic markers and multilineage capacities.
Differentiation potential of AT-MSCs was assessed for each patient. Adipose differentiation was characterized by the formation of lipid droplets that were positive on oil red O staining (A) compared to a control without differentiation media (B). Chondrogenic differentiation was confirmed after 7 culture days by safranin O staining (C) compared to a control without differentiation media (D). Osteogenic differentiation was confirmed after 21 culture days by alizarin red staining (E) compared to a control without differentiation media (F). Scale bar, 250 μm. (G) To assess immunophenotype, AT-MSCs from each patient and age/sex matched healthy donor cells were stained by fluorescent-conjugated antibodies against mesenchymal and hematopoietic stem cell markers and analyzed by flow cytometry. CKD AT-MSCs (red-filled histogram) and control cells (green-filled histogram) displayed positive expression for mesenchymal stromal cell markers (CD73, CD105, CD90) and were negative for other markers such as CD14, CD34, CD45 (data not shown). Isotype-matched controls are depicted as blue-filled histograms. Representative plots are shown in (G).
AT-MSCs, adipose tissue-derived mesenchymal stromal cells; CKD, chronic kidney disease.
Infusion safety and patient follow-up
All infusions were well-tolerated, and no treatment-related adverse events were reported. To assess disease progression, patients were stabilized with optimization and stabilization of therapy 12 months prior to study entry and then followed for one year. Individual clinical courses are summarized in Supplementary Table 1 (available online).
Proteinuria measurements
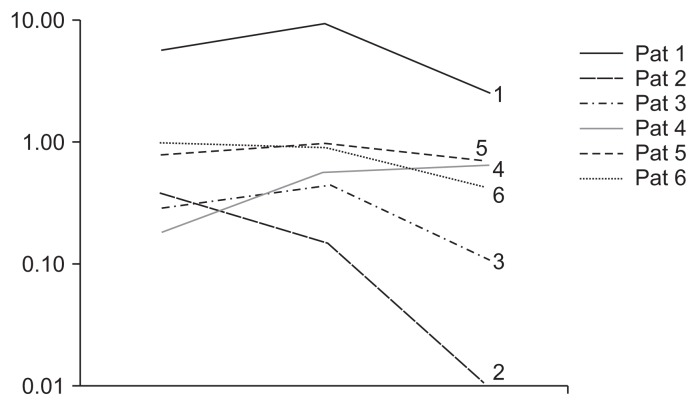
Over the 12 months prior to treatment, 4 of 6 patients demonstrated increased protein excretion (ranging from 20% to 205% increase). In contrast, in the 12 months after MSC infusion, a reduction of proteinuria occurred in all cases, with the exception of patient 4 with renal dysplasia, falling from a baseline median of 0.75 g/day (range, 0.15–9.57) to 0.54 (range, 0.01–2.66 g/day) at month 12 after infusion (P = 0.046) (Fig. 2 and Supplementary Table 1). One patient demonstrated a decrease of 10% and one demonstrated a decrease of 60% decrease post-infusion. Of note, although absolute levels of protein excretion did not decline in patient 4 after MSC treatment, the percent increase in proteinuria observed over the 12 months following MSC infusion was only 12% while it had been 205% in the 12 months prior to infusion, suggesting improvement.
Figure 2. Proteinuria before and after AT-MSC administration.
To assess changes in disease progression, patients (Pat) were stabilized with optimization and stabilization of therapy for 12 months prior to entry to the protocol and followed for one year after AT-MSC administration. A reduction in proteinuria occurred in all cases, with the exception of patient 4, from a median of 0.75 g/day (range, 0.15–9.57) at baseline to a median of 0.54 (range, 0.01–2.66 g/ day) at month 12 after infusion (P = 0.046). For visual simplification of curves only the main three time points are depicted (12 months prior to treatment, time of infusion, 12 months post-treatment).
AT-MSCs, adipose tissue-derived mesenchymal stromal cells.
Creatinine and MDRD eGFR
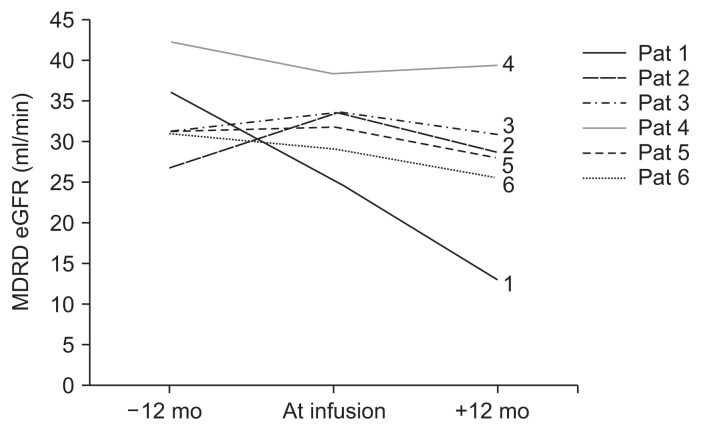
Overall, renal function as assessed by plasma creatinine levels and MDRD eGFR was decreased at 12 months following MSC infusion, although this decrease was not statistically significant (P = 0.065). All cases, with the exception of patient 4, showed a slight percent decrease in the eGFR ranging from 7.5% to 49.0%. Therefore, to analyze individual cases, we compared the slope of eGFR changes over the four quarterly visits in the year prior to infusion with the slope of eGFR changes over the twelve monthly visits following MSC infusion. The calculated eGFR slope, from the regression analysis for each patient, decreased from a median of −0.0049 mL/min/year (range, −0.6237 to 0.5528) to −0.3919 mL/min/year (range, −1.1075 to −0.0926), but this decrease was not significant (Wilcoxon paired test, P = 0.075). Interestingly, while it is difficult to reach conclusions with such a small, heterogeneous group of cases, the individuals demonstrating eGFR stabilization (patients 2, 3, 4; Fig. 3) were those with less inflammatory CKD types such as hypertensive nephrosclerosis, post-AKI CKD and renal dysplasia. This suggests that MSCs exert a range of effects that are not necessarily restricted to anti-inflammatory mechanisms.
Figure 3. Glomerular filtration rate (GFR) before and after AT-MSC administration.
Renal function estimated by plasma creatinine and MDRD estimated GFR (eGFR) at 12 months before and after the injection of AT-MSCs. All individual cases, with the exception of patient 4, demonstrated a slight percent decrease of the MDRD eGFR, ranging from 7.5% to 49%. The eGFR slopes from the regression analysis of each patient prior to therapy and after therapy were not significantly different. For visual simplification of curves only the main three time points are depicted (12 months prior to treatment, time of infusion, 12 months post-treatment). AT-MSCs, adipose tissue-derived mesenchymal stromal cells; MDRD, Modification of Diet in Kidney Disease.
The laboratory measurements regarding patient safety and measurements including coagulation (prothrombin and thromboplastin clotting times and platelet number), liver function (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatases, gama glutamil transpeptidase, total bilirubin, direct bilirubin, and serum albumin) and metabolic tests (glycaemia, uric acid, total cholesterol, high-density lipoprotein and low density lipoprotein cholesterol, and triglycerides) did not change between pre- and post-infusion periods (data not shown).
Discussion
Although not all six patients responded equally, these results indicate that autologous AT-MSC infusion reduced 24-hour proteinuria without recognizable adverse effects in six patients. However, this small and heterogeneous sample precludes wider generalization of these findings.
There is increasing evidence regarding the possible mechanisms of cell therapy in CKD. MSCs are capable of self-renewal, proliferation and migration to damaged areas, where they can differentiate into mature and functional cells [16]. Because low levels of persistent tissue engraftment can occur after i.v. MSC infusion [21], it is believed that therapeutic benefits are explained mainly by trophic and/or immune mediated effects [6,8]. Immunoactivity of MSCs is mediated by both secreted molecules and cell-cell contact and can involve dendritic cells, B cells, natural killer cells, and a variety of T helper cells [22]. Significant paracrine and trophic effects are mediated by the secretion of angiogenic and antiapoptotic factors such as vascular endothelial growth factor, insulin growth factor, and hepatocyte growth factor [23,24]. The broad repertoire of secreted and immunomodulatory factors produced by MSCs has potential to contribute to kidney regeneration, as MSC-conditioned culture media can confer renoprotective benefits comparable to those of direct cells [25]. Additional effects are mediated partially through microvesicles containing different protein and RNA cargo [26], and more recent data have suggested that stromal cell administration can rescue damaged cells even in the absence of MSC persistence in tissues and improve tissue regeneration through the transfer of active mitochondria [27]. In animal CKD models, such as 5/6 nephrectomy remnant kidney [28], diabetic nephropathy [7], and unilateral ureteral obstruction [29], MSC infusion has been associated with reduction in pro-fibrotic markers as well as with a decrease in inflammation, as evidenced by a decrease in interleukin (IL)-6 and tumor necrosis factor-α levels and heightened levels of the anti-inflammatory cytokines IL-4 and IL-10 [28,24,30]. Conditioned media from MSC cultures can also induce the migration and proliferation of kidney-derived epithelial cells [26].
Clinical trials recently demonstrated no safety signals in CKD patients treated with BM-MSCs [31–33]. In a single-arm phase I study of 6 patients with autosomal dominant polycystic kidney disease treated intravenously with autologous BM-MSCs (2 × 106/kg), there were no changes in eGFR or reductions in serum creatinine at 12 months compared to baseline [32]. This might be explained by evidence that suggests autologous BM-MSCs are defective in CKD individuals [13,15]. In contrast, Packham et al [31] published a report of a placebo controlled dose escalation study of allogeneic immunoselected BM-MSCs in a population of 35 diabetic kidney disease patients, randomized (2:1) with 150 × 106 MSCs, 300 × 106 MSCs, or placebo [31]. No adverse events or alloantibodies were detected. Additionally, they reported that median levels of IL-6 decreased, and there was a suggestion of a more pronounced treatment effect within the subgroup with higher baseline eGFR that received 150 × 106 cells. Urinary protein and albumin excretion remained unchanged [31].
Although AT-MSCs also have a favorable safety profile overall [34], adverse events have been reported, and renal deterioration has occurred with i.v. administration of extremely high doses [35]. The data we report here suggest that systemic AT-MSC infusion is feasible, safe, and reduces protein excretion in CKD patients. The observed reduction in proteinuria after AT-MSC infusion, which occurred in 5 of the 6 patients studied, is promising and contrasts with prior clinical reports [31,32]. In most ne-phropathies, proteinuria is a validated surrogate marker for progression of disease. Indeed, protective renin-angiotensin axis blockers that decrease proteinuria are titrated until protein excretion reaches less than 300 mg daily or adverse effects become intolerable [36].
It remains unclear at what level of GFR an individual may benefit most from cell therapy aimed at slowing CKD progression. Such a “therapeutic window” has been explored in clinical nephrology trials, including the RE-NAAL study of diabetic nephropathy [37] and fish oil for IgA nephropathy [38]. A well-controlled trial in diabetic kidney disease suggests individuals with major deterioration of their eGFR could be less responsive [31]. We chose to study patients with an eGFR ranging from 20 to 40 mL/ minute to avoid including individuals with end stage disease while still including patients at risk for deterioration. There are several evident limitations of our study. First, it is not a placebo-controlled trial, but rather an exploratory study comparing prolonged (12 month) pre- and post-treatment periods in CKD patients that underwent AT-MSC treatment. We utilized autologous AT-MSCs mainly for security reasons, but as suggested by trial results reported by Packham et al [31] and the increasing use of umbilical cord-derived MSCs [39], allogeneic cells could be explored. The main strength of this study is that it informs the safety and feasibility of a future controlled trial, which could assess additional serum and urinary biomarkers and morphological measurements on kidney biopsy samples that we were unable to assess.
In conclusion, we report an initial pilot study providing evidence that it is possible to generate autologous AT-MSCs for cell therapy in CKD patients and that the infusion treatments did not cause adverse events in the year following treatment. AT-MSCs demonstrated the potential to modify the progression of CKD, as the treatment reduced urinary protein excretion, which is a well-known surrogate marker of kidney disease progression. Such promising preliminary results justify designing and conducting future clinical trials that further evaluate the clinical response and molecular mechanisms of AT-MSC-based treatment of CKD and assess characteristics of responder patients to inform the best future candidates for treatment.
Supplementary Information
Acknowledgments
This work was supported by Cells for Cells (C4C).
Footnotes
Conflicts of interest
Valentina López G and Jorge Bartolucci received stipends from Cells for Cells. M. Khoury is the CSO of Cells for Cells and Consorcio Regenero. The other authors have no conflicts to report.
Authors’ contributions
Sandra Villanueva, Fernando González, Juan E. Carreño, Jorge Bartolucci, Fernando E. Figueroa, and Maroun Khoury participated in the study design. Fernando González performed the statistical analysis. Maroun Khoury, Fernando E. Figueroa, Eduardo Lorca, and Ricardo Valjalo participated in the conception, analysis, and interpretation of data. Valentina López, Manuel Lecanda, Rocío Strodthoff, and Francisca Fajre provided intellectual content of critical importance to the work and technical support. Andrés Tapia and César Vergar participated in the study coordination. Fernando González, Eduardo Lorca, and Ricardo Valjalo participated in patient selection and care. All authors read and approved the final manuscript.
References
- 1.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 2.Shishehbor MH, Oliveira LP, Lauer MS, et al. Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol. 2008;101:1741–1746. doi: 10.1016/j.amjcard.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;3:CD007004. doi: 10.1002/14651858.CD007004.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Alexandre CS, Volpini RA, Shimizu MH, et al. Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27:682–692. doi: 10.1634/stemcells.2008-0496. [DOI] [PubMed] [Google Scholar]
- 5.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 6.Semedo P, Palasio CG, Oliveira CD, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–682. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 9.Franquesa M, Herrero E, Torras J, et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. doi: 10.1089/scd.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerbini G, Piemonti L, Maestroni A, Dell’Antonio G, Bianchi G. Stem cells and the kidney: a new therapeutic tool? J Am Soc Nephrol. 2006;17(4 Suppl 2):S123–S126. doi: 10.1681/ASN.2005121339. [DOI] [PubMed] [Google Scholar]
- 11.de Groot K, Bahlmann FH, Sowa J, et al. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66:641–646. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhu JH, Chen JZ, Wang XX, Xie XD, Sun J, Zhang FR. Homocysteine accelerates senescence and reduces proliferation of endothelial progenitor cells. J Mol Cell Cardiol. 2006;40:648–652. doi: 10.1016/j.yjmcc.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 15.van Koppen A, Joles JA, Bongartz LG, et al. Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant. 2012;21:2299–2312. doi: 10.3727/096368912X636795. [DOI] [PubMed] [Google Scholar]
- 16.Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281–293. doi: 10.1242/dmm.017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi D, Wang D, Li X, et al. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 2012;31:841–846. doi: 10.1007/s10067-012-1943-2. [DOI] [PubMed] [Google Scholar]
- 18.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva S, Carreño JE, Salazar L, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond) 2013;125:199–210. doi: 10.1042/CS20120644. [DOI] [PubMed] [Google Scholar]
- 21.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 22.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 23.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Jiang H, Feng JM. Isogenic mesenchymal stem cells transplantation improves a rat model of chronic aristolochic acid nephropathy via upregulation of hepatic growth factor and downregulation of transforming growth factor β1. Mol Cell Biochem. 2012;368:137–145. doi: 10.1007/s11010-012-1352-5. [DOI] [PubMed] [Google Scholar]
- 25.Moghadasali R, Mutsaers HA, Azarnia M, et al. Mesenchymal stem cell-conditioned medium accelerates regeneration of human renal proximal tubule epithelial cells after gentamicin toxicity. Exp Toxicol Pathol. 2013;65:595–600. doi: 10.1016/j.etp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Dorronsoro A, Robbins PD. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. 2013;4:39. doi: 10.1186/scrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad T, Mukherjee S, Pattnaik B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanueva S, Ewertz E, Carrión F, et al. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond) 2011;121:489–499. doi: 10.1042/CS20110108. [DOI] [PubMed] [Google Scholar]
- 29.da Silva AF, Silva K, Reis LA, Teixeira VP, Schor N. Bone marrow-derived mesenchymal stem cells and their conditioned medium attenuate fibrosis in an irreversible model of unilateral ureteral obstruction. Cell Transplant. 2015;24:2657–2666. doi: 10.3727/096368915X687534. [DOI] [PubMed] [Google Scholar]
- 30.Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 31.Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–269. doi: 10.1016/j.ebiom.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makhlough A, Shekarchian S, Moghadasali R, et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. doi: 10.1186/s13287-017-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makhlough A, Shekarchian S, Moghadasali R, et al. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy. 2018;20:660–669. doi: 10.1016/j.jcyt.2018.02.368. [DOI] [PubMed] [Google Scholar]
- 34.Toyserkani NM, Jørgensen MG, Tabatabaeifar S, Jensen CH, Sheikh SP, Sørensen JA. Concise review: a safety assessment of adipose-derived cell therapy in clinical trials: a systematic review of reported adverse events. Stem Cells Transl Med. 2017;6:1786–1794. doi: 10.1002/sctm.17-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JS, Lee JH, Kwon O, et al. Rapid deterioration of pre-existing renal insufficiency after autologous mesenchymal stem cell therapy. Kidney Res Clin Pract. 2017;36:200–204. doi: 10.23876/j.krcp.2017.36.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito T, Ma LJ, Yang H, et al. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298:F683–F691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 38.Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 39.Bartolucci J, Verdugo FJ, González PL, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.