Escherichia fergusonii is a rare pathogen that has been isolated from the intestinal contents of warm-blooded animals [1]. It is closely related to Escherichia coli and causes infection of both wounds and the biliary and urinary tracts in humans [2]. Thrombotic microangiopathy includes thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), which share a similar pathogenicity. Neurological and renal complications significantly affect patient outcomes in these diseases.
A few case reports regarding infections caused by E. fergusonii are found in the literature. However, thrombotic microangiopathy associated with E. fergusonii bacteremia has not been reported previously. We present a case of a patient with E. fergusonii bacteremia and severe acute kidney injury. The condition of the patient was complicated by HUS, and the patient was advised to undergo dialysis.
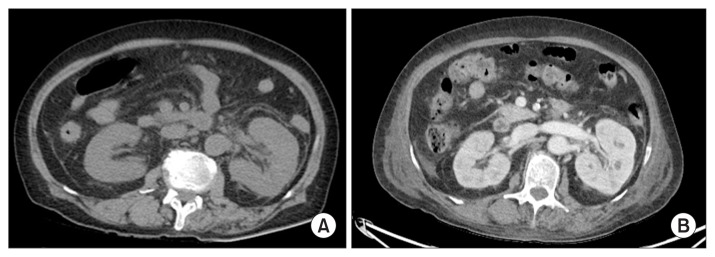
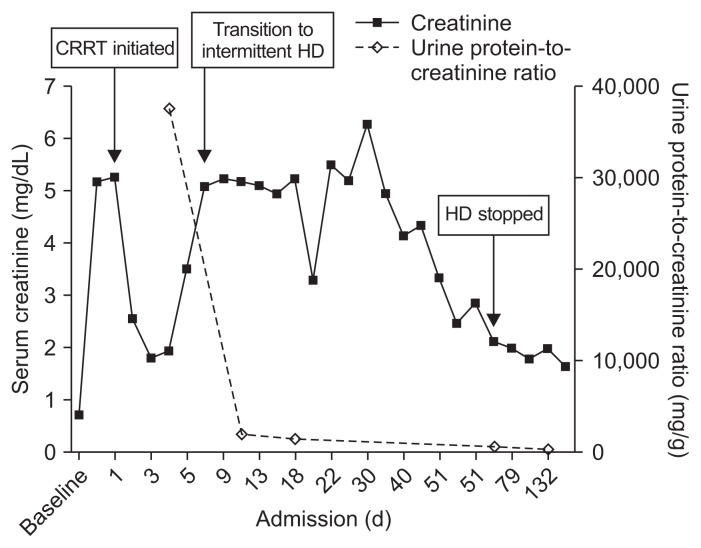
A 76-year-old female with a history of diabetes and stroke presented with general weakness for several days. On admission, her temperature was 36.2°C and her blood pressure was 147/80 mmHg. Physical examination showed pretibial pitting edema. Urinary bladder catheterization yielded purulent urine. The laboratory findings included the following: white blood cell (WBC) count 14,410/μL, hemoglobin 8.9 g/dL, platelet count 14,000/μL, C-reactive protein 22.8 mg/dL, blood urea nitrogen 92 mg/dL, creatinine 5.14 mg/dL, lactate dehydrogenase 282 U/L, prothrombin time 1.58 (normal, 0.9–1.08 international normalised ratio), and activated partial thromboplastin time 111 seconds (normal, 81–125 seconds). Urinalysis revealed the urine WBC count as > 100/high power field (HPF) and the red blood cell count as 31 to 50/HPF (dysmorphic red blood cell 85%) along with the presence of bacteria. The spot urine protein-to-creatinine ratio was 37,361 mg/g. Urine immunofixation was unremarkable. Chest radiography showed pulmonary edema, and non-enhanced computed tomography showed a swelling of the left kidney, indicating acute pyelonephritis (Fig. 1). A peripheral blood smear revealed anisopoikilocytosis. The plasma free hemoglobin was 25 mg/dL (normal, 0–14 mg/dL), and the Coombs test was negative. Autoantibodies, including antinuclear antibody, anti-neutrophil cytoplasmic antibody, and anti-glomerular basement membrane antibody, were absent. The levels of complement factors C3 and C4 were 73.77 mg/dL (normal, 90–180 mg/dL) and 18.10 mg/dL (normal, 10–40 mg/dL), respectively. The patient was administered ceftriaxone (intravenous 2 g daily) for a suspected urinary tract infection. On day 2, the patient developed progressive azotemia (creatinine 5.25 mg/dL), oliguria (5 mL/hour), and hypotension (80/50 mmHg), so continuous renal replacement therapy with a vasopressor infusion was initiated. Secondary HUS was diagnosed based on laboratory results showing micro-angiopathic hemolytic anemia, marked thrombocytopenia, and acute kidney injury. On day 3, two sets of blood and urine cultures yielded E. fergusonii which was susceptible to all antibiotics tested except fluoroquinolones. The E. fergusonii isolate was negative for both O157 serotyping and Shiga toxin. The patient recovered and was discharged on day 26. Hemodialysis was discontinued two months after discharge from the hospital. Her last follow-up laboratory examination showed the level of creatinine and the ratio of urine protein-to-creatinine as 1.79 mg/dL and 480.7 mg/g, respectively (Fig. 2).
Figure 1. Abdominal computed tomography (CT) findings.
(A) At admission, a non-enhanced CT showed swelling of the left kidney with perirenal fat stranding and renal fascial thickening, suggestive of acute pyelonephritis. (B) Follow-up enhanced CT on day 6 showed multiple small abscesses in the left kidney.
Figure 2. Courses of serum creatinine and urine protein-to-creatinine ratio.
CRRT, continuous renal replacement therapy; HD, hemodialysis.
In the present case, we believe that the urinary tract infection by E. fergusonii triggered secondary HUS. Thrombotic microangiopathy was a shared finding with TTP. Although the cleavage of von Willebrand factor by ADAMTS13 was not evaluated in our case, a lack of neurological deterioration, positive blood culture, and recovery without plasmapheresis were more indicative of HUS than TTP.
HUS is characterized by intravascular hemolysis, thrombocytopenia, and acute kidney injury [3,4]. Shiga toxin-producing E. coli (STEC) infections predominantly caused by serotype O157:H7 or O104:H4 are the most frequent and common causes of the syndrome. Inborn mutations in complement proteins or molecules not directly linked to the complement system are also associated with atypical HUS [3]. Drugs, cancer, and sepsis can also cause excessive activation of the complement system, leading to secondary HUS [3]. Unlike the current scenario, in TTP, several courses of anti-complement therapy might be needed to prevent disease recurrence and renal progression.
E. fergusonii is an unusual human pathogen [1]. Infections in wounds, the bloodstream, urinary tract, biliary tract, and pleura caused by E. fergusonii have been reported [2,5]. While E. fergusonii is closely related to E. coli, its ability to ferment adonitol, but not lactose, differentiates the two species [1]. Previously, the species which belonged to the Enterobacteriaceae family, but could not be classified under the serotype O157 and were associated with HUS, were of emerging clinical importance [6,7]. Although molecular serotyping was unavailable in the present case, the presence of the O157 antigen was ruled out using the latex reagent. An earlier report describing the presence of O157 somatic antigen in an isolate of E. fergusonii suggested that genetic transfer might be one of the probable reasons for this phenomenon [8]. Previously, multidrug-resistant E. fergusonii was reported in a patient with acute cystitis [2]. In our patient, the minimum inhibitory concentration showed resistance to fluoroquinolones, but it was sensitive to cephalosporins without evidence of extended-spectrum beta-lactamase production. Therefore, the patient in our case was successfully treated with ceftriaxone.
In conclusion, the present case demonstrated E. fergusonii bacteremia causing complicated HUS and acute kidney injury in a patient with a urinary tract infection. Although the serotype O157 was not detected, a similar pathogenicity as STEC infection might be responsible for the development of HUS.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Hariharan H, López A, Conboy G, Coles M, Muirhead T. Isolation of Escherichia fergusonii from the feces and internal organs of a goat with diarrhea. Can Vet J. 2007;48:630–631. [PMC free article] [PubMed] [Google Scholar]
- 2.Savini V, Catavitello C, Talia M, et al. Multidrug-resistant Escherichia fergusonii: a case of acute cystitis. J Clin Microbiol. 2008;46:1551–1552. doi: 10.1128/JCM.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jokiranta TS. HUS and atypical HUS. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trachtman H. HUS and TTP in children. Pediatr Clin North Am. 2013;60:1513–1526. doi: 10.1016/j.pcl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahapatra A, Mahapatra S, Mahapatra A. Escherichia fergusonii: an emerging pathogen in South Orissa. Indian J Med Microbiol. 2005;23:204. doi: 10.4103/0255-0857.16598. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KE, Thorpe CM, Sears CL. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin Infect Dis. 2006;43:1587–1595. doi: 10.1086/509573. [DOI] [PubMed] [Google Scholar]
- 7.Mariani-Kurkdjian P, Lemaître C, Bidet P, et al. Haemolyticuraemic syndrome with bacteraemia caused by a new hybrid Escherichia coli pathotype. New Microbes New Infect. 2014;2:127–131. doi: 10.1002/nmi2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fegan N, Barlow RS, Gobius KS. Escherichia coli O157 somatic antigen is present in an isolate of E. fergusonii. Curr Microbiol. 2006;52:482–486. doi: 10.1007/s00284-005-0447-6. [DOI] [PubMed] [Google Scholar]