Cinacalcet, an oral calcimimetic, can be used to treat secondary hyperparathyroidism (SHPT) in patients with non-dialysis-dependent chronic kidney disease (CKD-ND) even though most regulatory authorities limit approval of this compound for use in the chronic dialysis population. Randomized trials of cinacalcet for treating SHPT in CKD-ND have reported relatively poor parathyroid hormone (PTH) suppression and high rates of hypo-calcemia and hyperphosphatemia [1,2]. In this issue of Kidney Research and Clinical Practice, Pérez-Ricart and colleagues [3] report quite different results with long-term use of cinacalcet in 16 patients with SHPT and CKD-ND; most notably these results include PTH suppression at 50% below pretreatment levels and a very low rate of hypocalcemia. The present article compares the results of randomized trials to the observational data of Pérez-Ricart et al [3] and confirm that the findings of Pérez-Ricart et al [3] are due to active vitamin D (Active D; such as calcitriol, 1,25[OH]2D) that was used to treat the negative effects of cinacalcet, which include hypocalcemia and hyperphosphatemia.
Pérez-Ricart et al [3] performed a retrospective analysis that identified 41 CKD-ND patients that were treated with cinacalcet for SHPT. The authors had previously reported on these patients [4]. After a mean of 22 ± 12 months of treatment, 73.2% of patients achieved > 30% PTH suppression [4]. The authors now report data on 16 of these 41 patients that were treated with cinacalcet for an average of 32 ± 9 months, and at 36 months, the mean PTH was suppressed to 50.1% below pretreatment levels and the serum calcium decreased to 8.3%; however, two (12.5%) patients had hypocalcemia [3].
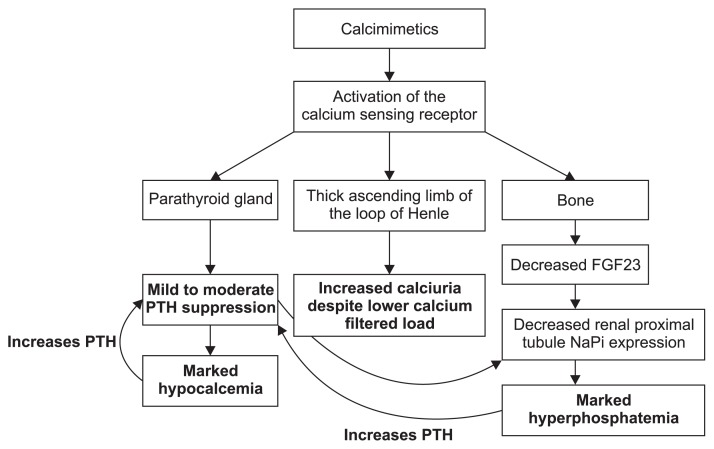
In CKD-ND, calcimimetics have distinctly different modes of action compared with active vitamin D (Fig. 1, 2), and the mechanism also differs compared to chronic dialysis patients. While active vitamin D and cinacalcet both suppress PTH, cinacalcet causes a rapid decline in PTH after each dose, followed by an increase towards baseline over the next 24 hours. On the other hand, active vitamin D downregulates PTH over the course of several days and can lead to more persistent suppression. These differing pharmacodynamic actions on serum PTH help explain why calcimimetics cause significantly lower serum calcium and frequent hypocalcemia, while active vitamin D is not associated with a decrease, but may cause a mild increase in serum calcium. The lower serum levels of PTH reduce phosphaturia, but calcimimetics also suppress fibroblast growth factor-23 (FGF23), resulting in a marked reduction in phosphaturia, leading to frequent hyperphosphatemia [1,5]. In contrast, active vitamin D stimulates FGF23, which likely accounts for the PTH suppression, with little or no change in serum phosphorus levels. However, low serum calcium and hyperphosphatemia are potent stimuli for PTH secretion, which helps explain why calcimimetics suppress PTH far less than active vitamin D in CKD-ND.
Figure 1. Mechanisms of calcimimetic that lead to moderate parathyroid hormone (PTH) suppression, hypocalcemia, hyperphosphatemia, and increased calciuria.
FGF23, fibroblast growth factor-23; NaPi, sodium-phosphate cotransporters 2a and 2c.
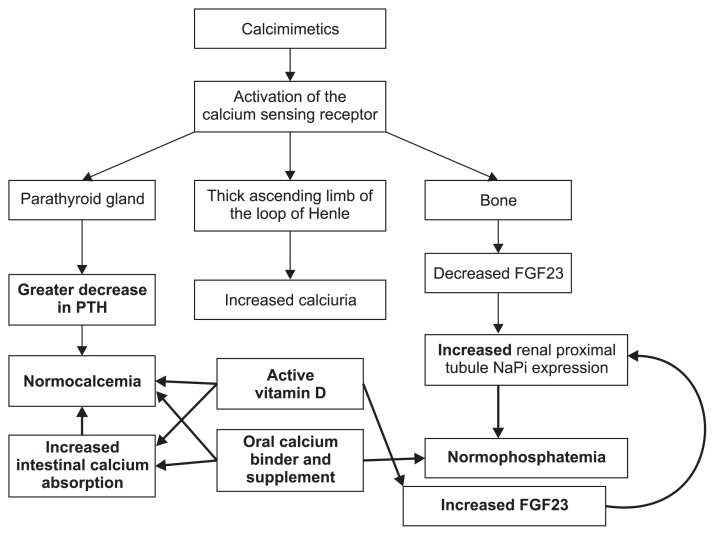
Figure 2. Mechanisms of active vitamin D and oral calcium enhancing parathyroid hormone (PTH) secretion (shown as bolded arrows), and mitigating the hypocalcemia and hyper-phosphatemia induced by using calcimimetic in patients with non-dialysis-dependent chronic kidney disease.
FGF23, fibroblast growth factor-23; NaPi, sodium-phosphate cotransporters 2a and 2c.
Calcimimetics also activate the calcium-sensing receptor (CaSR) in the thick ascending limb of the loop of Hen-le, causing marked calciuria, which is not observed with active vitamin D treatment. CaSR activation appears to stop potassium efflux through the renal outer medullary K+ (ROMK) channel and reduces Na-K-2Cl cotransporter activity [6]. CaSR activation may produce mild to moderate renal sodium chloride wasting, volume contraction, and increased aldosterone and renin levels with subsequent potassium wasting and hypokalemia, similar to type V Bartter syndrome [6].
Based on the mechanisms described above, when cal-cimimetics are used alone in CKD-ND they cause markedly lower serum calcium, frequent hypocalcemia and hyperphosphatemia, increased calciuria, and relatively meager PTH suppression (Fig. 1). Therefore, to treat the unwanted effects of calcimimetics, many clinicians, including Pérez-Ricart et al [3], have added active vitamin D, which further suppresses PTH, increases the calcium levels back towards normal, and stimulates phosphaturia by increasing FGF23 (Fig. 2).
Comparing the observations of Pérez-Ricart et al [3] to two double-blinded, placebo-controlled randomized clinical trials of cinacalcet in CKD-ND has revealed important insights. One 18-week trial randomized 54 CKD-ND patients to treatment with either cinacalcet or placebo, and titrated every 3 weeks from 30 mg up to 180 mg daily to suppress PTH ≥ 30% [2]. In that study, only 56% of cinacalcet-treated patients were able to achieve the PTH goal, while 19% of patients on placebo achieved the goal. Calcium used as a phosphorus binder or supplement increased from 37% to 60% in the cinacalcet arm [2]. Despite the oral calcium, cinacalcet caused hypocalcemia in more than half of patients and resulted in 15% of patients withdrawing [2].
In contrast to the study mentioned above, the controlled trials on CKD-ND have focused on active vitamin D, where calcitriol and paricalcitol achieve ≥ 30% PTH suppression in about 90% of patients. In these studies, about 4% of patients developed hypercalcemia, however, the hypocalcemia was resolved with treatment [7,8].
A larger cinacalcet trial treated hypocalcemia more aggressively with active vitamin D and oral calcium supplements [1]. This double-blind, placebo-controlled trial randomized 404 CKD-ND patients with SHPT. PTH suppression of ≥ 30% was achieved in 74% of cinacalcet-treated patients versus 28% on placebo, but active vitamin D use, which increased from 6% at baseline to 46%, accounted for much of this PTH suppression [1]. PTH suppression was also attributed to increased oral calcium use as a supplement and phosphate binder (19% use at baseline, 58% at end of trial) [1]. Despite the heavy use of active vitamin D and oral calcium, 62% of cinacalcet-treated patients developed hypocalcemia, and 25% had confirmed values that were less than 8.0 mg/dL [1]. Hypocalcemia was observed in just one placebo-treated patient [1].
In both of the controlled trials discussed, despite the increased use of phosphate binders, cinacalcet induced a marked placebo-subtracted increase in the serum phosphorus levels of 0.5 to 0.8 mg/dL, which was much more than the levels of 0.0 to 0.2 mg/dL observed with active vitamin D products in CKD-ND [1,2,7,8].
The observations of Pérez-Ricart et al [3] confirm aspects of both of these cinacalcet trials. Similar to these trials, Pérez-Ricart et al [3] reported that the mean dose of cinacalcet was about 30 mg/day. Pérez-Ricart et al [3] also found that calcium binder use increased from about 15% to 25%, and use of other binders increased, even though the percent increase was not given. At the time of cinacalcet initiation, 43.8% of patients were receiving active vitamin D, and this increased to 62.5% at 12 months, and 87.5% by the end of the observation period [3]. The frequent use of active vitamin D can just as easily account for the PTH suppression reported in the studies. active vitamin D also treats the hypocalcemic impact of cinacalcet by enhancing intestinal calcium absorption and increasing FGF23, which stimulates phosphaturia and mitigates the hyperphosphatemic actions of the calcimimetic [5,9]. Pérez-Ricart et al [3] used active vitamin D in 87.5% of patients, which likely accounts for the relatively low rate of hypocalcemia (12.5%) seen at 36 months.
Pérez-Ricart et al [3] concluded that cinacalcet was effective for at least 36 months in non-dialysis patients with SHPT. However, we suggest that these results were likely attributable to the fact that active vitamin D augments the less efficient PTH suppression by cinacalcet, which treats the problematic effects of hypocalcemia and hyperphosphatemia associated with calcimimetic.
Cinacalcet is also used to treat hyperparathyroidism after kidney transplantation (CKD-T). This patient population typically has enlarged parathyroid glands from pre-transplant CKD, which causes SHPT. Because kidney transplantation allows patients to experience near-normal renal function, the patients in this subgroup are effectively converted from SHPT characteristics to primary hyperparathyroidism [10]. Excessive PTH secretion leads to hypercalcemia, hypercalciuria, and hypophosphatemia. As in CKD-ND, the use of cinacalcet in CKD-T patients will lower serum calcium by suppressing PTH and raising urinary calcium; it will also raise serum phosphorus by lowering the phosphaturic hormones PTH and FGF-23 [11]. Because CKD-T patients with hyperparathyroidism have hypercalcemia and hypophosphatemia, cinacalcet will correct these biochemical abnormalities. However, the use of cinacalcet does not lead to improved bone mineral density or reduce the progression of hyperparathyroidism [11]. Consequently, the use of cinacalcet in CKD-T is controversial, and frequently this compound is not approved for use by regulatory authorities [10].
The goals of treating SHPT include stabilizing PTH levels and preventing further parathyroid gland hyperplasia, and also preventing or reversing osteitis fibrosa bone disease. It is currently unknown whether better SHPT management can lead to significant reduction in the risk of death or major cardiovascular events. The neutral results of the EVOLVE trial in hemodialysis patients, which compared cinacalcet to placebo, call into question whether marked improvements in PTH, calcium, and phosphorus have any cardiovascular benefits [12,13].
More frequent patient visits, close laboratory monitoring, heavy use of oral calcium and active vitamin D as adjuncts, and higher costs have all been associated with the use of cinacalcet in CKD-ND for SHPT. An easier approach would be to use nutritional vitamin D in the early stages of CKD-ND, which can increase endogenous calcitriol levels and lower PTH, active vitamin D, and phosphate binders in later stages. Notwithstanding the report of Pérez-Ricart and colleagues [3], randomized trials indicate cinacalcet is not a good choice for SHPT management in patients with CKD-ND.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Fizza Abbas developed the outline and first draft of the commentary. Daniel W. Coyne revised the first draft and created the figures.
References
- 1.Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197–207. doi: 10.1053/j.ajkd.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Charytan C, Coburn JW, Chonchol M, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyper-parathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46:58–67. doi: 10.1053/j.ajkd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Ricart A, Galicia-Basart M, Comas-Sugrañes D, Cruzado-Garrit JM, Segarra-Medrano A, Montoro-Ronsano JB. Long-term effectiveness of cinacalcet in non-dialysis patients with chronic kidney disease and secondary hyper-parathyroidism. Kidney Res Clin Pract. 2019;38:229–238. doi: 10.23876/j.krcp.18.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Ricart A, Galicia-Basart M, Alcalde-Rodrigo M, Segarra-Medrano A, Suñé-Negre JM, Montoro-Ronsano JB. Effectiveness of cinacalcet in patients with chronic kidney disease and secondary hyperparathyroidism not receiving dialysis. PLoS One. 2016;11:e0161527. doi: 10.1371/journal.pone.0161527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naveh-Many T, Silver J. The pas de trois of vitamin D, FGF23, and PTH. J Am Soc Nephrol. 2017;28:393–395. doi: 10.1681/ASN.2016090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmett M, Ellison DH. Bartter and Gitelman syndromes [Internet] Waltham: UpToDate; 2019. [cited 2019 Apr 10]. Available from: https://www.uptodate.com/contents/bartter-and-gitelman-syndromes. [Google Scholar]
- 7.Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3–4 CKD. Clin J Am Soc Nephrol. 2014;9:1620–1626. doi: 10.2215/CJN.10661013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47:263–276. doi: 10.1053/j.ajkd.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 10.Coyne DW, Delos Santos R. Evaluating the safety and rationale for cinacalcet posttransplant hyperparathyroidism and hypercalcemia. Am J Transplant. 2014;14:2446–2447. doi: 10.1111/ajt.12913. [DOI] [PubMed] [Google Scholar]
- 11.Evenepoel P, Cooper K, Holdaas H, et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am J Transplant. 2014;14:2545–2555. doi: 10.1111/ajt.12911. [DOI] [PubMed] [Google Scholar]
- 12.Moe SM, Chertow GM, Parfrey PS, et al. Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: The evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132:27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Parfrey PS. Cinacalcet for cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2013;368:1844–1845. doi: 10.1056/NEJMc1301247. [DOI] [PubMed] [Google Scholar]