Abstract
Objective
Neuropathic pain is a common complication of diabetes mellitus (DM). Patients may complain of several neuropathic symptoms including impaired peripheral sensation, numbness, tingling, burning, and pain. Because these symptoms may cross with symptoms of vitamin D deficiency, we hypothesized that neuropathic pain and vitamin D deficiency may be associated in patients with type 2 DM.
Research design and methods
This is a cross-sectional study that involved 239 participants with type 2 DM. Neuropathic pain was assessed using PainDETECT questionnaire. Serum 25-hydroxyvitamin D was measured by the electrochemiluminescence immunoassay, fasting blood glucose was measured by the hexokinase method and hemoglobin A1c was measured by the turbidimetric inhibition immunoassay.
Results
The prevalence of neuropathic pain among type 2 DM participants was 26.8%. Vitamin D deficiency was reported in 67.8% of type 2 DM participants. The neuropathy score for females was significantly higher than that for males (p<0.01). There was no significant difference in serum vitamin D between type 2 DM participants according to their gender and according to their neuropathy status (p>0.05). Ordinal logistic regression analysis has shown that female gender was the only significant predictor of neuropathic pain among type 2 DM participants (p<0.01 with an OR (95% CI) of 2.45 (1.29 to 4.67)).
Conclusions
Neuropathic pain was not associated with serum vitamin D but was associated with female gender in type 2 DM. Because our results were not consistent with other studies that used different neuropathy assessment tools, we suggest that further research should be conducted to check the validity of these tools in identifying subjects with neuropathy.
Keywords: neuropathic pain, vitamin d deficiency, type 2 diabetes mellitus, female gender
Significance of this study.
What is already known about this subject?
Neuropathic pain is a common complication of diabetes mellitus (DM). Vitamin D deficiency is very common among patients with type 2 DM and among the general population. There is increasing evidence suggesting that vitamin D deficiency could be associated with the development of neuropathic pain.
What are the new findings?
Neuropathic pain was not associated with serum vitamin D in patients with type 2 DM. Instead, it was associated with female gender.
How might these results change the focus of research or clinical practice?
Researchers should reconsider the association between vitamin D deficiency and neuropathic pain according to the method used for neuropathy determination. Further research could also be performed to confirm the association between neuropathic pain and gender in patients with type 2 DM.
Introduction
Neuropathic pain is a common complication of type 1 and type 2 diabetes mellitus (DM) with a lifetime prevalence of ~50%.1 The proposed pathophysiological mechanism behind the development of neuropathic pain is almost due to the toxic effects of chronic hyperglycemia.2 These include the formation of advanced glycation endproducts and reactive oxygen radicals, which can cause injuries in the microvasculature that supplies peripheral nerves.3–5 Therefore, patients may complain of various neuropathic symptoms including impaired peripheral sensation, numbness, tingling, burning, and pain.6 Unfortunately, the condition may deteriorate and lead to more serious problems such as foot ulcers and infections.7
Interestingly, there is a growing evidence suggesting vitamin D deficiency as a risk factor for diabetic neuropathy.8–13 Although vitamin D is known to be involved in calcium homeostasis and bone remodeling, it also has other systemic functions that could be mediated by its action on vitamin D receptors (VDRs), which are expressed on various cell types.14 So, vitamin D deficiency is not only involved in the pathogenesis of bone diseases but also it may be implicated in the development of other diseases including DM and cardiovascular diseases.15 16 Several researches have shown that vitamin D deficiency may predispose subjects to hyperglycemia and thus sufficient intake of vitamin D may improve their glycemic control.17 18 Additionally, complications of DM may be reduced or delayed by maintaining normal serum vitamin D levels.19 20 Increasing evidence suggests a role for vitamin D supplementation in improving symptoms of diabetic neuropathy. For instance, Lee et al 11 suggested that vitamin D could be used as an analgesic for pain resulting from diabetic neuropathy. Nadi et al 21 has also shown that vitamin D supplementation combined with training can improve symptoms of sensorimotor neuropathy in women with type 2 DM. Many other studies have also reported an improvement of symptoms of painful diabetic neuropathy on vitamin D supplementation.22–24 Regarding the association of vitamin D level with diabetic neuropathy, a recent meta-analysis has shown that vitamin D deficiency could be associated with the development of diabetic neuropathy in Caucasian patients with type 2 DM.13 A number of other studies conducted on different populations have also shown an association between the levels of serum vitamin D and diabetic neuropathy.12 25–27
However, our preliminary data showed that healthy vitamin D-deficient subjects usually experience peripheral neuropathic sensation including numbness, tingling, burning in addition to widespread musculoskeletal pain that resolved by vitamin D supplementation. The similarity in the clinical picture of both vitamin D deficiency and diabetic neuropathy lead us to the hypothesis that the two conditions may be related. Therefore, the aim of this study was to provide evidence that neuropathic pain is associated with vitamin D deficiency in patients with type 2 DM. In addition, this study aimed to find the prevalence of neuropathic pain in patients with type 2 DM.
Materials and methods
Participants
This study involved type 2 DM participants who were recruited from the outpatient endocrine clinic of King Abdullah University Hospital (KAUH), Ramtha, Jordan, between January and December 2017. Patients with history of chronic renal impairment, chronic hepatic disease and/or who were on recent vitamin D supplement were excluded from the study. All participants had signed appropriate consent forms before they had been informed about the purpose of the study and after obtaining ethical approval.
Assessment of neuropathy status
Neuropathy status was determined using the well-validated PainDETECT questionnaire28 that uses a scale from 0 to 38 to define neuropathy. Participants with a neuropathy score from 0 to 12 were considered as nociceptive (a neuropathic pain component is unlikely), participants with a neuropathy score from 13 to 18 were considered as having unclear neuropathy status (a neuropathic pain component can be present) and participants with a neuropathy score from 19 to 38 were considered as having neuropathic pain.28
Study design and sample size calculation
This is a cross-sectional study that involved a cohort of type 2 DM participants (n=239). Sample size was calculated using the formula (n= (t)2(p)(1 p)/(d)2),29 where t=1.96 (represents the 95% CI), p=0.20 (the approximate prevalence of neuropathy among patients with type 2 DM as determined by Ojo et al 30 using PainDETECT questionnaire28) and d=0.05 (the margin of error based on the 95% CI).29
Data collection
Data about age, gender, duration of type 2 DM, smoking, history of vitamin D supplements, current treatment with neuropathy medications, history of chronic renal impairment and history of chronic hepatic diseases were collected from participants’ medical records and by self-reporting. Body mass index (BMI) was calculated using the formula: BMI=weight (kg)/height (m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in mm Hg at rest using a mercury sphygmomanometer.
Blood sampling and lab assays
Appropriate fasting venous blood samples (10 mL) were collected in the biochemistry lab of KAUH by a qualified laboratory technician. Serum was prepared within 2 hours of blood collection by centrifugation at 2100×g for 8 min at room temperature using a high speed Jouan MR23i centrifuge (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Fasting blood glucose (FBG) was measured by the hexokinase method31 using a Hitachi 902 auto-analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Hemoglobin A1c (HbA1c) was measured by the turbidimetric inhibition immunoassay32 using a cobas b 101 system (Roche Diagnostics GmbH). 25-hydroxyvitamin D was measured by electrochemiluminescence immunoassay33 using a Roche Modular E170 Analyzer (Roche Diagnostics GmbH). Participants were classified as having deficient vitamin D level (<20 ng/mL), insufficient vitamin D level (20–30 ng/mL) or sufficient vitamin D level (>30 ng/mL).34
Statistical analysis
Data were analysed using the IBM SPSS statistics 20 software (IBM, Armonk, New York, USA). Continuous variables that were normally distributed were presented as mean±SD while continuous variables that were not normally distributed were presented as median (25th–75th percentiles). Qualitative variables were presented as frequency (%). Differences in the mean or median levels of continuous variables between male and female participants were determined using Student’s t test or Mann-Whitney test, respectively. Differences in the mean or median levels of continuous variables between nociceptive, neuropathic and participants with unclear neuropathy were determined using one-way analysis of variance test with post hoc analysis or Kruskal-Wallis H test, respectively. Differences in categorical variables between male and female participants, between participants who were on neuropathy medications and who were not on neuropathy medications and between nociceptive, neuropathic and participants with unclear neuropathy were determined using χ2 test or Fisher's exact test as appropriate. Ordinal logistic regression analysis was used to determine predictors of neuropathic pain. All p values were considered statistically significant at the level of <0.05.
Results
General and biochemical characteristics of type 2 DM participants
The mean age±SD was 56.51±9.03 years; the mean BMI±SD was 31.01±4.42; the median duration of type 2 DM (25th–75th percentiles) was 6 (3-10) years; the median FBG (25th–75th percentiles) was 8.55 (6.60–12.23) mmol/L; the median HbA1c (25th–75th percentiles) was 7.75 (6.81–9.43); the mean SBP±SD was 137.94±16.81 mm Hg; the mean DBP±SD was 82.97±10.23 mm Hg; the median 25-hydroxyvitamin D (25th–75th percentiles) was 14.77 (8.45–22.99) ng/mL and the percentage of current smoking was 18% . Characteristics of participants according to their gender are presented in table 1.
Table 1.
General and biochemical characteristics of type 2 DM participants according to their gender
| Variable | Gender | ||
| Male | Female | P value* | |
| N (%) | 99 (41.4) | 140 (58.6) | – |
| Age (year) | 57.39±9.58 | 55.90±8.61 | 0.21 |
| BMI (kg/m2) | 29.40±4.00 | 32.16±4.36 | <0.01 |
| Smoking | |||
| Yes | 39 (39.4) | 4 (2.9) | <0.01 |
| No | 60 (60.6) | 163 (97.1) | |
| Duration of type 2 DM (year) | 7 (4–13) | 5.50 (3-10) | 0.08 |
| FBG (mmol/L) | 8.70 (6.90–12.60) | 8 (6.5–12.1) | 0.29 |
| HbA1c (%) | 8.29 (6.94–9.80) | 7.53 (6.70–9.05) | 0.05 |
| SBP (mm Hg) | 139.82±15.99 | 136.60±17.31 | 0.15 |
| DBP (mm Hg) | 84.50±10.02 | 81.88±10.27 | 0.05 |
| 25-hydroxyvitamin D (ng/mL) | 14.80 (9.05–21.70) | 14.29 (8.04–24.30) | 0.79 |
| On neuropathic pain medication (gabapentin) | |||
| Yes | 8 (8.1) | 8 (5.7) | 0.60 |
| No | 91 (91.9) | 132 (94.3) | |
*Statistically significant differences (p<0.05) were determined using Student’s t test or Mann-Whitney test for continuous variables and χ2 test or Fisher‘s exact test for categorical variables. Data were expressed as frequency (%), mean±SD or median (25th–75th percentiles).
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose;; HbA1c, hemoglobin A1c; SBP, systolic blood pressure.
Prevalence of neuropathic pain among type 2 DM participants
The mean neuropathy score±SD for type 2 DM participants (n=239), as determined by the PainDETECT questionnaire,28 was 13.29±7.48 (range is 0–38). According to the questionnaire classification criteria, 26.8% of participants were classified as having neuropathy, 49% of participants were classified as nociceptive and 24.3% of participants were classified as having unclear neuropathy score. As shown in figure 1, neuropathy score was significantly higher in female participants compared with male participants (p<0.01).
Figure 1.
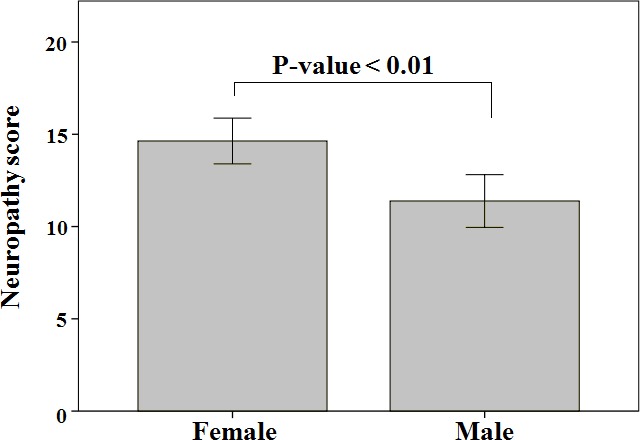
Difference in neuropathic score between male and female type 2 diabetes mellitus participants. P-value was determined using Student’s t-test (significance level was set at < 0.05). Bars represent mean neuropathy score +- Standard Deviation (SD).
Differences in 25-hydroxyvitamin D, glycemic measures and other variables according to neuropathy status among type 2 DM participants
Vitamin D deficiency and insufficiency were detected in 67.8% and 21.3% of type 2 DM participants (n=239), respectively. As shown in table 2, there were no significant differences in serum 25-hydroxyvitamin D, FBG, HbA1c, duration of type 2 DM, BMI, smoking status, SBP and DBP between participants who were classified as nociceptive, with unclear neuropathy score or with neuropathy (p>0.05). As well, there was no significant association between vitamin D status and neuropathy status (p=0.55). The age of type 2 DM participants with unclear neuropathy was significantly higher than the age of both nociceptive and neuropathic participants (p<0.01). The neuropathic status was only associated with the gender of type 2 DM participants (p<0.01); 76.6% of neuropathic participants were of female gender while the rest were males (23.4%).
Table 2.
Differences in study variables according to neuropathy status among participants with type 2 DM
| Variable | Neuropathy status | |||
| Nociceptive ( score ≤ 12,n = 117 ) | Unclear neuropathy (score 13 – 19,n = 58 ) | Neuropathy ( score ≥ 19,n = 64 ) | P value * | |
| Age (year) | 55.41±8.26† | 59.79±9.27†‡ | 55.55±9.57‡ | <0.01 |
| Gender | ||||
| Male | 59 (50.4) | 25 (43.1) | 15 (23.4) | <0.01 |
| Female | 58 (49.6) | 33 (56.9) | 49 (76.6) | |
| BMI (kg/m2) | 30.42±3.94 | 31.17±5.14 | 31.95±4.44 | 0.08 |
| Smoking | ||||
| Yes | 21 (17.9) | 11 (19) | 11 (17.2) | 0.97 |
| No | 96 (82.1) | 47 (81) | 53 (82.8) | |
| Duration of DM (year) | 6 (2–10) | 6.5 (4–10) | 6 (3–11) | 0.62 |
| FBG (mmol/L) | 8.6 (6.6–12.28) | 8.5 (6.98–11.05) | 8.5 (6–13.05) | 0.91 |
| HbA1c (%) | 7.68 (6.79–9.20) | 7.59 (6.84–9.48) | 8.02 (6.77–9.64) | 0.79 |
| SBP (mm Hg) | 138.67±18.08 | 139.67±17.41 | 135±13.38 | 0.25 |
| DBP (mm Hg) | 83.75±10.52 | 82.43±11.11 | 82.03±8.79 | 0.51 |
| 25-hydroxyvitamin D (ng/mL) | 14.34 (8.86–21.65) | 15.58 (8.29–25.73) | 13.77 (7.43–23.85) | 0.83 |
| Vitamin D status | ||||
| Deficient (<20 ng/mL) | 82 (70.1) | 36 (62.1) | 44 (68.8) | 0.55 |
| Insufficient (20–30 ng/mL) | 22 (18.8) | 17 (29.3) | 12 (18.8) | |
| Sufficient (>30 ng/mL) | 13 (11.1) | 5 (8.6) | 8 (12.5) | |
| On neuropathic pain medication (Gabapentin) | ||||
| Yes | 5 (4.3) | 6 (10.3) | 5 (7.8) | 0.26 |
| No | 112 (95.7) | 52 (89.7) | 59 (92.2) | |
*Statistically significant differences (p<0.05) were determined using one way analysis of variance test with post hoc analysis or Kruskal-Wallis H test for continuous variables and χ2 test or Fisher’s exact test for categorical variables. Data were expressed as frequency (%), mean±SD or median (25th–75th percentiles). Neuropathy status was determined using PainDETECT questionnaire.21
†Post-hoc analysis revealed significant difference in age between nociceptive and unclear neuropathy groups.
‡Post-hoc analysis revealed significant difference in age between unclear neuropathy and neuropathy groups.
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; SBP, systolic blood pressure.
Predictors of neuropathic pain among type 2 DM participants
Predictors of neuropathic pain were investigated using ordinal logistic regression model that involved ordinal neuropathic status (nociceptive, unclear neuropathy and neuropathy) as a dependent variable and other variables including age, gender, BMI, smoking, duration of type 2 DM, FBG, HbA1c, SBP, DBP and 25-hydroxyvitamin D as independent variables. As shown in table 3, female gender was the only significant predictor for neuropathic pain in participants with type 2 DM (p<0.01). For females, the odds of neuropathy category versus the combined unclear neuropathy and nociceptive categories were 2.45 times higher than that for males, adjusted to other variables in the model. Likewise, the odds of the combined neuropathy and unclear neuropathy categories versus nociceptive category were 2.45 times higher for females compared with males, adjusted to other variables in the model.
Table 3.
Ordinal logistic regression analysis for predictors of neuropathic pain among type 2 DM participants
| Variable | Estimate | SE | Wald | OR (95% CI) | P value |
| Neuropathy category 1 | 2.61 | 1.84 | 2.004.20 | 13.59 (0.37 to 504.87) | 0.16 |
| Neuropathy category 2 | 3.80 | 1.85 | 4.20 | 44.70 (1.18 to 1691.22) | 0.04 |
| Age (years) | 0.02 | 0.02 | 2.33 | 1.02 (0.99 to 1.06) | 0.13 |
| Female gender and male gender (Ref.) | 0.90 | 0.33 | 7.45 | 2.45 (1.29 to 4.67) | <0.01 |
| BMI (kg/m2) | 0.04 | 0.03 | 1.51 | 1.04 (0.98 to 1.10) | 0.22 |
| Smoking and non-smoking (Ref.) | 0.51 | 0.40 | 1.64 | 1.66 (0.76 to 3.62) | 0.20 |
| Duration of DM (years) | 0.02 | 0.02 | 0.78 | 1.02 (0.98 to 1.07) | 0.38 |
| FBG (mmol/L) | −0.06 | 0.04 | 2.81 | 0.94 (0.87 to 1.01) | 0.09 |
| HbA1c (%) | 0.18 | 0.09 | 3.79 | 1.19 (1.00 to 1.43) | 0.05 |
| SBP (mm Hg) | −0.01 | 0.01 | 0.92 | 0.99 (0.97 to 1.01) | 0.34 |
| DBP (mm Hg) | <−0.01 | 0.02 | 0.01 | 1.00 (0.97 to 1.03) | 0.91 |
| 25-hydroxyvitamin D (ng/mL) | <0.01 | 0.01 | 0.03 | 1.00 (0.98 to 1.03) | 0.86 |
Model fitting information: −2 Log likelihood=468.08 (intercept only) and 447.66 (final); χ2=20.42; p=0.03. Goodness of fit information: Pearson χ2=438.01; Pearson p value=0.41; deviance χ2=447.66; deviance p value=0.29. Neuropathy category 1: neuropathy score ≤12. Neuropathy category 2: neuropathy score 13–19. Neuropathy category 3: neuropathy score ≥19. P<0.05 was considered statistically significant.
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; Ref, reference; SBP, systolic blood pressure.
Association between vitamin D status and treatment with neuropathic pain medications among participants with type 2 DM
As shown in table 4, there were only 16 participants (6.69%) who were treated for neuropathy by gabapentin medication. Statistical analysis did not show any significant association between vitamin D status and treatment for neuropathic pain (p=0.78).
Table 4.
Association between vitamin D status and taking neuropathic pain medications among participants with type 2 DM
| Participants on neuropathic pain medications | P value | ||
| Vitamin D status | Yes (n=16) | No (n=223) | 0.78 |
| Deficient (<20 ng/mL) | 10 (62.5) | 152 (68.2) | |
| Insufficient (20–30 ng/mL) | 4 (25.0) | 47 (21.1) | |
| Sufficient (>30 ng/mL) | 2 (12.5) | 24 (10.8) | |
*Gabapentin was the only medication that was used to treat neuropathy.
†Statistically significant difference (p<0.05) was determined using Fisher’s exact test. Data were expressed as frequency (%).
DM, diabetes mellitus.
Discussion
In the current study, the prevalence of neuropathic pain among participants with type 2 DM was 26.8%. This was slightly higher than the prevalence of neuropathic pain among type 2 DM participants (21.6%) that was determined by Ojo et al 30 using the PainDETECT questionnaire28; the same assessment method used in our study. Importantly, figure 1 has shown that the neuropathy score in female participants was significantly higher than that for male participants. As well, table 1 has shown that 76.6% of neuropathic participants were females while 23.4% were males. This was almost similar to findings of Ojo et al’s30 study, in which 66.7% of neuropathic participants were females while 33.3% were males. These slight differences could be due to the differences between various populations as Ojo et al’s30 study was conducted in Nigeria and our study was conducted in Jordan. Therefore, these findings suggest that female patients with type 2 DM are more likely to complain of peripheral neuropathic symptoms compared with males.
The current study was also interested in investigating the association between vitamin D deficiency and peripheral neuropathic pain in participants with type 2 DM. The relative similarity in the clinical symptomatology of both conditions especially the feeling of tingling and numbness has driven us to the hypothesis that the two conditions could be associated. Notably, this study did not find any significant difference in vitamin D levels between type 2 DM participants who were nociceptive, with unclear neuropathy score and with neuropathy. Additionally, there was no significant association between vitamin D status (deficient, insufficient and sufficient vitamin D) and neuropathy status (nociceptive, unclear neuropathy and neuropathy) in participants with type 2 DM (table 2). Our findings were inconsistent with the results of the few studies reported in the literature that were interested in finding association between diabetic neuropathy and vitamin D deficiency. For instance, Orabi et al’s study35 has reported that patients with diabetic neuropathy were having significantly lower vitamin D levels compared with controls. Likewise, Shehab et al’s study8 had reported that patients with diabetic neuropathy were having significantly lower vitamin D levels compared with patients with type 2 DM without neuropathy. As well, Shillo et al’s study,12 has reported a significant lower vitamin D level in type 2 DM white Europeans with neuropathy compared with controls. This inconsistency could be due to the small sample size of Orabi et al’s35 and Shillo et al’s12 studies and the different neuropathy assessment methods used in our study. Unfortunately, there was no study in the literature that used the PainDETECT questionnaire28 to assess neuropathic pain in association with vitamin D deficiency although the questionnaire is well validated and was used by other researchers like Ojo et al 30 to investigate the prevalence of neuropathic pain in patients with type 2 DM as mentioned above. Even though, we believe that further studies are required to assess the relation between diabetic neuropathy and vitamin D levels using different methods of neuropathy assessment including both neuropathy questionnaires and clinical neuropathy assessment. This will expose the validity of each method in categorizing patients with neuropathy in relation to vitamin D status.
To find predictors of neuropathic pain among participants with Type 2 DM, ordinal logistic regression analysis has shown that female gender was the only significant predictor for neuropathic pain while vitamin D level, age, BMI, FBG, duration of type 2 DM, SBP and DBP were not. The significance level for the HbA1c in the model was on the borderline with a p value of 0.05 and OR (95% CI) of 1.19 (1.00 to 1.43). These results were interesting to us because most of other previous studies had reported different predictors for diabetic neuropathy including the long duration of DM, increased age and elevated HbA1c.36–38 As well, these studies did not find any gender-related differences in neuropathic pain among patients with type 2 DM.36–38 Again, the inconsistency between our results and other previous studies36–38 is almost because these studies used different methods to assess neuropathy. Interestingly, Gryz et al 36 tried to assess predictors of diabetic neuropathy in relation to different criteria of its diagnosis and they found that the predictors vary according to the criteria that were used for diagnosis. The only study that used PainDETECT questionnaire to assess neuropathy in patients with type 2 DM was Ojo et al’s study.30 As mentioned above, results of Ojo et al’s study30 were almost similar to our results in regard to the prevalence of diabetic neuropathy, frequency of neuropathy in females compared with males and the lack of any significant difference in FBG, HbA1c and duration of type 2 DM between patients with neuropathy and those without neuropathy. In contrast to Ojo et al’s study,30 age was not a predictor for neuropathic pain in our study. As mentioned above, this could be due to differences in the study populations as both studies were conducted on different populations.
In summary, this study did not find any association between neuropathic pain and vitamin D levels in participants with type 2 DM. So, this finding rejects our hypothesis that both vitamin D deficiency and neuropathic pain could be related. Instead, the current study has found that neuropathic pain in participants with type 2 DM can be predicted from the female gender but not from age, DM duration, FBG or HbA1c. The strengths of the current study comes from its suitable sample size and the method of neuropathy assessment which was well validated and used by other researchers to assess neuropathic pain.30 On the other hand, the current study has also some limitations that may affect its findings. For example, the neuropathy status was not determined clinically but was determined by self-reporting using the PainDETECT questionnaire.28 As well, we only used one assessment tool for neuropathy and we did not compare the validity of this tool compared with other questionnaires that were used previously to assess neuropathy.36–38 So, the comparison of our results with these studies36–38 could not be appropriate because of the differences in the methodology. Another possible limitation to the association between neuropathic pain and female gender is that females may have lower pain threshold and tolerance levels compared with males.39 Unfortunately, the difference in the pain threshold between males and females was not taken in consideration in the method that we used to assess neuropathic pain. Despite of these limitations, we believe that the current study is the first report that did not find any association between vitamin D levels and neuropathic pain in participants with type 2 DM. As well, this is the second study that used the PainDETECT questionnaire28 along with Ojo et al’s study30 to assess neuropathic pain in patients with type 2 DM. Because of the similarity in our findings and results of Ojo et al’s study30 and the inconsistency with other studies36–38 that used different neuropathy assessment methods, we think that it will be wrathful to do further research to compare the various neuropathy assessment tools on same populations and to compare their results with results of the clinical neuropathy assessment methods.
Conclusions
Neuropathy was not associated with serum vitamin D but was associated with female gender in participants with type 2 DM. This suggests that female patients with type 2 DM are more likely to complain of peripheral neuropathic symptoms compared with males. Because the lack of association between neuropathy and serum vitamin D was not consistent with other studies that used different neuropathy assessment tools, we suggest that further research should be conducted to check the validity of these tools in identifying subjects with neuropathy.
Acknowledgments
Authors would like to thank medical staff at the endocrine clinic of KAUH for their assistance in patients’ recruitment and Deanship of Scientific Research of JUST for supporting this research.
Footnotes
Contributors: MJK was responsible for the study design, patients’ recruitment, data analysis and manuscript writing while KKA was responsible for results interpretation and manuscript editing.
Funding: This research was financially supported by the Deanship of Scientific Research of Jordan University of Science and Technology (Grant number: 20150150).
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: Ethical approval was obtained from the Institutional Review Board (IRB) of King Abdullah University Hospital (KAUH) and Jordan University of Science and Technology (JUST), Irbid, Jordan (Approval number: 20150150).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1. Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000. Research 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schreiber AK, Nones CF, Reis RC, et al. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes 2015;6:432–44. 10.4239/wjd.v6.i3.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent AM, Russell JW, Low P, et al. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004;25:612–28. 10.1210/er.2003-0019 [DOI] [PubMed] [Google Scholar]
- 4. Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest 2003;111:431–3. 10.1172/JCI17862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim TKY, Shi XQ, Johnson JM, et al. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. Journal of Neuroscience 2015;35:3346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther 2018;40:828–49. 10.1016/j.clinthera.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 7. Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci 2016;17. doi: 10.3390/ijms17060917. [Epub ahead of print: 10 Jun 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shehab D, Al-Jarallah K, Mojiminiyi OA, et al. Does vitamin D deficiency play a role in peripheral neuropathy in type 2 diabetes? Diabet Med 2012;29:43–9. 10.1111/j.1464-5491.2011.03510.x [DOI] [PubMed] [Google Scholar]
- 9. Soderstrom LH, Johnson SP, Diaz VA, et al. Association between vitamin D and diabetic neuropathy in a nationally representative sample: results from 2001-2004 NHANES. Diabet Med 2012;29:50–5. 10.1111/j.1464-5491.2011.03379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell DSH. Reversal of the symptoms of diabetic neuropathy through correction of vitamin D deficiency in a type 1 diabetic patient. Case Rep Endocrinol 2012;2012:1–3. 10.1155/2012/165056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee P, Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med 2008;168:771–2. 10.1001/archinte.168.7.771 [DOI] [PubMed] [Google Scholar]
- 12. Shillo P, Selvarajah D, Greig M, et al. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet Med 2019;36:44–51. 10.1111/dme.13798 [DOI] [PubMed] [Google Scholar]
- 13. Qu G-B, Wang L-L, Tang X, et al. The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: an update systematic review and meta-analysis. J Clin Transl Endocrinol 2017;9:25–31. 10.1016/j.jcte.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys 2012;523:123–33. 10.1016/j.abb.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 15. Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci 2009;338:40–4. 10.1097/MAJ.0b013e3181aaee91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010;2010:1–18. 10.1155/2010/351385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kostoglou-Athanassiou I, Athanassiou P, Gkountouvas A, et al. Vitamin D and glycemic control in diabetes mellitus type 2. Ther Adv Endocrinol Metab 2013;4:122–8. 10.1177/2042018813501189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mirhosseini N, Vatanparast H, Mazidi M, et al. Vitamin D supplementation, glycemic control, and insulin resistance in Prediabetics: a meta-analysis. J Endocr Soc 2018;2:687–709. 10.1210/js.2017-00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bajaj S, Singh RP, Dwivedi NC, et al. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J Endocrinol Metab 2014;18:537–41. 10.4103/2230-8210.137512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Usluogullari CA, Balkan F, Caner S, et al. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr Disord 2015;15 10.1186/s12902-015-0029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nadi M, Marandi SM, Esfarjani F, et al. The comparison between effects of 12 weeks combined training and vitamin D supplement on improvement of sensory-motor neuropathy in type 2 diabetic women. Adv Biomed Res 2017;6:55 10.4103/2277-9175.205528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghadiri-Anari A, Mozafari Z, Gholami S, et al. Dose vitamin D supplementations improve peripheral diabetic neuropathy? A before-after clinical trial. Diabets Meta Syndr 2019;13:890–3. 10.1016/j.dsx.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 23. Alam U, Fawwad A, Shaheen F, et al. Improvement in neuropathy specific quality of life in patients with diabetes after vitamin D supplementation. J Diabetes Res 2017;2017:1–7. 10.1155/2017/7928083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basit A, Basit KA, Fawwad A, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care 2016;4:e000148 10.1136/bmjdrc-2015-000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan L, Zhang Y, Zhu J, et al. Association of vitamin D deficiency with diabetic peripheral neuropathy and diabetic nephropathy in Tianjin, China. Asia Pac J Clin Nutr 2018;27:599–606. 10.6133/apjcn.062017.11 [DOI] [PubMed] [Google Scholar]
- 26. Abdelsadek SE, El Saghier EO, Abdel Raheem SI. Serum 25(OH) vitamin D level and its relation to diabetic peripheral neuropathy in Egyptian patients with type 2 diabetes mellitus. Egypt J Neurol Psychiatr Neurosurg 2018;54 10.1186/s41983-018-0036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ozuguz U, Oruc S, Ulu MS, et al. Does vitamin D have any role in the improvement of diabetic peripheral neuropathy in type 1 diabetic patients? J Endocrinol Invest 2016;39:1411–7. 10.1007/s40618-016-0509-6 [DOI] [PubMed] [Google Scholar]
- 28. Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 29. Bartlett JE, Kotrlik JW, Higgins CC. Organizational research: determining appropriate sample size in survey research. Information Technology, Learning, and Performance Journal 2001;19:42–50. [Google Scholar]
- 30. Ojo O, Odeniyi I, Iwuala S, et al. Frequency of neuropathic pain in type 2 diabetes mellitus at the Lagos university teaching hospital: a questionnaire-based outpatient survey. J Clin Sci 2016;13:46–50. 10.4103/2408-7408.179648 [DOI] [Google Scholar]
- 31. Schmidt FH. [Blood glucose levels in capilary blood of adults assessed by the hexokinase method (author's transl)]. Klin Wochenschr 1973;51:520–2. [DOI] [PubMed] [Google Scholar]
- 32. Genc S, Omer B, Aycan-Ustyol E, et al. Evaluation of turbidimetric inhibition immunoassay (TINIA) and HPLC methods for glycated haemoglobin determination. J Clin Lab Anal 2012;26:481–5. 10.1002/jcla.21550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leino A, Turpeinen U, Koskinen P. Automated measurement of 25-OH vitamin D3 on the Roche modular E170 analyzer. Clinical Chemistry 2008;54:2059–62. 10.1373/clinchem.2008.111732 [DOI] [PubMed] [Google Scholar]
- 34. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009;19:73–8. 10.1016/j.annepidem.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oraby MI, Srie MA, Abdelshafy S, et al. Diabetic peripheral neuropathy: the potential role of vitamin D deficiency. Egypt J Neurol Psychiatr Neurosurg 2019;55 10.1186/s41983-019-0058-y [DOI] [Google Scholar]
- 36. Gryz EA, Szermer P, Galicka-Latała D, et al. [Predictors for diabetic neuropathy according to applied criteria of its diagnosis]. Przegl Lek 2002;59:881–4. [PubMed] [Google Scholar]
- 37. Khawaja N, Abu-Shennar J, Saleh M, et al. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bansal D, Gudala K, Muthyala H, et al. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014;5:714–21. 10.1111/jdi.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]


