Abstract
Objective
To determine whether a faecal immunochemical test (FIT) for faecal haemoglobin concentration (f-Hb) can be safely implemented in primary care as a rule-out test for significant bowel disease (SBD) (colorectal cancer (CRC), higher risk adenoma (HRA) and inflammatory bowel disease (IBD)) when used as an adjunct to the clinical assessment of new bowel symptoms.
Design
Single-centre prospective cohort study of all patients who attended primary care and submitted a FIT in the first calendar year of the service beginning December 2015. f-Hb was estimated using HM-JACKarc (Kyowa Medex) with a clinical cut-off of ≥10 µg Hb/g faeces. Incident cases of CRC were verified via anonymised record linkage to the Scottish Cancer Registry.
Results
5422 patients submitted 5660 FIT specimens, of which 5372 were analysed (positivity: 21.9%). 2848 patients were referred immediately to secondary care and three with f-Hb <10 µg/g presented acutely within days with obstructing CRC. 1447 completed colonoscopy in whom overall prevalence of SBD was 20.5% (95 CRC (6.6%), 133 HRA (9.2%) and 68 IBD (4.7%)); 6.6% in patients with f-Hb <10 µg/g vs 32.3% in patients with f-Hb ≥10 µg/g. One CRC was detected at CT colonoscopy. 2521 patients were not immediately referred (95.3% had f-Hb <10 µg/g) of which four (0.2%) later developed CRC. Record linkage identified no additional CRC cases within a follow-up period of 23–35 months.
Conclusion
In primary care, measurement of f-Hb, in conjunction with clinical assessment, can safely and objectively determine a patient’s risk of SBD.
Keywords: bowel disease, colorectal disease, faecal biomarkers, faecal immunochemical test, faecal haemoglobin, colorectal cancer, primary care
Summary box.
What is already known about this subject?
Symptom-based referral guidelines are poor predictors of underlying significant bowel disease (SBD) in patients presenting to primary care.
Previous studies have reported that a quantitative faecal immunochemical test (FIT) for faecal haemoglobin concentration (f-Hb) could be used in primary care as a ‘rule-out test’ to exclude SBD (cancer, higher risk adenoma, inflammatory bowel disease).
What are the new findings?
FIT can be introduced into routine clinical practice in primary care as an adjunct to clinical assessment and full blood count.
Record linkage to the Scottish Cancer Registry has confirmed that an f-Hb <10 µg Hb/g faeces, in the absence of iron deficient anaemia, rectal bleeding, a palpable mass or persistent diarrhoea, identifies patients with an extremely low risk of developing colorectal cancer.
In those patients who attended for colonoscopy, the prevalence of SBD is related to f-Hb; 32.3% of those with f-Hb ≥10 µg/g had SBD rising to 54.0% in those with f-Hb >400 µg/g.
How might it impact on clinical practice in the foreseeable future?
FIT should become integral to the assessment of all patients presenting to primary care with new bowel symptoms to objectively determine the risk of underlying SBD.
Introduction
It is estimated that 10% of all routine primary care visits are for bowel disorders1 but only a proportion of these are referred to secondary care. Referral guidelines from the National Institute for Health and Care Excellence (NICE) highlight symptoms and clinical features which may suggest serious bowel pathology, but concede that symptoms have a positive predictive value for colorectal cancer (CRC) of only 3%–4%,2 as also documented in a review and meta-analysis.3 Some patients may be at risk of false reassurance; a recent UK National Cancer Diagnosis Audit reported that only 41% of patients ultimately diagnosed with colon cancer had been referred from primary care via rapid access straight-to-test suspected cancer pathways.4 For those who are referred, colonoscopy is the gold standard test to exclude significant bowel disease (SBD); namely CRC, higher risk adenoma (HRA, defined as ≥3 adenomas or any adenoma ≥1 cm)5 and inflammatory bowel disease (IBD): low-risk adenomas (<1 cm) are excluded from this category.6 Drives to detect cancer early have resulted in increased demand for colonoscopy,7 but only 6%–13% of patients referred for colonoscopy from primary care have SBD.8 Unnecessary colonoscopy carries associated risks and costs.
A number of studies have investigated the utility of quantitative faecal immunochemical tests (FIT) for haemoglobin (Hb) to assist primary care in determining who may harbour SBD.8–15 FITs use antibodies to the globin moiety and are specific for intact human faecal Hb and its early degradation products.16 In a pilot study we explored the utility of a quantitative FIT, which examined faecal Hb concentrations (f-Hb) in samples taken in primary care at the point of referral in patients with new bowel symptoms8: f-Hb less than the limit of detection proved a good ‘rule-out test’ for SBD. These findings were replicated in similar smaller studies which used qualitative FIT17 18 and in one study using quantitative FIT.14 A very recent study has advocated the use of quantitative FIT to rule in CRC in patients presenting with the low-risk symptoms detailed in NICE NG12.19 As a result of the wealth of evidence and to clarify the application of FIT, NICE has recently developed diagnostic guidance (DG30) on quantitative FIT in primary care to guide referral for CRC, but suggested further studies were required.20 To date, there have been no published reports that describe implementation of FIT into routine practice in primary care, nor any which report long-term follow-up of patients with new bowel symptoms assessed in this way.
NHS Tayside serves a population of around 400 000. Each individual has a unique 10-digit identifier, the Community Health Index (CHI), which is associated with their patient record. Each year approximately 4000 patients are referred from primary care to secondary care for assessment of bowel symptoms via an electronic referral portal. This population has no access to guaiac faecal occult blood tests (gFOBT; out with the National Bowel Cancer Screening Programme) as they were withdrawn from routine laboratory services around 14 years ago due to concerns around diagnostic accuracy.21 These patients may be referred to either the direct-to-test colorectal service or to gastroenterology, but each service may redirect to the other at the discretion of the vetting consultant gastroenterologists. We wished to determine the impact of introducing quantitative FIT into routine practice within primary care on the outcome of patients presenting with new bowel symptoms if those found to have low f-Hb were not routinely referred,8 on the proviso that the f-Hb result should not replace good clinical acumen.
Methods
From December 2015, the gastroenterology and laboratory services collaborated to provide a quantitative FIT service to primary care (GP) surgeries, comprising written information detailing the rationale for measuring f-Hb, and FIT kits comprising one specimen collection device (Kyowa Medex, Tokyo, Japan) and a pictorial patient instruction sheet. If patients presented with new-onset bowel symptoms, general practitioners (Gps) were recommended to request f-Hb as an adjunct to history taking, as per the NICE NG12 guidelines in force at that time,2 not the updated in July 2017 version which included reference to DG30.20 In addition, a full blood count and renal function test were mandated as per standard clinical practice. This initiative had the full support of the GP Area Medical Committee. GP education was provided via a lecture on FIT and the relevance of f-Hb at a Protected Learning Time event hosted by Primary Care and attended by all GP surgeries, and was complemented by an educational newsletter distributed at the launch of the service. Practice nurses distributed a FIT kit to each patient. Patients were instructed to collect a single sample of faeces and to return the FIT device immediately in person to the GP surgery and from here they were delivered to Blood Sciences, Ninewells Hospital and Medical School, Dundee, at ambient temperature via the GP surgery routine sample collection service (a daily courier service) and stored at 4°C prior to analysis to ensure f-Hb stability. Analysis was performed from Monday to Friday, therefore the vast majority of samples were analysed on the day of arrival and results reported electronically to the requesting GP to provide rapid result turnaround. f-Hb was measured using an HM-JACKarc (Kyowa Medex) with an analytical working range of 7–400 µg Hb/g faeces. Samples with results above the upper analytical limit were not diluted and reassayed, but reported as ≥400 µg/g. Results with f-Hb ≥10 µg/g were defined as positive, exactly as recommended in NICE DG30.20 The reports also sign-posted Gps to the Gastroenterology website, which advised that f-Hb <10 µg/g, in the absence of iron deficiency anaemia (IDA), rectal bleeding, persistent diarrhoea, or a mass, would suggest that SBD was extremely unlikely. The gastroenterology and laboratory services sent quarterly newsletters to all GP surgery practice managers and Gps to reinforce the relevance of f-Hb, reminders to request FIT in all patients referred, and practical advice to ensure the correct labelling of completed FIT devices.
The laboratory is accredited by the UK Accreditation Service to ISO 15189 standards. Patients referred to endoscopy were investigated within 6 weeks of referral. The NHS Tayside endoscopy units participate in the accreditation scheme of the Joint Accreditation Group on GI Endoscopy. All findings were recorded on the endoscopy reporting system by the endoscopists. The diagnoses of CRC, HRA and IBD were confirmed by a gastrointestinal pathologist.
Data on all FIT specimens received in the first year of the service were retrieved from the laboratory database and manually linked using the CHI number with the Health Board’s electronic patient record to access comprehensive patient data including all correspondence, laboratory results, referrals to secondary care, colonoscopy findings, hospital admissions and any subsequent attendance at the primary care out-of-hours (OOH) service. Finally, in December 2018, the Health Informatics Centre (HIC), University of Dundee, used the CHI number of all patients who had submitted an FIT test to the laboratory to perform a post hoc anonymised record linkage with the Scottish Cancer Registry to identify all incident cases of CRC (International Classification of Diseases (ICD) codes C18, C19, and C20). Caldicott Guardian and ethical approvals were in place to cover the record linkage performed by HIC. MedCalc statistical software (MedCalc Software, Mariakerke, Belgium) was used for all calculations.
Results
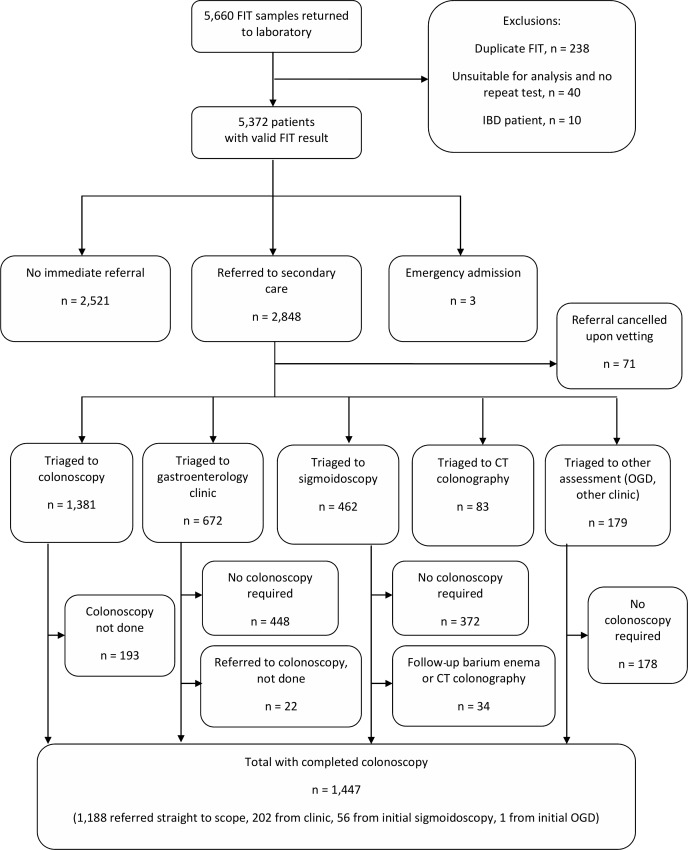
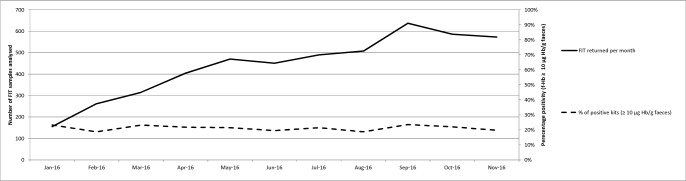
From 7 December 2015 to 7 December 2016, a total of 5422 patients submitted a total of 5660 FIT samples to the laboratory. The median age of patients was 65 years (range: 2–99, IQR: 51–75) and 56.4% were female. One hundred and fifty-two of the samples (2.7%) were unsuitable for analysis (most commonly due to faecal contamination) in whom 40 patients did not complete a repeat test. Ten patients had known IBD. In total, therefore, 50 patients were excluded from further analysis leaving a cohort of 5372 patients of whom 1175 had f-Hb ≥10 µg/g (positivity: 21.9%) (figure 1). Of these, 2848 patients were referred immediately by their GP to secondary care, and three patients were admitted acutely. The positivity rate in referred patients was 37.0%. The number of FIT samples received per month increased over time, but positivity was relatively constant (figure 2). In this year under study a total of 4072 patients were investigated by the colorectal service (equating to an FIT test uptake of 70.5% in referred patients). The influence of the addition of f-Hb on clinical decision-making in patients presenting with new bowel symptoms is summarised in table 1.
Figure 1.
The impact of the addition of a faecal immunochemical test (FIT) for haemoglobin as an adjunct to clinical assessment on the pathway of patients presenting to primary care with new bowel symptoms. IBD, inflammatory bowel disease; OGD, oesophagogastroduodenoscopy.
Figure 2.
Number of faecal immunochemical test (FIT) specimens received per month and percentage with faecal haemoglobin concentration (f-Hb) ≥10 µg/g.
Table 1.
Influence of faecal haemoglobin concentration (f-Hb) on clinical decision-making in patients (n, (%)) with new bowel symptoms (n=5372)
| Total n | f-Hb <10 µg/g n (%) |
f-Hb ≥10 µg/g n (%) |
P value† | |
| Patients with valid f-Hb result | 5372 | 4197 (78.1) | 1175 (21.9) | |
| Not referred by GP | 2521 | 2403 (95.3) | 118 (4.7) | <0.001 |
| Acute admission | 3 | 1 (33.3) | 2 (66.6) | |
| Referred to secondary care and triaged | 2848 | 1794 (63.0) | 1054 (37.0) | <0.001 |
| Referral rejected | 71 | 57 (80.3) | 14 (19.7) | <0.001 |
| Colonoscopy | 1381 | 621 (45.0) | 760 (55.0) | <0.001 |
| Routine | 345 | 258 (74.8) | 87 (25.2) | <0.001 |
| Urgent | 617 | 268 (43.4) | 349 (56.6) | 0.0011 |
| Urgent suspected cancer | 419 | 95 (22.7) | 324 (77.3) | <0.001 |
| Gastroenterology clinic | 672 | 521 (77.5) | 151 (22.5) | <0.001 |
| Sigmoidoscopy | 462 | 373 (80.7) | 89 (19.3) | <0.001 |
| Upper GI endoscopy only | 138 | 127 (92.0) | 11 (8.0) | <0.001 |
| CT colonoscopy | 83 | 62 (74.7) | 21 (25.3) | <0.001 |
| Other clinic* | 41 | 33 (80.5) | 8 (19.5) | <0.001 |
*Other clinic encompasses referrals to general surgical (25), liver (8), pelvic floor (3), inflammatory bowel disease (IBD, 3), paediatrics (1) and genetics (1) clinics.
†χ2 test.
GI, gastrointestinal; GP, general practitioner.
Knowledge of the f-Hb had a significant effect on primary and secondary care clinical decision-making. Patients with f-Hb ≥10 µg/g were more likely to be referred to secondary care, where on triage to colonoscopy they were also more likely to be categorised as urgent/urgent suspected cancer than patients with f-Hb <10 µg/g. On the other hand, patients with f-Hb <10 µg/g were less likely to be referred to secondary care, where on triage were more likely to be categorised to routine colonoscopy, or triaged to an outpatient clinic or a sigmoidoscopy than patients with f-Hb ≥10 µg/g. Of patients referred and triaged to colonoscopy, 55.0% had f-Hb ≥10 µg/g, whereas the majority of patients triaged to outpatient clinic or CT colonoscopy had f-Hb <10 µg/g. Seventy-one FIT samples were associated with a referral which was cancelled at the stage of vetting by the consultant gastroenterologist, most frequently due to insufficient clinical information; 80.3% of these had f-Hb <10 µg/g.
A total of 1447 patients ultimately completed full colonoscopy (table 2): 296 were found to have SBD (20.5%), comprising 95 CRC (6.6%), 133 HRA (9.2%) and 68 IBD (4.7%).
Table 2.
Prevalence of significant bowel disease (n; %) present at colonoscopy (colorectal cancer (CRC), higher risk adenoma (HRA), inflammatory bowel disease (IBD)), by category of faecal haemoglobin concentration (f-Hb) measured in primary care (n=1447)
| Colonoscopy completed (n=1447) |
<10 µg/g (n=667) | ≥10 µg/g (n=780) | P value† | |
| Colorectal cancer | 95 (6.6) | 9 (1.3) | 86 (11.0) | <0.001 |
| Higher risk adenoma | 133 (9.2) | 31 (4.6) | 102 (13.1) | <0.001 |
| Inflammatory bowel disease | 68 (4.7) | 4 (0.6) | 64 (8.2) | 0.003 |
| CRC+HRA+IBD | 296 (20.5) | 44 (6.6) | 252 (32.3) | <0.001 |
| Other* | 615 (42.5) | 304 (45.6) | 311 (39.9) | 0.038 |
| No pathology | 536 (37.0) | 319 (47.8) | 217 (27.8) | <0.001 |
*Low-risk adenoma, hyperplastic polyps, diverticular disease, angiodysplasia, haemorrhoids, microscopic colitis, radiation proctitis, and solitary rectal ulcer.
†χ2 test.
The prevalence of SBD detected at colonoscopy was associated with f-Hb measured at the point of referral; 6.6% in patients with f-Hb <10 µg/g, compared with 32.3% in patients with f-Hb ≥10 µg/g (p<0.001). Two hundred and fifty-two of 296 cases of SBD (85.1%) were associated with f-Hb ≥10 µg/g. The prevalence of SBD was highest in patients with f-Hb ≥400 µg/g (54.0%), compared with 24.2% in patients with f-Hb ≥10–399 µg/g.
Of the 44 patients with f-Hb, <10 µg/g found to have SBD at colonoscopy, there were 9 cases of CRC, 31 of HRA and 4 of IBD. Electronic patient record review of every individual who submitted an FIT sample but who did not have full colonic evaluation identified three further cases of CRC in patients with f-Hb <10 µg/g; all were admitted as emergencies with acute bowel obstruction within days of being referred and all had widespread metastatic disease on imaging (table 3). Of the eight patients who were fit enough for staging investigations, one was Dukes A and four were polyp cancers. Significantly, 10 of the 12 cases of CRC had been referred either as ‘urgent’ or ‘urgent suspected cancer’ despite their f-Hb result, including seven with coexisting IDA.
Table 3.
Cases of colorectal cancer presenting in patients with f-Hb <10 µg/g but who had been referred from primary care on clinical judgement (n=12)
| Urgency of referral | Age | Gender | Symptoms | Hb (g/L) | Tumour size (mm) | Tumour site | Dukes stage |
| USC | 78 | M | IDA, altered bowel habit | 120 | 26 | Descending | A |
| Urgent | 87 | F | IDA | 108 | 28 | Caecum | Polyp cancer |
| Urgent | 74 | F | IDA | 102 | 60 | Caecum | N/A |
| Urgent | 66 | M | IDA, weight loss | 94 | 57 | Transverse | B |
| Routine | 76 | F | Alternating diarrhoea/constipation | 132 | 13 | Caecum | Polyp cancer |
| Urgent | 76 | M | Abdominal pain, constipation | 138 | 22 | Sigmoid | C2 |
| Routine | 58 | M | Rectal bleeding | 134 | 8 | Descending | Polyp cancer |
| Urgent | 54 | F | IDA | 65 | 30 | Transverse | B |
| USC | 89 | M | IDA | 71 | 17 | Transverse | Polyp cancer |
| USC | 84 | F | IDA, weight loss | 98 | N/A | Caecum | N/A |
| Urgent | 67 | M | Alternating diarrhoea/constipation, abdominal pain, weight loss | 134 | N/A | Transverse | N/A |
| USC | 60 | F | Alternating diarrhoea/constipation | 139 | N/A | Transverse | N/A |
IDA, iron deficiency anaemia; N/A, not applicable; USC, urgent suspected cancer.
Furthermore, 17 of the 31 patients with HRA were referred on the basis of coexisting IDA, diarrhoea, rectal bleeding, or a palpable lesion on rectal examination, and one of the four IBD cases had diarrhoea. In summary, using f-Hb as an adjunct to history, examination and full blood count resulted in the identification of 278 out of the 296 (93.9%) cases of SBD.
Eighty-three patients (2.9%) were triaged to CT colonoscopy. The majority had altered bowel habit as the principal symptom (76.6%) and the remainder reported weight loss, anaemia, or rectal bleeding. Sixty-three patients (75.0%) had f-Hb <10 µg/g, and the remainder had a median f-Hb of 29 µg/g (IQR: 12–50). 51.0% had diverticulosis, 33.0% had no abnormality detected. Only one case of SBD was found (1.0%, namely a CRC in a patient with f-Hb ≥10 µg/g). Eleven polyps were detected, but all were <10 mm.
Referral rates to secondary care over the study period were compared with the corresponding time interval in the previous years. This revealed a reduction in referrals to the colorectal service from 4303 to 3905 (9.2%) and a reduction to gastroenterology outpatient clinics from 2796 to 2121 (24.1%), giving an overall referral reduction of 15.1% in the first year following the introduction of FIT.
Further subanalysis revealed that, at the vetting stage, 242 patients had the urgency of their referral altered by the consultant gastroenterologist prior to colonic investigation: 166 referrals were upgraded on the basis of the f-Hb ≥10 µg/g in which the yield of SBD was 33.7% (17 CRC, 14 IBD and 25 HRA). Forty-four patients had their referral downgraded based on f-Hb <10 µg/g from either ‘urgent’ or ‘urgent suspected cancer’ to ‘routine’; only two of these patients had SBD (both HRA). Thirty-two patients who had f-Hb <10 µg/g were upgraded on the basis of symptoms and patient history only; three had HRA and one patient had IBD.
The electronic patient record review of the 2521 FIT samples not associated with an immediate referral revealed 95.3% recorded f-Hb <10 µg/g. Median age 63 years (IQR: 2–99), 57.8% female. Over a median follow-up period of 11 months (range: 0–18), 183 (7.2%) were subsequently referred to the colorectal service; 27 (1.1%) attended the primary care OOH service with ongoing symptoms, but were not referred; 23 patients (0.9%) were admitted acutely; 9 patients tested positive on participation in the Scottish Bowel Screening Programme; 5 were recalled for a CRC surveillance colonoscopy, and 4 were referred to paediatric clinics. In total, 124 patients ultimately completed bowel investigations. There were 15 cases of SBD subsequently diagnosed (incidence: 0.6%); 4 cases of CRC (incidence: 0.2%), 5 cases of HRA and 6 new cases of IBD. All four CRC cases were associated with an initial f-Hb <10 µg/g. One 88-year-old patient with a progressive normocytic anaemia on rivaroxaban was referred 10 months later after a repeat FIT recorded f-Hb ≥400 µg/g: investigations revealed a Dukes C sigmoid cancer and locally advanced prostate cancer. Another patient who initially presented with abdominal pain was admitted 12 months later with obstructive symptoms with CT revealing a Dukes C CRC at the splenic flexure. Another with diarrhoea was admitted 6 weeks later with a palpable mass and a CT scan showed metastatic bowel cancer. The fourth patient was referred to the haematology clinic shortly after completion of the FIT on the basis of pelvic pain, hypercalcaemia, and a lytic bone lesion on pelvic X-ray. The patient was diagnosed with multiple myeloma and rectal cancer based on CT scan imaging alone as no histology was obtained prior to death.
Post hoc anonymised record linkage to the Scottish Cancer Registry using the CHI number of all patients who have submitted a FIT test to the laboratory generated a list of all incident cases of CRC since introduction of the FIT test. The most recent recorded registration of CRC was in November 2018. Confining the analysis to FIT test results recorded in the first year of the service generated a list of 103 incident cases of CRC with ICD 10 codes C18, C19, and C20 over a follow-up period between 23 and 35 months. All patients diagnosed with CRC were residents in the NHS Tayside catchment area. In other words, no additional cases of cancer were detected within the cohort described, over and above those identified by manual case note review.
Discussion
To our knowledge, this is the first report to describe the utility of providing FIT in primary care as a routine service to assist in the assessment of patients presenting with new bowel symptoms. In the first year, over 5600 samples were received in the laboratory with a positivity of 21.6% at the evidence-based recommended cut-off of ≥10 µg/g.20 Almost 50% of samples were associated with no immediate referral to secondary care (of which 95.0% had f-Hb <10 µg/g). Referral rates to secondary care simultaneously reduced by 15.1%. Of those patients referred and investigated, we confirmed that f-Hb <10 µg/g alone was a good rule-out test for SBD and, if used in conjunction with full blood count and clinical assessment, 93.9% of all SBD would have been correctly identified. We noted that the yield of SBD in those patients triaged to colonoscopy increased from 13.9% to 20.5%, suggesting that patients were more appropriately targeted for colonoscopy. Further, the subsequent incidence of SBD in those patients not immediately referred was very low at 0.7%. Ninety-nine (96%) of the 103 incident CRC cases were correctly identified with this strategy. Post hoc anonymised record linkage to the Scottish Cancer Registry identified no additional incident cases of CRC within the study cohort up to 35 months later and provides all clinicians with the necessary reassurance.
The strength of our data is that they were obtained from a single NHS Board in which all referrals from primary care enter through a common referral pathway, removing selection bias. Furthermore, patients were encouraged to complete an FIT irrespective of their presenting bowel symptoms. FIT samples were delivered to the laboratory via a daily delivery service and analyses performed every weekday. All patient data including referral symptoms, laboratory results, colonoscopy findings and admission notes are linked via their CHI number and, therefore, all outcome data were available. Importantly, we supplemented a manual case note review with a post hoc record linkage of all patients with an FIT test result to the Scottish Cancer Registry to identify any missed cases. A limitation is that we could neither capture the number of patients who were asked to complete an FIT test but chose not to, nor the number of further attendances to primary care of those patients who were not immediately referred and, thus, the true number of further contacts may be an underestimate.
Over the course of 1 year, we provided Gps and consultant gastroenterologists with supporting information (via newsletters and a link to the Gastroenterology website) on the importance of testing for f-Hb and the significance of a finding of f-Hb <10 µg/g, and this was assisted by a robust electronic test requesting and reporting system, and interim data analyses on the impact of the new service. Within 6 months of making FIT available, around two-thirds of all referrals to secondary care submitted FIT samples. The uptake of FIT in this symptomatic population proved better than the uptake of gFOBT in the Scottish Bowel Screening Programme in our region,22 suggesting good patient acceptability. Knowledge of f-Hb was of benefit to the gastroenterologists. Over the first year, it became clear there was an association between f-Hb and the prevalence of SBD detected at colonoscopy; 6.6% in patients with f-Hb <10 µg/g, 32.0% in all patients with f-Hb ≥10 µg/g, rising to 53.8% in patients with f-Hb ≥400 µg/g. It has previously been reported that f-Hb is directly related to the severity of underlying colorectal disease.23 At vetting, the urgency of investigation was adjusted in 210 referrals on the basis of f-Hb with clear benefits to these patients.
However, no test is perfect. As indicated by the negative predictive value, it is inevitable that some patients with f-Hb <10 µg/g will have underlying SBD. Of the patients referred with f-Hb <10 µg/g, 12 had CRC, but all had been referred on the basis of IDA, rectal bleeding, or clinical acumen—thus emphasising the thesis that FIT is an adjunct to current approaches for assessment and is not a diagnostic test on its own.24 Of the patients with f-Hb <10 µg/g who were not immediately referred, four were subsequently recorded to have CRC. However, these data have to be placed in context. Colonoscopy is considered as the gold standard test, but is associated with an interval cancer rate of 0.6% in patients under surveillance25 and a miss rate of 11.0% for advanced adenomas and up to 26% for all adenomas.26 27
In summary, we have demonstrated that FIT can be introduced into primary care as an adjunct to history, examination and blood tests in the assessment of patients with new bowel symptoms. The challenges and considerations required to set up such a service have recently been reviewed.28 Using a cut-off of f-Hb <10 µg/g, in the absence of red flag features of IDA, persistent diarrhoea, rectal bleeding, or palpable mass, provides an objective means of identifying patients with an extremely low risk of underlying SBD and thus avoids invasive investigation. Using this strategy, we have demonstrated that the referral rate to secondary care has decreased and that colonoscopy is more appropriately targeted. Furthermore, our approach demonstrates that knowledge of f-Hb, in conjunction with clinical assessment and full blood count, enables an objective assessment of a patient’s risk of underlying SBD which surpasses the predictive value of symptoms alone. These new data imply that FIT is of great value in the assessment of all patients presenting with new bowel symptoms and therefore should not be reserved simply for perceived ‘low-risk’ patients as currently suggested.29 Future research should focus on refining the predictive value of an elevated f-Hb, and on whether FIT could be used as a ‘rule-out’ test in any other patient groups referred for colonoscopy. Moreover, it might be that other approaches, including risk scoring using additional variables to the f-Hb, such as the Faecal Haemoglobin Concentration, Age and Sex Test score, have advantages in this clinical setting30: further research is required.
Acknowledgments
Lynne Taylor, NHS Tayside Blood Sciences, distributed FIT kits to GP practices.
Footnotes
Contributors: CM, JAS and RJCS designed, planned and conducted the study. RM analysed the faecal samples. CH and DH performed the record linkage. JD performed the statistical analysis and produced the figures and tables. All authors contributed to data interpretation and writing of the paper. CM wrote the final draft which was approved by all authors.
Funding: This study has been funded by Detect Cancer Early initiative (Scottish Government) and Chief Scientist Office.
Competing interests: CGF has undertaken paid consultancy with Immunostics, Ocean, NJ, USA, and Kyowa Medex, Tokyo, Japan, and has received support for attendance at conferences from Alpha Labs, Eastleigh, Hants, UK.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Seifert B, Rubin G, de Wit N, et al. . The management of common gastrointestinal disorders in general practice: a survey by the European Society for primary care gastroenterology (ESPCG) in six European countries. Dig Liver Dis 2008;40:659–66. [DOI] [PubMed] [Google Scholar]
- 2.NICE guideline NG12. Suspected cancer: recognition and referral 2018https://www.nice.org.uk/guidance/ng12 (accessed May 2018). [Google Scholar]
- 3.Jellema P, van der Windt DAWM, Bruinvels DJ, et al. . Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010;340:c1269 10.1136/bmj.c1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swann R, McPhail S, Witt J, et al. . Diagnosing cancer in primary care: results from the National cancer diagnosis audit. Br J Gen Pract 2018;68:e63–72. 10.3399/bjgp17X694169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman DA, Rex DK, Winawer SJ, et al. . Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer. Gastroenterology 2012;143:844–57. 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–62. 10.1056/NEJM199203053261002 [DOI] [PubMed] [Google Scholar]
- 7.Peacock O, Clayton S, Atkinson F, et al. . ‘Be Clear on Cancer’: the impact of the UK National Bowel Cancer Awareness Campaign. Colorectal Dis 2013;15:963–7. 10.1111/codi.12220 [DOI] [PubMed] [Google Scholar]
- 8.Mowat C, Digby J, Strachan JA, et al. . Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut 2016;65:1463–9. 10.1136/gutjnl-2015-309579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald PJ, Digby J, Innes C, et al. . Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis 2013;15:e151–9. 10.1111/codi.12087 [DOI] [PubMed] [Google Scholar]
- 10.Godber IM, Todd LM, Fraser CG, et al. . Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med 2016;54:595–602. 10.1515/cclm-2015-0617 [DOI] [PubMed] [Google Scholar]
- 11.Cubiella J, Salve M, Díaz-Ondina M, et al. . Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and sign referral criteria. Colorectal Dis 2014;16:O273–O282. 10.1111/codi.12569 [DOI] [PubMed] [Google Scholar]
- 12.Auge JM, Fraser CG, Rodriguez C, et al. . Clinical utility of one versus two faecal immunochemical test samples in the detection of advanced colorectal neoplasia in symptomatic patients. Clin Chem Lab Med 2016;54:125–32. 10.1515/cclm-2015-0388 [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Alonso L, Rodríguez-Moranta F, Ruiz-Cerulla A, et al. . An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis 2015;47:797–804. 10.1016/j.dld.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Widlak MM, Thomas CL, Thomas MG, et al. . Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther 2017;45:354–63. 10.1111/apt.13865 [DOI] [PubMed] [Google Scholar]
- 15.Auge JM, Rodriguez C, Espanyol O, et al. . An evaluation of the SENTiFIT 270 analyser for quantitation of faecal haemoglobin in the investigation of patients with suspected colorectal cancer. Clin Chem Lab Med 2018;56:625–33. 10.1515/cclm-2017-0605 [DOI] [PubMed] [Google Scholar]
- 16.Allison JE, Fraser CG, Halloran SP, et al. . Population screening for colorectal cancer means getting fit: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (fit). Gut Liver 2014;8:117–30. 10.5009/gnl.2014.8.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Högberg C, Karling P, Rutegård J, et al. . Immunochemical faecal occult blood tests in primary care and the risk of delay in the diagnosis of colorectal cancer. Scand J Prim Health Care 2013;31:209–14. 10.3109/02813432.2013.850205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias SG, Kok L, de Wit NJ, et al. . Is there an added value of faecal calprotectin and haemoglobin in the diagnostic work-up for primary care patients suspected of significant colorectal disease? A cross-sectional diagnostic study. BMC Med 2016;14 10.1186/s12916-016-0684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juul JS, Hornung N, Andersen B, et al. . The value of using the faecal immunochemical test in general practice on patients presenting with non-alarm symptoms of colorectal cancer. Br J Cancer 2018;119:471–9. 10.1038/s41416-018-0178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NICE Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care Diagnostics guidance [DG30], 2017. Available: https://www.nice.org.uk/guidance/dg30 [Accessed May 2018]. [PubMed]
- 21.Young GP, Sinatra MA, St John DJ. Influence of the delay in stool sampling on fecal occult blood test sensitivity. Clin Chem 1996;42:1107–8. [PubMed] [Google Scholar]
- 22.Quyn AJ, Fraser CG, Stanners G, et al. . Uptake trends in the Scottish bowel screening Programme and the influences of age, sex, and deprivation. J Med Screen 2018;25:24–31. 10.1177/0969141317694065 [DOI] [PubMed] [Google Scholar]
- 23.Digby J, Fraser CG, Carey FA, et al. . Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol 2013;66:415–9. 10.1136/jclinpath-2013-201445 [DOI] [PubMed] [Google Scholar]
- 24.Fraser CG. Faecal immunochemical tests (fit) in the assessment of patients presenting with lower bowel symptoms: concepts and challenges. Surgeon 2018;16:302–8. 10.1016/j.surge.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 25.Robertson DJ, Lieberman DA, Winawer SJ, et al. . Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 2014;63:949–56. 10.1136/gutjnl-2012-303796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heresbach D, Barrioz T, Lapalus M, et al. . Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40:284–90. 10.1055/s-2007-995618 [DOI] [PubMed] [Google Scholar]
- 27.Leufkens A, van Oijen M, Vleggaar F, et al. . Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy 2012;44:470–5. 10.1055/s-0031-1291666 [DOI] [PubMed] [Google Scholar]
- 28.Godber IM, Benton SC, Fraser CG. Setting up a service for a faecal immunochemical test for haemoglobin (fit): a review of considerations, challenges and constraints. J Clin Pathol 2018;71:1041–5. [DOI] [PubMed] [Google Scholar]
- 29.Westwood M, Lang S, Armstrong N, et al. . Faecal immunochemical tests (fit) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: a systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med 2017;15 10.1186/s12916-017-0944-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cubiella J, Digby J, Rodríguez-Alonso L, et al. . The fecal hemoglobin concentration, age and sex test score: development and external validation of a simple prediction tool for colorectal cancer detection in symptomatic patients. Int J Cancer 2017;140:2201–11. 10.1002/ijc.30639 [DOI] [PubMed] [Google Scholar]