Abstract
Background and aims
Non-invasive assessment of fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) is challenging, especially in resource-limited settings. MR or transient elastography and many patented serum scores are costly and not widely available. There are limited data on accuracy of serum-based fibrosis scores in urban slum-dwelling population, which is a unique group due to its dietary habits and socioeconomic environment. We did this study to compare the accuracy of serum-based fibrosis scores to rule out significant fibrosis (SF) in this population.
Methods
Histological and clinical data of 100 consecutive urban slum-dwelling patients with NAFLD were analysed. Institutional ethics committee permission was taken. Aspartate transaminase (AST) to platelet ratio index (APRI), fibrosis-4 index (FIB-4) and FIB-5 scores were compared among those with non-significant fibrosis (METAVIR; F0 to F1; n=73) and SF (METAVIR; F2 to F4; n=27).
Results
AST (IU/mL) (68.3±45.2 vs 23.9±10.9; p<0.0001), alanine transaminase (IU/mL) (76.4±36.8 vs 27.9±11.4; p<0.0001), FIB-4 (2.40±2.13 vs 0.85±0.52; p<0.0001) and APRI (1.18±0.92 vs 0.25±0.16; p<0.0001) were higher and platelets (100 000/mm3) (1.8±0.8 vs 2.6±0.7; p<0.0001), albumin (g/dL) (3.4±0.50 vs 3.7±0.4; p<0.0001), alkaline phosphatase (IU/L) (60.9±10.2 vs 76.4±12.9; p<0.0001) and FIB-5 (−1.10±6.58 vs 3.79±4.25; p<0.0001) were lower in SF group. APRI had the best accuracy (area under the receiver operating characteristic curve=0.95) followed by FIB-4 (0.78) and FIB-5 (0.75) in ruling out SF.
Conclusions
APRI but not FIB-5 or FIB-4 is accurate in ruling out SF in patients with NAFLD in an urban slum-dwelling population.
Keywords: non-alcoholic fatty liver disease, significant fibrosis, APRI, FIB-4, FIB-5
Summary box.
What is already known about this subject?
Non-invasive assessment of fibrosis in non-alcoholic fatty liver disease (NAFLD) in resource poor settings is challenging.
Non-invasive markers are moderately accurate in predicting significant fibrosis.
What are the new findings?
Aspartate transaminase to platelet ratio index (APRI) is an accurate predictor of absence of significant fibrosis in urban slum-dwelling population.
APRI is better than fibrosis-4 index (FIB-4) and FIB-5 at predicting absence of significant fibrosis in urban slum-dwelling subjects with NAFLD.
How might it impact on clinical practice in the foreseeable future?
APRI may be used to rule out significant fibrosis in urban slum dwellers with NAFLD.
Use of APRI may help in mass screening of population at risk of NAFLD complications.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting 17%–46% of adult population worldwide and 5%–31% in India.1–7 Presence of hepatic fibrosis is a predictor of complications in patients with NAFLD. Those with advanced fibrosis need to be on surveillance for complications of liver disease, such as varices and hepatocellular carcinoma. The current gold standard for diagnosis and staging of NAFLD is liver biopsy.8 9 However, high prevalence of the disease, sampling errors and risk of complications preclude its wide use.10–12
Other non-invasive alternatives involving liver imaging techniques such as FibroScan, transient elastography or MR elastography are accurate but have limited availability and are expensive. Transient elastography is less sensitive in patients who are obsese.13 This makes serum non-invasive markers of fibrosis an attractive alternative, especially if they are cheap, easily available and reliable.
Aspartate transaminase (AST) to platelet ratio index (APRI), fibrosis-4 index (FIB-4) and FIB-5 are three cheap and easily available scores for liver fibrosis assessment. FIB-4 is based on age, AST, alanine transaminase (ALT), and platelet count. It was initially described in the AIDS Pegasys Ribavirin International Coinfection Trial study to assess liver fibrosis in HIV and hepatitis C virus (HIV/HCV) coinfected patients,14 and later validated in a large cohort of HCV monoinfected patients.15 Attallah et al developed FIB-5 score, which is based on three biochemical markers (AST/ALT ratio, albumin, alkaline phosphatase (ALP)) and one haematological marker (platelet count). The score was validated on 604 patients with chronic HCV.16 17 Application of FIB-5 in NAFLD has not been studied previously. APRI has been found to have high accuracy to predict the severity of hepatic fibrosis in its first validation study.18
The objective of this study was to compare the performance of simple biochemical scores FIB-5, FIB-4 and APRI with liver biopsy to differentiate non-significant fibrosis (NSF; F0 to F1) and significant fibrosis (SF; F2 to F4) in urban slum-dwelling patients with NAFLD.
Methods
Clinical, biochemical and histological data of 100 consecutive outpatients and inpatients with NAFLD, at a tertiary care centre in India, collected during 2012–2013 was reanalysed. These patients were part of another study done previously at the same centre.19 APRI, FIB-4 and FIB-5 scores were calculated with formulas as shown in table 1.
Table 1.
Equations for scores
| Score | Equation |
| FIB-4 score19 | (Age [years]×AST(IU/L))/(platelet count(109/L)×(ALT (IU/L))1/2) |
| FIB-5 score16 | (albumin (g/L)×0.3+platelet count (109/L)×0.05)−(alkaline phosphatase (IU/L)×0.014+AST/ALT ratio×6+14) |
| APRI score14 | ((AST/ULN)/platelet count (109/L))×100 |
ALT, alanine transaminase;APRI, AST to platelet ratio index; AST, aspartate transaminase;FIB, fibrosis index; ULN, upper limit of normal.
Reanalysis of liver biopsies was done by a single expert histopathologist. SF was defined as a METAVIR score of F2 to F4 and NSF as a score of F0 or F1. The serum scores were compared with the liver biopsy METAVIR scores and the area under the receiver operating characteristic curve (AUROC) for APRI, FIB-4 and FIB-5 was calculated. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each of these scores using previously published cut-offs were calculated.14 16–18
Statistical analysis was done using SPSS software V.25. T-test and χ2 tests were used for assessing parametric data while Mann-Whitney test was used to analyse non-parametric data. P value <0.05 was considered as significant.
Patient and public involvement
Public and patients were not involved in the coproduction of research of this study.
Results
A total of 100 patients were included and analysed in this study. The demographic profile of patients is shown in table 2. Seventy-three patients had NSF while 27 patients had SF.
Table 2.
Demographical data
| Parameters | Non-significant fibrosis | Significant fibrosis |
| Cases, n | 73 (F0 to F24, F1 to F49) | 27 (F2 to F3, F3 to 17, F4 to F7) |
| Age (years) | ||
| Mean | 44.5 | 49.6 |
| SD | 13.3 | 11.4 |
| Range | 18–80 | 24–62 |
| Sex (%) | ||
| Male | 42 (57.5) | 11 (40.7) |
| Female | 31 (42.4) | 16 (59.2) |
SF group had lower platelet counts than NSF group (1.8±0.8 vs 2.6±0.7 × (x10ˆ9/L), respectively; p<0.0001). AST (68.3±45.2 vs 23.9±10.9 IU/mL, p<0.0001) and ALT (76.4±36.8 vs 27.9±11.4 IU/mL, p<0.0001) were higher in SF group as compared with NSF group. Albumin was lower in SF group (3.4±0.5 vs 3.7±0.4 g/dL, respectively; p<0.0001) than the NSF group. ALP was lower in SF group as compared with NSF group (IU/L) (60.9±10.2 vs 76.4±12.9; p<0.0001) (table 3). SF group had higher APRI scores (1.18±0.92 vs 0.25±0.16, respectively; p<0.0001) and FIB-4 scores (2.40±2.13 vs 0.85±0.52, respectively; p<0.0001). FIB-5 scores were lower in SF group (−1.10±6.58 vs 3.79±4.25, respectively; p<0.0001) (table 4).
Table 3.
Laboratory parameters
| Parameters | Mean laboratory parameters (X±SD) | P value | |
| Non-significant fibrosis (n=73) |
Significant fibrosis (n=27) |
||
| Platelet (100 000/mm3) | 2.6±0.7 | 1.8±0.8 | <0.0001 |
| Bilirubin* (mg/dL) | 0.9 (0.4–5) | 1.5 (0.4–4) | 0.06 |
| AST (IU/mL) | 23.9±10.9 | 68.3±45.2 | <0.0001 |
| ALT (IU/mL) | 27.9±11.4 | 76.4±36.8 | <0.0001 |
| Albumin (g/dL) | 3.7±0.4 | 3.4±0.5 | <0.0001 |
| Alkaline phosphate (IU/L) | 76.4±12.9 | 60.9±10.2 | <0.0001 |
*Median
ALT, alanine transaminase; AST, aspartate transaminase.
Table 4.
Mean FIB-4, median FIB-5 and mean APRI between SF and NSF groups
| Parameter | Non-significant fibrosis (n=73) |
Significant fibrosis (n=27) |
P value |
| Mean FIB-4 (X±SD) (range) |
0.85±0.52 (0.22–2.91) |
2.40±2.13 (0.41–8.21) |
<0.0001 |
| Median FIB-5 (X±SD) (range) |
3.79±4.25 (−11 to 14) |
−1.10±6.58 (−15 to 11) |
<0.0001 |
| Mean APRI (X±SD) (range) |
0.25±0.16 (0.07–0.87) |
1.18±0.92 (0.27–3.42) |
<0.0001 |
APRI, aspartate transaminase to platelet ratio index; FIB, fibrosis index; NSF, non-significant fibrosis; SF, significant fibrosis.
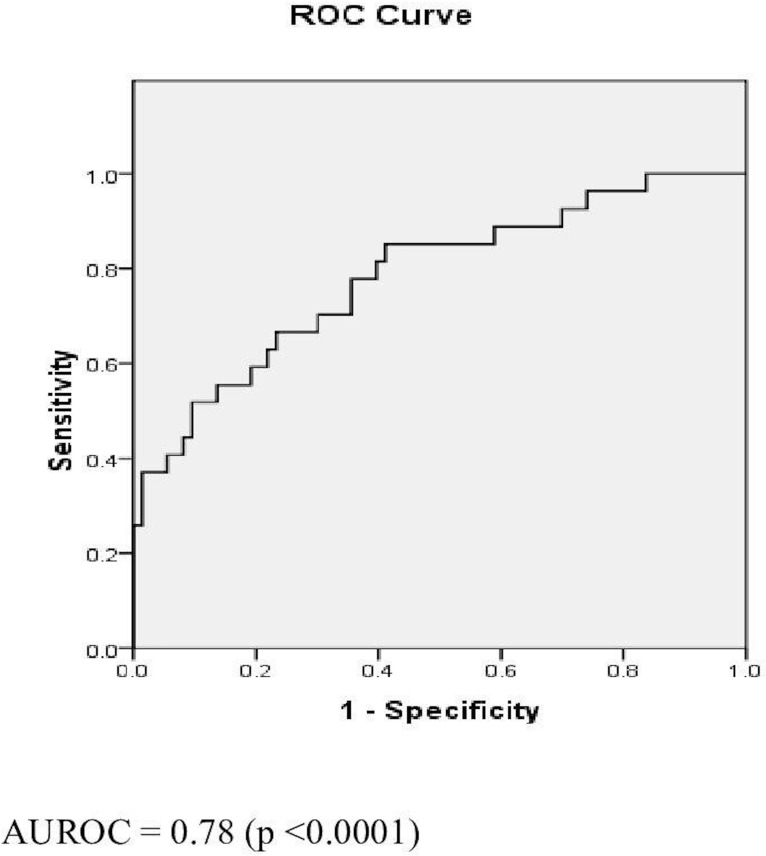
The ROCs of FIB-4, FIB-5 and APRI are as shown in figures 1–3. FIB-4 <1.45 had sensitivity, specificity, PPV, NPV and AUROC of 51.94%, 89.16%, 50%, 90.24% and 0.78, respectively. FIB-5 <0 had sensitivity, specificity, PPV, NPV and AUROC of 55.6%, 81.93%, 37.5%, 89.47% and 0.75, respectively. While FIB-5 ≥7.505 had sensitivity, specificity, PPV, NPV and AUROC of 88.24%, 21.69%, 18.75%, 90% and 0.75%, respectively, APRI <0.45 had sensitivity, specificity, PPV, NPV and AUROC of 85.2%, 87.7%, 58.33%, 96.05% and 0.95, respectively (table 5).
Figure 1.
Receiver operating characteristic (ROC) curve for fibrosis-4 index (FIB-4). AUROC, area under the ROC curve.
Figure 2.
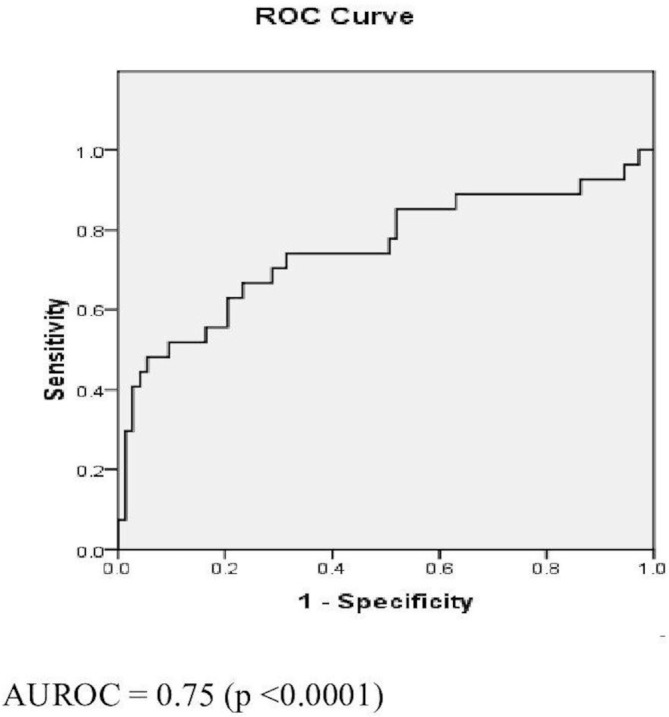
Receiver operating characteristic (ROC) curve for fibrosis-5 index (FIB-5). AUROC, area under the ROC curve.
Figure 3.
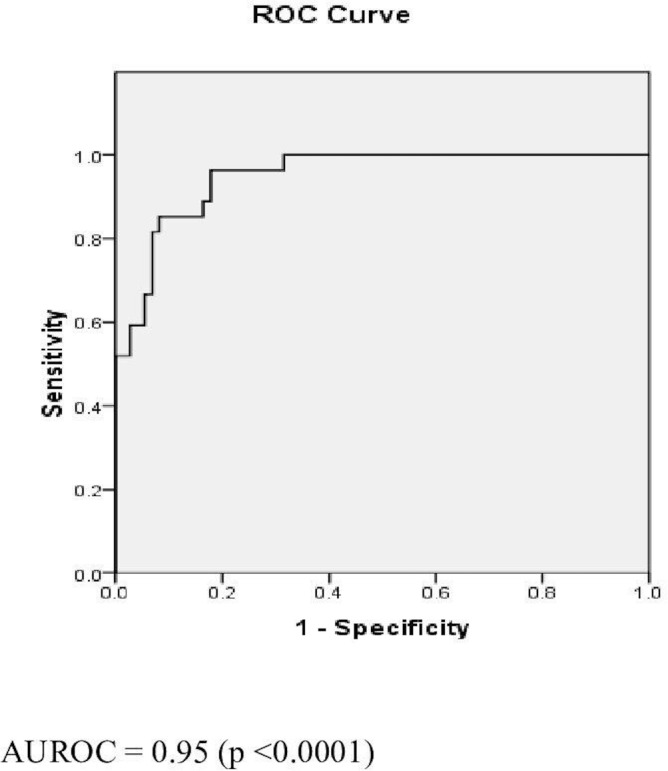
Receiver operating characteristic (ROC) curve for aspartate transaminase to platelet ratio index (APRI). AUROC, area under the ROC curve.
Table 5.
Performance characteristic of FIB-4, FIB-5 and APRI
| Parameters (cut-off) |
FIB-4 (%) (1.45) |
FIB-5 (%) (0) |
FIB-5 (%) (7.505) |
APRI (%) (0.45) |
| Sensitivity | 51.94 | 55.60 | 88.24 | 85.20 |
| Specificity | 89.16 | 81.93 | 21.69 | 87.70 |
| PPV | 50 | 37.50 | 18.75 | 58.33 |
| NPV | 90.24 | 89.47 | 90 | 96.05 |
| AUROC | 0.78 | 0.75 | 0.75 | 0.95 |
APRI, aspartate transaminase to platelet ratio index; AUROC, area under the receiver operating characteristic curve; FIB, fibrosis index; NPV, negative predictive value; PPV, positive predictive value.
Discussion
We evaluated the performance of simple non-invasive, easily available and cheap serum scores in ruling out SF in urban slum-dwelling patients with NAFLD. A little over quarter patients had SF. AST and ALT were higher in SF group while albumin, ALP and platelets were lower in SF group. APRI and FIB-4 were higher in the SF group. FIB-5 was lower in SF group. APRI <0.45 had the highest AUROC of 0.95 (p<0.0001) with sensitivity, specificity, PPV and NPV of 85.2%, 87.7%, 58.33%, and 96.05%, respectively. AUROC of FIB-4 <1.45 was 0.78 (p<0.0001) and that of FIB-5 <0 and ≥7.505 was 0.75 (p<0.0001).
In previous studies of APRI, AUROC was in the range of 0.67–0.87; while our study had an AUROC of 0.95 which is the best among these studies. Previous studies found a sensitivity of 27%–81% as compared with 85.2% in our study. Specificity in our study (87.7%) was similar to that of previous studies (80%–89%). PPV was in the range of 31%–62% in these studies as against 58.3% in current study and NPV was in the range of 84%–95% compared with 96.05% in present study. APRI had performed better in our study to rule out SF as compared with the previous studies.20–25 This may be due to a higher AST and lower platelet count in the SF group in our study, as compared with previous studies at the same stage of fibrosis.
The higher values of AST may be due to the unique pattern of the diet of this urban slum-dwelling population. It has been shown that the urban slum population of India tends to consume high-calorie high-carbohydrate diet.26 27 One of these studies is from the same slum population as in our study.27 It has been observed that serum transaminases activity increases (143% increase for ALT and 90% for AST) with high-calorie high-carbohydrate diet as compared with a balanced normal calorie diet. Increased flux of carbohydrate through glycolysis and related pathways leads to increased transaminase levels. The greater rise in ALT compared with AST may be due to its direct involvement in pyruvate metabolism.28 The differential effect of diet on findings of our studies underscores the need for dietary assessment while studying the NAFLD population.
In past studies of FIB-4, AUROC was in the range of 0.71–0.89 which was comparable with the present study (0.78). Sensitivity was in the range of 76.2%–90% (51.94% in the present study). Specificity was in the range of 54.9%–98% (89.16% in the present study). PPV was in the range of 24%–80% as against 50% in the current study. Similarly, NPV was in the range of 75.3%–98% compared with 90.24% in this study.17 20–25 29 As compared with previous studies, FIB-4 was not helpful for the prediction of SF in our study. This can be explained by the characteristics of our study cohort. Mean age of our study cohort is lower than that of other studies and with higher mean ALT levels resulting in lower mean FIB-4 values, which is reflected as narrow FIB-4 value range and low accuracy of the same.
In previous studies of FIB-5, two different cut-offs were used. AUROC was 0.71 and 0.90 which was 0.75 in the present study. Sensitivity was 18.4% and 98% (55.6% and 88.24%, respectively, at cut-off <0 and cut-off ≥7.505 in the present study). Specificity was 94.4% and 97% (81.93% and 21.69%, respectively, at cut-off <0 and cut-off ≥7.505 in the current study). PPV was 85.7% and 99% as against 18.75% (at cut-off ≥7.505) and 37.5% (at cut-off <0) in the current study. Similarly, NPV was 38.7% and 92% compared with 89.47% (at cut-off <0) and 90% (at cut-off ≥7.505) in this study.16 17 In our study, FIB-5 did not perform better as compared with previous studies. This heterogeneity may be attributable to different study population (HCV-infected patients) in these studies. There are no previous studies of FIB-5 in patients with NAFLD.
As the PPVs were in modest range (18.75%–58.33%) for all these scores, these are not accurate enough to predict SF. Therefore, they cannot replace liver biopsy for this purpose. However, all of these scores had better NPV close to almost 90%. So these scores, particularly APRI with AUROC of 0.95 and NPV of 96.05%, can rule out SF accurately.
A number of other panels of non-invasive serum markers of fibrosis such as the Enhanced Liver Fibrosis panel and FibroTest have been evaluated in patients with NAFLD.30 31 These tests are relatively expensive as some of them involve the measurement of matrix turnover markers.
Prevalence of NAFLD in rural population of India32 33 is 8.7%–30.7%, while the same in urban population of India34 35 is 16.6%–32%. The slum-dwelling population studied in our study is the unique one. This is a significant proportion of our population and yet it is underserved. This study demonstrates the usefulness of simple laboratory markers, particularly APRI, for ruling out SF. These are easily available and cheap. Therefore, they may have wider applicability. Using APRI, liver biopsy can be avoided in a significant proportion of patients with NAFLD. This would be the most cost-effective approach, especially while catering to urban slum-dwelling population. This is the first study to analyse novel parameter FIB-5 in patients with NAFLD, although it did not perform well in this population. One limitation of our study is the small sample size. However, it is difficult to perform a large study while dealing with a niche population like ours.
There are a lot of studies on non-invasive markers in the assessment of fibrosis in patients with NAFLD. These studies have different patient population groups and therefore their findings also vary in these studies. Different diet patterns and their consequent effects on non-invasive serum markers may be responsible for this discrepancy. We therefore recommend analysis of different diet patterns, while assessing the performance of these simple non-invasive markers in future studies. There is a need for a large prospective study to further assess the findings of our study.
Conclusion
APRI but not FIB-5 or FIB-4 is accurate in ruling out SF in patients with NAFLD. It is especially useful in urban slum-dwelling population, a resource-limited setting to identify patients at low risk for progression of NAFLD.
Footnotes
Contributors: AS, KMK, AA, PP, and AC contributed to performing literature searches, study design, data collection, data analysis, data interpretation, and writing of the manuscript. AS contributed to data analysis, data interpretation, and reviewing and writing of the manuscript, and is the study guarantor. SC, HK, SW, MI, and VP contributed to data collection and data analysis. KMK, AC and AS contributed to the study design, data collection, and writing of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved by the institutional ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 2. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Negro F, et al. . Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 2012;91:319–27. 10.1097/MD.0b013e3182779d49 [DOI] [PubMed] [Google Scholar]
- 4. Amarapurkar DN, Hashimoto E, Lesmana LA, et al. . How common is non-alcoholic fatty liver disease in the Asia?Pacific region and are there local differences? J Gastroenterol Hepatol 2007;22:788–93. 10.1111/j.1440-1746.2007.05042.x [DOI] [PubMed] [Google Scholar]
- 5. Pandit K, Goswami S, Ghosh S, et al. . Metabolic syndrome in South Asians. Indian J Endocr Metab 2012;16:44–55. 10.4103/2230-8210.91187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oommen A, Abraham V, George K, et al. . Prevalence of risk factors for non-communicable diseases in rural & urban Tamil Nadu. Indian J Med Res 2016;144:460–71. 10.4103/0971-5916.198668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pradeepa R, Anjana RM, Joshi SR, et al. . Prevalence of generalized & abdominal obesity in urban & rural India—the ICMR-INDIAB study (Phase-I) [ICMR-INDIAB-3]. Indian J Med Res 2015;142:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunt EM, Janney CG, Bisceglie AM, et al. . Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterology 1999;94:2467–74. 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 9. Neuschwander-Tetri B, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37:1202–19. 10.1053/jhep.2003.50193 [DOI] [PubMed] [Google Scholar]
- 10. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500. 10.1056/NEJM200102153440706 [DOI] [PubMed] [Google Scholar]
- 11. Regev A, Berho M, Jeffers LJ, et al. . Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterology 2002;97:2614–8. 10.1111/j.1572-0241.2002.06038.x [DOI] [PubMed] [Google Scholar]
- 12. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449–57. 10.1053/jhep.2003.09022 [DOI] [PubMed] [Google Scholar]
- 13. Pinzani M. Non-invasive evaluation of hepatic fibrosis: don't count your chickens before they're hatched. Gut 2006;55:310–2. 10.1136/gut.2005.068585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterling RK, Lissen E, Clumeck N, et al. . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 15. Vallet-Pichard A, Mallet V, Nalpas B, et al. . FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 16. Attallah AM, Shiha GE, Omran MM, et al. . A discriminant score based on four routine laboratory blood tests for accurate diagnosis of severe fibrosis and/or liver cirrhosis in Egyptian patients with chronic hepatitis C. Hepatol Res 2006;34:163–9. 10.1016/j.hepres.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 17. Shiha G, Seif S, Eldesoky A, et al. . A simple bedside blood test (Fibrofast; FIB-5) is superior to FIB-4 index for the differentiation between non-significant and significant fibrosis in patients with chronic hepatitis C. Hepatol Int 2017;11:286–91. 10.1007/s12072-017-9796-z [DOI] [PubMed] [Google Scholar]
- 18. Wai C, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 19. Parikh P, Ingle M, Patel J, et al. . An open-label randomized control study to compare the efficacy of vitamin E versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi J Gastroenterol 2016;22:192–7. 10.4103/1319-3767.182451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amorim TG, Staub GI, Lazzarotto C, et al. . Validation and comparison of simple non-invasive models for the prediction of liver fibrosis in chronic hepatitis C. Ann hepatol 2012;11:855–61. [PubMed] [Google Scholar]
- 21. McPherson S, Stewart SF, Henderson E, et al. . Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 22. Pissaia A, Borderie D, Bernard D, et al. . Apri and FIB-4 scores are useful after liver transplantation independently of etiology. Transplant Proc 2009;41:679–81. 10.1016/j.transproceed.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 23. Shah AG, Lydecker A, Murray K, et al. . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peleg N, Issachar A, Sneh-Arbib O, et al. . AST to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis 2017;49:1133–8. 10.1016/j.dld.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 25. Sumida Y, Yoneda M, Hyogo H, et al. . Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterology 2012;12 10.1186/1471-230X-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh A, Gupta V, Ghosh A, et al. . Quantitative estimates of dietary intake with special emphasis on snacking pattern and nutritional status of free living adults in urban slums of Delhi: impact of nutrition transition. BMC Nutr 2015;1 10.1186/s40795-015-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chopra H, Chheda P, Kehoe S, et al. . Dietary habits of female urban Slum-dwellers in Mumbai. Indian J Matern Child Health 2012;14:1–13. [PMC free article] [PubMed] [Google Scholar]
- 28. Purkins L, Love ER, Eve MD, et al. . The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a phase I unit. Br J Clin Pharmacol 2003;57:199–208. 10.1046/j.1365-2125.2003.01969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun W, Cui H, Li N, et al. . Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res 2016;46:862–70. 10.1111/hepr.12647 [DOI] [PubMed] [Google Scholar]
- 30. Ratziu V, Massard J, Charlotte F, et al. . Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6 10.1186/1471-230X-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guha IN, Parkes J, Roderick P, et al. . Non-invasive markers of fibrosis in non-alcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455e60. [DOI] [PubMed] [Google Scholar]
- 32. Das K, Das K, Mukherjee PS, et al. . Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 2010;51:1593–602. 10.1002/hep.23567 [DOI] [PubMed] [Google Scholar]
- 33. Majumdar A, Misra P, Sharma S, et al. . Prevalence of nonalcoholic fatty liver disease in an adult population in a rural community of Haryana, India. Indian J Public Health 2016;60:26–33. 10.4103/0019-557X.177295 [DOI] [PubMed] [Google Scholar]
- 34. Mohan V, Farooq S, Deepa M, et al. . Prevalence of non-alcoholic fatty liver disease in urban South Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract 2009;84:84–91. 10.1016/j.diabres.2008.11.039 [DOI] [PubMed] [Google Scholar]
- 35. Amarapurkar D, Kamani P, Patel N, et al. . Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol 2007;6:161–3. [PubMed] [Google Scholar]