Abstract
Background
Colorectal cancer is known to be an extrahepatic complication of non-alcoholic fatty liver disease (NAFLD). However, the interaction of NAFLD with obesity for incident colorectal cancer has not been clarified yet. Moreover, the effect of NAFLD and obesity for incident gastric cancer has not been clarified yet. Thus, we investigated whether NAFLD with or without obesity would be a risk factor for incident gastric cancer as well as colorectal cancer.
Methods
The study period was set from 2003 to 2016. NAFLD was diagnosed by abdominal ultrasonography using standardised criteria. We applied the Cox proportional hazards model to investigate the effect of NAFLD with or without obesity at baseline on incident gastric cancer as well as colorectal cancer. Age, sex, lifestyle factors including smoking states, alcohol consumption and exercise, and diabetes were used as covariates.
Results
During the study period, 27 944 individuals (16 454 men and 11 490 women) were registered in the NAfld in Gifu Area, Longitudinal Analysis study. During the mean (SD) observational period of 2357 (1458) days, incident gastric cancers were diagnosed in 48 individuals (incident rate 0.48 per 1000 person-years) and incident colorectal cancers were diagnosed in 52 individuals (incident rate 0.51 per 1000 person-years). The adjusted HR of NAFLD with obesity for incident gastric cancer was 3.58 (95% CI 1.73 to 7.38, p=0.001) and that for incident colorectal cancer was 2.96 (95% CI 1.73 to 7.38, p=0.003).
Conclusion
NAFLD with obesity was a risk factor for both incident gastric cancer and colorectal cancer in apparently healthy Japanese individuals.
Keywords: obesity, gastric cancer, colorectal cancer, epidemiology, NAFLD
Summary box.
What is already known about this subject?
Non-alcoholic fatty liver disease (NAFLD) has hepatic and extrahepatic complications. The risk of colorectal cancer has been known to increase in patients with NAFLD. However, the interaction of NAFLD and obesity for incident colorectal cancer as well as incident gastric cancer is unknown.
What are the new findings?
NAFLD with obesity is a high-risk state for incident gastric cancer as well as colorectal cancer.
How might it impact on clinical practice in the foreseeable future?
Obese individuals with NAFLD should be encouraged to receive screening examinations including upper gastrointestinal endoscopy for gastric cancer, in addition to occult blood tests for colorectal cancer.
Background
Non-alcoholic fatty liver disease (NAFLD) includes non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH).1 2 Hepatocellular carcinoma (HCC) as well as chronic hepatitis and liver cirrhosis are often observed in patients with NAFLD, especially in those with NASH. In addition to HCC, patients with NAFLD might be at a high risk for colorectal cancer.3 A recent meta-analysis of observational studies suggests that NAFLD is independently associated with a moderately increased prevalence and incidence of colorectal adenomas and cancer.4 This is because NAFLD is a hepatic manifestation of metabolic syndrome, and metabolic syndrome is a well-known risk factor for colorectal cancer.5–7 The mechanism underlying the link between NAFLD and colorectal cancer has not been fully elucidated, but it is likely to be similar to the putative mechanism underlying the link between metabolic syndrome and colorectal cancer.
Obesity is well-known risk factor for colorectal cancer.8–10 Because overnutrition is a cause of development of NAFLD, the major part of individuals with NAFLD are obese or over weight individuals.11 12 Thus, NAFLD with obesity are thought to be a risk for incident colorectal cancer. However, a part of individuals with NAFLD are lean.13 We previously reported that NAFLD without obesity, as well as NAFLD with obesity, are risk factor for incident diabetes.13 However, the association between NAFLD without obesity and incident colorectal cancer has not been clarified yet.
In addition to that, the risk of gastrointestinal cancer increases in individuals with metabolic syndrome.14–17 Thus, the risk of gastric cancer would increase in patients with NAFLD. Indeed, a recent cross-sectional study reported that the prevalence of NAFLD was higher in patients with gastric cancer than in the general population.18 Nonetheless, no study has revealed a direct association between NAFLD and incident gastric cancer. Moreover, the risk of NAFLD with or without obesity are still unknown.
To address this, we performed a longitudinal study to reveal the risk of NAFLD with or without obesity for the incidence of gastric cancer, as well as colorectal cancer, in apparently healthy Japanese individuals. We separated the study subjects according to the presence of NAFLD and/or obesity and investigated the incident rate of gastric cancer as well as colorectal cancer.
Methods
Study population and design
We previously performed a longitudinal cohort study known as the NAGALA study (NAfld in the Gifu Area, Longitudinal Analysis) to reveal the impact of NAFLD on several types of chronic diseases or cancers.7 In the NAGALA study, informed consent was obtained from individuals who participated in a nationwide health check-up programme known as Ningen Dokku, which translates roughly to ‘human dock’ (likening check-up patients to ships being repaired at dock). This nationwide programme promotes public health through the detection of chronic diseases—including gastrointestinal and other cancers—and their risk factors. Blood and urine examinations, upper gastrointestinal series or gastro-oesophageal endoscopy, abdominal ultrasonography and a faecal occult blood test are all part of the routine check-up.
For the NAGALA study, subjects participating in Ningen Dokku at the check-up centre of Murakami Memorial Hospital (Gifu, Japan), which was renamed to Asahi University Hospital since 2018, were recruited. This centre was founded in 1994, and more than 8000 individuals participated in the health check-up at this location each year.
Subjects were consisted with individuals who received health check-up programmes more than two times. Usually, they received health check-up programmes every year or every 2 years. We defined durations between the time when they registered and the time when they received the health-checkup programmes at the last time as observation period.
We provided an information packet to any check-up participant at Asahi University Hospital who was suggested to have a gastrointestinal cancer. The packet included a form that the doctors who performed the diagnostic examinations could use to provide their results to our team. The patients were notified by the information packet that a cancer was suggested by the health check-up programme and were encouraged to receive further examinations to diagnose it. We then collected the medical information on gastrointestinal cancers from the hospitals where the patients went for their additional examinations, again using standardised forms. Specialists in the field of gastrointestinal disease checked the collected information and defined each case as one of gastric cancer or colorectal cancer. We started this system on 1 January 2003 and set the study period as 1 January 2003–31 December 2016. The primary endpoint of the study was set to identify the hazard ratios of NAFLD with or without obesity at the baseline for the incident gastric cancer as well as colorectal cancer after adjusting sex, age and lifestyle factors including smoking habits, alcoholic consumption and physical activities and diabetes. In this study, if an individual was diagnosed with a cancer, the day when the individual was first suggested to have a cancer at the health check-up centre was defined as the incident day.
Individuals who participated in the health check-up programme from 1 January 2003 to 31 December 2016 and registered in the NAGALA study were included into the present study. The following individuals were excluded: (1) those with gastrointestinal cancer at baseline; (2) those receiving a medical treatment for diabetes, dyslipidaemia, hyperuricaemia or hypertension; (3) men who consumed alcohol of more than 210 g per week or females who consumed alcohol of more than 140 g per week19; and (4) those with known liver disease.7 20 Known liver disease was defined as positivity for hepatitis B antigen or hepatitis C antibody, or a history of known liver disease, including viral, genetic, autoimmune or drug-induced liver disease.21 We analysed the longitudinal data of individuals who participated in the health check-up programme more than two times.
Data collection and measurements
The detailed methods for data collection and measurements were described previously.19 Briefly, we used a standardised self-administered questionnaire to acquire information on the medical history and lifestyle factors, including smoking habits, alcoholic consumption and physical activity.7 19 Patients were categorised into three groups according to their smoking status (never smokers, ex-smokers and current smokers). Regarding exercise, if individuals participated in any kind of sports activity at least once a week on a regular basis, we categorised them as regular exercisers.22 Body mass index (BMI) was calculated as weight (kg)/height (m) squared. The conventional criteria for Asian obesity (BMI ≥25 kg/m2) were used.13 23
Definition of NAFLD
The definition of NAFLD was described previously.19 Briefly, the cut-off level of alcohol consumption was set as 210 g/week for men and as 140 g/week for women.19 Fatty liver was diagnosed based on the findings of ultrasonography. Among four known criteria, hepatorenal echo contrast and liver brightness are required for fatty liver.
Statistical analysis
P values of 0.05 or less were considered statistically significant. We analysed all data using SPSS software (V.25). We divided the individuals into four groups according to the presence of NAFLD and obesity. Categorical variables are expressed as percentages (n) and continuous variables are expressed as the means and SD. The HRs of the four groups for incident cancer were calculated by the Cox proportional hazards model, because there were censored cases and the follow-up duration was inconsistent. In the Cox proportional hazards model, the following potential cofactors were used as covariates: alcohol consumption, smoking status, exercise, sex and age at baseline examination.
Results
From 1 January 2003 to 31 December 2016, 27 944 individuals (16 454 men and 11 490 women) were registered in the NAGALA study. At the baseline examinations, 51 individuals were diagnosed with gastrointestinal cancers (25 gastric cancers and 26 colorectal cancers). A total of 15 926 individuals (8585 men and 7341 women) who had participated in the health check-up programme two or more times were analysed (figure 1).
Figure 1.
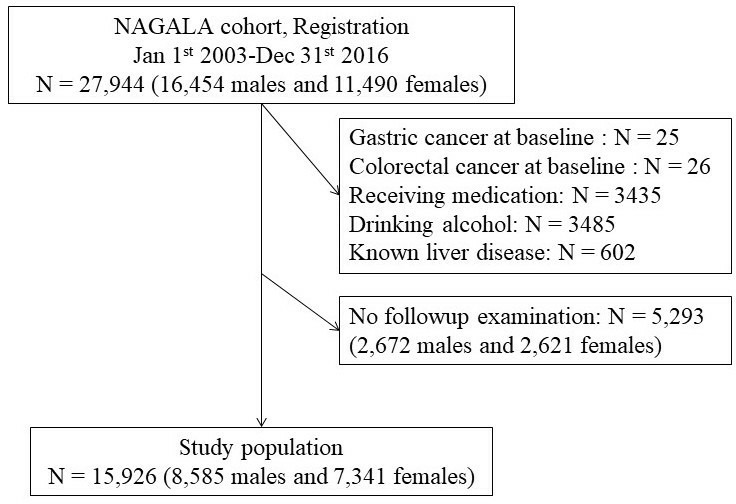
Inclusion and exclusion flow chart. NAGALA, NAfld in Gifu Area, Longitudinal Analysis.
The characteristics of the study population are shown in table 1. During the mean (SD) observation period of 2357 (1458) days, 48 gastric cancers and 52 colorectal cancers were newly diagnosed. The prevalence of never smoker were lower in individuals with incident gastric cancer as well as colorectal cancer (table 1). The metabolic abnormalities, including diabetes, total cholesterol levels, triglycerides levels, low-density lipoprotein cholesterol levels and uric acid levels were higher, and high-density lipoprotein cholesterol levels are lower in individuals with incident gastric cancer as well as colorectal cancer. However, the indicator for advanced forms of NAFLD, such as FIB4 index or NAFLD fibrosis score, was not significantly higher in individuals with incident gastric cancer as well as colorectal cancer.
Table 1.
Basic characteristics of the study population with or without incident cancer
| Non-gastric cancer | Incident gastric cancer | P value | Non-colorectal cancer | Incident colorectal cancer | P value | |
| Number | 15 878 | 48 | 15 874 | 52 | ||
| Male, % (N) | 53.9 (8553) | 66.7 (32) | 0.084 | 53.8 (8547) | 73.1 (38) | 0.006 |
| Exerciser, % (N) | 17.2 (2721) | 18.8 (9) | 0.70 | 17.2 (2722) | 15.7 (8) | 1.00 |
| Never smoker, % (N) | 59.5 (9419) | 37.5 (18) | 0.007 | 59.5 (9414) | 44.2 (23) | 0.08 |
| Ex smoker, % (N) | 18.8 (2979) | 27.1 (13) | 18.8 (2978) | 26.9 (14) | ||
| Current smoker, % (N) | 21.7 (3440) | 35.4 (17) | 21.7 (3442) | 28.8 (15) | ||
| Diabetes, % (N) | 1.9 (295) | 8.3 (4) | 0.012 | 1.9 (297) | 3.8 (2) | 0.26 |
| Alcohol consumption, mg/week | 32.5 (49.3) | 38.5 (51.3) | 0.41 | 32.4 (49.2) | 54.9 (54.9) | 0.002 |
| Age, year | 43.8 (9) | 52.2 (8.3) | <0.001 | 43.8 (9) | 45.3 (7.4) | 0.23 |
| BMI, kg/m2 | 22.3 (3.2) | 23 (3.5) | 0.10 | 22.3 (3.2) | 24.2 (3.8) | <0.001 |
| Systolic blood pressure, mm Hg | 114.7 (15.2) | 118 (15.8) | 0.13 | 114.7 (15.2) | 117.1 (13.4) | 0.25 |
| Diastolic blood pressure, mm Hg | 71.7 (10.5) | 73.7 (10) | 0.19 | 71.7 (10.5) | 74.4 (9.1) | 0.065 |
| AST, IU/L | 18.1 (9.4) | 18.5 (5.1) | 0.75 | 18.1 (9.4) | 17.8 (5.7) | 0.83 |
| ALT, IU/L | 20.7 (15.2) | 22.7 (11.3) | 0.35 | 20.7 (15.2) | 24.2 (12.5) | 0.097 |
| GGT, IU/L | 19.9 (17.5) | 21.4 (16.3) | 0.57 | 19.9 (17.5) | 20.5 (12.8) | 0.81 |
| Alb, g/dL | 4.33 (0.2) | 4.31 (0.18) | 0.53 | 4.33 (0.2) | 4.29 (0.29) | 0.13 |
| Platelet, 103/mL | 24.4 (5.6) | 24.9 (6.1) | 0.49 | 24.4 (5.6) | 25.3 (5.2) | 0.27 |
| FIB4 index | 0.79 (0.38) | 0.89 (0.33) | 0.057 | 0.79 (0.38) | 0.7 (0.25) | 0.11 |
| NAFLD fibrosis score | 1.07 (0.26) | 1.1 (0.31) | 0.34 | 1.07 (0.26) | 1.08 (0.27) | 0.78 |
| Total cholesterol, mg/dL | 200.8 (34.2) | 217.6 (32.1) | 0.001 | 200.8 (34.2) | 213.4 (31.4) | 0.009 |
| Triglycerides, mg/dL | 84 (64.7) | 114.2 (57.3) | 0.001 | 84 (64.5) | 123.1 (108.3) | 0.012 |
| LDL cholesterol, mg/dL | 128 (31.3) | 145 (30.5) | <0.001 | 128 (31.3) | 140.4 (34.8) | 0.004 |
| HDL cholesterol, mg/dL | 55.9 (15.3) | 49.7 (12.8) | 0.002 | 55.9 (15.3) | 51.3 (16.8) | 0.029 |
| Uric acid, mg/dL | 4.81 (1.34) | 5.27 (1.53) | 0.018 | 4.81 (1.34) | 5.62 (1.44) | <0.001 |
| Fasting blood glucose, mg/dL | 95.4 (13.2) | 101.1 (18) | 0.032 | 95.4 (13.2) | 97.4 (11.8) | 0.26 |
| HbA1c, % | 5.2 (0.49) | 5.26 (0.69) | 0.54 | 5.2 (0.49) | 5.2 (0.45) | 0.96 |
χ2 test is applied to categorical variables and t test is applied to continuous variables. Categorical variables are expressed as percentages (n). Continuous variables are expressed as the means (SD).
ALT, alanineaminotransferase; AST, aspartateaminotransferase; Alb, albumin; FIB4, fibrosis-4index; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; HbA1c, hemoglobinA1c; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease.
We evaluated the characteristics of subjects after stratification simultaneously for NAFLD and obesity status (table 2). The rate of male, exerciser, smoker or diabetes are not in the same level among these four groups separated by with or without NAFLD and obesity at the baseline.
Table 2.
Basic characteristics of the study population with or without NAFLD or obesity
| Non-NAFLD without obesity | Non-NAFLD with obesity | NAFLD without obesity | NAFLD with obesity | P value among all four groups* | |||
| Total number | 11 598 | 1117 | 1494 | 1717 | |||
| Incident gastric cancer, % (N) | 0.22 (25) | 0.18 (2) | 0.54 (8) | 0.76 (13) | |||
| Incident colorectal cancer, % (N) | 0.23 (27) | 0.45 (5) | 0.27 (4) | 0.93 (16) | |||
| Male, % (N) | 45.4 (5270) | 63.2 (706) | 81.4 (1216) | 81.1 (1393) | <0.001 | ||
| Exerciser, % (N) | 18.2 (2102) | 15.5 (172) | 14.9 (221) | 13.7 (235) | <0.001 | ||
| Never smoker, % (N) | 63.9 (7393) | 52.5 (586) | 45.8 (682) | 45.4 (776) | <0.001 | ||
| Ex smoker, % (N) | 16.6 (1916) | 20.8 (232) | 27.2 (405) | 25.7 (439) | <0.001 | ||
| Current smoker, % (N) | 19.5 (2261) | 26.7 (298) | 27.1 (403) | 29 (495) | <0.001 | ||
| Diabetes, %(N) | 0.6 (66) | 1.7 (19) | 4.6 (68) | 8.5 (146) | <0.001 | ||
| P value between two groups† | |||||||
| Non-NAFLD without obesity #1 | Non-NAFLD with obesity #2 | NAFLD without obesity #3 | NAFLD with obesity #4 | #1 and #2 | #1 and #3 | #1 and #4 | |
| Alcohol consumption, mg/week | 31.3 (48.4) | 39.6 (53.8) | 35.1 (51.3) | 33.6 (49.6) | 0.017 | <0.001 | 0.225 |
| Age, year | 43.4 (9.1) | 44.4 (8.7) | 46.6 (8.6) | 44.3 (8.3) | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 20.9 (2.1) | 26.6 (1.6) | 23.1 (1.4) | 27.9 (2.6) | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure, mm Hg | 111.3 (13.9) | 122.5 (13.5) | 120.3 (14.3) | 128.1 (15) | <0.001 | <0.001 | <0.001 |
| Diastolic blood pressure, mm Hg | 69.4 (9.7) | 76.8 (9.5) | 75.8 (9.7) | 80.8 (10.1) | <0.001 | <0.001 | <0.001 |
| AST, IU/L | 16.9 (8.2) | 18.4 (13.1) | 20 (7.8) | 24.4 (12) | <0.001 | <0.001 | <0.001 |
| ALT, IU/L | 16.9 (11.2) | 22.2 (12.2) | 28.4 (14.5) | 38.3 (23.5) | <0.001 | <0.001 | <0.001 |
| GGT, IU/L | 16.8 (13.5) | 23.3 (19.7) | 27.2 (21.6) | 32.6 (25.8) | <0.001 | <0.001 | <0.001 |
| Alb, g/dL | 4.32 (0.2) | 4.31 (0.19) | 4.39 (0.2) | 4.38 (0.2) | <0.001 | 0.247 | <0.001 |
| Platelet, 103/mL | 24.1 (5.6) | 25.1 (5.7) | 24.9 (5.3) | 25.2 (5.6) | <0.001 | <0.001 | <0.001 |
| FIB4 index | 0.8 (0.39) | 0.75 (0.4) | 0.76 (0.33) | 0.75 (0.34) | 0.001 | <0.001 | <0.001 |
| NAFLD fibrosis score | 1.05 (0.23) | 1.11 (0.32) | 1.07 (0.26) | 1.14 (0.35) | 0.010 | <0.001 | <0.001 |
| Total cholesterol, mg/dL | 196.9 (33.4) | 206.2 (33.8) | 212.1 (33.6) | 214.3 (33.9) | <0.001 | <0.001 | <0.001 |
| Triglycerides, mg/dL | 68.9 (45.4) | 97.9 (55.1) | 123 (72.3) | 144.5 (108.3) | <0.001 | <0.001 | <0.001 |
| LDL cholesterol, mg/dL | 123.7 (30.1 | 137 (30.7 | 140.1 (30.7 | 141.6 (32.3 | <0.001 | <0.001 | <0.001 |
| HDL cholesterol, mg/dL | 59.4 (15.1) | 49.5 (11.9) | 47.3 (11.8) | 44 (9.7) | <0.001 | <0.001 | <0.001 |
| Uric acid, mg/dL | 4.51 (1.22) | 5.22 (1.32) | 5.63 (1.14) | 5.9 (1.3) | <0.001 | <0.001 | <0.001 |
| Fasting blood glucose, mg/dL | 92.9 (9.9) | 97.7 (11.4) | 101.6 (16.6) | 105.1 (21.6) | <0.001 | <0.001 | <0.001 |
| HbA1c, % | 5.13 (0.39) | 5.23 (0.46) | 5.36 (0.63) | 5.5 (0.79) | <0.001 | <0.001 | <0.001 |
Categorical variables are expressed as percentages (n). Continuous variables are expressed as the means (SD).
*The difference of the ratio among all four groups is tested by χ2 test.
†The difference between each group (#2, #3 or #4) and non-NAFLD without obesity group (#1) is tested by to Dunnett's test.
ALT, alanineaminotransferase; AST, aspartateaminotransferase; Alb, albumin; BMI, body mass index; FIB4, fibrosis-4index; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; HbA1c, hemoglobinA1c; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease.
The incidence rate of gastric cancer was 0.34 per 1000 person years in the non-NAFLD without obesity, 0.29 in the non-NAFLD with obesity, 0.83 in the NAFLD without obesity and 1.21 in the NAFLD with obesity group (table 3). The incidence rate of colorectal cancer was 0.37 per 1000 person years in the non-NAFLD without obesity, 0.72 in the non-NAFLD with obesity, 0.41 in the NAFLD without obesity and 1.49 in the NAFLD with obesity group.
Table 3.
Incident rate of gastric cancer or colorectal cancer
| N | Incident gastric cancer | Incident colorectal cancer | |||
| Cases | Incidence rate (per 1000 person-year) | Cases | Incidence rate (per 1000 person-year) | ||
| Non-NAFLD without obesity | 11 598 | 25 | 0.34 | 27 | 0.37 |
| Non-NAFLD with obesity | 1117 | 2 | 0.29 | 5 | 0.72 |
| NAFLD without obesity | 1494 | 8 | 0.83 | 4 | 0.41 |
| NAFLD with obesity | 1717 | 13 | 1.21 | 16 | 1.49 |
The incidence rate are shown as cases per 1000 person-years.
NAFLD, non-alcoholic fatty liver disease.
The cumulative hazard curves for incident gastric cancer are shown in figure 2. The adjusted HR of NAFLD with obesity was 3.58 (95% CI 1.73 to 7.38, p=0.001) for gastric cancer and 2.96 (1.44–6.09, p=0.003) for colorectal cancer in comparison with non-NAFLD without obesity (table 4). In addition, the adjusted HR of NAFLD without obesity for gastric cancer was 1.96 (0.86–4.47, p=0.11), although it did not reach the level of statistical significance. Finally, the incidence rate of gastric cancer in the non-NAFLD patients with obesity was as low as that in the non-NAFLD patients without obesity.
Figure 2.
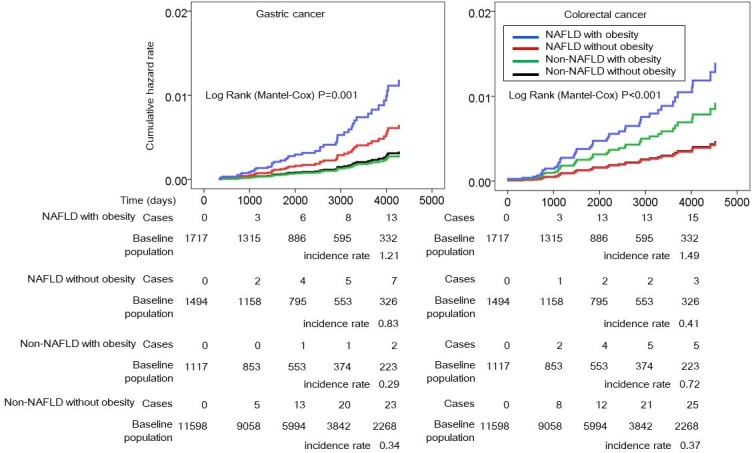
HRs of NAFLD with or without obesity for incident gastric cancer and colorectal cancer. The vertical axis shows the cumulative hazard rate for gastric cancer and colorectal cancer, and the horizontal axis indicates the observational time in days. The blue lines represent NAFLD with obesity, the red lines indicate NAFLD without obesity, the green lines indicate non-NAFLD with obesity and the black lines indicate non-NAFLD without obesity. NAFLD, non-alcoholic fatty liver disease.
Table 4.
The adjusted hazard risk of individuals with or without NAFLD and obesity for incident gastric cancer or colorectal cancer
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Incident gastric cancer | Model 1 | Model 2 | ||
| Non-NAFLD without obesity | Ref | Ref | ||
| NAFLD without obesity | 1.96 (0.86 to 4.47) | 0.11 | 1.84 (0.8 to 4.23) | 0.15 |
| Non-NAFLD with obesity | 0.88 (0.21 to 3.73) | 0.86 | 0.86 (0.2 to 3.67) | 0.84 |
| NAFLD with obesity | 3.58 (1.73 to 7.38) | 0.001 | 3.3 (1.57 to 6.9) | 0.002 |
| Exerciser | 1.00 (0.48 to 2.10) | 1.00 | 1.01 (0.48 to 2.13) | 0.97 |
| Alcohol consumption | 1.00 (0.99 to 1.00) | 0.6 | 1 (0.99 to 1) | 0.59 |
| Male | 0.7 (0.31 to 1.6) | 0.4 | 0.69 (0.3 to 1.57) | 0.37 |
| Age | 1.13 (1.1 to 1.17) | <0.001 | 1.13 (1.1 to 1.17) | 0 |
| Never smoker | Ref | Ref | ||
| Ex-smoker | 1.64 (0.67 to 4.01) | 0.28 | 1.65 (0.68 to 4.03) | 0.27 |
| Current smoker | 2.71 (1.2 to 6.11) | 0.016 | 2.75 (1.22 to 6.19) | 0.014 |
| Diabetes | 2.19 (0.76 to 6.31) | 0.15 | ||
| Incident colorectal cancer | Model 1 | Model 2 | ||
| Non-NAFLD without obesity | Ref | Ref | ||
| NAFLD without obesity | 0.98 (0.33 to 2.88) | 0.97 | 0.99 (0.34 to 2.93) | 0.99 |
| Non-NAFLD with obesity | 1.96 (0.74 to 5.17) | 0.17 | 1.97 (0.75 to 5.2) | 0.17 |
| NAFLD with obesity | 2.96 (1.44 to 6.09) | 0.003 | 3.04 (1.47 to 6.3) | 0.003 |
| Exerciser | 0.83 (0.37 to 1.89) | 0.66 | 0.83 (0.37 to 1.88) | 0.66 |
| Alcohol consumption | 1 (1 to 1.01) | 0.12 | 1 (1 to 1.01) | 0.12 |
| Male | 1.33 (0.57 to 3.14) | 0.51 | 1.34 (0.57 to 3.15) | 0.5 |
| Age | 1.04 (1 to 1.07) | 0.053 | 1.04 (1 to 1.07) | 0.049 |
| Never smoker | Ref | Ref | ||
| Ex smoker | 1.40 (0.62 to 3.15) | 0.42 | 1.39 (0.62 to 3.14) | 0.43 |
| Current smoker | 1.33 (0.6 to 2.94) | 0.48 | 1.33 (0.6 to 2.93) | 0.49 |
| Diabetes | 0.64 (0.09 to 4.81) | 0.67 |
The HRs of the four group for incident gastric cancer and colorectal cancer are calculated by the Cox proportional hazards model. In model 1, exerciser, alcohol consumption, male, age, smoking sates are used as covariates. In addition to them, diabetes is used as a covariate in model 2.
NAFLD, non-alcoholic fatty liver disease.
Discussion
This study clearly indicated that NAFLD with obesity at baseline was a high-risk state for gastric cancer, as well as colorectal cancer. There is no clear evidence that gastric cancer is one of the extrahepatic complications of NAFLD. Moreover, the risk of NAFLD with or without obesity for incident gastric cancer as well as colorectal cancer was unknown. However, this longitudinal study revealed, for the first time, that the risk of NAFLD with obesity for gastric cancer was statistically significantly high (HR 3.58, 95% CI 1.73 to 7.38, p=0.001). Additionally, that the risk of NAFLD with obesity for colorectal cancer was also statistically significantly high (HR 2.96, 95% CI 1.44 to 6.09, p=0.003).
Insulin resistance is pivotal for the progression of NAFLD.24 Insulin resistance was thought to be higher in obese individuals with NAFLD than those in lean individuals with NAFLD, but it has not been clarified yet. The high concentration of insulin caused by insulin resistance is thought to promote the proliferation of cancers.25 The insulin-like growth factor axis is upregulated in individuals with NAFLD, and this upregulation is thought to stimulate the formation of gastric cancer.3 The abnormal hormonal action of enlarged adipocytes in patients with NAFLD could also stimulate the formation of gastric cancer.26 Adipokines, inflammatory cytokines and chemokines produced by enlarged adipocytes in individuals with NAFLD is thought to modulate cellular proliferation and apoptosis.26 Among them, adiponectin has anticarcinogenic effects and is one of the downregulated adipokines in individuals with NAFLD.3 Adiponectin is thought to repress cell growth of carcinoma through AMPc-activated protein kinase.3 Adiponectin also induces apoptosis through a caspase-dependent pathway in endothelial cells.3 Tumour necrosis factor-alpha (TNF-α) is one of the upregulated adipokines in individuals with NAFLD. TNF-α was first identified as a cytotoxic factor produced by lymphocytes in the site of cancer.27–29 However, TNF-α is also known to promote carcinogenesis.30–32 Thus, insulin resistance, upregulated insulin-like growth factor axis and the abnormal hormonal actions accompanied by metabolic abnormality in individuals with NAFLD may be pivotal for the development of incident gastric cancer. However, future investigations will be needed to clarify whether there is a direct association between metabolic abnormality in individuals with NAFLD and incident gastric cancer.
The strengths of the present study include its population-based design and longitudinal analysis. In addition, the gastrointestinal cancer cases collected in this study were identified reliably through systemic surveillance. However, several limitations should also be noted. First, the state of Helicobacter pylori infection was not available as a potential confounder. Second, the diagnosis of NAFLD was done using ultrasonography, rather than liver biopsy. Nonetheless, ultrasonography has a high sensitivity and specificity for diagnosing fatty liver, has been validated and is reasonable noninvasive surrogate measure for use in clinical settings.33 34 Although it may provide a less accurate diagnosis than liver biopsy, it would be impossible to perform liver biopsy in such a large number of healthy individuals. Third, subjects received health check-up programmes every year or every 2 years. We defined durations between the time when they registered and the time when they received the health-checkup programmes at the last time as observation period. Gastric cancer or colorectal cancer might occur after the last visit. However, the mean observation period was not different with or without NAFLD or obesity. When cases were diagnosed and/or treated outside our facilities, we collected the medical information via letters. However, if cases died before carcinoma occurred, we treated them as censored cases. Fourth, we have no anatomopathological data. Thus, we have not assessed anatomopathological features of gastric cancer or colorectal cancer between subjects with or without NAFLD. Fifth, we have no detailed data regarding physical activity, body composition or diet. These states have remained as a potential confounders for incident colorectal cancer and gastric cancer. Lastly, the generalisability of our study to non-Japanese populations is uncertain.
In conclusion, NAFLD with obesity at baseline is a risk factor for gastric cancer as well as colorectal cancer. Obese individuals with NAFLD should be encouraged to receive screening examinations for gastric cancer, including upper gastrointestinal endoscopy, in addition to occult blood tests for colorectal cancer. Future investigations are needed to reveal the efficacy of lifestyle modification in obese individuals with NAFLD to prevent incident gastric cancer.
Acknowledgments
All authors would like to thank the medical staff in Asahi University Hospital.
Footnotes
Contributors: MH: conception and design, drafting of the manuscript and analysis and interpretation of data. YH: conception and design, and critical revision of the manuscript. AO: acquisition of data. TK: acquisition of data. MF: critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: The Ethics Committee of Asahi University Hospital approved the study (ID of the approval: 2018-05-03).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37:1202–19. 10.1053/jhep.2003.50193 [DOI] [PubMed] [Google Scholar]
- 2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 3. Sanna C, Rosso C, Marietti M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci 2016;17 10.3390/ijms17050717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantovani A, Dauriz M, Byrne CD, et al. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metabolism 2018;87:1–12. 10.1016/j.metabol.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 5. Tilg H, Diehl AM. NAFLD and extrahepatic cancers: have a look at the colon. Gut 2011;60:745–6. 10.1136/gut.2011.239392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanni E, Marengo A, Mezzabotta L, et al. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis 2015;35:236–49. 10.1055/s-0035-1562944 [DOI] [PubMed] [Google Scholar]
- 7. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–8. 10.7326/0003-4819-143-10-200511150-00009 [DOI] [PubMed] [Google Scholar]
- 8. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65. 10.1093/ajcn/86.3.556 [DOI] [PubMed] [Google Scholar]
- 9. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomark Prev 2007;16:2533–47. 10.1158/1055-9965.EPI-07-0708 [DOI] [PubMed] [Google Scholar]
- 10. Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19–30. 10.1111/j.1467-789X.2009.00613.x [DOI] [PubMed] [Google Scholar]
- 11. Wong VW-S, Chan W-K, Chitturi S, et al. Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. 10.1111/jgh.13857 [DOI] [PubMed] [Google Scholar]
- 12. Chitturi S, Wong VW-S, Chan W-K, et al. The Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-Part 2: management and special groups. J Gastroenterol Hepatol 2018;33:86–98. 10.1111/jgh.13856 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda T, Hamaguchi M, Kojima T, et al. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int 2016;36:275–83. 10.1111/liv.12912 [DOI] [PubMed] [Google Scholar]
- 14. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 2005;42:987–1000. 10.1002/hep.20920 [DOI] [PubMed] [Google Scholar]
- 15. Perseghin G. Viewpoints on the way to a consensus session: where does insulin resistance start? The liver. Diabetes Care 2009;32 Suppl 2:S164–S167. 10.2337/dc09-S303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol 2014;20:9217–28. 10.3748/wjg.v20.i28.9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin Y, Ness-Jensen E, Hveem K, et al. Metabolic syndrome and esophageal and gastric cancer. Cancer Causes Control 2015;26:1825–34. 10.1007/s10552-015-0675-4 [DOI] [PubMed] [Google Scholar]
- 18. Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 2012;35:2402–11. 10.2337/dc12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto Y, Hamaguchi M, Kojima T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol 2015;30:546–52. 10.1111/jgh.12786 [DOI] [PubMed] [Google Scholar]
- 20. Fukuda Y, Hashimoto Y, Hamaguchi M, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int 2016;36:713–20. 10.1111/liv.12977 [DOI] [PubMed] [Google Scholar]
- 21. McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis 2004;8:521–33. 10.1016/j.cld.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 22. Ryu S, Chang Y, Kim D-I, et al. Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 2007;53:71–7. 10.1373/clinchem.2006.078980 [DOI] [PubMed] [Google Scholar]
- 23. Hashimoto Y, Tanaka M, Kimura T, et al. Hemoglobin concentration and incident metabolic syndrome: a population-based large-scale cohort study. Endocrine 2015;50:390–6. 10.1007/s12020-015-0587-9 [DOI] [PubMed] [Google Scholar]
- 24. Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999;107:450–5. 10.1016/S0002-9343(99)00271-5 [DOI] [PubMed] [Google Scholar]
- 25. Gupta K, Krishnaswamy G, Karnad A, et al. Insulin: a novel factor in carcinogenesis. Am J Med Sci 2002;323:140–5. 10.1097/00000441-200203000-00004 [DOI] [PubMed] [Google Scholar]
- 26. Fan J-H, Wang J-B, Wang S-M, et al. Body mass index and risk of gastric cancer: a 30-year follow-up study in the Linxian general population trial cohort. Cancer Sci 2017;108:1667–72. 10.1111/cas.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolb WP, Granger GA. Lymphocyte in vitro cytotoxicity: characterization of human lymphotoxin. Proc Natl Acad Sci U S A 1968;61:1250–5. 10.1073/pnas.61.4.1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruddle NH, Waksman BH. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med 1968;128:1267–79. 10.1084/jem.128.6.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 1975;72:3666–70. 10.1073/pnas.72.9.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000;404:398–402. 10.1038/35006081 [DOI] [PubMed] [Google Scholar]
- 31. El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003;124:1193–201. 10.1016/S0016-5085(03)00157-4 [DOI] [PubMed] [Google Scholar]
- 32. Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 2003;125:364–71. 10.1016/S0016-5085(03)00899-0 [DOI] [PubMed] [Google Scholar]
- 33. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102:2708–15. 10.1111/j.1572-0241.2007.01526.x [DOI] [PubMed] [Google Scholar]
- 34. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–90. 10.1002/hep.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]


