Abstract
Knee osteoarthritis (OA) affects the joint beyond just the articular cartilage. Specifically, magnetic resonance imaging-identified bone marrow lesions (BML) in the subchondral bone have both clinical and pathophysiological significance. Compared to joint space narrowing on traditional radiographs, the presence of BMLs has been better correlated with severity of clinical symptoms as well as clinical deterioration. Presence of a BML increases the likelihood for progression to a total knee arthroplasty by up to nine fold. Histochemical analysis of BMLs has shown increased levels of tumor necrosis factor-alpha, matrix metalloproteinases and substance P, thought to stimulate pain receptors in osteoarthritis.
Level of evidence:
V
Key Words: Bone marrow lesions (BML), Knee osteoarthritis, Magnetic resonance imaging (MRI)
Introduction
Knee osteoarthritis (OA) is a leading cause of disability for those over the age of 65 and has been reported to be increasingly prevalent in younger individuals (1-3). In addition to loss of articular cartilage, OA also affects the entire joint and the associated pathologic changes have been recognized in synovial, meniscal, ligamentous, tendinous, and muscular periarticular tissues (4). Importantly, knee OA is also associated with substantial alterations in subchondral bone (SCB), including changes in histology, histochemistry, morphology and mechanical properties.
Historically, conventional radiography has been the accepted standard for describing the severity of OA in effected joints (5). Classification systems are typically based on quantification of joint space narrowing and osteophyte formation (6). Given the knowledge that OA has a global effect on the joint, it is not surprising that measuring loss of cartilage radiographically alone has limited clinical utility and only modest correlation with symptom severity (7). Advanced imaging modalities, such as magnetic resonance imaging (MRI), have the capacity to visualize soft tissue and quantitate inflammatory changes associated with OA. It has also been reported that MRI of SCB in patients with knee OA has important clinical relevance (8, 9).
Substantial evidence has revealed that SCB plays a critical role in both the pathophysiology and progression of knee OA (10-15). Alterations of SCB, in association with OA, have significant clinical implications including, correlation with severity of symptoms and prognosis (9-10). The purpose of this paper is to provide a concise literature review of articles describing the clinical and pathophysiologic significance of MRI identified bone marrow lesions (BML) associated with knee OA.
BML IDENTIFICATION WITH MRI
The term BML was first used by Wilson et al. to describe a specific MRI finding recognized in cancellous bone (14). A BML has decreased signal intensity when visualized by T1 weighted sequencing, but increased intensity if T2 weighted sequences are employed (14, 16, 17). Due to high signal on T2 weighted sequences, originally it was proposed that there was increased water content (edema) in the region of the BML. Hence, the term bone marrow edema (BME) is still commonly used when describing this finding (13, 18).
Numerous investigators have reported that a SCB BML is a common finding in patients with knee OA (18-21). A BML associated with knee OA may resolve, fluctuate in size or expand over a short period of time (15, 22). However, longitudinal studies have demonstrated that a BML is more likely to progress and increase in size over time rather than resolve in patients with knee OA (11, 23).
Subchondral bone attrition (SBA) is another term commonly used to describe a specific MRI finding, that being, subsidence of the subchondral bone plate (SCBP) (20). It has been confirmed by numerous investigators that SBA is closely associated with the presence of a BML in patients with knee OA (20, 24). Additionally, a large BML or one increasing in size is highly predictive of the rate at which SBA occurs and subsidence of the SCBP results in progressive joint malalignment (25). Therefore, both recognition of the presence and quantification of the size of a BML are important when evaluating an MRI in a patient with knee OA.
Hayashi et al. evaluated the role of intermediate weighted fat suppressed (IWFS) spin echo and dual echo steady state (DESS) sequences on 3T MRI for assessment of subchondral BMLs and subchondral cysts (26). It was determined that the IWFS sequence has an increased sensitivity to identify both the size and presence of subchondral BMLs compared with DESS sequences. Based on these findings, it is recommended that IWFS sequences be used for determining the size and extent of BMLs, while the DESS sequence should be used when focusing on the presence of subchondral cysts (26).
In addition to DESS sequences, Gradient recalled echo (GRE) type sequences are excellent for delineating subchondral cysts but insensitive for detecting BMLs; therefore, GRE sequences can be an accurate tool for identifying differences between subchondral cysts and BMLs (27).
BMLs can also be seen on T1-weighted images; however, they lack the sensitivity of fat suppressed T2-weighted images (27). Mayerhoefer et al. compared short tau inversion recovery (STIR) and T1-weighted contrast-enhanced MRI and noted that STIR was the optimal method for determining the signal contrast and size of BMLs (28).
More exotic MRI sequences aside, simple fat suppressed T2 or intermediate-weighted sequences or STIR sequences (i.e., any fluid-sensitive sequence), available on all commercial scanners, are satisfactory for characterization of size and location of BMLs.
Multiple scoring systems have been created and used to effectively measure structural abnormalities seen on MRI in knee OA. These scoring methodologies are typically divided into two major types: quantitative and semi-quantitative (29). Quantitative whole-joint measurements typically evaluate specific factors including bone volume, BML volume, meniscal position and volume, and synovial volume. These results are typically acquired through three-dimensional coverage of cartilaginous regions; however, the major limitation of this method is that the ill-defined shape and feathery margin of BMLs may make it difficult to evaluate their true volume (30-32).
It is crucial that BMLs are properly identified and segmented accurately, either by manual or automated threshold-based methods. Although both techniques are accessible and more accurate than using alternative quantitative measurements, manual methods can be subjective in volume calculation due to the irregular anatomical presence of BMLs. Moreover, the automatic threshold calculation has been reported to have increased intra- and inter-observer reliability (33, 34).
Semi-quantitative imaging analysis, introduced by Peterfy et al. in 1999, is more scale and gradient dependent (scoring volume) (32). Four major scoring systems have been created specifically for semi-quantitative analysis of knee OA: Whole-Organ Magnetic Resonance Imaging Score (WORMS), Knee Osteoarthritis Scoring System (KOSS), Boston Leeds Osteoarthritis Knee Score (BLOKS), and Magnetic Resonance Imaging Osteoarthritis Knee Score (MOAKS) [Table 1] (29, 32, 34, 35). These approaches are used to score most potentially involved articular features associated with OA, including articular bone marrow abnormality, bone attrition, cartilage morphology, osteophytes, menisci, anterior and posterior cruciate ligaments, medial and lateral collateral ligaments, effusions, periarticular cysts, bursitis, and intraarticular loose bodies. Because all of these systems have comparable reliability, choosing the most appropriate method for analysis remains a challenge.
Table 1.
MRI scoring systems for semi-quantiative analysis of BMLs associated with knee osteoarthritis
| 1. Whole-Organ Magnetic Resonance Imaging Score (WORMS) a. Subregional approach graded from 0-3 based on size as opposed to evaluating individual lesions |
| 2. Knee Osteoarthritis Scoring System (KOSS) a. Grades BMLs individually for each subregion and size of each lesion. Scores range from 0 to 3 with each score representing a BML that is absent, < 5mm, 5 mm to 2 cm, and > 2 cm |
| 3. Boston Leeds Osteoarthritis Knee Score (BLOKS) a. BMLs scored according to their size, percentage of subchondral surface area of the BML, and percentage of cystic BMLs |
| 4. Magnetic Resonance Imaging Osteoarthritis Knee Score (MOAKS) a. Divides the knee into 15 articular subregions and a single grade for size is generated for each region, incorporating all BMLs into one score |
Many studies have employed WORMS in evaluating knee OA (20, 36, 37). Given the close proximity and merging characteristics of neighboring BMLs, changes in a single lesion can be problematic, especially during periods of follow-up (38-40). Therefore, BML scoring using WORMS, a system designed based on a subregional approach graded from 0 to 3 as opposed to evaluating individual lesions, helps resolve the issue of individual BML assessment.
In contrast, the KOSS and BLOKS systems grade BMLs individually for each subregion and the size of each lesion. For KOSS, the scores range from 0 to 3, with each score representing a BML that is absent, diameter <5 mm; diameter 5 mm to 2 cm; and diameter >2 cm, respectively (38). The BLOKS system, which was introduced by Hunter et al., uses a similar approach to KOSS regarding the subregional division of the articular surfaces (35). BMLs in this scoring system are scored according to their size, percentage of subchondral surface area of the BML, and percentage of cystic BMLs.
MOAKS is a relatively new system, developed by Hunter et al., that attempts to rectify the limitations of other systems (34). In MOAKS, the knee is divided into 15 articular subregions for scoring BMLs, similar to the WORMS system. A single grade for size is generated for each region, incorporating all BMLs into one score (1, <33% of subregional volume; 2, 33%-66%; and 3, >66%). The number of BMLs is recorded in each subregion and the percentage of BMLs that are distinct from cysts is evaluated (1, <33% of lesions that are BML versus cyst; 2, 33%-66%; 3, >66%) (34, 39-41). The 15 subregions that are assessed in the WORMS and MOAKS systems are the medial and lateral patella, medial and lateral femur (anterior, central, and posterior), medial and lateral tibia (anterior, central, and posterior), and subspinous tibia [Figures 1-4].
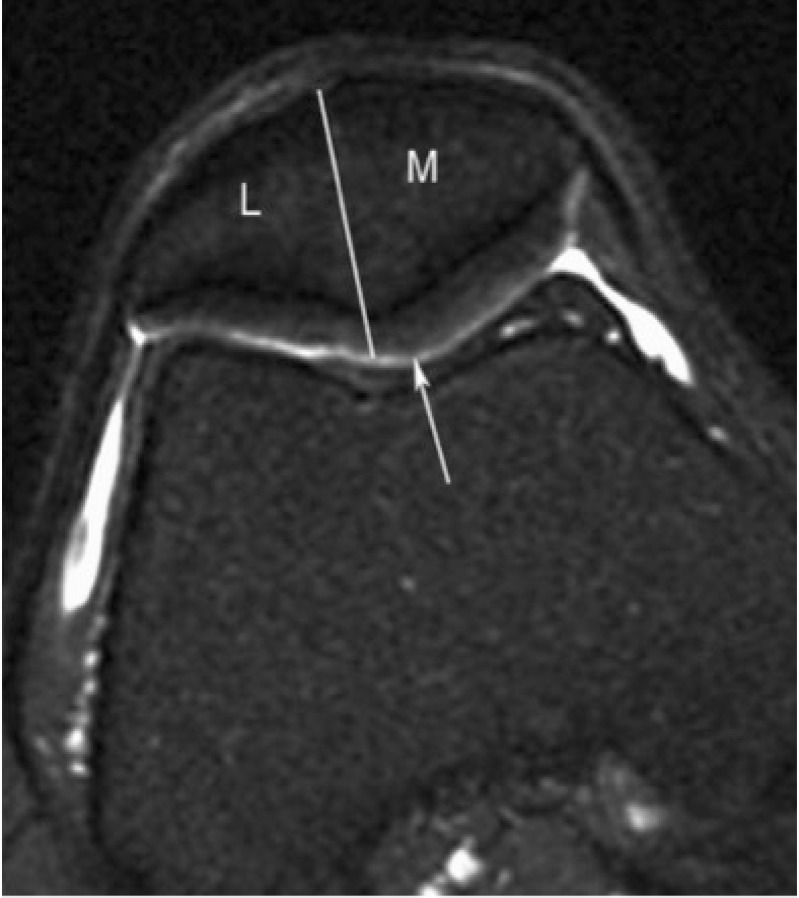
Figure 1.
Subregional division of the patella in axial plane with medial (M) and lateral (L) portions of patella as described in MOAKS. Patellar apex is part of medial subregion.
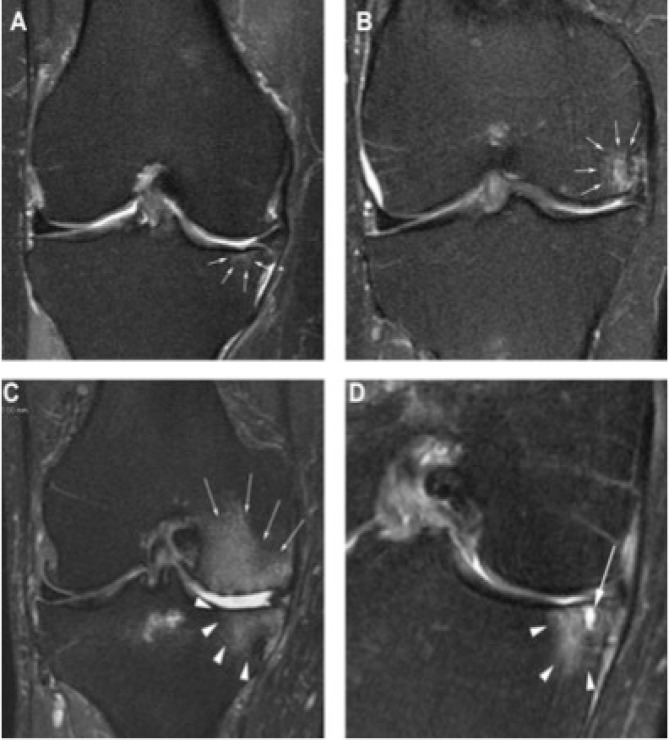
Figure 4.
BML grading. Grade 0=none, grade 1 <33% of subregional volume, grade 2=33–66% of subregional volume and grade 3 >66% of subregional volume. A. Coronal T2 w image shows small grade 1 BML in central subregion of medial tibia. B. A grade 2 BML is depicted in central subregion of medial femur. C. Grade 3 BMLs in the central sub-regions of the medial femur (arrows) and central medial tibia (arrowheads). D. Coronal image shows BML consisting of non-cystic/ill-defined portion (arrowheads) and cystic (arrow) part.
Unlike the WORMS and MOAKS, the BLOKS system evaluates the following subregions: medial and lateral patella, medial and lateral trochlea, medial and lateral weight bearing femur, medial and lateral weight bearing tibia, and subspinous tibia. In the KOSS system, the medial patella, patellar crest, lateral patella, medial and lateral trochlea, medial and lateral femoral condyle, and medial and lateral tibial plateau are evaluated (42).
The reliability of these scoring systems for the assessment of BMLs has been evaluated in different studies. Studies comparing BLOKS to WORMS have reported both advantages and disadvantages of each system; however, it was noted that WORMS was more promising. The subregional scoring approach of WORMS is not only easier to use, but also a much more accurate predictive tool for future cartilage loss and damage (40). These findings may be attributed to the fact that WORMS system not only reports on significantly smaller regions compared with BLOKS, but also is more sensitive at detecting changes in BML size.
CLINICAL SIGNIFICANCE OF OA WITH AN ASSOCIATED BML
In general, radiographic classification of knee OA based on radiographs has a moderate correlation with symptoms, joint function and reported quality of life (7). Given the lack of pain receptors in cartilage and the inability of radiographs to detect inflammatory bone changes, these findings are not surprising. Furthermore, radiographs have limited prognostic capacity and cannot reliably predict when and if a particular patient will require total knee arthroplasty (TKA) (29, 43).
Conversely, MRI has consistently been shown to have the capacity to be predictive of knee OA symptoms and if TKA will likely be needed (44). Specifically, the presence of a BML has been reported to correlate with OA symptoms and prognosis. The presence of a BML in patients with knee OA is associated with loss of joint function and decreased quality of life, the accepted indications for TKA. While MRI analysis of a knee with advanced OA demonstrates abnormalities of nearly every joint structure, the identification of a BML in the SCB, adjacent to an articular region of cartilage loss, has been shown to have particularly significant importance. A study by Link et al. found a strong correlation between the presence of a BML and WOMAC derived pain scores in 50 patients with knee OA (9). These same authors were not able to demonstrate a relationship for the presence of symptoms and ligament or meniscal abnormalities. Other studies have corroborated these results. Felson et al. noted that patients with knee OA are more likely to have pain if a BML is present (10). In this study, they reported 77.5% of patients with a BML had significant pain compared to only 30.0% reporting pain if no BML was detected (p<0.001). Large lesions were found almost exclusively in those patients with knee pain and a BML was the strongest MRI finding predictive of clinical symptoms. Additionally, in this same study, BML size correlated with the severity of knee pain. A separate longitudinal study reported that an increase in BML size was associated with both the onset and an increase in joint pain (45). Lo et al. also demonstrated that the presence of a BML was an independent factor predictive of the ability to bear weight and pain severity in a series of 160 patients with knee OA (46). Sowers et al. reported increased odds of pain, decreased ability to ambulate and loss of stair climbing performance in patients with knee OA and an associated BML (47). Together, these studies demonstrate a relationship between the presence of a BML and symptomatic knee OA. This has led investigators to hypothesize that an important source of knee OA pain is the SCB BML (48).
Additionally, the presence of a BML in patients with knee OA significantly increases the likelihood that TKA will be performed. Scher et al. reported that patients with knee OA and a BML had a nine-fold likelihood to progress to TKA when compared to controls without a BML (49). Stephanie et al. have also noted that the presence of a BML is a significant risk factor for clinical deterioration in patients with knee OA. In their investigation, BML severity related to the subsequent performance of TKA. Specifically, patients having a BML were 57% more likely to have TKA. Raynaud et al., based on analysis of their study data, concluded that a BML was the strongest recognized MRI finding that predicted TKA would be performed (50). More recently, Roemer et al. reported that the presence of a large BML or an increase in BML size prognosticated both clinical deterioration and performance of TKA in patients with OA (2.5x and 3.4x need for TKA respectively) (51). While a complete understanding of these relationships is lacking, it appears that the presence of a BML is associated with clinical deterioration, progression of symptoms and an increased likelihood that a patient will proceed to TKA.
BML HISTOLOGY, HISTOPATHOLOGY, MORPHO-LOGY, AND MECHANICAL PROPERTIES
Since many patients with OA and a BML eventually undergo joint replacement, a unique opportunity to study SCB related BML histology in retrieved bone specimens exists. Indeed a number of bone retrieval investigations have been performed and consistent findings reported (52, 53). Interestingly, despite the previously noted hyperintensity on T2-weighted MR images, edema in the region of the BML is usually described as minimal (16). However, increased vascularity and endothelial vascular neogenesis are noted histologic findings (54). It has been proposed that these vascular changes are responsible for the recognized signal on T2 imaging (16).
Histochemical analysis of SCB associated with a BML has demonstrated increased concentrations of inflammatory mediators when compared with unaffected, control specimens (55). Increased levels of tumor necrosis factor-alpha, matrix metalloproteinase-3 and -9 and substance P have been noted. These mediators may stimulate pain receptors (56).
Additionally, SCB morphology has been found to be altered in BML affected regions. Areas of trabecular bone fracture have also been reported in the BML region (52, 56-57). It has been found that extensive bone remodeling, suggesting high metabolic activity, is present in the BML effected SCB (58).
THE ROLE OF BML IN KNEE OA PATHOPHYSIOLOGY
The existing literature provides the basis for an enhanced understanding of the pathophysiology of knee OA. Cartilage loss redistributes joint forces leading to remodeling of the SCBP (and underlying SCB), eventually SBA (associated with subchondral bone plate collapse) occurs followed by progressive deformity and eventual joint failure (the need for TKA). In this section, these relationships will be examined.
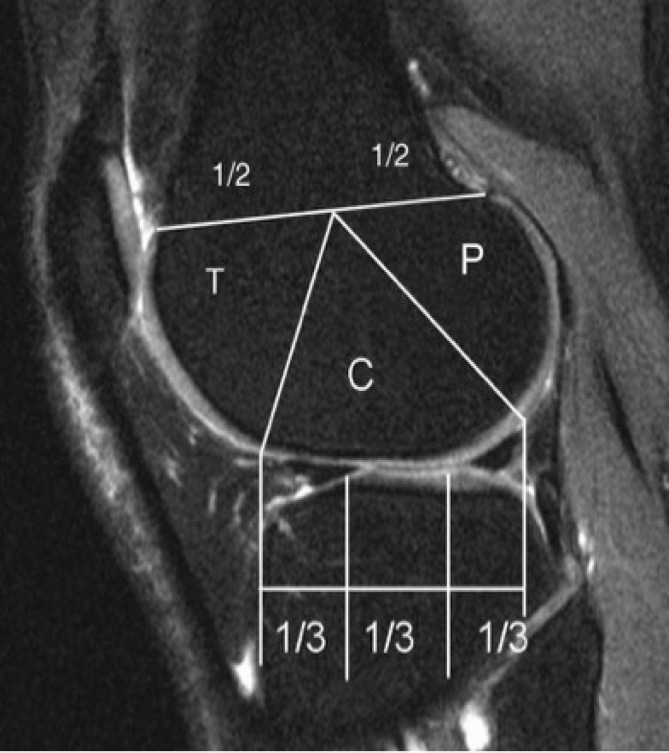
Figure 2.
Sagittal division of femur into trochlea (T), central (C) and posterior (P) regions and of tibia into anterior, central, and posterior subregions as described in MOAKS.
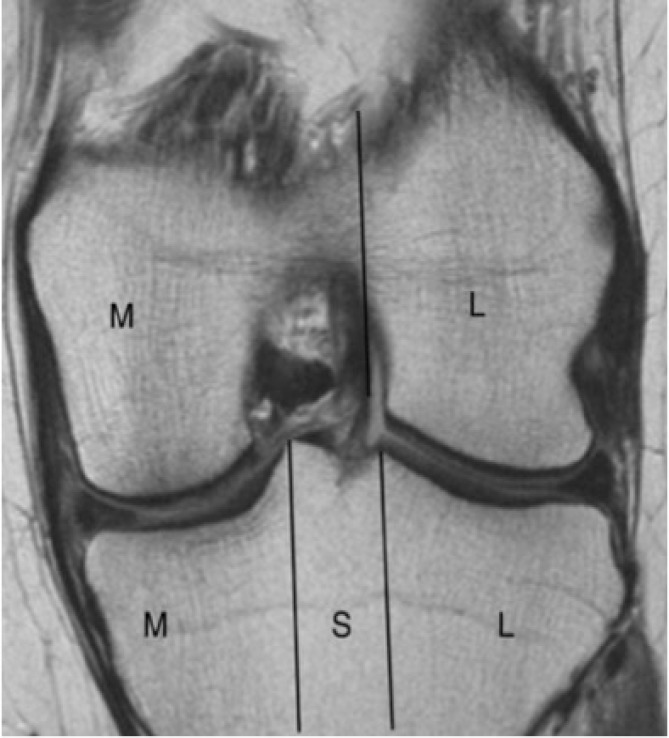
Figure 3.
Coronal division of femur into medial (M) and lateral (L) femoral condyle and of tibia into medial, subspinous (S) and lateral subregions as described in MOAKS. Intercondylar notch is considered part of medial femur.
CARTILAGE LOSS, JOINT FORCE REDISTRIBUTION AND SCB REMODELING
Review of literature related to cartilage loss associated with knee OA is not the purpose of this manuscript. However, it is relevant to consider the effect of articular cartilage loss on distribution of joint forces, the subchondral bone plate (SCBP) and the underlying SCB.
Work by Radin et al. in 1973 proposed that repetitive joint loading leads to asymptomatic SCB microfractures, stimulation of secondary centers of ossification, and thickening of the SCBP (59). This thickening or tidemark advancement occurs at the expense of the overlying cartilage, which over time, decreases in thickness. These changes often, at least initially, cause minimal or no symptoms. However, loss of cartilage does redistribute joint forces with concentration of force over the region of OA (60). Often these forces are localized to a small area and focused toward the periphery of the joint. The effect of force peripheralization leads to magnified articular stresses compared to a normal aligned knee joint exposed to more centralized joint forces.
In response to these alterations in joint mechanics, SCB remodels in accordance to the standard principles of Wolff’s law (61). Indeed it has been demonstrated that SCB below an arthritic region is a zone of high metabolic activity and increased vascularity (57-58). With physiologic remodeling and repair, the SCB becomes more densely mineralized and bone mechanical properties are enhanced (57).
DISEQUILIBRIUM OF SCB REMODELING
Repetitive loading of SCB leads to a cycle of bone damage followed by repair and if these processes are balanced, then the clinical consequences are insignificant. However, when damage exceeds reparative capacity, the normal architecture of SCB is altered and the mechanical properties are compromised (62). When the articular surface is loaded in the presence of inadequate support, SBA occurs with SCBP subsidence and progressive deformity.
The rate of SBA has been noted to increase exponentially following the development of a SCB BML. Recognition of a BML represents a critical point in the OA disease process. Retrieval analysis has demonstrated that the histology of a BML closely resembles the appearance of a stress fracture or bone non-union (53-56). These changes in histology are associated with significant loss of SCB mechanical integrity and inadequate support of the SCBP (16). Micro CT analysis of tibial plateau retrievals from our laboratory suggest that SCBP fracture may occur as OA progresses.
Failure of SCB to support the SCBP coupled with fracture of the SCBP likely explains the relationship between a BML, SBA, and onset of clinical symptoms. Quantitative distribution of bone pain receptors has been studied. The SCBP has no significant supply of pain receptors (63). However, SCB and the adjacent cortical bone periosteum are very well innervated.
Mechanically compromised SCB coupled with SCBP fracture likely is associated with deformation of the periosteum during weight bearing. Deformation of the periosteum has been demonstrated to be a very important source of bone related pain (64). Additionally, as previously noted, a significant increase in inflammatory mediators has been identified in BML affected SCB. The combination of bone inflammation coupled with structural compromise could explain why knee OA is associated with pain at rest, which increases significantly with weight bearing (65).
FUTURE DIRECTIONS
Knowledge of the integral relationship between formation of a BML and increased clinical symptoms with rapid disease progression is critical for understanding the pathophysiology of knee OA. Coupled with studies describing the histology, histochemistry and mechanical changes in SCB associated with a BML could provide the basis for developing methods, both pharmacologic and surgical, for attenuating the natural progression of OA symptoms.
Age associated cartilage loss could be a rate alterable process, but likely is inevitable. While loss of cartilage is perhaps an undesirable, consequence of aging, radiographic studies have shown a poor correlation between thinning of cartilage and OA symptoms (7, 29, 43). Therefore, cartilage loss alone may be a less important component of OA pathophysiology than SCB alterations that occur secondary to cartilage loss. Since onset and severity of OA symptoms correlate closely with SCB changes and are associated with disease progression, it is reasonable to contemplate interventions that could prevent or reverse the described alterations in SCB (10, 44, 46, 47).
For example, pharmacologic means or surgical procedures that mechanically or biologically enhance SCB before a BML forms could delay or even prevent the onset of severe OA symptoms. The histologic appearance of a BML has been well described with features similar to those found in stress fractures or bone non-unions.
Existing orthopaedic knowledge related to treatment of bone non-unions or stress fractures includes biologic stimulation and surgical stabilization of bone. Could the same principles be applied to a BML and lead to BML healing with improvement in OA symptoms and/or slow joint deterioration? Of course, it is important to also consider the need for later TKA. Treatment of a BML optimally should not compromise the performance or the outcome of TKA if it is eventually required.
It has long been recognized that loss of cartilage, as determined by radiographs, is a relatively inaccurate predictor of knee OA symptoms. Recent investigations have clearly demonstrated that formation of an MRI identified SCB BML closely correlates with severity of symptoms, functional limitations, disease/deformity progression (SBA) and the likelihood of TKA performance. This understanding of the pathophysiology of knee OA could potentially lead to interventions directed at prevention or reversal of SCB changes associated with BML formation. Successful prophylaxis to prevent BML formation or treatment to reverse a BML to normal SCB potentially could lead to relief of clinical symptoms and slowing of knee OA progression. If viable, these inventions would provide meaningful benefit to the increasingly large number of patients affected by knee OA.
Note: Figures 1-4 reprinted from Osteoarthritis and Cartilage, Vol. 19/1, Hunter DJ et al, Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score), 990-1002, Copyright (2011), with permission from Elsevier.
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–53. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18(2):147–56. doi: 10.1097/01.bor.0000209426.84775.f8. [DOI] [PubMed] [Google Scholar]
- 3.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med. 2011;2(2):205–12. [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 5.Roemer FW, Eckstein F, Hayashi D, Guermazi A. The role of imaging in osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):31–60. doi: 10.1016/j.berh.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Wright RW, Ross JR, Haas AK, Huston LJ, Garofoli EA, Harris D, et al. Osteoarthritis classification scales: interobserver reliability and arthroscopic correlation. J Bone Joint Surg Am. 2014;96(14):1145–51. doi: 10.2106/JBJS.M.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinds MB, Marijnissen AC, Bijlsma JW, Boers M, Lafeber FP, Welsing PM. Quantitative radiographic features of early knee osteoarthritis: development over 5 years and relationship with symptoms in the CHECK cohort. J Rheumatol. 2013;40(1):58–65. doi: 10.3899/jrheum.120320. [DOI] [PubMed] [Google Scholar]
- 8.Guymer E, Baranyay F, Wluka AE, Hanna F, Bell R, Davis S, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage. 2007;15(12):1437–42. doi: 10.1016/j.joca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 12.Kubota M, Ishijima M, Kurosawa H, Liu L, Ikeda H, Osawa A, et al. A longitudinal study of the relationship between the status of bone marrow abnormalities and progression of knee osteoarthritis. J Orthop Sci. 2010;15(5):641–6. doi: 10.1007/s00776-010-1512-y. [DOI] [PubMed] [Google Scholar]
- 13.Roemer FW, Hunter DJ, Guermazi A. MRI-based semiquantitative assessment of subchondral bone marrow lesions in osteoarthritis research. Osteoarthritis Cartilage. 2009;17(3):414–5. doi: 10.1016/j.joca.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Wilson AJ, Murphy WA, Hardy DC, Totty WG. Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167(3):757–60. doi: 10.1148/radiology.167.3.3363136. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63(3):691–9. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roemer FW, Frobell R, Hunter DJ, Crema MD, Fischer W, Bohndorf K, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17(9):1115–31. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Wluka AE, Hanna F, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68(6):850–5. doi: 10.1136/ard.2008.092221. [DOI] [PubMed] [Google Scholar]
- 18.Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niue J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68(9):1461–5. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 20.Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, et al. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage. 2008;16(9):1005–10. doi: 10.1016/j.joca.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanamas SK, Wluka AE, Pelletier JP, Pelletier JM, Abram F, Berry PA, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford) 2010;49(12):2413–9. doi: 10.1093/rheumatology/keq286. [DOI] [PubMed] [Google Scholar]
- 22.Garnero P, Peterfy C, Zaim S, Schoenharting M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: a three-month longitudinal study. Arthritis Rheum. 2005;52(9):2822–9. doi: 10.1002/art.21366. [DOI] [PubMed] [Google Scholar]
- 23.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol. 2006;16(3):608–18. doi: 10.1007/s00330-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Molina G, Neogi T, Hunter DJ, Niu J, Guermazi A, Reichenbach S, et al. The association of bone attrition with knee pain and other MRI features of osteoarthritis. Ann Rheum Dis. 2008;67(1):43–7. doi: 10.1136/ard.2007.070565. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi D, Englund M, Roemer FW, Niu J, Sharma L, Felson DT, et al. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage. 2012;20(11):1227–33. doi: 10.1016/j.joca.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi D, Guermazi A, Kwoh CK, Hannon MJ, Moore C, Jakicic J, et al. Semiquantitative assessment of subchondral bone marrow edema-like lesions and subchondral cysts of the knee at 3T MRI: a comparison between intermediate-weighted fat-suppressed spin echo and Dual Echo Steady State sequences. BMC Musculoskelet Disord. 2011;12(1):198. doi: 10.1186/1471-2474-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterfy CG, Gold G, Eckstein F, Cicuttini F, Dardzinski B, Stevens R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl A):A95–111. doi: 10.1016/j.joca.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Mayerhoefer ME, Breitenseher MJ, Kramer J, Aigner N, Norden C, Hofmann S. STIR vs T1-weighted fat-suppressed gadolinium-enhanced MRI of bone marrow edema of the knee: computer-assisted quantitative comparison and influence of injected contrast media volume and acquisition parameters. J Magn Reson Imaging. 2005;22(6):788–93. doi: 10.1002/jmri.20439. [DOI] [PubMed] [Google Scholar]
- 29.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):645–87. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19(5):435–43. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 31.Gray ML, Burstein D. Molecular (and functional) imaging of articular cartilage. J Musculoskelet Neuronal Interact. 2004;4(4):365–8. [PubMed] [Google Scholar]
- 32.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Schmid MR, Hodler J, Vienne P, Binkert CA, Zanetti M. Bone marrow abnormalities of foot and ankle: STIR versus T1-weighted contrast-enhanced fat-suppressed spin-echo MR imaging. Radiology. 2002;224(2):463–9. doi: 10.1148/radiol.2242011252. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28(2):453–61. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 36.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 37.Amin S, Guermazi A, Lavalley MP, Niu J, Clancy M, Hunter DJ, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(8):897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)--inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34(2):95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 39.Roemer FW, Eckstein F, Guermazi A. Magnetic resonance imaging-based semiquantitative and quantitative assessment in osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):521–55. doi: 10.1016/j.rdc.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, et al. Comparison of BLOKS and WORMS scoring systems part II Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18(11):1402–7. doi: 10.1016/j.joca.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conagahn PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemer FW, Guermazi A. MR imaging-based semiquantitative assessment in osteoarthritis. Radiol Clin North Am. 2009;47(4):633–54. doi: 10.1016/j.rcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004;12(1):3–31. doi: 10.1163/156856004773121347. [DOI] [PubMed] [Google Scholar]
- 44.Menashe L, Hirko K, Losina E, Kloppenburg M, Zhang W, Li L, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(1):13–21. doi: 10.1016/j.joca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 46.Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17(12):1562–9. doi: 10.1016/j.joca.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93(3):241–51. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharkey PF, Cohen SB, Leinberry CF, Parvizi J. Subchondral bone marrow lesions associated with knee osteoarthritis. Am J Orthop. 2012;41(9):413–7. [PubMed] [Google Scholar]
- 49.Scher C, Craig J, Nelson F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol. 2008;37(7):609–17. doi: 10.1007/s00256-008-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raynauld JP, Martel-Pelletier J, Haraoui B, Choquette D, Dorais M, Wildi LM, et al. Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70(8):1382–8. doi: 10.1136/ard.2010.146407. [DOI] [PubMed] [Google Scholar]
- 51.Roemer FW, Nevitt MC, Felson DT, Niu J, Lynch JA, Crema MD, et al. Predictive validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment in the tibio-femoral joint - the MOST Study. Osteoarthritis Cartilage. 2012;20(11):1391–8. doi: 10.1016/j.joca.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taljanovic MS, Graham AR, Benjamin JB, Gmitro AF, Krupinski EA, Schwartz SA, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 2008;37(5):423–31. doi: 10.1007/s00256-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 53.Leydet-Quilici H, Le Corroller T, Bouvier C, Giorgi R, Argenson JN, Champsaur P, et al. Advanced hip osteoarthritis: magnetic resonance imaging aspects and histopathology correlations. Osteoarthritis Cartilage. 2010;18(11):1429–35. doi: 10.1016/j.joca.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Saadat E, Jobke B, Chu B, Lu Y, Cheng J, Li X, et al. Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol. 2008;18(10):2292–302. doi: 10.1007/s00330-008-0989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hulejová H, Baresová V, Klézl Z, Polanská M, Adam M, Senolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38(3):151–6. doi: 10.1016/j.cyto.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Plenk H, Hofmann S, Eschberger J, Gstettner M, Kramer J, Schneider W, et al. Histomorphology and bone morphometry of the bone marrow edema syndrome of the hip. Clin Orthop Relat Res. 1997;334(1):73–84. [PubMed] [Google Scholar]
- 57.Hunter DJ, Gerstenfeld L, Bishop G, Davis AD, Mason , ZD , Einhorn TA, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11(1):R11. doi: 10.1186/ar2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter DJ, Lavalley M, Li J, Bauer DC, Nevitt M, DeGroot J, et al. Biochemical markers of bone turnover and their association with bone marrow lesions. Arthritis Res Ther. 2008;10(4):R102. doi: 10.1186/ar2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radin EL, Parker HG, Pugh JW, Steinberg RS, Paul IL, Rose RM. Response of joints to impact loading Relationship between trabecular microfractures and cartilage degeneration. J Biomech. 1973;6(1):51–7. doi: 10.1016/0021-9290(73)90037-7. [DOI] [PubMed] [Google Scholar]
- 60.Wu JZ, Herzog W, Epstein M. Joint contact mechanics in the early stages of osteoarthritis. Med Eng Phys. 2000;22(1):1–12. doi: 10.1016/s1350-4533(00)00012-6. [DOI] [PubMed] [Google Scholar]
- 61.Frost HM. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004;74(1):3–15. doi: 10.1043/0003-3219(2004)074<0003:AUOBPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 62.Poulet B, de Souza R, Kent AV, Saxon L, Barker O, Wilson A, et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthritis Cartilage. 2015;23(6):940–8. doi: 10.1016/j.joca.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frobell RB, Wirth W, Nevitt M, Wyman BT, Benichou O, Dreher D, et al. Presence, location, type and size of denuded areas of subchondral bone in the knee as a function of radiographic stage of OA - data from the OA initiative. Osteoarthritis Cartilage. 2010;18(5):668–76. doi: 10.1016/j.joca.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finocchietti S, Graven-Nielsen T, Arendt-Nielsen L. Bone hyperalgesia after mechanical impact stimulation: a human experimental pain model. Somatosens Mot Res. 2014;31(4):178–85. doi: 10.3109/08990220.2014.911171. [DOI] [PubMed] [Google Scholar]
- 65.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34(3):623–43. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]