Abstract
Background
Blood collection, transportation and storage remain a problem in countries where infrastructure, laboratory facilities and skilled manpower are scarce. This limits evaluation of immune responses in natural infections and vaccination in field studies. We developed methods to measure antigen specific antibody responses using dried blood spot (DBS) in cholera, ETEC and typhoid fever patients as well as recipients of oral cholera vaccine (OCV).
Methodology/Principle findings
We processed heparinized blood for preparing DBS and plasma specimens from patients with, cholera, ETEC and typhoid as well as OCV recipients. We optimized the conventional vibriocidal method to measure vibriocidal antibody response in DBS eluates. We measured responses in DBS samples and plasma (range of titer of 5 to 10240). Vibriocidal titer showed strong agreement between DBS eluates and plasma in cholera patients (ICC = 0.9) and in OCV recipients (ICC = 0.8) using the Bland-Altman analysis and a positive correlation was seen (r = 0.7, p = 0.02 and r = 0.6, p = 0.006, respectively). We observed a strong agreement of lipopolysaccharide (LPS) and cholera toxin B (CTB)-specific antibody responses between DBS eluates and plasma in cholera patients and OCV recipients. We also found agreement of heat labile toxin B (LTB) and membrane protein (MP)-specific antibody responses in DBS eluates and plasma specimen of ETEC and typhoid patients respectively.
Conclusion
Our results demonstrate that dried blood specimens can be used as an alternate method for preservation of samples to measure antibody responses in enteric diseases and vaccine trials and can be applied to assessment of responses in humanitarian crisis and other adverse field settings.
Introduction
Blood collection, processing and quick transportation to laboratories from remote areas in resource poor settings can be limitations in carrying out serological studies especially in developing countries where laboratory facilities and trained manpower as well as cold chain facilities are not optimal. Dried blood spot (DBS) is an alternate method for collecting blood specimens on paper. This lowers biological risks associated with conventional blood transportation, does not require maintaining cold-chain during transportation and DBS card can be shipped at ambient temperature and even by ordinary mail [1,2]. Blood collection and transportation from resource-limited settings remains an obstacle to follow up large vaccine trials, seroepidemiological studies and diagnosis of infectious diseases as well as large population based estimations of public health interventions. DBS can therefore be a solution to the cumbersome specimen collection and transportation that is generally used.
DBS has been used as an alternative method for diagnosis of viral [3–5], bacterial [6], fungal [7] and parasitic [6] infections. It has also been used to monitor HIV viral load and drug resistance in patients receiving antiretroviral therapy [1]. The DBS analysis platform is routinely used for estimation of DNA, protein and drugs [8–11] which have a great impact in clinical practice. DBS has been used for measuring immune responses after hepatitis A virus vaccination [12] with a positive agreement with plasma specimens. However, there is lack of information about the sensitivity of DBS to evaluate the immunogenicity in enteric infections.
In this study, we have used the DBS method to collect blood from patients with cholera, enterotoxigenic Escherichia coli (ETEC) diarrhea, typhoid fever as well as recipients of an oral cholera vaccine to evaluate feasibility and accuracy of immune responses. Immune responses measured using the DBS method was compared with results obtained with plasma specimens in the same cohort of patients and vaccinees. There was positive agreement between DBS and plasma specimen in the evaluation of immune responses in cholera, ETEC diarrhea and typhoid patients as well as OCV recipients.
Methods
Study participants
Blood specimens were obtained from patients with enteric infections which included cholera, ETEC, typhoid and as well as healthy participants who received two doses of heat-killed oral cholera vaccine (Shanchol, Shantha biotechnics, India). Cholera and ETEC-infected patients were culture confirmed by isolation of Vibrio cholerae O1 and enterotoxigenic Escherichia coli (ETEC) from stool specimens respectively. ETEC was confirmed by the presence of heat-labile toxin (LT) or heat-stable toxin (ST) or both by ELISA and PCR [13]. Typhoid fever was confirmed by isolation of Salmonella Typhi from blood of febrile patients. Patients were given appropriate antibiotics by physicians as was needed (S1 Table). Specimens were collected between January 2016 and December 2017.
Ethics statement
Specimen collection from cholera, ETEC and typhoid patients and healthy participants and were approved by the Institutional Research Board (IRB) of icddr,b. Written informed consent was obtained from the study participants (18–59 years), and consent from parents of those aged 5–10 years with assents from the minor participants (11–17 years) were obtained.
Collection of blood samples in DBS cards
Venous blood was collected (8–10 ml from adults and 3–5 ml from children; only 200 μl was used for preparation of DBS) from the study participants in sodium heparin tube (Beckton Dickinson, CA). Blood was collected from cholera and ETEC diarrheal patients on day 2, 7 and 30 of onset of disease. From typhoid fever patients, blood was collected after culture confirmation (day 1), 2–3 days later (day 2) and 18–22 days later (day 3). Healthy participants were vaccinated with oral cholera vaccine at two time points (days 0 and 14). Blood was collected prior to vaccination on day 0 and 14 (prior to second vaccination) and two more blood samples were collected at day 28 and 42 after the first vaccination. Approximately 50 μl of blood was adsorbed on each spot (200 μl for four spots) of DBS card (Whatman 903, GE Healthcare) and air dried at ambient temperature (15–25°C) for two hours by placing on a clean paper towel. The card was then transferred to a black card holder (Advantus 4X6 index card holder, Jacksonville, FL, USA) box and kept overnight at RT for adequate drying. The DBS card was placed in a single, gas-impermeable zipper bag containing desiccant sachets (2 desiccants per bag) to protect the cards from moisture. A humidity indicator card was added to monitor the moisture level in the zipper bag and it was checked monthly. If the indicator turned purple then the desiccant sachets were replaced with new desiccant sachets. The dried blood spotted cards were stored at -80°C until tested (1–6 months).
DBS elution
A 3.2 mm spot was punched with a DBS puncher (PerkinElmer, USA) from each blood-soaked circle of the DBS card. All punched spots from a single patient were transferred to one eppendorf tube and 0.5% bovine serum albumin (BSA) in phosphate buffered saline (PBS, 10 mM, pH 7.2) was added according to the desired dilution (18 punch in 270 μl; 1:10 for vibriocidal and 1 punch in 300 μl; 1:200 for ELISAs) and kept at RT overnight. The tube was centrifuged (2500 x g for 7 min) and eluate was collected in a fresh eppendorf tube.
Plasma separation
Plasma specimens were also separated from the same cohort of patients and vaccinees from the heparinized blood. Blood specimens were centrifuged, aliquoted (200 μl in eppondorf tube) and stored at -20°C until tested.
Vibriocidal assay
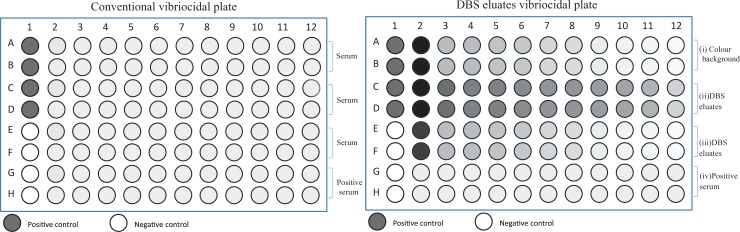
Vibriocidal antibody titer was measured according to the procedure described earlier [14]. Briefly, 25 μl of cold physiological saline (0.154 M) was dispensed in all wells except column #2 of 96-well microtiter plates (Nunc F, Denmark). Plasma and DBS eluates were heat inactivated at 56°C for 30 min. For plasma, 45 μl of cold saline and 5 μl of plasma and for DBS eluates 50 μl eluates were dispensed in corresponding wells in column #2. Both plasma and DBS eluates were serially diluted (initial dilution 1:10) in two fold dilutions. The dilution was accomplished by mixing the solution in column #2, aspirating 25 μl and dispensing and mixing the sample in column #3 and so on, until column #12 (1:10240 dilution). At the end, 25 μl was discarded from the last well in each row. V. cholerae O1 Inaba strain (T-19479) was cultured on blood agar plates at 37°C overnight. This strain was isolated from stool of cholera patient [15] in Bangladesh and well characterized [16–19] as well as used to measure vibriocidal antibody titer in plasma [14]. A loopful of bacteria was inoculated in Brain Heart Infusion (BHI) media for 2–3 hours at 37°C with shaking to mid-log phase of growth (OD to 0.4). Bacterial culture was centrifuged and resuspended in sterile saline (0.154 M) and the OD was adjusted to 0.3 at 600 nm using a spectrophotometer (Beckman DU 530 UV vis). For vibriocidal assay using plasma, 25 μl of bacterial suspension (21.25 μl normal saline, 1.25 μl bacteria and 2.5 μl guinea pig sera) was added to all wells except wells #E1, F1, G1 and H1 in column 1 of 96-well plates and 25 μl of cold saline was added to wells # E1, F1, G1 and H1 (Fig 1A). For vibriocidal assay using DBS, 25 μl of bacterial suspension (21.25 μl normal saline, 1.25 μl bacteria and 2.5 μl guinea pig sera) was added to all wells except wells row #A2-A12, B2-B12 wells and column #E1, F1, G1 & H1 wells and 25 μl of cold saline was added to wells # E1, F1, G1 and H1 (Fig 1B). The OD value of wells in row #A2-A12, B2-B12 was used as the color background control for DBS (Fig 1B). The plates were incubated at 37°C for 1 hour and 150 μl of BHI media was added to all wells and incubated again at 37°C. The plates were read spectrophotometrically (Eon, BioTek, Vermont, USA) until the positive control well reached OD around 0.20 to 0.28 at 595 nm.
Fig 1. Conventional vibriocidal assay and modified vibriocidal assay for DBS eluates.
A) The schematic diagram shows the conventional vibriocidal assay with plasma collected from cholera patients and cholera vaccinees using the 96-well plates (row A-F). Pooled sera obtained from cholera patients was used as positive control on each plate. B). The vibriocidal antibody assay was modified when DBS eluates were used to measure vibriocidal response in cholera patients/vaccinees. The first two rows (rows A-B) were used without adding bacterial inocula to calculate color background of heme in DBS eluates. Rows C to F were used for DBS eluates similar to the conventional vibriocidal assay and the last two rows (G-H) were used as positive control.
V. cholerae lipopolysaccharide (LPS) extraction
LPS was purified from V. cholerae strains as discussed previously [20]. Briefly, LPS was purified by the hot phenol-water procedure, ultracentrifugation and treatment with enzymes (proteinase K, DNase and RNase) from V. cholerae O1 Inaba (T-19479) strain.
V. cholerae LPS and CTB specific antibody responses in cholera patients and vaccines
For this assay, 96-well ELISA (Nunc F, Denmark) plates were coated with LPS of V. cholerae O1 in PBS (100 μl/well) at a concentration of 2.5 μg/ml and incubated at RT overnight. For anti-CTB, ELISA plates were coated (100 μl/well) with ganglioside GM1 (0.3 nM/ml) and incubated overnight at ambient temperature (15–25°C). The plates were washed with PBS and recombinant CTB (0.5 μg/ml) (gift of A.M Svennerholm, University of Gothenburg) added to the wells and incubated for 1 h at 37°C. The plates were washed for three times with PBS and blocked with 200 μl/well of 0.1% BSA-PBS and incubated at 37°C for 30–60 min. The plates were washed three times with PBS-0.05% Tween and once with PBS. Plasma and DBS eluates were added in the allocated wells (1:200 dilution) and incubated for 90 min at 37°C. The plates were washed three times with PBS-0.05% Tween and once with PBS. Horseradish peroxidase (HRP) conjugated anti-human antibody was added in each well (100 μl/well) and incubated for 90 min at 37°C. The plates were washed three times with PBS-0.05% Tween and once with PBS. For color development, orthophenylenediamine (OPD) (Sigma, St. Louis, MO) was added together with the substrate H2O2 (100 μl/well) [10 mg OPD in 10 mL 0.1M Citrate Buffer (pH-4.5), 4 μl 30% H2O2] to each well and OD measured at 450 nm using the kinetic method (Eon, BioTek, Vermont, USA).
Membrane protein antigen of S. Typhi specific antibody responses in typhoid fever patients
MP was prepared from S. Typhi by using a previously described procedure [21, 22]. Briefly, S. Typhi strain (Ty21a) was cultured on sheep blood agar plates and harvested in Tris buffer (10 mM Tris [pH 8.0], 5 mM MgCl2). Mixture was sonicated and centrifuged at 1400 x g for 10 min, supernatant transferred to fresh tubes, centrifuged again at 14900 x g for 30 min. The pellet was suspended in 10 ml of Tris buffer, and the protein content was determind [22]. Antibody responses in plasma as well as DBS of typhoid fever patients against MP [22, 23] were measured as described previously [22]. Briefly, 96-well ELISA (Nunc F, Denmark) plates were coated with MP of S.Typhi (Ty21a) in PBS at a concentration of 2.5 μg/ml and incubated at RT overnight. The plates were washed for three times with PBS and blocked with 200 μl/well of 0.1% BSA-PBS and incubated at 37°C for 30–60 min. The plates were washed three times with PBS-0.05% Tween and once with PBS. Plasma and DBS eluates were added in the allocated wells and incubated for 90 min at 37°C. The plates were washed three times with PBS-0.05% Tween and once with PBS. Horseradish peroxidase conjugated anti-human antibody was added in each well (100 μl/well) and incubated for 90 min at 37°C. The plates were washed three times with PBS-0.05% Tween and once with PBS. Orthophenylenediamine (OPD) with H2O2 (100 μl/well) [10 mg OPD in 10 mL 0.1M Citrate Buffer (pH-4.5), 4 μl 30% H2O2] was added to each well and OD was measured at 450 nm using the kinetic method [22].
Antibody responses against LTB in ETEC-infected patients
Plasma antibodies specific to LTB were measured using a previously described ELISA method [24]. Briefly, 96-well ELISA (Nunc F, Denmark) plates were coated (100 μl/well) with ganglioside GM1 (0.3 nM/ml) and incubated overnight at ambient temperature (15–25°C). The plates were washed with PBS and recombinant LTB (0.5 μg/ml) (gift of A.M Svennerholm, University of Gothenburg) added to the wells (100 μl/well) and incubated for 1 h at 37°C. The plates were washed with PBS-T (0.05%) for three times and once with PBS. The plasma samples (1:200 dilution) was added and incubated for 90 min at 37°C. The plates were washed three times with PBS-T(0.05%) and once with PBS. HRP-conjugated anti-human antibody was added to wells and incubated for 90 min at 37°C. The plates were washed three times with PBS-T (0.05%) and once with PBS. Substrate was added to the wells and plates were read at 450 nm using the kinetic method (Eon, BioTek, Vermont, USA).
Statistical analyses
We assessed differences in the magnitude of the responses using the Mann-Whitney U test using GraphPad prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Two-sided P values <0.05 were considered significant. Correlations were assessed using Spearman’s rank correlation. The Bland-Altman plots with 95% limits of agreement (LoA) were used to evaluate the agreement between results obtained with plasma and DBS. The 95% LoA was calculated as the average difference between the plasma and DBS ± 1.96SD (upper limit and lower limit) in SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA). The narrower the 95% LoA, the better is the agreement. In addition, the reliability of the DBS method was tested by intraclass correlation coefficient (ICC); if the ICCs were closer to 1, the reliability was higher. Figures were generated in GraphPad prism 6.
Results
Study participants
The patients and vaccines were followed up to day 30 and day 42 respectively after enrollment and were included in the analysis. Demographic features of the patients and vaccinees are provided in Table 1.
Table 1. Demographic features of patients and participants who were enrolled in this study.
| Age yrs (mean±SD) | Number (Female, Male) | |
|---|---|---|
| Cholera patients | 26.5±10.1 | 9, 8 |
| Cholera vaccines | 20.2±13.3 | 8, 9 |
| ETEC infected patients | 23.1±13.4 | 4, 5 |
| Typhoid fever patients | 12.4±9.2 | 7, 5 |
Optimization of vibriocidal assay for DBS eluates
We set up the vibriocidal assay procedure using DBS eluates so that the assay could be measured in an ELISA Reader (Eon, BioTek, Vermont, USA) similar to the way the conventional and existing vibriocidal assay was being used (Fig 1). The heme color was the main obstacle in setting up the vibriocidal assay to directly measure the antibody titer. To alleviate this problem, the color effect seen in the DBS specimens from each participant were used as DBS control to which no bacterial inoculum was added and this value was subtracted from the OD value of DBS vibriocidal row using an average value for negative controls. We defined the DBS blank as serial dilution (1:2) of DBS eluates and added physiological saline (0.154 M) instead of bacterial inoculum as described above. The optical density from the DBS blank was considered for the heme color and was deducted from DBS plus indicator wells. We defined the final OD of DBS plus indicator wells according the following formula:
OD value of DBS plus indicator well = (OD value of DBS plus indicator—OD value of DBS blank) + Average value of negative controls
If this final OD value was less than or equal to half of the average OD value of positive controls then it was considered as 50% killing of the V. cholerae O1 strain.
Vibriocidal responses in cholera patients and vaccines
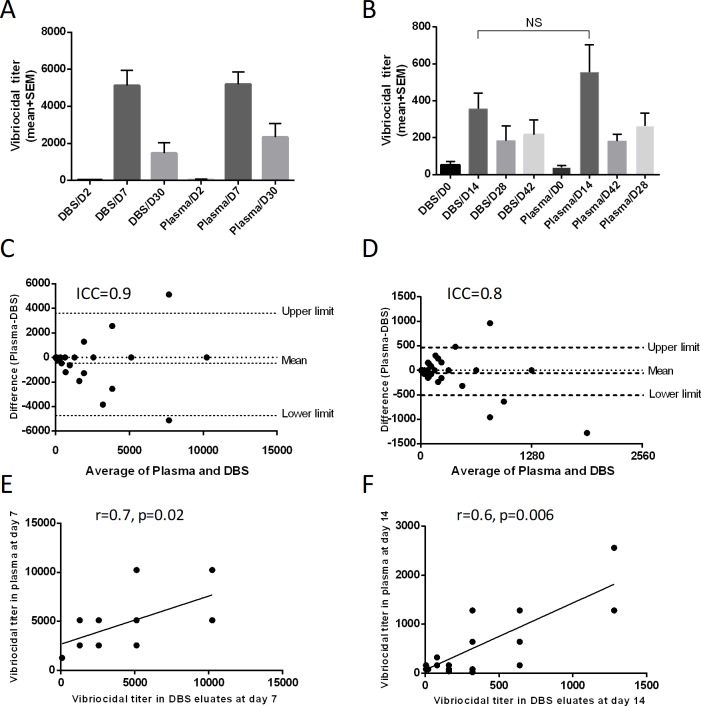
We analyzed the vibriocidal responses in DBS eluates, plasma of cholera patients (n = 17) and vaccinees (n = 17). We found similar magnitude of vibriocidal antibody titer in the corresponding day points of DBS and plasma of patients and vaccinees (Fig 2A and 2B). The responder frequency of vibriocidal titer (four-fold increase) in DBS eluates and plasma (two-fold increase) were similar (90%). There was a strong agreement (Bland-Altman agreement) of vibriocidal responses between DBS eluates and plasma in both patients and vaccinees (ICC = 0.9 and 0.8 respectively; Fig 2C and 2D).There was also a positive correlation of vibriocidal antibody responses at day 7 for patients (r = 0.73, p = 0.02; Fig 2E) and at day 14 for vaccinees (r = 0.6, p = 0.006; Fig 2F). Similar agreement and correlation were observed at other time points in patients and vaccinees (Parts A-D in S1 Fig and Parts A-F in S2 Fig).
Fig 2. DBS eluates and plasma vibriocidal responses in cholera patients and vaccinees.
Vibriocidal responses in cholera patients (A) and vaccinees (B) at different time points (n = 17). The Bland-Altman analysis of vibriocidal response in DBS eluates and plasma of cholera patients at day 7 (C) and vaccinees at day 14 (D) is shown. Spearman correlation of vibriocidal response in DBS eluates and plasma at day 7 of cholera patients (E) and day 14 of vaccinees was carried out (F).
Antibody responses in cholera patients and vaccines
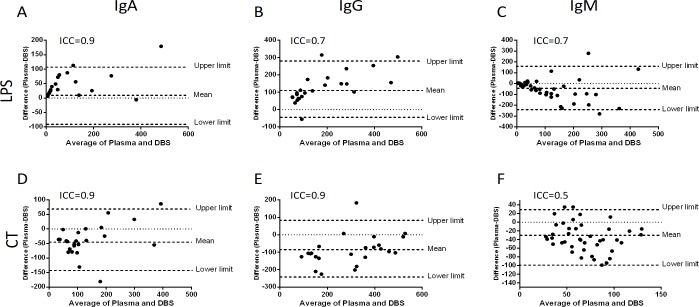
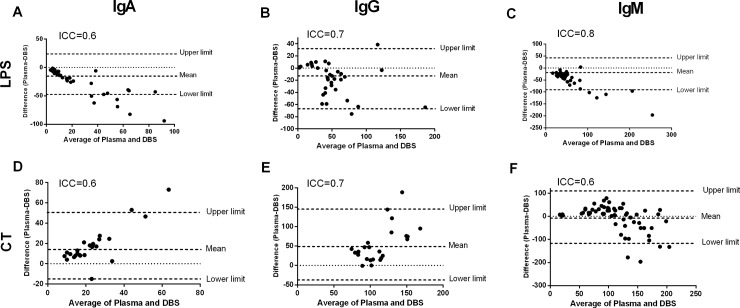
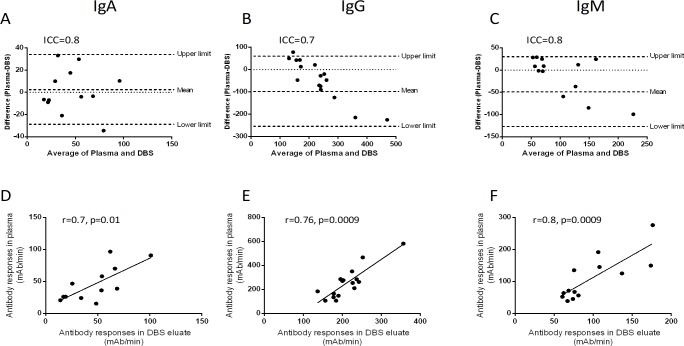
We investigated LPS and CTB-specific IgA, IgG and IgM antibody responses in the DBS eluates and plasma specimens of cholera patients and vaccinees. There was a strong agreement of LPS-specific antibody responses between DBS eluates and plasma of cholera patients (Intraclass correlation coefficient, ICC: IgA = 0.9, IgG = 0.7 and IgM = 0.7; Fig 3A–3C) and vaccinees (ICC: IgA = 0.6, IgG = 0.7 and IgM = 0.8; Fig 4A–4C). We also observed a strong agreement of CTB-specific antibody responses between DBS eluates and plasma of cholera patients (ICC: IgA = 0.9, IgG = 0.9 and IgM = 0.5; Fig 3D–3F) and vaccinees (ICC: IgA = 0.6, IgG = 0.7 and IgM = 0.6; Fig 4D–4F). We then investigated the correlation of LPS and CTB-specific antibody responses in DBS eluates and plasma specimens of cholera patients and vaccinees. There was a positive correlation of LPS-specific antibody responses in DBS eluates and plasma specimen of cholera patients (Parts A-C in S3 Fig) and vaccinees (Parts A-C in S4 Fig). Similarly, a positive correlation of CTB-specific antibody responses was observed in DBS eluates and plasma of cholera patients (Parts D-F in S3 Fig) and vaccinees (Parts D-F in S4 Fig).
Fig 3. LPS and CTB-specific antibody responses in DBS eluates and plasma in cholera patients.
The Bland-Altman analysis is shown for LPS-specific IgA (A), IgG (B), IgM (C) and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of cholera patients (n = 17) at day 7.
Fig 4. LPS and CTB-specific antibody responses in DBS eluates and plasma in OCV recipients.
The Bland-Altman analysis is shown for LPS-specific IgA (A), IgG (B), IgM (C) and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of OCV recipients (n = 17) at day 14.
Antibody responses in ETEC-infected patients
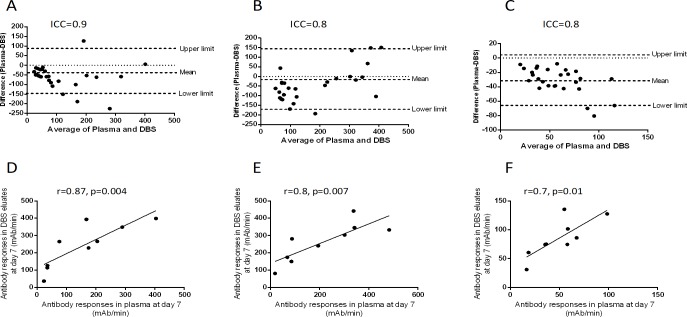
To investigate the immune responses in ETEC -infected patients we have measured IgA, IgG and IgM responses against LTB antigen by ELISA. There was a strong agreement of LTB-specific antibody responses between DBS eluates and plasma of ETEC-infected patients (ICC: IgA = 0.9, IgG = 0.8 and IgM = 0.8; Fig 5A–5C). There was also a positive correlation of LTB-specific antibody responses between DBS eluates and plasma of ETEC-infected patients at day 7 (IgA: r = 0.87, p = 0.004; IgG: r = 0.8, p = 0.007, and IgM: r = 0.7, p = 0.01). Similar correlations were observed at day 2 and day 30 (S5 Fig).
Fig 5. LTB-specific antibody responses in DBS eluates and plasma in ETEC-patients.
The Bland-Altman analysis of LTB-specific IgA (A), IgG (B), IgM (C) antibody responses between DBS eluates and plasma specimen of ETEC-patients are shown. Spearman correlation of LTB-specific IgA (D), IgG (E) and IgM (F) between DBS eluates and plasma specimen of ETEC patients.
Antibody responses in typhoid fever patients
We used S. Typhi membrane preparation (MP) [21] to measure antibody responses in DBS eluates and plasma specimens of typhoid fever patients. There was a strong agreement of MP-specific antibody responses between DBS eluates and plasma of typhoid fever patients (ICC: IgA = 0.8, IgG = 0.7 and IgM = 0.8; Fig 6A–6C). There was also a positive correlation of MP-specific antibody responses between DBS eluates and plasma of typhoid fever patients at day 1 (IgA: r = 0.7, p = 0.01; IgG: r = 0.76, p = 0.001, and IgM: r = 0.8, p = 0.001; Fig 6D–6F).
Fig 6. MP-specific antibody responses in DBS eluates and plasma in typhoid fever patients.
The Bland-Altman analysis of MP-specific IgA (A), IgG (B), IgM (C) antibody responses between DBS eluates and plasma specimen of typhoid fever patients (n = 12) are shown. Spearman correlation of MP-specific IgA (D), IgG (E) and IgM (F) between DBS eluates and plasma specimen are shown.
Discussion
Collection and transportation of blood specimen from field settings to laboratories is a problem in resource poor settings and especially in unpredicted epidemics and outbreaks, which often occur in places that have limited laboratory facilities. DBS can also be prepared using finger prick blood since about 50 μl of blood is only required per DBS spot. It is also a less invasive way of blood collection method that this does not necessitate the presence of skilled staff to perform phlebotomy [25]. Thus, the diagnosis and prognosis of diseases and evaluation of disease specific immune responses after natural infection and vaccination can become less difficult to assess and monitor using DBS as an alternative procedure for specimen collection. This procedure as used in this evaluation of enteric diseases and the cholera vaccine brings in an alternative specimen collection and storage method. Although the DBS method has existed for long for sampling for diagnosis of genetic disorders, recently there has been a resurgence of interest in the use of this procedure for carrying out immunological studies in vaccine trials and infectious diseases diagnosis. Enteric infections such as diarrheal diseases (cholera and ETEC diarrhea) as well as typhoid fever are endemic in Africa and Asia. Vaccination is a preventive measure to protect the vulnerable population in these regions. However, to evaluate immunological response to vaccines and natural infection requires collection of blood samples, and transportation to well-equipped laboratories in the cold. We therefore tested the possibility of the use of DBS blood collection method to evaluate immune responses in cholera, ETEC and typhoid patients as well as vaccinees in a country with high rates of enteric infections.
Vibriocidal antibody response is considered one of the key surrogate markers for both natural cholera infection and vaccination in the field of cholera immunology. For the vibriocidal assay, blood needs to be collected to obtain serum or plasma. The procedure requires blood collection and then separation using centrifuges and storage and transportation under cold conditions. All these requires such facilities to be present in remote field settings and peripheral laboratories which are mostly non-existent. In this study, we have demonstrated that the vibriocidal assay can be carried out using dried blood spot, DBS specimens which provide comparable responses to that obtained with plasma in the conventional method. Vibriocidal assay requires measurement of OD values to investigate the growth of V. cholerae strain. As the DBS eluates are reddish in color because of the presence of heme makes it impossible for measuring vibriocidal antibody in conventional assay. We were able to circumvent the effect of heme in the DBS eluates by subtracting the OD values of the DBS eluates from the color of the background from test wells. This allows us to measure vibriocidal antibody responses in the 96-well plates similar to that used for the conventional vibriocidal assay. This method does not require additional determination of bacterial CFU measurement checking in microbiological culture plates and this saves time, resources and also makes the procedure less costly. The DBS based optimized vibriocidal technique that we developed does not require DBS serum separator card and it is based on a one-step procedure with measurement of optical density of bacterial growth only. However, like the conventional assay it is programmable in 96-well plate ELISA plate reader (Gene 5). Recently Iyer et. al. used DBS serum separator card to separate serum and then performed vibriocidal assay using the drop-plate technique. The drop-plate technique is based on the assumption of determining bactericidal growth or killing using measurement of clumped colonies or serrated margins of growth [26]. However, the method present here does not require additional determination of CFU of bacteria on agar-based culture plate that saves time, resources and money. The method presented here does not require serum separator cards to separate serum for performing the vibriocidal assay [26]. In our opinion, the method described here can replace conventional assay method when only small amount of blood can be obtained from large population and also in field settings with limited resources.
Cholera is water borne disease that can be a cause of death within a few hours if not treated immediately. It has been shown that the oral cholera vaccines can protect people from three to five years from another episode [27–32]. By collecting blood samples from cholera patients and oral cholera vaccinees using the DBS procedure, we have shown that immune responses measured were similar to that seen using plasma. With the DBS usage using finger prick blood collection by venipuncture can be avoided. We also showed that responses to different diseases and antigens could be carried out using DBS specimens in ELISA procedures. In addition, the antibody responses measured in ETEC-infected diarrheal patients to LTB were also comparable when either DBS or plasma was used. Given that we observed a very positive correlation of immune responses in DBS compared to plasma specimen, we extended this observation to typhoid fever patients. The immune responses against membrane preparation (MP) in DBS eluates were also similar to that seen in plasma samples.
DBS has earlier been found to be useful for measuring antibody responses in viral infections e.g. hepatitis C, HIV and Measles [5, 33, 34]. The DBS specimens have also been used for analyzing antibody responses in bacterial, fungal and parasite infections and to our knowledge, there is no published data using DBS for assessing immune response in patients with cholera, ETEC diarrhea and typhoid fever [33–39]. Measuring antibody responses using DBS was found to strongly correlate with plasma antibodies [34, 40, 41]. We show here that the vibriocidal antibody assay which is an indirect correlate of protection in cholera [42] can be measured using the DBS eluates and we hope that this will make it easier to measure immune responses in mass vaccination campaigns as well as in serosurveys for estimating disease burden globally. Likewise large population studies on natural infection or vaccination in areas of typhoid and ETEC diarrhea both natural infection and vaccination as well as other infectious diseases will benefit with the DBS method of sample collection and testing described in this communication.
Supporting information
The Bland-Altman analysis of vibriocidal responses in plasma and DBS eluates of cholera patients at day 2 (A), day 30 (B) are shown. Spearman correlation of vibriocidal responses in plasma and DBS eluates at day 2 (C) and day 30 (D) are shown.
(TIF)
The Bland-Altman analysis of vibriocidal responses in plasma and DBS eluates of OCV recipients at day 0 (A), day 28 (B) and day 42 (C) are shown. Spearman correlation of vibriocidal responses in plasma and DBS eluates at day 0 (D), day 28 (E) and day 42 (F) are shown.
(TIF)
Spearman correlation of LPS-specific IgA (A), IgG (B), IgM (C), and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of cholera patients at day 7 are shown.
(TIF)
Spearman correlation of LPS-specific IgA (A), IgG (B), IgM (C), and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of cholera patients at day 14 are shown.
(TIF)
Spearman correlation of LTB-specific antibody responses at day 2 [IgA (A), IgG (B), IgM (C)] and day 30 [IgA (D), IgG (E), IgM (F)] between DBS eluates and plasma of ETEC-patients are shown.
(TIF)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors acknowledge the support of the dedicated field and laboratory workers as well as the study participants in this study at the icddr,b. This work was supported by the Bill & Gates foundation [MSB and FQ] and Emerging Global Leader Award; K43 TW010362, R01 AI130378 [TRB]. We also grateful to the Government of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monleau M, Aghokeng AF, Eymard-Duvernay S, Dagnra A, Kania D, Ngo-Giang-Huong N, et al. Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia. J Clin Microbiol. 2014;52(2):578–86. 10.1128/JCM.02860-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakhi AK, Bastani NE, Ellingjord-Dale M, Gundersen TE, Blomhoff R, Ursin G. Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study. BMC Cancer. 2015;15:265 10.1186/s12885-015-1275-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12(4):195–208. [PubMed] [Google Scholar]
- 4.Monleau M, Montavon C, Laurent C, Segondy M, Montes B, Delaporte E, et al. Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing. J Clin Microbiol. 2009;47(4):1107–18. 10.1128/JCM.02255-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzicanin A, Lubega I, Nanuynja M, Mercader S, Rota P, Bellini W, et al. Dried blood spots on filter paper as an alternative specimen for measles diagnostics: detection of measles immunoglobulin M antibody by a commercial enzyme immunoassay. J Infect Dis. 2011;204 Suppl 1:S564–9. [DOI] [PubMed] [Google Scholar]
- 6.Wihokhoen B, Dondorp AM, Turner P, Woodrow CJ, Imwong M. Use of Blood Smears and Dried Blood Spots for Polymerase Chain Reaction-Based Detection and Quantification of Bacterial Infection and Plasmodium falciparum in Severely Ill Febrile African Children. Am J Trop Med Hyg. 2016;94(2):322–6. 10.4269/ajtmh.15-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Elst KC, Span LF, van Hateren K, Vermeulen KM, van der Werf TS, Greijdanus B, et al. Dried blood spot analysis suitable for therapeutic drug monitoring of voriconazole, fluconazole, and posaconazole. Antimicrob Agents Chemother. 2013;57(10):4999–5004. 10.1128/AAC.00707-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers AG, Percy AJ, Yang J, Camenzind AG, Borchers CH. Multiplexed quantitation of endogenous proteins in dried blood spots by multiple reaction monitoring-mass spectrometry. Mol Cell Proteomics. 2013;12(3):781–91. 10.1074/mcp.M112.022442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deglon J, Versace F, Lauer E, Widmer C, Mangin P, Thomas A, et al. Rapid LC-MS/MS quantification of the major benzodiazepines and their metabolites on dried blood spots using a simple and cost-effective sample pretreatment. Bioanalysis. 2012;4(11):1337–50. 10.4155/bio.12.42 [DOI] [PubMed] [Google Scholar]
- 10.Leruez-Ville M, Ngin S, Guilleminot T, Kfutwah A, Moussa S, Tran T, et al. Detection of cytomegalovirus DNA on dried blood spots collected from infants infected with HIV: an in-house method adaptable in resource-limited settings. J Virol Methods. 2013;193(2):503–7. 10.1016/j.jviromet.2013.07.024 [DOI] [PubMed] [Google Scholar]
- 11.Fokkema MR, Bakker AJ, de Boer F, Kooistra J, de Vries S, Wolthuis A. HbA1c measurements from dried blood spots: validation and patient satisfaction. Clin Chem Lab Med. 2009;47(10):1259–64. 10.1515/CCLM.2009.274 [DOI] [PubMed] [Google Scholar]
- 12.Melgaco JG, Pinto MA, Rocha AM, Freire M, Gaspar LP, Lima SM, et al. The use of dried blood spots for assessing antibody response to hepatitis A virus after natural infection and vaccination. J Med Virol. 2011;83(2):208–17. 10.1002/jmv.21973 [DOI] [PubMed] [Google Scholar]
- 13.Begum YA, Baby NI, Faruque AS, Jahan N, Cravioto A, Svennerholm AM, et al. Shift in phenotypic characteristics of enterotoxigenic Escherichia coli (ETEC) isolated from diarrheal patients in Bangladesh. PLoS Negl Trop Dis. 2014;8(7):e3031 10.1371/journal.pntd.0003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qadri F, Mohi G, Hossain J, Azim T, Khan AM, Salam MA, et al. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2(6):685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang HA, Jonson G, Svennerholm AM, Palva ET. The maltose-inducible 43 kDa major outer membrane protein in Vibrio cholerae is immunogenic and common to different isolates. Microb Pathog. 1988;5(3):169–75. [DOI] [PubMed] [Google Scholar]
- 16.Jertborn M, Svennerholm AM, Holmgren J. Gut mucosal, salivary and serum antitoxic and antibacterial antibody responses in Swedes after oral immunization with B subunit-whole cell cholera vaccine. Int Arch Allergy Appl Immunol. 1984;75(1):38–43. [DOI] [PubMed] [Google Scholar]
- 17.Jonson G, Sanchez J, Svennerholm AM. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J Gen Microbiol. 1989;135(1):111–20. 10.1099/00221287-135-1-111 [DOI] [PubMed] [Google Scholar]
- 18.Osek J, Svennerholm AM, Holmgren J. Protection against Vibrio cholerae El Tor infection by specific antibodies against mannose-binding hemagglutinin pili. Infect Immun. 1992;60(11):4961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svennerholm AM, Jertborn M, Gothefors L, Karim AM, Sack DA, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149(6):884–93. 10.1093/infdis/149.6.884 [DOI] [PubMed] [Google Scholar]
- 20.Qadri F, Azim T, Chowdhury A, Hossain J, Sack RB, Albert MJ. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin Diagn Lab Immunol. 1994;1(1):51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhuiyan TR, Qadri F, Saha A, Svennerholm AM. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J. 2009;28(2):79–85. 10.1097/INF.0b013e31818a5d9d [DOI] [PubMed] [Google Scholar]
- 22.Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, et al. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol. 2009;16(11):1587–94. 10.1128/CVI.00311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanam F, Sayeed MA, Choudhury FK, Sheikh A, Ahmed D, Goswami D, et al. Typhoid fever in young children in Bangladesh: clinical findings, antibiotic susceptibility pattern and immune responses. PLoS Negl Trop Dis. 2015;9(4):e0003619 10.1371/journal.pntd.0003619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury F, Begum YA, Alam MM, Khan AI, Ahmed T, Bhuiyan MS, et al. Concomitant enterotoxigenic Escherichia coli infection induces increased immune responses to Vibrio cholerae O1 antigens in patients with cholera in Bangladesh. Infect Immun. 2010;78(5):2117–24. 10.1128/IAI.01426-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. J Vis Exp. 2014(83):e50973 10.3791/50973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer AS, Azman AS, Bouhenia M, Deng LO, Anderson CP, Graves M, et al. Dried Blood Spots for Measuring Vibrio cholerae-specific Immune Responses. PLoS Negl Trop Dis. 2018;12(1):e0006196 10.1371/journal.pntd.0006196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366(9479):44–9. 10.1016/S0140-6736(05)66550-6 [DOI] [PubMed] [Google Scholar]
- 28.Ali M, Sur D, You YA, Kanungo S, Sah B, Manna B, et al. Herd protection by a bivalent killed whole-cell oral cholera vaccine in the slums of Kolkata, India. Clin Infect Dis. 2013;56(8):1123–31. 10.1093/cid/cit009 [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13(12):1050–6. 10.1016/S1473-3099(13)70273-1 [DOI] [PubMed] [Google Scholar]
- 30.Khatib AM, Ali M, von Seidlein L, Kim DR, Hashim R, Reyburn R, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis. 2012;12(11):837–44. 10.1016/S1473-3099(12)70196-2 [DOI] [PubMed] [Google Scholar]
- 31.Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, et al. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med. 2014;370(22):2111–20. 10.1056/NEJMoa1312680 [DOI] [PubMed] [Google Scholar]
- 32.Qadri F, Wierzba TF, Ali M, Chowdhury F, Khan AI, Saha A, et al. Efficacy of a Single-Dose, Inactivated Oral Cholera Vaccine in Bangladesh. New Engl J Med. 2016;374(18):1723–32. 10.1056/NEJMoa1510330 [DOI] [PubMed] [Google Scholar]
- 33.Iversen J, Grebely J, Catlett B, Cunningham P, Dore GJ, Maher L. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int J Drug Policy. 2017;47:77–85. 10.1016/j.drugpo.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 34.Tran TN, de Vries PJ, Hoang LP, Phan GT, Le HQ, Tran BQ, et al. Enzyme-linked immunoassay for dengue virus IgM and IgG antibodies in serum and filter paper blood. BMC Infect Dis. 2006;6:13 10.1186/1471-2334-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boillot F, Peeters M, Kosia A, Delaporte E. Prevalence of the human immunodeficiency virus among patients with tuberculosis in Sierra Leone, established from dried blood spots on filter paper. Int J Tuberc Lung Dis. 1997;1(6):493–7. [PubMed] [Google Scholar]
- 36.Duarte EC, Gyorkos TW, Pang L, Avila S, Fontes CJ. Inter-test reliability of the anti-RESA indices based on ELISA tests using eluates from whole blood spots dried on filter paper. Epidemiol Infect. 2002;129(1):139–45. 10.1017/s0950268802007185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahne S, Schurink T, Wallinga J, Kerkhof J, van der Sande M, van Binnendijk R, et al. Mumps transmission in social networks: a cohort study. BMC Infect Dis. 2017;17(1):56 10.1186/s12879-016-2135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland WG, Thanh NG, My LN, Magnus E, Verloo D, Buscher P, et al. Evaluation of whole fresh blood and dried blood on filter paper discs in serological tests for Trypanosoma evansi in experimentally infected water buffaloes. Acta Trop. 2002;81(2):159–65. [DOI] [PubMed] [Google Scholar]
- 39.Torrella A, Solis RL, Rodriguez N, Medina Y, Pita M, Perez I, et al. [Ultramicro ELISA to the detection of IgM antibodies in Mycobacterium leprae using dry blood samples]. Rev Inst Med Trop Sao Paulo. 1994;36(2):131–8. [PubMed] [Google Scholar]
- 40.Yel L, Rabbat CJ, Cunningham-Rundles C, Orange JS, Torgerson TR, Verbsky JW, et al. A Novel Targeted Screening Tool for Hypogammaglobulinemia: Measurement of Serum Immunoglobulin (IgG, IgM, IgA) Levels from Dried Blood Spots (Ig-DBS Assay). J Clin Immunol. 2015;35(6):573–82. 10.1007/s10875-015-0184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colson KE, Potter A, Conde-Glez C, Hernandez B, Rios-Zertuche D, Zuniga-Brenes P, et al. Use of a commercial ELISA for the detection of measles-specific immunoglobulin G (IgG) in dried blood spots collected from children living in low-resource settings. J Med Virol. 2015;87(9):1491–9. 10.1002/jmv.24136 [DOI] [PubMed] [Google Scholar]
- 42.Losonsky GA, Yunyongying J, Lim V, Reymann M, Lim YL, Wasserman SS, et al. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun. 1996;64(1):10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Bland-Altman analysis of vibriocidal responses in plasma and DBS eluates of cholera patients at day 2 (A), day 30 (B) are shown. Spearman correlation of vibriocidal responses in plasma and DBS eluates at day 2 (C) and day 30 (D) are shown.
(TIF)
The Bland-Altman analysis of vibriocidal responses in plasma and DBS eluates of OCV recipients at day 0 (A), day 28 (B) and day 42 (C) are shown. Spearman correlation of vibriocidal responses in plasma and DBS eluates at day 0 (D), day 28 (E) and day 42 (F) are shown.
(TIF)
Spearman correlation of LPS-specific IgA (A), IgG (B), IgM (C), and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of cholera patients at day 7 are shown.
(TIF)
Spearman correlation of LPS-specific IgA (A), IgG (B), IgM (C), and CTB-specific IgA (D), IgG (E) and IgM (F) antibody responses between DBS eluates and plasma of cholera patients at day 14 are shown.
(TIF)
Spearman correlation of LTB-specific antibody responses at day 2 [IgA (A), IgG (B), IgM (C)] and day 30 [IgA (D), IgG (E), IgM (F)] between DBS eluates and plasma of ETEC-patients are shown.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.