Abstract
Objective:
In this review the authors discuss evidence from the literature concerning vitamin D and temporal bone diseases (benign paroxysmal positional vertigo (BPPV), Meniere´s disease (MD), vestibular neuritis, idiopathic facial paralysis, idiopathic acute hearing loss). Common features shared by Meniere´s disease, glaucoma and the possible influence by vitamin D are briefly discussed.
Data Sources, Study Selection:
Publications from 1970 until recent times have been reviewed according to a keyword search (see above) in PUBMED.
Conclusions:
MD, BPPV, vestibular neuritis, idiopathic facial paralysis, idiopathic acute hearing loss may all have several etiological factors, but a common feature of the current theories is that an initial viral infection and a subsequent autoimmune/autoinflammatory reaction might be involved. Additionally, in some of these entities varying degrees of demyelination have been documented. Given the immunomodulatory effect of vitamin D, we postulate that it may play a role in suppressing an eventual postviral autoimmune reaction. This beneficial effect may be enhanced by the antioxidative activity of vitamin D and its potential in stabilizing endothelial cells. The association of vitamin D deficiency with demyelination has already been established in other entities such as multiple sclerosis and experimental autoimmune encephalitis. Mice without vitamin D receptor show degenerative features in inner ear ganglia, hair cells, as well as otoconia. The authors suggest further studies concerning the role of vitamin D deficiency in diseases of the temporal bone. Additionally, the possible presence and degree of demyelination in these entities will have to be elucidated more systematically in the future.
Introduction
In 2013 we observed that vitamin D (VD) deficiency may be associated with benign paroxysmal positional vertigo (BPPV) (1). Since then this hypothesis has been supported by several laboratories (2-6). In the last 4 years we noted a trend that correcting VD deficiency in newly diagnosed cases of Meniere´s disease (MD) decreased the necessity of the ablative therapy with intratympanal gentamicin (7). These observations raise the question if VD influences inner ear pathology in general and if yes, by what mechanism. In this review we examine if there is a common mechanism by which VD may influence these pathogenetic processes.
Possible common pathogenic factors in diseases of the temporal bone
The etiology of the idiopathic peripheral (Bell´s) palsy is not entirely clear (with the exception of facial palsy caused by borrelia burgdorferi or herpes zoster, which are usually not called ‘idiopathic’). One proposed mechanism implicated reactivated herpes simplex viruses (HSV) (8,9), which might provoke a cell-mediated autoimmune response against myelin. Because of this demyelination, Bell´s palsy might be hypothetically regarded as a mononeuropathic form of Guillain-Barré syndrome (10).
After vestibular neuritis, histopathological changes are consistent with an isolated viral infection of the Scarpa´s ganglia, frequently without viral particles or antigens (11,12). Therefore either a direct viral damage or an immune-mediated process has been postulated analogous to the post-infectious encephalomyelitis seen in the central nervous system (11,13,14).
In BPPV, it has been shown that dislodgement of otoconia occurs frequently after vestibular neuritis, when only the superior vestibular nerve (which innervates the utriculus) is affected (15,16). In these cases the otoconia may be dislodged because the utricular nerve is involved along with the lateral and superior canal ampulla. The fact that parts of the labyrinth may remain unaffected after a bout of vestibular neuritis means that vestibular neuritis is a neuropathy/neuroepithelopathy. In principle, it is possible that idiopathic BPPV is also caused/exacerbated by a neuropathy of utricular nerve fibers. Indeed, in BPPV loss of vestibular ganglion cells could be shown together with histopathological features characteristic of reactivation of latent herpes simplex viruses (17).
Although sudden hearing loss (SHL) may be caused by infectious agents, such as herpes zoster, mumps virus; in most cases the pathogenesis remains unknown. Low frequency hearing loss should be considered separately from high frequency sudden hearing loss, because the former is possibly caused by increased endolymphatic pressure (hydrops) as in Meniere´s disease (18). In 2005 Merchant et al examined 17 temporal bones donated by patients with a history of sudden hearing loss (19). Unambiguous evidence of a concomitant direct viral invasion was lacking and the authors postulated that idiopathic SHL may be the result of pathologic activation of cellular stress pathways involving nuclear factor-kappaB (NF-kB) and release of inflammatory cytokines and other stress-related proteins within the cochlea, elicited by a systemic or distant viral infection, a systemic inflammatory disorder, physical, mental or metabolic stress that triggers an antibody response (19). Type II fibrocytes, which house the potassium ion recirculation system of the cochlea, could be targeted by such a process. In other studies, histopathologic changes after idiopathic SHL were indicative of infection with neurotropic viruses (20,21) or (herpes)viral cochleitis (22,23) . In animal experiments auditory nerve demyelination by transient loss of cochlear Schwann-cells caused auditory neuropathy associated with hidden hearing loss (24).
The etiology and pathogenesis of MD remain unknown. Proposed theories include viral infections and immune system-mediated mechanisms. The presence of anti-herpes simplex virus antibodies was more frequent than in controls in perilymph samples collected from patients with MD (25) and the virus could be more frequently isolated from vestibular ganglia in MD than from controls (26). Because endolymphatic hydrops is such a conspicuous histological change in cases of MD, histopathological examinations in the literature concentrated mainly on the end organ. However, in a light and electron microscopic study Spencer et al found moderate to severe demyelination in the vestibular nerve with microglia, which assumed a phagocytic role (27). Based on this, the authors postulated that viral and/or immune-mediated factors may play a role in MD, similar to those seen in other demyelinating diseases, such as multiple sclerosis and Guillain-Barre syndrome. Gacek observed a significant loss of vestibular ganglion cells in temporal bones from patients with history of MD with viral particles enclosed in transport vesicles (28). Kitamura et al demonstrated a small number of degenerated nerve fibers, found a correlation between reduced vestibular caloric response and the incidence /density of the abnormal nerve fibers (29). Considering the end organ in MD, degeneration of the blood-brain barrier (30) has been demonstrated, possibly caused by oxidative stress and pericyte pathology including degeneration and migration (31). Apart from perivascular microvascular damage also neuroepithelial damage with hair cell loss, basement membrane thickening (32), and diminished aquaporin 4 (AQP4) expression in the supporting cells have been shown (33,34). AQP6 exhibited a loss of polarity, being spread throughout the cell, rather than being localized to the apical region of the supporting cell (33). Cochlear sulcus cells in the apical cochlea, where usually the first signs of endolymphatic hydrops can be observed, contain AQP4 and AQP5 in their membranes (35,36). These channels are responsible for the water transport between endolymphatic and perilymphatic spaces. AQP4 is the most abundant water channel in the central nervous system. It is targeted by IgG in neuromyelitis optica, a syndrome characterized by optic neuritis and myelitis (37). It has been suggested that antibodies against AQP4 may also play a role in MD as it has many similarities to neuromyelitis optica in terms of age of onset, course characteristics, pathological features such as histological loss of AQP4 and treatment response to glucocorticoid therapy (38).
Cochlin (COCH, coagulation factor C homology), the most abundant extracellular matrix protein in the ear, has also been shown to be altered in MD. Immunolocalization showed increased levels of cochlin deposition and expression in the basilar membranes of the cristae ampullaris and maculae utricle in subjects with MD (39). Certain mutations of cochlin cause autosomal dominant nonsyndromic sensorineural hearing loss with variable vestibular symptoms (DFNA9). Although hearing deteriorates usually bilaterally in the high frequency region, this hereditary defect sometimes also causes vestibular symptoms like in MD (40,41). While the causative mechanism is not clear yet, it has been shown that the product of the mutant gene aggregates as abundant granulates of homogeneous acellular eosinophilic deposits in the cochlear and vestibular labyrinths, similar to protein aggregation in well-known neurodegenerative disorders such as Alzheimer’s disease (42,43). Therefore DFNA9 is listed among protein aggregate diseases along with Alzheimer´s and Parkinson´s diseases(44). One possible mechanism may be that mutant cochlin (when the mutation involves its LCCL domain) is cytotoxic due to prolonged or stable formation of dimers, whereas wildtype (normal) cochlin only forms transient dimers (45). This would explain why is it possible that aggregated cochlin molecules may have a role in MD although it has been shown that in sporadic ‘true’ MD the COCH gene is not mutated (46,47). In these sporadic cases accidentally misfolded cochlin molecules might ‘infect’ healthy ones, purely by changing their folding structure and make them resistant against degradation(45)2.
Is it possible that even without mutation, the cells of the inner ear start to produce such misfolded protein molecules? Apparently, this may be possible in chronic inflammation. During cellular stress or after harmful stimuli that damage the protein folding process, unfolded or misfolded proteins accumulate in the endoplasmatic reticulum (48), which in turn leads to an inflammatory reaction, involving transcription factors such as NF-kB and others. Thereby a vicious cycle ensues and exacerbates inflammatory reaction involving specialized cells such as macrophages (called microglia in the central nervous system) (48). As we already mentioned above, recently it has been shown that the inner ear (even its sensory cell areas) and the vicinity of the vestibulocochlear nerve are densely populated by macrophages/microglia (49). These cells cannot be differentiated from other fibrocyte-like cells by standard hematoxylin and eosin staining. It has also been shown that T cell responsiveness against cochlin may be a mechanism in cases of autoimmune-induced inflammation and hearing loss (50). According to a study, several Aspergillus species and penicillium molds share homology with the LCCL domain of cochlin, therefore fungal exposure might trigger autoimmunity in a subset of susceptible patients (51). A subset of MD patients have higher basal levels of proinflammatory cytokines and the exposure to Aspergillus and Penicillium extracts may trigger additional TNF-α release and exacerbate inflammation (52). In bilateral MD the TWEAK/Fn14 pathway, which is involved in the modulation of inflammation in several human autoimmune diseases (including multiple sclerosis, systemic lupus erythematosus or rheumatoid arthritis), may regulate cellular proliferation in lymphoid cells by increasing the translation of NF-κB (53). The authors suggest that carriers of a certain risk genotype (rs4947296) of a regulator of TWEAK/Fn14 may develop an NF-κB-mediated inflammatory response in MD.
However, systemic exposure to molds and subsequent autoimmune reaction should involve both ears, but sporadic MD is mostly unilateral. It has been shown that latent herpes viruses cause a localized, incomplete or low-level lytic infection leading to a persistent immune response (54), therefore unilateral, postinfectious immune reaction might occur without persistent viral presence by molecular mimicry and/or bystander activation (55) as in herpetic stromal keratitis (56). This may explain why in many cases of MD viral presence could not be demonstrated. Gacek and Gacek devised a complex anatomical ad pathological framework to explain all the features of BPPV, MD and neuritis, including the vertigo attacks, exclusively by neuronal pathology caused by herpetic ‘ganglionitis’ (57). The exact pattern of the post-herpetic degeneration may be determined by the local neural involvement. In some cases the demyelination may dominate, in others the end-organ pathology. For instance, Meniere´s disease may differ from all the other entities in that the chronic post-herpetic autoimmune inflammation may cause peripheral microvascular damage, dislocate aquaporin channels in the cell and/or lead to pathological aggregation and accumulation of cochlin molecules. From time to time, the endolymphatic pressure increases sharply, as has been recently convincingly demonstrated using measurements of the operating point of the outer hair cells during acute attacks (58), but chronic hydrops is certainly only a last common consequence of primary underlying pathologies.
In addition to the inner ear, cochlin has been identified in the glaucomatous trabecular meshwork (TM) (59). Primary open angle glaucoma is the most common form of glaucoma and is typically associated with elevated intraocular pressure. Over-expression and down-regulation of cochlin increases and decreases intraocular pressure (60). TM cells detect aqueous humor fluid shear stress via cochlin interactions with the cell surface bound and stretch-activated channel TREK-1 (61). It is not known if these pressure-sensing function operates in the inner ear. A rare cause of secondary glaucoma, the Posner-Schlossman syndrome, shows striking similarities to MD. This condition goes with recurrent, acute attacks of mild, unilateral, anterior uveitis accompanied by markedly elevated intraocular pressure. Although not much is known about this entity, it is believed that the initial event that leads to it is a viral infection in the anterior chamber, caused probably by cytomegalovirus (62) or herpes simplex virus (63). Apparently, vascular endothelial cell dysfunction is a risk factor for both normal-tension, open-angle glaucoma and for Posner-Schlossman syndrome as well, possibly by disturbing flow-mediated vasodilatation (62).
In conclusion, Bell´s palsy, BPPV, vestibular neuritis, sudden hearing loss and Meniere´s disease may all involve neuropathies/neuroepitheliopathies with variable demyelination and the pathogenetic role of viruses may be a common factor in subsets of these diseases. In the past decades, efforts to elucidate the pathogenetic mechanisms in these entities were concentrated on the pathologic features of the end-organs, and only a few studies addressed the frequency of the demyelination of the cochleo-vestibular nerve fibers or ganglia pathology.
Vitamin D: possible mechanisms of action
In the last few years it has been shown that VD regulates biological processes beyond its classical effects on skeletal mineral homeostasis. The most important non-classical effect is its immunomodulatory function (64), which has been confirmed by in vivo and in vitro studies including genome-wide analyses (65-67). VD upregulates the innate and inhibits the adaptive immune response and therefore its role in suppressing autoimmune/ autoinflammatory processes has been emphasized (68). In multiple sclerosis (MS) we see an example of this effect. MS is a heterogeneous, multifactorial disease influenced by both genetic and environmental factors. Pathological processes include breakdown of the blood-brain barrier, multifocal inflammation, demyelination, oligodendrocyte loss, reactive gliosis, and axonal degeneration (69), some of which have been observed in the above diseases of the temporal bone. Apparently infectious agents play a crucial role in inducing myelin-reactive pathogenic T cells (70). Potential mechanisms include cross reactivity with CNS myelin antigens, triggering an already expanded autoreactive immune repertoire (71). Increased VD levels, especially before the age of 20, are associated with a reduced risk of MS in later life and VD is not only linked to MS, but also to other autoimmune diseases, including rheumatoid arthritis, type 1 diabetes and systemic lupus erythematous (71).
Concerning increased intraocular pressure and glaucoma, in several studies the possibility has been raised that VD deficiency may be related to increased intraocular pressure (66). Krefting et al. found no statistical difference in intraocular pressure levels between individuals without eye disease with respect to VD levels (72). However, in patients with open angle glaucoma, VD deficiency was shown to be a potential risk factor for the development of the complaints (73). Also, gene expression studies showed that VD modulate genes regulating both aqueous humour outflow and production, as well as the architecture of the trabecular meshwork, thereby influencing intraocular pressure. By comprehensive microarray data analysis, Kutuzova et al. (74) found in cultured mouse calvarial cells and rat intestinal mucosa that VD altered the expression of genes known to be relevant to the regulation of intraocular pressure. In an interesting experiment the authors also examined the effect of VD and an active VD analogue on intraocular pressure in macaque monkeys (74). They demonstrated that topical VD transiently reduced intraocular pressure by 20 percent within hours. Until now these experiments have not been reproduced or followed upon (personal communication Prof Hector F. DeLuca), therefore the mechanism by which the impressive, acute decrease of the intraocular pressure was brought about is not clear.
A new mechanism, by which VD might influence the above processes, has been demonstrated by Gibson et al (75). The authors showed that dietary VD (which is usually considered an inactive precursor) had profound and immediate stabilizing effect on endothelial stability at physiologically relevant concentrations. The effects were independent of the canonical transcription-mediated VD pathway, which showed the presence of an alternative signaling modality by which VD acts directly on endothelial cells to prevent vascular leak. As seen above, increased permeability of the blood-brain-barrier and/or of the microvascular circulation has been hypothetically associated to the inflammatory processes of the inner ear.
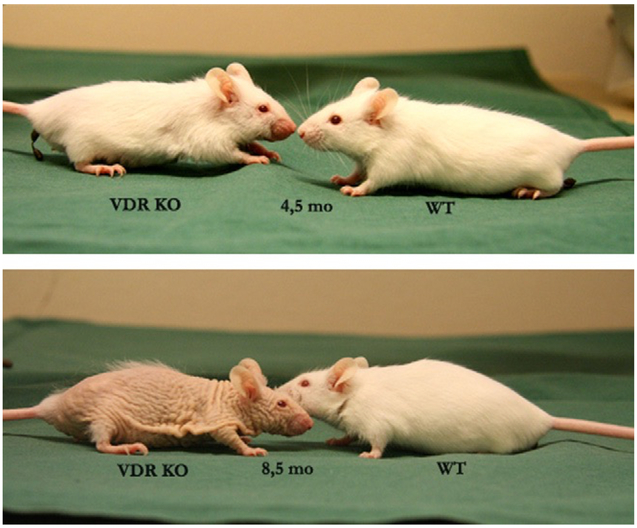
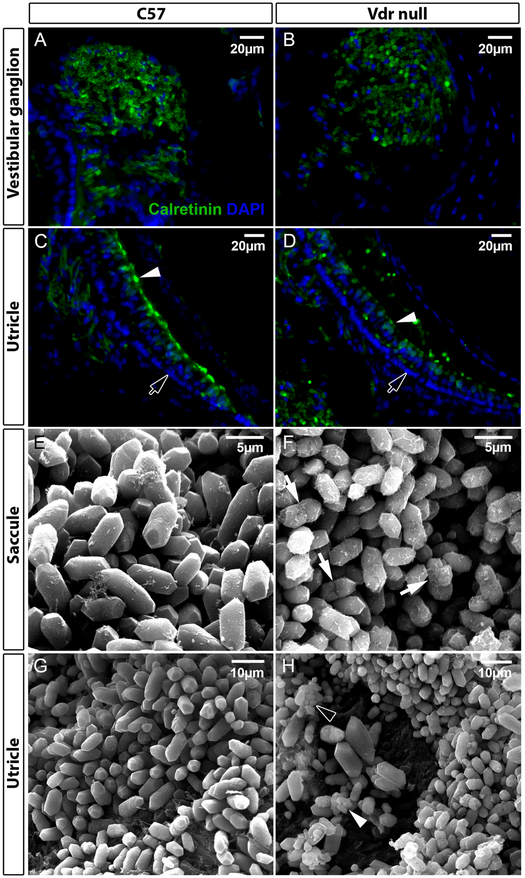
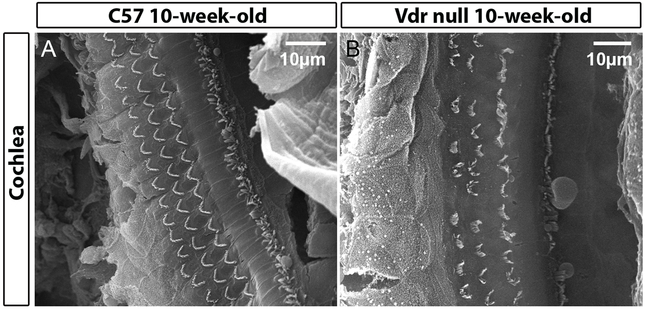
VD also influences the processes of aging (76). In the study of Keisala et al. (77) VD receptor knockout (Vdr null) mice, fed with a special rescue diet showed several aging related characteristics such as poorer survival, early alopecia, thickened skin (Figure 1), enlarged sebaceous glands and development of epidermal cysts. Intriguingly, the phenotype of aged VDR knockout mice was similar to mouse models with hypervitaminosis D. The authors suggested that VD homeostasis regulated physiological aging. In our laboratory (Vestibular Genetics Lab., Boys Town National Research Hospital, Omaha, US) a possible connection between VD deficiency and BPPV was found, because otoconia in Vdr null mice showed degenerative features such as fissure (arrows in Figure 2F), fusion (arrowhead in Figure 2H) and smaller particles (hollow arrowhead in Figure 2H). These features have been observed in aging otoconia (78,79). In addition, Vdr null mice also had compromised vestibular ganglia and hair cells in our material, as indicated by reduced signals of calretinin (Figure 2B vs. 2A, 2D vs. 2C, respectively). There was abnormal formation and/or loss of hair cells (Figure 3) in the cochlea of Vdr null mice as shown in our laboratory. Spiral ganglia loss in the mice has been previously reported by Zou et al, 2008. These mice have progressive hearing loss (80). In humans, VD deficiency has been associated with bilateral sensorineural hearing loss (81,82).
Figure 1.
Phenotype of vitamin D receptor knockout mouse (KO) compared to wildtype littermate (WT: wild type at the age of 4.5 (top) and 8.5 (bottom) months.
(Reprinted from (77) with permission from Elsevier).
Figure 2.
Effects of vitamin D receptor (Vdr) deletion in the vestibule. A-D: tissue sections were stained with an antibody against calretinin (green). Nuclei of cells were stained with DAPI (blue). The intensity of calretinin signals is reduced in the ganglion (B) and hair cells (D) of Vdr null mice as compared with age-matched wildtype controls (C57Bl/6J, labelled as C57) in A and C, respectively. Arrowheads in C-D denote hair cells, and arrows point to supporting cells. E-H: scanning electron micrographs of otoconia in C57 (E, G) and Vdr null mice (F, H) show degenerative features of otoconia in both the utricle and saccule of the Vdr null mice, such as fissure (arrows in F), fusion (arrowhead in H) and smaller particles (hollow arrowhead in H).
Figure 3.
Abnormal formation and/or loss of hair cells in the cochlea of Vdr null mice.
The direct effect of VD on otoconia would be controlling calcium concentration by regulating calcium absorption and uptake, and by influencing ion channel/pump expression which in turn affect calcium and subsequently otoconia formation/maintenance (Figure 4). In the intestine, the plasma membrane calcium pump (PMCA or Atp2b), isoform 1 is responsible for mediating the systemic effects of VD (83). Various PMCA isoforms are expressed in the inner ear [see NCBI GEO Profile], particularly PMCA2, which is essential for otoconial formation and hair cell function (84). In addition, calcium transport by transient receptor potential vanilloid 5 (TRPV5) is enhanced by VD (85). All TRPVs (TRPV1-6) are expressed in vestibular and cochlear sensory epithelia (86-88), and can regulate Ca2+ concentration in the endolymph (88), which would subsequently affect otoconia.
Figure 4.
Hypothetical mechanisms underlying the effects of vitamin D in the inner ear.
The effects of VD on ganglia and hair cells could also involve the above processes, i.e. via regulation of gene expression. In addition, VD is known to enhance antioxidant responses and reduce apoptosis in other organs, which would be beneficial to cellular function and survival in the inner ear as well (see also oxidative stress in MD (31) ). The protective effects of VD by enhancing antioxidant responses and reducing cell death have been shown at both cellular (89,90) and transcriptome levels (91). In human umbilical vein endothelial cell cultures it prevented cell death through modulation of the interplay between apoptosis and autophagy by inhibiting superoxide anion generation, maintaining mitochondria function and cell viability, activating survival kinases, and inducing NO production (67).
Concerning the immunomodulatory function of VD, the role of estrogen complicates the picture. In an earlier study we have shown that menopause increases the tendency of women to develop BPPV and that in the studied population there was a strong female:male preponderance (92) . A slight female:male preponderance is also known in patients with Meniere’s disease (1.3:1) and the peak incidence is in the 40 to 60-year age group (93,94). Recently it has been shown in animal experiments that low estrogen levels inhibit VD-mediated resistance to experimental autoimmune encephalomyelitis because estrogen controls VD metabolism and receptor expression (95). The findings in this study suggest that low estrogen levels might contribute to the increased female gender bias also in BPPV and MD by influencing VD metabolism.
Conclusion
While it is too early to draw a conclusion, it is possible that VD deficiency has a detrimental effect in BPPV and MD. Hypothetically, if after herpes simplex infection antigenic mimicry or bystander activation elicited a local autoimmune/autoinflammatory reaction in the temporal bone, this might be regulated/inhibited by physiological VD levels. The involvement of the different nerves would explain specific manifestations. MD would arise later if the chronic inflammatory reaction caused a faulty folding of cochlin in the endoplasmic reticulum, which could eventually cause accumulation of less degradable isoforms. Currently, the pathophysiologic mechanism of MD is yet unresolved and it is unclear to what extent neuropathy and demyelination exist in this disorder. However, the theory of antigenic mimicry or bystander activation, which may emerge years later, would explain the so called ‘late hydrops’ and the fact that it is often impossible to show dormant herpes viruses in the histological probes. Similarities with another pathological entity, glaucoma with intra-organ pressure increase, may be clarified in the future.
The role of decreasing estrogen levels in female patients with Meniere’s disease should also be examined. Given the known overall benefits of VD, we recommend the measurement of 25(OH)D in the serum and supplementation if necessary.
Acknowledgments
Conflicts of Interest and Source of Funding: Y.W.L. was supported by a grant (DC014748) from the National Institutes of Health. For the remaining authors none were declared.
References
- 1.Buki B, Ecker M, Junger H et al. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypotheses 2013;80:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong SH, Kim JS, Shin JW et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol 2013;260:832–8. [DOI] [PubMed] [Google Scholar]
- 3.Talaat HS, Abuhadied G, Talaat AS et al. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol 2015;272:2249–53. [DOI] [PubMed] [Google Scholar]
- 4.Talaat HS, Kabel AM, Khaliel LH et al. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx 2016;43:237–41. [DOI] [PubMed] [Google Scholar]
- 5.Meghji S, Murphy D, Nunney I et al. The Seasonal Variation of Benign Paroxysmal Positional Vertigo. Otol Neurotol 2017;38:1315–8. [DOI] [PubMed] [Google Scholar]
- 6.Whitman GT, Baloh RW. Seasonality of benign paroxysmal positional vertigo. JAMA Otolaryngol Head Neck Surg 2015;141:188–9. [DOI] [PubMed] [Google Scholar]
- 7.Buki B, Junger H, Lundberg YW. Vitamin D supplementation may improve symptoms in Meniere’s disease. Med Hypotheses 2018;116:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eviston TJ, Croxson GR, Kennedy PG et al. Bell’s palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry 2015;86:1356–61. [DOI] [PubMed] [Google Scholar]
- 9.Murakami S, Mizobuchi M, Nakashiro Y et al. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med 1996;124:27–30. [DOI] [PubMed] [Google Scholar]
- 10.Greco A, Gallo A, Fusconi M et al. Bell’s palsy and autoimmunity. Autoimmun Rev 2012;12:323–8. [DOI] [PubMed] [Google Scholar]
- 11.Ishiyama A, Ishiyama GP, Lopez I et al. Histopathology of idiopathic chronic recurrent vertigo. Laryngoscope 1996;106:1340–6. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF, Kitamura K. Second Louis H. Clerf Lecture. Vestibular neuritis. Ann Otol Rhinol Laryngol Suppl 1981;90:1–19. [PubMed] [Google Scholar]
- 13.Fenton JE, Shirazi A, Turner J et al. Atypical vestibular neuritis: a case report. Otolaryngol Head Neck Surg 1995;112:738–41. [DOI] [PubMed] [Google Scholar]
- 14.Lee SU, Kim HJ, Choi JY et al. Anti-ganglioside antibody-associated acute unilateral peripheral vestibulopathy. J Neurol 2019;266:250–2. [DOI] [PubMed] [Google Scholar]
- 15.Mandala M, Santoro GP, Awrey J et al. Vestibular neuritis: recurrence and incidence of secondary benign paroxysmal positional vertigo. Acta Otolaryngol 2010;130:565–7. [DOI] [PubMed] [Google Scholar]
- 16.Karlberg M, Hall K, Quickert N et al. What inner ear diseases cause benign paroxysmal positional vertigo? Acta Otolaryngol 2000;120:380–5. [DOI] [PubMed] [Google Scholar]
- 17.Gacek RR. Pathology of benign paroxysmal positional vertigo revisited. Ann Otol Rhinol Laryngol 2003;112:574–82. [DOI] [PubMed] [Google Scholar]
- 18.Plontke SK. Diagnostics and therapy of sudden hearing loss. GMS Curr Top Otorhinolaryngol Head Neck Surg 2017;16:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant SN, Adams JC, Nadol JB Jr. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2005;26:151–60. [DOI] [PubMed] [Google Scholar]
- 20.Linthicum FH Jr., Doherty J, Berliner KI. Idiopathic sudden sensorineural hearing loss: vascular or viral? Otolaryngol Head Neck Surg 2013;149:914–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khetarpal U, Nadol JB Jr., Glynn RJ Idiopathic sudden sensorineural hearing loss and postnatal viral labyrinthitis: a statistical comparison of temporal bone findings. Ann Otol Rhinol Laryngol 1990;99:969–76. [DOI] [PubMed] [Google Scholar]
- 22.Schuknecht HF, Donovan ED. The pathology of idiopathic sudden sensorineural hearing loss. Arch Otorhinolaryngol 1986;243:1–15. [DOI] [PubMed] [Google Scholar]
- 23.Stokroos RJ, Albers FW, Schirm J. The etiology of idiopathic sudden sensorineural hearing loss. Experimental herpes simplex virus infection of the inner ear. Am J Otol 1998;19:447–52. [PubMed] [Google Scholar]
- 24.Wan G, Corfas G. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat Commun 2017;8:14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold W, Niedermeyer HP. Herpes simplex virus antibodies in the perilymph of patients with Meniere disease. Arch Otolaryngol Head Neck Surg 1997;123:53–6. [DOI] [PubMed] [Google Scholar]
- 26.Vrabec JT. Herpes simplex virus and Meniere’s disease. Laryngoscope 2003;113:1431–8. [DOI] [PubMed] [Google Scholar]
- 27.Spencer RF, Sismanis A, Kilpatrick JK et al. Demyelination of vestibular nerve axons in unilateral Meniere’s disease. Ear Nose Throat J 2002;81:785–9. [PubMed] [Google Scholar]
- 28.Gacek RR. Meniere’s disease is a viral neuropathy. ORL J Otorhinolaryngol Relat Spec 2009;71:78–86. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura K, Kaminaga C, Ishida T et al. Ultrastructural analysis of the vestibular nerve in Meniere’s disease. Auris Nasus Larynx 1997;24:27–30. [DOI] [PubMed] [Google Scholar]
- 30.Ishiyama G, Lopez IA, Ishiyama P et al. The blood labyrinthine barrier in the human normal and Meniere’s disease macula utricle. Sci Rep 2017;7:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiyama G, Wester J, Lopez IA et al. Oxidative Stress in the Blood Labyrinthine Barrier in the Macula Utricle of Meniere’s Disease Patients. Front Physiol 2018;9:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCall AA, Ishiyama GP, Lopez IA et al. Histopathological and ultrastructural analysis of vestibular endorgans in Meniere’s disease reveals basement membrane pathology. BMC Ear Nose Throat Disord 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiyama G, Lopez IA, Sepahdari AR et al. Meniere’s disease: histopathology, cytochemistry, and imaging. Ann N Y Acad Sci 2015;1343:49–57. [DOI] [PubMed] [Google Scholar]
- 34.Ishiyama G, Lopez IA, Beltran-Parrazal L et al. Immunohistochemical localization and mRNA expression of aquaporins in the macula utriculi of patients with Meniere’s disease and acoustic neuroma. Cell Tissue Res 2010;340:407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckhard A, Muller M, Salt A et al. Water permeability of the mammalian cochlea: functional features of an aquaporin-facilitated water shunt at the perilymph-endolymph barrier. Pflugers Arch 2014;466:1963–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez IA, Ishiyama G, Lee M et al. Immunohistochemical localization of aquaporins in the human inner ear. Cell Tissue Res 2007;328:453–60. [DOI] [PubMed] [Google Scholar]
- 37.Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci 2016;1366:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He D, Zhang A, Li Y et al. Autoimmune aquaporin-4 induced damage beyond the central nervous system. Mult Scler Relat Disord 2017;18:41–6. [DOI] [PubMed] [Google Scholar]
- 39.Calzada AP, Lopez IA, Beltran Parrazal L et al. Cochlin expression in vestibular endorgans obtained from patients with Meniere’s disease. Cell Tissue Res 2012;350:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verstreken M, Declau F, Wuyts FL et al. Hereditary otovestibular dysfunction and Meniere’s disease in a large Belgian family is caused by a missense mutation in the COCH gene. Otol Neurotol 2001;22:874–81. [DOI] [PubMed] [Google Scholar]
- 41.Kim BJ, Kim AR, Han KH et al. Distinct vestibular phenotypes in DFNA9 families with COCH variants. Eur Arch Otorhinolaryngol 2016;273:2993–3002. [DOI] [PubMed] [Google Scholar]
- 42.Robertson NG, Cremers CW, Huygen PL et al. Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction. Hum Mol Genet 2006;15:1071–85. [DOI] [PubMed] [Google Scholar]
- 43.Burgess BJ, O’Malley JT, Kamakura T et al. Histopathology of the Human Inner Ear in the p.L114P COCH Mutation (DFNA9). Audiol Neurootol 2016;21:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefani M Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta 2004;1739:5–25. [DOI] [PubMed] [Google Scholar]
- 45.Yao J, Py BF, Zhu H et al. Role of protein misfolding in DFNA9 hearing loss. J Biol Chem 2010;285:14909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez E, Lopez-Escamez JA, Lopez-Nevot MA et al. Absence of COCH mutations in patients with Meniere disease. Eur J Hum Genet 2004;12:75–8. [DOI] [PubMed] [Google Scholar]
- 47.Usami S, Takahashi K, Yuge I et al. Mutations in the COCH gene are a frequent cause of autosomal dominant progressive cochleo-vestibular dysfunction, but not of Meniere’s disease. Eur J Hum Genet 2003;11:744–8. [DOI] [PubMed] [Google Scholar]
- 48.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008;454:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Malley JT, Nadol JB Jr., McKenna MJ Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol 2016;37:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baek MJ, Park HM, Johnson JM et al. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol 2006;177:4203–10. [DOI] [PubMed] [Google Scholar]
- 51.Pathak S, Hatam LJ, Bonagura V et al. Innate immune recognition of molds and homology to the inner ear protein, cochlin, in patients with autoimmune inner ear disease. J Clin Immunol 2013;33:1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frejo L, Gallego-Martinez A, Requena T et al. Proinflammatory cytokines and response to molds in mononuclear cells of patients with Meniere disease. Sci Rep 2018;8:5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frejo L, Requena T, Okawa S et al. Regulation of Fn14 Receptor and NF-kappaB Underlies Inflammation in Meniere’s Disease. Front Immunol 2017;8:1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol 1996;157:3542–9. [PubMed] [Google Scholar]
- 55.Fujinami RS, von Herrath MG, Christen U et al. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 2006;19:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2001;2:797–801. [DOI] [PubMed] [Google Scholar]
- 57.Gacek RR, Gacek MR. The three faces of vestibular ganglionitis. Ann Otol Rhinol Laryngol 2002;111:103–14. [DOI] [PubMed] [Google Scholar]
- 58.Avan P, Giraudet F, Chauveau B et al. Unstable distortion-product otoacoustic emission phase in Meniere’s disease. Hear Res 2011;277:88–95. [DOI] [PubMed] [Google Scholar]
- 59.Picciani R, Desai K, Guduric-Fuchs J et al. Cochlin in the eye: functional implications. Prog Retin Eye Res 2007;26:453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goel M, Sienkiewicz AE, Picciani R et al. Cochlin, intraocular pressure regulation and mechanosensing. PLoS One 2012;7:e34309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goel M, Sienkiewicz AE, Picciani R et al. Cochlin induced TREK-1 co-expression and annexin A2 secretion: role in trabecular meshwork cell elongation and motility. PLoS One 2011;6:e23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Megaw R, Agarwal PK. Posner-Schlossman syndrome. Surv Ophthalmol 2017;62:277–85. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto S, Pavan-Langston D, Tada R et al. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol 1995;119:796–8. [DOI] [PubMed] [Google Scholar]
- 64.Bikle D Nonclassic actions of vitamin D. J Clin Endocrinol Metab 2009;94:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munoz Garcia A, Kutmon M, Eijssen L et al. Pathway analysis of transcriptomic data shows immunometabolic effects of vitamin D. J Mol Endocrinol 2018;60:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reins RY, McDermott AM. Vitamin D: Implications for ocular disease and therapeutic potential. Exp Eye Res 2015;134:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uberti F, Lattuada D, Morsanuto V et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab 2014;99:1367–74. [DOI] [PubMed] [Google Scholar]
- 68.Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep 2009;7:58–63. [DOI] [PubMed] [Google Scholar]
- 69.Lemus HN, Warrington AE, Rodriguez M. Multiple Sclerosis: Mechanisms of Disease and Strategies for Myelin and Axonal Repair. Neurol Clin 2018;36:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hossain MJ, Tanasescu R, Gran B. Innate immune regulation of autoimmunity in multiple sclerosis: Focus on the role of Toll-like receptor 2. J Neuroimmunol 2017;304:11–20. [DOI] [PubMed] [Google Scholar]
- 71.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018;97:742–68. [DOI] [PubMed] [Google Scholar]
- 72.Krefting EA, Jorde R, Christoffersen T et al. Vitamin D and intraocular pressure--results from a case-control and an intervention study. Acta Ophthalmol 2014;92:345–9. [DOI] [PubMed] [Google Scholar]
- 73.Yoo TK, Oh E, Hong S. Is vitamin D status associated with open-angle glaucoma? A cross-sectional study from South Korea. Public Health Nutr 2014;17:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kutuzova GD, Gabelt BT, Kiland JA et al. 1alpha,25-Dihydroxyvitamin D(3) and its analog, 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D(3) (2MD), suppress intraocular pressure in non-human primates. Arch Biochem Biophys 2012;518:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibson CC, Davis CT, Zhu W et al. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLoS One 2015;10:e0140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mark KA, Dumas KJ, Bhaumik D et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep 2016;17:1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keisala T, Minasyan A, Lou YR et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol 2009;115:91–7. [DOI] [PubMed] [Google Scholar]
- 78.Ross MD, Peacor D, Johnsson LG et al. Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol 1976;85:310–26. [DOI] [PubMed] [Google Scholar]
- 79.Jang YS, Hwang CH, Shin JY et al. Age-related changes on the morphology of the otoconia. Laryngoscope 2006;116:996–1001. [DOI] [PubMed] [Google Scholar]
- 80.Zou J, Minasyan A, Keisala T et al. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol Neurootol 2008;13:219–30. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda K, Kobayashi T, Itoh Z et al. Evaluation of vitamin D metabolism in patients with bilateral sensorineural hearing loss. Am J Otol 1989;10:11–3. [PubMed] [Google Scholar]
- 82.Brookes GB. Vitamin D deficiency--a new cause of cochlear deafness. J Laryngol Otol 1983;97:405–20. [DOI] [PubMed] [Google Scholar]
- 83.Ryan ZC, Craig TA, Filoteo AG et al. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem Biophys Res Commun 2015;467:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kozel PJ, Friedman RA, Erway LC et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 1998;273:18693–6. [DOI] [PubMed] [Google Scholar]
- 85.van der Hagen EA, Lavrijsen M, van Zeeland F et al. Coordinated regulation of TRPV5-mediated Ca(2)(+) transport in primary distal convolution cultures. Pflugers Arch 2014;466:2077–87. [DOI] [PubMed] [Google Scholar]
- 86.Ishibashi T, Takumida M, Akagi N et al. Expression of transient receptor potential vanilloid (TRPV) 1, 2, 3, and 4 in mouse inner ear. Acta Otolaryngol 2008;128:1286–93. [DOI] [PubMed] [Google Scholar]
- 87.Takumida M, Ishibashi T, Hamamoto T et al. Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol 2009;129:1340–50. [DOI] [PubMed] [Google Scholar]
- 88.Nakaya K, Harbidge DG, Wangemann P et al. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol 2007;292:F1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfeffer PE, Lu H, Mann EH et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS One 2018;13:e0200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tohari AM, Zhou X, Shu X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem Funct 2016;34:82–94. [DOI] [PubMed] [Google Scholar]
- 91.Pasing Y, Fenton CG, Jorde R et al. Changes in the human transcriptome upon vitamin D supplementation. J Steroid Biochem Mol Biol 2017;173:93–9. [DOI] [PubMed] [Google Scholar]
- 92.Ogun OA, Buki B, Cohn ES et al. Menopause and benign paroxysmal positional vertigo. Menopause 2014;21:886–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minor LB, Schessel DA, Carey JP. Meniere’s disease. Curr Opin Neurol 2004;17:9–16. [DOI] [PubMed] [Google Scholar]
- 94.Buki B, Junger H, Avan P. Cochlear function in Meniere’s disease. Int J Audiol 2012;51:373–8. [DOI] [PubMed] [Google Scholar]
- 95.Nashold FE, Spach KM, Spanier JA et al. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol 2009;183:3672–81. [DOI] [PubMed] [Google Scholar]