Abstract
Inflammation plays an important role in tissue repair and regeneration. Recent work reveals that inflammatory signaling increases DNA accessibility so as to promote phenotypic fluidity in the response to injury.
Keywords: innate immunity, epigenetics, regeneration, nuclear reprogramming, endothelium, transdifferentiation
Introduction
Amidst the excitement regarding the success of novel anti-inflammatory strategies in the treatment of cardiovascular disease1, it is important to keep in mind that inflammation also plays a critical role in regeneration and repair. In the setting of tissue injury, there is a need for an optimal level of innate and adaptive immune activation for an adequate response to injury. Whereas there is a clear rationale for anti-inflammatory therapy for patients with atherosclerotic coronary artery disease, there remain unanswered questions. For example, what is the effect of anti-inflammatory therapy on physiological tissue repair and remodeling? This question follows logically from a consideration of recent findings regarding the role of inflammatory signaling in cellular plasticity and tissue regeneration.
Inflammatory Signaling in Response to Cellular Challenges
Cell-autonomous inflammatory signaling.
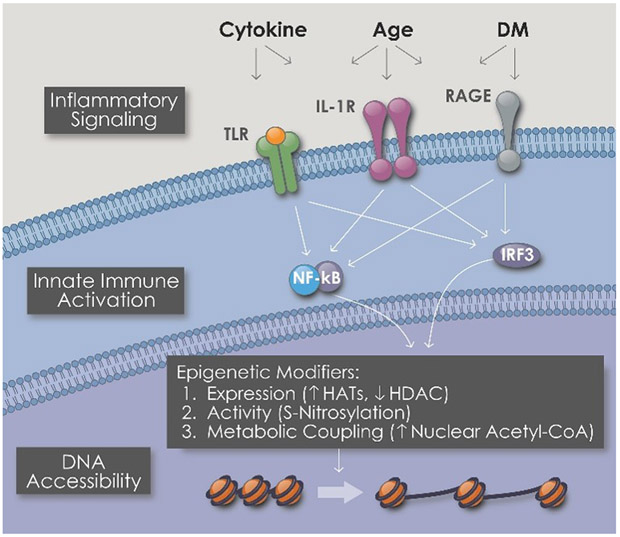
Mammalian cells have the ability to sense cellular challenges. Damage-associated molecular patterns (DAMPs) such as oxidized lipoproteins, or pathogen associated molecular patterns (PAMPs) such as uncapped viral RNA are detected by pattern recognition receptors (PRRs) on the cell surface or on endosomes in the cell cytoplasm2. These PRRs include the toll-like receptors (TLRs), the RIG1-like receptors (RLRs) and the receptor for advanced glycosylation endproducts (RAGE). The PRRs serve as sentinels that detect a cellular challenge and initiate an appropriate response. The stimulation of PRRs activates transcriptional effectors such as nuclear factor kappa beta (NFKb) that induce the expression of inflammatory cytokines. The cellular secretion of these cytokines engenders the next phase of the immune response.
Recruitment of professional immune cells.
Inflammatory cytokines that induce the expression of endothelial adhesion molecules (such as VCAM-1) and chemokines (such as MCP-1) that recruit circulating immune cells such as neutrophils and monocytes3. The infiltrating immune cells elaborate reactive oxygen and nitrogen species that defend against pathogens, but which may also contribute to tissue injury4. Monocytes transform into tissue macrophages that envelop and neutralize pathogens or cell debris within phagosomes, and which also participate in the repair process5. Dendritic monocytes process foreign proteins, displaying them to T cells to activate adaptive immunity, thus recruiting cytotoxic T cells and antibody-generating B cells.
To summarize, the PRRs of mammalian cells function as the sentinels of the immune system. However, the PRRs also subserve another function that has only recently been recognized. Specifically, these cellular sentinels also initiate epigenetic mechanisms to provide for the phenotypic fluidity required for an adaptive tissue response to an injurious agent.
Inflammatory Signaling Activates Epigenetic Mechanisms for Cellular Plasticity
Stimulation of PRRs and inflammatory signaling causes epigenetic alterations that increase DNA accessibility (Fig 1). In this way a cell opens up its genetic armamentarium in response to a threat. This is a critical cellular adaptation to tissue injury or invasion. A cell may need to transform itself from its quiescent basal state where it performs a specialized function, to a cell that is proliferating, migrating through extracellular matrix, generating new intercellular connections, or performing other new functions. Thus, cells in an area of injury undergo striking phenotypic changes. This cellular transformation requires the activation of genes that are often silenced in the basal state. The process by which cell-autonomous innate immune signaling induces epigenetic plasticity and phenotypic fluidity has been termed “transflammation”6.
When a cell is confronted by a local challenge such as pathogens (presenting pathogen-associated molecular patterns or PAMPs) or damage (presenting damage-associated molecular patterns or PAMPs), pattern recognition receptors (PRRs) on the cell surface or on endosomes within the cell are activated. The stimulation of PRRs triggers an inflammatory signaling cascade that leads to the activation of transcriptional effectors such as NFKb, IRF3 and IRF7. These effectors cause global changes in the expression and post-translational modification of epigenetic enzymes. Combined with a metabolic switch that supplies more acetylcoA to the nucleus, the epigenetic alterations increase DNA accessibility to increase the transcriptional repertoire of the cell so as to provide for phenotypic fluidity.
We find that activation of PRRs causes global changes in the expression of epigenetic enzymes that regulate the interaction of DNA with histone proteins. For example, the activation of TLR3 by viral RNA increases the expression of the histone acetyltransferase (HAT) family members while reducing the expression of the histone deacetylase (HDAC) family members7. The resulting change in the balance of histone acetylation favors the probability that chromatin exists in a more open configuration. Notably, the efficient induction of pluripotency by retroviral vectors encoding the Yamanaka factors (Oct 4, Sox2, KLF4 and cMYC) cannot occur without cell-autonomous inflammatory signaling7,8. Furthermore, the transdifferentiation of one somatic cell into a somatic cell of a different lineage also requires innate immune activation9.
A mediator of inflammation modulates epigenetic mechanisms
The enzyme inducible nitric oxide synthase (iNOS) generates nitric oxide, and is a master regulator of inflammatory response10. We have found that iNOS also has a major epigenetic role in facilitating cellular plasticity. During the transdifferentiation of fibroblasts to endothelial cells, iNOS is expressed and NO is generated11. The induction of iNOS expression occurs early in the transdifferentiation process. Pharmacological antagonism of iNOS, or its genetic knockdown, markedly suppresses the efficiency of transdifferentiation. Intriguingly, iNOS translocates to the nucleus during the transdifferentiation process. There, it binds directly to Ring1A, a component of the polycomb repressive complex 1 (PRC1). Ring1A is then S-nitrosylated by iNOS, which causes the PRC1 complex to disengage from the chromatin. This is significant, because PRC1 maintains the chromatin in a closed conformation. These effects of iNOS are associated with a global reduction in the trimethylation of lysine 27 on the histone protein H3 (H3K27me3)11, as well as an increase in the trimethylation of lysine 4 on histone protein H3 (H3K4me3). Such changes in histone markings are consistent with an open chromatin state. Thus there is a direct link between inflammatory signaling and epigenetic plasticity, to promote phenotypic fluidity.
Inflammatory signaling is coupled to a metabolic switch
At the onset of PRR activation, a metabolic switch is thrown that couples mitochondrial activity to epigenetic modifications12. Specifically, activation of innate immune signaling causes a metabolic switch in human fibroblasts from oxidative phosphorylation to glycolysis (a similar metabolic switch has been described for cancer cells and is known as the Warburg phenomenon). Pharmacological inhibitors of glycolysis (such as 2 deoxyglucose) block transdifferentiation, whereas as glycolytic activators enhance transdifferentiation. Intriguingly, the glycolytic switch is associated with mitochondrial export of citrate, its uptake by the nucleus, and metabolism to acetylcoA, the substrate for histone acetylation12. Thus, a metabolic switch is directly coupled to an epigenetic process that leads to an open chromatin configuration.
Role of circulating and resident immune cells in tissue regeneration.
These new insights into the role of cell-autonomous innate immune signaling in the plasticity of non-immune cells (such as fibroblasts) extend previous work revealing that circulating and resident immune cells play a critical role in the response to injury. Recent studies using contemporary techniques such as lineage tracing, parabiosis, and single cell RNAseq analyses indicate that there are multiple subpopulations of resident cardiac macrophages in the murine heart. These subsets of cardiac macrophages differ by transcriptional and functional profiles, and by whether they are derived during development, or from circulating monocytes over the lifetime of the individual. In general, the cardiac macrophages derived during development appear to be required for effective repair, whereas monocyte-derived macrophages and infiltrating monocytes are associated with adverse remodeling after ischemic injury13. Furthermore, tissue resident CCR2+ macrophages promote whereas tissue resident CCR2-macrophages inhibit monocyte recruitment14.
Physiological role of inflammation, and potential adverse effects of anti-inflammatories.
The concept that low levels of inflammatory signaling may have important physiological roles is not entirely new. For example, a cellular burst of oxygen-derived free radicals is cytotoxic to pathogens, and was traditionally thought to be a locally destructive inflammatory weapon. However, we now know that lower levels of oxygen derived free radicals play an important role in cell signaling, as in the vasodilation (and increased tissue perfusion) secondary to hydrogen peroxide generated in the vessel wall. Similarly, an initial spark of superoxide anion is required for a somatic cell to transform itself into a pluripotent cell15.
Clinicians recognize that patients with an impaired inflammatory response also have less regenerative capacity. Surgeons hesitate to perform major surgery on patients who are treated with high dose steroids, because of concerns that the anastomoses and incisions will fail to heal. Our preliminary observations indicate that there is a ‘Goldilock’s zone’ of optimal innate immune activation for the DNA accessibility that is required for phenotypic fluidity.
The recent findings that cell-autonomous inflammatory signaling is critical for cellular plasticity has clinical implications. Novel anti-inflammatory strategies in cardiovascular disease may have dose-dependent toxicity related to their effect on regenerative processes. Specifically, there may be a “J-curve” where intensification of anti-inflammatory therapy may impair recovery from ischemic injury or other tissue damage. Thus, more knowledge is required regarding the role of innate and adaptive immune responses to injury in patients with cardiovascular disease. We need improved methods to monitor inflammatory signaling regionally (as in the infarcted myocardium) to determine what may be adaptive versus maladaptive levels of tissue activation. Currently, our methods to monitor novel anti-inflammatory strategies are primitive, as they generally depend upon systemic measures (such as plasma C-reactive peptide) which do not provide information on the inflammatory response in the region of injury. Furthermore, because cellular plasticity is also required in normal tissue repair and remodeling, it is not known how these processes might be adversely affected by chronic administration of more potent anti-inflammatory medication. We need to have a greater understanding of the relative importance of different inflammatory signaling pathways for tissue repair. Such knowledge could be useful in development of anti-inflammatory strategies that do not adversely affect repair and regeneration. For example, a targeted anti-inflammatory therapy such as canakinumab (selectively inhibiting interleukin 1β) is likely preferable to broad spectrum anti-inflammatory agents such as methotrexate, for chronic treatment of atherosclerotic diseases. To conclude, there may be dose-limiting and/or time-dependent toxicities of novel anti-inflammatory agents related to their effects on tissue repair and regeneration. Future drug development and clinical studies should be designed with these concerns in mind. Finally, a comprehensive understanding of the role of inflammatory signaling in tissue regeneration and repair may lead to novel approaches to maintain an optimal level of immune activation after tissue injury.
Acknowledgements
This work was supported by grants from National Institutes of Health (R01HL133254; R01HL132155) and Cancer Prevention Institute of Texas (RP150611)
References
- 1.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, et al. ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017. September 21;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–84, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Cooke JP: Flow, NO, and atherogenesis. Proc Natl Acad Sci U S A. 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vannella KM, Wynn TA. Mechanisms of Organ Injury and Repair by Macrophages. Annu Rev Physiol. 2017;79:593–617.. [DOI] [PubMed] [Google Scholar]
- 6.Cooke JP. Therapeutic transdifferentiation: a novel approach for vascular disease. Circ Res. 2013;112:748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E and Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed N, Ospino F, Himmati F, Lee J, Chanda P, Mocarski ES and Cooke JP. Retinoic Acid Inducible Gene 1 Protein (RIG1)-Like Receptor Pathway Is Required for Efficient Nuclear Reprogramming. Stem Cells. 2017;35:1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ and Cooke JP. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131:300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–73 [PMC free article] [PubMed] [Google Scholar]
- 11.Meng S, Zhou G, Gu Q, Chanda PK, Ospino F and Cooke JP. Transdifferentiation Requires iNOS Activation: Role of RING1A S-Nitrosylation. Circ Res. 2016;119:e129–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai L, Reineke E, Hamilton DJ, Cooke JP. A Glycolytic Switch is Required for Transdifferentiation to Endothelial Lineage. Circulation. 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction.Nat Immunol. 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res. 2019;124:263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou G, Meng S, Li Y, Ghebre YT, Cooke JP. Optimal ROS Signaling Is Critical for Nuclear Reprogramming. Cell Rep 15: 919–25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]