Abstract
When extreme, anxiety can become debilitating. Anxiety disorders, which often first emerge early in development, are common and challenging to treat, yet the underlying mechanisms have only recently begun to come into focus. Here, we review new insights into the nature and biological bases of dispositional negativity, a fundamental dimension of childhood temperament and adult personality and a prominent risk factor for the development of pediatric and adult anxiety disorders. Converging lines of epidemiological, neurobiological, and mechanistic evidence suggest that dispositional negativity increases the likelihood of psychopathology via specific neurocognitive mechanisms, including attentional biases to threat and deficits in executive control. Collectively, these observations provide an integrative translational framework for understanding the development and maintenance of anxiety disorders in adults and youth and set the stage for developing improved intervention strategies.
Keywords: affective neuroscience, amygdala, attentional biases, developmental psychopathology, emotion, fear and anxiety, individual differences, neuroimaging
INTRODUCTION
Anxiety is a sustained state of elevated apprehension, arousal, and vigilance that occurs in the absence of clear and immediate danger (Davis, Walker, Miles, & Grillon, 2010; Grupe & Nitschke, 2013; LeDoux, 2015; Shackman & Fox, 2016a). Anxiety lies on a continuum and, when expressed in extreme ways or inappropriate contexts, can become debilitating (Conway et al., in press; Craske et al., 2017; Salomon et al., 2015; Shackman et al., 2016c). Anxiety disorders are the most prevalent family of mental illnesses (Global Burden of Disease Collaborators, 2016; U.S. Burden of Disease Collaborators, 2018; Wang, Gaitsch, Poon, Cox, & Rzhetsky, 2017). They typically emerge early in life, enabling greater cumulative damage, and can contribute to the development of depression, substance abuse, and other adverse outcomes (Bitsko et al., 2018; Fox & Kalin, 2014a; Kessler et al., 2007; Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012; Lee et al., 2014; McGorry, Purcell, Goldstone, & Amminger, 2011; Pratt, Druss, Manderscheid, & Walker, 2016; Shackman et al., 2016c). Existing treatments are underutilized, inconsistently effective, and, in the case of pharmaceuticals, associated with significant adverse effects (Craske et al., 2017; Gordon & Redish, 2016; Griebel & Holmes, 2013). In short, anxiety disorders impose a staggering burden on public health and the global economy, underscoring the urgency of developing a more complete understanding of the underlying mechanisms (DiLuca & Olesen, 2014; Global Burden of Disease Collaborators, 2016; Roehrig, 2016; U.S. Burden of Disease Collaborators, 2018).
We begin by describing new insights into the nature and the biological bases of dispositional negativity, a central dimension of mammalian temperament that confers elevated risk for the development of anxiety disorders and other stress-sensitive psychiatric diseases. Like anxiety disorders, dispositional negativity is a complex, multidimensional phenotype that encompasses variation in behavior, peripheral physiology, feelings, and cognition (Cavanagh & Shackman, 2015; Grupe & Nitschke, 2013; LeDoux, 2015; Shackman et al., 2016a; Shackman et al., 2016c). A key challenge is to identify the mechanisms underlying these features and discover how they contribute to the etiology of psychiatric disease in adults and youth. Here, we focus on recent advances in our understanding of threat-related1 attentional biases and deficits in executive control. These intermediate cognitive phenotypes are key features of dispositional negativity and there is compelling evidence that each can contribute to the development and course of anxiety disorders. While important strides have been made at delineating the neural underpinnings of attentional biases to threat, much less scientific attention has been devoted to executive deficits. In the final section, we highlight emerging evidence that these intermediate phenotypes can interact when threat-related cues are present, but unrelated to on-going goals. Although these new observations provide important insights, they also raise a number of interesting questions. We conclude by outlining some of the most important avenues for future research and some strategies for addressing them.
THE NATURE, CONSEQUENCES, AND NEUROBIOLOGY OF DISPOSITIONAL NEGATIVITY
The Nature of Dispositional Negativity
Dispositional negativity or ‘negative emotionality’—the propensity to experience and express more frequent, intense, or persistent fear, anxiety, and other negative emotions—is a fundamental dimension of childhood temperament and adult personality (Shackman, Stockbridge, LeMay, & Fox, 2018a; Shackman et al., 2016c). We conceptualize dispositional negativity as an extended family of closely related phenotypes that first emerge early in development, persist into adulthood, and reflect a combination of heritable and non-heritable factors (Kandler, Waaktaar, Mottus, Riemann, & Torgensen, in press; e.g., Kendler et al., in press; Roysamb, Nes, Czajkowski, & Vassend, 2018; Savage, Sawyers, Roberson-Nay, & Hettema, 2017; Soto & John, 2014; Vukasovic & Bratko, 2015). The psychometric structure of dispositional negativity is relatively invariant across cultures, languages, and ages, at least from elementary school onward (De Pauw, 2017; Kajonius & Giolla, 2017; McCrae, Terracciano, & Personality Profiles of Cultures, 2005; Schmitt, Allik, McCrae, & Benet-Martinez, 2007; Shiner, 2018; Soto & John, 2014; van Hemert, van de Vijver, Poortinga, & Georgas, 2002). Individual differences in dispositional negativity are highly reliable, show substantial agreement across instruments and informants, and predict objective behavioral and psychophysiological indices of anxiety in the laboratory, indicating that dispositional negativity is more than just a negative response bias (Back, Schmukle, & Egloff, 2009; Borkenau, Riemann, Angleitner, & Spinath, 2001; Brunson, Øverup, & Mehta, 2016; Buss, 1991; Connelly & Ones, 2010; Connolly, Kavanagh, & Viswesvaran, 2007; Costa & McCrae, 1988; Fetvadjiev, Meiring, van de Vijver, Nel, & De Kock, in press; Holland & Roisman, 2008; Kurtz, Puher, & Cross, 2012; McCrae & Costa, 1987; Mõttus, McCrae, Allik, & Realo, 2014; Pace & Brannick, 2010; Shackman et al., 2016c; Smith et al., 2016; Soto, John, Gosling, & Potter, 2011; Thielmann & Hilbig, in press; Vazire, 2010; Vazire & Carlson, 2010; Watson, Nus, & Wu, in press). Indeed, core features of this phenotypic family—including increased behavioral inhibition, heightened vigilance, and other signs of fear and anxiety—are expressed similarly across mammalian species, enabling ‘mechanistic’ (i.e., focal perturbation) studies to be performed in rodents and monkeys (Boissy, 1995; Capitanio, 2018; Fox & Kalin, 2014a; Mobbs & Kim, 2015; Oler, Fox, Shackman, & Kalin, 2016; Qi et al., 2010). Although the molecular pathways underlying dispositional negativity remain poorly understood, some promising candidates have been identified in humans and animals (Alisch et al., 2014; Alisch et al., 2017; Fox et al., 2012; Grotzinger et al., 2018; Hill et al., 2018; Kalin et al., 2016; Lo et al., 2017; Luciano et al., 2018; Nagel et al., 2018a; Nagel, Watanabe, Stringer, Posthuma, & van der Sluis, 2018b; Okbay et al., 2016; Oler et al., 2009; Rogers et al., 2013; Roseboom et al., 2014).
Dispositional Negativity Confers Risk for Anxiety Disorders and Other Psychiatric Diseases
Dispositional negativity is robustly associated with some of the most common and burdensome mental illnesses, including anxiety disorders, depression, and co-morbid substance abuse (e.g., Castellanos-Ryan et al., 2016; Davis et al., 2018; Hayes, Osborn, Lewis, Dalman, & Lundin, 2017; Hengartner, Tyrer, Ajdacic-Gross, Angst, & Rossler, 2018; Kendler et al., in press; Navrady et al., 2017; Paulus, Backes, Sander, Weber, & von Gontard, 2015; Seeboth & Mottus, 2018; Shackman et al., 2016c). Longitudinal work shows that individuals with elevated levels of dispositional negativity are more likely to develop internalizing (i.e., anxiety and mood) disorders in the future (e.g., Buzzell et al., 2017; Clark, Durbin, Hicks, Iacono, & McGue, in press; Goldstein, Kotov, Perlman, Watson, & Klein, 2018; Klein & Mumper, 2018; Luan et al., in press; Struijs et al., 2018; Wichstrom, Penelo, Rensvik Viddal, de la Osa, & Ezpeleta, 2018; Zinbarg et al., 2016). The magnitude of these prospective associations is substantial. A recent meta-analysis indicates that nearly half of children who show consistently elevated levels of shyness and behavioral inhibition—a core facet of dispositional negativity—were diagnosed with social anxiety disorder later in life (N = 692; risk ratio = 3.4; Clauss & Blackford, 2012). Among adults, data from the Zurich Cohort Study (N = 591) shows that a one standard-deviation increase in dispositional negativity at the time of the baseline assessment in 1988 increased the odds of developing an anxiety disorder by 32% and a major depressive episode by 41% during the twenty-year follow-up period (Hengartner, Ajdacic-Gross, Wyss, Angst, & Rossler, 2016a). Likewise, a recent meta-analysis of prospective longitudinal studies revealed medium-to-large relations between measures of dispositional negativity and future anxiety symptoms (Cohen’s d = .68), anxiety disorders (d = .48), depressive symptoms (d = .74), and major depressive disorder (MDD; d = .50) (N = 7,748 – 39,161; Jeronimus, Kotov, Riese, & Ormel, 2016). Relations between dispositional negativity and internalizing symptoms remain evident after eliminating overlapping item content or adjusting for baseline symptoms and they are magnified by social isolation, social exclusion, and stressor exposure (Frenkel et al., 2015; Gazelle & Rudolph, 2004; Hartley, Stritzke, Page, Blades, & Parentich, 2018; Hengartner et al., 2018; Jeronimus et al., 2016; Kendler, Kuhn, & Prescott, 2004; Kopala-Sibley et al., 2016a; Kopala-Sibley et al., 2016b; Lahey, Krueger, Rathouz, Waldman, & Zald, 2017; Markovic & Bowker, 2017; Uliaszek et al., 2009; Vinkers et al., 2014). Taken together, these observations suggest that high levels of dispositional negativity represent a diathesis for the internalizing spectrum of disorders (disposition × stressor → psychopathology). Other work suggests that dispositional negativity can promote mental illness by increasing the likelihood of experiences (e.g., loneliness, difficulty adjusting to university) and events (e.g., conflict, divorce, sickness) that, themselves, confer risk for internalizing illness in vulnerable individuals (disposition → stressor × disposition → psychopathology) (Abdellaoui et al., 2018; Clarke et al., 2018; Credé & Niehorster, 2012; Hengartner et al., 2018; Howland, Armeli, Feinn, & Tennen, 2017; Jocklin, McGue, & Lykken, 1996; Klimstra, Noftle, Luyckx, Goossens, & Robins, 2018; Matthews et al., in press; Overstreet, Berenz, Kendler, Dick, & Amstadter, 2017; Serrat, Villar, Pratt, & Stukas, 2018; Shackman et al., 2016c; Soto, in press; Tackett & Lahey, 2017). Among individuals with a history of internalizing illness, higher levels of dispositional negativity are associated with a greater number of diagnoses and a more pessimistic prognosis (e.g., Buckman et al., 2018; Bufferd et al., 2016; Hengartner, Kawohl, Haker, Rossler, & Ajdacic-Gross, 2016b; Shackman et al., 2016c; Spinhoven et al., 2016; Struijs et al., 2018).
Consistent with these phenotypic associations, family, twin, and genome-wide association studies (GWAS) show that dispositional negativity is genetically correlated with internalizing symptoms and disorders (Adams et al., 2019; Glahn et al., 2012; Gottschalk & Domschke, 2017; Hettema, 2008; Hill et al., 2018; Howard et al., 2018; Kendler & Myers, 2010; Lee et al., 2019; Levey et al., 2019; Li et al., 2018; Lo et al., 2017; Luciano et al., 2018; Meier et al., 2018; Nagel et al., 2018b; Navrady et al., 2018; Purves et al., 2017; Taylor et al., in press; Wray et al., 2018). For example, dispositional negativity is genetically associated with anxiety disorders (rG = .82, N = 17,310), depressive symptoms (rG = .79, N = 688,809), and MDD (rG = .68, N = 18,759) (Nagel et al., 2018a). These observations show that dispositional negativity, anxiety disorders, and depression are marked by similar patterns of intergenerational transmission: they ‘pass down the family tree’ in tandem. The sizable magnitude of these genetic correlations indicates strongly overlapping molecular genetic roots, dovetailing with psychometric and clinical evidence of continuity across the internalizing disorders and between normal phenotypic variation in personality in the population and psychopathology (Barlow, Sauer-Zavala, Carl, Bullis, & Ellard, 2013; Conway et al., in press; Sullivan et al., 2018; Waszczuk et al., 2018). Interestingly, ‘mendelian randomization’ analyses (Burgess, Butterworth, Malarstig, & Thompson, 2012; Burgess, Timpson, Ebrahim, & Davey Smith, 2015; Smith, 2010; Smith & Ebrahim, 2005; Smith et al., 2005a)—a family of genetic approaches that mitigate some of the most serious limitations of cross-sectional observational studies (e.g., confounding, reverse causation, reporting biases)—suggest that the causal pathways underlying these genetic correlations are similar, with a unidirectional pattern evident for both anxiety disorders and MDD (disposition → psychopathology) (Howard et al., in press; Nagel et al., 2018a; Speed, Hemani, Speed, Boerglum, & Oestergaard, 2018). In the case of depression, molecular genetic and longitudinal studies suggest that the experience of MDD can, over the course of a lifetime, enhance dispositional negativity (psychopathology → disposition), although this ‘scar’ effect appears to be substantially weaker than the reverse association (Howard et al., in press; Nagel et al., 2018a; Ormel et al., 2013).
Dispositional Negativity Causally Contributes to Psychopathology
Dispositional negativity is stable, but not immutable, and can change in response to experience. Like anxiety disorders and depression, dispositional negativity is amplified by exposure to stressors, trauma, and negative life events (Allen & Walter, 2018; Barlow et al., 2017; Bateson, Brilot, & Nettle, 2011; Bentley et al., 2017; Kandler & Ostendorf, 2016; Kornadt, Hagemeyer, Neyer, & Kandler, 2018; Milojev, Osbourne, & Sibley, 2014; Mueller, Wagner, Smith, Voelkle, & Gerstorf, in press; Roy, 2002; Shackman et al., 2016c; Wilson et al., 2006; Woods, Wille, Wu, Lievens, & De Fruyt, 2019), particularly when negative events occur prior to adulthood (Newton-Howes, Horwood, & Mulder, 2015; Ogle, Rubin, & Siegler, 2014; Shiner, Allen, & Masten, 2017). On the other hand, there is evidence that dispositional negativity can be attenuated by positive experiences, such as job promotions and marriage (Denissen, Luhmann, Chung, & Bleidorn, in press; Klimstra et al., 2018; Schalet et al., 2016; Shackman et al., 2016c). Likewise, genetic analyses of data gleaned from the UK Biobank (N = 328,917) suggest that increased educational attainment tends to reduce dispositional negativity (Nagel et al., 2018a). Other work demonstrates that dispositional negativity is sensitive to clinical interventions targeting anxiety and depression. In a comprehensive meta-analysis of clinical intervention studies (k = 199 studies), Roberts and colleagues showed robust reductions in dispositional negativity following psychosocial or pharmacological treatment for internalizing disorders (Cohen’s d = .59 for pre vs. post; d = .69 for treatment vs. controls; Roberts et al., 2017). Likewise, childhood interventions targeting heightened dispositional negativity reduce the likelihood of future internalizing problems (Rapee & Bayer, 2018). Taken together, these more mechanistic observations suggest that elevated levels of dispositional negativity causally contribute to the development and maintenance of internalizing disorders.
Relevance of the Amygdala to Dispositional Negativity
The neural circuits governing trait-like individual differences in dispositional negativity have only recently started to come into focus. Work by our group and others demonstrates that humans and monkeys with a more negative disposition show heightened responses to threat-relevant cues in a number of brain regions, including the amygdala, anterior hippocampus, anterior insula, bed nucleus of the stria terminalis (BST), mid-cingulate cortex, orbitofrontal cortex, and periaqueductal gray (Avery, Clauss, & Blackford, 2016; Cavanagh & Shackman, 2015; Fox & Kalin, 2014b; Fox & Shackman, 2019; Kalin, 2017; Kirlic et al., 2019; Lowery-Gionta, DiBerto, Mazzone, & Kash, 2018; Shackman & Fox, 2016b; Shackman et al., 2011b; Shackman et al., 2016c). While all of these regions are important, here we focus on the most intensely scrutinized component of this system, the amygdala, a heterogeneous collection of nuclei buried beneath the temporal lobe (Freese & Amaral, 2009; Yilmazer-Hanke, 2012) (Figure 1). Anatomically, the amygdala is poised to use information from sensory, contextual, and regulatory regions to assemble a range of reactions via dense mono- and poly-synaptic projections to the downstream regions that directly mediate the behavioral (e.g., passive and active avoidance), peripheral physiological (e.g., cardiovascular and neuroendocrine activity, startle), and cognitive (e.g., vigilance) features of momentary fear and anxiety (Davis & Whalen, 2001; Fox, Oler, Tromp, Fudge, & Kalin, 2015b; Freese & Amaral, 2009; Fudge et al., 2017; Lapate & Shackman, 2018) (Figure 1). Functional neuroimaging studies in monkeys and humans demonstrate that many of these downstream regions show robust connectivity with the amygdala, reinforcing the possibility that they represent coherent functional circuits that are relevant to human experience and disease (Birn et al., 2014; Fox et al., 2018c; Gorka, Torrisi, Shackman, Grillon, & Ernst, 2018; Tillman et al., 2018; Torrisi et al., 2018; Torrisi et al., 2015).
Figure 1. Simplified schematic of amygdala circuitry relevant to dispositional negativity, attentional biases, and hyper-vigilance to threat.
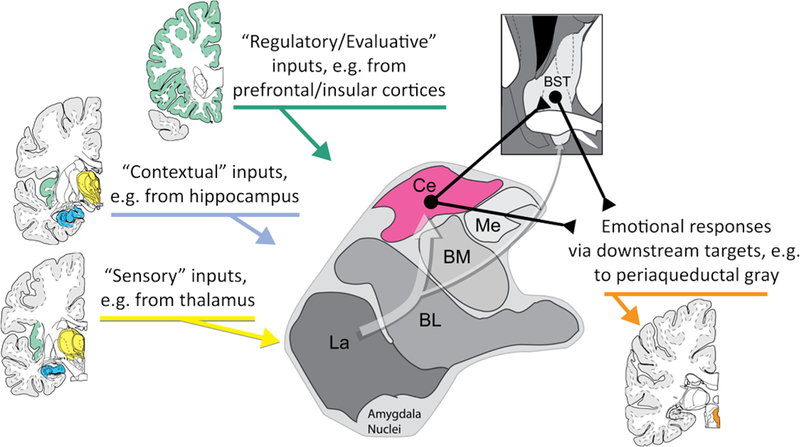
The amygdala is a heterogeneous collection of nuclei buried beneath the temporal lobe. It receives inputs from sensory (yellow), contextual (blue), and regulatory (green) systems and, as shown by the translucent white arrow at the center of the figure, information generally flows from the more ventral basal regions of the amygdala shown at the lower left toward the central (Ce) nucleus of the amygdala (magenta) and the neighboring bed nucleus of the stria terminalis (BST) at the upper right. The Ce and BST are, in turn, poised to orchestrate or trigger specific physiological, behavioral, and cognitive components of negative affect via their projections to downstream effector regions (orange). Prioritized processing of threat-related and other kinds of cues can occur through two mechanisms: directly, via projections from the basolateral (BL) nucleus to relevant areas of sensory cortex (e.g., fusiform face area) and indirectly, via projections from the Ce and BST to neuromodulatory systems in the basal forebrain and brainstem that, in turn, can modulate sensory cortex. Abbreviations—Basolateral (BL), Basomedial (BM), Central (Ce), Lateral (La), and Medial (Me) nuclei of the amygdala; Bed nucleus of the stria terminalis (BST). BM is often termed the ‘accessory basal’ (AB) nucleus. The term ‘basolateral amygdala’ (BLA) is often used to refer to the basal and lateral nuclei. Figure adapted with permission from (Tillman et al., 2018).
Human imaging research demonstrates that the amygdala is engaged by a broad range of unpleasant and potentially threat-relevant stimuli (Costafreda, Brammer, David, & Fu, 2008; Fox & Shackman, 2019; Fusar-Poli et al., 2009; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Naaz, Knight, & Depue, in press; Price et al., 2018; Sabatinelli et al., 2011; Sergerie, Chochol, & Armony, 2008). Recent high-resolution fMRI research indicates that the dorsal-posterior amygdala—in the approximate location of the central nucleus (Ce) (cf. Figure 1)—is particularly sensitive to aversive visual stimuli (Hrybouski et al., 2016). Increased activation in this region has, in turn, been associated with elevated signs and symptoms of arousal in response to threat (Fox & Shackman, 2019; Sjouwerman, Scharfenort, & Lonsdorf, 2017). More recent work has leveraged machine-learning approaches to show that the dorsal-posterior amygdala (in the region of the Ce) is a key component of circuits that underlie negative affect elicited by aversive photographs (Chang, Gianaros, Manuck, Krishnan, & Wager, 2015) and that distinguish conditioned threat (CS+) from safety (CS-) (Reddan, Wager, & Schiller, 2018).
Brain imaging studies provide compelling evidence that adults and youth with a more negative disposition are prone to increased or prolonged activity in the dorsal-posterior amygdala (Figure 2). This has been observed both at ‘rest’ (i.e., in the absence of an explicit task) and in response to novelty, negative emotional faces, unpleasant images, and conditioned threat cues (CS+) (e.g., Coombs, Loggia, Greve, & Holt, 2014; Gaffrey, Barch, & Luby, 2016; Kann, O’Rawe, Huang, Klein, & Leung, 2017; Shackman et al., 2016c; Sjouwerman et al., 2017; Stout, Shackman, Pedersen, Miskovich, & Larson, 2017). For example, Kaczkurkin and colleagues used a large peri-adolescent youth dataset (N = 875) to show that adolescent women are marked by a more negative disposition, on average, compared to adolescent men (consistent with other large-scales studies; Shackman et al., 2016c) and that this sex difference reflects elevated ‘resting’ perfusion in the dorsal amygdala (female-vs.-male → resting amygdala activity → disposition) (Kaczkurkin et al., 2016b). The association between dispositional negativity and task-related amygdala reactivity appears to be amplified among individuals with lower levels of perceived social support (Hyde, Gorka, Manuck, & Hariri, 2011), an important risk factor for the development of internalizing disorders (Kendler & Gardner, 2014; Shackman et al., 2018b).
Figure 2. Elevated dispositional negativity is associated with increased activity in the dorsal amygdala in the region of the Ce. Adults.
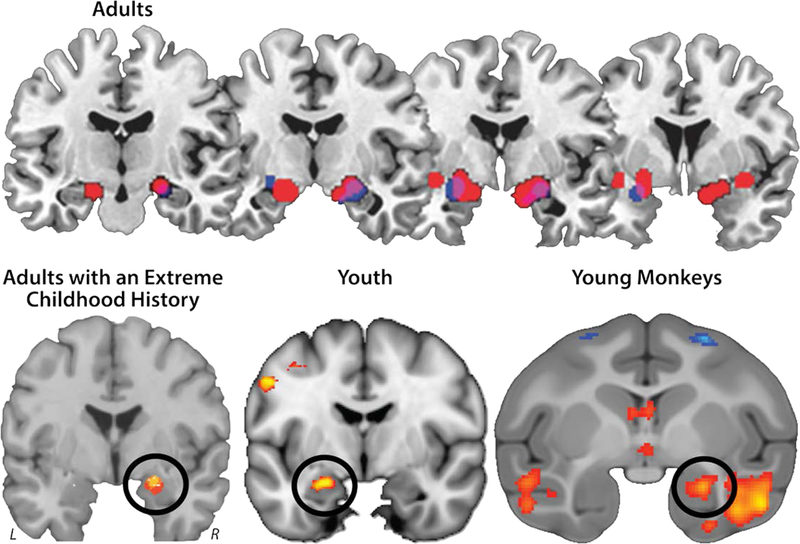
Meta-analysis of six published imaging studies reveals consistently elevated activation bilaterally in the dorsal amygdala among adults with a more negative disposition (Calder, Ewbank, & Passamonti, 2011). Significant relations with dispositional negativity (trait) are shown in blue; significant relations with momentary negative affect (state) are depicted in red; and the overlap is shown in purple. Adults with an extreme childhood history. Meta-analysis of seven published imaging studies reveals consistently elevated activation in the dorsal amygdala (black ring) in adults with a childhood history of elevated dispositional negativity (Fox & Kalin, 2014a). Six of eight amygdala peaks overlapped (yellow) in the dorsal amygdala; four of the peaks extended into the region shown in red. Youth. Using arterial spin labeled (ASL) functional MRI acquired in the absence of an explicit task (‘at rest’) from 878 youth (M = 16.5 years, range = 12–23 years), Kaczkurkin and colleagues (2016) demonstrated that individuals with a more negative disposition show elevated perfusion in the dorsal amygdala (black ring). Panel depicts the results of a voxelwise regression analysis. Young monkeys. Using high-resolution 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) acquired from 592 young rhesus monkeys, Fox and colleagues (2015) showed that threat-related metabolic activity in the dorsal amygdala (black ring) is increased among individuals with a more negative disposition. Abbreviations—L: left hemisphere, R: right hemisphere. Panel depicts the results of a voxelwise regression analysis. Portions of this figure were adapted with permission from (Calder et al., 2011; Fox & Kalin, 2014a; Fox et al., 2015a; Kaczkurkin et al., 2016a).
Studies of nonhuman primates afford an important opportunity to obtain concurrent measures of brain metabolism and naturalistic defensive responses to ethologically relevant threats—something that would be difficult to accomplish in humans, given the sensitivity of functional MRI to even modest amounts of motion artifact (Ciric et al., 2018), and the challenges of eliciting robust fear and anxiety in the laboratory (Shackman & Wager, 2019). Using fluorodeoxyglucose-positron emission tomography (FDG-PET) in samples encompassing as many as 592 individuals, we have demonstrated that metabolic activity in the Ce (Figure 2) is associated with heightened behavioral and neuroendocrine reactions to naturalistic threat (Fox & Kalin, 2014b; Fox et al., 2015a). Ce metabolism is moderately stable over time and context and, as such, represents a trait-like feature of brain function (Fox, Shelton, Oakes, Davidson, & Kalin, 2008). For example, Fox and colleagues showed that metabolic activity in the Ce during exposure to an unfamiliar human intruder’s profile showed an intra-class correlation (ICC) of 0.64 across three occasions over a 1.1 year span, similar to the concurrent re-test stability of dispositional negativity in young monkeys (ICC = 0.72; Fox et al., 2012; see also Shackman et al., 2013; Shackman et al., 2017) and the 5-year stability of dispositional negativity in humans (partial R = .60; N = 56,735; Hakulinen et al., 2015). Other work in nonhuman primates suggests that elevated activity in the Ce is a core substrate for different presentations of dispositional negativity (Figure 3). Like humans, individual monkeys have different ways of expressing their extreme disposition. Some characteristically respond to threat with high levels of the stress-sensitive hormone cortisol (and average levels of behavioral inhibition), whereas others show the reverse profile. Yet across these different phenotypes, we have observed a remarkably consistent pattern of elevated metabolism in the Ce (Shackman et al., 2013). This observation is broadly consistent with evidence suggesting that elevated amygdala reactivity to threat-related cues is a transdiagnostic marker of the internalizing disorders in humans (Etkin & Wager, 2007; Hamilton et al., 2012).
Figure 3. Elevated amygdala activity is a shared substrate for different phenotypic presentations of dispositional negativity.
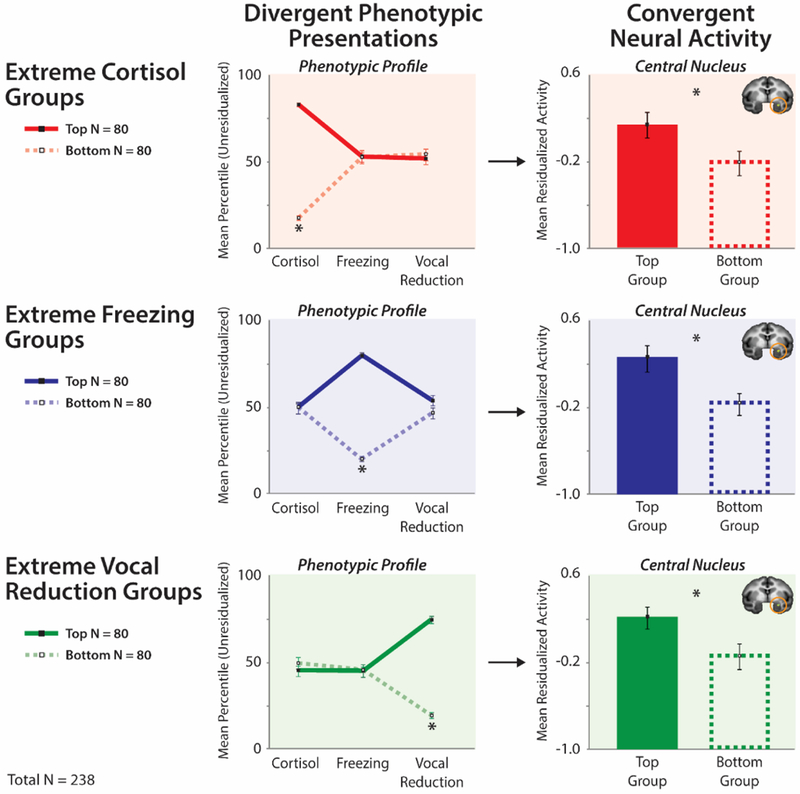
Shackman and colleagues (2013) used a well-established young nonhuman primate model of childhood dispositional negativity and high-resolution FDG-PET to demonstrate that individuals with divergent phenotypic presentations of their extreme disposition show increased activity in the Ce (orange rings). Divergent phenotypic presentations: To illustrate this, phenotypic profiles are plotted for groups (N = 80/group) selected to be extreme on a particular dimension of the phenotype (Top tercile: solid lines; Bottom tercile: broken lines). The panels on the left illustrate how this procedure sorts individuals into groups with divergent presentations of dispositional negativity. Convergent neural activity: To illustrate the consistency of Ce activity across divergent phenotypic presentations, mean neural activity for the extreme groups (± SEM) is shown on the right. Individuals with high levels of cortisol, freezing, or vocal reductions (and intermediate levels of the other two responses) were characterized by greater metabolic activity in the Ce. Figure adapted with permission from (Shackman et al., 2013).
Like the internalizing disorders, dispositional negativity is moderately heritable in humans and monkeys (Fox et al., 2015a; Shackman et al., 2016c). Recent work in nonhuman primates demonstrates that the neural circuitry underlying trait-like individual differences in dispositional negativity can be genetically fractionated. Metabolic activity in the Ce, while heritable, appears to be more relevant to understanding variation in dispositional negativity attributable to experience, such as stressor exposure (h2 = .26, rG = n.s., N = 592) (Fox et al., 2015a) (Figure 2). In contrast, functional connectivity between the Ce and BST (Figure 4a) appears to be more relevant to understanding heritable variation in dispositional negativity and, hence, to the intergenerational transmission of risk from parents to their offspring (h2 = .45, rG = .87, N = 378) (Fox et al., 2018c). Whether this pattern translates to humans remains unknown, making it a key challenge for future research.
Figure 4. Elevated dispositional negativity is associated with alterations in Ce functional connectivity.
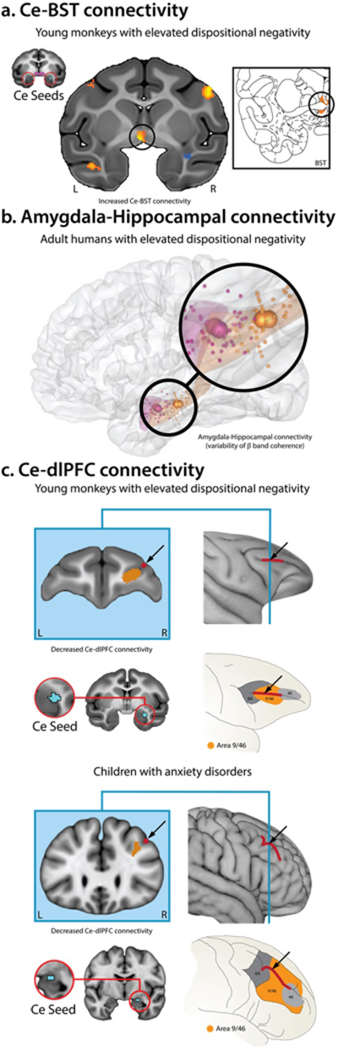
A. Ce-BST connectivity. Fox and colleagues (2018) used fMRI to demonstrate that functional connectivity between the Ce (red rings) and BST (black rings) is associated with elevated dispositional negativity in a sample of 378 young monkeys drawn from an extended 8-generation pedigree (N = 1,928). They also showed that Ce-BST functional connectivity is genetically correlated with individual differences in dispositional negativity, indicating an overlapping pattern of intergenerational transmission. Inset depicts the corresponding plane of the rhesus brain atlas. B. Amygdala-Hippocampal connectivity. Kirkby and colleagues (2018) used a combination of intracranial electrophysiological recordings, experience sampling, and machine learning techniques to identify an amygdala-hippocampal functional network (i.e. temporal variability of coherence in the β band; 13–30 Hz) that reliably predicted momentary fluctuations in negative mood among treatment-resistant, adult epilepsy patients with elevated levels of dispositional negativity. Figure depicts the spatially normalized centroid locations of amygdala (magenta) and hippocampal (orange) recording electrodes. C. Ce-dlPFC connectivity. Birn and colleagues (2014) demonstrated that young monkeys with elevated levels of dispositional negativity (top) and children with anxiety disorders (bottom) show a similar pattern of reduced functional connectivity between the Ce (red rings) and dorsolateral PFC (dlPFC; black arrows). Pediatric imaging data were collected while patients were quietly resting. Nonhuman primate data were collected under anesthesia, eliminating potential individual differences in scanner-elicited apprehension or neuroendocrine activation (cf. Shackman et al., 2016c). Abbreviations—L: left hemisphere, R: right hemisphere. Portions of this figure were adapted with permission from (Birn et al., 2014; Fox et al., 2018c; Kirkby et al., 2018).
Recent work has begun to move beyond the amygdala and clarify the architecture of the distributed neural circuitry underlying dispositional negativity (Fox & Shackman, 2019; Shackman et al., 2016c). For example, using a combination of chronic electrophysiological recordings, experience sampling, and machine learning, Kirkby and colleagues showed that momentary fluctuations in negative mood are reliably associated with the functional connectivity of a circuit linking the posterior-dorsal amygdala to the hippocampus, and that this association was only evident among individuals with a more negative disposition (Kirkby et al., 2018) (Figure 4b). Work using more conventional fMRI techniques demonstrates that young monkeys with elevated levels of dispositional negativity and children with anxiety disorders show reduced functional connectivity between the Ce and dorsolateral prefrontal cortex (dlPFC) at ‘rest’ (Figure 4c). Monkeys with a more negative disposition also showed reduced functional connectivity between the Ce and mesial prefrontal cortex (mPFC)—including regions of the pregenual anterior cingulate (pgACC)—broadly consistent with work in human adults (Kim, Gee, Loucks, Davis, & Whalen, 2011; Pezawas et al., 2005)2. Taken together, this suggests that alterations in these evolutionarily-conserved functional circuits may confer risk for the development of pathological anxiety (Birn et al., 2014; Oler et al., 2016). More broadly, these observations show that core features of personality and temperament—features that confer increased risk for mental illness—are embodied in the spontaneous activity of the brain, in the absence of overt trait-relevant challenges. An important avenue for future research will be to use focal perturbations, pharmacological interventions, or other mechanistic approaches to clarify the causal contribution of this circuitry to dispositional negativity and psychopathology (Dubois et al., in press; Grayson et al., 2016; Kalin et al., 2016). Prospective longitudinal studies will be required to understand the relevance of this circuitry to the emergence of psychopathology.
The Amygdala Causally Contributes to Extreme Fear and Anxiety
Mechanistic work in rodent models demonstrates that neuronal microcircuits encompassing the Ce are critical for orchestrating defensive responses to a wide variety of threats (Choi & Kim, 2010; Fox & Shackman, 2019; Gungor & Paré, 2016; Pliota et al., in press; Pomrenze et al., 2019; Tovote, Fadok, & Luthi, 2015). Other work indicates a role in dispositional negativity (Fadok, Markovic, Tovote, & Lüthi, 2018). For example, Ahrens and colleagues showed that anxious, behaviorally inhibited mice are characterized by tonically elevated activity in a specific type of Ce neurons—cells within the lateral division that express somatostatin and project to the BST (Ahrens et al., 2018)—consistent with the much coarser results revealed by FDG-PET and arterial spin labeling (ASL) perfusion fMRI studies of humans and monkeys (Abercrombie et al., 1998; Canli et al., 2006; Fox et al., 2008; Kaczkurkin et al., 2016b). In an elegant series of experiments, Ahrens and colleagues demonstrated that these neurons are sensitive to uncertain danger (i.e., unpredictable shock) and that they are both necessary and sufficient for heightened defensive responses (e.g., freezing) to novelty and diffuse threat (e.g., a brightly lit open-field). This and other recent studies that have exploited the cellular precision afforded by opto- and chemogenetic techniques make it abundantly clear that the Ce, like many other brain regions, harbors a variety of intermingled cell ‘types’—populations of neurons that can be distinguished based on their protein expression, firing characteristics, connectivity, and other features—and that different cell types within the Ce perform distinct, even opposing functional roles (Fox & Shackman, 2019; Pignatelli & Beyeler, 2018). The upshot is that research that relies on traditional lesion techniques, pharmacological interventions, or in vivo imaging techniques will necessarily reflect a mixture of cells and signals. Making sense of this complexity and identifying the circuit components most relevant to human experience and psychiatric disease represent important avenues for future research.
While our understanding of the primate amygdala lags behind that of rodents, work in monkeys and humans suggests that this region is crucial for extreme anxiety. In monkeys, fiber-sparing (excitotoxic) lesions of the amygdala—of the Ce in particular—have been shown to attenuate defensive behaviors and endocrine responses to a range of learned and innate threats (Davis, Antoniadis, Amaral, & Winslow, 2008; Kalin et al., 2016; Oler et al., 2016). These observations are consistent with studies of humans with disease-related amygdala damage (Bechara et al., 1995; Feinstein, Adolphs, Damasio, & Tranel, 2011; Feinstein, Adolphs, & Tranel, 2016; Klumpers, Morgan, Terburg, Stein, & van Honk, 2015; Korn et al., 2017). Patient SM, for example, is marked by near-complete bilateral destruction of the amygdala and shows a profound lack of fear and anxiety—whether measured objectively or subjectively—to both diffusely threatening contexts (e.g., a haunted house) and acute threat cues (e.g., spiders, snakes, clips of horror films, conditioned threat cues, ‘jump-scares’ in the haunted house, and even real-world assault) (Feinstein et al., 2011). Moreover, SM reports abnormally low levels of dispositional negativity when assessed using standard psychometric measures (Feinstein et al., 2011), consistent with clinical assessments of her temperament (Tranel, Gullickson, Koch, & Adolphs, 2006). An important caveat is that SM’s deficits may reflect damage to fibers of passage or more subtle functional disconnections (Davis & Whalen, 2001; Fox & Shackman, 2019) (R. Adolphs, personal communication, 24 July 2017). It also merits comment that SM and other patients with substantial amygdala damage can experience fear, even panic attacks, in the laboratory in response to breathing air enriched with CO2 (Feinstein et al., 2013; Khalsa et al., 2016). On balance, this body of work teaches us that the amygdala is not a fear or anxiety center, per se, but instead plays a critical role in assembling responses to threats encountered in the external environment.
Other research has examined the consequences of amplifying amygdala activity. Work in monkeys shows that genetic manipulations that increase metabolic activity in the Ce potentiate signs of anxiety (Kalin et al., 2016), in broad accord with rodent studies (Ahrens et al., 2018). Electrical stimulation studies in humans have revealed a more complex pattern of results (Inman et al., in press). Subjective responses to amygdala stimulation are infrequent, likely due to heterogeneity in electrode placement (C. Inman, personal communication, 24 March 2018). Nevertheless, when feelings are reported, they are typically described as a heightened state of negative affect and can be quite robust (Inman et al., in press). Inman and colleagues recently described an individual (‘subject 8’) who experienced intense fear and anxiety in response to 6V stimulation in the region of the right Ce: “It was, um, it was terrifying, it was just…it was like I was about to get attacked by a dog…like someone unleashes a dog on you, and it’s just like it’s so close, and you feel like you’re going to s*** your pants. It’s terrifying.” At 8V, he asked to terminate the stimulation, saying “that was so scary it was nauseating. It’s like, um, I went zip-lining a few weeks ago…and this was worse” (Inman et al., in press)3. Such feelings were never reported during intermixed sham trials. Taken together, the results of lesion and stimulation studies suggest that a circuit centered on the Ce is necessary and sufficient for many of the core signs and symptoms of anxiety.
Relevance of the Amygdala to Psychopathology
Four lines of evidence motivate the hypothesis that elevated amygdala reactivity contributes to the development and maintenance of mental illness. Amygdala activation:
Is elevated in children, adolescents, and adults with internalizing disorders and individuals with a positive family history (Shackman et al., 2016a). Heightened ‘resting’ activity has also been found in psychotic patients marked by elevated levels of paranoia and negative affect (Pinkham et al., 2015; Stegmayer et al., 2017). Amygdala activation has also been shown to co-vary with the severity of anxious symptoms, albeit less consistently (Thomas et al., 2001; van den Bulk et al., 2014).
Is amplified by exposure to the same kinds of stressors and psychological pathogens (e.g., combat, childhood maltreatment) that can precipitate acute mental illness in dispositionally vulnerable individuals (Hein & Monk, 2017; McCrory, Gerin, & Viding, 2017; Shackman et al., 2016a; Teicher, Samson, Anderson, & Ohashi, 2016).
Prospectively predicts heightened internalizing symptoms among adolescents and young adults exposed to stress, trauma, or negative life events (Admon et al., 2009; Stevens et al., 2017; Swartz, Knodt, Radtke, & Hariri, 2015). For example, McLaughlin and colleagues showed that adolescents marked by a more reactive amygdala at initial assessment experienced heightened posttraumatic symptoms 9 months later, following exposure to the terrorist attacks at the 2013 Boston Marathon (McLaughlin et al., 2014). Among preschool-aged children, amygdala activation prospectively predicts heightened negative affect (Gaffrey et al., 2016).
Is attenuated by clinically effective cognitive-behavioral and pharmacological (e.g., benzodiazepine) treatments for anxiety and depression in adults (Månsson et al., 2016; Shackman et al., 2016a). More recent work shows that amygdala reactivity is also dampened by low to moderate doses of ethyl alcohol (Hur et al., 2018), a well-established anxiolytic that, like the benzodiazepines, enhances inhibitory neurotransmission in the Ce (Bartholow, Henry, Lust, Saults, & Wood, 2012; Kaye, Bradford, Magruder, & Curtin, 2017; Sharko, Kaigler, Fadel, & Wilson, 2016). These observations suggest that the amygdala causally contributes to pathological anxiety in humans, consistent with the mechanistic work reviewed in the prior section.
Interim Conclusions
Dispositional negativity is a well-established diathesis for the internalizing spectrum of disorders. Children and adults with a more negative disposition are more likely to develop anxiety disorders and depression if they experience the appropriate precipitants (e.g., negative life events, chronic stress). Dispositional negativity can be conceptualized as an extended family of complex phenotypes that reflect multiple brain circuits and molecular pathways. Converging lines of epidemiological, imaging, mechanistic, and clinical evidence suggests that specific populations of neurons in the amygdala, particularly those harbored within the Ce: (a) underlie core features of dispositional negativity in humans and other mammals, (b) exert bi-directional control over defensive responses to threat and subjective feelings of fear and anxiety, and (c) causally contribute to the development of anxiety and mood disorders.
THE NATURE, CONSEQUENCES, AND NEUROBIOLOGY OF ATTENTIONAL BIASES TO THREAT
Alterations in vigilance, risk assessment, and other aspects of attention are hallmarks of dispositional negativity and anxiety (Blanchard, Griebel, & Blanchard, 2001; Grupe & Nitschke, 2013; Shackman et al., 2016a). Attention is a fundamental property of perception and cognition. Attentional mechanisms prioritize the most relevant sources of information while inhibiting or ignoring potential distractions and competing courses of action (Desimone & Duncan, 1995). Once a target is selected, attention determines how deeply it is processed, how quickly and accurately a response is executed, and how well it is remembered. Thus, attention involves both stimulus selection and the intensity of processing once a stimulus has been selected.
The Nature of Attentional Biases to Threat
Threat-related stimuli—whether learned (CS+) and unlearned (e.g., spiders)—can strongly influence feature selection and the depth of processing. Across a range of laboratory assays, they are more likely to be detected, to capture attention, and to be remembered (Shackman et al., 2016a). Threat-related stimuli are associated with enhanced processing in sensory regions of the brain and this amplified processing is associated with faster and more accurate detection of the stimuli (Shackman et al., 2016a).
Relevance of Attentional Biases to Dispositional Negativity and Anxiety Disorders
Heightened vigilance and exaggerated risk assessment behaviors to threat-related cues are hallmarks of dispositional negativity and pathological anxiety (Grupe & Nitschke, 2013). Like many patients with anxiety disorders, adults and youth with a more negative disposition tend to allocate excess attention to threat-related cues, even when they are task irrelevant (Pérez-Edgar et al., 2017; Shackman et al., 2016a; Silvers et al., 2017). On average, dispositionally negative adults are more likely to initially orient their gaze towards threat-related cues in free-viewing tasks; quicker to fixate threat-related targets in visual search tasks; and slower to disengage from threat-related distractors (Armstrong & Olatunji, 2012; Cisler & Koster, 2010; Rudaizky, Basanovic, & MacLeod, 2014; Sheppes, Luria, Fukuda, & Gross, 2013). Meta-analyses indicate that youth with elevated levels of dispositional negativity or anxiety disorders show a significantly greater attentional bias for threat-related stimuli when compared to typical youth (k = 44 studies; mean Cohen’s d = 0.21) or when compared to emotionally neutral stimuli (k = 16 studies; mean Cohen’s d = 0.54; Dudeney, Sharpe, & Hunt, 2015). Although the latter effect is similar to that reported in adult studies (k = 101 studies; mean Cohen’s d = 0.45; Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), recent large-scale studies suggest that the size of these effects is likely to be somewhat misleading. For example, a recent meta-analysis of clinical studies using various dot-probe4 tasks failed to uncover evidence of a significant threat bias in 1,005 anxiety patients (Kruijt, Parsons, & Fox, 2018). Eye-tracking studies have often failed to demonstrate enhanced threat detection or hypervigilance in pathologically anxious adults, although they have revealed consistent evidence of sustained attention to threat (e.g., increased dwell time) (Lazarov, Abend, & Bar-Haim, 2016; Lazarov et al., in press), consistent with evidence that adults with a more negative disposition are particularly impaired in disengaging from threat-related cues (Sheppes et al., 2013). Using data gleaned from a large (N = 1,291) international sample of youth, Abend and colleagues reported a zero-order correlation of r = .08 between anxiety symptoms and attentional biases to threat, again, indexed using the dot-probe (Abend et al., 2018). The modest size of this association likely reflects multiple factors, including the suboptimal psychometric properties of dot-probe tasks (McNally, in press; Price et al., 2015; Rodebaugh et al., 2016), an exclusive reliance on social threat (angry faces), and unmeasured heterogeneity in the nature of attentional biases (e.g., initial vigilance followed by avoidance; Armstrong & Olatunji, 2012; Di Simplicio et al., 2014; Mogg, Waters, & Bradley, 2017a; Naim et al., 2015; Onnis, Dadds, & Bryant, 2011; Roy, Dennis, & Warner, 2015; Waters et al., 2015; Weierich, Treat, & Hollingworth, 2008; Zvielli, Bernstein, & Koster, 2014). Developing tools for reliably quantifying these more nuanced cognitive biases represents a crucial direction for future research (Liu, Taber-Thomas, Fu, & Perez-Edgar, 2018; MacLeod, in press).
Attentional Biases to Threat Causally Contribute to Pathological Anxiety
Several lines of evidence suggest that attentional biases to threat-related cues can causally contribute to the development of pathological anxiety. Attentional biases to threat have been shown to promote inflated estimates of threat intensity or likelihood (Aue & Okon-Singer, 2015)—a key feature of extreme anxiety (Grupe & Nitschke, 2013; Stuijfzand, Creswell, Field, Pearcey, & Dodd, 2018)—and to foreshadow the development of social inhibition in children (Kiel & Buss, 2011). From a longitudinal perspective, attentional biases to threat-related cues have been shown to moderate the impact of dispositional negativity on the development of internalizing symptoms in youth. Among youth with an early history of extreme dispositional negativity, it is the subset who also show an attentional bias to threat who are most likely to exhibit social withdrawal and elevated anxiety symptoms in the future (Perez-Edgar et al., 2010a; Perez-Edgar et al., 2011; White et al., 2017). Moreover, there is some evidence that clinically effective cognitive-behavioral and pharmacological treatments for anxiety can reduce attentional biases to threat-related cues, with greater therapeutic gains among patients showing larger reductions in attentional biases (Hadwin & Richards, 2016; Reinholdt-Dunne, Mogg, Vangkilde, Bradley, & Esbjørn, 2015; Shackman et al., 2016a). Direct support for a causal role comes from meta-analyses of computer-based interventions aimed at reducing attentional biases to threat, often termed ‘attention bias modification’ (ABM). For example, Heeren and colleagues reported a small-to-medium reduction in social anxiety symptoms (g = .27) and reactivity to a public speaking challenge (g = .46) (N = 1,043; Heeren, Mogoase, Philippot, & McNally, 2015) Among adult clinical samples, small-to-medium treatment effects have been observed when ABM is compared to placebo training (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015; MacLeod & Clarke, 2015; Price et al., 2016b), although the precise size and consistency of such effects remain contentious (Cristea, 2018; Cristea, Kok, & Cuijpers, 2015; Grafton et al., 2017; Grafton et al., 2018; Kruijt, 2018; Mogg & Bradley, 2016a, 2018; Mogg et al., 2017a). Results have been less consistent in pediatric clinical samples (Hardee et al., 2013; Liu et al., 2018; Shackman et al., 2016a). Broadly speaking, across this literature the most promising clinical effects have been found in studies where ABM was delivered in the clinic or laboratory and produced evidence of ‘target engagement,’ that is, a demonstrable reduction in attentional biases to threat-related cues (Grafton et al., 2017; MacLeod & Grafton, 2016; Mogg & Bradley, 2018; Notebaert et al., 2018; Price et al., 2016b). Indeed, Heeren and colleagues reported a substantial between-study covariation (k = 8 studies, r = .90) between ABM-induced reductions in attentional biases and experimentally elicited anxiety (Heeren et al., 2015). On balance, these observations are consistent with the idea that attentional biases to threat can causally contribute to the development of anxiety disorders.
Relevance of the Amygdala to Attentional Biases to Threat
The neural mechanisms underlying attentional biases to threat remain poorly understood, particularly in youth. Nonetheless, there is compelling evidence that the prioritized processing of threat-related cues reflects the influence of neural circuits encompassing the amygdala. Imaging and single unit recording studies in humans and monkeys demonstrate that the amygdala is sensitive to a broad range of emotionally salient, attention-grabbing stimuli, including faces, aversive images, erotica, and food and drug cues (Méndez-Bértolo et al., 2016; Minxha et al., 2017; Shackman et al., 2016b). Increased amygdala activation is even observed using subliminal or task-irrelevant emotional stimuli (Brooks et al., 2012; Cromheeke & Mueller, 2014; Hung, Gaillard, Yarmak, & Arsalidou, 2018; Krug & Carter, 2010) and has been associated with more severe symptoms in pediatric anxiety patients (Monk et al., 2008b). Among children, heightened amygdala activation is associated with enhanced detection of threat-related faces in a crowed array and, among those exposed to early deprivation, greater amygdala activation is associated with elevated anxiety symptoms (Silvers et al., 2017). Among adults, individuals with a more negative disposition show heightened amygdala activation and enhanced attentional capture (i.e., response slowing) to threat-related cues, even when they are task-irrelevant (Ewbank et al., 2009). Likewise, adults and children with anxiety disorders have been shown to exhibit increased amygdala activation and exaggerated behavioral interference when performing standard ‘emotional attention’ tasks (e.g., emotional Stroop, dot-probe; Boehme et al., 2015; Price et al., 2016a).
As shown in Figure 5a, anatomical tracing studies in nonhuman primates and mechanistic studies in rodents indicate that the amygdala is well-positioned to prioritize the processing of threat and other salient stimuli. Enhanced attention can occur via at least two mechanisms: directly, via excitatory projections from the basolateral (BL) nucleus of the amygdala (Figure 1) to the relevant areas of sensory cortex (e.g., fusiform face area) and indirectly, via projections from the basal nuclei and Ce to neuromodulatory systems in the basal forebrain and brainstem that, in turn, can modulate sensory cortex (i.e., increase the neuronal signal-to-noise ratio; Davis & Whalen, 2001; Freese & Amaral, 2009). Consistent with this perspective, adult imaging research shows that trial-by-trial fluctuations in amygdala activity predict whether degraded threat stimuli are detected—consistent with single unit recording studies in monkeys (Peck, Peck, & Salzman, 2014)—and demonstrate that this association is statistically mediated by enhanced activation in the relevant areas of sensory cortex (Lim, Padmala, & Pessoa, 2009) (Figure 5b). Determining whether this distributed amygdalo-cortical circuitry is altered in individuals with a negative disposition or anxiety disorder remains an important challenge for the future.
Figure 5. The amygdala plays a key role in enhancing attention to threat-relevant information.
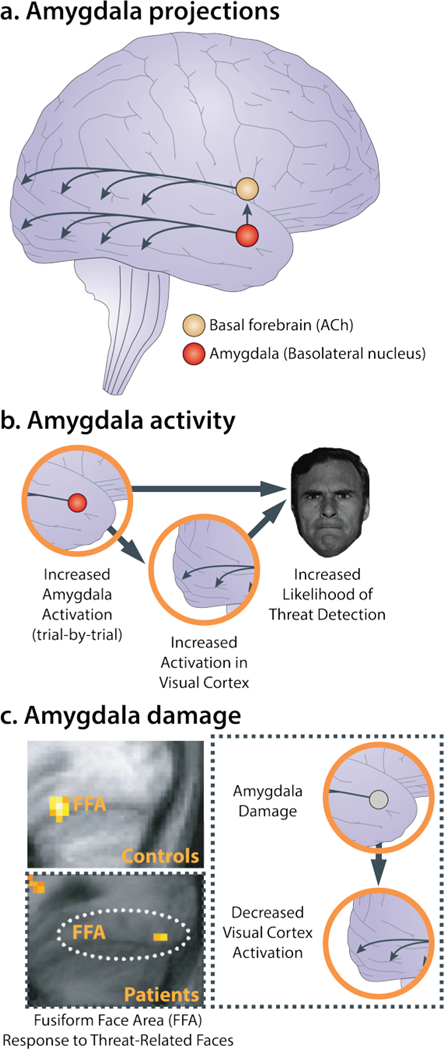
a. Amygdala projections. Anatomical tracing studies in monkeys and mechanistic studies in rodents indicate that the amygdala can enhance vigilance and prioritize the processing of threat-relevant information directly, via monosynaptic projections from the basolateral nucleus (BL; see Figure 1) to sensory cortex, and indirectly, via projections from the basal nuclei and central nucleus (Ce) to ascending neuromodulatory systems in the basal forebrain and brain stem. In turn, these transmitter systems can enhance the signal-to-noise ratio of neuronal processing in cortical sensory regions. In this simplified illustration, select projections from the basal forebrain cholinergic (ACh) system to the visual cortex are depicted. b. Amygdala activity. Using fMRI, Lim and colleagues demonstrated that amygdala activation predicts trial-by-trial fluctuations in threat detection (Lim et al., 2009). Mediation analyses revealed that relations between amygdala activation and detection performance were explained by increased activation in the visual cortex, consistent with work in animals. c. Amygdala damage. In a seminal study, Vuilleumier and colleagues (2004) showed that individuals with amygdala damage do not show increased activation to threat-related facial expressions in the fusiform face area (FFA) of the visual cortex, indicating that the amygdala causally contributes to the enhanced processing of threat-related stimuli in humans. This observation has since been replicated using more selective chemical lesions in monkeys (Hadj-Bouziane et al., 2012). Abbreviations—ACh: acetylcholine; FFA: fusiform face area. Portions of this figure were adapted with permission from (Tang, Holzel, & Posner, 2015; Vuilleumier et al., 2004).
A growing body of research in human adults and monkeys indicates that the amygdala plays a mechanistically important role in biasing attention to threat-related cues. Manipulations that potentiate amygdala reactivity also enhance attentional biases to threat-related information (Herry et al., 2007). For example, Herry and colleagues demonstrated that exposure to an emotionally neutral, temporally unpredictable train of auditory pulses activates the lateral and BL amygdala (cf. Figure 1) and amplifies attentional biases to angry faces in a dot-probe task. Conversely, patients with amygdala damage and monkeys with selective amygdala lesions do not show enhanced processing of threat-related cues (i.e., fearful or threatening faces) in sensory cortex (Hadj-Bouziane et al., 2012; Rotshtein et al., 2010; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). In particular, amygdala insults markedly reduce ‘valence’ effects for facial expressions (i.e., Threat > Neutral) in the fusiform face area in humans (Vuilleumier et al., 2004) (Figure 5c) and inferior temporal cortex in monkeys (Hadj-Bouziane et al., 2012). In humans, amygdala damage disrupts the prioritized processing of threat-related faces in crowded stimulus arrays (Bach, Hurlemann, & Dolan, 2015). Likewise, monkeys’ normal preference for viewing conspecific faces is disrupted by fiber-sparing (excitotoxic) lesions of the amygdala (Taubert et al., 2018)5.
Other work suggests that the amygdala can actively tune attention. In addition to biasing selection and increasing the depth of processing, there is compelling evidence that the amygdala plays a key role in redirecting gaze (i.e., overt attention) to those features of the face, such as the eyes and brow, that are most diagnostic of threat, trustworthiness, anger, and fear (Oosterhof & Todorov, 2008, 2009; Smith, Cottrell, Gosselin, & Schyns, 2005b). Using a combination of eye tracking and brain imaging, Gamer and colleagues have demonstrated that human adults are biased to reflexively attend the eye and brow region of the face, that this bias is most pronounced for threat-related (i.e., fearful) facial expressions, and that individuals with greater amygdala activation are more likely to shift their gaze to the eyes (Gamer & Buchel, 2009; Scheller, Buchel, & Gamer, 2012) (Figure 6a, b). Similar effects have been obtained for complex non-social cues; subjects are biased to fixate the visual features most predictive of threat and this tendency co-varies with trial-by-trial fluctuations in amygdala activation (Eippert, Gamer, & Buchel, 2012). With regard to faces, this kind of attentional bias is exaggerated among adults with a more negative disposition (Perlman et al., 2009) and those with social anxiety disorder (Boll, Bartholomaeus, Peter, Lupke, & Gamer, 2016). Importantly, patients with circumscribed amygdala damage do not show reflexive saccades to the eyes (Gamer, Schmitz, Tittgemeyer, & Schilbach, 2013) (Figure 6c). Instead, they tend to fixate the mouth, both in laboratory assessments and real-world social interactions (Adolphs et al., 2005; Spezio, Huang, Castelli, & Adolphs, 2007), and this impairs the ability to recognize facial expressions of fear (Adolphs et al., 2005). Likewise, monkeys with selective lesions of the amygdala show markedly reduced detection of threat-diagnostic facial features (i.e., enhanced capture) and spend more time visually exploring the mouth region of the face (Dal Monte, Costa, Noble, Murray, & Averbeck, 2015). These converging lines of neurophysiological and mechanistic evidence indicate that the amygdala is crucial for re-allocating attention to threat-diagnostic social cues in adults. A key challenge for the future is establishing whether the amygdala performs a similar role IN other clinical populations and youth.
Figure 6. The amygdala plays a key role in orienting overt attention to potentially threat-diagnostic information in the environment. a. Attentional exploration of faces.
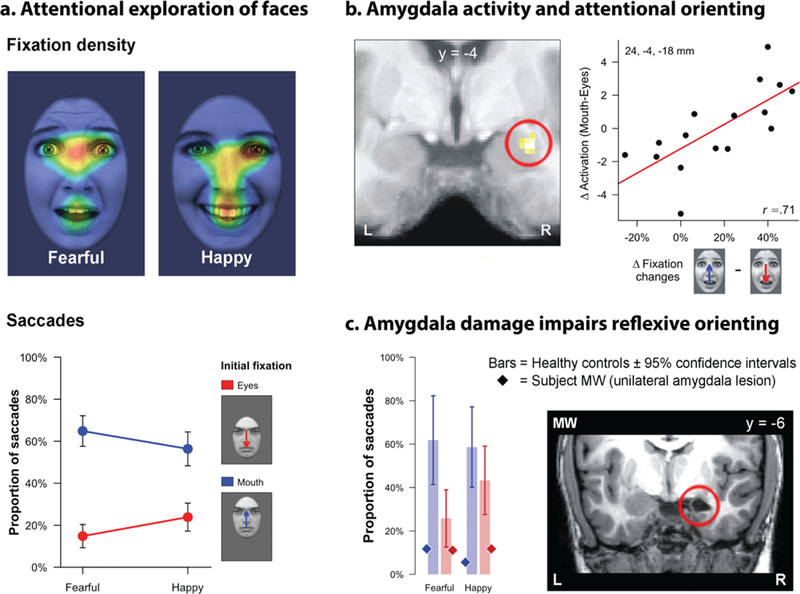
Eye tracking data reveal a strong bias for scanning the eye and brow region, particularly for fearful faces (Scheller et al., 2012). This bias is evident in both the density of fixations over time (top panel: warmer colors indicate higher density) and the likelihood of reflexive saccades toward the facial feature presented in the visual periphery (bottom panel). b. Amygdala activation and attentional orienting. Individuals with increased activation in the right amygdala (indicated by the red ring) are more likely to orient their gaze to the eye and brow region of fearful faces (Gamer & Buchel, 2009). C. Amygdala damage impairs reflexive orienting. Patient MW has selective damage to the right amygdala (red ring) and shows a profound reduction in reflexive saccades to the eye region of the face (Gamer et al., 2013). Abbreviations—L: left hemisphere, R: right hemisphere. Portions of this figure were adapted with permission from (Shackman et al., 2016a).
Pervasive Hypervigilance May Reflect Stress-Induced Sensitization of the Amygdala
Hypervigilance in inappropriate or maladaptive settings is a core feature of extreme anxiety (Grupe & Nitschke, 2013; Notebaert et al., in press; Notebaert, Tilbrook, Clarke, & MacLeod, 2017). Persistent, contextually inappropriate vigilance or attentional biases to threat-related information may reflect stress-induced sensitization of the amygdala. Recent work in adult humans shows that acute experimental stressors (e.g., threat-of-shock, aversive film clips) potentiate defensive reactions (i.e., startle) to threat-related facial expressions (Grillon & Charney, 2011a), cause persistent increases in spontaneous amygdala activity (Cousijn et al., 2010)—consistent with rodent studies (Ahrens et al., 2018)—and potentiate amygdala reactivity to threat-related faces (Pichon, Miendlarzewska, Eryilmaz, & Vuilleumier, 2015; van Marle, Hermans, Qin, & Fernandez, 2009). Acute stressors produce even longer-lasting changes (i.e., minutes to hours) in amygdala functional connectivity (Hermans et al., 2017; Vaisvaser et al., 2013; van Marle, Hermans, Qin, & Fernandez, 2010). Moreover, these kinds of neurobiological ‘spill-over’ effects are amplified among individuals with a more negative disposition. For example, a large-scale imaging study (n = 120) showed that dispositionally negative individuals exhibit potentiated activation to threat-related faces following acute stressor exposure (Everaerd, Klumpers, van Wingen, Tendolkar, & Fernandez, 2015). Persistent amygdala sensitization could promote pervasive anxiety and ‘spillover’ of negative affect by increasing the likelihood that attention is allocated to threat-related cues in the environment (Grupe & Nitschke, 2013; Macatee, Albanese, Schmidt, & Cougle, 2017; Shackman et al., 2016c). Understanding the relevance of these pathways to the development of anxiety disorders is important because the roots of anxiety disorders often extend into childhood (Kessler et al., 2007) and mental illnesses that emerge before adulthood impose a substantially higher economic burden than those that emerge in mid or later life (Lee et al., 2014; WHO, 2007).
Interim Conclusions
Hypervigilance is a core feature of the anxiety disorders and dispositional negativity and, on average, adults and youth with a more negative disposition tend to allocate excess attention to potentially threat-related cues, even when they are task irrelevant (Shackman et al., 2016a). Like other candidate biomarkers, the magnitude of this cross-sectional association is too small to be clinically useful, at least when assessed using the popular, but psychometrically flawed dot-probe task (Abend et al., 2018; Fu & Pérez-Edgar, 2019; Kruijt et al., 2018; Rodebaugh et al., 2016). Preliminary work using new paradigms and new behavioral measures, including eyetracking, suggests that patients with anxiety disorders and individuals with a more negative disposition are more likely to dwell on threat-related cues and are more likely to shift attention to potentially threat-diagnostic features of the environment (Boll et al., 2016; Lazarov et al., 2016; Lazarov et al., in press; Perlman et al., 2009; Sheppes et al., 2013). Attentional biases to threat prospectively predict the first emergence of anxiety symptoms in youth and interventions that attenuate attentional biases to threat have been shown to reduce pathological anxiety in adults, indicating a causal contribution (Grafton et al., 2017; White et al., 2017).
Converging lines of neuroimaging, electrophysiological, and mechanistic research indicate that the amygdala plays a crucial role in prioritizing the processing of threat-related cues (Bach et al., 2015; Gamer et al., 2013; Hadj-Bouziane et al., 2012; Lim et al., 2009; Peck et al., 2014; Vuilleumier et al., 2004). Individuals with a more negative disposition and patients with anxiety disorders show exaggerated behavioral interference and elevated amygdala activation when performing emotional attention tasks (Boehme et al., 2015; Ewbank et al., 2009; Price et al., 2016a). Exposure to acute stressors increases the on-going activity of the amygdala and potentiates reactivity to threat-related cues encountered in the future, suggesting a substrate for the kinds of mood spillover effects and inappropriate deployment of attentional resources that characterize individuals with a more negative disposition and many anxiety patients (Cousijn et al., 2010; Everaerd et al., 2015; Shackman et al., 2016c).
THE NATURE, CONSEQUENCES, AND NEUROBIOLOGY OF EXECUTIVE DEFICITS
The Nature of Executive Function and Cognitive Control
Lapses in concentration and problems with cognitive function are clinically significant features of anxiety disorders and other psychiatric illnesses (American Psychiatric Association, 2013). Yet the contributions of executive function and cognitive control—the basic building blocks of intelligence and complex everyday cognition—to pathological anxiety have received considerably less empirical attention than attentional biases to threat. Executive function refers to the processes involved in shifting between mental sets or tasks, updating and monitoring working memory (e.g., n-back continuous performance task), and inhibiting prepotent responses (Banich, 2009; Miyake & Friedman, 2012; Miyake et al., 2000). Cognitive control encompasses a range of processes—including attention, inhibition, and learning—that are engaged when automatic or habitual responses are insufficient to sustain goal-directed behavior, as with the inhibitory facet of executive function (Shackman et al., 2011b). Like fear and anxiety, cognitive control is a component of the NIMH Research Domain Criteria (RDoC) (Clark, Cuthbert, Lewis-Fernandez, Narrow, & Reed, 2017; Kozak & Cuthbert, 2016). Common assays of cognitive control include variants of the Anti-Saccade, Eriksen Flanker, Go/No-Go, Simon, Stop-Signal, and Stroop tasks. Here, we use ‘executive control’ as a rubric for executive function and cognitive control.
Relevance of Executive Control Deficits to Dispositional Negativity and Anxiety Disorders
Converging lines of educational, epidemiological, developmental, and experimental research suggest that dispositional negativity is associated with deficits in executive control. Increased dispositional negativity is associated with reduced educational attainment (Damian, Su, Shanahan, Trautwein, & Roberts, 2015; Hengartner et al., 2016b; Hill, Weiss, McIntosh, Gale, & Deary, 2017; Nagel et al., 2018a; Nagel et al., 2018b) and fluid intelligence (Dubois, Galdi, Han, Paul, & Adolphs, 2018). Dispositional negativity is genetically correlated with reduced educational attainment (rG = −.22, N = 328,917) (Nagel et al., 2018a) and lower intelligence (rG = −.21, N = 170,911) (Savage et al., 2018), suggesting shared molecular underpinnings. In laboratory settings, children and adults with a more negative disposition are prone to executive control deficits when performing standard emotionally neutral tasks, that is, in the absence of threat-related cues (Basten, Stelzel, & Fiebach, 2011; Beaudreau, MacKay-Brandt, & Reynolds, 2013; Berggren & Derakshan, 2013; Bishop, 2009; Derakshan & Eysenck, 2009; Derryberry & Reed, 2002; Eysenck & Derakshan, 2011; Eysenck, Derakshan, Santos, & Calvo, 2007b; Gustavson, Altamirano, Johnson, Whisman, & Miyake, 2017; Gustavson & Miyake, 2016; Muris, de Jong, & Engelen, 2004; Muris, van der Pennen, Sigmond, & Mayer, 2008; Osinsky, Gebhardt, Alexander, & Hennig, 2012). For example, adults with a more negative disposition tend to commit more errors in task-switching and inhibitory control paradigms (Ansari & Derakshan, 2011; Basten et al., 2011; Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009a; Derakshan, Smyth, & Eysenck, 2009b; Garner, Ainsworth, Gould, Gardner, & Baldwin, 2009; Goodwin & Sher, 1992; Orem, Petrac, & Bedwell, 2008; Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, 2010; Pacheco-Unguetti, Lupiáñez, & Acosta, 2009; Wieser, Pauli, & Mühlberger, 2009). Clinical samples reveal a broadly similar pattern (Aupperle, Melrose, Stein, & Paulus, 2012; Hallion, Tolin, Assaf, Goethe, & Diefenbach, 2017; Polak, Witteveen, Reitsma, & Olff, 2012; Scott et al., 2015; Stefanopoulou, Hirsch, Hayes, Adlam, & Coker, 2014; Wright, Lipszyc, Dupuis, Thayapararajah, & Schachar, 2014). Nevertheless, the limited number, breadth, and quality of clinical studies signals the need for additional research (McTeague, Goodkind, & Etkin, 2016; Snyder, Miyake, & Hankin, 2015).
Executive Control Deficits Causally Contribute to Pathological Anxiety
Longitudinal studies show that executive control difficulties are prospectively associated with greater anxiety, worry, and rumination in the future (Aupperle et al., 2012; Bredemeier & Berenbaum, 2013; Crowe, Matthews, & Walkenhorst, 2007; De Lissnyder et al., 2012; Duchesne, Larose, Vitaro, & Tremblay, 2010; Pérez-Edgar, Taber-Thomas, Auday, & Morales, 2014; Snyder et al., 2014; Whitmer & Banich, 2007; Zhang et al., 2015). In a nationally representative sample of 2,605 American adults, decrements in set shifting, updating, and inhibition conferred robust risk of developing generalized anxiety disorder (GAD) across the 9-year follow-up period (e.g., Odds Ratios for Updating > 6.00; Zainal & Newman, 2018). Likewise, a recent meta-analysis uncovered evidence of cognitive impairment—including lower IQ (−0.19 SD) and academic performance—in first-degree relatives of individuals with MDD (N = 8,468) (MacKenzie, Uher, & Pavlova, in press), suggesting a causal role. Conversely, there is emerging evidence that interventions targeting cognitive control can ameliorate anxiety symptoms, reinforcing the conclusion that executive control deficits causally contribute to the development of pathological anxiety (e.g., Cohen, Daches, Mor, & Henik, 2014; Cohen, Mor, & Henik, 2015).
The Neurobiology of Executive Control
Executive control is often associated with the prefrontal cortex (PFC). Historically, this view was motivated by early evidence of impairments in goal-directed behavior and complex cognition in monkeys and humans with selective damage to the lateral PFC (Bianchi & Macdonald, 1922; Duncan, 1986; Ferrier, 1886; Grafman, 1994; Knight, 1984; Passingham, 1993). Recent meta-analyses of the functional neuroimaging literature have extended this perspective, suggesting that executive control reflects the coordinated function of several large-scale brain circuits, including the frontoparietal network (dlPFC, intraparietal sulcus) and cingulo-opercular network (midcingulate cortex, anterior insula, frontal operculum) (Chen et al., 2018; Hung et al., 2018; Li et al., 2017; McKenna, Rushe, & Woodcock, 2017) (Figure 7).
Figure 7. Executive control networks.
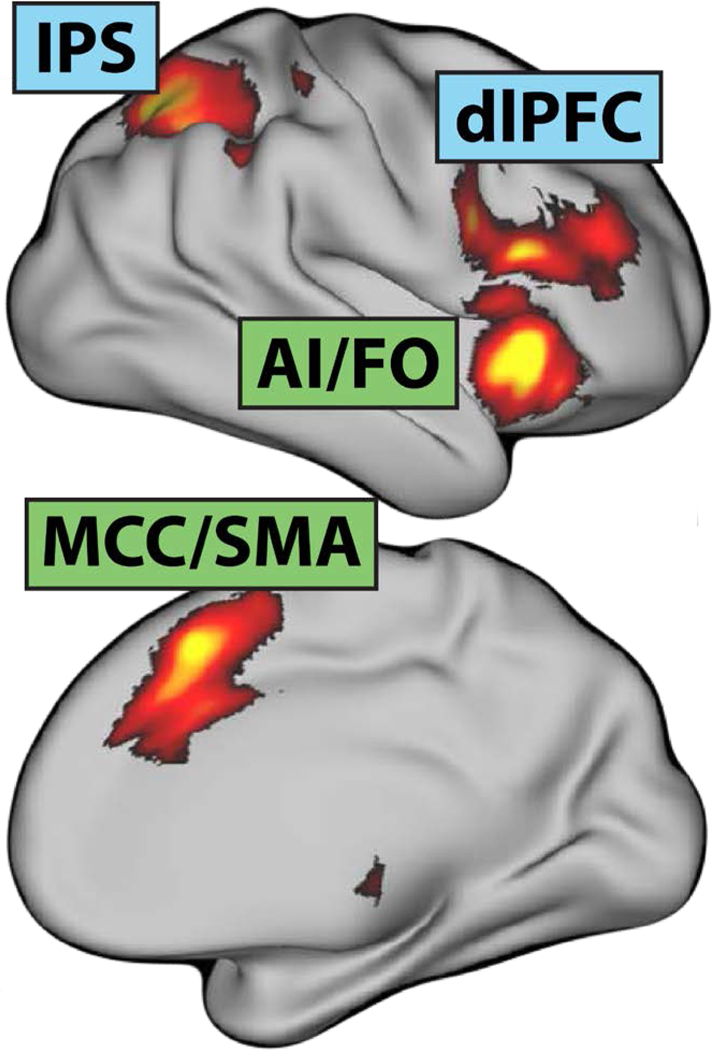
The frontoparietal (blue) and cingulo-opercular (green)networks are sensitive to a broad spectrum of executive function and cognitive control tasks. Abbreviations—AI: anterior insula; dlPFC: dorsolateral prefrontal cortex; FO: frontal operculum; IPS: intraparietal sulcus; MCC: midcingulate cortex; SMA: supplementary motor area. This figure were adapted with permission from (Li et al., 2017)
Relevance of Executive Control Networks to Dispositional Negativity and Anxiety Disorders
To our knowledge, only three functional neuroimaging studies have examined relations between dispositional negativity and executive control. Two studies reported increased engagement of frontoparietal control networks, while a third, much smaller study reported decreased engagement. Fales and colleagues showed that dispositional negativity enhanced activation of frontoparietal regions on control-demanding trials of a complex n-back task in the absence of overt differences in performance (N = 96; Fales et al., 2008). Likewise, Basten and colleagues found enhanced dlPFC activation on control-demanding (i.e. incongruent) trials of the widely used Stroop task (N = 46; Basten et al., 2011). Here, the positive association between dispositional negativity and dlPFC activation remained significant after controlling for performance decrements among subjects with a more negative disposition. Finally, Bishop used a compound attentional-search/response-conflict paradigm to reveal reduced dlPFC activation and slower target identification among individuals with a more negative disposition (N = 17; Bishop, 2009). While the results of these three studies preclude strong conclusions, the overall pattern aligns with the hypothesis that dispositionally negative individuals tend to inefficiently allocate executive control resources, requiring greater effort or neural engagement to achieve similar (or worse) ends (Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck, Derakshan, Santos, & Calvo, 2007a). At present, even less is known about the relevance of executive control networks to clinical anxiety. A comprehensive recent meta-analysis of neuroimaging studies failed to uncover any significant regional differences in activation during the performance of emotionally neutral executive control tasks, although this may reflect the disproportionate representation of obsessive-compulsive compulsive disorder (OCD) samples (k = 32 studies; McTeague et al., 2016; McTeague et al., 2017). Given the consequences of executive control deficits for the development of pathological anxiety (Zainal & Newman, 2018), additional research in adults and youth is clearly warranted
EMERGING EVIDENCE FOR THE INTERPLAY OF ATTENTIONAL BIASES AND EXECUTIVE CONTROL
While most research has focused on attentional biases to threat or deficits in executive control in isolation, an emerging body of data and theory suggests that these processes are intimately related and can reciprocally interact (Bishop, 2008b, 2009; Bishop & Forster, 2013; Derakshan et al., 2009a; Eysenck & Derakshan, 2011; Eysenck et al., 2007b; Iordan, Dolcos, & Dolcos, 2013; Mogg & Bradley, 2016b; Mogg & Bradley, 2018; Mogg, Waters, & Bradley, 2017b; Tottenham & Gabard-Durnam, 2017). From a conceptual perspective, such interactions are most likely to occur when there is competition between task-irrelevant threat-related cues and on-going goals, as with a variety of ‘emotional conflict’ tasks (e.g., emotional Stroop). Monitoring and adjudicating this conflict demands executive control resources, rendering them less available for on-going cognitive performance (Shackman et al., 2011b) or anxiety regulation (Buhle et al., 2014). Consistent with this view, there is evidence that excessive allocation of attention and working memory capacity to threat disrupts on-going performance and hijacks regions of the frontoparietal network, and that these adverse consequences are more pronounced among dispositionally negative individuals (Hur et al., 2015; Moran, 2016; Robinson, Vytal, Cornwell, & Grillon, 2013; Shackman, Maxwell, McMenamin, Greischar, & Davidson, 2011a; Shackman et al., 2006; Stout, Shackman, & Larson, 2013). Other work demonstrates that attentional biases to threat are enhanced among dispositionally negative individuals with poor cognitive control and that they are reduced under conditions that facilitate cognitive control (Derryberry & Reed, 2002; Hadwin & Richards, 2016; Hur, Iordan, Berenbaum, & Dolcos, 2016; Lonigan & Vasey, 2009; Susa, Pitică, Benga, & Miclea, 2012; Taylor, Bomyea, & Amir, 2010).
Given the many ways in which attentional biases to threat and executive control can potentially interact, and the numerous mono- and polysynaptic pathways linking the amygdala to regions involved in executive control, the underlying neural circuitry is likely to be complex and at least somewhat task-dependent (Benarroch, 2015; Etkin, Buchel, & Gross, 2015; Freese & Amaral, 2009; Mogg & Bradley, 2018). Nevertheless, recent meta-analyses of the functional neuroimaging literature reveal a remarkably consistent engagement of regions within the frontoparietal and cingular-opercular networks (Figure 7) across a wide range of emotional interference tasks (k = 10–48 studies; Chen et al., 2018; Cromheeke & Mueller, 2014; Hung et al., 2018; Song et al., 2017; Xu, Xu, & Yang, 2016). Work focused on adult anxiety patients has begun to document aberrant functional connectivity between the amygdala and these control regions, as well as diminished mPFC responses to threat distractors (Bishop, 2008a; Blackford & Pine, 2012; Carpenter et al., 2015; Ding et al., 2011; Etkin et al., 2015; Kim, Gee, Loucks, Davis, & Whalen, 2010; Liao et al., 2010; Monk et al., 2006; Monk et al., 2008a; Price, Eldreth, & Mohlman, 2011; Shin et al., 2005; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002; Sussman, Jin, & Mohanty, 2016; Sylvester et al., 2012; Tillfors et al., 2001). While most of the work remains undone, these observations suggest that dispositional negativity and anxiety disorders disrupt the balance between attention and control—amplifying the attentional salience of threat and attenuating executive control—leading to less effective or less efficient performance (Berggren & Derakshan, 2013; Eysenck et al., 2007b; Snyder et al., 2015).
FUTURE CHALLENGES
The data that we have reviewed provide new insights into the neurocognitive mechanisms that support individual differences in dispositional negativity and that link this disposition to the development of anxiety disorders and other psychiatric diseases. Yet, it is clear that our understanding remains far from complete. Throughout the review, we highlighted a number of specific conceptual and methodological challenges for future research in this area. Here, we outline some broader questions for the field and offer some strategies for starting to address them (for general recommendations about best practices, see Fox, Lapate, Davidson, & Shackman, 2018a).
How do different aspects of attention contribute to the development of anxiety disorders? In this review, we have treated hypervigilance and attentional biases to threat-related information as virtually synonymous. Yet, there is a growing recognition that the amount of attention allocated to threat-related cues can be decomposed into several constituents: (i) the likelihood that task-relevant threat will be detected and attention will be re-oriented, (ii) the likelihood that task-irrelevant threat will capture attention or bias behavior (i.e., reduced attentional control or selectivity), (iii) the rapidity of disengagement from threat, and (iv) the degree of attentional avoidance (or maintenance) during sustained, free-viewing tasks (Mogg & Bradley, 2018; Richards, Benson, Donnelly, & Hadwin, 2014). Although work by Gamer and colleagues demonstrates that the amygdala plays a crucial role in the initial re-orienting to threat-diagnostic features of the face (Gamer & Buchel, 2009; Gamer et al., 2013), much less is known about the clinical relevance or neurobiology of these other biases in adults or youth. Addressing this question will require the integration of eye tracking with brain imaging or electrophysiological assays in individuals with anxiety disorders or varying levels of familial or dispositional risk. Longitudinal studies in high-risk populations would be especially valuable.
How do different components of the amygdala contribute to risk? Like attention, the amygdala can be divided into meaningful sub-components or nuclei (Freese & Amaral, 2009; Yilmazer-Hanke, 2012) (Figure 1). Developing a deeper understanding of this heterogeneity and its relevance to the development of anxiety disorders and other stress-sensitive mental illnesses requires that we first acknowledge it. Although investigators need to be cautious when assigning specific labels (e.g., Ce) to activation clusters in imaging studies, we encourage them to describe the relative position of activation peaks (e.g., dorsal-posterior amygdala) and interpret their results on the basis of the most likely sub-component of the amygdala (e.g., ‘in the region of the Ce’). The use of high-field MRI or specialized analytic approaches (e.g., spatially unsmoothed data) may also prove useful (Fox & Shackman, 2019; Hur et al., 2018; Tillman et al., 2018).
Which brain circuits are associated with individual differences in risk? There is widespread consensus that dispositional negativity, hyper-vigilance for threat, and executive control deficits—like other psychologically and psychiatrically relevant processes—reflect the coordinated activity of distributed brain circuits (Okon-Singer, Hendler, Pessoa, & Shackman, 2015; Pessoa, 2013; Shackman, Fox, & Seminowicz, 2015). Yet most imaging investigators (including our team) have relied heavily on localization strategies, where function is mapped to isolated brain structures. Unfortunately, this approach tends to promote the development of models in which a small number of territories—the amygdala, dlPFC, and MCC, for example—do all or most of the ‘heavy lifting.’ Overcoming this important barrier requires that we accelerate the transition from localization strategies to network-based approaches (Fornito, Zalesky, & Breakspear, 2015; Guloksuz, Pries, & Van Os, 2017; McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Servaas et al., 2014). Information-based approaches, such as multivoxel classifier approaches, provide another powerful tool for discovering the distributed functional networks associated with emotional states, traits, and disorders (Kragel, Koban, Barrett, & Wager, 2018; Woo, Chang, Lindquist, & Wager, 2017). Developing robust and generalizable (i.e., task- and sample-general) classifiers that are firmly grounded in overt behavior or subjective report are more likely to be useful for therapeutics development and more likely to successfully translate to the clinic (Hur, Tillman, Fox, & Shackman, in press; Shackman & Fox, 2018; Shackman & Wager, 2019).
How relevant are individual differences in brain function to anxiety-related experience and behavior in the real world? Most psychophysiological and imaging studies of anxiety and anxiety-relevant cognitive mechanisms (e.g., attention bias, attention control) rely on a limited number of well-controlled, but highly artificial manipulations (e.g., static emotional faces, unpleasant images, threat-of-shock), collected under unnatural conditions (Coan & Allen, 2007; Fox, Lapate, Shackman, & Davidson, 2018b). Although this approach has afforded many important insights, the real-world or ‘translational’ significance of the circuits identified in the laboratory often remain unclear. Given the limitations of ambulatory measures of brain activity—there is no ‘fMRI helmet’ as yet—addressing this fundamental question requires integrating assays of brain function and behavior (e.g., eye tracking) acquired in the scanner with thoughts, feelings, and behavior assessed under naturalistic conditions in the field (Anderson, Monroy, & Keltner, 2018) or in the laboratory (e.g., during semi-structured interactions or using commercially available virtual reality techniques; Creed & Funder, 1998; Kroes, Dunsmoor, Mackey, McClay, & Phelps, 2017; Perez-Edgar et al., 2010b; Stolz, Endres, & Mueller, in press; Thomson et al., 2019, in press). Work combining fMRI with ecological momentary assessment (EMA) and other experience-sampling techniques highlights the value of this approach for identifying the neural systems underlying naturalistic variation in mood and behavior in adults, adolescents, and older children (Berkman & Falk, 2013; Forbes et al., 2009; Heller et al., 2015; Lopez, Hofmann, Wagner, Kelley, & Heatherton, 2014; Price et al., 2016a; Wilson, Smyth, & MacLean, 2014). The development of robust mobile eye trackers (Liu et al., 2018), the emergence of commercial software for automated facial analytics (Olderbak, Hildebrandt, Pinkpank, Sommer, & Wilhelm, 2014), and the widespread dissemination of ‘smart’ mobile technologies afford new opportunities for intensively quantifying social attention, arousal, behavior, mood, and anxiety-relevant features of the environment (Boukhechba, Chow, Fua, Teachman, & Barnes, 2018; Chow et al., 2017; Mohr, Zhang, & Schueller, 2017; Picard, 2018; Saeb, Lattie, Schueller, Kording, & Mohr, 2016; Shackman et al., 2018b; Stingone et al., 2017). Networked sensors in smartphones and other wearables are already woven into the fabric of our lives. In the U.S., 77% of adults and 94% of young adults (<30 years) own smartphones (Pew Research Center, 2018). Because data are repeatedly captured in the real world, smartphone-based EMA circumvents the mnemonic biases that can distort daily diaries, clinical assessments, and other retrospective ‘snapshots’ (Ebner-Priemer & Trull, 2009; Kanning, Ebner-Priemer, & Schlicht, 2013; Shiffman, Stone, & Hufford, 2008; Solhan, Trull, Jahng, & Wood, 2009; Stone, Shiffman, Atienza, & Nebeling, 2007; Tost, Champagne, & Meyer-Lindenberg, 2015). Smartwatches and other wearable sensors (e.g., actigraphy, GPS) go a step further, eliminating the need for subjects to repeatedly respond to surveys and providing continuous and objective measures (‘movies’) of anxiety-relevant behaviors (Gambhir, Ge, Vermesh, & Spitler, 2018). Moreover, digital tracking can provide behavioral phenotypes (e.g., locomotion, sleep, social avoidance) that are directly comparable between humans and animals (Freimer & Mohr, in press; Hong et al., 2015), facilitating the development of cross-species models and enhancing opportunities for mechanistic insight (Fox & Shackman, 2019; Shackman & Fox, 2016b). Combining these measures with laboratory assays of brain function would open the door to discovering the neural systems underlying maladaptive experiences and pathology-promoting behaviors (e.g., social withdrawal, avoidance) in the real world, close to clinical end-points (Price et al., 2016a). This approach promises a depth of understanding that cannot be achieved using either animal models or isolated measures of brain function and represents a key step to establishing the clinical and therapeutic relevance of these brain circuits.
What mechanisms underlie individual differences in risk? Much of the data that we have reviewed comes from brain imaging studies. Aside from unresolved questions about the origins and significance of the measured signals (Logothetis, 2008), the most important limitation of imaging studies is that they cannot address necessity or sufficiency. A crucial challenge for the future is to develop a mechanistic understanding of the brain regions and functional circuits that confer increased risk for the development of anxiety disorders in adults and youth. Addressing this fundamental question requires coordinated research efforts in humans and nonhuman animal models. This could be achieved by combining mechanistic techniques in animals with the same whole-brain imaging strategies routinely used in humans, enabling the development of bidirectional translational models (Birn et al., 2014; Fox & Shackman, 2019; Kalin, 2017; Terburg et al., 2018). Nonhuman primate models are likely to be particularly useful for modeling and understanding the molecular and cellular neurobiology of dispositional negativity because monkeys and humans share similar genes and brains, which endow the two species with a shared repertoire of complex social, emotional, and cognitive behaviors (Fox & Shackman, 2019). Furthermore, well-established techniques already exist for studying both dispositional negativity and attention in nonhuman primates (Hadj-Bouziane et al., 2012; Noudoost, Albarran, & Moore, 2014; Oler et al., 2016).
Human studies will also be crucial. After all, anxiety disorders are defined and diagnosed on the basis of subjective symptoms and human studies are essential for understanding the neural mechanisms supporting the experience of fear and anxiety (LeDoux & Hofmann, 2018; Pankevich, Altevogt, Dunlop, Gage, & Hyman, 2014; Pine & LeDoux, 2017; Zoellner & Foa, 2016). Human studies are also important for identifying the features of animal models that are conserved and, hence, most relevant to understanding human disease and to developing improved interventions for human suffering (‘forward translation;’ Birn et al., 2014; Hyman, 2016; Pankevich et al., 2014). In humans, imaging approaches can be applied to patients with circumscribed brain damage (Adolphs, 2016; Motzkin et al., 2015a; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2014, 2015b; Spunt et al., 2015). Alternatively, fMRI or EEG can be combined with noninvasive perturbation techniques (Bestmann & Feredoes, 2013; Dubois et al., in press; Reinhart & Woodman, 2014), neurofeedback (deBettencourt, Cohen, Lee, Norman, & Turk-Browne, 2015; Greer, Trujillo, Glover, & Knutson, 2014; Stoeckel et al., 2014), cognitive-behavioral interventions (Britton et al., 2015; Schnyer et al., 2015), pharmacological interventions (Paulus, Feinstein, Castillo, Simmons, & Stein, 2005; Wager et al., 2013), or more passive psychological manipulations (i.e., temporally unpredictable auditory stimuli; Herry et al., 2007). Prospective longitudinal imaging studies represent another important approach to identifying candidate mechanisms, especially in relation to the development of internalizing disorders (Admon, Milad, & Hendler, 2013; Burghy et al., 2012; Herringa et al., 2013; McLaughlin et al., 2014; Swartz, Williamson, & Hariri, 2015).
CONCLUSIONS
The work that we have reviewed highlights the importance of amygdala, frontoparietal, and cingular-opercular circuits to individual differences in dispositional negativity and two prominent intermediate phenotypes: threat-related attentional biases, and deficits in executive control. Collectively, these observations provide an integrative translational framework for understanding the development and maintenance of anxiety and mood disorders in adults and youth and set the stage for developing improved strategies for preventing or treating them.
ACKNOWLEDGEMENTS
Authors acknowledge assistance from M. Barstead, K. DeYoung, L. Friedman, M. Gamer, S. Haas, C. Kaplan, K. Rubin, J. Smith, R. Tillman and financial support from the California National Primate Center; National Institute of Health (DA040717, MH107444); University of California, Davis; and University of Maryland, College Park. Authors declare no conflicts of interest
Footnotes
The terms ‘threat-related’ or ‘threat-relevant’ encompass a broad range of stimuli, including clear and immediate dangers (e.g., cues paired with shock), novel situations or individuals, uncertain or diffuse dangers (e.g., darkness), aversive stimuli (e.g., unpleasant images or films), and angry and fearful facial expressions. Angry faces signal a direct threat to the observer and prompt the mobilization of defensive responses, as indexed by potentiation of the startle reflex (Dunning, Auriemmo, Castille, & Hajcak, 2010; Hess, Sabourin, & Kleck, 2007; Springer, Rosas, McGetrick, & Bowers, 2007b), facilitation of avoidance-related movements (Marsh, Ambady, & Kleck, 2005), and increased fear ratings (Dimberg, 1988). In contrast, fearful faces signal the presence, but not the source of potential threat, and can promote heightened vigilance in the absence of defensive mobilization (Whalen, 1998). Static images of fearful faces typically do not amplify the startle reflex (Grillon & Charney, 2011b; Springer, Rosas, McGetrick, & Bowers, 2007a) or autonomic measures (Dunsmoor, Mitroff, & LaBar, 2009). But they can increase subjective feelings of anxiety (Blairy, Herrera, & Hess, 1999) and are perceived as more threatening and arousing than neutral or happy faces (Grillon & Charney, 2011b; Wieser & Keil, 2014). Fearful faces elicit a more cautious, inhibited behavioral response style (Tipples, 2018). They also increase vigilance for potentially threat-relevant cues, particularly for low spatial-frequency (LSF) information. Indeed, the mere presentation of fearful faces has been shown to enhance subsequent contrast sensitivity (Phelps, Ling, & Carrasco, 2006), LSF orientation sensitivity (Bocanegra & Zeelenberg, 2009; Nicol, Perrotta, Caliciuri, & Wachowiak, 2013), and context memory (Davis et al., 2011); boost the temporal resolution of subsequent visual processing (Bocanegra & Zeelenberg, 2011a, 2011b); and increase the efficiency of visual search (Becker, 2009).
Although other studies in youth have identified relations between amygdala-mPFC functional connectivity and anxiety, the sign of the association (i.e., hyper- vs. hypo-connectivity) has proven inconsistent, potentially reflecting differences in sample age, analytic approach, or the specific amygdala nuclei examined (Gee et al., 2013; Jalbrzikowski et al., 2017; Qin et al., 2014).
A video record of the stimulation is available at http://dx.doi.org/10.1016/j.neuropsychologia.2018.03.019
In the ‘dot-probe’ paradigm, subjects view two lateralized cues (e.g., words, faces), one threat-related, the other emotionally neutral. A short time following the offset of the cues (e.g., 500 msec), a probe (e.g., a dot) is presented in either the same location as the threat-related (‘congruent’) or neutral cue (‘incongruent’) with equal probability. Bias scores are computed by subtracting the mean reaction time for congruent trials from the mean reaction time for incongruent trials. Positive scores indicate faster engagement or slower disengagement from the threat-related cue.
Opposing effects have been reported for a rare group of patients with selective BLA damage (i.e., sparing Ce). BL patients have difficulty ignoring task-irrelevant threat, show prolonged attention to potentially threat-diagnostic facial features, and exhibit enhanced recognition of dynamic fearful facial expressions (de Gelder et al., 2014; Terburg et al., 2012). Building on mechanistic work in rodents, these observations have been interpreted as evidence that BL normally inhibits vigilance-related processes orchestrated by the Ce (Terburg et al., 2018).
REFERENCES
- Abdellaoui A, Sanchez-Roige S, Sealock J, Treur JL, Dennis J, Fontanillas P, … Boomsma DI. (2018). Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend R, de Voogd L, Salemink E, Wiers RW, Perez-Edgar K, Fitzgerald A, … Bar-Haim Y (2018). Association between attention bias to threat and anxiety symptoms in children and adolescents. Depression and Anxiety, 35, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, … Davidson RJ (1998). Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport, 9, 3301–3307. [DOI] [PubMed] [Google Scholar]
- Adams MJ, Howard DM, Luciano M, Clarke T-K, Davies GM, Hill WD, … McIntosh AM. (2019). Stratifying depression by neuroticism: revisiting a diagnostic tradition using GWAS data. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, & Hendler T (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America, 106, 14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, & Hendler T (2013). A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci, 17, 337–347. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2016). Human lesion studies in the 21st century. Neuron, 90, 1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, & Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Ahrens S, Wu MV, Furlan A, Hwang GR, Paik R, Li H, … Li B (2018). A central extended amygdala circuit that modulates anxiety. Journal of Neuroscience, 38, 5567–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisch RS, Chopra P, Fox AS, Chen K, White AT, Roseboom PH, … Kalin NH (2014). Differentially methylated plasticity genes in the amygdala of young primates are linked to anxious temperament, an at risk phenotype for anxiety and depressive disorders. Journal of Neuroscience, 34, 15548–15556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisch RS, Van Hulle C, Chopra P, Bhattacharyya A, Zhang SC, Davidson RJ, … Goldsmith HH (2017). A multi-dimensional characterization of anxiety in monozygotic twin pairs reveals susceptibility loci in humans. Transl Psychiatry, 7, 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MS, & Walter EE (2018). Linking big five personality traits to sexuality and sexual health: A meta-analytic review. Psychological Bulletin, 144, 1081–1110. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Anderson CL, Monroy M, & Keltner D (2018). Emotion in the wilds of nature: The coherence and contagion of fear during threatening group-based outdoors experiences. Emotion, 18, 355–368. [DOI] [PubMed] [Google Scholar]
- Ansari TL, & Derakshan N (2011). The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia, 49(5), 1146–1153. [DOI] [PubMed] [Google Scholar]
- Armstrong T, & Olatunji BO (2012). Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clinical Psychology Review, 32, 704–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aue T, & Okon-Singer H (2015). Expectancy biases in fear and anxiety and their link to biases in attention. Clinical Psychology Review, 42, 83–95. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, & Paulus MP (2012). Executive function and PTSD: disengaging from trauma. Neuropharmacology, 62, 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Hurlemann R, & Dolan RJ (2015). Impaired threat prioritisation after selective bilateral amygdala lesions. Cortex, 63, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back MD, Schmukle SC, & Egloff B (2009). Predicting actual behavior from the explicit and implicit self-concept of personality. Journal of Personality and Social Psychology, 97, 533–548. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18(2), 89–94. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Bullis JR, Gallagher MW, Murray-Latin H, Sauer-Zavala S, … Cassiello-Robbins C (2017). The unified protocol for transdiagnostic treatment of emotional disorders compared with diagnosis-specific protocols for anxiety disorders: A randomized clinical trial. JAMA Psychiatry, 74, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, & Ellard KK (2013). The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science, 2. [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, & Wood PK (2012). Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology, 121, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, & Fiebach CJ (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23, 3132–3145. [DOI] [PubMed] [Google Scholar]
- Bateson M, Brilot B, & Nettle D (2011). Anxiety: an evolutionary approach. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 56, 707–715. [DOI] [PubMed] [Google Scholar]
- Beaudreau SA, MacKay-Brandt A, & Reynolds J (2013). Application of a cognitive neuroscience perspective of cognitive control to late-life anxiety. Journal of Anxiety Disorders, 27, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, & Damasio AR (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–1118. [DOI] [PubMed] [Google Scholar]
- Becker MW (2009). Panic search: fear produces efficient visual search for nonthreatening objects. Psychological science, 20(4), 435–437. [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2015). The amygdala: functional organization and involvement in neurologic disorders. Neurology, 84, 313–324. [DOI] [PubMed] [Google Scholar]
- Bentley KH, Boettcher H, Bullis JR, Carl JR, Conklin LR, Sauer-Zavala S, … Barlow DH (2017). Development of a single-session, transdiagnostic preventive intervention for young adults at risk for emotional disorders. Behavior Modification, 145445517734354. [DOI] [PubMed] [Google Scholar]
- Berggren N, & Derakshan N (2013). Attentional control deficits in trait anxiety: why you see them and why you don’t. Biological Psychology, 92, 440–446. [DOI] [PubMed] [Google Scholar]
- Berkman ET, & Falk EB (2013). Beyond brain mapping: Using neural measures to predict real-world outcomes. Curr Dir Psychol Sci, 22, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, & Feredoes E (2013). Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Annals of the New York Academy of Sciences, 1296, 11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, & Macdonald JH (1922). The mechanism of the brain: and the function of the frontal lobes: E. & S. Livingstone. [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, … Kalin NH. (2014). Evolutionarily conserved dysfunction of prefrontal‐amygdalar connectivity in early‐life anxiety. Molecular Psychiatry, 19, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ (2008a). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129(1), 141–152. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2008b). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129, 141–152. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12, 92–98. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, & Forster S (2013). Trait anxiety, neuroticism and the brain basis of vulnerability to affective disorder In Armony J & Vuilleumier P (Eds.), Cambridge handbook of human affective neuroscience (pp. 553–574). New York, NY: Cambridge University Press. [Google Scholar]
- Bitsko RH, Holbrook JR, Ghandour RM, Blumberg SJ, Visser SN, Perou R, & Walkup JT (2018). Epidemiology and impact of health care provider-diagnosed anxiety and depression among US children. Journal of Developmental and Behavioral Pediatrics, 39(5), 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, & Pine DS (2012). Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child and Adolescent Psychiatric Clinics, 21(3), 501–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blairy S, Herrera P, & Hess U (1999). Mimicry and the judgment of emotional facial expressions. Journal of Nonverbal behavior, 23(1), 5–41. [Google Scholar]
- Blanchard DC, Griebel G, & Blanchard RJ (2001). Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neuroscience and Biobehavioral Reviews, 25, 205–218. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, & Zeelenberg R (2009). Emotion improves and impairs early vision. Psychological science, 20(6), 707–713. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, & Zeelenberg R (2011a). Emotion-induced trade-offs in spatiotemporal vision. Journal of Experimental Psychology: General, 140(2), 272–282. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, & Zeelenberg R (2011b). Emotional cues enhance the attentional effects on spatial and temporal resolution. Psychon Bull Rev, 18(6), 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WH, & Straube T (2015). Neural correlates of emotional interference in social anxiety disorder. PLoS ONE, 10, e0128608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy A (1995). Fear and fearfulness in animals. Quarterly Review of Biology, 70, 165–191. [DOI] [PubMed] [Google Scholar]
- Boll S, Bartholomaeus M, Peter U, Lupke U, & Gamer M (2016). Attentional mechanisms of social perception are biased in social phobia. Journal of Anxiety Disorders, 40, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkenau P, Riemann R, Angleitner A, & Spinath FM (2001). Genetic and environmental influences on observed personality: evidence from the German Observational Study of Adult Twins. Journal of Personality and Social Psychology, 80, 655–668. [DOI] [PubMed] [Google Scholar]
- Boukhechba M, Chow P, Fua K, Teachman BA, & Barnes LE (2018). Predicting social anxiety from global positioning system traces of college students: feasibility study. JMIR Ment Health, 5, e10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeier K, & Berenbaum H (2013). Cross-sectional and longitudinal relations between working memory performance and worry. Journal of Experimental Psychopathology, 4(4), jep. 032212. [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, & Bar-Haim Y (2015). Neural changes with attention bias modification for anxiety: a randomized trial. Soc Cogn Affect Neurosci, 10, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Savov V, Allzen E, Benedict C, Fredriksson R, & Schioth HB (2012). Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage, 59, 2962–2973. [DOI] [PubMed] [Google Scholar]
- Brunson JA, Øverup CS, & Mehta PD (2016). A social relations examination of neuroticism and emotional support. Journal of Research in Personality, 63, 67–71. [Google Scholar]
- Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, & Pilling S (2018). Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clinical Psychology Review, 64, 13–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Olino TM, Dyson MW, Carlson GA, & Klein DN (2016). Temperament distinguishes persistent/recurrent from remitting anxiety disorders across early childhood. Journal of Clinical Child and Adolescent Psychology, 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Malarstig A, & Thompson SG (2012). Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ, 345, e7325. [DOI] [PubMed] [Google Scholar]
- Burgess S, Timpson NJ, Ebrahim S, & Davey Smith G. (2015). Mendelian randomization: where are we now and where are we going? International Journal of Epidemiology, 44, 379–388. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, … Birn RM (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15, 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM (1991). Conflict in married couples: Personality predictors of anger and upset. Journal of Personality, 59, 663–703. [DOI] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, … Fox NA (2017). A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Ewbank MP, & Passamonti L (2011). Personality influences the neural responses to viewing facial expressions of emotion. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 1684–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, … Lesch KP (2006). Neural correlates of epigenesis. Proceedings of the National Academy of Sciences of the United States of America, 103(43), 16033–16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP (2018). Behavioral inhibition in nonhuman primates: The elephant in the room In Pérez-Edgar K & Fox NA (Eds.), Behavioral inhibition: Integrating theory, research, and clinical perspectives (pp. 17–33). Cham, Switzerland: Springer. [Google Scholar]
- Carpenter KL, Angold A, Chen N-K, Copeland WE, Gaur P, Pelphrey K, … Egger HL (2015). Preschool anxiety disorders predict different patterns of amygdala-prefrontal connectivity at school-age. PLoS ONE, 10(1), e0116854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Briere FN, O’Leary-Barrett M, Banaschewski T, Bokde A, Bromberg U, … Consortium I (2016). The structure of psychopathology in adolescence and its common personality and cognitive correlates. Journal of Abnormal Psychology, 125, 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology, Paris, 109, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, & Wager TD (2015). A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol, 13, e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Becker B, Camilleri J, Wang L, Yu S, Eickhoff SB, & Feng C (2018). A domain-general brain network underlying emotional and cognitive interference processing: evidence from coordinate-based and functional connectivity meta-analyses. Brain Struct Funct, 223, 3813–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, & Kim JJ (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America, 107, 21773–21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow PI, Fua K, Huang Y, Bonelli W, Xiong H, Barnes LE, & Teachman BA (2017). Using mobile sensing to test clinical models of depression, social anxiety, state affect, and social isolation among college students. Journal of Medical Internet Research, 19, e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Rosen AFG, Erus G, Cieslak M, Adebimpe A, Cook PA, … Satterthwaite TD (2018). Mitigating head motion artifact in functional connectivity MRI. Nat Protoc, 13, 2801–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, & Koster EHW (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Durbin CE, Hicks BM, Iacono WG, & McGue M (in press). Personality in the age of industry: Structure, heritability, and correlates of personality in middle childhood from the perspective of parents, teachers, and children. Journal of Research in Personality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, & Reed GM (2017). Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol Sci Public Interest, 18, 72–145. [DOI] [PubMed] [Google Scholar]
- Clarke T-K, Zeng Y, Navrady L, Xia C, Haley C, Campbell A, … McIntosh AM (2018). Genetic and environmental determinants of stressful life events and their overlap with depression and neuroticism. Wellcome Open Research, 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2007). Handbook of emotion elicitation and assessment. NY: Oxford University Press. [Google Scholar]
- Cohen N, Daches S, Mor N, & Henik A (2014). Inhibition of negative content—A shared process in rumination and reappraisal. Front Psychol, 5, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N, Mor N, & Henik A (2015). Linking executive control and emotional response: A training procedure to reduce rumination. Clinical Psychological Science, 3(1), 15–25. [Google Scholar]
- Connelly BS, & Ones DS (2010). An other perspective on personality: meta-analytic integration of observers’ accuracy and predictive validity. Psychological Bulletin, 136, 1092–1122. [DOI] [PubMed] [Google Scholar]
- Connolly JJ, Kavanagh EJ, & Viswesvaran C (2007). The convergent validity between self and observer ratings of personality: A meta-analytic review. International Journal of Selection and Assessment, 15, 110–117. [Google Scholar]
- Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, … Eaton NR (in press). A Hierarchical Taxonomy of Psychopathology can reform mental health research. Perspectives on Psychological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G 3rd, Loggia ML, Greve DN, & Holt DJ (2014). Amygdala perfusion is predicted by its functional connectivity with the ventromedial prefrontal cortex and negative affect. PLoS ONE, 9, e97466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT Jr., & McCrae RR (1988). Personality in adulthood: a six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. Journal of Personality and Social Psychology, 54, 853–863. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, & Fu CH (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58, 57–70. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, … Fernandez G (2010). Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences of the United States of America, 107, 9867–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, & Wittchen HU (2017). Anxiety disorders. Nat Rev Dis Primers, 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credé M, & Niehorster S (2012). Adjustment to college as measured by the student adaptation to college questionnaire: A quantitative review of its structure and relationships with correlates and consequences. Educational Psychology Review, 24, 133–165. [Google Scholar]
- Creed AT, & Funder DC (1998). Social anxiety: from the inside and outside. Personality and Individual Differences, 25, 19–33. [Google Scholar]
- Cristea IA (2018). Author’s reply. Kruijt AW (2018). British Journal of Psychiatry, 212, 248. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, & Cuijpers P (2015). Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. The British Journal of Psychiatry, 206, 7–16. [DOI] [PubMed] [Google Scholar]
- Cromheeke S, & Mueller SC (2014). Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Struct Funct, 219, 995–1008. [DOI] [PubMed] [Google Scholar]
- Crowe SF, Matthews C, & Walkenhorst E (2007). Relationship between worry, anxiety and thought suppression and the components of working memory in a non-clinical sample. Australian Psychologist, 42(3), 170–177. [Google Scholar]
- Dal Monte O, Costa VD, Noble PL, Murray EA, & Averbeck BB (2015). Amygdala lesions in rhesus macaques decrease attention to threat. Nat Commun, 6, 10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian RI, Su R, Shanahan M, Trautwein U, & Roberts BW (2015). Can personality traits and intelligence compensate for background disadvantage? Predicting status attainment in adulthood. Journal of Personality and Social Psychology, 109, 473–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Somerville LH, Ruberry EJ, Berry AB, Shin LM, & Whalen PJ (2011). A tale of two negatives: differential memory modulation by threat-related facial expressions. Emotion, 11, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, … Hotopf M (2018). Mental health in UK Biobank: development, implementation and results from an online questionnaire completed by 157 366 participants. BJPsych Open, 4, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Davis M, Antoniadis EA, Amaral DG, & Winslow JT (2008). Acoustic startle reflex in rhesus monkeys: a review. Reviews in the Neurosciences, 19, 171–185. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, & van Honk J (2014). The role of human basolateral amygdala in ambiguous social threat perception. Cortex, 52, 28–34. [DOI] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EH, Goubert L, Onraedt T, Vanderhasselt M-A, & De Raedt R (2012). Cognitive control moderates the association between stress and rumination. Journal of Behavior Therapy and Experimental Psychiatry, 43(1), 519–525. [DOI] [PubMed] [Google Scholar]
- De Pauw S (2017). Childhood personality and temperament In Widiger TA (Ed.), The Oxford Handbook of the Five Factor Model (pp. 243–280). New York, NY: Oxford University Press. [Google Scholar]
- deBettencourt MT, Cohen JD, Lee RF, Norman KA, & Turk-Browne NB (2015). Closed-loop training of attention with real-time brain imaging. Nature Neuroscience, 18, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissen JJA, Luhmann M, Chung JM, & Bleidorn W (in press). Transactions between life events and personality traits across the adult lifespan. Journal of Personality and Social Psychology. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, & Eysenck MW (2009a). Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. Experimental Psychology, 56(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Derakshan N, & Eysenck MW (2009). Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist, 14(2), 168–176. [Google Scholar]
- Derakshan N, Smyth S, & Eysenck MW (2009b). Effects of state anxiety on performance using a task-switching paradigm: An investigation of attentional control theory. Psychonomic Bulletin & Review, 16(6), 1112–1117. [DOI] [PubMed] [Google Scholar]
- Derryberry D, & Reed MA (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111(2), 225. [DOI] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Doallo S, Costoloni G, Rohenkohl G, Nobre AC, & Harmer CJ (2014). ‘Can you look me in the face?’ Short-term SSRI administration reverts avoidant ocular face exploration in subjects at risk for psychopathology. Neuropsychopharmacology, 39(13), 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLuca M, & Olesen J (2014). The cost of brain diseases: a burden or a challenge? Neuron, 82, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Dimberg U (1988). Facial electromyography and the experience of emotion. Journal of Psychophysiology, 2, 277–282. [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, … Gong Q (2011). Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magnetic Resonance Imaging, 29(5), 701–711. [DOI] [PubMed] [Google Scholar]
- Dubois J, Galdi P, Han Y, Paul LK, & Adolphs R (2018). Resting-state functional brain connectivity best predicts the personality dimension of openness to experience. bioRxiv, 215129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Oya H, Tyszka JM, Howard M 3rd, Eberhardt F, & Adolphs R (in press). Causal mapping of emotion networks in the human brain: Framework and initial findings. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne S, Larose S, Vitaro F, & Tremblay RE (2010). Trajectories of anxiety in a population sample of children: Clarifying the role of children’s behavioral characteristics and maternal parenting. Development and Psychopathology, 22(2), 361–373. [DOI] [PubMed] [Google Scholar]
- Dudeney J, Sharpe L, & Hunt C (2015). Attentional bias towards threatening stimuli in children with anxiety: A meta-analysis. Clinical Psychology Review, 40, 66–75. [DOI] [PubMed] [Google Scholar]
- Duncan J (1986). Disorganisation of behaviour after frontal lobe damage. Cognitive Neuropsychology, 3(3), 271–290. [Google Scholar]
- Dunning JP, Auriemmo A, Castille C, & Hajcak G (2010). In the face of anger: Startle modulation to graded facial expressions. Psychophysiology, 47, 874–878. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, & LaBar KS (2009). Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory, 16(7), 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner-Priemer UW, & Trull TJ (2009). Ecological momentary assessment of mood disorders and mood dysregulation. Psychol Assess, 21(4), 463–475. [DOI] [PubMed] [Google Scholar]
- Eippert F, Gamer M, & Buchel C (2012). Neurobiological mechanisms underlying the blocking effect in aversive learning. Journal of Neuroscience, 32, 13164–13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nature Reviews. Neuroscience(11), 693–700. [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D, Klumpers F, van Wingen G, Tendolkar I, & Fernandez G (2015). Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage, 112, 218–224. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, & Calder AJ (2009). Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage, 44, 1144–1151. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, & Derakshan N (2011). New perspectives in attentional control theory. Personality and Individual Differences, 50(7), 955–960. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007a). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007b). Anxiety and cognitive performance: attentional control theory. Emotion, 7(2), 336. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Markovic M, Tovote P, & Lüthi A (2018). New perspectives on central amygdala function. Current Opinion in Neurobiology, 49, 141–147. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, & Braver TS (2008). Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci, 8, 239–253. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, & Tranel D (2011). The human amygdala and the induction and experience of fear. Current Biology, 21, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, & Tranel D (2016). A tale of survival from the world of Patient S.M In Amaral DG & Adolphs R (Eds.), Living without an amygdala. New York: Guilford. [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, … Wemmie JA (2013). Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience, 16, 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier D (1886). The functions of the brain: Smith, Elder. [Google Scholar]
- Fetvadjiev VH, Meiring D, van de Vijver FJR, Nel JA, & De Kock F (in press). Self–other agreement in personality traits and profiles across cultures: A multirater, multiscale study in Blacks and Whites in South Africa. Journal of Personality. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, & Breakspear M (2015). The connectomics of brain disorders. Nature Rev Neurosci, 16, 159–172. [DOI] [PubMed] [Google Scholar]
- Fox AS, & Kalin NH (2014a). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Kalin NH (2014b). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Lapate RC, Davidson RJ, & Shackman AJ (2018a). The nature of emotion: A research agenda for the 21st century In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion. Fundamental questions (2nd ed, pp. 403–417). New York, NY: Oxford University Press. [Google Scholar]
- Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (2018b). The nature of emotion Fundamental questions (2nd ed). New York, NY: Oxford University Press. [Google Scholar]
- Fox AS, Oler JA, Birn RM, Shackman AJ, Alexander AL, & Kalin NH (2018c). Functional connectivity within the primate extended amygdala is heritable and predicts early-life anxious temperament. Journal of Neuroscience, 38, 7611–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, … Kalin NH (2015a). Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences USA, 112, 9118–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, & Kalin NH (2012). Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proceedings of the National Academy of Sciences of the United States of America, 109, 18108–18113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DP, Fudge JL, & Kalin NH (2015b). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2008). Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE, 3, e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, & Amaral DG (2009). Neuroanatomy of the primate amygdala In Whalen PJ & Phelps EA (Eds.), The human amygdala (pp. 3–42). NY: Guilford. [Google Scholar]
- Freimer NB, & Mohr DC (in press). Integrating behavioural health tracking in human genetics research. Nature Reviews. Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel TI, Fox NA, Pine DS, Walker OL, Degnan KA, & Chronis-Tuscano A (2015). Early childhood behavioral inhibition, adult psychopathology and the buffering effects of adolescent social networks: a twenty-year prospective study. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, & Pérez-Edgar K (2019). Threat-related attention bias in socioemotional development: A critical review and methodological considerations. Developmental Review, 51, 31–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kelly EA, Pal R, Bedont JL, Park L, & Ho B (2017). Beyond the classic VTA: Extended amygdala projections to DA-striatal paths in the primate. Neuropsychopharmacology, 42, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, … Politi P (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, & Luby JL (2016). Amygdala reactivity to sad faces in preschool children: An early neural marker of persistent negative affect. Dev Cogn Neurosci, 17, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir SS, Ge TJ, Vermesh O, & Spitler R (2018). Toward achieving precision health. Sci Transl Med, 10(430). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, & Buchel C (2009). Amygdala activation predicts gaze toward fearful eyes. Journal of Neuroscience, 29, 9123–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Schmitz AK, Tittgemeyer M, & Schilbach L (2013). The human amygdala drives reflexive orienting towards facial features. Current Biology, 23, R917–918. [DOI] [PubMed] [Google Scholar]
- Garner M, Ainsworth B, Gould H, Gardner H, & Baldwin D (2009). P. 4. b. 005 Impaired attentional control in high and low anxious healthy volunteers: evidence from the antisaccade task. European Neuropsychopharmacology, 19, S599. [Google Scholar]
- Gazelle H, & Rudolph KD (2004). Moving toward and away from the world: social approach and avoidance trajectories in anxious solitary youth. Child Development, 75, 829–849. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 201307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW Jr., Charlesworth JC, … Blangero J (2012). High dimensional endophenotype ranking in the search for major depression risk genes. Biological Psychiatry, 71, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Collaborators. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388, 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Kotov R, Perlman G, Watson D, & Klein DN (2018). Trait and facet-level predictors of first-onset depressive and anxiety disorders in a community sample of adolescent girls. Psychological Medicine, 48, 1282–1290. [DOI] [PubMed] [Google Scholar]
- Goodwin AH, & Sher KJ (1992). Deficits in set-shifting ability in nonclinical compulsive checkers. Journal of Psychopathology and Behavioral Assessment, 14(1), 81–92. [Google Scholar]
- Gordon JA, & Redish AD (2016). On the cusp. Current challenges and promises in psychiatry In Redish AD & Gordon JA (Eds.), Computational psychiatry: New perspectives on mental illness (pp. 3–14). Cambridge, MA: MIT Press. [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, & Ernst M (2018). Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage, 168, 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk MG, & Domschke K (2017). Genetics of generalized anxiety disorder and related traits. Dialogues Clin Neurosci, 19, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J (1994). Alternative frameworks for the conceptualization of prefrontal lobe functions. Handbook of neuropsychology, 9(7), 187–199. [Google Scholar]
- Grafton B, MacLeod C, Rudaizky D, Holmes EA, Salemink E, Fox E, & Notebaert L (2017). Confusing procedures with process when appraising the impact of cognitive bias modification on emotional vulnerability. British Journal of Psychiatry, 211, 266–271. [DOI] [PubMed] [Google Scholar]
- Grafton B, MacLeod C, Rudaizky D, Salemink E, Fox E, & Notebaert L (2018). Authors’ reply. British Journal of Psychiatry, 212, 246–247. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, … Amaral DG (2016). The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron, 91, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SM, Trujillo AJ, Glover GH, & Knutson B (2014). Control of nucleus accumbens activity with neurofeedback. Neuroimage, 96, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, & Holmes A (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nature Reviews. Drug Discovery, 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, & Charney DR (2011a). In the face of fear: anxiety sensitizes defensive responses to fearful faces. Psychophysiology, 48, 1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, & Charney DR (2011b). In the face of fear: anxiety sensitizes defensive responses to fearful faces. Psychophysiology, 48(12), 1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, … Tucker-Drob EM (2018). Genomic SEM provides insights into the multivariate genetic architecture of complex traits. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews. Neuroscience, 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guloksuz S, Pries L, & Van Os J (2017). Application of network methods for understanding mental disorders: pitfalls and promise. Psychol Med, 47(16), 2743–2752. [DOI] [PubMed] [Google Scholar]
- Gungor NZ, & Paré D (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36, 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Altamirano LJ, Johnson DP, Whisman MA, & Miyake A (2017). Is set shifting really impaired in trait anxiety? Only when switching away from an effortfully established task set. Emotion, 17, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, & Miyake A (2016). Trait worry is associated with difficulties in working memory updating. Cogn Emot, 30, 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Liu N, Bell AH, Gothard KM, Luh WM, Tootell RB, … Ungerleider LG (2012). Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America, 109, E3640–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwin JA, & Richards HJ (2016). Working memory training and CBT reduces anxiety symptoms and attentional biases to threat: A preliminary study. Front Psychol, 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Raback L, Virtanen M, Kivimaki M, & Jokela M (2015). Personality and depressive symptoms: Individual participant meta-analysis of 10 cohort studies. Depression and Anxiety, 32, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Tolin DF, Assaf M, Goethe J, & Diefenbach GJ (2017). Cognitive control in generalized anxiety disorder: relation of inhibition impairments to worry and anxiety severity. Cognitive Therapy and Research, 41, 610–618. [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry, 169, 693–703. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, … Perez-Edgar K (2013). Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry, 74, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley EL, Stritzke WG, Page AC, Blades CA, & Parentich KT (2018). Neuroticism confers vulnerability in response to experimentally induced feelings of thwarted belongingness and perceived burdensomeness: Implications for suicide risk. Journal of Personality. [DOI] [PubMed] [Google Scholar]
- Hayes JF, Osborn DPJ, Lewis G, Dalman C, & Lundin A (2017). Association of late adolescent personality with risk for subsequent serious mental illness among men in a Swedish nationwide cohort study. JAMA Psychiatry, 74, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A, Mogoase C, Philippot P, & McNally RJ (2015). Attention bias modification for social anxiety: A systematic review and meta-analysis. Clinical Psychology Review, 40, 76–90. [DOI] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58, 222–230. [DOI] [PubMed] [Google Scholar]
- Heller AS, Fox AS, Wing E, Mayer K, Vack NJ, & Davidson RJ (2015). The neurodynamics of affect in the laboratory predicts persistence of real-world emotional responses. Journal of Neuroscience, 35, 10503–10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MP, Ajdacic-Gross V, Wyss C, Angst J, & Rossler W (2016a). Relationship between personality and psychopathology in a longitudinal community study: a test of the predisposition model. Psychological Medicine, 46, 1693–1705. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, Kawohl W, Haker H, Rossler W, & Ajdacic-Gross V (2016b). Big Five personality traits may inform public health policy and preventive medicine: Evidence from a cross-sectional and a prospective longitudinal epidemiologic study in a Swiss community. Journal of Psychosomatic Research, 84, 44–51. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, Tyrer P, Ajdacic-Gross V, Angst J, & Rossler W (2018). Articulation and testing of a personality-centred model of psychopathology: evidence from a longitudinal community study over 30 years. European Archives of Psychiatry and Clinical Neuroscience, 268, 443–454. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Kanen JW, Tambini A, Fernández G, Davachi L, & Phelps EA (2017). Persistence of amygdala–hippocampal connectivity and multi-voxel correlation structures during awake rest after fear learning predicts long-term expression of fear. Cerebral Cortex, 27(5), 3028–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, … Seifritz E (2007). Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience, 27, 5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess U, Sabourin G, & Kleck RE (2007). Postauricular and eyeblink startle responses to facial expressions. Psychophysiology, 44, 431–435. [DOI] [PubMed] [Google Scholar]
- Hettema JM (2008). What is the genetic relationship between anxiety and depression? Am J Med Genet C Semin Med Genet, 148C, 140–146. [DOI] [PubMed] [Google Scholar]
- Hill WD, Arslan RC, Xia C, Luciano M, Amador C, Navarro P, … Penke L (2018). Genomic analysis of family data reveals additional genetic effects on intelligence and personality. Molecular Psychiatry, 23, 2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Weiss A, McIntosh AM, Gale CR, & Deary IJ (2017). Genetic contribution to two factors of neuroticism is associated with affluence, better health, and longer life. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AS, & Roisman GI (2008). Big Five personality traits and relationship quality: Self-reported, observational, and physiological evidence. Journal of Social and Personal Relationships, 25, 811–829. [Google Scholar]
- Hong W, Kennedy A, Burgos-Artizzu XP, Zelikowsky M, Navonne SG, Perona P, & Anderson DJ (2015). Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proceedings of the National Academy of Sciences of the United States of America, 112, E5351–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, … McIntosh AM (in press). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, … McIntosh AM (2018). Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun, 9, 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland M, Armeli S, Feinn R, & Tennen H (2017). Daily emotional stress reactivity in emerging adulthood: temporal stability and its predictors. Anxiety Stress Coping, 30, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, Shafer AT, Baron CA, Seres P, … Malykhin NV (2016). Amygdala subnuclei response and connectivity during emotional processing. Neuroimage, 133, 98–110. [DOI] [PubMed] [Google Scholar]
- Hung Y, Gaillard SL, Yarmak P, & Arsalidou M (2018). Dissociations of cognitive inhibition, response inhibition, and emotional interference: Voxelwise ALE meta-analyses of fMRI studies. Human Brain Mapping, 39, 4065–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Iordan AD, Berenbaum H, & Dolcos F (2016). Emotion–attention interactions in fear conditioning: Moderation by executive load, neuroticism, and awareness. Biological Psychology, 121, 213–220. [DOI] [PubMed] [Google Scholar]
- Hur J, Kaplan CM, Smith JF, Bradford DE, Fox AS, Curtin JJ, & Shackman AJ (2018). Acute alcohol administration dampens central extended amygdala reactivity. Scientific Reports, 8, 16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Miller GA, McDavitt JR, Spielberg JM, Crocker LD, Infantolino ZP, … Heller W (2015). Interactive effects of trait and state affect on top-down control of attention. Social Cognitive and Affective Neuroscience, 10, 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Tillman RM, Fox AS, & Shackman AJ (in press). The value of clinical and translational neuroscience approaches to psychiatric illness. Behavioral and Brain Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, & Hariri AR (2011). Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia, 49, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE (2016). Back to basics: luring industry back into neuroscience. Nature Neuroscience, 19, 1383–1384. [DOI] [PubMed] [Google Scholar]
- Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, & Willie JT (in press). Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan AD, Dolcos S, & Dolcos F (2013). Neural signatures of the response to emotional distraction: a review of evidence from brain imaging investigations. Frontiers in Human Neuroscience, 7, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, & Luna B (2017). Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biological Psychiatry, 82, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimus BF, Kotov R, Riese H, & Ormel J (2016). Neuroticism’s prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychological Medicine, 46(14), 2883–2906. [DOI] [PubMed] [Google Scholar]
- Jocklin V, McGue M, & Lykken DT (1996). Personality and divorce: a genetic analysis. Journal of Personality and Social Psychology, 71, 288–299. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, … Satterthwaite TD (2016a). Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry, 80, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, … Sattherthwaite TD (2016b). Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry, 80, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajonius PJ, & Giolla EM (2017). Personality traits across countries: Support for similarities rather than differences. PlosOne, 12, e0179646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH (2017). Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. European Neuropsychopharmacology, 27, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, … Oler JA (2016). Overexpressing corticotropin-releasing hormone in the primate amygdala increases anxious temperament and alters its neural circuit. Biological Psychiatry, 80, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler C, & Ostendorf F (2016). Additive and synergetic contributions of neuroticism and life events to depression and anxiety in women. European Journal of Personality, 30, 390–405. [Google Scholar]
- Kandler C, Waaktaar T, Mottus R, Riemann R, & Torgensen S (in press). Unravelling the interplay between genetic and environmental contributions in the unfolding of personality differences from early adolescence to young adulthood. European Journal of Personality. [Google Scholar]
- Kann SJ, O’Rawe JF, Huang AS, Klein DN, & Leung H-C (2017). Preschool negative emotionality predicts activity and connectivity of the fusiform face area and amygdala in later childhood. Social Cognitive and Affective Neuroscience, 12, 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning MK, Ebner-Priemer UW, & Schlicht WM (2013). How to Investigate Within-Subject Associations between Physical Activity and Momentary Affective States in Everyday Life: A Position Statement Based on a Literature Overview. Front Psychol, 4, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Magruder KP, & Curtin JJ (2017). Probing for neuroadaptations to unpredictable stressors in addiction: Translational methods and emerging evidence. J Stud Alcohol Drugs, 78, 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Gardner CO (2014). Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. American Journal of Psychiatry, 171, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Aggen S, Heath A, Colodro-Conde L, … Gillespie NA (in press). Shared and specific genetic risk factors for lifetime major depression, depressive symptoms and neuroticism in three population-based twin samples. Psychological Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, & Prescott CA (2004). The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. American Journal of Psychiatry, 161, 631–636. [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Myers J (2010). The genetic and environmental relationship between major depression and the five-factor model of personality. Psychological Medicine, 40, 801–806. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, & Ustun TB (2007). Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry, 20, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res, 21, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, & Hurlemann R (2016). Panic anxiety in humans with bilateral amygdala lesions: Pharmacological induction via cardiorespiratory interoceptive pathways. Journal of Neuroscience, 36, 3559–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel EJ, & Buss KA (2011). Toddlers’ duration of attention toward putative threat. Infancy, 16, 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2010). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 21(7), 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 7, 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, … Sohal VS (2018). An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell, 175, 1688–1700. [DOI] [PubMed] [Google Scholar]
- Kirlic N, Aupperle RL, Rhudy JL, Misaki M, Kuplicki R, Sutton A, & Alvarez RP (2019). Latent variable analysis of negative affect and its contributions to neural responses during shock anticipation. Neuropsychopharmacology, 44, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, & Mumper EE (2018). Behavioral inhibition as a precursor to psychopathology In Pérez-Edgar K & Fox NA (Eds.), Behavioral inhibition: Integrating theory, research, and clinical perspectives (pp. 283–307). Cham, Switzerland: Springer. [Google Scholar]
- Klimstra TA, Noftle EE, Luyckx K, Goossens L, & Robins RW (2018). Personality development and adjustment in college: A multifaceted, cross-national view. Journal of Personality and Social Psychology, 115, 338–361. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Morgan B, Terburg D, Stein DJ, & van Honk J (2015). Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Social Cognitive and Affective Neuroscience, 10, 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT (1984). Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 59(1), 9–20. [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Danzig AP, Kotov R, Bromet EJ, Carlson GA, Olino TM, … Klein DN (2016a). Negative emotionality and its facets moderate the effects of exposure to hurricane Sandy on children’s postdisaster depression and anxiety symptoms. Journal of Abnormal Psychology, 125, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Kotov R, Bromet EJ, Carlson GA, Danzig AP, Black SR, & Klein DN (2016b). Personality diatheses and Hurricane Sandy: effects on post-disaster depression. Psychological Medicine, 46, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Vunder J, Miró J, Fuentemilla L, Hurlemann R, & Bach DR (2017). Amygdala lesions reduce anxiety-like behavior in a human benzodiazepine-sensitive approach-avoidance conflict test. Biological Psychiatry, 82, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornadt AE, Hagemeyer B, Neyer FJ, & Kandler C (2018). Sound body, sound mind? The interrelation between health change and personality change in old age. European Journal of Personality, 32, 30–45. [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH research domain criteria initiative: Background, issues, and pragmatics. Psychophysiology, 53, 286–297. [DOI] [PubMed] [Google Scholar]
- Kragel PA, Koban L, Barrett LF, & Wager TD (2018). Representation, pattern information, and brain signatures: From neurons to neuroimaging. Neuron, 99, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MCW, Dunsmoor JE, Mackey WE, McClay M, & Phelps EA (2017). Context conditioning in humans using commercially available immersive Virtual Reality. Sci Rep, 7, 8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug MK, & Carter CS (2010). Adding fear to conflict: A general purpose cognitive control network is modulated by trait anxiety. Cognitive, Affective & Behavioral Neuroscience, 10, 357–371. [DOI] [PubMed] [Google Scholar]
- Kruijt A-W, Parsons SJ, & Fox E (2018). No evidence for attention bias towards threat in clinical anxiety: a meta-analysis of baseline bias in attention bias modification RCTs-preprint. PsyArXiv. [DOI] [PubMed] [Google Scholar]
- Kruijt AW (2018). British Journal of Psychiatry, 212, 246. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Puher MA, & Cross NA (2012). (2012) Prospective prediction of college adjustment using self- and informant-rated personality traits. Journal of Personality Assessment, 94, 630–637. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin, 143, 142–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapate RC, & Shackman AJ (2018). What is an emotion? In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion. Fundamental questions (2nd ed, pp. 38–43). New York, NY: Oxford University Press. [Google Scholar]
- Lazarov A, Abend R, & Bar-Haim Y (2016). Social anxiety is related to increased dwell time on socially threatening faces. Journal of Affective Disorders, 193, 282–288. [DOI] [PubMed] [Google Scholar]
- Lazarov A, Suarez-Jimenez B, Tamman A, Falzon L, Zhu X, Edmondson DE, & Neria Y (in press). Attention to threat in posttraumatic stress disorder as indexed by eye-tracking indices: a systematic review. Psychological Medicine, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2015). Anxious. Using the brain to understand and treat fear and anxiety. New York, NY: Viking. [Google Scholar]
- LeDoux JE, & Hofmann SG (2018). The subjective experience of emotion: a fearful view. Current Opinion in Behavioral Sciences, 19, 1–6. [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, & Casey BJ (2014). Mental health. Adolescent mental health--opportunity and obligation. Science, 346, 547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, … Smoller JW (2019). Genome wide meta-analysis identifies genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. bioRxiv, 528117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey DF, Gelernter J, Polimanti R, Zhou H, Cheng Z, Aslan M, … Stein M (2019). Reproducible risk loci and psychiatric comorbidities in anxiety: Results from ~200,000 Million Veteran Program participants. bioRxiv, 540245. [Google Scholar]
- Li JJ, Hilton EC, Lu Q, Hong J, Greenberg JS, & Mailick MR (2018). Validating psychosocial pathways of risk between neuroticism and late life depression using a polygenic score approach. Open Science Framework. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang G, Li Z, Qi Y, Cole MW, & Liu X (2017). Conflict detection and resolution rely on a combination of common and distinct cognitive control networks. Neuroscience and Biobehavioral Reviews, 83, 123–131. [DOI] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, … Chen H (2010). Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS ONE, 5(12), e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, Padmala S, & Pessoa L (2009). Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proceedings of the National Academy of Sciences of the United States of America, 106, 16841–16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Barrett LF (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, & Bar-Haim Y (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety, 32, 383–391. [DOI] [PubMed] [Google Scholar]
- Liu P, Taber-Thomas BC, Fu X, & Perez-Edgar KE (2018). Biobehavioral markers of attention bias modification in temperamental risk for anxiety: A randomized control trial. Journal of the American Academy of Child and Adolescent Psychiatry, 57, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MT, Hinds DA, Tung JY, Franz C, Fan CC, Wang Y, … Chen CH (2017). Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nature Genetics, 49, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008). What we can do and what we cannot do with fMRI. Nature, 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, & Vasey MW (2009). Negative affectivity, effortful control, and attention to threat-relevant stimuli. Journal of Abnormal Child Psychology, 37(3), 387–399. [DOI] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, & Heatherton TF (2014). Neural predictors of giving in to temptation in daily life. Psychol Sci, 25(7), 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, DiBerto J, Mazzone CM, & Kash TL (2018). GABA neurons of the ventral periaqueductal gray area modulate behaviors associated with anxiety and conditioned fear. Brain Struct Funct, 223, 3787–3799. [DOI] [PubMed] [Google Scholar]
- Luan Z, Poorthuis AMG, Hutteman R, Denissen JJA, Asendorpf JB, & van Aken MAG (in press). Unique predictive power of other‐rated personality: An 18‐year longitudinal study. Journal of Personality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Hagenaars SP, Davies G, Hill WD, Clarke T-K, Shirali M, … Deary IJ (2018). Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nature Genetics, 50, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Albanese BJ, Schmidt NB, & Cougle JR (2017). Attention bias towards negative emotional information and its relationship with daily worry in the context of acute stress: An eye-tracking study. Behaviour Research and Therapy, 90, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie LE, Uher R, & Pavlova B (in press). Cognitive performance in first-degree relatives of individuals with vs without major depressive disorder: A meta-analysis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C (in press). Anxiety-linked attentional bias: backward glances and future glimpses. Cogn Emot [DOI] [PubMed] [Google Scholar]
- MacLeod C, & Clarke PJF (2015). The attentional bias modification approach to anxiety intervention. Clinical Psychological Science, 3, 58–78. [Google Scholar]
- MacLeod C, & Grafton B (2016). Anxiety-linked attentional bias and its modification: Illustrating the importance of distinguishing processes and procedures in experimental psychopathology research. Behaviour Research and Therapy, 86, 68–86. [DOI] [PubMed] [Google Scholar]
- Månsson KN, Salami A, Frick A, Carlbring P, Andersson G, Furmark T, & Boraxbekk CJ (2016). Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry, 6, e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic A, & Bowker JC (2017). Friends also matter: Examining friendship adjustment indices as moderators of anxious-withdrawal and trajectories of change in psychological maladjustment. Developmental Psychology, 53(8), 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, & Kleck RE (2005). The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion, 5, 119–124. [DOI] [PubMed] [Google Scholar]
- Matthews T, Danese A, Caspi A, Fisher HL, Goldman-Mellor S, Kepa A, … Arseneault L (in press). Lonely young adults in modern Britain: findings from an epidemiological cohort study. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, & Costa PT Jr. (1987). Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology, 52, 81–90. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Terracciano A, & Personality Profiles of Cultures, P (2005). Universal features of personality traits from the observer’s perspective: data from 50 cultures. Journal of Personality and Social Psychology, 88, 547–561. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Gerin MI, & Viding E (2017). Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58, 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Purcell R, Goldstone S, & Amminger GP (2011). Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Curr Opin Psychiatry, 24, 301–306. [DOI] [PubMed] [Google Scholar]
- McKenna R, Rushe T, & Woodcock KA (2017). Informing the structure of executive function in children: A meta-analysis of functional neuroimaging data. Front Hum Neurosci, 11, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety, 31, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, & Pessoa L (2014). Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience, 34, 11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ (in press). Attentional bias for threat: Crisis or opportunity? Clinical Psychology Review. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Goodkind MS, & Etkin A (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of Psychiatric Research, 83, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, & Etkin A (2017). Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. American Journal of Psychiatry, 174, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Trontti K, Als TD, Laine M, Pedersen MG, Bybjerg-Grauholm J, … Mors O (2018). Genome-wide Association Study of Anxiety and Stress-related Disorders in the iPSYCH Cohort. bioRxiv, 263855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Bértolo C, Moratti S, Toledano R, Lopez-Sosa F, Martinez-Alvarez R, Mah YH, … Strange BA (2016). A fast pathway for fear in human amygdala. Nature Neuroscience, 19, 1041–1049. [DOI] [PubMed] [Google Scholar]
- Milojev P, Osbourne D, & Sibley CG (2014). Personality resilience following a natural disaster. Social Psychological and Personality Science, 5, 760–768. [Google Scholar]
- Minxha J, Mosher C, Morrow JK, Mamelak AN, Adolphs R, Gothard KM, & Rutishauser U (2017). Fixations gate species-specific responses to free viewing of faces in the human and macaque amygdala. Cell Rep, 18, 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Mobbs D, & Kim JJ (2015). Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Current Opinion in Behavioral Sciences, 5, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (2016a). Anxiety and attention to threat: Cognitive mechanisms and treatment with attention bias modification. Behaviour Research and Therapy, 87, 76–108. [DOI] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (2016b). Anxiety and attention to threat: Cognitive mechanisms and treatment with attention bias modification. Behaviour Research and Therapy, 87, 76–108. [DOI] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (2018). Anxiety and threat-related attention: Cognitive-motivational framework and treatment. Trends Cogn Sci, 22, 225–240. [DOI] [PubMed] [Google Scholar]
- Mogg K, Waters AM, & Bradley BP (2017a). Attention bias modification (ABM): Review of effects of multisession ABM training on anxiety and threat-related attention in high-anxious individuals. Clin Psychol Sci, 5, 698–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Waters AM, & Bradley BP (2017b). Attention bias modification (ABM): Review of effects of multisession ABM training on anxiety and threat-related attention in high-anxious individuals. Clinical Psychological Science, 5(4), 698–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Zhang M, & Schueller SM (2017). Personal sensing: Understanding mental health using ubiquitous sensors and machine learning. Annual Review of Clinical Psychology, 13, 23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, … Ernst M (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry, 163(6), 1091–1097. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, … Pine DS (2008a). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, … Pine DS (2008b). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65, 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychological Bulletin, 142, 831–864. [DOI] [PubMed] [Google Scholar]
- Mõttus R, McCrae RR, Allik J, & Realo A (2014). Cross-rater agreement on common and specific variance of personality scales and items. Journal of Research in Personality, 52, 47–54. [Google Scholar]
- Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, & Koenigs M (2015a). Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex, 64, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2014). Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. Journal of Neuroscience, 34(31), 10430–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015b). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wagner J, Smith J, Voelkle MC, & Gerstorf D (in press). The interplay of personality and functional health in old and very old age: Dynamic within-person interrelations across up to 13 years. Journal of Personality and Social Psychology. [DOI] [PubMed] [Google Scholar]
- Muris P, de Jong PJ, & Engelen S (2004). Relationships between neuroticism, attentional control, and anxiety disorders symptoms in non-clinical children. Personality and Individual Differences, 37(4), 789–797. [Google Scholar]
- Muris P, van der Pennen E, Sigmond R, & Mayer B (2008). Symptoms of anxiety, depression, and aggression in non-clinical children: Relationships with self-report and performance-based measures of attention and effortful control. Child Psychiatry and Human Development, 39(4), 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz F, Knight LK, & Depue BE (in press). Explicit and ambiguous threat processing: Functionally dissociable roles of the amygdala and bed nucleus of the stria terminalis. Journal of Cognitive Neuroscience, 1–17. [DOI] [PubMed] [Google Scholar]
- Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, … Posthuma D (2018a). Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nature Genetics, 50, 920–927. [DOI] [PubMed] [Google Scholar]
- Nagel M, Watanabe K, Stringer S, Posthuma D, & van der Sluis S (2018b). Item-level analyses reveal genetic heterogeneity in neuroticism. Nat Commun, 9, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim R, Abend R, Wald I, Eldar S, Levi O, Fruchter E, … Bar-Haim Y (2015). Threat-related attention bias variability and posttraumatic stress. American Journal of Psychiatry, appiajp201514121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navrady LB, Adams MJ, Chan SWY, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Ritchie SJ., & McIntosh AM. (2018). Genetic risk of major depressive disorder: the moderating and mediating effects of neuroticism and psychological resilience on clinical and self-reported depression. Psychological Medicine, 48, 1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navrady LB, Ritchie SJ, Chan SWY, Kerr DM, Adams MJ, Hawkins EH, … McIntosh AM. (2017). Intelligence and neuroticism in relation to depression and psychological distress: Evidence from two large population cohorts. Eur Psychiatry, 43, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Howes G, Horwood J, & Mulder R (2015). Personality characteristics in childhood and outcomes in adulthood: findings from a 30 year longitudinal study. Australian and New Zealand Journal of Psychiatry, 49, 377–386. [DOI] [PubMed] [Google Scholar]
- Nicol JR, Perrotta S, Caliciuri S, & Wachowiak MP (2013). Emotion-specific modulation of early visual perception. Cogn Emot, 27, 1478–1485. [DOI] [PubMed] [Google Scholar]
- Notebaert L, Georgiades JV, Herbert M, Grafton B, Parsons S, Fox E, & MacLeod C (in press). Trait anxiety and the alignment of attentional bias with controllability of danger. Psychological Research. [DOI] [PubMed] [Google Scholar]
- Notebaert L, Grafton B, Clarke PJF, Rudaizky D, Chen NTM, & MacLeod C (2018). Emotion-in-Motion, a novel approach for the modification of attentional bias: An experimental proof-of-concept s`tudy. JMIR Serious Games, 6, e10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notebaert L, Tilbrook M, Clarke PJF, & MacLeod C (2017). When a bad bias can be good: Anxiety-linked attentional bias to threat in contexts where dangers can be avoided. Clinical Psychological Science, 5, 485–496. [Google Scholar]
- Noudoost B, Albarran E, & Moore T (2014). Neural signatures, circuitry, and modulators of selective attention In Gazzaniga MS & Mangun GR (Eds.), The cognitive neurosciences (5th ed., pp. 233–243). Cambridge, MA: MIT Press. [Google Scholar]
- Ogle CM, Rubin DC, & Siegler IC (2014). Changes in neuroticism following trauma exposure. Journal of Personality, 82, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, … Cesarini D (2016). Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics, 48, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, & Shackman AJ (2015). The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olderbak S, Hildebrandt A, Pinkpank T, Sommer W, & Wilhelm O (2014). Psychometric challenges and proposed solutions when scoring facial emotion expression codes. Behav Res Methods, 46, 992–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shackman AJ, & Kalin NH (2016). The central nucleus of the amygdala is a critical substrate for individual differences in anxiety In Amaral DG & Adolphs R (Eds.), Living without an amygdala (pp. 218–251). NY: Guilford. [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, … Kalin NH (2009). Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. Journal of Neuroscience, 29, 9961–9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnis R, Dadds MR, & Bryant RA (2011). Is there a mutual relationship between opposite attentional biases underlying anxiety? Emotion, 11, 582–594. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, & Todorov A (2008). The functional basis of face evaluation. Proceedings of the National Academy of Sciences of the United States of America, 105, 11087–11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof NN, & Todorov A (2009). Shared perceptual basis of emotional expressions and trustworthiness impressions from faces. Emotion, 9, 128–133. [DOI] [PubMed] [Google Scholar]
- Orem DM, Petrac DC, & Bedwell JS (2008). Chronic self-perceived stress and set-shifting performance in undergraduate students. Stress, 11(1), 73–78. [DOI] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, … Oldehinkel AJ (2013). Neuroticism and common mental disorders: meaning and utility of a complex relationship. Clinical Psychology Review, 33, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinsky R, Gebhardt H, Alexander N, & Hennig J (2012). Trait anxiety and the dynamics of attentional control. Biological Psychology, 89(1), 252–259. [DOI] [PubMed] [Google Scholar]
- Overstreet C, Berenz EC, Kendler KS, Dick DM, & Amstadter AB (2017). Predictors and mental health outcomes of potentially traumatic event exposure. Psychiatry Research, 247, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace VL, & Brannick MT (2010). How similar are personality scales of the “same” construct. Personality and Individual Differences, 49, 669–676. [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, & Lupiáñez J (2010). Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science, 21(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Lupiáñez J, & Acosta A (2009). Attention and anxiety: relationship between alertness and cognitive control with trait anxiety. Psicologica, 30(1), 1–25. [Google Scholar]
- Pankevich DE, Altevogt BM, Dunlop J, Gage FH, & Hyman SE (2014). Improving and accelerating drug development for nervous system disorders. Neuron, 84, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R (1993). The Frontal Lobes and Voluntary Action. Oxford Psychology Series. [Google Scholar]
- Paulus FW, Backes A, Sander CS, Weber M, & von Gontard A (2015). Anxiety disorders and behavioral inhibition in preschool children: a population-based study. Child Psychiatry and Human Development, 46, 150–157. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, & Stein MB (2005). Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry, 62, 282–288. [DOI] [PubMed] [Google Scholar]
- Peck EL, Peck CJ, & Salzman CD (2014). Task-dependent spatial selectivity in the primate amygdala. Journal of Neuroscience, 34, 16220–16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, & Fox NA (2010a). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion, 10, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, McDermott JN, Korelitz K, Degnan KA, Curby TW, Pine DS, & Fox NA (2010b). Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology, 46, 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Morales S, LoBue V, Taber-Thomas BC, Allen EK, Brown KM, & Buss KA (2017). The impact of negative affect on attention patterns to threat across the first 2 years of life. Developmental Psychology, 53(12), 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, … Fox NA (2011). Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology, 39(6), 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Taber-Thomas B, Auday E, & Morales S (2014). Temperament and attention as core mechanisms in the early emergence of anxiety In Children and Emotion (Vol. 26, pp. 42–56): Karger Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Morris JP, Vander Wyk BC, Green SR, Doyle JL, & Pelphrey KA (2009). Individual differences in personality predict how people look at faces. PLoS ONE, 4, e5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2013). The cognitive-emotional brain: From interactions to integration. Cambridge, MA: MIT Press. [Google Scholar]
- Pew Research Center (Producer). (2018, Janury 21, 2019). Mobile fact sheet. Retrieved from http://www.pewinternet.org/fact-sheet/mobile/
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS,. Weinberger DR (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience, 8, 828–834. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, & Carrasco M (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological science, 17(4), 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard RW (2018). How are emotions physically embodied? In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion. Fundamental questions. (pp. 287–291). New York, NY: Oxford University Press. [Google Scholar]
- Pichon S, Miendlarzewska EA, Eryilmaz H, & Vuilleumier P (2015). Cumulative activation during positive and negative events and state anxiety predicts subsequent inertia of amygdala reactivity. Soc Cogn Affect Neurosci, 10, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, & Beyeler A (2018). Valence coding in amygdala circuits. Current Opinion in Behavioral Sciences, 26, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, & LeDoux JE (2017). Elevating the role of subjective experience in the clinic: Response to Fanselow and Pennington. American Journal of Psychiatry, 174, 1121–1122. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, & Tamminga C (2015). Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. American Journal of Psychiatry, 172, 784–792. [DOI] [PubMed] [Google Scholar]
- Pliota P, Bohm V, Grossl F, Griessner J, Valenti O, Kraitsy K, … Haubensak W (in press). Stress peptides sensitize fear circuitry to promote passive coping. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, & Olff M (2012). The role of executive function in posttraumatic stress disorder: a systematic review. Journal of Affective Disorders, 141, 11–21. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, … Messing RO (2019). A corticotropin releasing factor network in the extended amygdala for anxiety. Journal of Neuroscience, 39, 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Druss BG, Manderscheid RW, & Walker ER (2016). Excess mortality due to depression and anxiety in the United States: results from a nationally representative survey. General Hospital Psychiatry, 39, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R, Eldreth D, & Mohlman J (2011). Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry, 1(10), e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, … Siegle GJ (2016a). Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev Cogn Neurosci, 19, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Cummings L, Gilchrist D, Graur S, Banihashemi L, Kuo SS, & Siegle GJ (2018). Towards personalized, brain-based behavioral intervention for transdiagnostic anxiety: Transient neural responses to negative images predict outcomes following a targeted computer-based intervention. Journal of Consulting and Clinical Psychology, 86, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, … Amir N (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychol Assess, 27, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, … Bar-Haim Y (2016b). Pooled patient-level meta-analysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clinical Psychology Review, 50, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves KL, Coleman JRI, Rayner C, Hettema JM, Deckert J, McIntosh AM, … Eley TC (2017). The common genetic architecture of anxiety disorders. bioRxiv, 203844. [Google Scholar]
- Qi C, Roseboom PH, Nanda SA, Lane JC, Speers JM, & Kalin NH (2010). Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes Brain Behav, 9, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, & Menon V (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological Psychiatry, 75, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, & Bayer JK (2018). Behavioural inhibition and the prevention of internalising distress in early childhood In Pérez-Edgar K & Fox NA (Eds.), Behavioral inhibition: Integrating theory, research, and clinical perspectives (pp. 337–355). Cham, Switzerland: Springer. [Google Scholar]
- Reddan MC, Wager TD, & Schiller D (2018). Attenuating neural threat expression with imagination. Neuron, 100, 994–1005 e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, & Woodman GF (2014). Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. Journal of Neuroscience, 34(12), 4214–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Vangkilde SA, Bradley BP, & Esbjørn BH (2015). Attention control and attention to emotional stimuli in anxious children before and after cognitive behavioral therapy. Cognitive Therapy and Research, 39(6), 785–796. [Google Scholar]
- Richards HJ, Benson V, Donnelly N, & Hadwin JA (2014). Exploring the function of selective attention and hypervigilance for threat in anxiety. Clinical Psychology Review, 34, 1–13. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Luo J, Briley DA, Chow PI, Su R, & Hill PL (2017). A systematic review of personality trait change through intervention. Psychological Bulletin, 143, 117–141. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, & Grillon C (2013). The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci, 7, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, … Lenze EJ (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology, 125, 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig C (2016). Mental disorders top the list of the most costly conditions in the United States: $201 billion. Health Affairs, 35, 1130–1135. [DOI] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, … Kalin NH (2013). CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Molecular Psychiatry, 18, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, … Kalin NH (2014). Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biological Psychiatry, 76, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshtein P, Richardson MP, Winston JS, Kiebel SJ, Vuilleumier P, Eimer M, … Dolan RJ (2010). Amygdala damage affects event-related potentials for fearful faces at specific time windows. Human Brain Mapping, 31, 1089–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A (2002). Childhood trauma and neuroticism as an adult: possible implication for the development of the common psychiatric disorders and suicidal behaviour. Psychological Medicine, 32, 1471–1474. [DOI] [PubMed] [Google Scholar]
- Roy AK, Dennis TA, & Warner CM (2015). A critical review of attentional threat bias and its role in the treatment of pediatric anxiety disorders. Journal of Cognitive Psychotherapy, 29, 171–184. [DOI] [PubMed] [Google Scholar]
- Roysamb E, Nes RB, Czajkowski NO, & Vassend O (2018). Genetics, personality and wellbeing. A twin study of traits, facets and life satisfaction. Sci Rep, 8, 12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaizky D, Basanovic J, & MacLeod C (2014). Biased attentional engagement with, and disengagement from, negative information: independent cognitive pathways to anxiety vulnerability? Cogn Emot, 28, 245–259. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, … Jeffries J (2011). Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage, 54, 2524–2533. [DOI] [PubMed] [Google Scholar]
- Saeb S, Lattie EG, Schueller SM, Kording KP, & Mohr DC (2016). The relationship between mobile phone location sensor data and depressive symptom severity. PeerJ, 4, e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, … Vos T (2015). Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health, 3, e712–723. [DOI] [PubMed] [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, … Posthuma D (2018). Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nature Genetics, 50(7), 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JE, Sawyers C, Roberson-Nay R, & Hettema JM (2017). The genetics of anxiety-related negative valence system traits. Am J Med Genet B Neuropsychiatr Genet, 174, 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalet BD, Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam JD, & Shelton RC (2016). Specific pharmacological effects of paroxetine comprise psychological but not somatic symptoms of depression. PLoS ONE, 11, e0159647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller E, Buchel C, & Gamer M (2012). Diagnostic features of emotional expressions are processed preferentially. PLoS ONE, 7, e41792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt DP, Allik J, McCrae RR, & Benet-Martinez V (2007). The geographic distribution of Big Five personality traits: Patterns and profiles of human self-description across 56 nations. J Cross Cult Psychol, 38, 173–212. [Google Scholar]
- Schnyer DM, Beevers CG, deBettencourt MT, Sherman SM, Cohen JD, Norman KA, & Turk-Browne NB (2015). Neurocognitive therapeutics: from concept to application in the treatment of negative attention bias. Biol Mood Anxiety Disord, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, … Schweinsburg BC (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141, 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeboth A, & Mottus R (2018). Successful explanations start with accurate descriptions: Questionnaire items as personality markers for more accurate predictions. European Journal of Personality, 32, 186–201. [Google Scholar]
- Sergerie K, Chochol C, & Armony JL (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32, 811–830. [DOI] [PubMed] [Google Scholar]
- Serrat R, Villar F, Pratt MW, & Stukas AA (2018). On the quality of adjustment to retirement: The longitudinal role of personality traits and generativity. Journal of Personality, 86, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Geerligs L, Renken RJ, Marsman JB, Ormel J, Riese H, & Aleman A (2014). Connectomics and neuroticism: An altered functional network organization. Neuropsychopharmacology, 40, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016a). Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36, 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016b). Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36, 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2018). Getting serious about variation: Lessons for clinical neuroscience. Trends in Cognitive Sciences, 22, 368–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, & Kalin NH (2013). Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America, 110, 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2017). Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Molecular Psychiatry, 22, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, & Seminowicz DA (2015). The cognitive-emotional brain: Opportunities and challenges for understanding neuropsychiatric disorders. Behavioral and Brain Sciences, 38, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Kaplan CM, Stockbridge MD, Tillman RM, Tromp DPM, Fox AS, & Gamer M (2016a). The neurobiology of anxiety and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology, 7, 311–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Maxwell JS, McMenamin BW, Greischar LL, & Davidson RJ (2011a). Stress potentiates early and attenuates late stages of visual processing. Journal of Neuroscience, 31, 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011b). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience, 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, & Davidson RJ (2006). Anxiety selectively disrupts visuospatial working memory. Emotion, 6, 40–61. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Stockbridge MD, LeMay EP, & Fox AS (2018a). The psychological and neurobiological bases of dispositional negativity In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion. Fundamental questions (2nd ed., pp. 67–71). New York, NY: Oxford University Press. [Google Scholar]
- Shackman AJ, Stockbridge MD, Tillman RM, Kaplan CM, Tromp DP, Fox AS, & Gamer M (2016b). The neurobiology of dispositional negativity and attentional biases to threat: implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology, 7(3), 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016c). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142, 1275–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Wager TD (2019). The emotional brain: Fundamental questions and strategies for future research. Neuroscience Letters, 693, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Weinstein JS, Hudja SN, Bloomer CD, Barstead MG, Fox AS, & Lemay EP (2018b). Dispositional negativity in the wild: Social environment governs momentary emotional experience. Emotion, 18, 707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, & Wilson MA (2016). Ethanol-induced anxiolysis and neuronal activation in the amygdala and bed nucleus of the stria terminalis. Alcohol, 50, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Luria R, Fukuda K, & Gross JJ (2013). There’s more to anxiety than meets the eye: Isolating threat-related attentional engagement and disengagement biases. Emotion, 13, 520–528. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, … Krangel TS (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62(3), 273–281. [DOI] [PubMed] [Google Scholar]
- Shiner RL (2018). Personality as lasting individual differences in emotions In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion. Fundamental questions. (2nd ed., pp. 61–64). NY: Oxford University Press. [Google Scholar]
- Shiner RL, Allen TA, & Masten AS (2017). Adversity in adolescence predicts personality trait change from childhood to adulthood. Journal of Research in Personality, 67, 171–182. [Google Scholar]
- Silvers JA, Goff B, Gabard-Durnam LJ, Gee DG, Fareri DS, Caldera C, & Tottenham N (2017). Vigilance, the amygdala, and anxiety in youths with a history of institutional care. Biol Psychiatry Cogn Neurosci Neuroimaging, 2, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjouwerman R, Scharfenort R, & Lonsdorf TB (2017). Individual differences in fear learning: Specificity to trait-anxiety beyond other measures of negative affect, and mediation via amygdala activation. BioRxiv. [Google Scholar]
- Smith DJ, Escott-Price V, Davies G, Bailey ME, Colodro-Conde L, Ward J, … O’Donovan MC (2016). Genome-wide analysis of over 106 000 individuals identifies 9 neuroticism-associated loci. Molecular Psychiatry, 21, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD (2010). Mendelian randomization for strengthening causal inference in observational studies: Application to gene x environment interactions. Perspect Psychol Sci, 5, 527–545. [DOI] [PubMed] [Google Scholar]
- Smith GD, & Ebrahim S (2005). What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? British Medical Journal, 330, 1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Ebrahim S, Lewis S, Hansell AL, Palmer LJ, & Burton PR (2005a). Genetic epidemiology and public health: hope, hype, and future prospects. Lancet, 366, 1484–1498. [DOI] [PubMed] [Google Scholar]
- Smith ML, Cottrell GW, Gosselin F, & Schyns PG (2005b). Transmitting and decoding facial expressions. Psychol Sci, 16, 184–189. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Whisman MA, Turner AE, Guild RM, & Munakata Y (2014). Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. Cognition & Emotion, 28(5), 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol, 6, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solhan MB, Trull TJ, Jahng S, & Wood PK (2009). Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychol Assess, 21(3), 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Zilverstand A, Song H, d’Oleire Uquillas F, Wang Y, Xie C, … Zou Z (2017). The influence of emotional interference on cognitive control: A meta-analysis of neuroimaging studies using the emotional Stroop task. Sci Rep, 7, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CJ (in press). How replicable are links between personality traits and consequential life outcomes? The life outcomes of personality replication project. Psychological Science. [DOI] [PubMed] [Google Scholar]
- Soto CJ, & John OP (2014). Traits in transition: the structure of parent-reported personality traits from early childhood to early adulthood. Journal of Personality, 82, 182–199. [DOI] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, & Potter J (2011). Age differences in personality traits from 10 to 65: Big Five domains and facets in a large cross-sectional sample. Journal of Personality and Social Psychology, 100(2), 330–348. [DOI] [PubMed] [Google Scholar]
- Speed D, Hemani G, Speed MS, Boerglum AD, & Oestergaard SD (2018). Does neuroticism cause depression? A mendelian randomization study. bioRxiv. [Google Scholar]
- Spezio ML, Huang PY, Castelli F, & Adolphs R (2007). Amygdala damage impairs eye contact during conversations with real people. Journal of Neuroscience, 27, 3994–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, Batelaan N, Rhebergen D, van Balkom A, Schoevers R, & Penninx BW (2016). Prediction of 6-yr symptom course trajectories of anxiety disorders by diagnostic, clinical and psychological variables. Journal of Anxiety Disorders, 44, 92–101. [DOI] [PubMed] [Google Scholar]
- Springer US, Rosas A, McGetrick J, & Bowers D (2007a). Differences in startle reactivity during the perception of angry and fearful faces. Emotion, 7(3), 516. [DOI] [PubMed] [Google Scholar]
- Springer US, Rosas A, McGetrick J, & Bowers D (2007b). Differences in startle reactivity during the perception of angry and fearful faces. Emotion, 7, 516–525. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Elison JT, Dufour N, Hurlemann R, Saxe R, & Adolphs R (2015). Amygdala lesions do not compromise the cortical network for false-belief reasoning. Proceedings of the National Academy of Sciences of the United States of America, 112, 4827–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanopoulou E, Hirsch CR, Hayes S, Adlam A, & Coker S (2014). Are attentional control resources reduced by worry in generalized anxiety disorder? Journal of Abnormal Psychology, 123, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer K, Strik W, Federspiel A, Wiest R, Bohlhalter S, & Walther S (2017). Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophrenia Research, 190, 96–101. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, & Brown GG (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry, 59(11), 1027–1034. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, … Ressler KJ (2017). Amygdala reactivity and anterior cingulate habituation predict Posttraumatic Stress Disorder symptom maintenance after acute civilian trauma. Biological Psychiatry, 81, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone JA, Buck Louis G. M., Nakayama SF, Vermeulen RC, Kwok RK, Cui Y, … Teitelbaum SL (2017). Toward greater implementation of the exposome research paradigm within environmental epidemiology. Annual Review of Public Health, 38, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, Gilman JM, … Evins AE (2014). Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuroimage Clin, 5, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz C, Endres D, & Mueller EM (in press). Threat-conditioned contexts modulate the late positive potential to faces-A mobile EEG/virtual reality study. Psychophysiology, e13308. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Atienza AA, & Nebeling L (2007). The science of real-time data capture. NU: Oxford University Press. [Google Scholar]
- Stout DM, Shackman AJ, & Larson CL (2013). Failure to filter: Anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience, 7, doi: 10.3389/fnhum.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Pedersen WS, Miskovich TA, & Larson CL (2017). Neural circuitry governing anxious individuals’ mis-allocation of working memory to threat. Scientific Reports, 7, 8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs SY, Lamers F, Rinck M, Roelofs K, Spinhoven P, & Penninx B (2018). The predictive value of Approach and Avoidance tendencies on the onset and course of depression and anxiety disorders. Depression and Anxiety, 35, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuijfzand S, Creswell C, Field AP, Pearcey S, & Dodd H (2018). Research Review: Is anxiety associated with negative interpretations of ambiguity in children and adolescents? A systematic review and meta-analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines, 59, 1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, … Psychiatric Genomics, C. (2018). Psychiatric genomics: An update and an agenda. American Journal of Psychiatry, 175, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susa G, Pitică I, Benga O, & Miclea M (2012). The self regulatory effect of attentional control in modulating the relationship between attentional biases toward threat and anxiety symptoms in children. Cognition & Emotion, 26(6), 1069–1083. [DOI] [PubMed] [Google Scholar]
- Sussman TJ, Jin J, & Mohanty A (2016). Top-down and bottom-up factors in threat-related perception and attention in anxiety. Biological psychology, 121, 160–172. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, & Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, & Hariri AR (2015). Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. American Journal of Psychiatry, 172, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C, Corbetta M, Raichle M, Rodebaugh T, Schlaggar B, Sheline Y, … Lenze E (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, & Lahey B (2017). Neuroticism In Widiger TA (Ed.), The Oxford handbook of the five factor model (pp. 39–56). New York, NY: Oxford University Press. [Google Scholar]
- Tang YY, Holzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation. Nature Reviews. Neuroscience, 16, 213–225. [DOI] [PubMed] [Google Scholar]
- Taubert J, Flessert M, Wardle SG, Basile BM, Murphy AP, Murray EA, & Ungerleider LG (2018). Amygdala lesions eliminate viewing preferences for faces in rhesus monkeys. Proceedings of the National Academy of Sciences, 115, 8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Bomyea J, & Amir N (2010). Attentional bias away from positive social information mediates the link between social anxiety and anxiety vulnerability to a social stressor. Journal of Anxiety Disorders, 24(4), 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Martin J, Lu Y, Brikell I, Lundström S, Larsson H, & Lichtenstein P (in press). Association of genetic risk factors for psychiatric disorders and traits of these disorders in a Swedish population twin sample. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews. Neuroscience, 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, … van Honk J (2012). Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry, 2, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, Scheggia D, Triana Del Rio R., Klumpers F, Ciobanu AC, Morgan B, … van Honk J (2018). The basolateral amygdala is essential for rapid escape: A human and rodent study. Cell, 175, 723–735 e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielmann I, & Hilbig BE (in press). Nomological consistency: A comprehensive test of the equivalence of different trait indicators for the same constructs. Journal of Personality. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, … Casey BJ (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Thomson ND, Aboutanos M, Kiehl KA, Neumann C, Galusha C, & Fanti KA (2019). Physiological reactivity in response to a fear-induced virtual reality experience: Associations with psychopathic traits. Psychophysiology, 56, e13276. [DOI] [PubMed] [Google Scholar]
- Thomson ND, Aboutanos M, Kiehl KA, Neumann C, Galusha C, & Fanti KA (in press). Physiological reactivity in response to a fear-induced virtual reality experience: Associations with psychopathic traits. Psychophysiology, e13276. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, & Fredrikson M (2001). Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. American Journal of Psychiatry, 158(8), 1220–1226. [DOI] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, & Shackman AJ (2018). Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping, 39, 1291–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipples J (2018). Caution follows fear: Evidence from hierarchical drift diffusion modelling. Emotion, 18, 237–247. [DOI] [PubMed] [Google Scholar]
- Torrisi S, Gorka AX, Gonzalez-Castillo J, O’Connell K, Balderston N, Grillon C, & Ernst M (2018). Extended amygdala connectivity changes during sustained shock anticipation. Transl Psychiatry, 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, … Ernst M (2015). Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Human Brain Mapping, 36, 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Champagne FA, & Meyer-Lindenberg A (2015). Environmental influence in the brain, human welfare and mental health. Nature Neuroscience, 18, 4121–4131. [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Gabard-Durnam LJ (2017). The developing amygdala: a student of the world and a teacher of the cortex. Curr Opin Psychol, 17, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Luthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews. Neuroscience, 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, & Adolphs R (2006). Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry, 11, 219–232. [DOI] [PubMed] [Google Scholar]
- U.S. Burden of Disease Collaborators. (2018). The state of US health, 1990–2016. Burden of diseases, injuries, and risk factors among US states. JAMA, 319, 1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliaszek AA, Hauner KK, Zinbarg RE, Craske MG, Mineka S, Griffith JW, & Rose RD (2009). An examination of content overlap and disorder-specific predictions in the associations of neuroticism with anxiety and depression. J Res Pers, 43, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, … Hendler T (2013). Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci, 7, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bulk BG, Meens PH, van Lang ND, de Voogd EL, van der Wee NJ, Rombouts SA, … Vermeiren RR (2014). Amygdala activation during emotional face processing in adolescents with affective disorders: the role of underlying depression and anxiety symptoms. Front Hum Neurosci, 8, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert DA, van de Vijver FJR, Poortinga YH, & Georgas J (2002). Structural and functional equivalence of the Eysenck Personality Questionnaire within and between countries. Personality and Individual Differences, 33, 1229–1249. [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, & Fernandez G (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66, 649–655. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, & Fernandez G (2010). Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage, 53, 348–354. [DOI] [PubMed] [Google Scholar]
- Vazire S (2010). Who knows what about a person? The self-other knowledge asymmetry (SOKA) model. Journal of Personality and Social Psychology, 98, 281–300. [DOI] [PubMed] [Google Scholar]
- Vazire S, & Carlson EN (2010). Self-knowledge of personality: Do people know themselves? Social and Personality Psychology Compass, 2010, 605–620. [Google Scholar]
- Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, & Boks MP (2014). Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depression and Anxiety, 31, 737–745. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, & Dolan RJ (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7, 1271–1278. [DOI] [PubMed] [Google Scholar]
- Vukasovic T, & Bratko D (2015). Heritability of personality: A meta-analysis of behavior genetic studies. Psychological Bulletin, 141, 769–785. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, & Kross E (2013). An fMRI-based neurologic signature of physical pain. New England Journal of Medicine, 368, 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gaitsch H, Poon H, Cox NJ, & Rzhetsky A (2017). Classification of common human diseases derived from shared genetic and environmental determinants. Nature Genetics, 49, 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Eaton NR, Krueger RF, Shackman AJ, Waldman ID, Zald DH, … Watson D (2018). Redefining phenotypes to advance psychiatric genetics: Implications from the Hierarchical Taxonomy of Psychopathology. PsyArXiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Zimmer-Gembeck MJ, Craske MG, Pine DS, Bradley BP, & Mogg K (2015). Look for good and never give up: A novel attention training treatment for childhood anxiety disorders. Behaviour Research and Therapy, 73, 111–123. [DOI] [PubMed] [Google Scholar]
- Watson D, Nus E, & Wu KD (in press). Development and validation of the faceted inventory of the five-factor model (FI-FFM). Assessment. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Treat TA, & Hollingworth A (2008). Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion, 22, 985–1018. [Google Scholar]
- Whalen PJ (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current directions in psychological science, 7(6), 177–188. [Google Scholar]
- White LK, Degnan KA, Henderson HA, Pérez-Edgar KA, Walker OL, Shechner T, … Fox NA (2017). Developmental relations between behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development, 88, 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer AJ, & Banich MT (2007). Inhibition versus switching deficits in different forms of rumination. Psychological Science, 18(6), 546–553. [DOI] [PubMed] [Google Scholar]
- WHO WHO (2007). Economic aspects of mental health in children and adolescents. Geneva: WHO. [Google Scholar]
- Wichstrom L, Penelo E, Rensvik Viddal K, de la Osa N, & Ezpeleta L (2018). Explaining the relationship between temperament and symptoms of psychiatric disorders from preschool to middle childhood: hybrid fixed and random effects models of Norwegian and Spanish children. Journal of Child Psychology and Psychiatry and Allied Disciplines, 59, 285–295. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, & Keil A (2014). Fearful faces heighten the cortical representation of contextual threat. NeuroImage, 86, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, & Mühlberger A (2009). Probing the attentional control theory in social anxiety: An emotional saccade task. Cognitive, Affective, & Behavioral Neuroscience, 9(3), 314–322. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Barnes LL, Mendes de Leon CF, Bienias JL, & Bennett DA (2006). Childhood adversity and psychosocial adjustment in old age. American Journal of Geriatric Psychiatry, 14, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Smyth JM, & MacLean RR (2014). Integrating ecological momentary assessment and functional brain imaging methods: new avenues for studying and treating tobacco dependence. Nicotine and Tobacco Research, 16 Suppl 2, S102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Chang LJ, Lindquist MA, & Wager TD (2017). Building better biomarkers: brain models in translational neuroimaging. Nature Neuroscience, 20, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SA, Wille B, Wu C. h., Lievens F, & De Fruyt F (2019). The influence of work on personality trait development: The demands-affordances TrAnsactional (DATA) model, an integrative review, and research agenda. Journal of Vocational Behavior, 110, 258–271. [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, … Major Depressive Disorder Working Group of the Psychiatric Genomics, C. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, & Schachar R (2014). Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. Journal of Abnormal Psychology, 123, 429–439. [DOI] [PubMed] [Google Scholar]
- Xu M, Xu G, & Yang Y (2016). Neural systems underlying emotional and non-emotional interference processing: An ALE meta-analysis of functional neuroimaging studies. Front Behav Neurosci, 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM (2012). Amygdala. In Mai JK & Paxinos G (Eds.), The human nervous system (pp. 759–834). San Diego: Academic Press. [Google Scholar]
- Zainal NH, & Newman MG (2018). Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychological Medicine, 48, 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Norton J, Carriere I, Ritchie K, Chaudieu I, & Ancelin ML (2015). Risk factors for late-onset generalized anxiety disorder: results from a 12-year prospective cohort (The ESPRIT study). Transl Psychiatry, 5, e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg RE, Mineka S, Bobova L, Craske MG, Vrshek-Schallhorn S, Griffith JW, … Anand D (2016). Testing a hierarchical model of neuroticism and its cognitive facets: Latent structure and prospective prediction of first onsets of anxiety and unipolar mood disorders during 3 years in late adolescence. Clinical Psychological Science, 4, 805–824. [Google Scholar]
- Zoellner LA, & Foa EB (2016). Applying Research Domain Criteria (RDoC) to the study of fear and anxiety: A critical comment. Psychophysiology, 53, 332–335. [DOI] [PubMed] [Google Scholar]
- Zvielli A, Bernstein A, & Koster EH (2014). Dynamics of attentional bias to threat in anxious adults: bias towards and/or away? PLoS ONE, 9, e104025. [DOI] [PMC free article] [PubMed] [Google Scholar]


