Abstract
Application of 4-aminopyridine (4AP, 50 μm) to combined slices of adult rat hippocampus–entorhinal cortex-induced ictal and interictal epileptiform discharges, as well as slow field potentials that were abolished by the μ-opioid agonist [d-Ala2,N-Me-Phe4,Gly-ol5] enkephalin (DAGO, 10 μm) or the GABAA receptor antagonist bicuculline methiodide (BMI, 10 μm); hence, they represented synchronous GABA-mediated potentials. Ictal discharges originated in the entorhinal cortex and propagated to the hippocampus, whereas interictal activity of CA3 origin was usually recorded in the hippocampus. The GABA-mediated potentials had no fixed site of origin or modality of propagation; they closely preceded (0.2–5 sec) and thus appeared to initiate ictal discharges. Only ictal discharges were blocked by the antagonist of the NMDA receptor 3,3-(2-carboxypiperazine-4-yl)propyl-1-phosphonate (CPP, 10 μm), whereas the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm) abolished all epileptiform activities. The GABA-mediated potentials continued to occur synchronously in all regions even after concomitant application of CNQX and CPP. [K+]o elevations were recorded in the entorhinal cortex during the ictal discharge (peak values = 13.9 ± 0.9 mm) and the synchronous GABA-mediated potentials (peak values = 4.2 ± 0.1 mm); the latter increases were presumably attributable to postsynaptic GABAA-receptor activation because they were abolished by DAGO or BMI. Their role in initiating ictal activity was demonstrated by using DAGO, which abolished both GABA-mediated synchronous potentials and ictal discharges. These data indicate that NMDA-mediated ictal discharges induced by 4AP originate in the entorhinal cortex; such a conclusion is in line with clinical evidence obtained in temporal lobe epilepsy patients. 4AP also induces GABA-mediated potentials that spread within the limbic system when excitatory transmission is blocked and may play a role in initiating ictal discharge by increasing [K+]o.
Keywords: entorhinal cortex, seizures, GABA, excitatory amino acids, rat, 4-aminopyridine
The in vitro hippocampal slice preparation is a valuable tool for identifying the fundamental mechanisms that are involved in neuronal synchronization, including the generation of epileptiform discharges recorded in patients with temporal lobe epilepsy (Schwartzkroin, 1993). This experimental approach takes advantage of the lamellar organization of the hippocampus (Andersen et al., 1966a,b, 1971). However, as some structures of the temporal lobe (e.g., the amygdala and the entorhinal cortex) are removed during slicing, phenomena such as the initiation and the spread of epileptiform activity within the limbic system may not be fully appreciated in this preparation.
An additional limitation encountered in studies performed in the “isolated” hippocampal slice relates to the type of epileptiform activity that occurs in this preparation. Spontaneous epileptiform activity recorded in medium containing physiological [K+] most often consists of brief events that resemble interictal discharges observed in situ, whereas prolonged epileptiform discharges (which are the equivalent of the status epilepticus seen in humans or in vivo animal models) are only rarely observed (Schwartzkroin and Prince, 1978; Rutecki et al., 1985, 1987; Dingledine et al., 1986). Clinical and experimental findings indicate that status epilepticus may cause hippocampal neuronal loss resembling the histopathological pattern of hippocampal sclerosis found in patients with temporal lobe epilepsy (Ben-Ari, 1985;Babb and Brown, 1987; Gloor, 1991).
The absence of prolonged epileptiform discharges has also been documented by analyzing the effects induced by the convulsant drug 4-aminopyridine (4AP) in isolated hippocampal slices obtained from adult rodent brain (Voskyul and Albus, 1985; Rutecki et al., 1987;Perreault and Avoli, 1991, 1992). 4AP induces interictal epileptiform activity that is abolished by non-NMDA receptor antagonists at a time when GABA-mediated inhibitory mechanisms are potentiated (Rutecki et al., 1989; Perreault and Avoli, 1991). In addition, 4AP also induces synchronous GABA-mediated potentials, which continue to occur and to spread within the hippocampal slice when excitatory synaptic transmission is blocked by specific antagonists of NMDA and non-NMDA receptors (Michelson and Wong, 1991; Perreault and Avoli, 1991,1992).
To avert some of the limitations inherent in the classic hippocampal slice preparation, we have used in the present study combined slices of the rat hippocampus–entorhinal cortex with field potential recording techniques to analyze the modalities of initiation and propagation, as well as the pharmacological profile of the different types of synchronous activity induced by 4AP in the limbic system maintainedin vitro. Here we report that bath application of 4AP induces different patterns of spontaneous activity, including a novel, ictal-like epileptiform discharge that originates in the entorhinal cortex and is caused by an NMDA-dependent mechanism. In addition, we used ion-selective recordings to measure the extracellular K+ concentration ([K+]o) associated with the 4AP-induced synchronous activity recorded in the entorhinal cortex.
Some of these findings have appeared in abstract form (Barbarosie et al., 1995; Köhling et al., 1995).
MATERIALS AND METHODS
Adult male Sprague–Dawley rats (200–250 gm) were decapitated under halothane anesthesia, and the brains were quickly removed. A block of brain tissue containing the retrohippocampal region was placed in cold (1–3°C), oxygenated artificial CSF (ACSF). Horizontal slices (500 μm thick) were cut using a vibratome and were then transferred to a tissue chamber where they lay in an interface between ACSF and humidified gas (95% O2/5% CO2) at 34–35°C, pH 7.4. The composition of the ACSF was (in mm): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. 4AP (50 μm), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm), 3,3-(2-carboxypiperazine-4-yl)-propyl-1-phosphonate (CPP, 10 μm), bicuculline methiodide (BMI, 10 μm), and [d-Ala2,N-Me-Phe4,Gly-ol5] enkephalin (DAGO, 10 μm) were applied to the bath. All chemicals were acquired from Sigma except CNQX and CPP, which were obtained from Tocris Cookson.
Slices consisted of both entorhinal cortex and hippocampal formation, including the subicular cortex. Field potential recordings were made with glass microelectrodes that were filled with 2 m NaCl or ACSF (resistance 2–10 MΩ) and were positioned in the medial portion of the entorhinal cortex (see Fig.8A), in the granule layer or in the dendritic region of the dentate gyrus, and in the stratum radiatum of CA3 and CA1 subfields. Signals were fed to high-impedance amplifiers. The recorded signals were displayed on a Gould chart recorder and were digitized and stored on videotape for subsequent analysis. Time-delay measurements were performed using a digital oscilloscope. For each trace, the onset of the different types of 4AP-induced synchronous potentials was determined by visual inspection as the time of the earliest deflection of the baseline recording. Measurements of the [K+]o were obtained from the medial portion of the entorhinal cortex using double-barreled ion-selective microelectrode based on the valinomycin ion exchanger Fluka 60398. The ion-selective microelectrode had tip diameters of 2–6 μm. The reference channel was backfilled with 150 mm NaCl and the ion-selective channel with 100 mm KCl. In calibration solutions containing 124 mm NaCl, the electrode showed a potential change of ∼57 mV to a 10-fold increase in [K+]. In this series of experiments, field potential activity was recorded through the reference channel of the ion-selective microelectrode.
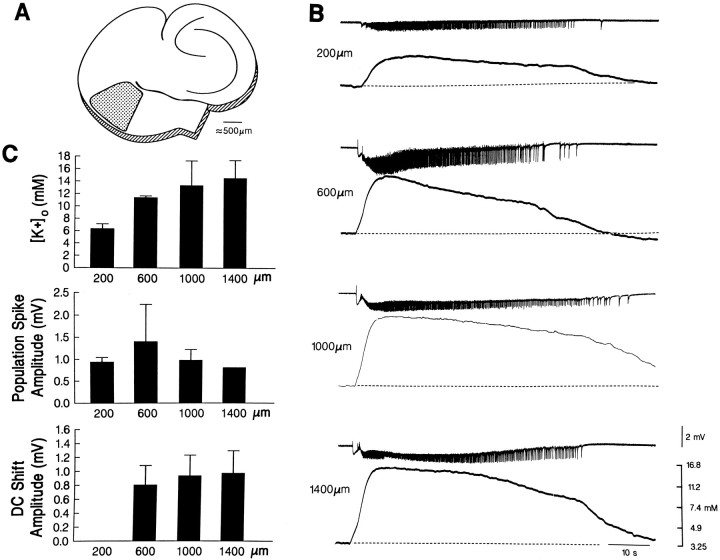
Fig. 8.
A, Schematic drawing of a hippocampus–entorhinal cortex slice; the entorhinal region where recordings were made is indicated by the dotted area.B, Simultaneous field potential (top trace in each pair) and [K+]orecordings (bottom trace in each pair) performed at different depths of the entorhinal cortex as indicated by thenumbers to the left of each pair. C, Plots of the maximal values of the [K+]o increases and of the amplitude of the population spikes and of the DC shift associated with the ictal discharges recorded at different depths in three slices.
A series of cutting experiments was performed using a microknife to establish the origin and the pathway(s) involved in the propagation of the synchronous activity induced by 4AP. To further establish the efficacy of this procedure, the tissue on both sides of the cut was gently separated by a distance of 100–500 μm.
The data base for the results presented here includes more than 120 combined hippocampus–entorhinal cortex slices. In 30 slices, field potential activity and the [K+]o were analyzed at different sites along an axis that was normal to the pial aspect of the entorhinal cortex. The data obtained in the course of these experiments were segregated into four different groups according to the distance from the pia (i.e., 0–400, 400–800, 800-1200, and 1200–1600 μm). Measurements throughout the text are expressed as mean ± SEM unless otherwise stated, and n indicates the number of slices or neurons studied under each experimental procedure. The results obtained were compared with the Student’s t test or the ANOVA test and were considered significantly different if p < 0.05.
RESULTS
Synchronous field potentials induced by 4AP
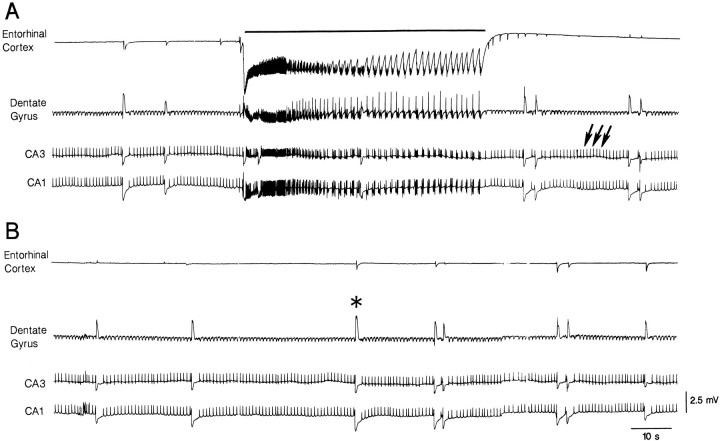
Simultaneous field potential recordings from entorhinal cortex and hippocampus during 4AP application revealed several types of spontaneous synchronous activity. Figure 1 illustrates a typical pattern that was seen in more than 80 slices and consisted of the following three types of field potential activity. (1) Ictal-like epileptiform discharges (hereafter termed ictal discharges) that occurred in both entorhinal cortex and all hippocampal areas, and showed electrographic features that were reminiscent of tonic-clonic seizures (continuous line in Fig. 1A). These ictal discharges lasted 20–480 sec, had intervals of occurrence of 71–1000 sec, and were characterized by high-frequency trains of population spikes riding on a steady shift of negative polarity that was of larger amplitude in entorhinal recordings. (2) Brief (80–150 msec) interictal-like epileptiform discharges (hereafter termed interictal discharges) that were recorded only in the hippocampus proper at intervals of 0.7–11.5 sec (arrows in Fig.1A). (3) “Slow” events (asterisk in Fig.1B) that occurred synchronously at intervals of 22–103 sec in the different areas of the hippocampus–entorhinal cortex slice and were mainly of negative polarity both in the entorhinal neocortex and in the dendritic regions of the hippocampus proper. When the recording microelectrodes were positioned in the pyramidal or granule layer, these slow events were of positive polarity (e.g., dentate gyrus recording in Figs. 1, 2, 3; cf. Perreault and Avoli, 1992). Because these “slow” field potentials were abolished by application of BMI or DAGO (see below), they will be referred to as GABA-mediated potentials. The GABA-mediated events recorded in the entorhinal cortex had durations of 0.9–3.9 sec.
Fig. 1.
Field potential recordings performed simultaneously in the entorhinal cortex (middle layers) and in different hippocampal areas (apical dendrites of CA1 and CA3, and dentate granule layer) during application of 4AP demonstrate the occurrence of three different types of activity. The first (continuous line in A) is recorded synchronously in all areas and consists of a sustained ictal-like epileptiform discharge. The second type (arrows in A) is seen in the hippocampal regions only and consists of continuous interictal-like events. The third type of synchronous activity, referred to as GABA-mediated potential, is recorded in all areas and is characterized by a slow field potential that is of negative polarity in all areas except dentate gyrus (asterisk in B).A and B are continuous recordings.
Fig. 2.
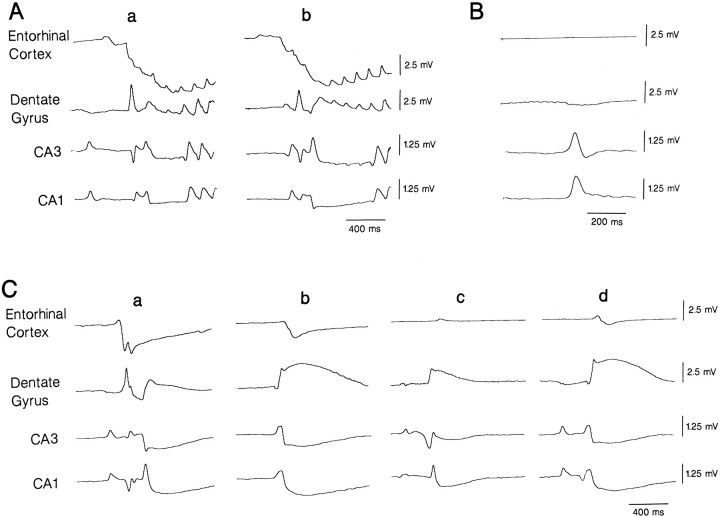
Expanded traces of simultaneous field potential recordings performed in the entorhinal cortex and in different hippocampal areas (same experimental conditions as in Fig. 1) show the modalities of onset and spread of ictal discharges (A), hippocampal interictal events (B), and GABA-mediated potentials (C). Note that the onset of the ictal discharges occurs first in the entorhinal cortex (a and b inpanel A), and different sites of origin characterize the different examples of GABA-mediated potentials (a–d inpanel C).
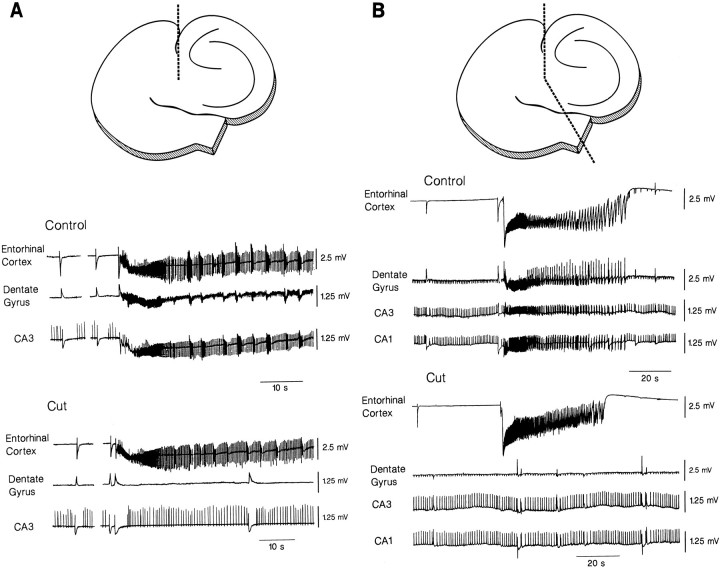
Fig. 3.
Effects induced by sectioning the perforant pathway (A) or both the perforant pathway and the subicular area to achieve a complete separation between the entorhinal cortex and the hippocampus proper (B). The location and the extent of the cuts are shown at the top of each panel. A, Lesioning the perforant pathway only abolishes ictal discharges in all hippocampal areas; under these conditions, the interictal activity of hippocampal origin continues to occur, and GABA-mediated potentials can be recorded synchronously in both entorhinal cortex and hippocampus.B, The complete surgical separation of the entorhinal cortex and hippocampus makes the GABA-mediated potentials occur asynchronously in entorhinal cortex and hippocampal areas.
As shown in Figures 1, 2, 3, 4, 5, the ictal discharge was preceded (and thus it appeared to be initiated) by the GABA-mediated potential, which occurred 0.2–5 sec before the onset of the ictal event. However, only 11.7 ± 1.2% of the GABA-mediated events (data obtained from 9 slices) were followed by an ictal discharge. A further type of interictal activity occurred in the entorhinal cortex in 13 of the 89 slices studied during 4AP application (see Fig. 5C). These interictal discharges lasted longer than those recorded in the hippocampus proper only, and were observed simultaneously in the dentate gyrus and CA3 and CA1 sectors (not illustrated). GABA-mediated events were hardly distinguishable in the entorhinal cortex whenever this type of interictal discharge occurred (see Fig. 5C). The values of the duration and of the rate of occurrence of the different types of spontaneous activity recorded in the entorhinal cortex and the CA3 subfield of hippocampus during 4AP application are summarized in Table 1.
Fig. 4.
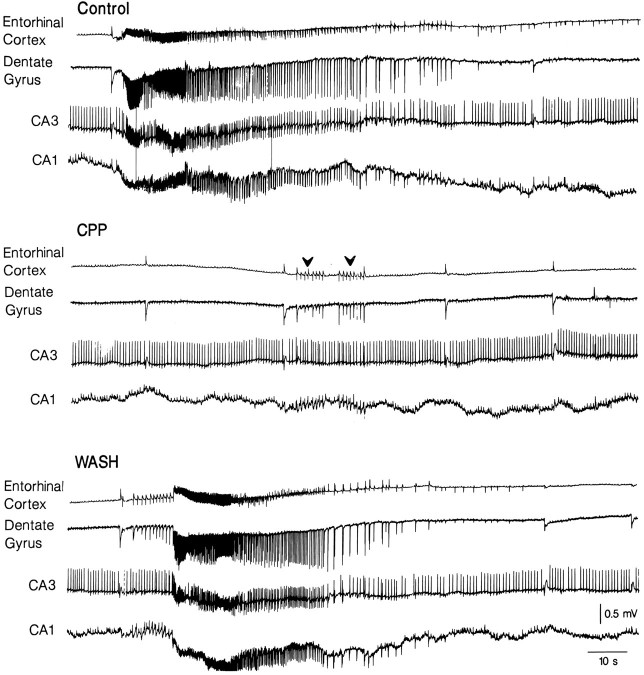
Effects induced by the NMDA receptor antagonist CPP (10 μm) on the spontaneous field potential activity induced by 4AP. Note that the ictal discharges are abolished and replaced by a sequence of interictal events (arrowheads) that appear synchronously in the entorhinal cortex and hippocampus proper; by contrast, the interictal activity recorded in the hippocampus proper continues to occur at a higher rate than in control. Note also that the synchronous GABA-mediated potentials are not affected by CPP application.
Fig. 5.
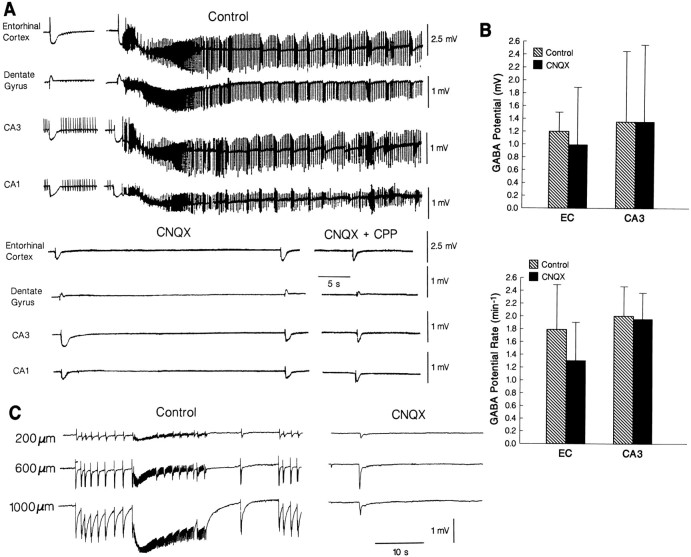
Effects induced by the non-NMDA receptor antagonist CNQX on the spontaneous synchronous activity induced by 4AP.A, CNQX abolishes all types of epileptiform discharges in both entorhinal cortex and hippocampus, whereas GABA-mediated field potentials continue to occur synchronously in most of the cases, even after further addition of the NMDA receptor antagonist CPP.B, Plot of the changes induced by CNQX on the amplitude and rate of occurrence of the GABA-mediated potential analyzed in the entorhinal cortex and CA3 of six slices. C, Effects induced by CNQX on the epileptiform activity recorded in the entorhinal cortex at different depths (as indicated by the numbers on theleft) from the pia; in this experiment, both interictal and ictal discharges were recorded under control conditions, whereas after CNQX application only a synchronous event reminiscent of the GABA-mediated potential is seen.
Table 1.
Duration and intervals of occurrence of the different types of synchronous activity induced by 4AP
| Ictal discharge | Entorhinal interictal discharge | Hippocampal interictal discharge | GABA-mediated field potential | |||||
|---|---|---|---|---|---|---|---|---|
| Duration (sec) | Interval (sec) | Duration (sec) | Interval (sec) | Duration (msec) | Interval (sec) | Duration (sec) | Interval (sec) | |
| Entorhinal cortex | 109.8 ± 19.4 | 459.6 ± 47.6 | 2.1 ± 0.3 | 3.8 ± 0.5 | – | – | 1.6 ± 0.9 | 36.8 ± 17.7 |
| n = 25 | n = 18 | n = 5 | n = 5 | – | – | n = 24 | n = 26 | |
| CA3 area | 106 ± 26.8 | 444.5 ± 45.4 | 2.0 ± 0.4 | 3.8 ± 0.6 | 111.4 ± 9.9 | 0.9 ± 0.06 | 1.9 ± 0.5 | 45.0 ± 14.4 |
| n = 18 | n = 13 | n = 4 | n = 4 | n = 7 | n = 6 | n = 10 | n = 10 | |
Time-delay analysis of the onset of the three types of spontaneous activity recorded in the hippocampus–entorhinal cortex slices suggested that the ictal discharges originated in the entorhinal cortex from where they propagated successively to the dentate area and CA3 and CA1 subfields (Fig. 2Aa,b). This type of analysis also indicated that hippocampal interictal discharges occurred in the CA3 subfield earlier than those simultaneously recorded in the CA1 area (Fig. 2B). In agreement with a previous study (Perreault and Avoli, 1992), this type of interictal activity was of very small amplitude (or not detectable at all) in the dentate gyrus compared with that recorded in the CA3 or CA1 areas. Moreover, each dentate interictal event did not have any time delay when compared with the corresponding field potential observed in either CA3 or CA1 area. Therefore, the interictal events recorded in the dentate area did represent a volume conduction phenomenon (cf. Perreault and Avoli, 1992). As illustrated in Figure 2C, there was no single area of origin for the GABA-mediated field potentials; in 23% of the cases (n = 90 events analyzed in 6 slices), these field potentials appeared first in the entorhinal cortex (Fig. 2Ca), whereas in 15, 23, and 18% of the cases they started in dentate, CA3, and CA1, respectively (Fig. 2Cb–d). In the remaining cases, the GABA-mediated field potentials appeared in more than one area at the same time, suggesting a site of origin that was located somewhere between the recorded areas, and then propagated toward them. A summary of the time delays of the onset of the ictal events of entorhinal origin and of the interictal discharges seen in the hippocampus proper is shown in Table 2.
Table 2.
Time delays of the onset for the epileptiform activities induced by 4-aminopyridine in entorhinal cortex and hippocampus
| Entorhinal cortex | Dentate gyrus | CA3 | |
|---|---|---|---|
| ↓ | ↓ | ↓ | |
| Dentate gyrus | CA3 | CA1 | |
| Entorhinal ictal | 44.2 ± 24.2 | 43.3 ± 19.1 | 28.3 ± 6.8 |
| discharge (msec) | n = 6 | n = 6 | n = 6 |
| Hippocampal interictal | – | – | 12.3 ± 3.5 |
| discharge (msec) | – | – | n = 4 |
Effects of fiber pathway cuts on the 4AP-induced synchronous activity
The origin and the modalities of propagation of the different types of synchronous activity induced by 4AP was also studied in sectioning experiments, where fiber pathways were severed, thus isolating some areas of the slice from one or more input pathways. Cut performed at the level of the subiculum only (a procedure that should interrupt most of the connections between the CA1 subfield and the entorhinal cortex) did not modify the rate of occurrence, the duration, or the degree of synchronization of any of the spontaneous activities recorded under control conditions (not illustrated; n = 3 slices).
Sectioning the perforant pathway (n = 5 slices) made ictal discharges disappear in the hippocampus, but did not affect the occurrence of interictal epileptiform activity recorded in the CA1 and CA3 areas (Fig. 3A). This procedure induced a 15–32% decrease in the rate of occurrence of the GABA-mediated field potential and modified the degree of synchronicity. Although some of these events remained synchronous in all the areas of the hippocampus–entorhinal cortex slice, 46 ± 5% of them occurred independently in the entorhinal cortex compared with the hippocampus proper. However, when the cut was extended to the subiculum and the two portions of the slice were gently separated (n = 2 slices), all GABA-mediated field potentials were generated in an asynchronous fashion in the entorhinal cortex and in the different hippocampal areas (Fig. 3B).
Hippocampal interictal discharges disappear in CA1 after a cut of the Schaffer collateral (n = 4 slices), whereas ictal discharges of entorhinal origin could still be recorded in the dentate gyrus and CA3 subfield, not in the CA1 area (not illustrated). This type of lesion did not influence the synchronicity of the GABA-mediated field potentials recorded in the different sectors of the hippocampus and entorhinal cortex (not illustrated).
Pharmacological properties of the spontaneous synchronous activity
Bath application of the NMDA receptor antagonist CPP (n = 5 slices) abolished the ictal discharges of entorhinal origin in all experiments. In the presence of CPP, series of interictal-like events could be recorded in the entorhinal cortex after some of the GABA-mediated field potentials (Fig. 4,arrowheads in the CPP panel). This type of recurrent interictal activity propagated to the hippocampus proper. In agreement with previous studies (Perreault and Avoli, 1991, 1992), the interictal activity recorded in the hippocampus proper was not influenced by CPP. Moreover, neither the rate of occurrence nor the duration and shape of the GABA-mediated field potentials were modified by this NMDA receptor antagonist. The effects of CPP were reversible after wash (Fig. 4, WASH).
Non-NMDA receptor antagonist CNQX application (n = 6 slices) made all types of epileptiform discharge disappear, whereas GABA-mediated field potentials persisted in both entorhinal cortex and hippocampus (Fig. 5A;CNQX). CNQX induced a nonsignificant decrease in the amplitude of the negative component of the GABA-mediated field potentials recorded in both entorhinal cortex and hippocampal areas, while markedly reducing the initial positive component of this potential whenever present (e.g., Fig. 5A,B; CNQX). The occurrence rate of the GABA-mediated events recorded in the entorhinal cortex during CNQX application decreased in 2 of 6 experiments, but remained unchanged in the hippocampus proper (Fig.5B); this effect probably reflected a decrease of synchronicity for these field potentials between the entorhinal cortex and the hippocampus. Further application of CPP to medium containing CNQX and 4AP did not induce additional changes (Fig. 5A;n = 5 slices). The interictal activity recorded in the entorhinal cortex of two slices was abolished by CNQX along with the ictal discharges (Fig. 5C).
In line with previous experiments performed in isolated hippocampal slices (Perreault and Avoli, 1989, 1992; Barbarosie et al., 1994), the synchronous GABA-mediated potentials recorded in the hippocampus–entorhinal cortex slice during application of 4AP, CNQX, and CPP were abolished by application of the GABAA receptor antagonist BMI (n = 8 slices, not illustrated) or of the μ-opioid receptor agonist DAGO, which is expected to prevent GABA release from inhibitory interneurons (Madison and Nicoll, 1988; Lambert et al., 1991; Cohen et al., 1992;Capogna et al., 1993) (n = 3 slices, not illustrated). These findings, therefore, indicate that the synchronous field potentials recorded in the different areas of the hippocampus–entorhinal cortex during application of 4AP and ionotropic excitatory amino acid receptor antagonists represent mainly a GABAA-type, postsynaptic response of principal neurons to GABA released from inhibitory interneurons.
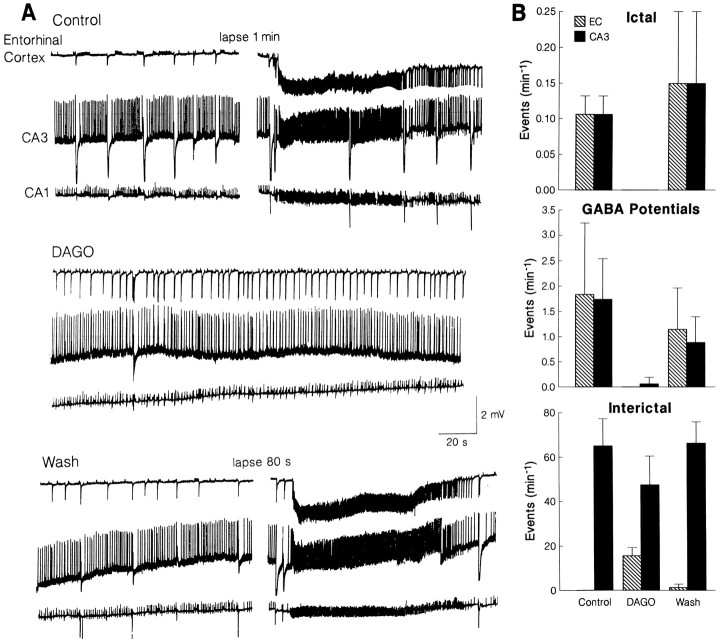
In six experiments, we also studied the effects induced by DAGO on the synchronous activity recorded in the combined hippocampus–entorhinal cortex slice during application of 4AP only. Also in these experiments, the μ-opioid receptor agonist abolished the occurrence of GABA-mediated field potentials in all areas, an effect that was accompanied by the disappearance of ictal discharges (Fig.6A). These changes were associated with the appearance of entorhinal interictal discharges (duration 0.2–1 sec) that propagated to the hippocampus proper and made interictal activity of hippocampal origin decrease in rate (Fig. 6A). A quantitative summary of these effects is provided in Figure6B.
Fig. 6.
Effects induced by DAGO on the synchronous activity recorded in the entorhinal cortex and CA3 area during application of 4AP. Note in A that during DAGO, both GABA-mediated field potentials and ictal discharges disappear, whereas interictal discharges propagating to the hippocampus proper appear in the entorhinal cortex. This effect is accompanied by a decrease in the rate of occurrence of hippocampal interictal discharges. B, Plot of the changes induced by DAGO on the rate of occurrence of GABA-mediated potentials, ictal discharges, and interictal events in five slices.
Potassium measurements in the entorhinal cortex
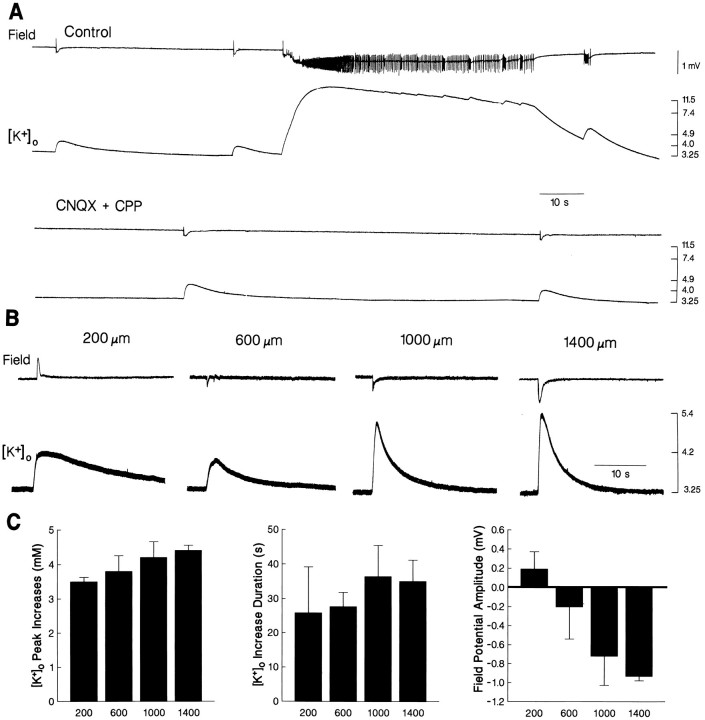
The spontaneous activity induced by 4AP was also analyzed in the entorhinal cortex with simultaneous field potential and [K+]o recordings. As illustrated in Figure 7A (left panel), the GABA-mediated field potentials recorded 600–800 μm from the pial surface were accompanied by transient increases in [K+]o that attained peak values of 3.5–9.3 mm (4.2 ± 0.1 mm) and had overall durations of 5–50 sec (25.3 ± 1.42 sec; n = 14 slices). These [K+]o elevations were not followed by undershoots. Ictal discharges recorded at the same depths were associated with sustained increases in [K+]o that attained peak values of 11–17 mm (13.9 ± 0.9 mm; n = 6 slices) in coincidence with the tonic phase, 4–7 sec after the discharge onset (Fig.7A). These [K+]o elevations slowly declined over time, but additional, transient increases in [K+]o (up to 13 mm) coincided with each clonic-type discharge (Fig. 7B, asterisk). The overall duration of the [K+]o increases recorded throughout the ictal discharge was 97–250 sec (147 ± 17.5 sec;n = 6 slices); in most cases, [K+]o undershoots to 2.6–2.8 mm followed the termination of the discharge (Fig. 7B).
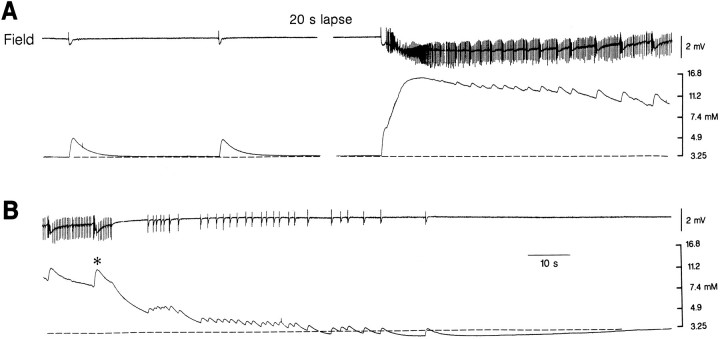
Fig. 7.
Field potential (top trace) and [K+]o (bottomtrace) recordings in the middle layers of the entorhinal cortex during application of 4AP. The GABA-mediated field potentials are associated with transient increases in [K+]o (left panel in A), while a sustained elevation is seen during the tonic phase of the ictal discharge; note that the ictal discharge is initiated by an increase in [K+]o that is larger than the elevations associated with the isolated GABA-mediated field potential. Note in B that distinct increases in [K+]o accompany clonic discharges during return of [K+]o toward baseline values (asterisk) and that a pronounced undershoot follows the termination of the ictal discharge.
The [K+]o elevation coinciding with the GABA-mediated field potential preceding the onset of the ictal discharge appeared to initiate such event. Thus, as illustrated in Figure 7A, [K+]o increased up to 6.5 mm during the initial GABA-mediated field potential, and ictal activity appeared shortly (0.5–1 sec) after. The [K+]o increases associated with the GABA-mediated potentials temporally related with the ictal discharges were larger than those observed in isolation. For instance, in the experiment of Figure 7, the GABA-mediated [K+]o elevations recorded between ictal events reached peak values of 4.4 ± 0.3 mm (n = 17 events), whereas those preceding the ictal discharges had values of 6.0 ± 0.2 mm (n = 6 events).
Field potential and [K+]orecordings made at different depths of the entorhinal cortex (n = 3 slices) revealed that [K+]o increases associated with the ictal discharge reached their maximal values in the deep layers of the entorhinal cortex (at ∼1000–1400 μm from the pia), which was also the site where the field potential, DC shift showed maximal negative values (Fig. 8). By contrast, the negative-going population spikes that overrode the negative DC shift were of maximal amplitude at 600 μm from the pia (Fig. 8).
In keeping with the findings reported above, application of CNQX and CPP (n = 17 slices) abolished the spontaneous epileptiform discharges recorded in the entorhinal cortex with field potential and K+-selective microelectrodes (Fig.9A). Under these experimental conditions, both GABA-mediated events and concomitant [K+]o increases still occurred, although at a decreased rate (from 1.8 ± 0.7/min under control conditions to 1.4 ± 0.6/min during CNQX and CPP application;n = 14 and 17 slices, respectively). Comparison of the [K+]o elevations recorded at equivalent depth of the entorhinal cortex under these two experimental conditions did not yield any significant difference. Therefore, CNQX and CPP only induced a significant decrease of the rate of occurrence of the GABA-mediated potentials, but no change in the amplitude or duration of the associated [K+]o increases.
Fig. 9.
A, Effects induced by the excitatory amino acid receptor antagonists CNQX and CPP on the 4AP-induced synchronous activity recorded in the entorhinal cortex with simultaneous field potential and [K+]o recordings.B, Field potential and [K+]o recordings performed in the entorhinal cortex at different depths from the pia (as indicated by the numbers at top of each panel) during application of 4AP, CNQX, and CPP. C, Plots of the peak values and duration of the [K+]o increases as well as of the amplitude of the GABA-mediated field potentials recorded at different depths in five entorhinal cortex slices during application of 4AP, CNQX, and CPP.
Field potential and [K+]orecordings performed at different depths of the entorhinal cortex during application of 4AP and excitatory amino acid receptor antagonists indicated that, in most cases, the largest [K+]o increases associated with the GABA-mediated events occurred in the deep layers (1000–1400 μm) (Fig. 9B). However, these differences were not significant when data obtained from 17 slices were pooled together. The synchronous GABA-mediated field potentials and the associated increases in [K+]o were abolished by application of BMI (n = 1 slice, not illustrated) or DAGO (n = 5 slices, not illustrated). Both pharmacological procedures did not induce any evident change in the baseline [K+]o.
DISCUSSION
Origin and spread of the 4AP-induced epileptiform activity
Application of 4AP to slices of the hippocampus–entorhinal cortex induces prolonged epileptiform discharges that are seen in all regions and resemble the electrographic seizure activity seen in animal models of epilepsy in situ (Matsumoto and Ajmone-Marsan, 1964). These ictal discharges originate in the entorhinal cortex, from which they propagate to the different areas of the hippocampus proper following the well known trisynaptic loop (Andersen et al., 1966a,b, 1971). This evidence rests on both time-delay measurements of ictal discharge onset and sectioning experiments, which show that severing the perforant path can abolish the occurrence of the ictal discharges in the hippocampus proper. Moreover, cutting the Schaffer collateral makes ictal discharges disappear in the CA1 area, indicating that excitatory inputs from the CA3 area are necessary for entraining CA1 pyramidal cells to generate the ictal epileptiform discharge.
As the ictal discharge propagates from the entorhinal cortex to the hippocampus proper via the perforant path, such activity appears to overcome the gating function of the dentate gyrus; this filtering mechanism has been documented in other models of limbic discharge (Dreier and Heinemann, 1991; Lothman et al., 1993), including those recorded in the isolated guinea pig brain preparation (Paré et al., 1992). However, in contrast to findings obtained in the isolated guinea pig brain preparation, we could not document any reentering activity from the CA1 area to the entorhinal cortex via the subiculum. This finding is in line with the lack of changes in discharge pattern seen in the entorhinal cortex after a subicular cut. Similar data have been reported for the ictal activity induced by pilocarpine (Nagao et al., 1996) and for interictal discharges induced by GABAA receptor antagonists (Jones and Lambert, 1990a,b) in combined slices of hippocampus–entorhinal cortex. However, this experimental evidence should be regarded with caution when extrapolating to the mechanisms of limbic seizure in vivo, because connections from the hippocampus to the entorhinal cortex are functional in temporal lobe epilepsy patients (Rutecki et al., 1989).
Profile analysis of the field potentials recorded in the entorhinal cortex during the ictal discharge induced by 4AP indicates that the largest negative shifts and [K+]o increases occur in the deep layers (i.e., ∼1000–1600 μm from the pia), although population spikes have maximal negative amplitudes at depths of ∼600–800 μm. These [K+]o elevations had values similar to those reported in the entorhinal cortex during Mg2+-free-induced ictal discharges (Dreier and Heinemann, 1991); in this study as well, the largest negative shifts and [K+]o increases were seen in the entorhinal deep layers.
The role of the entorhinal cortex in generating robust epileptiform activity that resembles ictal discharges is in keeping with experiments performed in combined hippocampus–entorhinal cortex slices that were bathed in Mg2+-free medium (Walther et al., 1986;Wilson et al., 1988; Jones and Lambert, 1990a,b; Dreier and Heinemann, 1991), GABAA receptor antagonists (Jones and Lambert, 1990a,b), pilocarpine (Nagao et al., 1996), or elevated [K+] (Bear and Lothman, 1993). Moreover, the entorhinal cortex plays a prominent role in the generation of limbic seizures induced in vivo by electrical stimulation of the hippocampus (Stringer and Lothman, 1992). A dysfunction of the entorhinal cortex has been documented in temporal lobe epilepsy patients (Rutecki et al., 1989; Deutsch et al., 1991; Spencer and Spencer, 1994) in whom surgical removal of the entorhinal cortex is essential for achieving seizure control (Goldring et al., 1992).
Interictal epileptiform discharges were recorded in the hippocampus proper during application of 4AP. In agreement with previous studies performed in the isolated hippocampal slice preparation (Perreault and Avoli, 1991, 1992), these interictal epileptiform discharges originate in the CA3 area and propagate to CA1 via the Schaffer collaterals. This type of interictal activity does not propagate to the entorhinal cortex, further indicating that synchronous discharges of hippocampal origin do not excite the entorhinal cortex neurons in our slice preparation. Pilocarpine induces an analogous type of interictal discharge in the CA3 and CA1 areas, not in the entorhinal cortex (Nagao et al., 1996).
Pharmacology of the 4AP-induced epileptiform activity
Activation of ionotropic non-NMDA receptors plays a primary role in the generation of all types of epileptiform discharge induced by 4AP in the hippocampal–entorhinal cortex slice, as indicated by the experiments performed with CNQX. The results observed in the hippocampus proper are identical to those reported for the interictal epileptiform activity induced by 4AP or tetraethylammonium in isolated hippocampal slices (Perreault and Avoli, 1991, 1992; Fueta and Avoli, 1993), whereas those obtained in the entorhinal cortex are analogous to the effects of non-NMDA receptor antagonists on the epileptiform discharges induced by GABAA receptor antagonists (Jones and Lambert, 1990a,b) or pilocarpine (Nagao et al., 1996).
Our results also reveal that activation of NMDA receptors is instrumental for the occurrence of the ictal discharges of entorhinal origin. The involvement of NMDA-mediated mechanisms in the initiation and propagation of seizure activity has been reported in many experimental models of epileptiform discharge (Dingledine et al., 1986;Hwa and Avoli, 1991). However, with the obvious exception of the epileptiform discharges induced by Mg2+-free medium (Mody et al., 1987; Tancredi et al., 1990; Avoli et al., 1991), these previous studies have shown that blockade of the NMDA receptor only decreases the duration of the epileptiform discharge. Hence, the present findings, along with those obtained in the pilocarpine model (Nagao et al., 1996), indicate that in the entorhinal cortex NMDA receptor may play a unique role in seizure generation. This conclusion is in line with the demonstration of prolonged NMDA-mediated depolarizations in entorhinal neurons (Jones and Heinemann, 1988;Jones, 1994). Our results also confirm that the interictal activity recorded in the hippocampus proper during application of 4AP is not influenced by NMDA-receptor antagonists (cf. Perreault and Avoli, 1991).
GABA-mediated synchronous potentials and the initiation of ictal discharges
4AP also induces slow potentials that occur simultaneously in all hippocampal areas and in the entorhinal cortex, which represent GABA-mediated, synchronous events (cf. Perreault and Avoli, 1989, 1992;Barbarosie et al., 1994). Time-delay analysis indicates that there is no single area of origin for these GABA-mediated potentials, because they can appear first in any of the regions simultaneously recorded in the hippocampus–entorhinal cortex slice or in more than one area at the same time. Therefore, our findings extend to those reported byPerreault and Avoli (1992) in the isolated hippocampal slice and indicate that the pacemaker of the 4AP-induced, synchronous GABA-mediated potential is not confined to a single region of the limbic system. Moreover, the GABA-mediated potentials do not propagate to the other regions of the hippocampus or of the entorhinal cortex in a constant and predictable pattern, as is the case for the different types of epileptiform discharge induced by 4AP. Such a conclusion also rests on the findings obtained by sectioning different pathways of the hippocampus–entorhinal cortex slice.
GABA-mediated potentials still occur during application of CNQX (as well as CPP), although at a reduced rate in the entorhinal cortex. Because this decrease in rate of occurrence was not seen in the hippocampus, we are inclined to conclude that it reflects the failure of some GABA-mediated synchronous potentials originating in the hippocampus to propagate to relatively distant sites, such as the entorhinal cortex. Therefore, similar to that reported in the isolated hippocampal slice (Perreault and Avoli, 1992) and in the human neocortex (Avoli et al., 1994), the spread of the GABA-mediated field potentials within the combined hippocampus–entorhinal cortex slice does not depend solely on a “classic” mechanism of pathway excitatory transmission.
It has been proposed (Perreault and Avoli, 1992) that the GABA-mediated event is initiated by the synchronous firing of inhibitory interneurons located in any region of the hippocampus (and in the present experiments of the entorhinal cortex) from where it propagates after the recruitment of other interneurons located nearby, and in other regions via the spatial dispersion of [K+]o, which increases after the initial process of interneuron-firing GABA release and the subsequent postsynaptic activation of GABAAreceptors that may be located on neurons and glial cells. According to this hypothesis, the [K+]o elevation depolarizes and makes neighboring interneurons fire, thus producing a positive feedback mechanism through which the GABA-mediated phenomenon can spread. This K+ accumulation mechanism is supported by our pharmacological data as well as by the occurrence of a transient [K+]o elevation in coincidence with the GABA-mediated event. Increases in [K+]o have been recorded during synchronous GABA-mediated potentials induced by 4AP in adult or juvenile rat hippocampus (Louvel et al., 1994; Köhling et al., 1996; Morris et al., 1996) and entorhinal cortex (Lücke et al., 1995).
Several mechanisms may contribute to the increase in [K+]o caused by the activation of GABAA receptors that are located postsynaptically on neuronal and glial elements. These processes include an outward counter/cotransport of K+ with Cl−/HCO−3 anion shift (Kaila et al., 1992; Kaila, 1994), voltage-dependent K+currents (Rudy, 1988), and Na+-dependent GABA uptake (Kaila et al., 1992). A GABAA-mediated depolarization attributable to an inward Cl−current has been recorded in glial cells (MacVicar et al., 1989;Steinhäuser et al., 1994). It has been shown that application of GABA to hippocampal slices induces a GABA receptor-mediated [K+]o augmentation (Barolet and Morris, 1991).
The GABA-mediated synchronous potential plays a facilitory role in the initiation of the 4AP-induced ictal activity of entorhinal origin. Moreover, this mechanism may rely on the increase in [K+]o that is caused by GABAA receptor activation. These conclusions rest on two main pieces of evidence. First, the pattern of ictal discharge initiates during the [K+]o elevation associated with the GABA-mediated potential, and whenever ictal activity appears, it is preceded by GABA-dependent [K+]o increases that are of larger amplitude than those seen to occur in isolation between ictal events. Second, application of the μ-opioid agonist DAGO to medium containing 4AP abolishes both GABA-mediated potentials and concomitant [K+]o increases, along with the subsequent ictal discharges; this pharmacological procedure does not abolish interictal epileptiform activity.
These results, therefore, indicate that the GABA-mediated potential may be instrumental in initiating ictal discharges and suggest that in this model, at least, [K+]oincreases in the adult rat entorhinal cortex represent a causal factor for the occurrence of ictal epileptiform activity. Several studies have demonstrated that [K+]oincreases during seizure activity (Heinemann et al., 1977; Benninger et al., 1980; Heinemann and Dietzel, 1984; Somjen and Giacchino, 1985). It was unclear, however, whether these [K+]o elevations were the result of seizure activity or its causal factor. A mechanism similar to that proposed here for the initiation of ictal discharge in the entorhinal cortex may also be operant in the CA3 area of isolated hippocampal slices obtained from young rats during application of a similar concentration of 4AP (Avoli et al., 1993).
Footnotes
This study was supported by the Medical Research Council of Canada (Grant MT-8109 to M.A.) and travel grants from the Wellcome GmBH to A.L. and R.K. M.A. was an FRSQ Chercheur-Boursier and M.B. a Savoy Foundation Fellow. We thank Mr. V. Epp for clerical assistance.
Correspondence should be addressed to Dr. Massimo Avoli, 3801 University, Room 794, McGill University, Montreal, Quebec, Canada H3A 2B4.
REFERENCES
- 1.Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966a;66:448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Holmqvist B, Voorhoeve PE. Excitatory synapses on hippocampal apical dendrites activated by entorhinal stimulation. Acta Physiol Scand. 1966b;66:461–472. doi: 10.1111/j.1748-1716.1966.tb03224.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Bliss VP, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 4.Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure J-G. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–596. doi: 10.1002/ana.410300412. [DOI] [PubMed] [Google Scholar]
- 5.Avoli M, Psarropoulou C, Tancredi V, Fueta Y. On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J Neurophysiol. 1993;70:1018–1029. doi: 10.1152/jn.1993.70.3.1018. [DOI] [PubMed] [Google Scholar]
- 6.Avoli M, Mattia D, Siniscalchi A, Perreault P, Tomaiuolo F. Pharmacology and electrophysiology of a synchronous GABA-mediated potential in the human neocortex. Neuroscience. 1994;62:655–666. doi: 10.1016/0306-4522(94)90467-7. [DOI] [PubMed] [Google Scholar]
- 7.Babb T, Brown W. Pathological findings in epilepsy. In: Engel J, editor. Treatment of the epilepsies. Raven; New York: 1987. pp. 511–540. [Google Scholar]
- 8.Barbarosie M, Nagao T, Avoli M. Control of 4-aminopyridine-induced synchronous activity by adenosine A1 and μ-opioid receptor agonists in adult rat hippocampus. Neurosci Lett. 1994;182:208–212. doi: 10.1016/0304-3940(94)90799-4. [DOI] [PubMed] [Google Scholar]
- 9.Barbarosie M, Köhling R, Lücke A, Nagao T, Avoli M. [K+]omeasurements during synchronous activity induced by 4-aminopyridine in the rat entorhinal cortex. Soc Neurosci Abstr. 1995;21:981. [Google Scholar]
- 10.Barolet AW, Morris ME. Changes in extracellular K+ evoked by GABA, THIP and baclofen in the guinea-pig hippocampal slice. Exp Brain Res. 1991;84:591–598. doi: 10.1007/BF00230971. [DOI] [PubMed] [Google Scholar]
- 11.Bear J, Lothman EW. An in vitro study of focal epileptogenesis in combined hippocampal-parahippocampal slices. Epilepsy Res. 1993;14:183–193. doi: 10.1016/0920-1211(93)90043-7. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 13.Benninger C, Kadis J, Prince DA. Extracellular calcium and potassium changes in hippocampal slices. Brain Res. 1980;187:165–182. doi: 10.1016/0006-8993(80)90502-8. [DOI] [PubMed] [Google Scholar]
- 14.Capogna M, Gahwiler BH, Thompson SM. Mechanism of μ-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol (Lond) 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 16.Deutsch C, Spencer S, Robbins R, Cicchetti D, Spencer D. Interictal spikes and hippocampal somatostatin levels in temporal lobe epilepsy. Epilepsia. 1991;32:174–178. doi: 10.1111/j.1528-1157.1991.tb05241.x. [DOI] [PubMed] [Google Scholar]
- 17.Dingledine R, Ynes MA, King GL. Involvement of N -methyl-d-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol (Lond) 1986;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreier JP, Heinemann U. Regional and time-dependent variations of low Mg2+-induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- 19.Fueta Y, Avoli M. Tetraethylammonium-induced epileptiform activity in young and adult hippocampus. Dev Brain Res. 1993;72:51–58. doi: 10.1016/0165-3806(93)90158-7. [DOI] [PubMed] [Google Scholar]
- 20.Gloor P. Mesial temporal sclerosis: historical background and an overview from a modern perspective. In: Lüders H, editor. Epilepsy surgery. Raven; New York: 1991. pp. 689–703. [Google Scholar]
- 21.Goldring S, Edwards I, Harding GW, Bernardo KL. Results of anterior temporal lobectomy that spares the amygdala in patients with complex partial seizures. J Neurosurg. 1992;77:185–193. doi: 10.3171/jns.1992.77.2.0185. [DOI] [PubMed] [Google Scholar]
- 22.Heinemann U, Dietzel I. Extracellular potassium concentration in chronic alumina cream foci of cats. J Neurophysiol. 1984;52:421–434. doi: 10.1152/jn.1984.52.3.421. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- 24.Hwa GGC, Avoli M. The involvement of excitatory amino acids in neocortical epileptogenesis: NMDA and non-NMDA receptors. Exp Brain Res. 1991;86:248–256. doi: 10.1007/BF00228949. [DOI] [PubMed] [Google Scholar]
- 25.Jones RSG. Synaptic and intrinsic properties of neurons of origin of the perforant path in layer II of the rat entorhinal cortex in vitro. Hippocampus. 1994;4:335–353. doi: 10.1002/hipo.450040317. [DOI] [PubMed] [Google Scholar]
- 26.Jones RSG, Heinemann U. Synaptic and intrinsic responses of medial entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988;59:1476–1496. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- 27.Jones RSG, Lambert JDC. The role of excitatory amino acid receptors in the propagation of epileptiform discharges from the entorhinal cortex to the dentate gyrus in vitro. Exp Brain Res. 1990a;80:310–322. doi: 10.1007/BF00228158. [DOI] [PubMed] [Google Scholar]
- 28.Jones RSG, Lambert JDC. Synchronous discharges in the rat entorhinal cortex in vitro : site of initiation and the role of excitatory amino acid receptors. Neuroscience. 1990b;34:657–670. doi: 10.1016/0306-4522(90)90172-z. [DOI] [PubMed] [Google Scholar]
- 29.Kaila K. Ionic basis of GABAAreceptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 30.Kaila K, Rydqvist B, Pasternack M, Voipio J. Inward current caused by sodium-dependent uptake of GABA in the crayfish stretch-receptor neurone. J Physiol (Lond) 1992;453:627–645. doi: 10.1113/jphysiol.1992.sp019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köhling R, Nagao T, Lücke A, Mattia D, Avoli M. Field-potential activity induced by 4-aminopyridine in the rat hippocampal/entorhinal slice preparation. Soc Neurosci Abstr. 1995;21:981. [Google Scholar]
- 32.Köhling R, Lücke A, Nagao T, Speckmann E-J, Avoli M. Extracellular potassium clearance in the hippocampus of rats with long-term seizures. Neurosci Lett. 1996;201:87–91. doi: 10.1016/0304-3940(95)12136-r. [DOI] [PubMed] [Google Scholar]
- 33.Lambert NA, Borroni AM, Grover LM, Teyler TJ. Hyperpolarizing and depolarizing GABAA receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J Neurophysiol. 1991;66:1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- 34.Lothman EW, Stringer JL, Bertram EH. Cambridge UP; CambridgeUK: 1993. Rapidly recurring seizures and status epilepticus: ictal density as a factor in epileptogenesis. In Epilepsy: models, mechanisms and concepts (Schwartzkroin PA, ed), pp 323–355. . [Google Scholar]
- 35.Louvel J, Avoli M, Kurcewicz I, Pumain R. Extracellular free potassium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. Neurosci Lett. 1994;167:97–100. doi: 10.1016/0304-3940(94)91036-7. [DOI] [PubMed] [Google Scholar]
- 36.Lücke A, Nagao T, Köhling R, Avoli M. Synchronous potentials and elevations in [K+]o in the adult rat entorhinal cortex maintained in vitro. Neurosci Lett. 1995;185:155–158. doi: 10.1016/0304-3940(95)11248-u. [DOI] [PubMed] [Google Scholar]
- 37.MacVicar BA, Tse FWY, Crichton SA, Kettenmann H. GABA-activated Cl− channels in astrocytes of hippocampal slices. J Neurosci. 1989;9:3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol. 1964;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- 40.Michelson HB, Wong RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- 41.Mody I, Lambert JDC, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- 42.Morris ME, Obrocea GV, Avoli M (1996) Extracellular K+ accumulations and synchronous GABA-mediated potentials evoked by 4-aminopyridine in the adult rat hippocampus. Exp Brain Res, in press. [DOI] [PubMed]
- 43.Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in combined slices of the rat hippocampus-entorhinal cortex. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- 44.Paré D, deCurtis M, Llinás R. Role of the hippocampal-entorhinal loop in temporal lobe epilepsy: extra- and intracellular study in the isolated guinea pig brain in vitro . J Neurosci. 1992;12:1867–1881. doi: 10.1523/JNEUROSCI.12-05-01867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perreault P, Avoli M. Effects of low concentrations of 4-aminopyridine of CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989;67:953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- 46.Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- 47.Perreault P, Avoli M. 4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 49.Rutecki PA, Lebeda F, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54:1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- 50.Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- 51.Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzkroin PA. Cambridge UP; CambridgeUK: 1993. Epilepsy: models, mechanisms, and concepts, p 544. . [Google Scholar]
- 53.Schwartzkroin PA, Prince DA. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978;147:117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- 54.Somjen GG, Giacchino JL. Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. J Neurophysiol. 1985;53:1098–1108. doi: 10.1152/jn.1985.53.4.1098. [DOI] [PubMed] [Google Scholar]
- 55.Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 56.Steinhäuser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–36. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- 57.Stringer JL, Lothman EW. Reverberatory seizure discharges in hippocampal-parahippocampal circuits. Exp Neurol. 1992;116:198–203. doi: 10.1016/0014-4886(92)90168-p. [DOI] [PubMed] [Google Scholar]
- 58.Tancredi V, Hwa GGC, Zona C, Brancati A, Avoli M. Low magnesium epileptogenesis in the rat hippocampal slice: electrophysiological and pharmacological features. Brain Res. 1990;511:280–290. doi: 10.1016/0006-8993(90)90173-9. [DOI] [PubMed] [Google Scholar]
- 59.Voskyul RA, Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 1985;342:54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- 60.Walther H, Lambert JDC, Jones RSG, Heinemann U, Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986;69:156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- 61.Wilson WA, Swartzwelder HS, Anderson WW, Lewis DV. Seizure activity in vitro: a dual focus model. Epilepsy Res. 1988;2:289–293. doi: 10.1016/0920-1211(88)90036-8. [DOI] [PubMed] [Google Scholar]