Abstract
A cDNA clone is described that encodes a novel G-protein-coupled dopamine receptor (DopR99B) expressed in Drosophila heads. The DopR99B receptor maps to 99B3–5, close to the position of the octopamine/tyramine receptor gene at 99A10–B1, suggesting that the two may be related through a gene duplication. Agonist stimulation of DopR99B receptors expressed in Xenopus oocytes increased intracellular Ca2+ levels monitored as changes in an endogenous inward Ca2+-dependent chloride current. In addition to initiating this intracellular Ca2+ signal, stimulation of DopR99B increased cAMP levels. The rank order of potency of agonists in stimulating the chloride current is: dopamine > norepinephrine > epinephrine > tyramine. Octopamine and 5-hydroxytryptamine are not active (<100 μm). This pharmacological profile plus the second-messenger coupling pattern suggest that the DopR99B receptor is a D1-like dopamine receptor. However, the hydrophobic core region of the DopR99B receptor shows almost equal amino acid sequence identity (40–48%) with vertebrate serotonergic, α1- and β-adrenergic, and D1-like and D2-like dopaminergic receptors. Thus, thisDrosophila receptor defines a novel structural class of dopamine receptors. Because DopR99B is the second dopamine receptor cloned from Drosophila, this work establishes dopamine receptor diversity in a system amenable to genetic dissection.
Keywords: cloned dopamine receptor, Drosophila melanogaster, Xenopus oocyte expression, adenylyl cyclase, calcium, gene mapping
Dopamine is a neurotransmitter in both the central and the peripheral nervous systems and is involved in a variety of important physiological and behavioral processes, such as neuroendocrine function, emotion, and motor control (Civelli et al., 1993). Moreover, dopaminergic systems have been implicated in several neurological disorders, including Parkinson’s disease and schizophrenia (Seeman et al., 1984; Goldstein and Deutch, 1992;Wichmann and DeLong, 1993). Dopamine exerts its effects by interacting with G-protein-coupled, heptahelical membrane receptors on the cell surface (Jackson and Westlind-Danielsson, 1994).
Numerous agonists and antagonists of dopamine receptors have been discovered in the search for new drugs to battle neurological disorders. Using these drugs, dopamine receptors were originally classified into D1 and D2 subtypes based on their pharmacological specificity with neuroleptics (Kebabian and Calne, 1979), a class of dopamine antagonists used to alleviate the main symptoms of schizophrenia (Levinson, 1991). Molecular cloning of dopamine receptors has revealed at least five distinct subtypes (D1–D5) that can be divided into two classes, the D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors based on their sequence similarity and pharmacological profile (Gingrich and Caron, 1993). These two classes of dopamine receptors are linked to distinct cascades for signal transduction. Activation of D1-like receptors stimulates adenylyl cyclase and phosphatidylinositol-4,5-bisphosphate (PI) metabolism, whereas D2-like receptor activation inhibits adenylyl cyclase and activates potassium channels (Gingrich and Caron, 1993; Jackson and Westlind-Danielsson, 1994).
Dopamine and dopamine receptors have long been thought to play an important role in the invertebrate nervous system (Weiss and Drummond, 1981; Sonetti et al., 1987; Walker and Holden-Dye, 1989; Ali and Orchard, 1994; Hall, 1994). We report here the molecular cloning and functional characterization of a novel Drosophila dopamine receptor that shows a pharmacology and second-messenger coupling pattern similar to D1-like receptors. However, thisDrosophila receptor differs structurally from the D1-like group of dopamine receptors that includes mammalian D1 and D5 subtypes, and it also differs from a previously reported D1-like receptor fromDrosophila (Gotzes et al., 1994; Sugamori et al., 1995). Northern blot analysis suggests that this novel Drosophilareceptor is expressed in both central and peripheral nervous systems. Stimulation of these receptors, expressed in Xenopusoocytes, generates a calcium signal and increases cAMP levels. Gene mapping provides a first step toward future studies aimed at mutant dissection of the physiological roles of dopamine receptor subtypes in the intact organism, including their possible involvement in memory and learning.
MATERIALS AND METHODS
PCR amplification for initial isolation of new receptor genomic DNA. The forward primer (OPS3: CATAGCCCTCGACCGGTACT) encodes the cytoplasmic end of the third transmembrane domain of theDrosophila octopamine/tyramine receptor (OctyR99AB) (Arakawa et al., 1990). The reverse primer (OPS4: GGCAGCCAGCAGATGACGAA) encodes the middle region of transmembrane domain VI from this receptor. The PCR template was 300 ng of Drosophila genomic DNA prepared from wild-type Canton-S adult flies (Jowett, 1986). The 100 μl PCR mixture contained 0.2 mm each of deoxyribonucleotide triphosphate, 10 mm Tris-HCl buffer, pH 8.3, 50 mm KCl, 1.5 mm MgCl2, 0.001% gelatin, 0.1 μm of each primer, and 2.5 units of AmpliTaq DNA polymerase (Perkin-Elmer, Norwalk, CT). PCR was performed under reduced stringency annealing conditions: 94°C for 2 min, 33°C for 2 min, 72°C for 2 min for 6 cycles followed by an additional 31 cycles with an annealing temperature of 42°C instead of 33°C. Final extension was 10 min at 72°C. PCR products were analyzed by electrophoresis of 10 μl of reaction mixture on a 1% agarose gel.
DNA sequencing. Direct sequencing of the PCR product was performed as described previously (Feng et al., 1995; Zheng et al., 1995) using a Taq Dye Primer Cycle Sequencing kit (Applied Biosystems, Foster City, CA). To facilitate the sequencing of the cDNA clone, nested deletions were used (Henikoff, 1987). Each segment of DNA was sequenced at least twice in both directions. The contig was assembled using Geneworks software (Intelligenetics, Mountain View, CA).
Screening for cDNA clones. The 0.68 kb fragment from the initial PCR amplification experiment was labeled with [α-32P]dCTP (∼110 TBq/mmol) using the Multiprime DNA labeling system (Amersham, Arlington Heights, IL) and was used to screen a Drosophila head cDNA library (Itoh et al., 1985) in λgt11, generously provided by Dr. Paul Salvaterra (Beckman Research Institute, Duarte, CA). Four positive clones were identified by high-stringency (Sambrook et al., 1989) screening of 4 × 105 plaque-forming units (pfu). The longest insert (4 kb) was cut out with EcoRI and subcloned into pBluescript II SK(−) for further analysis. This insert and the gene that encodes it are referred to as DopR99B throughout this paper.
Northern blots. Heads, bodies, and appendages (legs and antennae) were isolated from frozen adult flies (Schmidt-Nielsen et al., 1977). Poly(A)+ RNA and blots were prepared as described previously (Feng et al., 1995; Zheng et al., 1995). Poly(A)+ RNA (10 μg/lane) was run on a denaturing, formaldehyde agarose (0.8%) gel. Blots were probed with the 32P-labeled 0.68 kb PCR fragment added to a final concentration of 106 cpm/ml. After high-stringency washing (Feng et al., 1995; Zheng et al., 1995), blots were exposed to x-ray film for 24 hr at −70°C.
In situ hybridization to salivary gland chromosome squashes.The 0.68 kb PCR fragment was biotinylated, hybridized to larval salivary gland polytene chromosome squashes, and localized using the Gibco (Gaithersburg, MD) Bluegene detection kit as described by Engels et al. (1985) with minor modifications (Feng et al., 1995; Zheng et al., 1995).
Expression in Xenopus oocytes. Sense cRNA was prepared in vitro from the DopR99B clone in the pBluescript II SK(−) vector using T7 RNA polymerase (Stratagene, La Jolla, CA) after linearizing the plasmid with NotI (Promega, Madison, WI). Transcripts were capped by adding 0.75 units of m7G(5′)ppp(5′)G (Boehringer Mannheim, Indianapolis, IN) to a standard 150 μl transcription reaction (Stratagene kit).
Stage V and VI oocytes from virgin female adult Xenopus laevis were manually separated and placed in sterile ND96 medium [in mm: NaCl 96, KCl 2, CaCl2 1.8, MgCl2 1, HEPES buffer (pH 7.6) 5, containing 2.4 mm sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.2 mg/ml gentamycin]. The oocytes were defolliculated enzymatically by incubation in ND96 containing collagenase (2 mg/ml) for 30 min. Oocytes were then injected with 50 ng of Drosophila DopR99B receptor sense cRNA and incubated at 19°C for 2–5 d. Uninjected oocytes were used as controls.
Electrophysiological recordings were made from oocytes using a two-microelectrode voltage-clamp technique, at a −60 mV holding potential, to measure oocyte currents (Van Renterghem et al., 1987). Oocytes were continuously superfused with ND96 during the experiments at room temperature, and drugs were added to the superfusate.
cAMP assays. To monitor cAMP levels, individual oocytes were preincubated for 30 min in ND96 plus 100 μmisobutylmethylxanthine (IBMX). Experimental oocytes were incubated for a further 30 min with the desired concentration of agonist in the same medium while control oocytes (to measure basal cAMP levels) were incubated in parallel in the same medium without agonist. After the incubations, each oocyte was homogenized in 500 μl of acidified ethanol, centrifuged to remove particulate matter, and the supernatant was evaporated to dryness in a vacuum centrifuge (Savant, Farmingdale, NY). Each sample was taken up in 60 μl of assay buffer and assayed for cAMP using a commercial assay kit (Amersham).
Drugs. The drugs used in the classification of the expressed receptor were obtained from the following sources: dopamine hydrochloride, (−)-norepinephrine hydrochloride, (−)-epinephrine, tyramine hydrochloride,dl-octopamine hydrochloride, 5-hydroxytryptamine hydrochloride, (±)-isoproterenol hydrochloride, phentolamine hydrochloride, and dl-propranolol were from Sigma (Poole, Dorset, UK); R(+)-SKF-38393 [R(+)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol], quineloranedihydrochloride, (−)-quinpirole hydrochloride, PD-128,907 [(+)-(4aR,10bR)-3,4,4a,10b-tetrahydro4-propyl-2H,5H-(1)benzopyrano-(4,3-b)-1,4-oxazin-9-ol-hydrochloride],cis-(Z)-flupenthixol dihydrochloride,R(+)SCH-23390 [R(+)-7chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzepine hydrochloride], S(−)-sulpiride, spiperone hydrochloride, (+)-butaclamol hydrochloride, S(−)-eticlopride hydrochloride, domperidone, (+)-bromocriptine methanesulfonate, (±)-6-chloro-APB [(±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3benzazepine hydrobromide], R(+)-6-bromo-APB (R(+)-6-bromo7, 8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydro-bromide), (±)-6-chloro-PB [(±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide], and (±)-PPHT [(±)-2-(N-phenylethyl-N-propyl)amino-5-hydroxytetralin hydrochloride] were from Research Biochemicals (Natick, MA).
RESULTS
Cloning of a novel Drosophila dopamine receptor
When we began these studies, cDNAs encoding several G-protein-coupled receptors had been cloned from Drosophila, including a muscarinic acetylcholine receptor (Onai et al., 1989), several serotonergic receptors (Witz et al., 1990; Saudou et al., 1992), and an adrenergic receptor homolog (OctyR99AB) (Arakawa et al., 1990). However, there were no representatives of the dopaminergic receptor family cloned from invertebrates even though the role of dopamine was well established in the invertebrate nervous system.
To expand the group of cloned G-protein-coupled receptors fromDrosophila, we used reduced stringency PCR with primers from regions conserved in all biogenic amine receptors. To facilitate the identification of those PCR products that were likely to encode new receptors, we amplified across a region including cytoplasmic loop 3 (between transmembrane domains V and VI). This nonconserved loop varies greatly in length among different G-protein-coupled receptors (Probst et al., 1992), so different size amplification products are expected and would serve to distinguish fragments encoding new receptors from those encoding previously cloned ones. In addition, the amplified region also includes the conserved transmembrane domains IV, V, and part of VI. Because these domains are easily recognized, this allows rapid identification of these amplified fragments encoding G-protein-coupled receptors.
PCR primers were chosen by aligning the amino acid sequence of theDrosophila octopamine/tyramine receptor (OctyR99AB) (Arakawa et al., 1990) with sequences of a subset of previously cloned vertebrate biogenic amine receptors. A forward primer sequence (OPS3) was selected from the region of the Drosophilaoctopamine/tyramine receptor cDNA that encodes the cytoplasmic end of the third transmembrane domain (amino acids IALDRYW). This included the DRY sequence that is found in almost all cloned G-protein-coupled receptors. A reverse primer sequence (OPS4) was from the conserved middle region of transmembrane domain VI of OctyR99AB cDNA (encoding amino acids FVICWLP). Genomic DNA was used as the template to avoid assumptions concerning the time- and tissue-specific expression of receptors.
Using the OPS3 and OPS4 PCR primers, five fragments (1.7, 1.0, 0.68, 0.60, and 0.46 kb) were amplified from Drosophila genomic DNA. Direct sequencing of the PCR products revealed that the 1.7 kb fragment is the genomic equivalent of OctyR99AB with a 0.7 kb intron in the region encoding cytoplasmic loop 3. The 0.68 kb fragment had high sequence similarity to OctyR99AB and other G-protein-coupled receptors in the transmembrane domains, but was different in cytoplasmic loop 3. These data suggested that this 0.68 kb fragment encodes part of a novel G-protein-coupled receptor gene inDrosophila. Using this 0.68 kb PCR fragment as a probe, four overlapping cDNA clones were isolated from an adult head cDNA library. The clone with the longest insert (4 kb) was completely sequenced and has been designated DopR99B.
Structural features of the DopR99B cDNA sequence
The 4 kb cDNA insert contains an open reading frame encoding 538 amino acids with a predicted molecular mass of 59.5 kDa (Fig.1A). The open reading frame is defined by the first in-frame ATG that is preceded by an in-frame stop codon. Although the flanking sequence preceding this ATG (−4 to −1, TGCA) does not match well with the Drosophila consensus sequence for a translation start site [C/A A A A/C (Cavener, 1987)], a potential ribosome-binding site around this ATG was identified (Fig.1A). A comparison of the cDNA sequence in the regions used for the original PCR primers shows that in the forward primer region there are 4 base mismatches concentrated in the first 7 nucleotides from the 5′ end. The reverse primer had only 1 base mismatch in the fourth base position from the 3′ end. The robust amplification of the 0.68 kb product indicates that these mismatches were all tolerated at the initial annealing temperature of 33°C.
Fig. 1.

The DopR99B sequence. A, Nucleotide sequence and deduced amino acid sequence of theDrosophila dopamine receptor DopR99B. In-frame stop codons are indicated by asterisks. The sequence of a potential ribosome binding site is boxed. The seven transmembrane domains are underlined and numbered. Potential PKC phosphorylation sites are indicated by Δ. Potential N-glycosylation sites are marked by ♦. B, Hydrophobicity plot of the deduced DopR99B amino acid sequence. Regionsabove the line are hydrophobic. Transmembrane domains arenumbered. The hydropathy plot was done using the method ofKyte and Doolittle (1982) and the Geneworks software (Intelligenetics). The Genbank accession number for DopR99B is U34383.
Sequence comparison with other G-protein-coupled receptors
The deduced amino acid sequence for DopR99B shows many standard characteristics of the G-protein-coupled receptor gene family. The hydropathy plot (Fig. 1B) reveals the 7 transmembrane domains diagnostic of all members of this group of receptors (Probst et al., 1992). At the cytoplasmic end of transmembrane domain III is the highly conserved DRY sequence. This amino acid triplet is thought to be important in G-protein coupling (Dixon et al., 1987; Fraser et al., 1988). Single cysteine residues (at 182 and 261) in extracellular loops 1 and 2, respectively, are also conserved. These residues form a disulfide bond that stabilizes the functional receptor structure (Dixon et al., 1987; Karnik et al., 1988; Fraser, 1989). Two aspartate residues in TM II and TM III that are conserved in all catecholamine receptors are also conserved in DopR99B (D154 and D189). These two aspartates are thought to play a direct role in binding the amine groups on catecholamines (Strader et al., 1988). The three serine residues in TM V that are postulated to interact with the catecholamine ring hydroxyl groups (Strader et al., 1989; Pollock et al., 1992) are also conserved in DopR99B (S273, S274, S277). Thus, this new receptor has appropriately placed amino acid side chains for binding catecholamines.
There are other, more general structural motifs that are found in thisDrosophila receptor as well as in other G-protein-coupled receptors. For example, mammalian dopamine receptors typically have one to four N-linked glycosylation sites in their extracellular domains (Jackson and Westlind-Danielsson, 1994). This Drosophilareceptor has four potential N-linked glycosylation sites in the amino terminus (positions 5, 31, 47, and 68). This region is likely to be an extracellular domain. All G-protein-coupled receptors have protein kinase C sites in cytoplasmic loop 3 (between TM V and VI) and in the C-terminal tail, but the exact positions of these sites are not strictly conserved. This newly cloned Drosophila receptor has eight potential protein kinase C phosphorylation sites in cytoplasmic loop 2 (position 222), cytoplasmic loop 3 (positions 302, 320, 328, and 405), and in the C terminus (positions 504, 509, and 514). Finally, many G-protein-coupled receptors undergo a post-translational palmitoylation at a conserved cysteine residue in the C-terminal tail (Gingrich and Caron, 1993). This modification serves to anchor the tail to the internal face of the plasma membrane, creating a fourth cytoplasmic loop. The Drosophila receptor has several cysteines that could serve this function (at 490, 492, 493, 517, 521, and 534). The conserved structural motifs, along with the fact that this gene product has, overall, a high sequence similarity with other G-protein-coupled receptors, suggest that it encodes a novel G-protein-coupled receptor in Drosophila.
Comparison of the predicted hydrophobic core region of the DopR99B deduced amino acid sequence revealed significant sequence similarity with vertebrate and invertebrate dopamine receptors (41–47% identity), serotonin receptors (40–47% identity), α1- and β-adrenergic receptors (46–48% identity), as well as theDrosophila octopamine/tyramine receptor from which the primers were derived (47% identity). Detailed sequence comparison of either the hydrophobic core region or the full-length deduced amino acid sequence showed that the DopR99B receptor clearly belonged to the biogenic amine class of receptors, but it was not possible to predict its receptor type based on sequence comparisons alone. The dendrogram in Figure 2 shows that DopR99B did not fall clearly within one of the biogenic amine receptor groups, although a previously described (Gotzes et al., 1994; Sugamori et al., 1995)Drosophila dopamine receptor (DopR35EF) consistently grouped with the vertebrate D1-like dopamine receptors. To define the DopR99B receptor-type pharmacologically, we expressed it in Xenopusoocytes.
Fig. 2.

Structural relationship of DopR99B with other G-protein-coupled receptors. Sequence alignment was done with the PILEUP program of the Wisconsin Genetic Computer Group (GCG) software (Devereux et al., 1984) using the hydrophobic cores of each receptor listed. The hydrophobic core was defined as the region between and including transmembrane domains I and V plus the region between and including transmembrane domains VI and VII. The lengths of the horizontal lines are inversely proportional to the percentages of sequence similarity between receptors or groups of receptors. In the receptor names listed, the first three to four letters refer to the general receptor type: Adr, adrenergic; Dop, dopamine; 5ht, serotonin; Octy, octopamine/tyramine. The next letters in the name refer to the species:dro, Drosophila; hum, human;rat, rat; xen, Xenopus. The exceptions to this nomenclature are three Drosophilareceptors (DopR35EF, DopR99B,OctyR99AB). For these, the type name is followed byR (receptor) and then the salivary gland chromosome map position. Pathway refers to the second-messenger coupling reported in the literature for each receptor: PLC, phospholipase C;AC, adenylyl cyclase. An upward arrowindicates stimulation of the indicated second-messenger system, and adownward arrow indicates inhibition.Accession # refers to the Genbank database locator number.
Expression studies in Xenopus oocytes
Xenopus oocytes translate exogenous mRNAs encoding neurotransmitter receptors and incorporate the receptor proteins into their cell membranes (Sumikawa et al., 1981; Barnard et al., 1982). To define which ligands activate the DopR99B receptor, we expressed its cRNA in Xenopus oocytes and tested the ability of a range of biogenic amines to initiate responses. The application of dopamine initiated transient inward current responses in injected oocytes (Fig.3A). Uninjected, control oocytes showed no responses (data not shown). The responses have a reversal potential of −21.7 ± 2.9 mV (n = 3) estimated from current–voltage (I–V) plots, consistent with their mediation via the activation of the endogenous, inward calcium-dependent chloride current of the oocyte. This inward current is presumably generated by the same mechanism originally reported byMasu et al. (1987) after stimulation of another G-protein-coupled receptor (the bovine substance-K receptor) expressed in oocytes; i.e., receptor-mediated activation of phospholipase C increases PI hydrolysis and stimulates release of intracellular calcium which, in turn, activates the endogenous calcium-dependent chloride current of the oocyte.
Fig. 3.
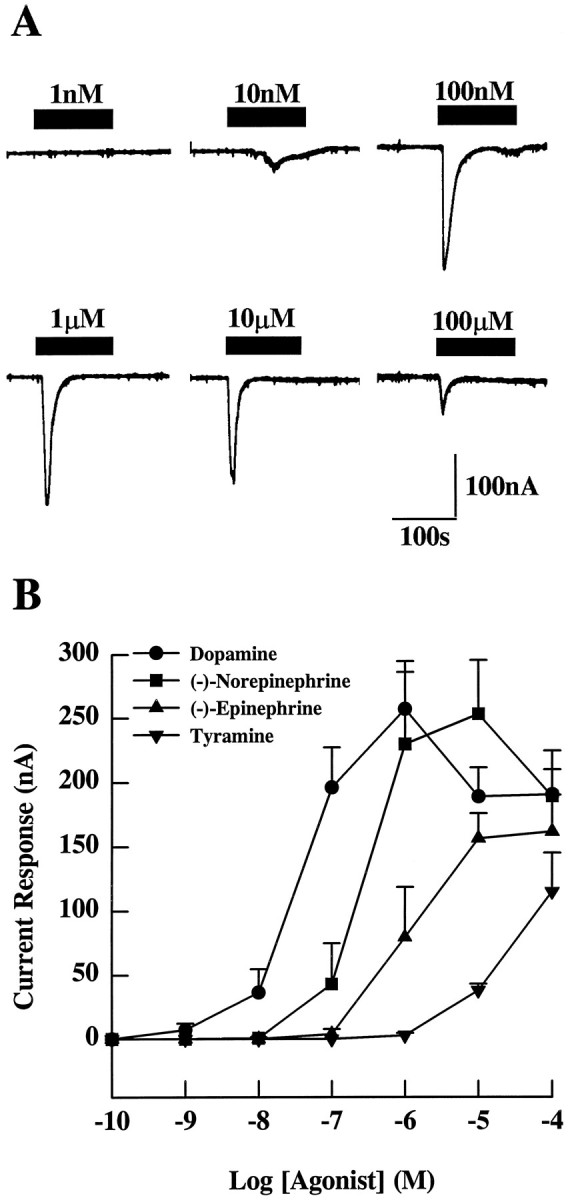
Inward current response after agonist stimulation mediated by the DopR99B receptor expressed in Xenopusoocytes. Two minute pulses of agonists were given to Xenopusoocytes 3 d after the injection of DopR99B cRNA. A, Typical responses of a single oocyte to various concentrations of dopamine.B, Dose–response curves for dopamine, (−)-norepinephrine, (−)-epinephrine, and tyramine. The results (from at least 6 oocytes) are expressed as the mean peak inward current ± SE initiated by each amine.
Dose–response curves (Fig. 3B) indicate that the threshold response to dopamine occurred in the region of 1 nm, whereas the maximal response occurred at 1 μm. Exposure of injected oocytes to concentrations of dopamine >1 μm consistently produced smaller responses (Fig. 3A,B). Figure3B also shows that the catecholamines norepinephrine and epinephrine induce inward currents in injected oocytes. However, these catecholamines are less effective than dopamine. The injected oocytes also showed inward currents when exposed to tyramine (Fig.3B), but much higher concentrations were required and the average current response was smaller at the maximum concentration tested (100 μm) than for the other amines. Oocytes showed no response to eitherdl-octopamine or 5-hydroxytryptamine at concentrations up to 100 μm. Taken together, these results suggest that the DopR99B cDNA clone encodes aDrosophila dopamine receptor.
To determine whether this Drosophila dopamine receptor falls into the D1-like or D2-like pharmacological category, the ability of antagonists (10 μm) to block the responses to 1 μm dopamine in oocytes expressing the DopR99B receptor was determined (Table 1A). The rank order potency of the dopaminergic antagonists tested was: flupenthixol > R(+)-SCH-23390 > S(−)-sulpiride > spiperone > (+)butaclamol > S(−)eticlopride = domperidone. The D1-like receptor blockers were, in general, more effective than those with D2-like receptor specificity. The α-adrenergic blocker phentolamine and the β-adrenergic blockerdl-propranolol were poor blockers, falling within the same range as the weaker D2-like receptor blockers. Thus, in terms of antagonist responses, the DopR99B receptor would be classed with the D1-like dopamine receptor group.
Table 1.
Effects of agonists and antagonists on inward currents inXenopus oocytes expressing the DrosophilaDopR99B receptor
| A | |||
|---|---|---|---|
| Antagonists (10 μm) | % of Response to 1 μmdopamine | Receptor-type specificity | |
| Flupenthixol | 3.9 ± 1.6% | (n = 5) | D1/D2-like |
| R(+) SCH23390 | 34.2 ± 8.9% | (n = 6) | D1/D5 |
| S(−)-sulpiride | 56.8 ± 16.1% | (n = 5) | D2-like |
| Spiperone | 63.0 ± 9.2% | (n = 7) | D2-like |
| (+)Butaclamol | 66.7 ± 4.5% | (n = 6) | D2/D1-like |
| Phentolamine | 71.6 ± 7.1% | (n = 6) | α-Adrenergic |
| dl-Propranolol | 89.6 ± 5.9% | (n = 7) | β-Adrenergic |
| S(−)-eticlopride | 103.2 ± 10.9% | (n = 4) | D2-like |
| Domperidone | 118.3 ± 7.0% | (n = 3) | Peripheral D2 |
| B | |||
|---|---|---|---|
| Agonists (10 μm) | % of Response to 1 μmdopamine | Receptor-type specificity | |
| (±)-6-Chloro-APB | 89.4 ± 3.8% | (n = 6) | D1-like |
| R(+)-6-bromo-APB | 73.3 ± 9.1% | (n = 6) | D1-like |
| (±)-6-Chloro-PB | 19.6 ± 7.7% | (n = 5) | D1-like |
| Quinelorane | 11.3 ± 3.0% | (n = 8) | D2-like |
| (±)-Bromocriptine | 7.5 ± 2.6% | (n = 5) | D2-like |
| Quinpirole | 4.1 ± 2.3% | (n = 6) | D2/D3 |
| R(+)-SKF-38393 | 1.8 ± 1.2% | (n = 6) | D1-like |
| (±)-Isoproterenol | 1.3 ± 0.9% | (n = 6) | β-Adrenergic |
| PD-128,907 | 0 | (n = 6) | D3 |
| (±)-PPHT | 0 | (n = 4) | D2-like |
Inward currents were initiated by 2 min pulses of 1 μm dopamine. The mean response to a 2 min control pulse of 1 μm dopamine was 331.4 ± 15.6 nA (n = 73).
A, The size of the response to a dopamine pulse given in the presence of 10 μm antagonist is expressed as a percentage ± SE of the response to a control dopamine pulse given to the same oocyte. Antagonists alone did not initiate any currents.
B, Agonists were applied as 2 min pulses. The inward currents generated are expressed as the percentage ± SE of the response of the same oocyte to a control dopamine pulse.
When a range of synthetic dopamine agonists specific for various vertebrate receptor subtypes was tested (Table 1B), both D1-like and D2-like agonists mimicked the dopamine responses in oocytes expressing the DopR99B receptor. However, with the exception of the relatively ineffective D1-like agonist SKF-38393, the D1-like agonists were more effective than the D2-like agonists. Thus, these pharmacological studies of the inward currents generated by dopamine application indicate that the receptor encoded by the DopR99B cDNA, again, has a pharmacological profile closer to D1-like dopamine receptors.
Another way to categorize receptors is in terms of the second-messenger systems to which they couple. Therefore, we have also investigated second-messenger coupling for the DrosophilaDopR99B receptor. This receptor, when expressed in Xenopusoocytes, appears to couple to multiple second-messenger systems. As described above, receptor activation initiates a calcium response. In addition, dopamine activation of the DopR99B receptor also increases cAMP levels in oocytes (Fig. 4). This response is mimicked by the application of epinephrine and norepinephrine to expressing oocytes, but not by phenylethylamine, 5-hydroxytryptamine,dl-octopamine, phenylethanolamine, or tyramine when these agonists were tested at a concentration of 10 μm (Table 2). Stimulation of cAMP levels is characteristic of second-messenger coupling by D1-like receptors, whereas inhibition of cAMP synthesis is characteristic of D2-like receptors (see Fig. 2). Therefore, in this respect, DopR99B also resembles D1-like receptors. We have named this receptor DopR99B (for Dopamine Receptor fromDrosophila mapping to 99B to distinguish it from another Drosophila D1-like dopamine receptor (which we designate as DopR35EF) described previously (Gotzes et al., 1994;Sugamori et al., 1995).
Fig. 4.
Dose–response curves for increase in cAMP levels in oocytes expressing DopR99B. Five days after injection withDrosophila DopR99B receptor cRNA, injected and uninjected (control) oocytes were treated with the indicated dopamine concentrations for 30 min in the presence of 100 μm IBMX after preincubation for 30 min in 100 μm IBMX. The results are expressed as the mean oocyte cAMP level (pmol/oocyte) ± SE (n = 5 oocytes).B, Basal levels.
Table 2.
Effects of biogenic amines on cAMP levels inXenopus oocytes expressing the DrosophilaDopR99B receptor
| Agonist | DopR99B cRNA-injected | Uninjected | ||
|---|---|---|---|---|
| Basal | 0.82 ± 0.05 | (n = 9) | 0.87 ± 0.08 | (n = 10) |
| Dopamine | 1.46 ± 0.10 | (n = 10) | 0.77 ± 0.09 | (n = 10) |
| (−)-Epinephrine | 1.28 ± 0.15 | (n = 8) | 0.76 ± 0.08 | (n = 10) |
| (−)-Norepinephrine | 1.15 ± 0.06 | (n = 8) | 0.71 ± 0.07 | (n = 9) |
| Phenylethylamine | 0.86 ± 0.09 | (n = 10) | 0.69 ± 0.10 | (n = 10) |
| 5-Hydroxytryptamine | 0.83 ± 0.09 | (n = 5) | 0.96 ± 0.05 | (n = 5) |
| dl-Octopamine | 0.78 ± 0.07 | (n = 10) | 0.69 ± 0.08 | (n = 10) |
| Phenylethanolamine | 0.76 ± 0.06 | (n = 10) | 0.89 ± 0.06 | (n = 10) |
| Tyramine | 0.71 ± 0.08 | (n = 10) | 0.71 ± 0.09 | (n = 9) |
cAMP levels were measured 5 d after cRNA injection. Before measurement, oocytes were preincubated for 30 min in 100 μm IBMX and then exposed to 10 μm biogenic amine for 30 min in the presence of 100 μm IBMX. The results are expressed as the mean oocyte cAMP level (pmol/oocyte) ± SE.
mRNA distribution and chromosomal localization of DopR99B
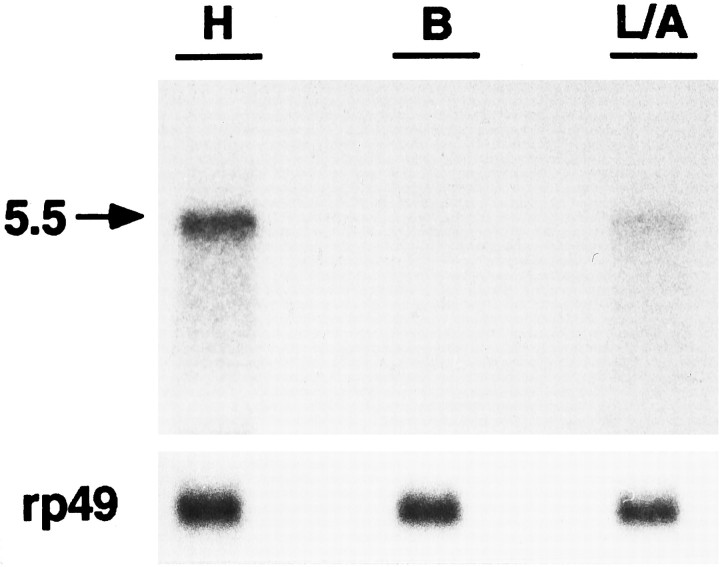
To determine the distribution of the DopR99B transcript in different body parts, we did Northern blot analysis with poly(A)+ RNA isolated from heads, bodies, and appendages (mostly legs and antennae). The blot was probed with the original 0.68 kb PCR fragment that contains the nonconserved cytoplasmic loop 3. As shown in Figure 5, a single band of 5.5 kb mRNA was detected predominantly in heads with a lighter signal in appendages. No expression was detected in bodies. This distribution is consistent with a role for these dopamine receptors in the central and peripheral nervous systems. The absence of major expression in bodies suggests that this receptor is not highly expressed in flight muscle.
Fig. 5.
Northern blot analysis of DopR99B. A Northern blot of poly(A)+ RNA isolated from heads (H), bodies (B), and legs/antennae (L/A) was probed with the32P-labeled 0.68 kb PCR fragment. To control for mRNA recovery, the blot was reprobed with ribosomal protein cDNA (rp49), which is expressed throughout the organism (O’Connell and Robash, 1984).
To determine where DopR99B maps in the Drosophila genome, the 0.68 kb PCR fragment was biotinylated and hybridized toDrosophila salivary gland polytene chromosomes (Fig.6). The probe hybridized to a single location on the right arm of chromosome 3 at 99B3–5. This location is close to, but distinct from, the Drosophila octopamine/tyramine receptor gene that maps to 99A10–B1 (Arakawa et al., 1990). The structural relationship between DopR99B and this Drosophilaoctopamine/tyramine receptor (OctyR99AB, Fig. 2) suggests that these receptors may be related by a local gene duplication followed by independent evolution.
Fig. 6.
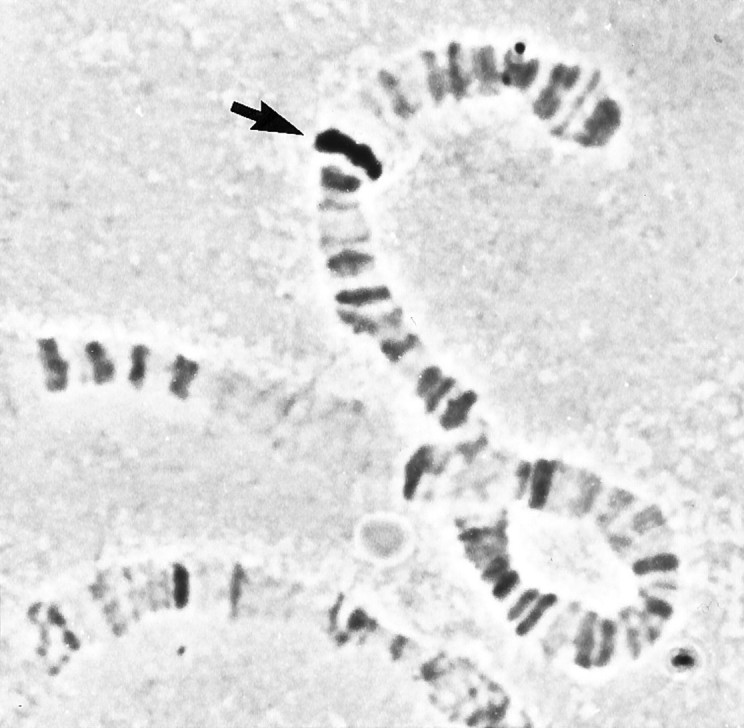
Chromosome localization of DopR99B. In situ hybridization to Drosophila salivary gland polytene chromosomes using the biotinylated 0.68 kb PCR fragment. Thearrow indicates the site of hybridization at 99B3–5 on the right arm of chromosome 3.
DISCUSSION
The present study establishes, for the first time, the presence of multiple dopamine receptor types in insects. These receptors, which we have designated as DopR35EF [cloned in previous studies (Gotzes et al., 1994; Sugamori et al., 1995)] and DopR99B (present study), are similar in that both stimulate adenylyl cyclase when activated. Although both resemble the vertebrate D1-like dopamine receptor class, there are differences. For example, vertebrate D1-like receptors generally have long C-terminal tails ranging from 113 to 117 amino acids in length, whereas D2-like receptors have short tails in the range of 16–18 amino acids (Jackson and Westlind-Danielsson, 1994). The two Drosophila dopamine receptors have C-terminal tails that are intermediate in length (62 amino acids for DopR35EF and 64 for DopR99B).
Although the two Drosophila receptors resemble each other with respect to C-terminal tail length, in overall sequence similarity DopR35EF is less similar to DopR99B (43% sequence identity in the hydrophobic core region) than it is to the vertebrate D1-like class of dopamine receptors (46–48% identity). When dendrograms are constructed based on structural relatedness (e.g., Fig. 2), DopR35EF consistently falls into a group with vertebrate D1-like dopamine receptors regardless of whether the entire sequence or only the hydrophobic core is used for comparison. DopR99B does not group with the D1-like receptors, but instead shows almost equal sequence identity to a number of different biogenic amine receptor groups. This suggests that the dopamine receptor subtypes in Drosophila did not arise from each other but, rather, may represent convergent evolution from different precursors.
Although the Drosophila DopR99B receptor does not fall into the D1-like structural group, it does show pharmacological properties similar to D1-like receptors (Jackson and Westlind-Danielsson, 1994;Seeman and VanTol, 1994) when expressed in Xenopus oocytes. Agonists and antagonists with D1-like selectivity are, in general, more effective than those with D2-like receptor selectivity. A comparison of our pharmacological data with the limited information on DopR35EF (Gotzes et al., 1994; Sugamori et al., 1995) suggests that these twoDrosophila dopamine receptors differ in their pharmacological specificity. For example, the dopamine receptor agonist SKF-38393 is 30% as effective as dopamine on DopR35EF (Gotzes et al., 1994), but is almost inactive on DopR99B. With respect to antagonists, SCH-23390 is less effective than flupenthixol and butaclamol on DopR35EF, but is intermediate between flupenthixol and butaclamol on DopR99B.
Agonist stimulation of DopR99B activates adenylyl cyclase to increase cAMP levels and also generates a calcium signal presumably through stimulation of phospholipase C. Both of these responses have been reported for vertebrate D1-like receptors (Figure 2) (Mahan et al., 1990). In contrast, vertebrate D2-like receptors usually show opposite effects on these second-messenger systems because they inhibit cAMP synthesis (Jackson and Westlind-Danielsson, 1994) and are associated with a decline in intracellular Ca2+ levels. Structural features, presumably in the second and third intracellular loops and in the C terminus, must underlie the ability of thisDrosophila dopamine receptor to couple to two different second-messenger systems. Because the DopR99B receptor is structurally distinct from classical vertebrate dopamine receptors, the use of chimeras and the application of in vitro mutagenesis may produce novel insights into how dopamine interacts with this receptor and how this interaction results in the stimulation of different systems.
Our results leave unanswered the question of whetherDrosophila has a D2-like dopamine receptor homolog. Dopamine and the enzymes involved in its synthesis are widely distributed in the insect nervous system (Klemm, 1976; Evans, 1980; Livingstone and Tempel, 1983; Brown and Nestler, 1985; Budnik and White, 1987). Previous studies demonstrating the presence of dopamine-sensitive adenylyl cyclases in insect nervous tissue (Nathanson and Greengard, 1973; Bodnaryk, 1979; Uzzan and Dudai, 1982; Orr et al., 1987) and salivary glands (House and Ginsborg, 1979; Lafon-Cazal and Bockaert, 1984; Evans and Green, 1990a,b; Ali and Orchard, 1994), together with radioligand binding studies on cockroach brains (Notman and Downer, 1987), suggested the presence of D1-like dopamine receptors in insects. These D1-like receptor responses could correspond to either the DopR99B receptor described in this report or the DopR35EF receptor (Gotzes et al., 1994; Sugamori et al., 1995) or both. There are no reports yet of D2-like dopamine receptor activity in insects, but this is not a well studied area. None of the other PCR amplification products found in our search for new receptor types in Drosophila encode additional dopamine receptors, although at least one product (the 0.60 kb fragment) represents another G-protein-coupled receptor (G. Feng, R. Venard, and L. M. Hall, unpublished results). Thus, a modification of our cloning strategy will be necessary to search for additional dopamine receptor cDNAs in Drosophila.
In insects, the dense innervation of the mushroom bodies by dopamine-immunoreactive neurons (Budnik and White, 1988; Schafer and Rehder, 1989: Nassel and Elkes, 1992), together with studies on behavioral mutants of Drosophila (Tempel et al., 1984;Buchner, 1991), has suggested roles for dopamine in both memory and learning, and in neuronal development. Analysis of theDrosophila mutant Ddc, which is deficient in dopa decarboxylase (therefore lacking dopamine and serotonin), reveals learning deficits (Tempel et al., 1984) and an abnormal pattern of neuronal arborization (Budnik et al., 1989) that can be partially rescued by feeding flies dopamine, but not serotonin. These studies suggest possible roles for cloned dopamine receptors fromDrosophila.
Our work, in conjunction with that of others (Gotzes et al., 1994;Sugamori et al., 1995), establishes that there are at least two genetically distinct dopamine receptors in Drosophila that both stimulate adenylyl cyclase when activated. The DopR99B receptor is also capable of generating a calcium signal. Are either of these receptors involved in the signal transduction role that dopamine plays in learning and memory? Because the DopR35EF receptors are preferentially expressed in the optic lobes (Gotzes et al., 1994), they are unlikely to play a role in learning and memory that involves mushroom body-mediated functions. In contrast, DopR99B is good candidate for dopaminergic modulation of learning and memory because this receptor is expressed preferentially in mushroom bodies (K. Han and R. Davis, personal communication). Genetic studies aimed at identifying dopamine receptor mutations in Drosophila will be useful in defining their physiological and behavioral roles.
Footnotes
This work was supported by National Institutes of Health Jacob Javits Neuroscience Investigator Award NS16204 to L.M.H. and NATO Collaborative Research Grant 900709 to P.D.E. and L.M.H. G.F. was supported, in part, by a predoctoral fellowship from the Pharmaceutical Manufacturers Association Foundation. V.R. was supported, in part, by a grant from the Isaac Newton Trust. C.T.K. was supported by National Institutes of Health training Grant GM07145 and by a Howard Hughes Predoctoral Fellowship. We thank Jane Pursey-Lee for outstanding technical assistance in the initial stages of cloning and sequencing the 0.68 PCR fragment, and Dr. Todd R. Jackman for helpful comments.
Correspondence should be addressed to Linda M. Hall, Department of Biochemical Pharmacology, State University of New York at Buffalo, 329 Hochstetter Hall, North Campus, Buffalo, NY 14260-1200 (please note that Dr. Hall is on sabbatical until 9/30/96. Her sabbatical address should be used for correspondence concerning this manuscript during that time).
Dr. Hall’s sabbatical address (9/95 to 9/30/96): Department of Microbiology and Molecular Genetics, Medical Sciences I Bldg C, Room 266, University of California-Irvine, Irvine, CA 92717-4025.
Dr. Feng’s current address: Department of Anatomy and Neurobiology, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110.
REFERENCES
- 1.Ali DW, Orchard I. Characterization of dopamine and serotonin receptors on the salivary glands of the locust, Locusta migratoria . Biog Amines. 1994;10:195–212. [Google Scholar]
- 2.Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Fraser CM, Venter JC. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Miledi R, Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond [Biol] 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 4.Bodnaryk RP. Identification of specific dopamine- and octopamine-sensitive adenylate cyclases in the brain of Mamestra configurata Wlk. Insect Biochem. 1979;9:155–162. doi: 10.1139/o79-028. [DOI] [PubMed] [Google Scholar]
- 5.Brown CS, Nestler C. Catecholamines and indolalkylamines. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology, Vol. 11. Pergamon; Oxford: 1985. pp. 436–497. [Google Scholar]
- 6.Budnik V, White K. Genetic dissection of dopamine and serotonin synthesis in the nervous system of Drosophila melanogaster . J Neurogenet. 1987;4:309–314. [PubMed] [Google Scholar]
- 7.Budnik V, White K. Catecholamine-containing neurons in Drosophila melanogaster distribution and development. J Comp Neurol. 1988;268:400–413. doi: 10.1002/cne.902680309. [DOI] [PubMed] [Google Scholar]
- 8.Budnik V, Wu C-F, White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989;9:2866–2877. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchner E. Genes expressed in the adult brain of Drosophila and effects of their mutations on behavior: a survey of transmitter- and second messenger-related genes. J Neurogenet. 1991;7:153–192. doi: 10.3109/01677069109167432. [DOI] [PubMed] [Google Scholar]
- 10.Cavener DR. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987;15:1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon RAF, Sigal IS, Candelore MR, Register RB, Scattergood W, Rands E, Strader CD. Structural features required for ligand binding to the β-adrenergic receptor. EMBO J. 1987;6:3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels WR, Preston CR, Thompson P, Eggleston WB. In situ hybridization to Drosophila salivary chromosomes with biotinylated DNA probes and alkaline phosphatase. Focus. 1985;8:6–8. [Google Scholar]
- 15.Evans PD. Biogenic amines in the insect nervous system. Adv Insect Physiol. 1980;15:317–473. [Google Scholar]
- 16.Evans AM, Green KL. The action of dopamine receptor antagonists on the secretory response of the cockroach salivary gland in vitro . Comp Biochem Physiol. 1990a;97C:283–286. doi: 10.1016/0742-8413(90)90142-v. [DOI] [PubMed] [Google Scholar]
- 17.Evans AM, Green KL. Characterization of the dopamine receptor mediating the hyperpolarization of cockroach salivary gland acinar cells in vitro . Br J Pharmacol. 1990b;101:103–108. doi: 10.1111/j.1476-5381.1990.tb12097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng G, Deák P, Kasbekar DP, Gil DW, Hall LM. Cytogenetic and molecular localization of tipE: a gene affecting sodium channels in Drosophila melanogaster . Genetics. 1995;139:1679–1688. doi: 10.1093/genetics/139.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser CM. Site-directed mutagenesis of β-adrenergic receptors: identification of conserved cysteine residues that independently affect ligand binding and receptor activation. J Biol Chem. 1989;264:9266–9270. [PubMed] [Google Scholar]
- 20.Fraser CM, Chung FZ, Wang CD, Venter JC. Site-directed mutagenesis of human β-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci USA. 1988;85:5478–5482. doi: 10.1073/pnas.85.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992;6:2413–2421. [PubMed] [Google Scholar]
- 23.Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5receptors. Receptors Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- 24.Hall H. Dopamine receptors: radioligands for pharmacological and biochemical characterization. In: Niznik HB, editor. Dopamine receptors and transporters. Marcel Dekker; New York: 1994. pp. 3–35. [Google Scholar]
- 25.Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- 26.House CR, Ginsborg BL. Pharmacology of cockroach salivary secretion. Comp Biochem Physiol. 1979;63C:1–6. [Google Scholar]
- 27.Itoh N, Salvaterra P, Itakura K. Construction of an adult Drosophila head cDNA expression library with lambda gt11. Dros Inform Serv. 1985;61:89. [Google Scholar]
- 28.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. J Pharmacol Exp Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 29.Jowett T. Preparation of nucleic acids. In: Roberts DB, editor. Drosophila : a practical approach. IRL; Washington, DC: 1986. [Google Scholar]
- 30.Karnik SS, Sakmar TP, Chen HB, Khorana HG. Cysteine residues 110 and 187 are essential for the formation of the correct structure in bovine rhodopsin. Proc Natl Acad Sci USA. 1988;85:8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 32.Klemm N. Histochemistry of putative transmitter substances in the insect brain. Prog Neurobiol. 1976;7:99–169. doi: 10.1016/0301-0082(76)90014-9. [DOI] [PubMed] [Google Scholar]
- 33.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 34.Lafon-Cazal M, Bockaert J. Pharmacological characterization of dopamine-sensitive adenylate cyclase in the salivary glands of Locusta migratoria L. Insect Biochem. 1984;14:541–545. [Google Scholar]
- 35.Levinson DF. Pharmacological treatment of schizophrenia. Clin Ther. 1991;13:326–352. [PubMed] [Google Scholar]
- 36.Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila . Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- 37.Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+mobilization in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 1987;329:836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- 39.Nassel DR, Elkes K. Aminergic neurons in the brain of blowflies and Drosophila : dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 40.Nathanson JA, Greengard P. Octopamine-sensitive adenylate cyclase: evidence for a biological role of octopamine in nervous tissue. Science. 1973;180:308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- 41.Notman HJ, Downer RGH. Binding of [3H]pifluthixol, a dopamine antagonist, in the brain of the American cockroach, Periplaneta americana . Insect Biochem. 1987;17:587–590. [Google Scholar]
- 42.O’Connell P, Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onai T, FitzGerald MG, Arakawa S, Gocayne JD, Urquhart DA, Hall LM, Fraser CM, McCombie WR, Venter JC. Cloning, sequence analysis and chromosome localization of a Drosophila muscarinic acetylcholine receptor. FEBS Lett. 1989;255:219–225. doi: 10.1016/0014-5793(89)81095-6. [DOI] [PubMed] [Google Scholar]
- 44.Orr GL, Gole JWD, Notman HJ, Downer RGH. Pharmacological characterization of the dopamine-sensitive adenylate cyclase in cockroach brain: evidence for a distinct dopamine receptor. Life Sci. 1987;41:2705–2715. doi: 10.1016/0024-3205(87)90463-2. [DOI] [PubMed] [Google Scholar]
- 45.Pollock NJ, Manelli AM, Hutchins CW, Steffey ME, MacKenzie RG, Frail DE. Serine mutations in transmembrane-V of the dopamine D1-receptor affect ligand interactions and receptor activation. J Biol Chem. 1992;267:17780–17786. [PubMed] [Google Scholar]
- 46.Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992;11:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch EF, Maniatis T. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. Molecular cloning: a laboratory manual, 2nd ed. . [Google Scholar]
- 48.Saudou F, Boschert U, Amlaiky N, Plassat J-L, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer S, Rehder V. Dopamine-like immunoreactivity in the brain and suboesophageal ganglion of the honey bee. J Comp Neurol. 1989;280:43–58. doi: 10.1002/cne.902800105. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Nielsen BK, Gepner JI, Teng NNH, Hall LM. Characterization of an α-bungarotoxin binding component from Drosophila melanogaster . J Neurochem. 1977;29:1013–1029. doi: 10.1111/j.1471-4159.1977.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 51.Seeman P, VanTol HHM. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 52.Seeman P, Ulpian C, Bergeron C, Riederer P, Jellinger K, Gabriel E, Reynolds GP, Tourtellotte WW. Bimodal distribution of dopamine receptor densities in brains of schizophrenics. Science. 1984;225:728–730. doi: 10.1126/science.6147018. [DOI] [PubMed] [Google Scholar]
- 53.Sonetti D, Biondi C, Ferretti ME, Portolan A, Brunelli M. Effects of serotonin, dopamine and prostaglandin-E2 on adenylate-cyclase activity and cyclic AMP levels in different ganglia of the fresh water snail Planorbis corneus L. Neurochem Int. 1987;11:119–126. doi: 10.1016/0197-0186(87)90158-6. [DOI] [PubMed] [Google Scholar]
- 54.Strader CD, Sigal IS, Candelore MR, Rands E, Hill WS, Dixon RAF. Conserved aspartic acid residue-79 and residue-113 of the β-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988;263:10267–10271. [PubMed] [Google Scholar]
- 55.Strader CD, Candelore MR, Hill WS, Sigal IS, Dixon RAF. Identification of two serine residues involved in agonist activation of the β-adrenergic receptor. J Biol Chem. 1989;264:13572–13578. [PubMed] [Google Scholar]
- 56.Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylate cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- 57.Sumikawa K, Houghton M, Emtage JS, Richards BM, Barnard EA. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981;292:862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- 58.Tempel BL, Livingstone MS, Quinn WG. Mutations in the dopa decarboxylase gene affect learning in Drosophila . Proc Natl Acad Sci USA. 1984;81:3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uzzan A, Dudai Y. Aminergic receptors in Drosophila melanogaster : responsiveness of adenylate cyclase to putative neurotransmitters. J Neurochem. 1982;38:1542–1550. doi: 10.1111/j.1471-4159.1982.tb06631.x. [DOI] [PubMed] [Google Scholar]
- 60.Van Renterghem C, Bilbe G, Moss S, Smart TG, Constanti A, Brown DA, Barnard EA. GABA receptors induced in Xenopus oocytes by chick brain messenger RNA: evaluation of TBPS as a use dependent channel blocker. Mol Brain Res. 1987;2:21–31. doi: 10.1016/0169-328x(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 61.Walker RJ, Holden-Dye L. Commentary on the evolution of transmitters, receptors and ion channels in invertebrates. Comp Biochem Physiol. 1989;93A:25–39. doi: 10.1016/0300-9629(89)90188-6. [DOI] [PubMed] [Google Scholar]
- 62.Weiss S, Drummond GI. Dopamine-sensitive and serotonin-sensitive adenylate-cyclase in the gill of Aplysia californica . Mol Pharmacol. 1981;20:592–597. [PubMed] [Google Scholar]
- 63.Wichmann T, DeLong MR. Pathophysiology of parkinsonian motor abnormalities. Adv Neurol. 1993;60:53–61. [PubMed] [Google Scholar]
- 64.Witz P, Amlaiky N, Plassat J-L, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci USA. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel α1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15:1132–1143. doi: 10.1523/JNEUROSCI.15-02-01132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]