Abstract
We examined the massive early cell death that occurs in the ventral horn of the cervical spinal cord of the chick embryo between embryonic days 4 and 5 (E4 and E5). Studies with immunohistochemistry, in situ hybridization, and retrograde-tracing methods revealed that many dying cells express Islet proteins and Lim-3 mRNA (motoneuron markers) and send their axons to the somatic region of the embryo before cell death. Together, these data strongly suggest that the dying cells are somatic motoneurons. Cervical motoneurons die by apoptosis and can be rescued by treatment with cycloheximide and actinomycin D. Counts of motoneuron numbers between E3.5 and E10 revealed that, in addition to cell death between E4 and E5, motoneuron death also occurs between E6 and E10 in the cervical cord. Studies with [3H]thymidine autoradiography and morphological techniques revealed that in the early cell-death phase (E4–E5), genesis of motoneurons, axonal elongation, and innervation of muscles is still ongoing. However, studies with [3H]thymidine autoradiography also revealed that the cells dying between E4 and E5 become postmitotic before E3.5. Increased size of peripheral targets, treatment with neuromuscular blockade, and treatment with partially purified muscle or brain extracts and defined neurotrophic agents, such as NGF, BDNF, neurotrophin-3, CNTF, bFGF, PDGF, S100-β, activin, cholinergic differentiation factor/leukemia inhibitory factor, bone morphogenetic protein-2, IGF-I, interleukin-6, and TGF-β1, were all ineffective in rescuing motoneurons dying between E4 and E5. By contrast, motoneurons that undergo programmed cell death atlater stages (E6–E10) in the cervical cord are target-dependent and respond to activity blockade and trophic factors. Experimental approaches revealed that early cell death also occurs in a notochord-induced ectopic supernumerary motoneuron column in the cervical cord. Transplantation of the cervical neural tube to other segmental regions failed to alter the early death of motoneurons, whereas transplantation of other segments to the cervical region failed to induce early motoneuron death. These results suggest that the mechanisms that regulate motoneuron death in the cervical spinal cord between E4 and E5 are independent of interactions with targets. Rather, this novel type of cell death seems to be determined by signals that either are cell-autonomous or are derived from other cellswithin the cervical neural tube.
Keywords: motoneuron, sympathetic, cell death, apoptosis, neurotrophic factors, cervical, chicken, quail, avian, development
It has long been known that massive cell death occurs in the ventral region of the early chick-embryo cervical spinal cord (Levi-Montalcini, 1950, 1964; Shieh, 1951; O’Conner and Wyttenbach, 1974; Oppenheim et al., 1982, 1989). Striking features of this cell death are precocity of onset, short duration, and large numbers of synchronously dying cells. Cell death begins on embryonic day E4 and lasts <1 d (Levi-Montalcini, 1950). Although the morphology of dying cells is the same as dying cells in other regions of the nervous system (O’Conner and Wyttenbach, 1974), a detailed examination of this novel form of neuronal cell death, including the identification of the phenotype of the dying cells, has not been performed.Levi-Montalcini (1950, 1964) suggested that these degenerating cells represent a transient population of abortive or vestigial visceral motoneurons, that is, sympathetic preganglionic neurons (SPNs). However, the results of a recent study indicate that the SPNs in the thoracolumbar region are not originally intermixed with the somatic motoneurons (Prasad and Hollyday, 1991), a finding inconsistent with the argument that the dying cells in the cervical cord are preganglionic neurons.
Considerable evidence now exists showing that regulation of neuronal death during embryonic development depends on interactions with synaptic targets. In several cases it has been shown that virtually all neurons send their axons to the target before cell death, and it is generally believed that competition for target-associated factors determines how many neurons survive the cell death period (for review, see Purves, 1988; Oppenheim, 1989, 1991). However, it is also known that in the very early stages of avian development (e.g., E2–E3) there are regions containing many pyknotic cells in the CNS (Glücksmann, 1951; Källén, 1955; Silver and Hughes, 1973; Silver, 1978; Cuadros and Rios, 1988; Navascués et al., 1988; Homma et al., 1994). Because the onset of this early cell death occurs before either axonogenesis or the establishment of synaptic connections, it is obvious that in this case cell death cannot be regulated by target-derived signals. A recent study has suggested that this early cell death may represent a kind of negative selection of inappropriate phenotypes or precursor cells (Homma and Oppenheim, 1992;Homma et al., 1994). In contrast to this early form of cell death, the death of ventral neurons in the E4 cervical neural tube occurs 1–2 d later, at a time when axonogenesis and peripheral motoneuron projections have begun. The major objectives of the present investigation were to determine the phenotype of the dying cervical cells, to examine their differentiation, and to begin to examine the cellular and molecular signals that control this novel type of programmed cell death.
MATERIALS AND METHODS
Fertilized eggs
Normal fertilized chicken eggs were obtained from Kasumigaura Farm (Tsuchiura, Japan), Daiichi Farm (Akagi, Gunma, Japan), and Hubbard Farm (Statesville, NC). Crooked neck eggs were obtained from the Department of Animal Genetics, University of Connecticut, andtalpid2 eggs were supplied by the Poultry Science Department at the University of Wisconsin. Fertilized quail eggs were obtained from Tokai Yuki Farm (Toyohashi, Japan). Eggs were incubated in the laboratory (37.6°C, 60% humidity) until they reached the desired stages. Eggs from a single source were used for individual studies.
Counting dying cells and healthy motoneurons by light microscopy
Chick and quail embryos were removed from the shell, placed in a Petri dish containing saline, and carefully staged through a dissecting microscope by the Hamburger–Hamilton morphological stage series (Hamburger and Hamilton, 1951). After staging, embryos were eviscerated, pinned to a small piece of cardboard in an extended position, and placed in Carnoy’s or Bouin’s fixative overnight. After routine processing and embedding in paraffin, transverse serial sections were cut at 8 μm from the brainstem through the brachial region and stained with thionin or hematoxylin eosin. The number of pyknotic cells in the ventral horn region was counted in every sixth section, and the average number of pyknotic cells per section was obtained.
The number of healthy motoneurons in the ventral horn was counted on E6, E8, and E10 in sections with thionin staining. Counts were made in every 10th or 20th section, and cell counts were multiplied by either 20 or 10. Because of the criteria used for these cell counts (Oppenheim, 1989; Clarke and Oppenheim, 1995), it was not necessary to use correction factors. In some experiments, Islet-1-immunopositive neurons in the ventral region of the spinal cord were counted as healthy motoneurons.
Immunohistochemistry
Monoclonal anti-neurofilament antibody was obtained from Bio-Science Products (Emmenbrücke, Switzerland). SC1 monoclonal antibody was a kind gift from Dr. H. Tanaka at Kumamoto University (Kumamoto, Japan). Monoclonal anti-Islet-1 antibody (40.2D6) was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore, MD), and the Department of Biology, University of Iowa (Iowa City, IA). A pan-Islet monoclonal antibody (4D5) that recognizes both Islet-1 and Islet-2 (Tsuchida et al., 1994) was a kind gift from Drs. T. Jessell and T. Tsuchida at Columbia University (New York, NY). Secondary antibodies and an ABC kit were obtained from commercial sources. DAB was used as chromogen for the peroxidase reaction. For neurofilament immunohistochemistry, embryos were immersion-fixed in 4% paraformaldehyde in 0.1 m phosphate buffer overnight at 4°C. Serial transverse sections (100 μm thick) were cut on a freezing microtome. Anti-neurofilament antibody (1:100 dilution) was applied overnight at 4°C. Biotin-labeled anti-mouse IgG (1:200 dilution) was applied for 1 hr and then ABC solution for 1 hr. Sections were collected onto gelatin-chrome alum-coated slides, dehydrated, and mounted with Eukitt. For immunohistochemistry of SC1, embryos fixed by 4% paraformaldehyde for several hours were cut into 10-μm-thick sections on cryostat. Antibody (supernatant of hybridoma undiluted or diluted at 1:2–4) was applied for 30–60 min at room temperature. After washing, the FITC-conjugated secondary antibody was applied for 30 min. For immunohistochemistry of Islet-1, sections were prepared by the same procedure as for SC1. Antibody (supernatant of hybridoma, 40.2D6) without dilution was applied for 1 hr at room temperature. Biotin-labeled anti-mouse IgG (1:200 dilution) was applied for 1 hr and then ABC solution for 1 hr. For colocalization studies of Islet antigens and Lim-3 (see below), a pan-Islet monoclonal antibody (4D5) was used.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method
For visualizing DNA fragmentation, the TUNEL method described byGavrieli et al. (1992) was used. For double staining with SC1 or Islet-1 antibody after immunohistochemistry, a reaction solution, composed of 1 mm CoCl2, 50 μg/ml gelatin, 10 nmol/ml biotin-16 deoxyuridine triphosphate (dUTP) (Boehringer Mannheim, Mannheim, Germany), 100 U/ml terminal deoxynucleotidyl transferase (Takara Shuzou, Otsu, Japan), and 100 mm sodium cacodylate buffer, pH 7.0, was applied directly to the sections. Streptavidin–Texas Red conjugate was used for detecting incorporated biotin-16 dUTP by the TUNEL reaction. Observations were made with a Leica TCS 4D confocal microscope. The TUNEL method was also used to stain all cell nuclei. For this purpose, sections were processed by DNase I solution, composed of 1 μg/ml DNase I in DN buffer, for 10 min before the TUNEL reaction.
Injection of FITC–latex beads
A window was made in the shell, and the embryonic membranes over the embryo were gently opened with forceps. After staging of the embryo, a solution of 0.2% FITC-labeled latex beads (Magsphere, Pasadena, CA) was injected with air pressure through a glass micropipette into peripheral muscle masses in the cervical (neck) region of the embryo. FITC-labeled latex beads of two different diameters (0.126 and 0.052 μm) were used. The latex beads were diluted in a 5% glucose solution, because saline or Tyrode’s solution causes the beads to aggregate. The injection was made at stage (st.) 22 to st. 23. After injection, the window in the shell was closed with Parafilm, and the egg was returned to the incubator. After 12–18 hr, the embryo was fixed at st. 24 to st. 25. For light microscopy, the embryo was immersed overnight in phosphate-buffered 4% paraformaldehyde. The cervical region of the embryo was dissected, cryoprotected, and embedded in Tissue-Tek ornithine transcarbamylase compound. The block was cut into transverse sections on a cryostat, and the sections were collected on silane-coated slides. Slides were processed for the TUNEL method, coverslipped with glycerol, and observed with a confocal microscope. For electron microscopy, the embryo was immersed overnight in the same fixative used for the electron microscopic preparation described below. After it was embedded in the gelatin-egg yolk mixture, the cervical region of the embryo was cut into transverse sections (100 μm) on a vibratome. Sections were osmificated, dehydrated, and embedded in Epon 812. Thin sections were cut on an ultramicrotome. To facilitate the observation of the latex beads within cells, staining with uranyl acetate and lead citrate was omitted.
DiI labeling
Embryos were fixed by immersion in 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.3, overnight and then placed on a SYLGARD-coated Petri dish with a small amount of buffer. To facilitate the injections, the dorsal half of the cervical spinal cord was removed on one side. DiI (1,1′-diocadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) (Molecular Probes, Eugene, OR), dissolved at 0.1% in a mixture of ethanol and DMSO (9:1), was pressure-injected into the ventral region of the cervical spinal cord on the intact side. After the injection, the embryo was returned to the fixative and left for 3–7 d at 37°C and then embedded in a gelatin–egg yolk mixture as described previously (Oppenheim et al., 1988b). Serial transverse sections (100 μm thick) were cut on a vibratome and observed with a Zeiss Axiophot epifluorescent microscope.
Electron microscopy
Embryos were placed in a fixative composed of 3% glutaraldehyde and 2% paraformaldehyde in 0.1 m phosphate buffer, pH 7.3. They were stored in the same fixative at 4°C overnight. Desired regions were dissected, osmificated, dehydrated through a graded ethanol series, and embedded in Epon 812 (TAAB, Berks, UK). Thin sections were cut on an ultramicrotome, stained with uranyl acetate and lead citrate, and examined on a Hitachi H-7000 electron microscope. For counting axon numbers, serial semithin sections (4–5 μm thick) were cut perpendicular to the ventral root of the 10th cervical segment. Sections containing the desired portion of the ventral root were mounted on the faced-off block with epoxy resin and thin-sectioned. Photomontages of the entire ventral root were made, and the profiles of axons were counted.
Colocalization of Lim-3 mRNA and Islet proteins
Lim-3 mRNA was localized using in situhybridization. The plasmid containing the Lim-3 cDNA, a kind gift from Drs. T. Jessell and T. Tsuchida of Columbia University (New York, NY), was linealized and transcribed in both the sense and antisense orientations. Digoxigenin-UTP was added to the transcription mixture of label and transcript. Tissue for in situhybridization was fixed overnight at 4°C in 4% paraformaldehyde in PBS, postfixed in 20% sucrose, embedded in Tissue-Tek, and stored at −70°C. Frozen sections (15 μm) were mounted on polylysine-coated slides and dried at room temperature for 3–6 hr. Sections were prehybridized overnight at room temperature in a solution containing 50% formamide, 5× SSC, 5× Denhardt’s solution, 250 μg/ml tRNA, and 50 μg/ml sheared Herring sperm DNA. Probes for hybridization were suspended in the same solution and applied to sections under sealed coverslips. Hybridization was carried out for 12–18 hr at 65°C. Sections were washed in 0.2× SSC at hybridization temperatures for 1 hr. Digoxigenin-labeled nucleotides were detected with an alkaline phosphatase-conjugated anti-digoxigenin antibody. The antibody was localized with nitroblue tetrazolium and X-Phos. The addition of 0.24 mg/ml levamisole to the reaction mixture inhibited endogenous alkaline phosphatase activity. For colocalization of Lim-3 and Islet proteins, the tissue was incubated with both anti-digoxigenin and the pan-Islet antibody 4D5 (Tsuchida et al., 1994) at the same time. The anti-digoxigenin antibody was localized first, and the pan-Islet antibody was localized later, using an ABC kit.
[3H]thymidine autoradiography
Chick embryos ranging in age from 60 hr to 4.5 d were treated with 10 μCi of tritiated thymidine at 12 hr intervals. Embryos were killed at either E4.5 or E5.5 by immersing them in 4% paraformaldehyde in phosphate buffer (0.1 m), pH 7.3, at 4°C overnight. Lower cervical segments (C9–C11) were removed and embedded in Epon 812 resin without osmification. Semithin sections (1.0 μm thick) were cut and affixed to gelatin-chrome alum-coated glass slides. Sections were prepared for autoradiography with a 1:1 dilution of Kodak NTB-2 emulsion and distilled water. After an exposure time of 2–6 weeks, the sections were developed and stained with Mayer’s hematoxylin overnight at 37°C. After the stain was washed off, sections were allowed to dry and coverslipped with Eukitt.
Microsurgery
At appropriate developmental stages, windows were made in the shell, and embryos were visualized by injecting India ink diluted by saline beneath the embryos. Manipulations were performed using fine tungsten needles.
Transplantation of cervical segments to the brachial region.Lower cervical segments (C9–C11) and adjacent notochord were removed from donor embryos at st. 11–12 (12–16 somites) and transplanted into either brachial (C14–C16: experimental) or cervical (C9–C11: control) segments of host embryos at st. 12–13 (16–20 somites). The number of pyknotic cells in the transplanted grafts was counted at E4.5 (st. 24), and surviving (healthy) Islet-1-immunopositive motoneurons were counted at E5 and E9.
Transplantation of thoracic segments to the cervical region.Thoracic segments (T2–T4) of st. 13 embryos were transplanted into the cervical region (C9–C11) of st. 12 embryos. The number of pyknotic cells was counted at E4.5, and the formation of a nucleus of Terni was examined on E7 by using retrograde tracing after injection of DiI into the ventral roots of fixed embryos (see below).
Transplantation of cervical segments to the thoracic region.For cervical to thoracic neural tube transplants, donor chick embryos were st. 10–12 and host embryos, st. 13–15. Using tungsten needles, 4–5 segments of the rostral (C2–C6) or caudal (C8–C12) cervical neural tube were excised and placed into a previously prepared gap spanning T2–T6 in the host thoracic region. Controls consisted of cervical to cervical or thoracic to thoracic transplants. Embryos were allowed to survive until E4.5 or E7.5. The region of the spinal cord containing the transplant was dissected and immersion-fixed in Bouin’s solution, processed, embedded in paraffin, serially sectioned (8–10 μm), and stained with hematoxylin and eosin. On E4.5, the number of pyknotic cells in cervical to thoracic and cervical to cervical transplants was counted in every 10th section. On E7–E8, cervical to thoracic transplants were examined for the presence of a nucleus of Terni (sympathetic preganglionic nucleus) using retrograde tracing with DiI.
Transplantation of cervical segments between chick and quail. Incubation of chick and quail eggs was started at the same time. At E1.5, cervical segments adjacent to somites 13–16 (C9–C12) were transplanted from the chick to the quail or from the quail to the chick. Cases with transplantation of cervical segments in the same species served as controls. Embryos were killed at E3.5, E4, E4.5, or E5 and processed histologically; the number of pyknotic cells in cervical segments was counted.
Induction of an ectopic supernumerary motoneuron column. The notochord transplant procedure described by Yamada et al. (1991) was used for inducing a supernumerary motoneuron column in the lateral neural tube. At E1.5 (st. 10), notochords were removed from donor chick embryos and placed adjacent to the lateral wall of the neural tube in either the cervical or brachial region of host embryos. Embryos were fixed at E4.5 by immersion in 4% paraformaldehyde and processed for double staining with SC1 and the TUNEL method, as described above.
Curare treatments
Curare, (d-tubocurarine chloride) (Sigma, St. Louis, MO) was dissolved in PBS at 1% concentration. A window was made in the shell, and 50 μl of a 1% curare solution (0.5 mg) was dropped onto the chorioallantoic membrane through a window in the shell at st. 18 (E3) and again at st. 22 (E3.5–E4). Embryos were killed at st. 24 (E4.5). Before they were killed, they were observed through the window in the shell with a dissecting microscope for several minutes to confirm that all neuromuscular activity was inhibited by the curare treatment. The number of pyknotic cells in the 10th cervical segment was counted. For long-term treatment, the administration of curare was started at E3.5, E4.5, or E5.5. Between E3.5 and E5, 50 μl of a 1% curare solution (0.5 mg) was administered at 12 hr intervals; between E5.5 and E9.5, 200 μl of 1% solution (2 mg) was administered at 24 hr intervals. For controls, PBS was administered. Embryos were killed at E10, and axon numbers in the C10 ventral root were counted in the electron microscope. In some embryos treated from E5.5 to E9, motoneurons were counted in every 10th section through cervical segments C1–C12.
Cycloheximide, actinomycin D, and neurotrophic agents
Cycloheximide (0.25 μg) and actinomycin D (0.5 μg) in 50 μl Tyrode’s solution were administered into the egg through a window in the shell every 3 hr, beginning at st. 22–23 and ending at st. 24 (3–4 injections). Embryos were fixed in Bouin’s solution 8–10 hr after the first injection and processed for paraffin histology; sections (6 μm) were stained with hematoxylin and eosin. Cell counts of pyknotic profiles were made in every 10th section from C8 through C12. The rate of protein synthesis in the nervous system of some embryos after treatment was measured as previously described (Oppenheim et al., 1990).
Muscle and brain extracts (MEX, BEX) were prepared from E10 chick embryos as described previously (Oppenheim et al., 1988a), and astrocyte-conditioned medium (A-CM) was prepared as described in Yin et al. (1994). The human recombinant growth factors and neurotrophic agents were obtained from Amgen Inc. (Thousand Oaks, CA), except for activin, which was a gift from Dr. Ueno of Hokkaido University (Sapporo, Japan), and BMP-2, which was a gift from the Genetics Institute Inc. (Cambridge, MA). MEX, BEX, and A-CM were administered as a single dose of 150 μg of total protein in 50 μl of PBS with 3 μg BSA or cytochrome c as a carrier. Growth factors and trophic agents were also administered as a single dose (3 μg/50 μl). Embryos were treated once on E3.5, fixed and staged at E4.5, and processed for histology and cell counts as described above.
One group of embryos was treated with either MEX or BEX once daily from E6 to E9 and killed on E10 as described in Oppenheim et al. (1988a,1993). The cervical spinal cord was dissected, fixed in Bouin’s fixative, embedded in paraffin, sectioned (10 μm), and stained with thionin. Cell counts of cervical motoneurons were made in every 10th section through segments C2–C12.
Crooked neck (cn/cn) and talpid2(ta2/ta2) mutant avian embryos
Both mutants are autosomal recessive. Embryos from thecn and ta2 flocks that had a normal phenotype were used as controls. cn Embryos have a defect in excitation–contraction coupling (Airey et al., 1993) and thus are completely paralyzed. Mutant cn embryos were identified by the total absence of neuromuscular activity (motility) when observed for 5 min through a window in the shell on E4.5 (Oppenheim et al., 1996). ta2 Embryos fail to exhibit programmed cell death in the limb mesenchyme (Dvorak and Fallon, 1991) and can be identified on E4.5 by a distinctly enlarged limb bud (Abbott et al., 1960; Hinchliffe and Thorogood, 1974). Mutant and control embryos were fixed, staged, and processed for paraffin histology on E4.5 as described above for normal embryos. Cell counts of pyknotic cervical motoneurons were made from every 5th or 10th section through cervical segments C2–C12.
RESULTS
Spatiotemporal distribution and morphology of dying cells
Many dead cells exhibiting nuclear pyknosis (Clarke and Oppenheim, 1995) were observed in the ventral region of the cervical segments of E4.5 (st. 24) chick embryos (Fig. 1A). Dead cells also expressed positive reactivity to the TUNEL method, which can detect fragmentation of DNA (Gavrieli et al., 1992) (Fig.1B,C). Double staining with the TUNEL method and SC1 immunohistochemistry, which defines ventral horn neurons (Tanaka and Obata, 1984), revealed that dead cells were located throughout the ventral horn region (Fig. 1B–D). Although some dead cells were observed in the marginal zone adjacent to the ventral horn, most of the dead cells were observed within the ventral horn.
Fig. 1.
Photomicrographs of transverse sections through the cervical spinal cord of a st. 24 embryo showing the distribution of dying neurons in the ventral horn. A, Hematoxylin eosin staining. B–D, Double staining with the TUNEL method and SC1 (marker for ventral horn neurons) immunohistochemistry.B, Double staining; C, TUNEL; D, SC1. Scale bars, 50 μm. Arrows in A indicate pyknotic profiles of degenerating neurons.
To quantify the timing, distribution, and extent of the degeneration, we counted pyknotic cells in the cervical cord from st. 22 (E3.5–E4) to st. 27 (E5–E5.5) (Fig. 2). In the chicken, there are 15 cervical nerves, and the cervical enlargement consists of C13, C14, C15, and T1 segments. Pyknotic cells were found extending from the caudal portion of the C1 segment to the rostral portion of segment C14. Dying cells were first observed at st. 23 (E4). In all segments the greatest number of pyknotic cells was observed at st. 24 (E4.5), after which the number of pyknotic cells decreased from st. 25 to 27 (E4.5–E5.5). There was a significant rostro–caudal gradient in the magnitude of cell death, with approximately twice as much degeneration occurring in the caudal cervical segments (Fig. 2). At st. 23, when cell death begins, there are ∼60 and 80 Islet-1–positive healthy ventral horn neurons per 8-μm-thick section in rostral–cervical segments (C3–C4) and caudal–cervical segments (C10–C11), respectively. Accordingly, the observed rostro–caudal gradient is partially explained by differences in cell number before the onset of cell death.
Fig. 2.
Line graphs showing numbers (mean ± SD) of pyknotic (dying and dead) cells in the ventral horn per 8-μm-thick section in the third, seventh, and eleventh cervical segments at st. 22 (E3.5) to st. 27 (E5.5). *C3 st. 24 vs C11 st. 24, p < 0.001; t test. Sample size = 4 at all stages.
Detailed descriptions of the morphology of degenerating cells in the cervical ventral horn have been reported by O’Conner and Wyttenbach (1974). Although our observations are in agreement with these authors (Fig. 3A), we have extended their observations to the morphology of pyknotic profiles observed in the marginal zone adjacent to the motor column. It was often observed that dead cells in this region were contained within large, round macrophage-like cells (Fig. 3B). Phagosomes containing cell debris were also often found in the endfeet of neuroepithelial cells (Fig. 3C). These findings suggest that pyknotic profiles observed in the marginal zone by light microscopy (see above) are cell debris that were previously removed from the motor columns by the phagocytic activity of neuroepithelial cells and macrophage-like cells. Recently, macrophages have been confirmed to be present in the ventral marginal zone and the ventral horn during this early cell death period (Cuadros et al., 1993).
Fig. 3.

Electron micrographs showing dying cells and other phagocytic cells that contain fragments (apoptotic bodies) of dead cells in the cervical ventral horn. A, Dying cells.B, Macrophage-like cell containing cell debris in the marginal zone. C, Cell debris contained in a neuroepithelial cell (arrow) in the marginal zone. Scale bars, 1 μm.
Determination of the identity of dying cells by specific markers and retrograde tracing
Colocalization of TUNEL positivity and a neuronal marker, Islet-1
To identify dying cells in the ventral horn, an Islet-1 antibody was used as a marker for ventral horn neurons (Ericson et al., 1992;Tsuchida et al., 1994). At E4 (st. 23), virtually all of the cells in the ventral horn region expressed Islet-1 immunoreactivity (Fig.4A). To demonstrate localization of Islet-1 in dying cells, sections of E4–E4.5 (st. 23+) embryos were double stained with TUNEL and anti-Islet-1 antibody and observed with confocal microscopy. Colocalization of TUNEL and Islet-1 were often observed in the optical thin sections of confocal microscopy. In a representative case at st. 23+ (Fig. 4B), an average 4 of 11 TUNEL positive profiles showed colocalization with Islet-1 in a 10-μm-thick section.
Fig. 4.

Top. A, A fluorescent, double-exposed photomicrograph showing cell nuclei (red) and Islet-1 immunopositive neurons in the ventral horn (green) at E4 (st. 23). Virtually all the cells in the ventral horn region express Islet-1 immunoreactivity. B, A fluorescent double-exposed photomicrograph taken by a confocal microscope showing colocalization of TUNEL positivity (red) and Islet-1 immunopositivity (green) in the nucleus of a dying neuron (arrow). Scale bars, 10 μm. The double-labeled cells are shown in yellow.
A reduction in the number of Islet-1-positive neurons during the cell death period was also observed. In C10–C11 segments, on average, 80 per 8-μm-thick section and ∼3200 per one segment of Islet-1-positive cells were counted at E4 (st. 23) (Table1). At E4.5, the number of Islet-1-positive neurons was reduced to 60 per 8-μm-thick section and ∼2600 neurons per one segment (Table 1). Because generation of motoneurons still continues between E4 and E4.5 (see below), the actual reduction of Islet-1-positive cells is very likely much more than the 25% indicated by these data.
Table 1.
The number of Islet-1-positive neurons in the ventral horn of C10–C11 segments
| Embryonic days | Number of Islet-1positive neurons in 8-μm-thick sections1_a | Number of Islet-1positive neurons in one segment1_b | n |
|---|---|---|---|
| E4 (st. 23) | 80.5 ± 5.1 | 3221 ± 243 | 5 |
| E4.5 (st. 24) | 60.4 ± 3.0 | 2604 ± 141* | 5 |
| E5 (st. 26) | 53.6 ± 4.6 | 2547 ± 221 | 5 |
Number of Islet-1-positive cell nuclei in 10-μm-thick sections were counted, and the raw numbers were adjusted by average diameter of nuclei and section thickness following the formula of Abercrombie (1946).
Number of Islet-1-positive neurons in 8-μm-thick sections were multiplied by length of sections (E4: 320 μm; E4.5: 345 μm; E5: 380 μm)/8 μm.
*p < 0.001 versus E4 (t test).
Colocalization of Lim-3 mRNA and Islet proteins
In studies of early motoneuron development, Ericson et al. (1992)have shown that Islet proteins are expressed by differentiating visceral and somatic motoneurons. More recently, Tsuchida et al. (1994)have found that the transcription factor Lim-3 is expressed in somatic motoneurons and ventral interneurons. Although neither Islet proteins nor Lim-3 mRNA alone can distinguish somatic from visceral motoneurons, expression of both markers in the same cell implies a somatic motoneuron phenotype for cervical neurons located in the medial motoneuron column (Tsuchida et al., 1994). Therefore, a procedure allowing colocalization of Lim-3 by in situ hybridization and of Islet proteins (Islet-1 and Islet-2) by pan-Islet immunohistochemistry was performed. Lim-3 mRNA was found in a subpopulation of ventral neurons, as shown in Figure5. Islet immunoreactivity is present in a distinct but partially overlapping population. The position of Islet andLim-3 colocalization corresponds closely to the area in which dying cells are observed. It was not possible to counterstain these preparations with a Nissl stain to directly observe pyknotic nuclei without obscuring the results of the in situhybridization study. To determine whether dying cells were confined to the region of colocalization, this region was drawn (20×) using a camera lucida (Fig. 5C). After making the drawings, the sections were stained with hematoxylin, and the pyknotic nuclei were drawn in over the original drawing of Lim-3 and Islet colocalization. Most of the dying cells (85%) were within the region of colocalization. Many of those dying cells outside the region of colocalization were closely apposed to cells expressing both markers and may represent cells with low levels of Lim-3 expression. Other pyknotic cells outside the region of colocalization were located close to the marginal zone. Electron microscopic observations indicate that apparent pyknotic nuclei observed near the marginal zone actually are cell debris contained within phagocytic processes (Fig. 3) and thus would no longer be expected to express phenotypic markers.
Fig. 5.
Bottom. Colocalization ofLim-3 mRNA and Islet proteins in the cervical spinal cord at E4.5. Stars indicate cells that express only Islet proteins.A, Transverse section through cervical (C10) spinal cord. Whereas some cells (stars) only express Islet proteins (brown), most cells in the ventral horn (arrowheads in A, B, C) express both Islet proteins and Lim-3 (purple). Scale bar, 25 μm. B, D, Higher magnifications of the boxed area inA. Scale bar in B, D, 20 μm. C, Drawings of two representative sections showing the area of colocalization of Islet proteins and Lim-3 in the ventral horn (solid line) and individual pyknotic cells (small circles) observed within this region. Arrowheadsindicate the lateral boundary between the ventral horn and marginal zone. Scale bar, 100 μm.
Results of the above experiments, taken together, suggest that many, if not all, dying cells in the ventral horn of the cervical ventral horn express Islet proteins and Lim-3 mRNA before cell death, consistent with their being somatic motoneurons. Finally, previous studies on the localization of spinal interneurons by retrograde labeling failed to observe labeled cells in the cervical ventral horn region (Oppenheim et al., 1988b). Therefore, it seems highly likely that the dying cervical cells are somatic motoneurons that constitute the medial motor column.
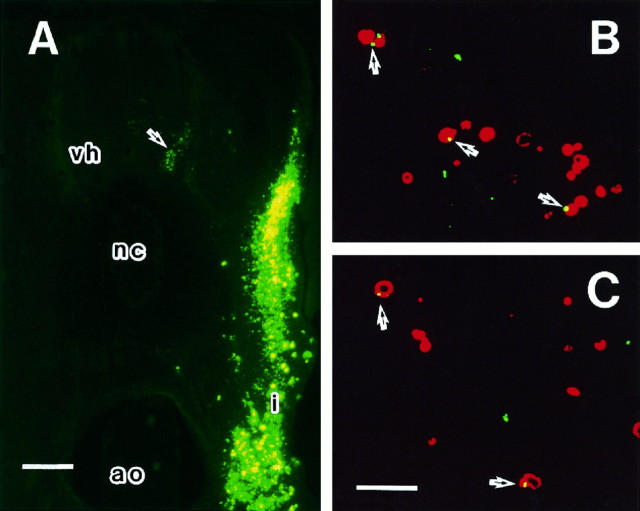
Retrograde labeling of dying cells by FITC–latex beads
To confirm that dying cells send their axons to the peripheral somatic region of the embryo before the onset of degeneration, we retrogradely labeled cells in the ventral horn with FITC-labeled latex beads. These beads are nontoxic to living cells and are recognizable by both light and electron microscopy (Katz et al., 1984; Egensperger and Holländer, 1988). The beads were injected into the cervical somatic region of embryos at st. 22–23, and the embryos were fixed at st. 24–25. FITC–latex beads were observed in cells in the ventral horn region and in the spinal ganglion (Fig.6A). Because no fluorescence was observed on the side contralateral to the injection site or in other adjacent segments, it is unlikely that the FITC–latex beads were transported through the circulation or by other means not involving retrograde transport. Observations of sections double labeled by TUNEL and FITC–latex beads by confocal microscopy revealed that there was close colocalization of TUNEL positive profiles and FITC–latex beads (Fig. 6B,C). Observations with electron microscopy revealed that the latex beads within healthy cells were surrounded by a cellular membrane (Fig. 7A), suggesting that the beads had been taken into the cell by pinocytosis. We also observed some dying cells that contained latex beads (Fig. 7B,C). In a representative case shown in Figure 6, an average of 28 TUNEL-positive profiles were counted per 10-μm-thick section. On average, four of these were found to be colocalized with the FITC–latex beads. In the same sections, an average of 21 clusters of FITC–latex beads was observed. This relatively low rate of colocalization of FITC–latex beads and TUNEL positivity may be partially explained by technical limitations (e.g., efficiency of the retrograde tracing method). It is also possible that some axons of degenerating cells do not reach the target region before the onset of cell death or that axons of some degenerating cells do not even project through the spinal nerve into the periphery.
Fig. 6.
Photomicrographs showing neurons retrogradely labeled with FITC–latex beads. A, Fluorescent micrograph showing the injection site (i) and retrogradely labeled cells in the ipsilateral ventral horn. Dorsal toward thetop. Scale bar, 100 μm. vh, Contralateral ventral horn; nc, notochord; ao, dorsal aorta.B, C, Fluorescent confocal micrographs showing colocalization of FITC–latex beads and TUNEL positivity. Scale bar inC, 10 μm for B, C. Arrows indicate FITC–latex beads located within TUNEL-positive profiles. Double labeling is shown in yellow.
Fig. 7.

Electron micrographs showing healthy and degenerating neurons retrogradely labeled by FITC–latex beads. The diameter of the beads shown here is 0.126 μm. To increase relative electron density of the beads, staining with uranyl acetate and lead citrate was omitted. A, The latex beads contained in a healthy cell. Note that the beads are surrounded by a cellular membrane (arrowheads). Scale bar, 0.5 μm. B, C, Latex beads observed in dying cells or in debris from dead cells (arrowheads). In C, the cell membrane around the latex bead can still be discerned. Scale bars, 1 μm.
Examination of motoneuron death between E5.5 and E10 in the cervical cord
The above results indicate that many of the dying cells in the cervical cord at E4–E5 are somatic motoneurons. This raises the question of whether this cell death is simply an early onset of the type of motoneuron death that occurs later in other regions between E6 and E10 (Hamburger, 1975; Chu-Wang and Oppenheim, 1978a,b; Oppenheim and Majors-Willard, 1978; Laing, 1982; Lanser and Fallon, 1984;O’Brien and Oppenheim, 1990; O’Brien et al., 1990). To address this question, we examined whether cell death also occurs in the ventral cervical cord after E5. We counted healthy and pyknotic cells in the ventral horn and axons in the ventral root from E5.5 to E10. Between E5.5 and E10, ∼40–50% of the axons in the ventral root of cervical segments disappear (Fig. 8). Counts of healthy cells in the ventral horn revealed that between E6 and E10, ∼50% of the cells also disappear (Fig. 8, Table 2). These data were confirmed by counts of Islet-immunopositive cells on E6 and E10 showing a 50–60% reduction in the number of immunoreactive cells in the ventral horn (data not shown). During this period, significant numbers of pyknotic motoneurons were also observed by light (Table 2) and electron microscopy (data not shown). Taken together, these results indicate that motoneuron death occurs in the cervical cord between E5.5 and E10 at a similar rate to other spinal segments.
Fig. 8.
The number (mean ± SD) of healthy cells in the ventral horn of lower cervical segments (C9–C12) at E6, E8, and E10, and axons in the ventral root of C10 at E6 and E10.
Table 2.
Cell number in the cervical motor column (C2–C12)
| Embryonic days | Cell number (mean ± SD) | n | Pyknosis2_a | Axon number (C10) (mean ± SD) | n |
|---|---|---|---|---|---|
| E6 | 14020 ± 2300 | 5 | 3631 ± 374 | 6 | |
| E8 | 8940 ± 2400 | 5 | 30/1000 cells | ||
| E10 | 7590 ± 630 | 5 | 1744 ± 190 | 5 |
The number of degenerating (pyknotic) cells per 1000 healthy motoneurons.
Temporal relationship between early cell death and development of motoneurons
The results described above suggest that motoneuron death in the cervical cord occurs in two phases: an initial short phase (E4–E5) and a later, more extended phase (E5.5–E10). To begin to further characterize motoneuron death during the early phase, we examined the development of spinal nerves and the timing of formation (birth dates) of cervical motoneurons.
Development of spinal nerves
Development of cervical spinal nerves was examined by immunohistochemistry, using a neurofilament antibody and an anterograde-labeling method using DiI (Fig. 9). At E3.5, bundles of peripheral spinal nerve axons run ventrolaterally along the ventromedial side of the dermamyotome, and the leading edge of the axon bundles has already reached the ventral end of the dermamyotome (Fig.9A). At E4, the leading edge of the axon bundles still remains at the ventral end of the dermamyotome, and a small number of axons have begun to project from the bundle to enter the dermamyotome (Fig. 9B). At E5, the dorsal branches of the spinal nerve become distinct, and most have entered the dermamyotome (Fig.9C); the leading edges of the ventral branches also now begin to enter the somatopleural region of the embryo; sprouts from the ventral branch also enter the dermamyotome. At E6, the leading edge of the spinal nerve reaches the subcutaneous region, and myotomes are now heavily innervated by many axons. Small axonal sub-branches also can be observed running toward the muscles in front of the vertebra (Fig.9D).
Fig. 9.
A–D, Camera lucida drawings showing the development of cervical spinal nerves from E3.5 to E6. dm: Somite (dermamyotome); drg: dorsal root ganglion;nc: notochord; sg: sympathetic ganglia;ao: aorta. Scale bars, 250 μm. E, F, Fluorescent photomicrographs of transverse sections through the cervical region of st. 21 (E) and st. 23 (F) embryos following DiI injection into the ventral region of the cervical cord. Dorsal is toward the top. Scale bars, 100 μm. Note that there is an apparent communicating branch (arrow inE) projecting from the spinal nerve toward the sympathetic ganglion. Virtually all such communicating branches between the spinal nerve and the sympathetic trunk disappear by st. 23 (F).
The existence of transient preganglionic communicating branches from the cervical spinal nerves to the sympathetic ganglia has been controversial (Tello, 1925; Terni, 1931; Levi-Montalcini, 1950). We observed apparent communicating branches in >50% (10/17) of the cervical spinal nerves at E3.5 (st. 21). These communicating branches consisted of only a very small number of axons (Fig. 9E). By E4 (st. 23), the number of communicating branches was reduced to <2% (1/53) (Fig. 9F). No preganglionic communicating branches were observed at E4.5 or at later stages.
Axons in the ventral root
We examined the C10 ventral root in the electron microscope and counted axons during the early cell death period (E3.5–E5.5). Figure10 shows the changes in axon numbers in the ventral root during this time. The number of axons increased rapidly from st. 21/22 (E3.5) to st. 23 (E4). Between st. 23 (E4) and st. 26 (E5), when early cell death occurs, there was no significant change in axon number. From st. 26 (E5) to st. 28 (E5.5–E6), there was again an increase in axon number. Between st. 23 and st. 26, we often observed degenerating axons in the ventral root (Fig.11A). Growth cone-like profiles also often were observed in the ventral root during this period (Fig.11B). These observations suggest that in the period of early cell death (E4–E5) axonal elongation and innervation of muscles are still ongoing.
Fig. 10.
Axon numbers (mean ± SD) in the C10 ventral root between st. 21/22 (E3.5) and st. 28 (E5.5–6).
Fig. 11.

A, An electron micrograph of a transverse section through the ventral root of the cervical cord of a st. 25 embryo showing a degenerating axon (arrows). B, An electron micrograph of a section perpendicular to the C10 ventral root at st. 26. An apparent healthy-growth cone profile can be seen (g). Scale bars, 1 μm.
Timing of formation of motoneurons
To determine the birth dates of surviving motoneurons versus motoneurons that die during the early cell death period, we examined the timing of the final mitoses of neurons by using [3H]thymidine (Figs. 12,13).
Fig. 12.
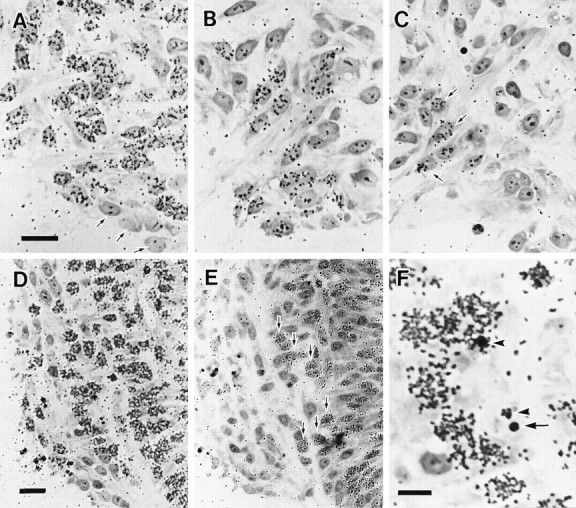
Micrographs showing transverse sections through the cervical–ventral horn of chick embryos treated with [3H]thymidine and processed for autoradiography. Dorsal is toward the top and medial is toward the right.A–C, Motoneurons that survived until E5.5. [3H]thymidine was administered at E3 (A), E3.5 (B), and E4 (C). Most of the neurons were labeled by [3H]thymidine administered at E3. The earliest developing unlabeled postmitotic motoneurons were located in the most ventromedial region of the ventral horn (arrows inA). Some motoneurons were labeled by [3H]thymidine administered at E4 (arrows inC). D, E, Dying and healthy neurons in the ventral horn region at E4.5. [3H]thymidine was administered at E3 (D) and E3.5 (E). Many cells in the ventral horn were labeled by [3H]thymidine administered at E3. After [3H]thymidine treatment at E3.5, most of the cells, including dying cells, in the lateral portion of ventral horn (lateral to the commissural fiber bundles) were not labeled. Labeled cells were located in the medial portion of the ventral horn (arrows). F, Higher magnification ofD, showing labeled (arrowheads) and unlabeled (arrow) pyknotic profiles. Scale bar in A, 10 μm for A–C. Scale bar in D, 20 μm forD, E. Scale bar in F, 10 μm.
Fig. 13.

A, Bar graph showing birth dates of healthy cells in the ventral horn of the cervical cord at E5.5. B, Bar graph showing birth dates of those cells that die (are pyknotic) at E4.5, compared with the cells that are apparently healthy at E4.5 in the ventral horn. Abscissa indicates periods of development.Ordinate indicates percentage of cells that become unable to incorporate [3H]thymidine during each period, which was obtained by subtracting average percentage of cells unlabeled by [3H]thymidine treatment at the beginning of each period from that at the end of each period. Sample size = 2 for all stages. Note that most of the dying cells at E4.5 fail to incorporate [3H]thymidine (i.e., have become postmitotic) before E3.5, primarily between E3 and E3.5. Similar results were obtained with the use of another S-phase marker, bromodeoxyuridine (data not shown).
Motoneurons that survive until E5.5. Most motoneurons in the cervical–ventral horn were generated between E2 and E4.5 (Fig.13A). Early generated neurons tend to be located in the ventro-medial region of the ventral horn (Fig.12A), whereas the later generated motoneurons were located in ventro-lateral regions (Fig. 12B,C), reflecting a kind of inside–outside sequence of motoneuron production that has been observed in other spinal regions (Hollyday and Hamburger, 1977).
Dying cells and apparently healthy cells at E4.5. More than 90% of dying cervical neurons became postmitotic before E3.5, with over 60% of these being generated between E3 and E3.5. On the other hand, apparently healthy motoneurons at E4.5 were generated between E2 and E4.5 (Figs. 12, 13B). Healthy motoneurons that became postmitotic after E3.5 were located in more medial portions of the ventral horn at this stage (Fig. 12E).
These data indicate that the generation of motoneurons that survive the early cell death period is more prolonged than that of neurons that die during this time and that most of the dying cells at E4.5 have withdrawn from the mitotic cycle more than 12 hr before the initiation of cell death.
Experimental approaches
Curare treatment
It is known that treatment with curare and other neuromuscular-blocking agents can rescue motoneurons from programmed motoneuron death (Pittman and Oppenheim, 1978, 1979). Neuromuscular activity begins in the cervical region by E3.5–E4.0 (Oppenheim, 1974), and this activity is blocked by curare, indicating that at least some cervical motoneurons have established functional synaptic contacts by E3.5. We examined the effects of curare treatment on the early cell death as well as on cervical motoneuron death at later developmental stages. Two kinds of experiments were performed. In the first experiment, curare treatment was started at E3 (st. 18), and pyknosis in the ventral horn of the cervical cord was quantified at E4.5 (st. 24). In the second experiment, curare treatment was started at E3.5, E4.5, or E5.5 and continued until E9.5. Axons in the ventral root of the C10 segment were counted at E10. Curare treatment from E3 did not alter the number of pyknotic cells in the ventral horn of the cervical cord at E4.5 (Fig. 14A). After curare treatment from E5.5 to E9.5, ∼40% more axons were counted in the ventral root as compared with controls (Fig. 14B). However, when curare treatment started at E4.5 or E3.5, axon numbers in the ventral root on E10 did not differ from that seen after curare treatment had begun on E5.5. Also, curare treatment from E3 to E4.5 did not increase the number of healthy cervical motoneurons present on E5, whereas treatment from E5.5 to E9.5 resulted in a significant (40%) increase in surviving motoneurons on E10 (data not shown). Collectively, these results suggest that curare treatment is effective in rescuing cervical motoneurons from cell death between E5.5 and E10 but is ineffective in preventing early cell death between E4 and E5.
Fig. 14.

Pyknotic cells and axon numbers following curare treatment. A, Bar graph showing the numbers (mean ± SD) of pyknotic cells per 8-μm-thick section in the C10 segment at E4.5 following curare treatment. No significant differences were observed.B, Bar graph showing the number (mean ± SD) of axons in the C10 ventral root at E10 after continuous curare treatment from E3.5, E4.5, or E5.5 to E9.5. All experimental groups showed significant increases in axon numbers compared to controls (p < 0.001, ANOVA). However, no significant differences were observedbetween experimental groups.
Transplantation of cervical segments to the brachial region
To examine whether the size of peripheral muscle targets affects cell death in the cervical cord, we transplanted the cervical cord to the brachial (wing) region, counted pyknotic cells in the transplanted graft at E4.5 (st. 24), and also compared the number of healthy Islet-1-immunopositive cells in the ventral horn of the transplant at E5 and E9. This kind of manipulation is known to rescue motoneurons from cell death in the medial motor column of the thoracic region, even though the targets of the transplanted motoneurons are not appropriate. (O’Brien and Oppenheim, 1990; O’Brien et al., 1990). Used in this analysis were only those cases in which peripheral nerves from the transplant were confirmed to enter the limb by light microscopy. Figure15A shows no significant differences on E4.5 in the number of pyknotic cells between control and experimental cases (Fig. 15A). Additionally, the number of healthy cells in the ventral horn of experimental cases was not significantly different from controls on E5 (Fig. 15B). However, at E9 ∼30% more healthy motoneurons were present in experimental cases (Fig.15C). These results suggest that increasing target size can rescue motoneurons that normally would die between E5 and E9 but that similar increases in target size do not rescue cervical cells during the early cell death period.
Fig. 15.

Bar graphs showing the results of transplantation of cervical segments to the cervical region (control) and to the brachial region. A, The number (mean ± SD) of pyknotic cells at E4.5 after experimental (cervical to brachial transplant) and control (cervical to cervical transplant) transplants was not significantly different. B, The number of Islet-1-immunopositive neurons in the ventral horn in 10-μm-thick section at E5 was not significantly different between control and experimental groups. C, Transplantation of the cervical neural tube to the brachial region resulted in approximately 30% more Islet-1-immunopositive neurons on E9. p < 0.001, t test.
Transplantation of cervical segments to the thoracic region and thoracic segments to the cervical region
To examine the possibility that the cervical environment is somehow involved in inducing early cell death in the cervical ventral horn, transplantations of cervical segments to the thoracic region and thoracic segments to the cervical region were performed.
Transplantation of either rostral (n = 6) or caudal (n = 7) segments of the cervical neural tube to the thoracic region on E2 failed to alter the amount of cell death observed in the ventral horn on E4.5 (Sham control = 18 ± 9 dying cells per section,n = 8 vs 21 ± 7 per section in transplant embryos,n = 13). When thoracic segments (T2–T4) of st. 13 embryos were transplanted into the cervical region (C9–C11) of st. 12 embryos, cell death between E4 and E5 was not observed in the ventral horn of the transplanted thoracic segments (data not shown). Additionally, examination of labeled neurons by the retrograde DiI technique showed that a distinct nucleus of Terni, which is a thoracic-specific structure, had failed to develop in cervical segments transplanted into the thoracic region (Fig. 16A). By contrast, in thoracic segments transplanted into the cervical region, a normal-appearing nucleus of Terni was formed (Fig. 16B), and communicating rami projecting from the spinal nerves to the sympathetic ganglia were maintained (data not shown).
Fig. 16.
A, A fluorescent photomicrograph of a transverse section of an E7.5 chick embryo spinal cord showing the absence of a nucleus of Terni (sympathetic preganglionic neurons) in cervical segments transplanted into the thoracic region. B, A fluorescent photomicrograph of a transverse section of the E7.5 chick embryo spinal cord showing a nucleus of Terni (sympathetic preganglionic neurons) in thoracic segments transplanted into the cervical region. The nucleus of Terni (arrows) and motoneurons were retrogradely labeled by injection of DiI into the ventral root. Scale bar, 100 μm. VH, Ventral horn;FP, floor plate.
Transplantation of cervical segments between the chick and the quail
We examined cell death in the cervical cord of quail embryos and confirmed that, whereas early cell death also occurs in this avian species, the onset was ∼0.5 d earlier than that in the chick embryo, reflecting the generally accelerated development of the quail embryo (Fig. 17A). We used this time difference between the two species to determine whether circulating systemic signals affect the time of initiation of cell death. Cervical segments were transplanted either from chick to quail or from quail to chick. The number of pyknotic cells was counted in the cervical segments at E3.5, E4, E4.5, and E5, in both sham control embryos and in embryos with transplanted grafts. Although a small (but statistically significant) difference was seen at E4.5 in cases with quail-to-chick transplantation (Fig. 17B), in general after both chick-to-quail and quail-to-chick transplantations, the timing of initiation and the rate of cell death were similar to controls (Fig.17B,C). These results suggest that, even after transplantation between different species, the temporal schedule of development of ventral horn neurons is preserved and that the initiation of early cervical cell death in avian embryos is determined by cues intrinsic to the cells themselves or by signals derived from other cells within the cervical neural tube.
Fig. 17.

A, A line graph showing the difference in timing of cell death in the lower cervical cord (C11) between chick and quail. Sample size = 4 for both chick and quail. B, The number (mean ± SD) of pyknotic cells in cervical segments transplanted from chick to quail (experimental) and chick to chick (control). C, The number (mean ± SD) of pyknotic cells in cervical segments transplanted from quail to chick (experimental) and quail to quail (control). *p < 0.001, t test.
Cell death in an induced ectopic supernumerary ventral horn
A supernumerary ventral horn can be induced in avian embryos by transplanting the notochord or floor plate to an ectopic site lateral or dorsal to the neural tube (Placzek et al., 1991; Yamada et al., 1991, 1993). We examined whether early cell death also occurs in such an induced ectopic supernumerary ventral horn in either the cervical or brachial cord. Cell death occurred on schedule at E4.5 in the induced motor column in the cervical cord (Fig.18A), whereas no cell death was observed in the induced motor column in the brachial segments (Fig.18B).
Fig. 18.
Fluorescent photomicrographs of transverse sections through the cervical (A) and brachial region (B) of E4.5 chick embryos that received a lateral notochord graft placed between the neural tube and somites on the right side at E1.5. Double labeling for SC1 (green) and TUNEL (red). In addition to the presence of normal ventral motoneurons [VH(L) and VH(R)], supernumerary motoneurons were induced laterally (VH′) by the implanted notochord. Cell death occurred in the supernumerary motoneuron column of the cervical spinal cord (A) but not in the brachial region (B). Scale bar, 50 μm.
Effect of metabolic inhibitors and neurotrophic agents
Cycloheximide and actinomycin D. To determine whether protein or RNA synthesis is required for the initiation of the early cervical cell death, embryos were treated with cycloheximide or actinomycin D. Treatment was started at st. 23, and after 8–10 hr of survival, the number of pyknotic cells was counted in the C10 segment. A total of 0.75 μg of cycloheximide and 1.5 μg of actinomycin D was administered. It was confirmed that in this situation protein synthesis was reduced by >60%, without any apparent delay of general embryonic development (data not shown). As shown in Table 3, the number of pyknotic cells in the cervical segments was considerably reduced by both cycloheximide and actinomycin D treatment.
Table 3.
Effects of cycloheximide and actinomycin D on early neuronal death in the cervical spinal cord
| Group | Pyknotic cells/section (Mean ± SD) | n |
|---|---|---|
| PBS | 31.8 ± 7.5 | 13 |
| Cycloheximide | 3.0 ± 2.2* | 10 |
| Actinomycin D | 1.5 ± 1.0* | 6 |
*p < 0.001; t tests.
Neurotrophic factors. To determine whether growth factors or trophic molecules are involved in early cervical cell death, we examined the effect of treatment with various defined neurotrophic factors and partially purified tissue extracts, which are known to promote the survival of motoneurons and other neuronal populations during avian development in vitro and in vivo(Arakawa et al., 1990; Sendtner et al., 1990, 1991, 1992a,b;Bhattacharyya et al., 1992; Oppenheim et al., 1992, 1993; Yan et al., 1992, 1993; Henderson et al., 1993; Li et al., 1994; Yin et al., 1994). Treatment with these agents was started at E3.5, and pyknosis was quantified on E4.5 (st. 24). In addition to MEX, BEX, and A-CM, NGF, BDNF, NT-3, activin, BMP-2, TGF-β1, CNTF, bFGF, IGF-I, IL-6, CDF/LIF, S100-β, and PDGF were also tested. The amount of each factor used and the results are summarized in Table 4. None of the agents that were examined had any effect on the early death of cervical motoneurons on E4.5. Although many of these factors have been shown to rescue motoneurons in lumbar segments from normal cell death between E5 and E10, with the exception of MEX and BEX (Table 4), their effectiveness in vitro or in vivo oncervical cell death between E5 and E10 has not yet been examined.
Table 4.
Effects of conditioned media, tissue extracts, and growth factors on early neuronal death in the cervical spinal cord
| Group | n | Pyknotic cells E4.54_a (% control) | Healthy cells E10 (Mean ± SD) |
|---|---|---|---|
| Control4_b | |||
| PBS | 33 | 100.0 | |
| Cytochrome c | 5 | 96.2 | 8100 ± 937 (n = 7)4_e |
| Conditioned media | |||
| Astrocyte-conditioned media | 8 | 87.1 | |
| Tissue Extracts4_c | |||
| E9 chick muscle extract (MEX) | 6 | 96.9 | 11,678 ± 871* (n = 4)4_e |
| E9 chick brain extract (BEX) | 9 | 98.9 | 10,707 ± 635* (n = 4)4_e |
| Growth Factors4_d | |||
| Nerve growth factor | 3 | 95.1 | |
| Brain-derived neurotrophic factor | 5 | 81.8 | |
| Neurotrophin-3 | 7 | 99.1 | |
| Ciliary neurotrophic factor | 5 | 99.6 | |
| IL-6 | 7 | 94.8 | |
| CDF/LIF | 5 | 97.0 | |
| Platelet-derived growth factor | 7 | 106.5 | |
| S100-β | 7 | 86.5 | |
| Activin | 4 | 91.5 | |
| Transforming growth factor-β1 | 7 | 85.7 | |
| BMP-2 | 5 | 96.3 | |
| bFGF | 5 | 93.6 | |
| IGF-1 | 6 | 102.3 |
For clarity, the number of pyknotic cells/section in treated groups is presented as a percentage of the number of pyknotic cells/section in the corresponding PBS control group. However, statistical analysis (Student’s t test) was performed using the raw data (pyknotic cells/section). No significant differences were observed for any of the tested factors.
Embryos treated with 3 μg/50 μl cytochrome C were compared with PBS-treated animals to monitor any nonspecific effect of treatment with large doses of protein.
150 μg total protein/50 μl.
3 μg/50 μl.
Embryos were treated daily from E6 to E9 with 150 μg total MEX or BEX protein/50 μl saline or only saline (control) and killed on E10.
*p < 0.01 versus control (t test).
Cervical cell death in mutant (cn andta2) embryos
Although spinal motoneuron death between E6 and E10 is greatly decreased in muscular dysgenic cn-mutant embryos (Oppenheim et al., 1996), early cervical cell death was not altered incn embryos examined on E4.5 (Table 4). Inta2-mutant embryos, programmed cell death of mesenchyme fails to occur in the developing limb (Dvorak and Fallon, 1991). We examined early cervical cell death inta2 embryos to determine whether this mutation might also affect the programmed cell death of neurons. However, as shown in Table 5, the death of cervical motoneurons inta2 embryos occurred on schedule (E4.5) and at the same rate as in the controls. These results suggest that the molecular defects in the cell death process in these mutants does not affect cell death in the cervical spinal cord between E4 and E5.
Table 5.
Cervical cell death in cn andta2 embryos on E4.5
| Group | n | Pyknotic cells/section (Mean ± SD) |
|---|---|---|
| ta control | 7 | 16.7 ± 2.6 |
| ta mutant | 6 | 14.9 ± 5.1 |
| cn control | 3 | 13.0 ± 2.2 |
| cnmutant | 4 | 14.0 ± 3.8 |
DISCUSSION
Identity of the dying cells
The present study provides two major lines of evidence that dying cells in the cervical–ventral horn of chick embryo between E4 and E5 are somatic motoneurons. First, we demonstrated that many of the dying cells express Islet proteins and Lim-3 mRNA before cell death. Colocalization of both markers in the same cells indicates that the cells are somatic motoneurons in the medial motor column that innervate axial muscles (Tsuchida et al., 1994). Second, we confirmed that at least some of the dying cells in the ventral horn of the cervical cord of the E4–E5 chick embryo send axons to the cervical–somitic region before cell death.
Dying cells in the cervical–ventral horn have been suggested to be transient visceral motoneurons, sympathetic preganglionic neurons (SPNs) (Levi-Montalcini, 1950, 1964). This suggestion was based on the following observations. (1) Communicating branches appear to exist between cervical spinal nerves and sympathetic ganglia before the cell death period, and these disappear concomitant with cell loss (Levi-Montalcini, 1950). (2) In thoracic segments, the SPNs initially seem to be intermixed with somatic motoneurons in the ventral horn but then later migrate from this region to form a distinct nucleus. (3) The notion that the dying cells in the cervical–ventral horn are vestigial and transient SPN-like cells intermixed with somatic motoneurons was given further support by an experimental study in which the cervical cord was transplanted to the thoracic level. Following transplantation, a SPN-like nucleus of Terni was observed in a few cases (Shieh, 1951).
The existence of transient preganglionic communicating branches in the cervical region has been controversial. Both Tello (1925) and Terni (1931) concluded that the communicating branches are aberrant, whereasLevi-Montalcini (1950) concluded that they are a normal, albeit transient, phenomenon. Our results after anterograde tracing with DiI and immunohistochemistry of neurofilament revealed that >50% of the cervical spinal nerves have apparent communicating branches consisting of only a small number of axons and projecting toward the sympathetic ganglia at E3.5. However, most of such projecting axons had disappeared by E4, when cell death begins. Because we could not identify the parent neurons of axons projecting toward the sympathetic ganglia or determine whether they actually innervate the ganglia, it is unclear whether these are transient, sympathetic preganglionic neurons or transient aberrantly projecting axons or axonal branches of somatic motoneurons. In any event, because the number of axons in the communicating branches that are observed is far too few to account for the numerous dying cells and because these disappear before rather than during the cell death period, it is unlikely that many of the dying cells have axons projecting toward the sympathetic ganglia before cell death. In the present study, we did not examine the development of SPNs in thoracic segments. However, in a recent study, Prasad and Hollyday (1991) used a retrograde tracing method to show that most SPNs in the thoracic segments remain close to the ventricular epithelium until the start of the dorsal migration and thus are never intermixed with somatic motoneurons in the ventral horn, as originally claimed byLevi-Montalcini (1950).
Finally, we have been unable to replicate the report by Shieh (1951)that, after transplantation of cervical segments to the thoracic region, SPN-like cells are found and that these represent rescued cervical neurons (i.e., transient preganglionic cells) that would have died if the neural tube had remained in its normal location. Although we cannot exclude the possibility that under some circumstances the thoracic environment can induce or maintain the development of SPN-like cells in transplanted cervical segments, our experiments provide no evidence for this notion. Additional evidence that the dying cervical motoneurons on E4.5 do not represent preganglionic cells that die because of a nonpermissive environment comes from our observation that preganglionic cells survive and differentiate after transplantation of the thoracic neural tube to the cervical region on E2.
The mode of early cervical cell death is by apoptosis
A previous morphological study suggested that the early cell death in the cervical cord occurs by apoptosis (O’Conner and Wyttenbach, 1974). Our own observations support this idea. First, we demonstrated, using the TUNEL method, that DNA fragmentation occurs in the dying cells. Second, it was confirmed that this cell death process requires protein and RNA synthesis. Finally, ultrastructural examination confirmed that the dying cells exhibit the morphology of apoptosis.
There are two types of motoneuron death in the cervical region
Cell death of cervical motoneurons occurs in two phases: an initial short phase (E4–E5) and a second, more extended (E5–E10) phase. The present study has revealed that, although the mode of cell death in the early and late phases is the same (apoptotic) (O’Conner and Wyttenbach, 1974; Chu-Wang and Oppenheim, 1978a,b), the regulation of motoneuron death in the two phases is different.
Two lines of evidence indicate that the late phase of cell death corresponds to naturally occurring motoneuron death that also occurs in other segments of the spinal cord between E6 and E10. First, the time and rate of this second phase of cervical cell death are similar to motoneuron death in other segments. In other segments (e.g., lumbar, thoracic, brachial) of the chick spinal cord, ∼40–60% of motoneurons are known to die between E6 and E10 (Hamburger, 1975;Chu-Wang and Oppenheim 1978a,b; Oppenheim and Majors-Willard, 1978;Laing, 1982; Lanser and Fallon, 1984; O’Brien and Oppenheim, 1990;O’Brien et al., 1990). Second, activity–blockade (curare) treatment and increasing the size of peripheral targets, both of which are known to prevent naturally occurring motoneuron death (Hollyday and Hamburger, 1976; Pittman and Oppenheim, 1978, 1979; O’Brien and Oppenheim, 1990), are also effective in modulating cell death in the cervical region between E6 and E10. It has been suggested that neurons are programmed originally to die at a certain stage in development and that their survival is contingent on successful competition for limiting sources of trophic factors. Access to sufficient trophic factor is thought to suppress an active cell death program (the neurotrophic theory) (for review, see Purves, 1988; Oppenheim, 1989,1991). In early stages of motoneuron development, target muscles are a particularly important source of trophic factors.
Our present results suggest that the early and late phases of cell death in the cervical cord are regulated differently. First, we observed that axonal outgrowth and the genesis of motoneurons continue during the early phase of cell death. We also observed that some dying cells were retrogradely labeled by latex beads injected into the presumptive target region of the cells before the onset of cell death. These observations indicate that at least some neurons in the cervical–ventral horn can survive the early phase of cell death before their axons have reached the target, whereas other neurons whose axons have already reached the target region undergo cell death. Second, neither activity blockade (curare) nor increasing the size of peripheral targets was effective in altering the early phase of cell death. Third, the early phase of cervical cell death was not altered in the cn mutant, whereas motoneuron death in the late phase was decreased. Finally, treatment with exogenous, partially purified MEX, which is known to rescue motoneurons between E6 and E10 (Oppenheim et al., 1988a), did not rescue the dying cells in the early phase. These results are difficult to explain within the context of the target-derived neurotrophic theory (Oppenheim, 1989), and they also tend to exclude the possibility that there are two motoneuron populations in the ventral horn of the cervical cord of the chick, one of which becomes target dependent early, and another that becomes target dependent later (Mettling et al., 1993).
Recent studies also suggest that the intracellular mechanisms mediating the early phase of cervical motoneuron death may differ from those acting between E6–E10. Interleukin 1β-converting enzyme (ICE), a mammalian homolog of the Caenorhabditis elegans cell death gene, ced-3, has been shown to induce apoptosis of neurons (Miura et al., 1993). Recently, it was reported that administration of ICE inhibitors prevents cell death of avian lumbar motoneurons in vitro and in vivo (Milligan et al., 1995). By contrast, these same inhibitors were ineffective in preventing the early phase of cell death in the cervical cord (Milligan et al., 1995). Another apparent difference in the intracellular mechanisms of cell death in the early and late phases is the possible role of oxidative stress.N-acetylcysteine (NAC), an agent that raises intracellular levels of the major antioxidant glutathione, rescues rat sympathetic neurons from cell death in vitro after NGF deprivation (Ferrari et al., 1995). Treatment of chick embryos with NAC during the late period of programmed cell death (E6–E9) significantly reduces the number of pyknotic motoneurons on E8 and increases the number of healthy cells on E10. By contrast, treatment with NAC on E3.5 and E4.0 does not prevent the early phase of cervical motoneuron death (Oppenheim et al., 1995). Because the intracellular events that mediate the death of avian motoneurons are unknown, the significance of the different results after treatment with ICE inhibitors andN-acetylcysteine is not entirely clear. However, they suggest that the molecular mechanisms that mediate early and late phases of cervical cell death may differ.
What regulates early cell death in the cervical cord?
Induction of an ectopic supernumerary ventral horn in the cervical (but not brachial) spinal cord results in the occurrence of an early phase of motoneuron death in the induced ventral horn region. This suggests that perhaps all of the neuroepithelial cells comprising the cervical neural tube contain a potential early cell death program that is activated once the induction and differentiation of motoneurons begin.
After transplantation of cervical segments to other spinal regions on E2, the rate of early cell death was not altered. Furthermore, after transplantation of the thoracic segments to the cervical region, early cell death did not occur in the ventral horn of the transplanted thoracic segments. These results provide strong evidence that, after E2, the local environment outside the neural tube does not regulate early cell death; rather, the transplanted neural tube develops according to its site of origin. For example, a sympathetic preganglionic nucleus specific for thoracic segments was retained in thoracic segments transplanted to the cervical region. Together with previous studies (O’Brien and Oppenheim, 1990; O’Brien et al., 1990), these findings suggest that the rostro–caudal identity of the avian spinal cord is determined before E2 and can not be altered by transplantation after this time, although some modification of motoneuron development by target-derived signals occurs at later developmental stages (Yin and Oppenheim, 1992).
We have confirmed that early cervical cell death also occurs in the quail embryo ∼0.5 d earlier than in the chick embryo, probably reflecting the faster rate of development of the quail embryo. After reciprocal transplantation of the cervical segments at E1.5 between quail and chick, early cell death in the transplanted segments occurred according to the schedule of the donor tissue. This indicates that, after transplantation, a species-specific program for cell death is maintained for at least 2.5–3 d despite differences in putative systemic signals in the host embryo. By contrast, in some other examples of cell death, alterations in the concentration of systemic hormones trigger cell death (Fahrbach and Truman, 1987; Kimura and Truman, 1990; Truman et al., 1990; Fahrbach et al., 1994). Also, these data from quail–chick transplants are not consistent with the possibility that signals derived from ascending or descending fiber tracts induce early cervical cell death. The occurrence of these signals would have been accelerated (chick to quail) or retarded (quail to chick) in these experiments. Preliminary studies with the chick tend to support these data, in that prevention of the ingrowth of ascending or descending fiber tracts does not seem to alter the rate of early cervical cell death (Yaginuma et al., unpublished observation).
Taken together, these results suggest that early cell death in the avian cervical cord may be determined by a cell-autonomous program. The intrinsic program would be initiated in motoneurons in the cervical cord after at least two steps: first, differentiation into acervical-specific neural tube; and second, differentiation into somatic, medial motoneurons. Once this cell death program is initiated at a certain developmental stage, environmental factors would not be able to suppress it. However, because of inherent technical limitations of our in vivo experiments, we could not determine whether local cell–cell signals derived fromwithin the cervical neural tube play a role in regulating cell death. Transplantation of cervical segments to ectopic locations was always done with the organization of, and local interactions within, the neural tube being maintained. Therefore, we cannot exclude the possibility that other cervical cells in close proximity to motoneurons are involved in the cell death process. The posterior necrotic zone (PNZ) of the chick wing bud is a well known example of programmed cell death in which such local signals may regulate survival (Saunders et al., 1962; Saunders, 1966; Fallon and Saunders, 1968). When tissue in this region is excised later than st. 21 and transplanted to an ectopic region, mesenchymal cells in the PNZ die on schedule at st. 24 (Saunders et al., 1962; Fallon and Saunders, 1968). However, a recent study has shown that cell death in the PNZ can be inhibited by removing the overlying ectodermal ridge (Brewton and MacCabe, 1988). This suggests that signals from the immediate cellular environment can alter the cell death program in the PNZ. The same thing may be true for early cervical cell death. Recently, it was reported that a vertebrate homolog of the Drosophila segment polarity gene hedgehog (vhh-1) contributes to the floor plate- and motoneuron-inducing activities of the notochord (Roelink et al., 1994). It will be of considerable interest to determine whethervhh-1 is also involved in the determination or the initiation of early cervical cell death.
Our present results also do not address the issue of whether the selection of which specific neurons will die is linked to particular cell lineages, as occurs in the nervous systems of nematoda and some insects (for review, see Ellis et al., 1991; Driscoll, 1992; Truman et al., 1992), or whether cell death is determined stochastically by cell–cell interactions among motoneurons or between motoneurons and other local cells. Although motoneurons in the ventral horn of the cervical cord at E4 are known to be a homogeneous population based on morphological criteria (Levi-Montalcini, 1950, 1964; Oppenheim et al., 1989), an analysis of cell birth dates revealed that most of the dying cells in the early phase become postmitotic before E3.5, whereas motoneurons that survive the cell death period are generated as early as E2 and as late as E4.5. This difference in the birth dates indicates that neurons dying during the early phase of cell death may comprise a discrete subpopulation, most of which are destined to die.
Neurotrophic factors were not effective
One of the striking results of this study is that tissue extracts and a wide variety of neurotrophic factors, which have been shown to rescue motoneurons from programmed cell death during later development or after injury (Arakawa et al., 1990; Sendtner et al., 1990, 1991,1992a,b; Bhattacharyya et al., 1992; Oppenheim et al., 1992, 1993;Yan et al., 1992, 1993; Henderson et al., 1993; Li et al., 1994; Yin et al., 1994), were ineffective in preventing early cervical cell death. This suggests that the cells that die in the early phase of cell death either do not express functional receptors for these neurotrophic factors or that receptors, if present, do not regulate survival at these stages. Preliminary results indicate that the low-affinity neurotrophin receptor, p75, is present on the axons of E4.5 cervical spinal nerves. In addition, mRNA for the high-affinity neurotrophin receptors trkA and trkC are present at E4 in the ventral–cervical spinal cord, and the radiolabeled-trkA and trkC ligands, NGF and NT-3, respectively, are taken up by E4 ventral–cervical neurons after systemic administration (S. McKay and R. Oppenheim, unpublished data). However, none of these neurotrophins rescued cervical motoneurons from the early phase of cell death. Another possible explanation for the negative results of the neurotrophic experiments is that, although receptors may have been expressed, treatment was begun too late to influence the cell death program. Although treatment with neurotrophic factors was started at E3.5 12–24 hr before the initiation of cell death, because most of the cells that die at E4.5 become postmitotic before E3.5, it is possible that by E3.5 the cells are already irreversibly committed to a cell death program and, therefore, could not be rescued. Although we can not exclude this possibility, because preliminary studies using MEX treatment begun on E2.5 also failed to rescue cells on E4.5, we consider this an unlikely alternative. Finally, we also can not exclude the possibility that other known or novel growth factors not tested here may prove to be effective in rescuing these cells from death.
The negative results with NGF also provide additional evidence against the argument that the dying cells are SPNs. Treatment of chick embryosin vivo with NGF during the normal period of cell death of thoracic SPNs (E7–E11) rescues these cells by an indirect route, i.e., by increasing the size of sympathetic ganglia (Oppenheim et al., 1982). However, neither this previous study nor the present one found any effect of NGF on the early phase of cervical cell death despite the fact that NGF does increase the size of cervical sympathetic ganglia on E4.5 (Oppenheim et al., 1982).
Comparison with cell death in the earliest stages of neural tube development
Cell death in very early developmental stages has been reported in various regions of the central nervous systems of vertebrates (Glücksmann, 1951; Silver, 1978). For example, between E2.5 and E3.5 many pyknotic cells are observed in four distinct regions of the chick embryo caudal–neural tube, excluding the ventral horn (Homma et al., 1994). This early cell death is thought to represent a kind of negative selection for inappropriate phenotypes or precursor cells, because cell death occurs during differentiation and pattern formation of the neural tube along the dorso–ventral axis (Homma et al., 1994), and perturbations that alter putative signals for dorso–ventral differentiation (e.g., notochord removal) also alter the amount and distribution of cell death (Homma and Oppenheim, 1992). Although the early death of cervical motoneurons may also be involved in some aspects of differentiation along the dorso–ventral axis, there are important differences in these two types of early cell death. Most of the dying cells in the E4.5 cervical spinal cord have been postmitotic for 12 hr to 2 d and have begun axonogenesis and target innervation, whereas dying cells in the early neural tube (E2–E3) die shortly after becoming postmitotic or even during the cell cycle (Homma et al., 1994) and before extending the neuronal processes.
In conclusion, we have identified an early and massive form of programmed cell death of cervical motoneurons that is distinct from spinal motoneuron death at later stages, primarily by virtue of its independence from regulation by target-derived signals and its occurrence only in cervical segments of the spinal cord. At present neither the biological functions nor the specific mechanisms regulating this novel type of programmed neuronal death are known. Because the early death of cervical neurons is the only example of programmed cell death in vertebrates known to us in which motoneuron loss and survival occur independent of regulation by target-derived trophic signals, further studies are needed to attain a deeper understanding of this novel phenomenon.
Footnotes
This study was supported by the University of Tsukuba Research Projects, Grant-in-Aid from the Japanese Ministry of Education, Science, and Culture (Nos. 03770024, 05780560, and 07780671) to H.Y., and by National Institutes of Health Grant NS20402 and a grant from the Amyotrophic Lateral Sclerosis Society to R.W.O.
We thank Drs. M. Matsushita, N. Okado, S. Homma, M. Watanabe, and H. Kimata for their help and advice, Dr. H. Tanaka for providing SC1 antibody, Drs. T. Jessell and T. Tsuchida for Islet antibodies and theLim-3 probe, Dr. Ueno for providing activin, and H. Yamada for his unfailing technical assistance. Monoclonal anti-Islet protein antibody (40.2D6) was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biology, University of Iowa, Iowa City, IA, under contract NO1-HD-2–3144 from the National Institute of Child Health and Human Development.
Correspondence should be addressed to Dr. Yaginuma at the above address.
REFERENCES
- 1.Abbott UK, Taylor LW, Abplanalp H. Studies with talpid, an embryonic lethal of the fowl. J Hered. 1960;51:195–202. [Google Scholar]
- 2.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 3.Airey JA, Baring MD, Beck CF, Chelliah Y, Deerink TJ, Ellisman MH, Houenou LJ, McKenny DD, Sutko JL, Talvenheimo J. Failure to make normal alpha-ryanodine receptor is an early event associated with the crooked neck dwarf (cn ) mutation. Dev Dyn. 1993;197:169–188. doi: 10.1002/aja.1001970303. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya A, Oppenheim RW, Prevette D, Moore BW, Brackenbury R, Ratner N. S100 is present in developing chicken neurons and Schwann cells and promotes motor neuron survival in vivo. J Neurobiol. 1992;23:451–466. doi: 10.1002/neu.480230410. [DOI] [PubMed] [Google Scholar]
- 6.Brewton RG, MacCabe JA. Ectodermal influence on physiological cell death in the posterior necrotic zone of the chick wing bud. Dev Biol. 1988;126:327–330. doi: 10.1016/0012-1606(88)90142-x. [DOI] [PubMed] [Google Scholar]
- 7.Chu-Wang I-W, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. I. A light and electron microscopic study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978a;177:33–58. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- 8.Chu-Wang I-W, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. II. A quantitative and qualitative analysis of degeneration in the ventral root, including evidence for axon outgrowth and limb innervation prior to cell death. J Comp Neurol. 1978b;177:59–86. doi: 10.1002/cne.901770106. [DOI] [PubMed] [Google Scholar]
- 9.Clarke PGH, Oppenheim RW. Neuron death in vertebrate development: in vivo methods. In: Schwartz L, Osborne B, editors. Methods in cell biology: cell death. Academic; New York: 1995. pp. 277–323. [PubMed] [Google Scholar]
- 10.Cuadros MA, Rios A. Spatial and temporal correlation between early nerve fiber growth and neuroepithelial cell death in the chick embryo retina. Anat Embryol. 1988;178:543–551. doi: 10.1007/BF00305042. [DOI] [PubMed] [Google Scholar]
- 11.Cuadros MA, Martin C, Coltey P, Almendros A, Navascués J. First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J Comp Neurol. 1993;330:113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- 12.Driscoll M. Molecular genetics of cell death in the nematode Caenorhabditis elegans. J Neurobiol. 1992;23:1327–1351. doi: 10.1002/neu.480230919. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak L, Fallon JF. Talpid2mutant chick limb has anteroposterior polarity and altered patterns of programmed cell death. Anat Rec. 1991;231:251–260. doi: 10.1002/ar.1092310213. [DOI] [PubMed] [Google Scholar]
- 14.Egensperger R, Holländer H. Electron microscopic visualization of fluorescent microspheres used as a neuronal tracer. J Neurosci Methods. 1988;23:181–186. doi: 10.1016/0165-0270(88)90001-5. [DOI] [PubMed] [Google Scholar]
- 15.Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 16.Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 17.Fahrbach SE, Truman JW. Possible interactions of a steroid hormone and neural inputs in controlling the death of an identified neuron in the moth Manduca sexta. J Neurobiol. 1987;18:497–508. doi: 10.1002/neu.480180603. [DOI] [PubMed] [Google Scholar]
- 18.Fahrbach SE, Choi MK, Truman JW. Inhibitory effects of actinomycin D and cycloheximide on neuronal death in adult Manduca sexta. J Neurobiol. 1994;25:59–69. doi: 10.1002/neu.480250106. [DOI] [PubMed] [Google Scholar]
- 19.Fallon JF, Saunders JJ. In vitro analysis of the control of cell death in a zone of prospective necrosis from the chick wing bud. Dev Biol. 1968;18:553–570. doi: 10.1016/0012-1606(68)90026-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari G, Yan CYI, Greene LA. N -acetylcysteine (d- andl-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995;15:2857–2866. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–502. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glücksmann A. Cell deaths in normal vertebrate ontogeny. Biol Rev. 1951;26:59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 25.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillip HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 26.Hinchliffe JR, Thorogood PV. Genetic inhibition of mesenchymal cell death and the development of form and skeletal pattern in the limbs of talpid3( ta3) mutant chick embryos. J Embryol Exp Morphol. 1974;31:747–760. [PubMed] [Google Scholar]
- 27.Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- 28.Hollyday M, Hamburger V. An autoradiographic study of the formation of the lateral motor column in the chick embryo. Brain Res. 1977;132:197–208. doi: 10.1016/0006-8993(77)90416-4. [DOI] [PubMed] [Google Scholar]
- 29.Homma S, Oppenheim RW. Notochord-dependent cell survival in the ventral half of the chick neural tube. Soc Neurosci Abstr. 1992;18:43. [Google Scholar]
- 30.Homma S, Yaginuma H, Oppenheim RW. Cell death during the earliest stages of spinal cord development in the chick embryo: a possible means of early phenotypic selection. J Comp Neurol. 1994;345:377–395. doi: 10.1002/cne.903450305. [DOI] [PubMed] [Google Scholar]
- 31.Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- 32.Kimura KI, Truman JW. Postmetamorphic cell death in the nervous and muscular systems of Drosophila melanogaster. J Neurosci. 1990;10:403–411. doi: 10.1523/JNEUROSCI.10-02-00403.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Källén B. Cell degeneration during normal ontogenesis of rabbit brain. J Anat. 1955;89:153–161. [PMC free article] [PubMed] [Google Scholar]
- 34.Laing NG. Timing of motoneuron death in the brachial and lumbar regions of the chick embryo. Dev Brain Res. 1982;5:181–186. doi: 10.1016/0165-3806(82)90155-9. [DOI] [PubMed] [Google Scholar]
- 35.Lanser ME, Fallon JF. Development of the lateral motor column in the limbless mutant chick embryo. J Neurosci. 1984;4:2043–2050. doi: 10.1523/JNEUROSCI.04-08-02043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levi-Montalcini R. The origin and development of the visceral system in the spinal cord of the chick embryo. J Morphol. 1950;86:253–284. [Google Scholar]
- 37.Levi-Montalcini R. Events in the developing nervous system. Prog Brain Res. 1964;4:1–29. [Google Scholar]
- 38.Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- 39.Mettling C, Camu W, Henderson CE. Embryonic wing and leg motoneurons have intrinsically different survival properties. Development (Camb) 1993;118:1149–1156. doi: 10.1242/dev.118.4.1149. [DOI] [PubMed] [Google Scholar]
- 40.Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM. Peptide inhibitors of the interleukin-1β-converting enzyme (ICE) protease family arrest programmed cell death of motoneurons. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 41.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1β-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 42.Navascués J, Martin-Partido G, Alvarez IS, Rodriguez-Gallar do L. Cell death in suboptic necrotic centers of chick embryo diencephalon and their topographic relationship with the earliest optic fiber fascicles. J Comp Neurol. 1988;278:34–46. doi: 10.1002/cne.902780103. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien MK, Oppenheim RW. Development and survival of thoracic motoneurons and hindlimb musculature following transplantation of thoracic neural tube to the lumbar region in the chick embryo: anatomical aspects. J Neurobiol. 1990;21:313–340. doi: 10.1002/neu.480210207. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien MK, Landmesser L, Oppenheim RW. Development and survival of thoracic motoneurons and hindlimb musculature following transplantation of thoracic neural tube to the lumbar region in the chick embryo: functional aspects. J Neurobiol. 1990;21:341–355. doi: 10.1002/neu.480210208. [DOI] [PubMed] [Google Scholar]
- 45.O’Conner TM, Wyttenbach CR. Cell death in the embryonic chick spinal cord. J Cell Biol. 1974;60:448–459. doi: 10.1083/jcb.60.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oppenheim RW. The ontogeny of behavior in the chick embryo. In: Rosenblatt J, Shaw F, editors. Advances in the study of behavior. Academic; New York: 1974. [Google Scholar]
- 47.Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 48.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 49.Oppenheim RW, Majors-Willard C. Neuronal cell death in the brachial spinal cord of the chick is unrelated loss of polyneuronal innervation in wing muscle. Brain Res. 1978;154:148–152. doi: 10.1016/0006-8993(78)91062-4. [DOI] [PubMed] [Google Scholar]
- 50.Oppenheim RW, Maderdrut J, Wells D. Nerve growth factor-induced hypertrophy of the column of Terni in the chick embryo. Dev Brain Res. 1982;3:134–139. doi: 10.1016/0165-3806(82)90081-5. [DOI] [PubMed] [Google Scholar]
- 51.Oppenheim RW, Haverkamp LJ, Prevette D, McManaman JL, Appel SN. Reduction of naturally occurring motoneuron death in vivo by a target-derived trophic factor. Science. 1988a;240:919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- 52.Oppenheim RW, Schneiderman A, Shimizu I, Yaginuma H. Onset and development of intersegmental projections in the chick embryo spinal cord. J Comp Neurol. 1988b;275:159–180. doi: 10.1002/cne.902750202. [DOI] [PubMed] [Google Scholar]
- 53.Oppenheim RW, Cole T, Prevette D. Early regional variations in motoneuron numbers arise by differential proliferation in the chick embryo spinal cord. Dev Biol. 1989;133:468–474. doi: 10.1016/0012-1606(89)90050-x. [DOI] [PubMed] [Google Scholar]
- 54.Oppenheim RW, Prevette D, Tytell M, Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990;138:104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- 55.Oppenheim RW, Yin Q-W, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992;360:755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- 56.Oppenheim RW, Prevette D, Haverkamp LJ, Houenou L, Yin Q-W, McManaman J. Biological studies of a putative avian muscle-derived neurotrophic factor that prevents naturally occurring motoneuron death in vivo. J Neurobiol. 1993;24:1065–1079. doi: 10.1002/neu.480240806. [DOI] [PubMed] [Google Scholar]
- 57.Oppenheim RW, Hong J, Caldero J, Milligan C, Newsome A, Lloyd L, Prevette D. Pharmacological agents that modify oxidative stress promote motoneuron survival during development. Soc Neurosci Abstr. 1995;21:1789. [Google Scholar]
- 58.Oppenheim RW, Prevette D, Houenou LJ, Pincon-Raymond M, Dimitriadou V (1996) Neuromuscular development in the avian paralytic mutant, crooked neck dwarf (cn/cn): Muscle activity and motoneuron survival. J Comp Neurol, in press. [DOI] [PubMed]
- 59.Pittman RH, Oppenheim RW. Neuromuscular blockade increases motoneuron survival during normal cell death in the chick embryo. Nature. 1978;271:364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- 60.Pittman RH, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- 61.Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development [Suppl] 1991;2:105–122. [PubMed] [Google Scholar]
- 62.Prasad A, Hollyday M. Development and migration of avian sympathetic preganglionic neurons. J Comp Neurol. 1991;307:237–258. doi: 10.1002/cne.903070207. [DOI] [PubMed] [Google Scholar]
- 63.Purves D. Harvard; Cambridge, MA: 1988. Body and brain, a trophic theory of neural connections. . [DOI] [PubMed] [Google Scholar]
- 64.Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, Dodd J. Floor plate and motor neuron induction by vhh-1 , a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 65.Saunders JJ. Death in embryonic systems. Science. 1966;154:604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- 66.Saunders JWJ, Gasseling MT, Saunders LC. Cellular death in morphogenesis of the avian wing. Dev Biol. 1962;5:147–178. doi: 10.1016/0012-1606(62)90008-8. [DOI] [PubMed] [Google Scholar]
- 67.Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 68.Sendtner M, Arakawa Y, Stockli KA, Kreutzberg GW, Thoenen H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J Cell Sci. 1991;15:103–109. doi: 10.1242/jcs.1991.supplement_15.14. [DOI] [PubMed] [Google Scholar]
- 69.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992a;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 70.Sendtner M, Schmalbruch H, Stockli KA, Carroll P, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992b;358:502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- 71.Shieh P. The neoformation of cells of preganglionic type in the cervical spinal cord of the chick embryo following its transplantation to the thoracic level. J Exp Zool. 1951;117:359–395. [Google Scholar]
- 72.Silver J (1978) Cell death during development of the nervous system. In: Handbook of sensory physiology, Vol. IX, Development of sensory systems (Jacobson M, ed), pp 419–436. Berlin: Springer.
- 73.Silver J, Hughes AFW. The role of cell death during morphogenesis of the mammalian eye. J Morphol. 1973;140:159–170. doi: 10.1002/jmor.1051400204. [DOI] [PubMed] [Google Scholar]
- 74.Tanaka H, Obata K. Developmental changes in unique cell surface antigens of chick embryo spinal motoneurons and ganglion cells. Dev Biol. 1984;106:26–37. doi: 10.1016/0012-1606(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 75.Tello JF. Sur la formation des chaines des primaires et secondaires du grand sympathique dans l’embryon de poulet. Trav Lab Rech Biol Univ Madrid. 1925;23:1–28. [Google Scholar]
- 76.Terni T. Il simpatico cervicale degli amnioti. (Ricerche di morfologia comparata). Z Anat Entwicklungsgesch. 1931;96:289–426. [Google Scholar]
- 77.Truman JW, Fahrbach SE, Kimura K. Hormones and programmed cell death: insights from invertebrate studies. Prog Brain Res. 1990;86:25–35. doi: 10.1016/s0079-6123(08)63164-7. [DOI] [PubMed] [Google Scholar]
- 78.Truman JW, Thorn RS, Robinow S. Programmed neuronal death in insect development. J Neurobiol. 1992;23:1295–1311. doi: 10.1002/neu.480230917. [DOI] [PubMed] [Google Scholar]
- 79.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 80.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 81.Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- 82.Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 83.Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- 84.Yin Q-W, Oppenheim RW. Modifications of motoneuron development following transplantation of thoracic spinal cord to the lumbar region in the chick embryo: evidence for target-derived signals that regulate differentiation. J Neurobiol. 1992;23:376–395. doi: 10.1002/neu.480230405. [DOI] [PubMed] [Google Scholar]
- 85.Yin Q-W, Johnson J, Prevette D, Oppenheim RW. Cell death of spinal motoneurons in the chick embryo following deafferentation: rescue effects of tissue extracts, soluble proteins and neurotrophic agents. J Neurosci. 1994;14:7629–7640. doi: 10.1523/JNEUROSCI.14-12-07629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]