Abstract
Objective:
Determine the extent to which bilateral cochlear implantation increases patient-reported benefit as compared to unilateral implantation and no implantation.
Data Sources:
PubMed, Scopus, CINAHL, and Cochrane databases searches were performed using the keywords (“Cochlear Implant” or “Cochlear Implantation”) and (“bilateral”).
Study Selection:
Studies assessing hearing/CI-specific (CI) and general-health-related (HR) quality of life (QOL) in adult patients after bilateral cochlear implantation were included.
Data Extraction:
Of the 31 articles meeting criteria, usable QOL data were available for 16 articles (n=355 bilateral CI recipients).
Data Synthesis:
Standardized mean difference (Δ) for each measure and weighted effects were determined. Meta-analysis was performed for all QOL measures and also independently for hearing/CI-specific QOL and HRQOL.
Conclusion:
When measured using hearing/CI-specific QOL instruments, patients reported very large improvements in QOL comparing before cochlear implantation to bilateral CI (Δ=2.07 [1.76 to 2.38]) and medium improvements comparing unilateral CI to bilateral CI (Δ=0.51 [0.32 to 0.71]). Utilization of parallel vs. crossover study design did not impact QOL outcomes (χ2= 0.512, p=0.47). No detectable improvements were observed in either CI transition when using HRQOL instruments (no CI to bilateral CI: Δ=0.40 [−0.02 to 0.81]; unilateral CI to bilateral CI: Δ=0.22 [−0.02 to 0.46]).
The universal nature of HRQOL instruments may render them insensitive to the medium to large QOL improvements reported by patients using hearing/CI-specific QOL instruments. Given that HRQOL instruments are used to determine the economic benefit of health interventions, these measurement differences suggest that the health economic value of bilateral cochlear implantation has been underestimated.
Keywords: cochlear implant, cochlear implantation, hearing, sensorineural hearing loss, patient reported outcome measure, quality of life, speech recognition
INTRODUCTION
Unilateral cochlear implantation has traditionally been the standard treatment for patients with bilateral moderate-to-profound sensorineural hearing loss. The increased costs associated with a second cochlear implant (CI), the potential loss of residual hearing in the contralateral ear, and preclusion of future therapies were previously thought to outweigh the benefits of bilateral implantation to communication.(1,2) However, as outcomes data accrued showing significant benefits, bilateral cochlear implantation has become a favored therapy for bilateral moderate-to-profound sensorineural hearing loss and is now considered routine in children.(3) Conversely, adoption in adults has been less common with less than 50% receiving bilateral CIs.(4)
Restoration of binaural hearing has several functional advantages. Central processing of duplicate auditory stimuli increases the apparent loudness of sounds through summation(5) and leads to sharper source segregation, known as binaural squelch.(6,7) With bilateral implants, patients may selectively focus on sounds presented to either ear, minimizing the impact of the head shadow effect.(8) Improvements in summation and the decreased impact of the head shadow effect are realized soon after activation of the second implant, while central source segregation capabilities continue to improve up to four years after surgery.(6) These combined benefits translate to superior sound localization and speech recognition in complex listening environments for bilateral CI recipients.(9–16)
The vast majority of research evaluating outcomes in bilateral CI users has focused on objective measures such as those described earlier, but QOL improvement after bilateral implantation has received less attention. Patient-reported outcome measures (PROMs) are instruments devised to capture patient perspectives about their overall health or treatment, which allows direct input from the impacted population about how disease processes and interventions impact patients’ lives. These instruments provide patients the means to report their outcomes using a validated tool. For interventions where survival is not the primary outcome, such as cochlear implantation, QOL instruments have become increasingly important and accepted means to understand the impact of a procedure on a patient’s life. The importance of PROMs is best underscored by the Center for Medicaid and Medicare Services (CMS) targeting QOL improvement as a primary outcome measure(17) and the FDA requiring that PROMs be included in all clinical trials where an intervention seeks FDA approval.(18)
PROMs can be classified into two main categories—general health-related QOL (HRQOL) and disease-specific QOL. General HRQOL instruments are meant to be applied to large, diverse populations to provide a broad overview of QOL. These instruments are the most commonly used for economic analysis to determine the cost effectiveness of a particular treatment through measurement of total health.(19,20) In contrast, disease-specific instruments are typically validated and applied in a particular population that share a health condition or disability. The disease-specific QOL instruments that have been applied in CI research can be further divided into hearing-specific instruments that have been validated in individuals with hearing loss, but not CI users, and CI-specific instruments that have been validated in the CI population.
Previous work has shown a clear improvement in QOL after unilateral implantation with differences in effect size magnitude dependent on whether general health-related or hearing/CI-specific QOL instruments were applied.(21–23) Pre to post-CI QOL improvement using hearing/CI-specific instruments showed a very large positive effect, which likely results from the inclusion of communication-related items in these instruments, but only a medium positive effect was observed using HRQOL instruments. QOL data on the addition of a second implant are mixed and appear to be similarly related to the category of QOL instrument.(16,24,25) Prior attempts to aggregate QOL outcomes in bilateral CI users have been limited by heterogeneity in both the QOL outcome measures used and study design.(10–12,26) To determine the degree to which instrument selection influences QOL results, we assessed outcome differences between studies that use general HRQOL and hearing/CI-specific instruments. In addition, we compared the outcome differences in studies that we have termed “parallel” versus “crossover” design. Parallel studies are those where QOL outcomes were compared between two groups who differed based on unilateral versus bilateral implantation. Crossover studies are those where each subject serves as his or her own control and QOL is measured before and after implantation.
METHODS
Literature Search
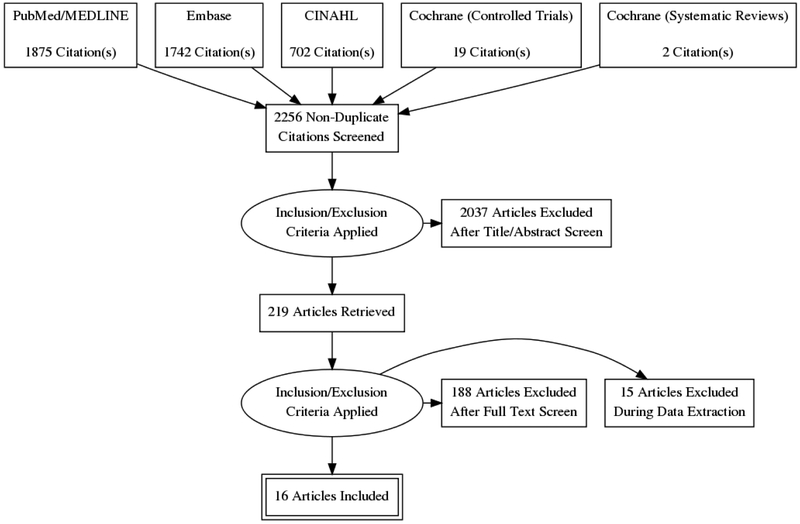
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.(27) Two authors independently searched PubMed, Embase, CINAHL, and Cochrane databases using the search terms “cochlear implantation” or “cochlear implant” and “bilateral”. With these criteria, 2256 unique manuscripts published before December 2017 were identified. Studies assessing QOL in adults after bilateral cochlear implantation were included. Articles published in English that reported QOL PROMs translated into languages other than their native format were also included. No date range limitations prior to December 2017 were placed on the search. Case reports, letters to the editor, abstracts, book chapters, articles not published or translated into English, and articles involving CI recipients less than 18 years old were excluded. After screening by title and abstract for inclusion and exclusion criteria, 219 articles were selected for full text review (Fig. 1). Disagreements over inclusion/exclusion fulfillment were mediated by the senior author.
FIG. 1.
Literature review process utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) search method.
Data Extraction
Author, year of publication, patient demographics, sample size, and data mean and standard deviation were recorded for articles selected for comparison. Data points were not estimated based on graphical plots and were only extracted if numerical values were reported. If data were recorded at multiple intervals, the last time point available was used for comparison. Authors were contacted if reported data did not permit comparison, and supplementary data were obtained for 9 of the included articles. Study populations were divided according to their implant status (pre-implant, unilateral CI, bilateral CI) and according to study design. QOL data were divided into HRQOL, hearing-specific, and CI-specific PROMs (specifically created and validated for CI users). PROMs that use a reverse scale (lower scores represent a better QOL) had values multiplied by −1 for analysis. Questionnaires were adjusted to have a score from 0 to +100 so that all data could be measured on the same proportional scale. Level of evidence for each selected article was evaluated with the Oxford Center for Evidence-Based Medicine.(28)
Statistical Methods
Meta-analysis of included studies evaluating the impact of bilateral cochlear implantation on QOL with a continuous measure (comparison of means and standard deviations between unilateral implantation and bilateral implantation) performed with Cochrane Review Manager (RevMan) version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, 2011, Copenhagen, Denmark). Both the fixed effects model and the random effects model were used in this study. Under the fixed effects model, it is assumed that all studies come from a common population, and that the effect size (standardized mean difference) is not significantly different among the different trials. This assumption is tested by the “heterogeneity test.” If this test yields a low probability value (p < .05), then the fixed effects model may be invalid. In this case, the random effects model may be more appropriate, in which both the random variation within the studies and the variation between the different studies are incorporated. Under the random effects model, the true effects in the studies are assumed to vary between studies, and the summary effect is the weighted average of the effects reported in the different studies.(29) The random effects model provides a more conservative estimate (i.e., with a wider confidence interval), but the results from the two models typically agree when there is no heterogeneity. When heterogeneity was present, the random effects model was the preferred model. Additionally, the Sterne and Egger tests were performed for further assessment of risk of publication bias.(30,31) For this study, the null hypothesis was that there was no difference between the two groups with respect to QOL data. Data are presented as mean ± standard deviation (95% confidence interval) and as standardized mean difference (SMD). SMD (or Cohen’s d) is a unitless numerical measure of effect size which assesses the magnitude and certainty of benefit.(31) Positive values indicate treatment has a positive effect on outcome measures with thresholds of 0.2–0.49 = small effect, 0.5–0.79 = medium effect, and ≥0.8 = large effect.(31) The total SMD with 95% confidence interval is given for both the fixed effects model and the random effects model. If the value 0 is not inside the 95% confidence interval, then the SMD is statistically significant at the 5% level (p < .05).
In addition, a comparison of weighted means among the three groups (preimplant, unilateral implant, and bilateral implants was done for QOL data and speech recognition data. The program MedCalc 18.2.1 (MedCalcSoftware, Oostende, Belgium) lists the standardized mean difference, 95% confidence interval, and p-value for all statistical tests. A p value of < 0.05 is considered significant for all statistical tests. Finally, potential publication bias was evaluated by visual inspection of the funnel plot and Egger’s regression test, which statistically examines the asymmetry of the funnel plot.(30) For this analysis, the null hypothesis was that there is no difference between QOL of unilateral and bilateral implant recipients.
RESULTS
Included studies
31 articles met criteria for analysis; however, 15 could not be included due to incomplete statistical reporting. Data were available or obtained for the remaining 16 studies totaling 660 patients. 3 articles(16,24,32,33) examined the same population of patients, and data extracted from these studies were combined for the purpose of analysis. Demographic data were available for all but one study (96.1% of included patients). Patients were 53.8% female, and the average age at implantation was 52.9±14.2 years (range, 18–89 years). Subjects from 5 studies(16,24,32–37) were asked to complete more than one type of QOL questionnaire, and their responses were counted separately for each PROM completed. Table 1 summarizes the studies included in the meta-analysis.
Table 1.
Studies Included in Meta-analysis
| Article | Level of Evidence | Treatment Group | Mean ± SD (Range) | Male %/Female % | Follow-up (months) |
|---|---|---|---|---|---|
| Roux 2017(43) | 4 | 1 | 45.24 ± 16.75 | 59/41 | >12 |
| 2 | 42.91 ± 16.85 | 54/46 | >12 | ||
| Sousa 2017(44) | 4 | 1 | N/A | N/A | >12 |
| 2 | >12 | ||||
| Nahm 2017(45) | 4 | 2 | 59 ± 16 | 41/59 | 24–60 |
| Capretta 2016(36) | 4 | 1 | 69 (54–88) | 53/47 | N/A |
| 2 | 57.25 (53–62) | 50/50 | N/A | ||
| van Zon(24), Kraaijenga(16), Smulders(33) | 1 | 0 | 52.5 ± 12.5 (26–67) | 58/42 | - |
| 1 | >12 | ||||
| 2 | >12 | ||||
| (Simultaneous) | 1 | 0 | 47.7 ± 15.9 (18–70) | 42/58 | >12 |
| 2 | >12 | ||||
| Zhang 2015(34) | 3 | 0 | 63 ± 13 (20–81) | 42/58 | - |
| 2 | 24 | ||||
| Ramos-Miguel 2015(46) | 4 | 1 | 51 ± 13 | 40/60 | N/A |
| 2 | N/A | ||||
| Harkonen 2015(35) | 4 | 1 | 41 (19–58) | 40/60 | 12–168 |
| 2 | 12 | ||||
| Potts 2014(47) | 4 | 1 | 45.8 ± 8.6 (38–58) | 50/50 | >24 |
| 2 | 6 | ||||
| Perreau 2014(48) | 4 | 1 | 55.3 ± 15.2 | 51/49 | 105.3 ± 70.9 |
| 2 | 43/57 | 69.8 ± 44.5 | |||
| Bonnard 2013 | 4 | 2 | 44.3 ± 11.3 (31–58) | 17/83 | 7–74 |
| Olze 2012(49) | 4 | 0 | 50.3 ± 14.5 (18–71) | 27/73 | - |
| 1 | >6 | ||||
| 2 | >6 | ||||
| Tyler 2009(37) | 4 | 1 | 55.7 ± 15.5 (18–89) | 48/52 | >12 |
| 2 | 52.8 ± 15.6 (20–81) | 50/50 | >12 | ||
| Noble 2008(50) | 4 | 1 | 60.6 ± 15.1 | 51/49 | N/A |
| 2 | 64.3 ± 15.5 | 43/57 | N/A |
Pre-implant, unilateral CI, and bilateral CI are abbreviated as treatment groups 0, 1, and 2, respectively.
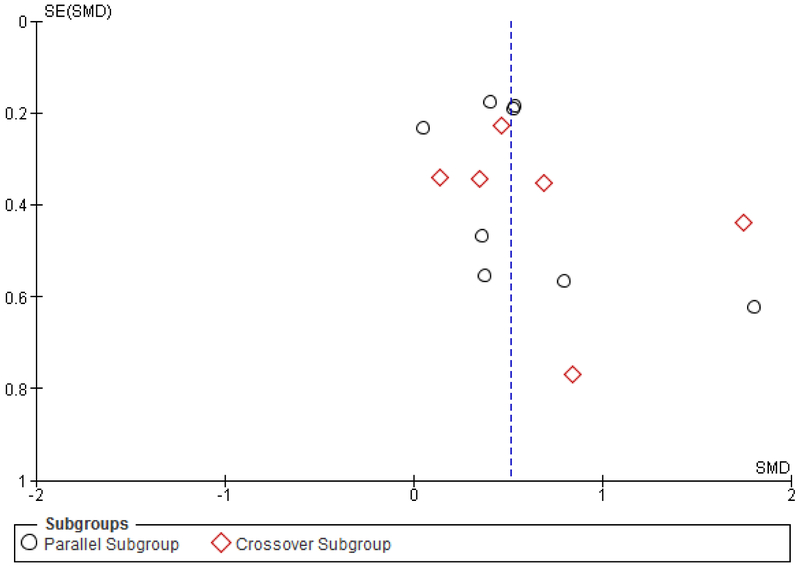
To investigate the presence of publication bias, inspection of the funnel plot of effects calculated from individual studies was performed. According to funnel plots and the Egger’s test, there was no indication of publication bias (p=0.17) among the set of studies included in this meta-analysis (Fig. 2). Results from HRQOL and hearing/CI-specific PROMs are reported separately based on prior research showing differences in QOL outcomes depending on the category of PROM used.(21,22)
FIG. 2.
Funnel plot of hearing and cochlear implant–specific QOL PROMs unilateral to bilateral, including subset analysis of crossover and parallel patient groups; SE = standard error; SMD = standardized mean difference.
Comparison of no CI to bilateral CI
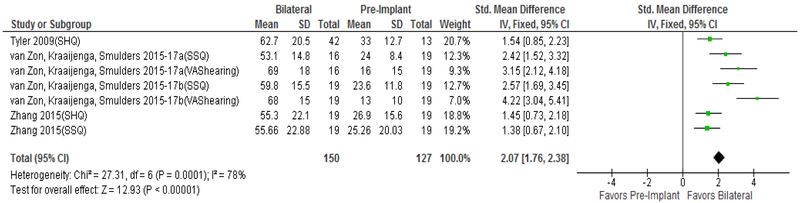
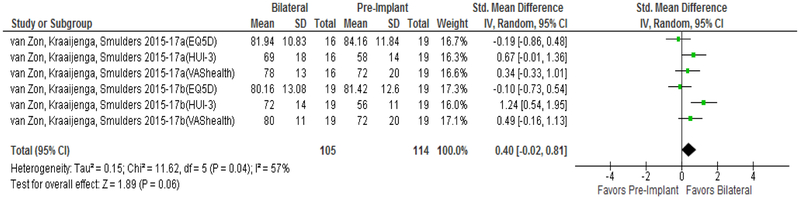
Improvement in QOL from pre-implantation to unilateral CI is well reported in prior meta-analyses(21,22) and is not the focus of the current study. No studies used CI-specific PROMs to compare QOL from no CI to bilateral CIs. As seen in Figure 3, patients showed large QOL improvement when comparing no CI to bilateral CI using hearing-specific QOL instruments (Δ=2.07 [1.76 to 2.38]). In contrast, general HRQOL instruments measured no significant QOL improvement when comparing no CI to bilateral CIs (Figure 4, Δ=0.40 [−0.02 to 0.81]). Both analyses reported significant heterogeneity in the data with I2 values of 78% (p=0.0001) and 57% (p=0.047) for hearing-specific and general HRQOL instruments, respectively.
FIG. 3.
Forest plot of hearing–specific PROMs comparing pre-implant to bilateral; CI = confidence interval; SD = standard deviation; QOL = quality of life; PROMs = patient-reported outcome measurement studies; SHQ = Spatial Hearing Questionnaire; SSQ = Speech, Spatial, and Qualities of Hearing Questionnaire; VAS = Visual Analogue Scale.
FIG. 4.
Forest plot of HRQOL PROMs comparing pre-implant to bilateral; CI = confidence interval; SD = standard deviation; QOL = quality of life; PROMs = patient-reported outcome measurement studies; EQ-5D = EuroQol 5 Dimensions; HUI-3 = Health Utilities Index 3; VAS = Visual Analogue Scale.
Comparison of unilateral CI to bilateral CI
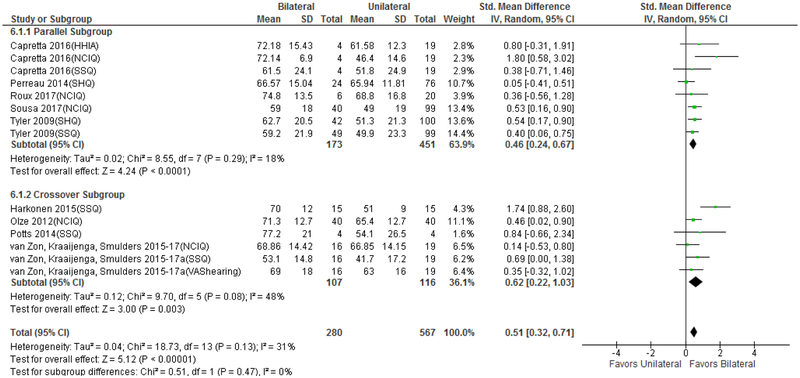
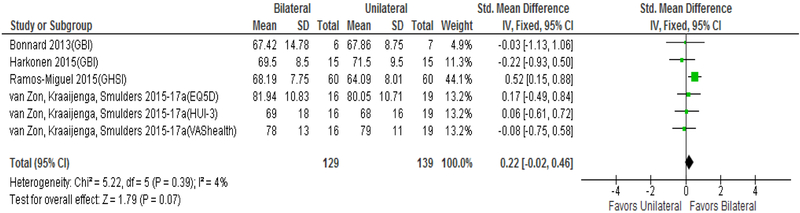
Figures 5 and 6 display change in QOL from unilateral CI to bilateral CI. Hearing/CI-specific PROMs revealed medium improvement in QOL (Δ=0.51 [0.32 to 0.71]), which was smaller than the improvement seen from no CI to bilateral CI. There was no difference in QOL improvement detected based on parallel (Δ=0.46 [0.24,0.67]) vs. crossover (Δ=0.52 [0.22,1.03]) study design (Figure 5, χ2= 0.512, p=0.47) nor was there heterogeneity detected between the subgroup I2=0% (p=0.47). HRQOL PROMs detected no significant improvement in QOL between unilateral CI to bilateral CI (Δ=0.22 [−0.02 to 0.46]). Five of the six HRQOL studies included in the analysis used a crossover design, which precluded a separate analysis based on study design. No significant heterogeneity was detected in the hearing/CI-specific PROM analysis (I2=31%, p=0.13) or HRQOL PROM analysis (I2=4%, p=0.39).
FIG. 5.
Forest plot of hearing and cochlear implant–specific QOL PROMs unilateral to bilateral, including subset analysis of crossover and parallel study design; CI = confidence interval; SD = standard deviation; QOL = quality of life; PROMs = patient-reported outcome measurement studies; HHIA = Hearing Handicap Inventory for Adults; NCIQ = Nijmegen Cochlear Implant Questionnaire; SSQ = Speech, Spatial, and Qualities of Hearing Questionnaire; SHQ = Spatial Hearing Questionnaire; VAS = Visual Analogue Scale.
FIG. 6.
Forest plot of HRQOL PROMs comparing unilateral to bilateral; CI = confidence interval; SD = standard deviation; QOL = quality of life; PROMs = patient-reported outcome measurement studies; GBI = Glasgow Benefit Inventory; GHSI = Global Health State Inventory; EQ-5D = EuroQol 5 Dimensions; HUI-3 = Health Utilities Index 3; VAS = Visual Analogue Scale.
Analysis of SSQ Subdomains
The SSQ is a hearing-specific PROM that aims to discern speech in competing sounds with multiple types of background noise, determine direction and distance of sound, and the ability to recognize, listen to, and segregate simultaneous sounds.(38) Seven studies published subdomains of the SSQ Hearing Scale (speech hearing, spatial hearing, and qualities of hearing), but several did not report all three subdomain scores. Therefore, a meta-analysis was not able to be performed and weighted means were computed to compared change after bilateral implantation. All subdomains and the total SSQ score demonstrated significant benefit after bilateral implantation whether measured from no CI to bilateral CI or unilateral CI to bilateral CI (Tables 2 and 3). The spatial domain showed larger improvement compared to the other domains with the addition of a second CI.
Table 2:
Weighted Mean SSQ Subdomains Pre-Implant vs. Bilateral
| Variable | Pre-Implant | Bilateral CI | Difference | p-value |
|---|---|---|---|---|
| SSQ Total | 24.80±11.90 | 60.20±16.78 | 35.40±15.36 | <0.0001* |
| Speech | 17.07±11.33 | 55.36±18.65 | 38.29±16.62 | <0.0001* |
| Spatial | 20.13±13.77 | 58.68±20.25 | 38.55±18.39 | <0.0001* |
| Qualities | 33.75±15.00 | 66.13±16.06 | 32.38±15.77 | <0.0001* |
SSQ indicates the Speech, Spatial, and Qualities of Hearing Questionnaire; QOL indicates quality of life
Table 3:
Subdomain Weighted Mean Unilateral vs. Bilateral
| Variable | Unilateral CI | Bilateral CI | Difference | p-value |
|---|---|---|---|---|
| SSQ Total | 48.12±17.48 | 60.20±16.78 | 12.08±17.18 | <0.0001* |
| Speech | 48.04±19.89 | 55.36±18.65 | 7.32±19.37 | 0.0021* |
| Spatial | 39.08±19.79 | 58.68±20.25 | 19.60±19.99 | <0.0001* |
| Qualities | 56.51±18.82 | 66.13±16.06 | 9.62±17.81 | <0.0001* |
| NCIQ Total | 65.77±12.90 | 71.59±13.18 | 5.82±13.02 | 0.0001* |
| Basic sound perception | 61.20±16.33 | 68.64±16.91 | 7.45±16.54 | 0.0046* |
| Advanced sound perception | 73.02±16.00 | 68.94±17.38 | -4.08±16.50 | 0.1166 |
| Speech production | 62.62±17.71 | 75.15±14.78 | 12.53±16.73 | <0.0001* |
| Self-esteem | 60.83±16.73 | 65.29±14.84 | 4.46±16.09 | 0.0794 |
| Activity limitations | 70.49±18.72 | 73.78±17.59 | 3.29±18.33 | 0.2547 |
| Social interactions | 64.06±13.25 | 66.29±11.75 | 2.23±12.74 | 0.2670 |
SSQ indicates the Speech, Spatial, and Qualities of Hearing Questionnaire; NCIQ, Nijmegan Cochlear Implant Questionnaire; BSP, Basic Sound Perception; ASP, Advanced Sound Perception; SP, Speech Production; SE, Self-Esteem; AL, Activity Limitations; SI, Social Interactions
Analysis of NCIQ Subdomains
The NCIQ is a CI-specific PROM with six subdomains: basic sound perception (BSP), advanced sound perception (ASP), speech production (SP), self-esteem (SE), activity limitations (AL), and social interactions (SI).(39) None of the studies that met inclusion criteria measured NCIQ scores from no CI to bilateral CI. Therefore, only unilateral to bilateral CI results were compared. Significant improvements following bilateral implantation were seen for the BSP and SP subdomains (all p<0.005) with no change in ASP, SE, AL, and SI subdomains (Table 3). It is important to note that when the NCIQ was initially published, the advanced sound perception (ASP) and speech production (SP) subdomains were incorrectly coded (swapped) and a corrected code was later released.(40) The articles by van Zon,(24) Kraaijenga,(16,32) and Smulders(33) were verified to have used the corrected code; however, data from other articles were analyzed as presented (Table 3).
DISCUSSION
The current study represents the first comprehensive meta-analysis focused on QOL improvement after bilateral cochlear implantation. Two prior studies were unable to perform this analysis due to inadequate data at the time of publication.(10,11) One advantage to our methodology was splitting HRQOL and hearing/CI-specific QOL analyses into separate analyses given the known measurement effect differences between these categories of PROMs in this patient population (discussed later). In addition, we performed subgroup analyses, when possible, for studies that used a parallel vs. cross-over study design. This revealed a positive medium improvement in hearing/CI-specific QOL regardless of the study design, which may have implications regarding the design of future studies on this topic.
There are clear known benefits of binaural hearing that have been shown to be present even when auditory stimuli are presented through CIs. Bilateral cochlear implantation allows users to take advantage of binaural squelch (6,7) and summation(5) and help eliminate the head shadow effect. (41,42) Together, these are the primary physiological factors that are hypothesized to drive improved sound localization and speech recognition in complex listening environments for bilateral CI recipients.(9–16,32) The relationship between these factors and improved QOL is not currently known. However, if bilateral implantation does return a patient to a more “normal” neurophysiological functional status and real-world communication is improved, it is reasonable to assume that QOL would improve. This was most clearly shown in our subdomain analysis where the largest improvement from unilateral to bilateral CI was in the SSQ spatial subdomain.
The known measurement differences between HRQOL and hearing/CI-specific QOL instruments(21,22,24) were accentuated in the current analysis. Here, HRQOL PROMs showed no QOL improvement whether comparing no CI to bilateral CIs or unilateral CI to bilateral CIs, while hearing/CI-specific PROMs showed a very large and medium QOL improvement, respectively. This is likely due to general HRQOL PROMs inclusion of items, such as mobility and bodily pain, that are seemingly unrelated to cochlear implantation, which may render these instruments insensitive to more specific improvement in QOL that occur when transitioning to bilateral CIs. These differences are significant because HRQOL PROMs are currently used to estimate the economic benefit of cochlear implantation. The current results showing larger effect sizes of the QOL improvement after bilateral cochlear implantation with hearing/CI-specific rather than HRQOL instruments demonstrate that using HRQOL instruments may greatly underestimate the health economic benefit of bilateral cochlear implantation.
An additional limitation of HRQOL PROMs is that they are considered indirect measures of healthy utility. Other more direct measures using standard gamble and time trade-off methods are preferred and can be specific to a particular intervention. Similar to HRQOL instruments, hearing/CI-specific QOL instruments can also indirectly evaluate health utility by correlating outcomes with direct measures. However, additional research is needed to provide the evidence to thoroughly assess the economic benefits of bilateral cochlear implantation.
The results of the current study showed differences in the magnitude of hearing/CI-specific QOL improvement between no CI to bilateral CIs and unilateral to bilateral CIs. Here, there was a four-fold difference in hearing/CI-specific QOL improvement when transitioning from essentially no hearing to bilateral CI vs. unilateral CI to bilateral CI. A previous meta-analysis revealed very large effect size from unilateral implantation when measured with hearing/CI-specific PROMs (Δ=1.77 [1.28 to2.26]).(21) This suggests that unilateral cochlear implantation imparts nearly 3.5-fold improvement in QOL compared to bilateral implantation.
Interpretation of these data is difficult as some routine measurement characteristics of the hearing/CI-specific PROMs are not known. The most important is the minimal important difference (MID), which allows researchers and clinicians to distinguish when PROM score change corresponds to a clinically significant change. This allows interpretation of PROM score change to move beyond statistical evaluation (i.e., 95% confidence intervals and p-values) and focus on the point at which a PROM score change is associated with the self-perception of patient improvement, which may or may not be the same. None of the hearing/CI-specific PROMs included in the meta-analysis has established MIDs. Therefore, while our data showed a medium effect size QOL improvement from unilateral CI to bilateral CI, self-perception of QOL improvement remains unknown, making it difficult to interpret the added value to QOL of a second CI.
Our study is limited by the reporting bias that is inherent to all meta-analyses. In addition, the PROMs most commonly used to assess QOL in the included studies were not validated in or developed for the adult CI population. Moreover, the hearing/CI-specific PROMs used were not developed using the most rigorous standards, which could impact the magnitude, reliability, and interpretation of the reported outcomes. With regard to the NCIQ, although we are confident in the overall score of this assessment, we are hesitant to make conclusions about the advanced sound perception and speech production domains. Much of the literature does not clearly define whether these domains were measured according to the instrument’s original protocol or the code book later released as a corrigendum, which complicates data interpretation. However, differences in the code book utilized would not impact the overall score.
CONCLUSIONS
Meta-analysis of hearing and CI-specific PROMs showed a very large QOL improvement when transitioning from no CI to bilateral CIs and medium QOL improvement when receiving a second implant. However, HRQOL PROMs failed to show improvement in either comparison. The universal nature of HRQOL instruments may render them insensitive to the medium to large QOL improvements reported by patients using hearing/CI-specific QOL instruments. Given that HRQOL instruments are used to determine the economic benefit of health interventions, these measurement differences suggest that the health economic value of bilateral cochlear implantation has been underestimated. Additional research is needed to demonstrate the benefits of bilateral cochlear implantation, including QOL improvements using CI-specific instruments. These results can then be used to provide more appropriate estimates of the health economic value of bilateral cochlear implantation in adults with moderate-to-profound sensorineural hearing loss.
Acknowledgments
The authors thank Talita le Roux, Aline Faria de Sousa, Aaron Moberly, Yvette Smulders, Véronique Kraaijenga, Alice van Zon, Kati Härkönen, Lisa Potts, and Ann Perreau for providing additional data from their published articles for this study.
This publication was supported by a K12 award through the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number UL1TR001450, and grants from the Doris Duke Foundation and the American Cochlear Implant Alliance. In addition, this work was supported by the Otology and Neurotology Cochrane Scholars Program.
Footnotes
The authors have no conflicts of interest to declare.
The authors have no financial disclosures to make.
REFERENCES
- 1.McDermott HJ, Lech M, Kornblum MS et al. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: possible correlates of neural plasticity. J Acoust Soc Am 1998;104:2314–25. [DOI] [PubMed] [Google Scholar]
- 2.Peters BR, Litovsky R, Parkinson A et al. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol 2007;28:649–57. [DOI] [PubMed] [Google Scholar]
- 3.Balkany T, Hodges A, Telischi F et al. William House Cochlear Implant Study Group: position statement on bilateral cochlear implantation. Otol Neurotol 2008;29:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McRackan TR, Hand BN, Consortium CIQoL et al. Development of the Cochlear Implant-Quality of Life (CI-QOL) Item Bank. Ear and Hearing 2018. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorman MF, Gifford RH. Combining acoustic and electric stimulation in the service of speech recognition. International journal of audiology 2010;49:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eapen RJ, Buss E, Adunka MC et al. Hearing-in-noise benefits after bilateral simultaneous cochlear implantation continue to improve 4 years after implantation. Otol Neurotol 2009;30:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buss E, Pillsbury HC, Buchman CA et al. Multicenter U.S. bilateral MED-EL cochlear implantation study: speech perception over the first year of use. Ear and hearing 2008;29:20–32. [DOI] [PubMed] [Google Scholar]
- 8.Schleich P, Nopp P, D’Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear and hearing 2004;25:197–204. [DOI] [PubMed] [Google Scholar]
- 9.Berrettini S, Baggiani A, Bruschini L et al. Systematic review of the literature on the clinical effectiveness of the cochlear implant procedure in adult patients. Acta Otorhinolaryngol Ital 2011;31:299–310. [PMC free article] [PubMed] [Google Scholar]
- 10.Crathorne L, Bond M, Cooper C et al. A systematic review of the effectiveness and cost-effectiveness of bilateral multichannel cochlear implants in adults with severe-to-profound hearing loss. Clin Otolaryngol 2012;37:342–54. [DOI] [PubMed] [Google Scholar]
- 11.Gaylor JM, Raman G, Chung M et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2013;139:265–72. [DOI] [PubMed] [Google Scholar]
- 12.van Schoonhoven J, Sparreboom M, van Zanten BG et al. The effectiveness of bilateral cochlear implants for severe-to-profound deafness in adults: a systematic review. Otol Neurotol 2013;34:190–8. [DOI] [PubMed] [Google Scholar]
- 13.Rana B, Buchholz JM, Morgan C et al. Bilateral Versus Unilateral Cochlear Implantation in Adult Listeners: Speech-On-Speech Masking and Multitalker Localization. Trends in hearing 2017;21:2331216517722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verschuur CA, Lutman ME, Ramsden R et al. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol 2005;26:965–71. [DOI] [PubMed] [Google Scholar]
- 15.Mosnier I, Sterkers O, Bebear JP et al. Speech performance and sound localization in a complex noisy environment in bilaterally implanted adult patients. Audiology & neuro-otology 2009;14:106–14. [DOI] [PubMed] [Google Scholar]
- 16.Kraaijenga VJC, Ramakers GGJ, Smulders YE et al. Objective and Subjective Measures of Simultaneous vs Sequential Bilateral Cochlear Implants in Adults: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2017;143:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services: Quality Strategy.
- 18.Patrick DL, Burke LB, Powers JH et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007;10 Suppl 2:S125–37. [DOI] [PubMed] [Google Scholar]
- 19.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271–92. [DOI] [PubMed] [Google Scholar]
- 20.Horsman J, Furlong W, Feeny D et al. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McRackan TR, Bauschard M, Hatch JL et al. Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope 2018;128:982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McRackan TR, Bauschard M, Hatch JL et al. Meta-analysis of Cochlear Implantation Outcomes Evaluated With General Health-related Patient-reported Outcome Measures. Otol Neurotol 2018;39:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakers GG, Smulders YE, van Zon A et al. Agreement between health utility instruments in cochlear implantation. Clin Otolaryngol 2016;41:737–43. [DOI] [PubMed] [Google Scholar]
- 24.van Zon A, Smulders YE, Stegeman I et al. Stable benefits of bilateral over unilateral cochlear implantation after two years: A randomized controlled trial. Laryngoscope 2017;127:1161–8. [DOI] [PubMed] [Google Scholar]
- 25.Smulders YE, van Zon A, Stegeman I et al. Cost-Utility of Bilateral Versus Unilateral Cochlear Implantation in Adults: A Randomized Controlled Trial. Otol Neurotol 2016;37:38–45. [DOI] [PubMed] [Google Scholar]
- 26.Bond M, Mealing S, Anderson R et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model Health technology assessment (Winchester, England: ) 2009;13:1–330. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Available at: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed March 29, 2018.
- 29.Borenstein M. Introduction to meta-analysis. Chichester, U.K.: John Wiley & Sons, 2009:xxviii, 421 p. [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates, 1988:xxi, 567 p. [Google Scholar]
- 32.Kraaijenga VJC, Ramakers GGJ, Smulders YE et al. Objective and Subjective Measures of Simultaneous vs Sequential Bilateral Cochlear Implants in Adults: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smulders YE, van Zon A, Stegeman I et al. Comparison of Bilateral and Unilateral Cochlear Implantation in Adults: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2016;142:249–56. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Tyler R, Ji H et al. Speech, Spatial and Qualities of Hearing Scale (SSQ) and Spatial Hearing Questionnaire (SHQ) Changes Over Time in Adults With Simultaneous Cochlear Implants. American journal of audiology 2015;24:384–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harkonen K, Kivekas I, Rautiainen M et al. Sequential bilateral cochlear implantation improves working performance, quality of life, and quality of hearing. Acta oto-laryngologica 2015;135:440–6. [DOI] [PubMed] [Google Scholar]
- 36.Capretta NR, Moberly AC. Does quality of life depend on speech recognition performance for adult cochlear implant users? Laryngoscope 2016;126:699–706. [DOI] [PubMed] [Google Scholar]
- 37.Tyler RS, Perreau AE, Ji H. Validation of the Spatial Hearing Questionnaire. Ear and hearing 2009;30:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). International journal of audiology 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinderink JB,KP, Van Den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg 2000;123:756–65. [DOI] [PubMed] [Google Scholar]
- 40.Corrigendum. Otolaryngology–Head and Neck Surgery 2016;156:391-.28145830 [Google Scholar]
- 41.Senn P, Kompis M, Vischer M et al. Minimum audible angle, just noticeable interaural differences and speech intelligibility with bilateral cochlear implants using clinical speech processors. Audiology & neuro-otology 2005;10:342–52. [DOI] [PubMed] [Google Scholar]
- 42.Laszig R, Aschendorff A, Stecker M et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol 2004;25:958–68. [DOI] [PubMed] [Google Scholar]