Abstract
Single-unit discharge was recorded from cells in the posterior hypothalamic nucleus (PH), supramammillary nucleus (SuM), and medial mammillary nucleus (MM) during hippocampal theta (θ) elicited by stimulation of the reticular nucleus pontis oralis (RPO). In agreement with previously published work, θ-related cells in the PH (12 cells) were all classified as tonic θ-ON (increased tonic discharge rate during hippocampal θ), whereas those in the SuM (9 cells) and MM (15 cells) were all classified as phasic θ-ON (rhythmic discharge, in phase with ongoing θ). The effect of RPO stimulation on cell discharge was tested after hippocampal θ was abolished by infusion of procaine into the medial septum/vertical limb of the diagonal band. The RPO-elicited discharge patterns of all PH tonic θ-ON cells and all SuM phasic θ-ON cells survived septal procaine infusion. Further, the discharge rate of PH cells and the frequency of burst discharge of SuM cells during RPO stimulation both increased after the infusion. In contrast, septal procaine infusion abolished the RPO-elicited rhythmic discharge pattern in MM phasic θ-ON cells and attenuated their discharge rates. These results indicate that the PH and SuM form parts of an ascending system mediating hippocampal θ, whereas the MM receives (and perhaps relays to other parts of the limbic system) rhythmic input descending from the septo-hippocampal system. In addition, PH and SuM receive descending inputs that limit the discharge rates of their θ-related cells during hippocampal θ.
Keywords: intraseptal, procaine, hippocampal theta, supramammillary nucleus, posterior hypothalamic nucleus, medial mammillary nucleus, rhythmic cell discharge
Hippocampal theta (θ) activity is a large-amplitude, almost sinusoidal slow-wave activity, the occurrence of which has been postulated to be involved in processes of hippocampal synaptic plasticity (Pavlides et al., 1988; Buzsáki, 1989; Huerta and Lisman, 1993) and related behavioral functions (O’Keefe and Nadel, 1978; Bland et al., 1986; Miller, 1991). There is evidence that θ may be particularly important in hippocampal processing of spatial information (O’Keefe and Recce, 1993; Skaggs and McNaughton, 1996). It is well established that activation of an ascending system of projections that have their putative origin in the nucleus reticularis pontis oralis (RPO) elicits θ activity in the hippocampus (for review, see Vertes, 1986). These “synchronizing” effects are mediated via the medial septum/vertical limb of the diagonal band of Broca (MS/vDBB) (for review, see Bland, 1986; Stewart and Fox, 1990). However, there is a variety of recent evidence suggesting that, rather than directly influencing MS/vDBB activity, as had been proposed previously (O’Keefe and Nadel, 1978; Bland, 1986), reticular influences are relayed to the MS/vDBB via a nuclei in the posterior hypothalamic region.
Anatomical work has suggested that fibers from the RPO are relayed to the MS/vDBB after first synapsing in the supramammillary nucleus (SuM) (Veazy et al., 1982; Vertes, 1982, 1992; Vertes and Martin, 1988). Procaine mapping of the system (Kirk and McNaughton, 1993) and rhythmic multiunit (Kirk and McNaughton, 1991) and single unit (Bland et al., 1993, 1995; Kirk et al., 1994; Kocsis and Vertes, 1994) activity in the SuM during θ suggest that, in addition to acting as a relay, the SuM transduces the intensity of RPO activity into the frequency of θ.
Lesions of, or procaine infused into the region of, the posterior hypothalamus (PH) abolish hippocampal or septal θ activity (Kawamura et al., 1961; Anchel and Lindsley, 1972; Robinson and Whishaw, 1974;Bland et al., 1994, 1995; Oddie et al., 1994), and high-frequency (100 Hz) stimulation in the PH has been shown to be particularly effective in eliciting hippocampal θ field activity (Bland and Vanderwolf, 1972) and θ pattern discharge in medial septal cells (Bland et al., 1990). Thus, the PH has also been proposed to be a relay in the ascending synchronizing system. Further, there are dense projections from the posterior hypothalamic nucleus (PH) to the MS/vDBB (Vertes et al., 1993a). In contrast to SuM, however, θ-related discharge in the PH is tonic (Bland et al., 1995).
Neurons in the mammillary nuclei have also been shown to discharge rhythmically at θ frequencies (Mignard et al., 1987; Kocsis and Vertes, 1994; Bland et al., 1995), and repetitive bursts have been recorded in vitro from the medial (MM) or lateral (LM) mammillary nucleus (Alonso and Llinás, 1992; Llinás and Alonso, 1992). As with hippocampal θ, this activity in the mammillary nuclei may subserve their proposed role in memory in general (Mair et al., 1979), and spatial memory in particular (Sziklas and Petrides, 1993). In contrast to the SuM and PH, however, it has been suggested (although not unequivocally demonstrated) that the mammillary nuclei may receive θ-frequency information descending from the septo-hippocampal system (Alonso and Llinás, 1992; Llinás and Alonso, 1992; Kocsis and Vertes, 1994). This information is likely of hippocampal origin (Kocsis and Vertes, 1994) and may be relayed to the mammillary bodies via the subiculum (Swanson and Cowan, 1975,1977), i.e., via part of the circuit originally proposed by Papez (1937).
In the current experiments, θ-related unit discharge was recorded from PH, SuM, and MM during hippocampal θ elicited by RPO stimulation (Vertes, 1982, 1986). Subsequently, hippocampal θ was abolished by septal infusion of procaine. Thus, the contribution of θ-coded input descending from the septo-hippocampal system (relative to input ascending from the reticular formation) on the θ-related unit discharge in the PH, SuM, and MM was determined.
MATERIALS AND METHODS
Thirty-four male Long–Evans rats (0.25–0.5 kg) were initially anesthetized with a mixture of Halothane (M.T.C. Pharmaceuticals, Cambridge, Ontario, Canada) in oxygen (∼1.5% MAC) for jugular cannula insertion. Halothane was subsequently discontinued, and urethane (0.8 gm/ml) was administered via a jugular cannula as required for the remaining surgical and experimental procedures. Rats were secured in a stereotaxic apparatus, core temperature was maintained at 37°C, and heart rates were monitored constantly throughout the experiments. Recording electrodes consisted of Kynar-insulated tungsten (etched to 0.5–1.0 MΩ tip resistance) for hippocampal field activity, and glass electrodes (5.0–10 MΩ) were filled with 0.5 m sodium acetate and 2% pontamine sky blue for unit recording. Bipolar stimulating electrodes consisted of two insulated stainless steel wires (250 μm) twisted together. Glass electrode tip locations were marked by passing 50 μA of current for 10 min (5 min cathodal, 5 min anodal). Procaine hydrochloride [20% (w/v) in saline, 2.0 μl, 0.5 μl/min] was infused into the MS/vDBB region by a microinfusion pump via a 5 μl Hamilton syringe connected to a 30 gauge cannula by SILASTIC tubing (Dow Corning, Midland, MI). Hippocampal recording electrodes were placed in the stratum moleculare of the dentate gyrus (AP, Bregma −3.3 mm; L, midline 2.4 mm; DV, dura 2.5–3.0 mm). The tip of the infusion cannula was placed in the medial septum (AP, Bregma +0.0 to 0.5 mm; L, midline 0.0 mm; DV, 5.0 to 6.0 mm). Glass unit recording electrodes were lowered through the PH/SuM/MM region (AP, Bregma −3.0 to −5.0 mm; L, midline 0.0–0.2 mm DV, dura 7.0–9.5 mm) as described previously (Bland et al., 1995). Stimulating electrodes were placed in the RPO (AP, Bregma −8.0 to −9.0 mm; L, 1.0–1.5 mm; DV, dura 8.0–9.0 mm). After perfusion and fixation of the brain, frozen sections (40 μm) were taken serially, mounted on glass slides, and stained with thionine for subsequent verification of hippocampal field electrode and septal cannula placement and glass electrode tip location.
Field and cell signals were stored on FM tape. Samples (20 sec) of cell and field activity were taken during spontaneously occurring large-amplitude irregular field activity (LIA), and subsequently during 4–5 Hz θ field activity elicited by 100 Hz (100–300 μA) stimulation of the RPO. Sampling during RPO stimulation was from the onset of stimulation. In contrast to higher frequencies of stimulation-elicited θ (>6 Hz) (Bland and Vanderwolf, 1972), intensities of RPO stimulation resulting in 4–5 Hz θ resulted in a relatively stationary waveform over the 20 sec sample. Samples (20 sec from stimulation onset) of field and cell activity were subsequently taken during the same level of RPO stimulation after hippocampal θ was abolished by infusing procaine (2.0 μl) into the MS/vDBB region (Brücke et al., 1959; Mizumori et al., 1989; Kirk and McNaughton, 1993; Lawson and Bland, 1993). Cell discharge (spontaneous and stimulated every 2 min) was sampled until hippocampal θ recovered.
Data analysis and separation of cell discharge from stimulation artifact was accomplished off-line using a PC microcomputer and a software acquisition and analysis package (DataWave Technologies, Longmount, CO). Unit cell activity was digitized through a 12 bit analog-to-digital converter and sampled at a frequency of ∼1.6 kHz. Field activity was simultaneously sampled at a frequency of 133 Hz. Each data segment was subjected to a real-time fast Fourier analysis and classified as either θ or LIA by the following criteria: (1) θ was defined as a sinusoidal-like waveform with a peak frequency of 2–8 Hz and a small bandwidth, and (2) LIA was defined as a large-amplitude irregular activity with a broad frequency band (0.5–25 Hz) (see Leung et al., 1982). Analysis of digitized data samples provided the mean discharge rate of a cell (in Hz) and an auto-correlation histogram (ACH) of the discharge pattern of the cell. For cross-correlation analysis, both the digitized field activity and the spike train were converted to ASCII files and split into 512 bit (3.85 sec) segments. Each spike train segment was convolved with a Gaussian kernel [δ = 15 msec; the resulting spike density function (Ahmed and Rao, 1975;Richmond et al., 1987) reflected the inter- rather than the intraburst spike intervals] and cross-correlated relative to the hippocampal field activity using a frequency-domain algorithm (Press et al., 1986). Five segments were averaged to produce the final cross-correlation function (CCF).
RESULTS
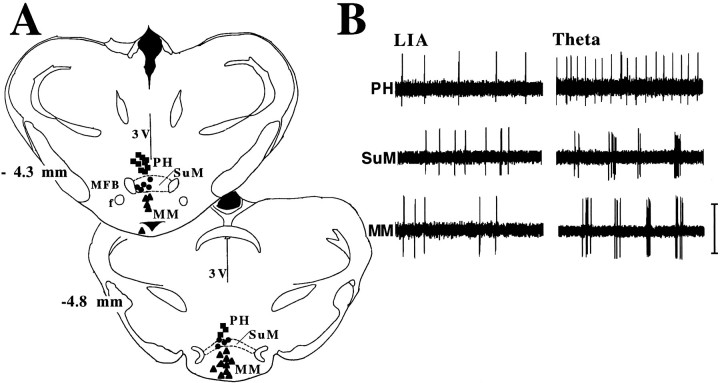
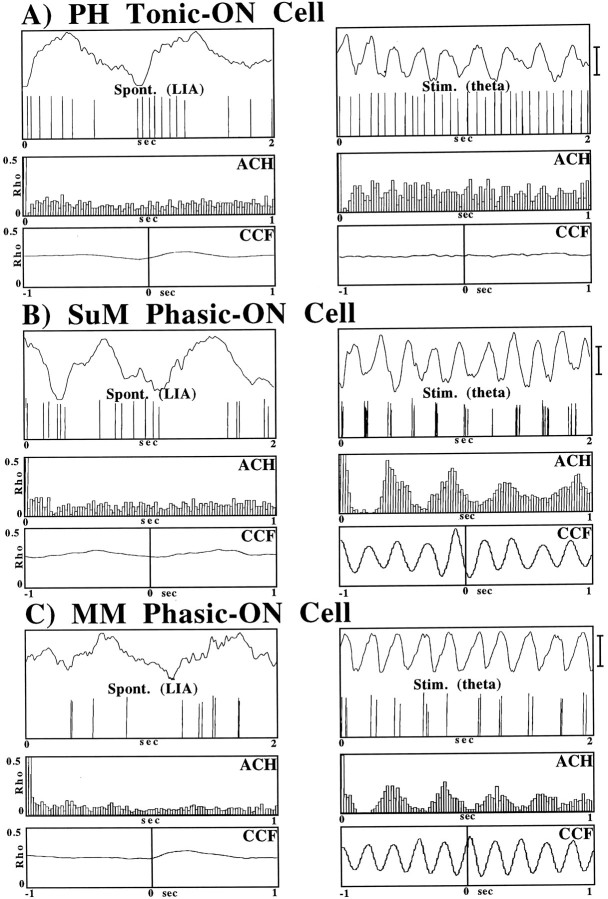
Cells localized to the PH, SuM, and MM were shown to have distinct θ-related discharge patterns. The location of cells and examples of their discharge patterns (analog oscilloscope traces) in LIA and θ are shown in Figure 1. The relationship of the discharge of each type of cell to hippocampal EEG is shown in Figure2. PH, SuM, and MM cells were classified in relation to hippocampal field activity according to criteria that have been used previously to classify cells in the hippocampal formation (Colom and Bland, 1987; Bland and Colom, 1989), the entorhinal cortex (Dickson et al., 1995), the MS/vDBB (Ford et al., 1989), and cingulate cortex (Colom et al., 1988). All θ-related cells recorded in the PH (n = 12) were found to be of the tonic θ-ON type (i.e., they discharged tonically and at higher rates during hippocampal θ elicited by RPO stimulation than during LIA; see Figs.1B, 2A). All θ-related cells in the SuM (n = 9) and MM (n = 15) were found to be of the phasic θ-ON type (i.e., they discharge in rhythmic bursts phase-locked to concurrent hippocampal θ activity and nonrhythmically during LIA; see Figs. 1B, 2B,C).
Fig. 1.
A, Diagrammatic reconstruction (Paxinos and Watson, 1982) of the location of classified cells in the caudal diencephalon. Cells were localized in the various nuclei by an examination of the histology showing pontamine sky blue deposits ejected from the glass microelectrodes used to record the cells. Tonic θ-ON cells (shown as black squares) were found in the posterior hypothalamic nucleus (PH), phasic θ-ON cells were found in the supramammillary nucleus (SuM; black circles), and medial mammillary nucleus (MM; black triangles).B, Discharge patterns (1 sec oscilloscope traces) of PH, SuM, and MM cells during spontaneous LIA (left side) and during theta elicited by RPO stimulation. Calibration bar, 0.5 mV.
Fig. 2.
A–C, Analyses used to classify PH tonic θ-ON cells and SuM and MM phasic θ-ON cells. Shown are the digitized field and cell activity (top panels), auto-correlation histograms (ACH;middle panels), and cross-correlation functions (CCF; bottom panels) during hippocampal LIA (left column) and RPO-elicited θ (right column). A, PH cells show a nonrhythmic discharge pattern during both θ and LIA conditions with a higher discharge rate during the θ condition. The ACHs also indicate that the cell discharged in a nonrhythmic pattern during the simultaneous occurrence of either LIA or θ, and the CCF shows that the cell did not discharge preferentially with respect to the phase of the extracellular θ field activity. B, C, The ACHs of SuM and MM cells show that they discharge in a nonrhythmic pattern during LIA and a rhythmic pattern during RPO-elicited hippocampal θ. The CCFs show that the cells discharged preferentially on a particular phase of the extracellular θ wave. Calibration bar, 1.0 mV.
Effects of septal procaine infusion
An illustrative example of the effect of septal procaine infusion on the discharge of a PH tonic θ-ON cell is shown in Figure3A. The mean of the responses (in terms of discharge rate) of all PH tonic θ-ON cells to septal procaine infusion is shown in Figure 4A. In the preprocaine condition, hippocampal θ was elicited by RPO stimulation. The rate of PH tonic θ-ON cell discharge increased relative to that during LIA (see Figs. 2A, 3A,Pre-Procaine). In the period immediately after the procaine infusion, in which suppression of hippocampal θ was maximal, not only was PH tonic θ-ON cell discharge still observed, but the same level of RPO stimulation significantly (t(1,11) = 3.6; p < 0.05; Scheffé post hoc pairwise comparison) increased their mean discharge rate relative to the preprocaine condition (Figs.3A, 4A, Post-Procaine). This was particularly evident in the illustrated cell (Fig.3A). After 10–20 min, RPO stimulation again elicited clear θ activity in the hippocampal field and the rate of tonic cell discharge under stimulation returned to preprocaine levels (Figs.3A, 4A, Recovery).
Fig. 3.
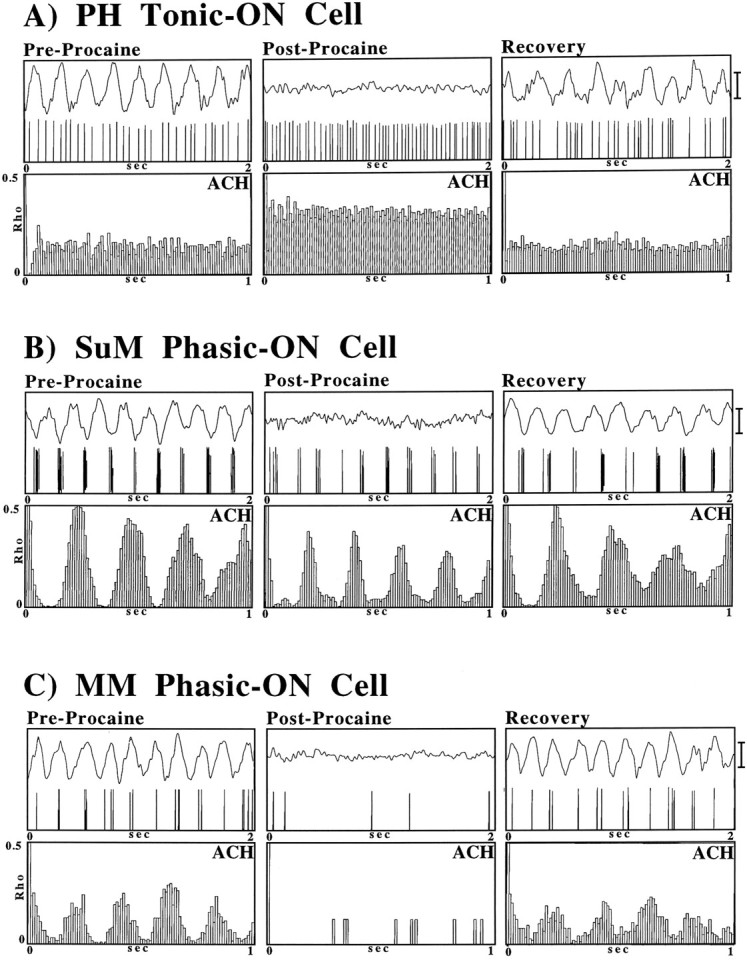
The effect of septal infusions of procaine on RPO stimulation elicited hippocampal θ and θ-related PH (A), SuM (B), and MM (C) cell discharge. Shown are the digitized field and cell activity (top panels) and auto-correlation histograms (ACH; middle panels) in the preprocaine condition (Pre-Procaine) during the abolition of hippocampal θ immediately (1–3 min) after septal procaine infusion (Post-Procaine) and when hippocampal θ returns, 10–30 min after the end of the procaine infusion (Recovery). Calibration bar, 1.0 mV.
Fig. 4.
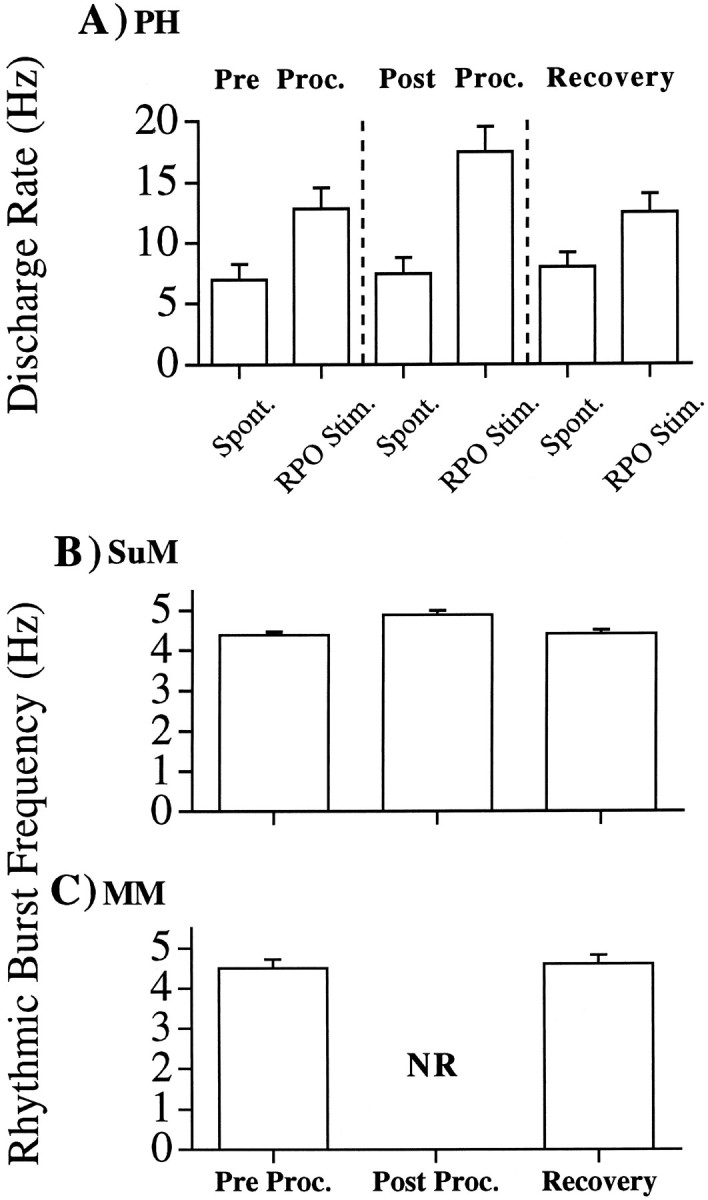
A, Mean discharge rate of PH cells (in the spontaneous condition and under RPO stimulation) before the septal infusion of procaine (Pre Proc.), within a 5 min period after the end of the septal procaine infusion in which RPO-elicited hippocampal θ was completely abolished (Post Proc.), and after RPO-elicited hippocampal θ had recovered to preprocaine levels (Recovery). Spontaneous levels of PH cell discharge were not significantly different across the preprocaine, postprocaine, and recovery conditions. Under RPO stimulation, however, significant differences in discharge rates were observed across conditions (F(2,11) = 10.1;p < 0.005). The discharge rate of PH cells under RPO stimulation was significantly higher in the postprocaine condition relative to that in the preprocaine or recovery conditions (bothp < 0.005; Scheffé post hoc pairwise comparisons). B, Mean rhythmic burst frequency of SuM cells during RPO stimulation before the septal infusion of procaine (Pre Proc.), within a 5 min period after the end of the septal procaine infusion in which RPO-elicited hippocampal θ was completely abolished (Post Proc.), and after RPO-elicited hippocampal θ had recovered to preprocaine levels (Recovery). Significant differences in discharge rates were observed across conditions (F(2,10) = 23.5; p < 0.0005). The rhythmic burst frequency of SuM cells under RPO stimulation was significantly higher in the postprocaine condition relative to that in the preprocaine or recovery conditions (both p < 0.001; Scheffépost hoc pairwise comparisons). C, Mean rhythmic burst frequency of MM cells during RPO stimulation before the septal infusion of procaine (Pre Proc.), within a 5 min period after the end of the septal procaine infusion in which RPO-elicited hippocampal θ was completely abolished (Post Proc.), and after RPO-elicited hippocampal θ had recovered to preprocaine levels (Recovery). There was no significant difference in rhythmic burst frequency between the preprocaine and recovery conditions. In the postprocaine period, rhythmic discharge was not observed (NR).
An illustrative example of the response of an SuM phasic θ-ON cell to septal procaine infusion is shown in Figure 3B. In the preprocaine condition, RPO stimulation elicits hippocampal θ and phase-locked rhythmical SuM cell discharge. After septal procaine infusion, hippocampal θ activity is abolished but RPO stimulation continued to elicit rhythmical activity in SuM phasic θ-ON cells. This is evident from the ACF of the cell (Fig. 3B,Post-Procaine). Although SuM phasic θ-ON cells survived septal procaine infusion, this manipulation was not without effect. As can be seen in the example in Figure 2B (compare ACH pre- vs postprocaine) and from the means in Figure 4B(pre- vs postprocaine), RPO stimulation postprocaine elicited a slightly higher burst frequency in SuM phasic θ-ON cells than the same level of stimulation in the preprocaine condition. Although small, the increase was found to be significant (t(1,8) = 5.1; p < 0.001; Scheffé post hoc pairwise comparison). After 10–20 min postprocaine, clear hippocampal θ was again elicited in response to RPO stimulation, and the frequency of burst discharge returned to preprocaine rates (Figs. 3B,4B, Recovery).
The response of MM phasic θ-ON cells to septal procaine infusion is illustrated in Figure 3C. As with the SuM phasic θ-ON cells, in the preprocaine condition RPO stimulation elicits rhythmical MM cell discharge phase-locked to hippocampal θ. In contrast to PH and SuM θ-ON cells in which discharge rates but not discharge patterns were altered after septal procaine, septal procaine infusion radically altered the discharge rate and pattern in MM cells. As can be seen in Figures 3C and 4C, in the first 5 min after septal procaine infusion (when the procaine block was maximally effective) MM cell discharge was severely attenuated (and in some cases abolished completely). This was the case for both spontaneous discharge and that during RPO stimulation. After the initial attenuation (or abolition) of MM cell discharge rates, spontaneous cell discharge rates increased toward preprocaine levels. However, RPO stimulation did not elicit rhythmical MM cell discharge patterns until clear θ-activity was again observed in the hippocampal record.
DISCUSSION
In the present report, the dependence of θ-related single-unit discharge of cells in the caudal hypothalamus on θ activity in the septo-hippocampal system was assessed. Hippocampal θ activity was elicited by RPO stimulation, and θ-related discharge patterns of cells were classified according to previously used criteria. In accord with the findings of our previous study (Bland et al., 1995), θ-related PH cells were all found to be of the tonic θ-ON type (i.e., they discharged tonically and at higher rates during hippocampal θ than during LIA), whereas all θ-related SuM and MM cells were of the phasic θ-ON type (i.e., they discharge rhythmically and phase-locked to concurrent hippocampal θ activity, and nonrhythmically during LIA). Subsequently, the discharge patterns of the cells in response to RPO stimulation were tested after hippocampal θ was abolished by septal procaine infusion. It was found that θ-related cells in different nuclei were differentially affected by the abolition of θ.
The response of tonic PH θ-ON cell discharge to RPO stimulation (i.e., increases in tonic discharge rate) survived after the abolition of θ by infusion of procaine into the septum. Furthermore, there was a significant increase in discharge rate during stimulation after procaine infusion relative to that during stimulation in the preprocaine condition. Similarly, rhythmic SuM phasic θ-ON cell discharge in response to RPO stimulation was still observed after the abolition of hippocampal θ by septal procaine infusion. Again, however, septal procaine was not without effect. The burst frequency of SuM phasic θ-on cells during stimulation after procaine was slightly (but significantly) higher than that seen during stimulation before procaine.
These data indicate that the increases in discharge rate of PH tonic θ-ON cells and the rhythmic discharge of SuM phasic θ-ON cells during hippocampal θ are independent of θ activity in the septo-hippocampal system. This supports previous suggestions that the PH and SuM are both relays in an ascending “θ-synchronizing” system from the RPO to the MS/vDBB, and that PH tonic θ-ON and SuM phasic θ-ON cells act in synergy in the generation of hippocampal θ. It has been suggested (Kirk and McNaughton, 1991, 1993) that the level of ascending tonic RPO input is transduced into the frequency of θ in the SuM (reflected in the frequency of SuM cell burst discharge), and that this frequency-coded information is then fed via the medial forebrain bundle (Veazy et al., 1982; Vertes, 1992) to the MS/vDBB. The PH, however, may provide tonic input (possibly cholinergic; see Brazhnik and Vinogradova, 1986; Oddie et al., 1994;Bland et al., 1995) to the MS/vDBB and hippocampus that accentuates rhythmic firing in septal cells (Brazhnik and Vinogradova, 1986; Bland et al., 1994) and is required for the expression of θ field activity in the hippocampus (Oddie et al., 1994).
The fact that after septal procaine infusion the rate of PH tonic θ-ON cell discharge and the frequency of bursts of SuM phasic θ-ON cells both increase during stimulation suggests that θ-related PH and SuM cell discharge are normally subject to descending modulatory or rate-limiting influences from the septo-hippocampal system (on the basis of SuM multiunit activity after septal procaine, we have suggested previously that this may be true for the SuM (Kirk and McNaughton, 1991). Phasic θ-ON cells in the SuM may also be involved in a mutual resonant interaction with those in the septo-hippocampal system during hippocampal θ. This may result in phasic SuM cells being entrained to a lower burst frequency than would be the case if they were solely under the influence of input ascending from the midbrain. Miller (1991) has suggested previously an involvement of resonant loops in θ activity in other parts of the system (see alsoBland and Colom, 1993).
Neither the origin nor the hypothalamic targets of the descending influence from the septo-hippocampal system are known. Because of diffusion (Myers, 1966), the volume of septal procaine infused (2.0 μl) is likely to abolish or severely attenuate θ-related discharge in a considerable region of the septal complex and, because of its dependence on septal projections, that in the hippocampus and parahippocampal areas as well (for review, see Miller, 1991). However, the lateral septum, which receives projections from hippocampal CA3 region (Swanson and Cowan, 1977; Leranth et al., 1992), sends descending projections to the SuM (Swanson and Cowan, 1979) and other diencephalic nuclei (Swanson and Cowan, 1979; Leranth et al., 1992). Thus, the lateral septal area may relay descending modulatory signals to PH and SuM during hippocampal θ. As has been suggested previously, the descending influences may target a population of tonic θ-OFF cells found in the border region between PH and SuM (Bland et al., 1995). It is also possible that the MM (in receipt of descending influences from the septo-hippocampal system, see below) mediates activity in PH and SuM. MM has been shown to project to surrounding hypothalamic regions including SuM and PH (Gonzalo-Ruiz et al., 1991). Clearly, more work is needed to distinguish between these possibilities.
It should be noted that whereas procaine infused into the SuM attenuated the amplitude and frequency of reticularly elicited hippocampal θ in urethane-anesthetized (Kirk and McNaughton, 1993; Thinschmidt et al., 1995) and unanesthetized (McNaughton et al., 1995) rats, lesions of the SuM did not appear to affect spontaneous and movement-related θ in the unanesthetized animal (Thinschmidt et al., 1995). It seems likely, therefore, that, as has been suggested previously, rhythmical activity in SuM is not necessary for the expression of θ per se (Kirk and McNaughton, 1993; Bland et al., 1995; Thinschmidt et al., 1995). Further, it has been demonstrated that lesions of the RPO produced little obvious change in hippocampal θ in freely moving animals (Farris and Sainsbury, 1990). Hence, it is possible that the SuM is involved in the modulation of θ frequencies only when in receipt of high levels of activation from the RPO that may normally only occur in particular behavioral states (Vertes, 1982,1986) or, as in the present study, during RPO stimulation. Input from the PH may be particularly important for the expression of θ generally (see above). However, a variety of other pathways may be involved also. For example, cells of the pedunculopontine tegmentum (PPT) also project directly to the MS/vDBB (Woolf and Butcher, 1986), and stimulation of (Vertes, 1982), or infusion of carbachol into (Vertes et al., 1993b) the PPT has been shown to effectively elicit θ.
In contrast to PH and SuM θ-ON cells, the discharge properties of MM phasic θ-ON cells were considerably affected by septal procaine infusion. Not only was the discharge of MM phasic-ON cells nonrhythmic during RPO stimulation after septal procaine, but discharge rates were severely attenuated immediately after septal procaine infusion. The loss of rhythmic discharge of MM cells after septal procaine supports the results of partial coherence analysis suggesting that rhythmical, θ-related MM discharge (but not that of SuM) is driven by descending inputs originating in the hippocampal formation (Kocsis and Vertes, 1994). As noted above, the MM is likely to receive descending input originating in the hippocampus and relayed via the subiculum (Swanson and Cowan, 1975, 1977; Allen and Hopkins, 1989). The MM projects heavily to the anterior thalamus (Veazy et al., 1982) and may be involved in relaying θ-frequency activity from the septo-hippocampal system to the anterior nucleus of the thalamus (Alonso and Llinás, 1992). In turn, the anterior thalamus may relay, asKocsis and Vertes (1994) note, θ-frequency activity back to the hippocampus via the cingulate and entorhinal cortices. Thus, θ activity may be transmitted around the circuit originally described byPapez (1937). However, the MM is also in receipt of projections from the entorhinal cortex (Shibata, 1988) and medial septum (Swanson and Cowan, 1979).
The attenuation of MM discharge rates after septal procaine indicates that MM cell discharge per se is largely dependent on descending input from the septo-hippocampal system. During θ-activity, descending input from the septo-hippocampal system may counteract inhibitory GABAergic influences to MM ascending from the midbrain (Tappaz and Brownstein, 1977; Stratford and Wirtshafter, 1989) or from the tuberomammillary nuclei (Gonzalo-Ruiz et al., 1992). MM cells in vitro (lacking extrinsic sources of modulation) can discharge in rhythmic (albeit sub-θ-range) bursts (Alonso and Llinás, 1992). During θ-activity in vivo, descending septo-hippocampal influences may act to overcome any tonic inhibitory input to MM and to entrain the endogenous rhythmic activity of MM cells to θ frequencies.
In summary, the single-unit work reported here has demonstrated that tonic θ-ON cell discharge in PH, and phasic θ-ON cell discharge in SuM, is not dependent on input descending from the septum or hippocampus during θ activity. This supports the idea that the PH and SuM form relays in the ascending θ-synchronizing system. The present study has also documented for the first time that MM phasic θ-ON cell rhythmic discharge, and discharge per se, is largely dependent on input descending from the septo-hippocampal system during θ. Finally, although descending input from the septo-hippocampal system to PH and SuM is not required for θ-related activity in these structures, we provide evidence for descending, modulatory influences from the septo-hippocampal system to both PH and SuM.
Footnotes
This work was supported by Natural Sciences and Engineering Research Council (NSERC) Grant A9935 to B.H.B., a University of Calgary Post-Doctoral Fellowship to I.J.K., and an Alberta Heritage Foundation for Medical Research Post-Graduate Scholarship to S.D.O. J.K. was a visiting scientist supported by funds from NSERC. We thank Karen Waldie for comments on this manuscript.
Correspondence should be addressed to Brian H. Bland, Department of Psychology, Behavioral Neuroscience Research Group, The University of Calgary, Calgary, Alberta, Canada T2N 1N4.
Dr. Konopacki’s current address: Department of Neurobiology, University of Lódź, 66 Rewolucji 1905r Street, 90-222 Lódź, Poland.
REFERENCES
- 1.Ahmed N, Rao KR. Springer; Berlin: 1975. Orthogonal transforms for digital signal processing. . [Google Scholar]
- 2.Allen GV, Hopkins DA. Mammillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal complex, and midbrain tegmentum. J Comp Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Llinás RR. Electrophysiology of the mammillary complex in vitro. II. Medial mammillary neurons. J Neurophysiol. 1992;68:1321–1331. doi: 10.1152/jn.1992.68.4.1321. [DOI] [PubMed] [Google Scholar]
- 4.Anchel H, Lindsley B. Differentiation of two reticulo-hypothalamic systems regulating hippocampal activity. Electroenceph Clin Neurophysiol. 1972;32:209–226. doi: 10.1016/0013-4694(72)90171-x. [DOI] [PubMed] [Google Scholar]
- 5.Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 6.Bland BH, Colom LV. Preliminary observations on the physiology and pharmacology of hippocampal theta-off cells. Brain Res. 1989;505:333–336. doi: 10.1016/0006-8993(89)91463-7. [DOI] [PubMed] [Google Scholar]
- 7.Bland BH, Colom LV. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol. 1993;41:157–208. doi: 10.1016/0301-0082(93)90007-f. [DOI] [PubMed] [Google Scholar]
- 8.Bland BH, Vanderwolf CH. Diencephalic and hippocampal mechanisms of motor activity in the rat: effects of posterior hypothalamic stimulation on behavior and hippocampal slow wave activity. Brain Res. 1972;43:67–88. doi: 10.1016/0006-8993(72)90275-2. [DOI] [PubMed] [Google Scholar]
- 9.Bland BH, Colom LV, Ford RD. Responses of septal θ-on and θ-off cells to activation of the dorsomedial-posterior hypothalamic region. Brain Res Bull. 1990;24:71–79. doi: 10.1016/0361-9230(90)90289-c. [DOI] [PubMed] [Google Scholar]
- 10.Bland BH, Oddie SD, Dickson CT, Trepel C. Discharge patterns of posterior-supramammillary hypothalamic cells in relation to hippocampal field activity. Soc Neurosci Abstr. 1993;19:355. [Google Scholar]
- 11.Bland BH, Oddie SD, Colom LV, Vertes RP. The extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:649–660. doi: 10.1002/hipo.450040604. [DOI] [PubMed] [Google Scholar]
- 12.Bland BH, Konopacki J, Kirk IJ, Oddie SD, Dickson CT. Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethane anesthetized rat. J Neurophysiol. 1995;74:322–333. doi: 10.1152/jn.1995.74.1.322. [DOI] [PubMed] [Google Scholar]
- 13.Brazhnik ES, Vinogradova OS. Control of the neuronal rhythmic bursts in the septal pacemaker of the theta rhythm: effects of anaesthetics and anticholinergic drugs. Brain Res. 1986;380:94–106. doi: 10.1016/0006-8993(86)91433-2. [DOI] [PubMed] [Google Scholar]
- 14.Brücke F, Petsche H, Pillat B, Deisenhammer E. Über veränderungen des hippocampus-elektrencephalogrammes beim kaninchen nach novocaininjektion in die septumregion. Arch Exp Pathol Pharmakol. 1959;273:276–284. [PubMed] [Google Scholar]
- 15.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 16.Colom LV, Bland BH. State-dependent spike train dynamics of hippocampal formation neurons: evidence for theta-on and theta-off cells. Brain Res. 1987;422:277–286. doi: 10.1016/0006-8993(87)90934-6. [DOI] [PubMed] [Google Scholar]
- 17.Colom LV, Christie BR, Bland BH. Cingulate cell discharge patterns related to hippocampal EEG and their modulation by muscarinic and nicotinic agents. Brain Res. 1988;460:329–338. doi: 10.1016/0006-8993(88)90377-0. [DOI] [PubMed] [Google Scholar]
- 18.Dickson CT, Kirk IJ, Oddie SD, Bland BH. The classification of theta-related cells in the entorhinal cortex: evidence that the ascending brainstem hippocampal synchronising pathway exerts parallel control of the EC theta cell discharges. Hippocampus. 1995;5:320–328. doi: 10.1002/hipo.450050404. [DOI] [PubMed] [Google Scholar]
- 19.Farris PD, Sainsbury RS. The role of the pontis oralis in the generation of RSA activity in the hippocampus of the guinea pig. Physiol Behav. 1990;47:1193–1199. doi: 10.1016/0031-9384(90)90372-b. [DOI] [PubMed] [Google Scholar]
- 20.Ford R, Colom LV, Bland BH. The classification of medial septum-diagonal band cells as theta-on or theta-off in relation to hippocampal EEG states. Brain Res. 1989;493:269–282. doi: 10.1016/0006-8993(89)91162-1. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinás RR. Afferent projections to the mammillary complex of the rat, with special reference to those from surrounding hypothalamic regions. J Comp Neurol. 1991;321:277–299. doi: 10.1002/cne.903210208. [DOI] [PubMed] [Google Scholar]
- 22.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura H, Nakamura Y, Tokizane T. Effect of acute brain lesions on the electrical activities of the limbic system and neocortex. Jpn J Neurophysiol. 1961;11:564–575. doi: 10.2170/jjphysiol.11.564. [DOI] [PubMed] [Google Scholar]
- 24.Kirk IJ, McNaughton N. Supramammillary cell firing and hippocampal rhythmical slow activity. NeuroReport. 1991;2:723–725. doi: 10.1097/00001756-199111000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Kirk IJ, McNaughton N. Mapping the differential effects of procaine on the frequency and amplitude of reticularly elicited rhythmical slow activity. Hippocampus. 1993;3:517–526. doi: 10.1002/hipo.450030411. [DOI] [PubMed] [Google Scholar]
- 26.Kirk IJ, Konopacki J, Bland BH. Differential effects of septal procaine infusion on theta-related discharge patterns of posterior-hypothalamic, supramammillary, and medial mammillary neurons. Soc Neurosci Abstr. 1994;20:346. doi: 10.1523/JNEUROSCI.16-17-05547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocsis B, Vertes RP. Characterization of neurons in the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine and procaine. Exp Neurol. 1993;120:132–144. doi: 10.1006/exnr.1993.1047. [DOI] [PubMed] [Google Scholar]
- 29.Leranth C, Deller T, Buzsáki G. Intraseptal connections redefined: lack of a lateral septum to medial septum path. Brain Res. 1992;583:1–11. doi: 10.1016/s0006-8993(10)80004-6. [DOI] [PubMed] [Google Scholar]
- 30.Leung L-WS, Lopes da Silva FH, Wadman WJ. Spectral characteristics of the hippocampal EEG in the freely moving rat. Electroenceph Clin Neurophysiol. 1982;54:303–319. doi: 10.1016/0013-4694(82)90162-6. [DOI] [PubMed] [Google Scholar]
- 31.Llinás RR, Alonso A. Electrophysiology of the mammillary complex in vitro. I. Tuberomammillary and lateral medial mammillary neurons. J Neurophysiol. 1992;68:1307–1320. doi: 10.1152/jn.1992.68.4.1307. [DOI] [PubMed] [Google Scholar]
- 32.Mair WGP, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: a neuropathological and neuropsychological investigation of two cases. Brain. 1979;102:749–783. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- 33.McNaughton N, Logan B, Panickar KS, Kirk IJ, Pan W-X, Brown NT, Heenan A. Contribution of synapses in the medial supramammillary nucleus to the frequency of hippocampal theta rhythm in freely moving rats. Hippocampus. 1996;5:534–545. doi: 10.1002/hipo.450050605. [DOI] [PubMed] [Google Scholar]
- 34.Mignard M, Bentzinger D, Bender N, Gabriel M. Type I and type II theta-like unit activity in structures of the Papez circuit during differential avoidance conditioning in rabbits. Soc Neurosci Abstr. 1987;13:305. [Google Scholar]
- 35.Miller R. Springer; Berlin: 1991. Cortico-hippocampal interplay and the representation of contexts in the brain. . [Google Scholar]
- 36.Mizumori SYJ, Barns CA, McNaughton BL. Reversible inactivation of the medial septum: selective effects on the spontaneous unit activity of different hippocampal cell types. Brain Res. 1989;500:99–106. doi: 10.1016/0006-8993(89)90303-x. [DOI] [PubMed] [Google Scholar]
- 37.Myers RD. Injection of solutions into cerebral tissue: relation between volume and diffusion. Physiol Behav. 1966;1:171–174. [Google Scholar]
- 38.Oddie SD, Bland BH, Colom LV, Vertes RP. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:454–473. doi: 10.1002/hipo.450040408. [DOI] [PubMed] [Google Scholar]
- 39.O’Keefe J, Nadel L. Oxford UP; Oxford: 1978. The hippocampus as a cognitive map. . [Google Scholar]
- 40.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 41.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychol. 1937;38:725–743. [Google Scholar]
- 42.Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. Academic; Sydney: 1982. The rat brain in stereotaxic coordinates. . [DOI] [PubMed] [Google Scholar]
- 44.Press WH, Flannery BB, Teukolsky SA, Vetterling WT. Cambridge UP; Cambridge: 1986. Numerical recipes: the art of scientific computing. [Google Scholar]
- 45.Richmond BJ, Optican LM, Podell M, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J Neurophysiol. 1987;57:132–146. doi: 10.1152/jn.1987.57.1.132. [DOI] [PubMed] [Google Scholar]
- 46.Robinson TE, Whishaw IQ. Effects of posterior hypothalamic lesions on voluntary behavior and electroencephalograms in the rat. J Comp Physiol Psychol. 1974;86:768–786. doi: 10.1037/h0036397. [DOI] [PubMed] [Google Scholar]
- 47.Shibata HA. A direct projection from the entorhinal cortex to the mammillary nuclei in the rat. Neurosci Lett. 1988;90:6–10. doi: 10.1016/0304-3940(88)90777-x. [DOI] [PubMed] [Google Scholar]
- 48.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 49.Statford TR, Wirtshafter D. Gudden’s tegmental nuclei are the major source of GABA found in the mammillary body of the rat. Soc Neurosci Abstr. 1989;2:1244. [Google Scholar]
- 50.Stewart M, Fox S. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- 51.Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex not Ammon’s horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 53.Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–656. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- 54.Sziklas V, Petrides M. Memory impairments following lesions of the mammillary region. Eur J Neurosci. 1993;5:525–540. doi: 10.1111/j.1460-9568.1993.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 55.Tappaz ML, Brownstein MJ. Origin of glutamate-decarboxylase (GAD)-containing cells in discrete hypothalamic nuclei. Brain Res. 1977;132:95–106. doi: 10.1016/0006-8993(77)90708-9. [DOI] [PubMed] [Google Scholar]
- 56.Thinshmidt JS, Kinney GG, Kocsis B. The supramammillary nucleus: is it necessary for the mediation of hippocampal theta rhythm? Neuroscience. 1995;67:301–312. doi: 10.1016/0306-4522(95)00045-k. [DOI] [PubMed] [Google Scholar]
- 57.Veazy RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis ). II. Efferent connections. J Comp Neurol. 1982;207:135–156. doi: 10.1002/cne.902070204. [DOI] [PubMed] [Google Scholar]
- 58.Vertes RP. Brainstem generation of hippocampal EEG. Prog Neurobiol. 1982;19:159–186. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 59.Vertes RP. Brainstem modulation of the hippocampus. In: Isaacson RL, Pribram KH, editors. The hippocampus, Vol. 4. Pribram; New York: 1986. [Google Scholar]
- 60.Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- 61.Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe in the rat. J Comp Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- 62.Vertes RP, Crane AM, Colom LV, Bland BH. PHAL-L analysis of ascending projections from the posterior hypothalamus in the rat. Soc Neurosci Abstr. 1993a;19:1442. [Google Scholar]
- 63.Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993b;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- 64.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain. III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]