Abstract
There are a total of eight sensory organs in the chick inner ear. Each sensory organ has a distinct structure tailored for its function, and its morphology is well characterized. However, the origin of these sensory organs and the lineage relationships among them are largely unknown. In this report, we show that BMP4 (bone morphogenetic protein), a secreted protein of the TGF-β gene family, is the earliest sensory marker identified to date for the chick inner ear. In addition to BMP4, we show that Msx-1 is a sensory marker for the three cristae, the lagena, and macula neglecta. P75NGFR (nerve growth factor receptor) is a marker for the three cristae only. Based on the expression pattern of these three genes—BMP4, Msx-1, and p75NGFR—it is estimated that the first sensory organs to be generated were the superior and posterior cristae at stage 19, followed by the macula sacculi at stage 20, the lateral crista at stage 22, the basilar papilla and lagena at stage 23, the macula utriculi at stage 24, and the macula neglecta at stage 29. The age of generation of each sensory organ as defined by the first appearance of these molecular markers is well in advance of the histological differentiation. In addition, the differential gene expressions in each presumptive sensory organ may contribute to the distinct structure of the mature organ.
Keywords: inner ear development, Msx-1, p75NGFR, BMP4, placode-derived, sensory organ
In the chick inner ear, there is one auditory sensory organ known as the basilar papilla, and seven vestibular organs that include three crista ampullae, two maculae, one lagena, and one macula neglecta. The decision to become sensory versus nonsensory tissue occurs early during development (Swanson et al., 1990). However, the generation of the sensory organ topology within the inner ear is not clear. Based on morphological analysis of nonmammalian vertebrates (Knowlton, 1967; Norris, 1892), it was postulated that all sensory organs of the inner ear are derived exclusively from the ventromedial wall of the otocyst, a region that first gives rise to the eighth cranial ganglion. More recent data suggest that cells in the anteroventrolateral portion of the otocyst, rather than in the ventromedial wall, give rise to the eighth ganglion (Carney and Couve, 1989). However, the origin of the sensory organs remains unclear.
To gain a better understanding of inner ear development and to identify genes that are important in its pattern formation, one of the gene families we focused on was transforming growth factor-β (TGF-β). Many members of this gene family have been shown to be important signaling molecules during embryogenesis, especially those that belong to the DVR ( decapentaplegic- veg- related) subgroup (for review, see Kingsley, 1994; Wall and Hogan, 1994). Within the DVR subgroup, BMP4 is important for mesoderm patterning as well as the development of many organs such as heart, teeth, and limbs (Vainio et al., 1993; Fainsod et al., 1994; Suzuki et al., 1994; Winnier et al., 1995; Zou and Niswander, 1996). It has been reported that BMP4 is also expressed in the otocyst of mice (Jones et al., 1991).
Another class of genes that is important in patterning during embryogenesis is the homeobox-containing genes. Among them, Msx-1, previously known as Hox-7, is a mouse homolog of theDrosophila muscle segment homeobox (msh) gene. Msx genes have been shown to be important signaling factors in epithelial–mesenchyme interactions during embryogenesis (Suzuki et al., 1991; Takahashi et al., 1991). Msx-1-deficient mice have an abnormality in one of the middle ear ossicles (Satokata and Maas, 1994). In the chick inner ear, Msx-1 is first detected in the dorsal one-third of the invaginating otic placode (Suzuki et al., 1991), an area that we found eventually gives rise to the endolymphatic apparatus. Thus far, there has been no report of Msx-1 gene expression in later stages of inner ear development.
As reported here, we demonstrate that BMP4 is a marker for all presumptive sensory organs in the chick inner ear using in situ hybridization. Msx-1, in addition to being an early marker for the endolymphatic apparatus, was found to be expressed in the three cristae, the lagena, and the macula neglecta. Based on the gene expression patterns of BMP4, Msx-1, and the previously reported p75NGFR, the origin and time of generation for each presumptive sensory organ in the chick inner ear were determined.
MATERIALS AND METHODS
Embryos. Fertilized White Leghorn eggs (Truslow Farm, MD) were incubated for designated times, and embryos were staged according to Hamburger and Hamilton (1951).
Probes. The following chicken cDNA sequences were used to generate digoxigenin-labeled sense and antisense RNA probes: a full-length BMP4 cDNA (Roberts et al., 1995), 3.1 kb of p75NGFR cDNA, which included ∼2 kb of 3′-untranslated region (Large et al., 1989), and a 550 bp fragment of Msx-1 cDNA downstream from the homeobox (Suzuki et al., 1991). Corresponding sense probes were carried out in each experiment as controls and yielded little hybridization signal.
Whole-mount in situ hybridization. Whole-mountin situ hybridization was performed as described previously (Riddle et al., 1993) with the following modifications. All embryos were permeabilized with proteinase K, and enzyme dosages used were optimized for different embryo stages. Proteinase K concentrations ranging from 1 to 20 μg/ml were used for embryos ranging from stage 8 [embryonic day 1 (E1)] to stage 24 (E4), respectively. Hybridization conditions, washings, and detection procedures were carried out as described previously (Riddle et al., 1993). Embryos that were selected for preparation of frozen sections were dehydrated in 30% sucrose, embedded in gelatin, and subsequently sectioned.
In situ hybridization of frozen sections. Frozen sections of chick embryos were processed for in situ hybridization essentially as described above. Briefly, embryos were fixed overnight with 4% paraformaldehyde in PBS, dehydrated in 30% sucrose, and embedded in OCT. Embryos were sectioned at 12–14 μm thickness onto SuperFrost slides (VWR) and stored in a dessicator at −80°C. Forin situ hybridization, slides were first rehydrated, post-fixed, and permeabilized with 10 μg/ml proteinase K for 2 min. Hybridization was carried out in “seal-a-meal” bags (Kapak). Each bag contained no more than 5 glass slides and 5 ml of hybridization solution with a probe concentration of ∼0.2 μg/ml.
Three-dimensional reconstruction. Images of serial sections of the chick inner ear after in situ hybridization were captured directly from a Zeiss Axiophot microscope onto a Macintosh computer using a charge-coupled device camera and National Institutes of Health Image software. Image files were transferred to a Silicon Graphics Manta workstation. Areas of interest from each section were traced and subsequently aligned and reconstructed into three-dimensional images using ROSS software (Biocomputation Center, Ames Research Center, NASA). Alignment of sections for reconstruction was also aided by the atlas of the developing chick inner ear (Bissonnette and Fekete, 1996).
RESULTS
BMP4 mRNA expression in the chick inner ear
E1 (stage 8) to E4 (stage 24)
The following whole-mount in situ hybridization results are summarized from a total of 87 chick embryos ranging from stage 8 to stage 24 over 11 experiments. BMP4 mRNA was not detected in the otic placode of chick embryos at stage 10 but was expressed in rhombomere 3 and 5 of the hindbrain as reported previously (Graham et al., 1994) (data not shown). However, as soon as the otic placode started to invaginate, BMP4 mRNA was found in the medial and posterior margin of the invaginating placode (stage 11, E1.5, Fig.1A, arrowheads). When the otic placode deepened to form the otic cup, the medial part of the invaginating placode became the dorsal portion of the otic cup and remained in close association with the hindbrain (black arrowheads in Fig. 1B). At this stage, BMP4 transcripts were found in the rim of the otic cup (arrowheads in Fig. 1B). The positive area was broad at the dorsal and posterior rim of the otic cup (black arrowheads in Fig. 1B), but small at the ventral rim (white arrowheads in Fig.1B,C). The majority of the hybridization signal at the ventral rim, observed in whole-mount embryos, was contributed by ectoderm immediately adjacent to the otic epithelium rather than by the otic epithelium itself (compare white arrow and white arrowheads in Fig. 1C). At stage 16 (26–28 somites, E2.5), the otic cup was closing rapidly and was closed by the beginning of stage 17 (E2.5). At the beginning of stage 16 (26 somites), the BMP4-positive area at the ventral rim of the otic cup had expanded (Fig. 1D,G,arrowheads). During stage 16 (28 somites), two concentrations of the hybridization signal started to appear, a posterior focus (arrow in Fig. 1E) and an anterior streak (arrowhead, Fig. 1E). Both of these concentrations were more apparent by stage 17 (arrow and arrowhead in Fig.1F; n = 8; 5 separate experiments), and the hybridization signals were located within the otic epithelium (Fig. 1H,I). By stage 19 (E3), two principal foci of BMP4 hybridization signals were evident in the otocyst, an anterior and a posterior (Fig.1J). The anterior streak of BMP4 expression (black arrowheads in Fig.1E,F; white arrowin Fig. 1H) was replaced by a single focus. Whereas the restriction of the posterior focus occurred gradually and was quite evident from our results (Fig.1E,F,J), the origin of the anterior focus is less clear. Even though the presence of the anterior streak (arrowhead, Fig.1E,F) before the appearance of the restricted anterior spot (Fig. 1J) was obvious, the transition from the streak to a single focus appeared abrupt. Among eight stage 18 embryos (pool of 5 experiments), four had a positive anterior streak, two had an anterior spot, and two were negative for both hybridization signals. Because the hybridization signal in the anterior focus was always lower than that of the posterior focus (Fig. 1J), the transition from the streak to a single spot may have been difficult to detect because of technical limitations. Alternatively, the anterior focus arises independently and bears no relationship to the positive streak.
Fig. 1.
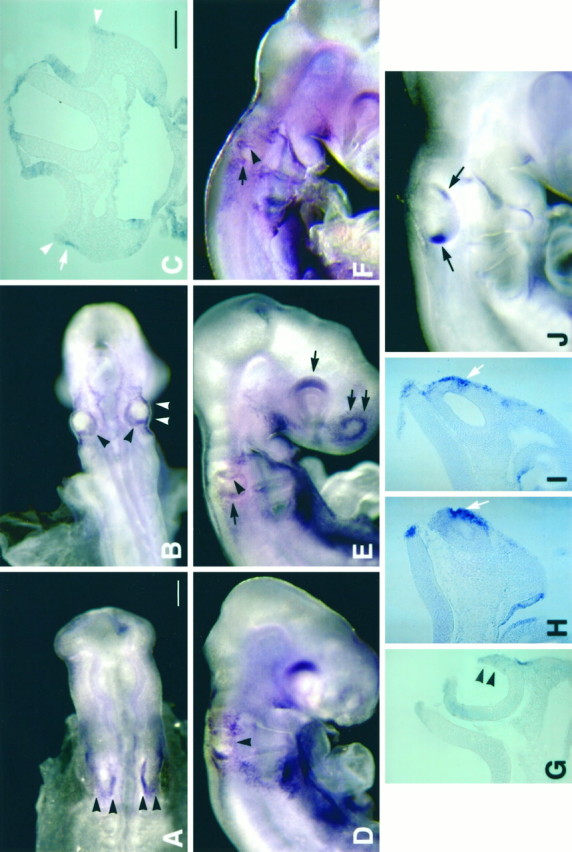
Gene expression of BMP4 in developing chick inner ear, stages 11–19, by whole-mount in situhybridization. The stages of embryos illustrated are as follows: (A) 12 somites, stage 11; (B,C) 21 somites, stage 13; (D,G) 26 somites, stage 16; (E) 28 somites, stage 16; (F, H, I) 30 somites, stage 17; and (J) 40 somites, stage 19. At stage 11 (A), BMP4 expression was detected in the medial and posterior margin of the otic placode (arrowheads). By stage 13 (B), BMP4 expression was present in the dorsal and posterior margin of the otic cup (black arrowheads). Some transcripts were detected in the ventral margin of the otic cup (white arrowheadsin B), but the majority of this hybridization signal was found to be contributed by ectoderm adjacent to the otic cup shown in a transverse section (C) of an embryo the same age asB (stage 13). Arrowheads inC point to hybridization signal within the otic epithelium, and the arrow points to signal in the adjacent ectoderm. At 26 somites (stage 16; D), the expression in the dorsal and posterior margin remained whereas the expression in the ventral portion of the otic epithelium had expanded (arrowheads in D, G). By 28 somites (stage 16; E), a positive streak appeared in the anterior otocyst (arrowhead) and the posterior hybridization signal became more restricted (black arrow). In addition, the dorsal portion of retina (single arrow) and the olfactory placode (double arrows) were also positive. A slightly older embryo (30 somites, stage 17; F) showed a similar pattern, with the arrowhead pointing to the anterior streak and the arrow pointing to the beginning of the posterior focus. In this embryo (F), the otocyst was closed as indicated by the notch shown in a transverse section (H). The hybridization signal from the anterior streak was present within the otic epithelium (arrow inH). Hybridization signal in the posterior focus was also within the otic epithelium, as shown in a more posterior section (arrow in I). By stage 19, two BMP4-positive foci were evident in the otocysts (arrowsin J). Scale bars: A,B, D–F, J, 200 μm; C, G–I, 100 μm.
Whereas more BMP4 expression appeared in other parts of the otic epithelium as development continued, the two initial foci of BMP4-positive areas persisted and remained in the same relative positions within the otocyst (based on whole-mount embryos; data not shown). These two positions, the anterior and the posterior, corresponded to the areas that give rise to superior and posterior crista ampullaris, respectively (Romanoff, 1960; Von Bartheld et al., 1991). These results suggested to us that BMP4 may be an early marker for sensory organs in the chick inner ear, and further analysis of BMP4 expression pattern in older embryos was carried out using frozen sections.
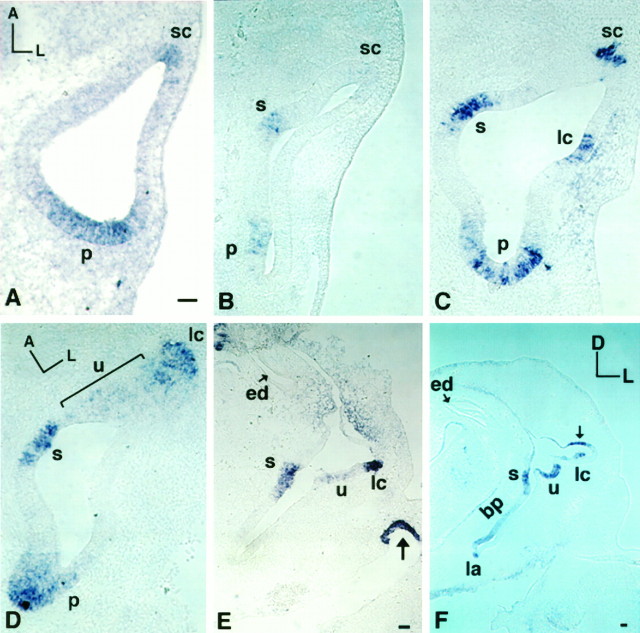
Results from in situ hybridization performed on frozen sections confirmed the observations made in whole-mount embryos: only one predominant posterior focus of BMP4 expression was seen in the stage 18 otocyst (data not shown), and two foci of BMP4 expression were noted by stage 19 (Fig. 2A,sc and p). Over the next five stages, three more foci appeared near the anterior focus (i.e., presumptive superior crista) as new sensory organs were generated. Identification of each presumptive sensory organ was determined by carefully tracing each respective area of BMP4 expression through different stages of development until histology of the sensory tissue was distinct, as well as by comparing the relative relationship between different areas of BMP4 expression using three-dimensional reconstructions (Fig.3) (see also Fig. 3 in Oh et al., 1996). At stage 20, a new area of BMP4 expression appeared in the medial part of the otocyst that was the beginning of the presumptive macula sacculi (n = 6), and the pattern became more apparent by stage 21 (E3.5) (Fig. 2B, see s). By stage 22/23, the presumptive lateral crista ampullaris appeared in the anterolateral wall of the otocyst (Fig. 2C, lc). By stage 24 (E4), the presumptive macula utriculi also appeared and had a broad and diffuse domain of BMP4 expression located between the macula sacculi and the lateral crista ampullaris (Fig.2D, u).
Fig. 2.
Gene expression of BMP4 in developing inner ear, stages 19 (E3) to stage 30 (E6.5), by in situhybridization of frozen sections. A–Dare horizontal sections of chick otocysts. At stage 19 (E3;A) only two BMP4-positive areas were present [anterior (sc) and posterior (p)]. The BMP4 expression in macula sacculi (s) is shown at stage 21 (B); lateral crista (lc) at stage 22 (C) and macula utriculi (u) at stage 24 (D). E and F are transverse sections of chick otocysts of stage 27 (E5) and stage 30 (E6.5), respectively. In addition to the BMP4-positive sensory organs as indicated in E and F, BMP4 was also expressed in the mesenchyme surrounding the dorsal portion of the inner ear (E), the tip of the first pharyngeal pouch (arrow in E), and the roof of the ampulla (arrow in F). Orientation:A, anterior; D, dorsal; L, lateral. ed, Endolymphatic apparatus; bp, basilar papilla; la, lagena. Scale Bar, 50 μm.
Fig. 3.
Three-dimensional reconstruction of BMP4 gene expression of a stage 27 (E5) chick inner ear. The right inner ear is shown from an anterior (A) and a posterior (B) view. BMP4-positive areas are displayed in different colors, which include superior crista (sc) inblue, lateral crista (lc) inyellow, macula utriculi (u) inred, macula sacculi (s) inorange, basilar papilla (bp) and lagena (la) traced as one object in fuchsia, and posterior crista (pc) inbluish-green. Image in Awas tilted dorsally to reveal all of the positive areas. This specimen was reconstructed from 20 alternate sections of 12 μm thickness. Only the inner borders of the otic epithelium from each section were traced to give the contour of the inner ear. However, the entire outlines of each positive area were traced, which included the inner and outer borders of the positive epithelium. As a result, positive areas appear as bulged objects situated on the reconstructed fluid ducts of the inner ear. ed, Endolymphatic apparatus;psc, primordia for the superior and posterior semicircular canals. Scale bar, 100 μm.
The area of BMP4 expression in the posterior region of the otocyst was observed to give rise to four presumptive sensory organs: the posterior crista, macula neglecta, lagena, and basilar papilla. By stage 20, this posterior domain started to expand ventromedially. Because all four sensory organs were positive for BMP4 and hybridization signal from each sensory organ was contiguous with that of the other, it was difficult to ascribe the time of generation for these sensory organs based on BMP4 gene expression alone. However, using a combination of BMP4 with other markers, such as Msx-1 and p75NGFR, a closer approximation of the time of generation for each of these four sensory organs was feasible (see section on Msx-1 and p75NGFR expression below).
E5 (stage 27) and later
By stage 27 (E5), the inner ear had undergone substantial expansion in the dorsoventral dimension. Even though histological differentiation of sensory tissues had just begun at this age, most of the sensory organs in the inner ear were already discrete entities based on BMP4 mRNA distribution. Figure 2E is a transverse section through the middle of the inner ear showing discrete BMP4 expression at the lateral crista (lc), maculae utriculi (u), and sacculi (s). In addition, BMP4 transcripts were detected in the mesenchyme surrounding the primordium for the anterior and posterior semicircular canals. A posterior section from a stage 30 (E6.5) embryo is shown in Figure 2F. BMP4 was expressed in the same sensory organs as seen in stage 27 (E5;s, u, and lc in Fig.2E) and in the basilar papilla and lagena (Fig.2F, bp and la). In addition, BMP4 expression was found in the roof of the ampullae (arrowin Fig. 2F), which most likely arose independently from that in the presumptive crista (Oh et al., 1996). Even though the histology of the sensory organs was much more distinct at stage 30 (E6.5), most of the sensory organs could be discerned by stage 27 (E5) based on BMP4 expression pattern. Three-dimensional reconstruction of the in situ data helps illustrate the localization of hybridization signals to each respective sensory organ (Fig. 3). At stage 27 (E5), the morphological structure of the inner ear was still rudimentary. The semicircular canals have not yet formed, and the basilar papilla has not acquired its mature length (Fig. 3) [also refer to the atlas of the developing chick inner ear (Bissonnette and Fekete, 1996)]. However, seven of the eight sensory organs were already evident at stage 27 (E5) based on BMP4 expression. The macula neglecta was not identifiable until stage 29 (E6.0; see section below). Also, based on BMP4 expression alone, the presumptive lagena and papilla were continuous with each other at stage 27 (E5) and did not become separate entities until stage 30 (E6.5; Fig.2F, see bp and la). However, the combination of expression of BMP4 and other markers indicate that the lagena and the papilla were clearly separate units at least by stage 23 (see section below).
Msx-1 and p75NGFR expression in the developing chick inner ear
As described earlier, the first BMP4 area to become restricted in the otocyst was the posterior focus at stage 18. Its location corresponded to the future posterior crista ampullaris. However, this posterior focus quickly expanded medially and ventrally and subsequently included more than one sensory organ. In an effort to identify other molecular markers that would help distinguish the different presumptive sensory tissues within the posterior cluster, two available markers proved useful: p75NGFR and Msx-1. P75NGFR mRNA was reported to appear first in the E3–E4.5 chick otocyst as two foci, one anterior and one posterior, which presumably are the anlages of the anterior (i.e., superior) and posterior crista ampullaris, respectively (Von Bartheld et al., 1991). By E7, p75NGFR mRNA was reported to be present in the three cristae and was restricted to only the planum semilunatum area of each crista by E11 (Von Bartheld et al., 1991). In general, our findings were in agreement with published results, except that we detected p75NGFR expression earlier than E3, during formation of the otic cup (data not shown). The initial expression of p75NGFR in the presumptive cristae was broader than that of BMP4 (Fig.4C,D). Both the presumptive superior (compare Fig. 4C and 4D,sc) and lateral cristae (compare Fig. 4G and 4H, lc), based on BMP4 expression, were included in one broad anterior hybridization signal of p75NGFR (Fig.4D,H). As sensory tissue differentiation began, p75NGFR expression segregated from the main sensory areas starting at E5 (data not shown), and remained in the peripheral portion of the crista known as the transitional zone at E12. (arrow in Fig. 5A). On the other hand, Msx-1 (also known as G-Hox 7) was reported to be expressed in the dorsal one-third of the otic cup at stage 13 (E2) embryos, and this expression persisted in the otocyst (Suzuki et al., 1991). We determined that this dorsal area of the otocyst gave rise to the endolymphatic apparatus by tracing Msx-1 expression in later stages of development. In addition to the endolymphatic apparatus, we found Msx-1 expression in all three cristae, the lagena, macula neglecta, as well as some portions of the semicircular canals (Fig. 5B) (see also Fig. 6F for expression in macula neglecta).
Fig. 4.
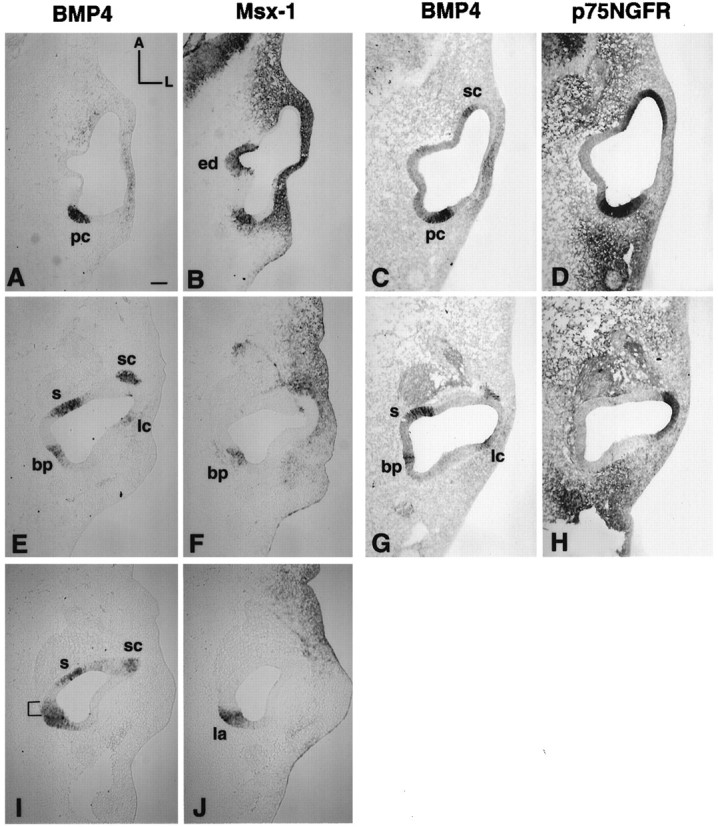
Gene expression pattern of BMP4, Msx-1, and p75NGFR in a stage 23 (E4) chick otocyst. A andB, E and F, andI and J are pairs of adjacent 12 μm sections from one embryo, with A and Bbeing the most dorsal pair and I and Jthe most ventral. C and D andG and H are pairs from another embryo. The C/D pair was chosen from a level similar to the A/B pair, and theG/H to E/F.A, C, E, G, and I were probed for BMP4 mRNA; B, F, and J were probed for Msx-1; and D and H were probed for p75NGFR. In the most dorsal sections, the posterior BMP4-positive area was Msx-1-positive (B) as well as p75NGFR-positive (D), which marked the presumptive posterior crista (pc) area. More ventral sections were still positive for Msx-1 (F) but negative for p75NGFR (H), indicating the presumptive basilar papilla (bp) region. The positive p75NGFR area inH included the presumptive lateral crista area and was continuous with the superior crista area in D. In the ventral portion of this broad BMP4-positive area (I, J), part of the area was strongly positive for Msx-1 marking the presumptive lagena. Area marked with bracketin I, which was positive only for BMP4, was part of the presumptive basilar papilla. Scale bar, 100 μm.
Fig. 5.
Gene expression of p75NGFR and Msx-1 in the developing inner ear. Transverse sections of an E12 (stage 38;A) and an E6 (stage 29; B) inner ear were probed for p75NGFR and Msx-1, respectively. At E12, p75NGFR expression was expressed in the three cristae of the inner ear. A part of the lateral crista ampullaris is shown in A. P75NGFR expression was located in the peripheral portion of the crista (arrows in A) as well as many areas of mesenchyme next to the otic epithelium (arrowheads). At E6 (stage 29), as illustrated in B, Msx-1 was expressed in the lagena (la), three cristae ampullaris [lateral crista is shown (lc)], the endolymphatic apparatus (ed), and some portions of the semicircular canals (ssc). Orientation: D, dorsal;M, medial; hb, hind brain. Scale bars:A, 50 μm; B, 100 μm.
Fig. 6.
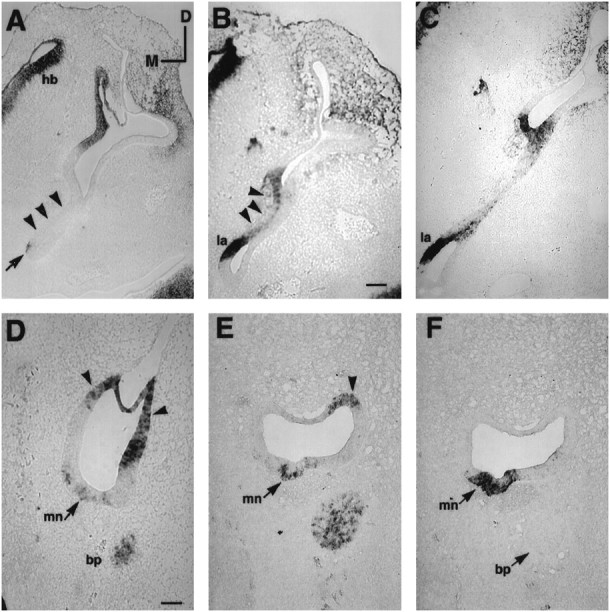
BMP4 and Msx-1 expression in the presumptive basilar papilla and macula neglecta. A–Cand F were probed for Msx-1. D andE were probed for BMP4. A andB are transverse sections of a stage 26 embryo (E4.5–E5) showing that the Msx-1 expression was absent in the anterior portion of basilar papilla (arrowhead inA) but present in the posterior portion of the papilla (arrowhead in B). The arrow inA points to part of the positive area in the lagena, which is more obvious in a posterior section shown in B(la). The Msx-1 expression in the papilla decreased over time (stage 27, E5; C) and eventually disappeared with transcripts remaining in the lagena (la) only (see Fig.5B). D and E are transverse sections indicating BMP4 expression in macula neglecta (mn) at stage 29 (E6) and stage 31 (E7), respectively.F is an adjacent section of E probed for Msx-1. Arrowheads in D andE point to positive hybridization signals from the posterior ampulla. The proximal tip of the basilar papilla (bp), which was BMP4-positive, is also shown inD–F. Orientations: D, dorsal; M, medial; hb, hind brain. Scale bars: A–C, 100 μm;D–F = 50 μm.
Both Msx-1 and p75NGFR were used to help distinguish among the four presumptive sensory organs in the posterior otocyst: the posterior crista, basilar papilla, lagena, and macula neglecta. Within this posterior cluster, p75NGFR was a marker for the posterior crista only (Fig. 4C,D). On the other hand, Msx-1 is a marker for all four sensory organs initially, including most of the papilla (Fig.4B,F,J). Even though the first restriction of BMP4 expression in the otocyst was located in the area of the future posterior crista at stage 18, we estimate the generation age for the posterior crista to be stage 19. This is based on the time when primordia for other sensory organs can also be identified using the differential expression of p75NGFR and BMP4. At stage 19, p75NGFR overlapped with the posterior area of BMP4 except for a small medioventral portion that was positive for BMP4 only and was presumed to be the primordium for the papilla and lagena (data not shown; n = 3). The portion of otic epithelium that was positive for both markers was the anlage for the posterior crista. By the end of stage 19 and the beginning of stage 20, Msx-1 gene transcription was activated within this presumptive sensory area and its expression pattern overlapped with BMP4, including the ventromedial portion, which was p75NGFR-negative (data not shown). This ventromedial portion was the beginning of the presumptive papilla and lagena, and this area expanded and became more evident by stage 23. Figure 4 is a series of horizontal sections of otocysts at stage 23 and illustrates the complexity of the broad posterior BMP4-positive area. The most dorsal portion of this posterior area was the presumptive posterior crista, which was positive for BMP4, Msx-1, and p75NGFR (Fig.4A–D, pc). The p75NGFR-positive area was always broader and more extensive than that of BMP4 (compare Fig. 4C and 4D). A few sections more ventrally, the otic epithelium became p75NGFR-negative (compare Fig. 4G and 4H, seebp) while remaining positive for BMP4 and Msx-1 (compare Fig. 4E and 4F, bp). This area was part of the presumptive basilar papilla. At the most ventral portion of this broad posterior area, the otic epithelium was strongly positive for Msx-1 and BMP4 (Fig.4I,J, see la), negative for p75NGFR (data not shown), and marked the presumptive lagena.
Furthermore, at E4 (stage 23/24), the otocyst started to extend in both a ventral and a medial direction, forming a tube-like structure to achieve the shape of a mature inner ear. This tube-like structure formed the basilar papilla, and the tip of the tube was the lagena. The medial surface of this tube-like structure developed into the sensory organ of the basilar papilla (for review, see Romanoff, 1960). However, the entire medial surface of the basilar papilla was not positive for BMP4 but, rather, the positive BMP4 area was in somewhat of a V-shape with a short anterior arm and a longer posterior arm joined ventrally by the lagena at stage 27 (E5; see fuchsia color area in Fig. 3B). The posterior arm was Msx-1-positive (stage 26, E4.5–E5.0) and gradually became negative by stage 29 (E6; compare Fig.6B and 6C with Fig. 5B). The short anterior arm that was slightly anterior to the lagena was always BMP4-positive but Msx-1-negative at stage 26 (E4.5;arrowhead in Fig. 6A). Before the appearance of a clear V-shaped pattern of BMP4 expression in the papilla, a portion of otic epithelium anterior to the presumptive lagena was always BMP4-positive and Msx-1-negative (area marked bybracket in Fig. 4I), which presumably is part of the presumptive sensory tissue in the papilla. Therefore, we ascribe the generation age for lagena and basilar papilla anlages to be stage 23, an age when these sensory organs can be identified separately from each other as well as from the posterior crista.
The macula neglecta is the smallest of the eight sensory organs. It is located on the floor of the utricle immediately anterior to the posterior crista ampullaris (Correia et al., 1974). Based on BMP4 and Msx-1 expression, the macula neglecta was the last sensory organ to form its own discrete unit. In the presumptive location where macula neglecta developed, BMP4 and Msx-1 were expressed at least by stage 23. Because of the proximity of these expressions to the presumptive posterior crista and the posterior arm of papilla, which were both BMP4- and Msx-1-positive, the generational age of macula neglecta could not be ascribed until stage 29 (E6), when BMP4 expression became discrete (arrow in Fig. 6D,mn). However, at this age results from adjacent sections analyzed for Msx-1 mRNA indicated that the Msx-1 expression was not yet restricted and was still continuous with that of the posterior crista as well as the papilla (data not shown). By stage 31 (E7), both BMP4 (Fig. 6E) and Msx-1 (Fig. 6F) expressions in the macula neglecta were discrete.
DISCUSSION
Origin of sensory organs
Based on morphological studies by Knowlton (1967), it was postulated that the ventromedial wall of the otic cup gave rise to the eight sensory organs of the inner ear. However, several lines of evidence argue against this hypothesis. First, crude fate mapping studies of the mouse otocyst at the 11th day of gestation indicate that the topology of the inner ear, including the sensory organs, is basically established by this age of development (Li et al., 1978). The 11th day of gestation in the mouse is equivalent to approximately stage 24 in the chick, a time, according to Knowlton (1967), when sensory organs have not yet split away from their origin. However, different portions of the mouse otocyst give rise to appropriate sensory and nonsensory structures after 10 d of culturing in vitro(Li et al., 1978). Sensory organs were not found to arise from a single area of the otocyst as Knowlton’s hypothesis might predict.
Another line of evidence that argues against sensory organs of the inner ear originating from the ventromedial wall of the otocyst comes from studies of other sensory organ markers, such as brain-derived nerve growth factor (BDNF) and p75NGFR. At E4, BDNF and p75NGFR mRNAs were not localized to the medial part of the otocyst as might be expected but, rather, in an anterior and posterior focus similar to the pattern of BMP4 (Von Bartheld et al., 1991; Hallbook et al., 1993). Even though we detected BDNF and p75NGFR mRNA earlier than previously reported, we did not detect any transcripts in the ventromedial wall of the otic cup or otocyst (data not shown). One possible explanation is that sensory organs may indeed originate from the ventromedial wall of the otocyst but at an earlier time than Knowlton (1967) had described, with transcription of sensory-specific genes such as BDNF, p75NGFR, or BMP4 only activated as each sensory organ is generated. This may be argued in the case of BDNF because BDNF transcripts were not detected until stage 17/18 (data not shown). However, this does not hold for BMP4 because it was expressed when the otic placode began to invaginate, before the formation of the eighth ganglion. The initial expression of BMP4 was along the dorsal and posterior portions of the invaginating otic placode. Based on the initial pattern of BMP4 expression from the invaginating placode to the otocyst, we postulate that induction of early presumptive sensory tissues most likely occurs at the junction of the otic epithelium and the adjacent ectoderm. Specifically, the portion of otic epithelium that is in close association with the hindbrain, rather than the ventromedial wall of the otocyst. Intimate interaction of the hindbrain with the otocyst is important for normal inner ear development (for review, see Noden and Van De Water, 1992). Furthermore, because the distribution of BMP4 mRNA was broad to begin with and became restricted later as two foci in the otocyst, it is conceivable that the area with the potential to develop into sensory tissue may also be broad initially and becomes restricted to specific locations, possibly by other genes such as the proneural or neurogenic genes. Therefore, depending on how the otic cup closes to form the otocyst, it is possible that cells within the anterior and posterior foci may share the same lineage. It is also possible that cells in the anterior focus and cells that give rise to the ganglia (Carney and Couve, 1989) may share one lineage, because they are located in close proximity to each other. These possibilities need to be examined with detailed fate-mapping studies. Nevertheless, it is interesting to note that hair cells develop autonomously when the otocyst is transplanted to ectopic locations as early as stage 13 (E2), whereas other morphogenetic changes fail to occur normally (Swanson et al., 1990). These data suggest that induction of sensory tissue is an early event. Because BMP4 mRNA was already present in the otic cup by stage 11 (E1.5), activation of BMP4 gene transcription may be associated with sensory tissue induction.
Order of sensory organ initiation
Based on the pattern of BMP-4, Msx-1, and p75NGFR mRNA distribution, we postulate that the first presumptive sensory organs to generate in the inner ear are the anterior and posterior cristae ampullaris at stage 19, the macula sacculi at stage 20, the lateral crista at stage 22, the papilla and lagena at stage 23, the macula utriculi at stage 24, and the macula neglecta at stage 29 (Table1). It is interesting that the anterior and posterior cristae are also the first sensory organs to show histological differentiation (Knowlton, 1967), suggesting that early discrete BMP4 expression may be correlated with early sensory differentiation.
Table 1.
Summary of sensory organ generation based on BMP4, Msx-1, and p75NGFR gene expression patterns
| Sensory organ | BMP4 | Msx-1 | p75NGFR | Stage of generation1_a |
|---|---|---|---|---|
| Superior crista | + | + | + | 19 |
| Macula sacculi | + | − | − | 20 |
| Lateral crista | + | + | + | 22 |
| Macula utriculi | + | − | − | 24 |
| Posterior cluster | + | 18 | ||
| Posterior crista | + | + | + | 19 |
| Basilar papilla | ||||
| Anterior arm | + | − | − | 23 |
| Posterior arm | + | +1_b | − | 23 |
| Lagena | + | + | − | 23 |
| Macula neglecta | + | + | − | 29 |
The generation age for each sensory organ is defined when discrete expression of each molecular marker listed was activated for that particular sensory organ. The only exception is the macula neglecta: at stage 29 (E6), only BMP4 expression was restricted, and Msx-1 expression did not become restricted until stage 31 (E7).
Msx-1 expression in the posterior arm of the basilar papilla disappeared by stage 29.
As far as we can determine, the three sensory organs—superior crista, macula sacculi, and lateral crista—appear to arise de novoas independent units based on the discrete appearance of BMP4 hybridization signals. However, our data cannot exclude the possibility that a small number of cells split from the anterior focus (the presumptive superior crista) to form the next two organs (the lateral crista and the macula sacculi) and that these primordia are undetectable using in situ hybridization techniques until the sensory tissues became more developed. On the other hand, the origin of the macula utriculi is different. This sensory organ appeared at stage 24 as an area of diffuse BMP4 expression between the presumptive lateral crista and macula sacculi with no clear demarcation of boundaries. Therefore, macula utriculi could arise independently or split from its neighboring sensory tissues, namely, the lateral crista, macula sacculi, and/or superior crista. By the same token, our data also cannot exclude the possibility that the four sensory organs in the posterior cluster arise de novo, independently from each other.
In addition, the macula neglecta is thought to split from the posterior crista ampullaris at embryonic day 6 (Knowlton, 1967). Macula neglecta was positive for BMP4 and Msx-1, and the posterior crista was positive for both of these markers as well as p75NGFR. However, we were not able to identify the macula neglecta component within the presumptive posterior crista area and confirm the origin of this sensory organ. Macula neglecta could arise either from the posterior crista directly or from the primordium of lagena and papilla after its separation from the posterior crista. A more specific marker for the macula neglecta is needed before we can address this issue in more detail.
In summary, our study provides a more in-depth understanding of the origin and the developmental organization of sensory organs in the inner ear. At the moment, the functions of BMP4 and Msx-1 remain elusive. In other systems, both BMP4 and Msx-1 are important molecules mediating epithelial–mesenchymal interactions (Takahashi et al., 1991;Vainio et al., 1993). The interaction between mesenchymal and epithelium is also an important component of the normal morphogenesis of the inner ear (for review, see Van Der Water et al., 1980). Both BMP-4 and Msx-1 may function solely in the development of the sensory organs within the otic epithelium and/or they may mediate a coordinated differentiation between epithelia and mesenchyme. The type I BMP4 receptors identified so far, alk-3 and alk-6, are both present in the developing inner ear (Dewulf et al., 1995). Alk-3 is ubiquitously expressed while alk-6 is associated with the mesenchyme surrounding the otic capsule as well as sensory tissues of the cochlea and saccule (Dewulf et al., 1995). Therefore, both models appear to be possible at this time. In BMP4-deficient mice, the consequence of the lack of this gene product in development of the ear cannot be pursued because mutant embryos do not survive beyond 9.5 d of gestation (Winnier et al., 1995). Nevertheless, our results will provide a good basis to address commitment and plasticity of presumptive sensory organs, as well as to decipher the functions of BMP4 and Msx-1 in the development of the inner ear.
Footnotes
We are indebted to the staff in the Biocomputation Center, Ames Research Center, NASA, for their help with ROSS software. We also thank Dr. Donna Fekete for providing the atlas of the developing chick inner ear before publication, Drs. L. Reichardt and M. Solursh for providing plasmids, Drs. Connie Cepko, James Battey, Susan Wray, and Daniel Choo for reviewing this manuscript, and Ms. Mirene Boerner for editing.
Correspondence should be addressed to Doris K. Wu, NIDCD, 5 Research Court, Room 2B34, Rockville, MD 20850.
Dr. Oh’s present address: Department of Otolaryngology, Seoul National University, 28 Yongon-Dong Chono-Gu, Seoul, Korea 110-744.
REFERENCES
- 1.Bissonnette JP, Fekete DM. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J Comp Neurol. 1996;174:1–11. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Carney PR, Couve E. Cell polarity changes and migration during early development of the avian peripheral auditory system. Anat Rec. 1989;225:156–164. doi: 10.1002/ar.1092250211. [DOI] [PubMed] [Google Scholar]
- 3.Correia MJ, Landolt JP, Young ER. The sensura neglecta in the pigeon: a scanning electron and light microscopy study. J Comp Neurol. 1974;154:303–316. doi: 10.1002/cne.901540306. [DOI] [PubMed] [Google Scholar]
- 4.Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Spiegle KV, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- 5.Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 7.Hallbook F, Ibanez CF, Ebendal T, Persson H. Cellular localization of brain-derived neurotrophic factor and neurotrophin-3 mRNA expression in the early chicken embryo. Eur J Neurosci. 1993;5:1–14. doi: 10.1111/j.1460-9568.1993.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–52. [PubMed] [Google Scholar]
- 9.Jones MC, Lyons KM, Hogan BLM. Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–542. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley DM. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 11.Knowlton VY. Correlation of the development of membranous and bony labyrinths, acoustics ganglia, nerves, and brain centers of the chick embryos. J Morphol. 1967;121:179–208. [Google Scholar]
- 12.Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989;2:1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- 13.Li CW, Van Der Water TR, Ruben RJ. The fate mapping of the eleventh and twelfth day mouse otocyst: an in vitro study of the sites of origin of the embryonic inner ear sensory structures. J Morphol. 1978;157:249–268. doi: 10.1002/jmor.1051570302. [DOI] [PubMed] [Google Scholar]
- 14.Noden DM, Van De Water TR. Genetics analyses of mammalian ear development. Trends Neurosci. 1992;15:235–237. doi: 10.1016/0166-2236(92)90056-e. [DOI] [PubMed] [Google Scholar]
- 15.Norris HW. Studies on the development of the ear of amblystoma. J Morphol. 1892;7:23–34. [Google Scholar]
- 16.Oh SH, Johnson R, Wu DK. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J Neurosci. 1996;16:0000–0000. doi: 10.1523/JNEUROSCI.16-20-06463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanoff AL. The avian embryo structure and function, pp 365–381. MacMillan; New York: 1960. The organ of special sense. [Google Scholar]
- 18.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 20.Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakamm K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki HR, Padanilam BJ, Vitale E, Ramirez F, Solursh M. Repeating developmental expression of G-Hox 7, a novel Homeobox-containing gene in the chicken. Dev Biol. 1991;148:375–388. doi: 10.1016/0012-1606(91)90345-4. [DOI] [PubMed] [Google Scholar]
- 23.Swanson GJ, Howard M, Lewis J. Epithelial autonomy in the development of the inner ear of a bird embryo. Dev Biol. 1990;137:243–257. doi: 10.1016/0012-1606(90)90251-d. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Bontoux M, Le Douarin NM. Epithelio-mesenchymal interactions are critical for Quox 7 expression and membrane bone differentiation in the neural crest derived mandibular mesenchyme. EMBO J. 1991;10:2387–2393. doi: 10.1002/j.1460-2075.1991.tb07777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 26.Van Der Water TR, Li CW J RR, Shea CA. Ontogenic aspects of mammalian inner ear development. In: Gorlin RJS, editor. Birth defects, Vol 16. Liss; New York: 1980. pp. 5–45. [PubMed] [Google Scholar]
- 27.Von Bartheld CS, Patterson SL, Heuer JG, Wheeler EF, Bothwell M, Rubel EW. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development. 1991;113:455–470. doi: 10.1242/dev.113.2.455. [DOI] [PubMed] [Google Scholar]
- 28.Wall NA, Hogan BLM. TGF-β related genes in development. Curr Opin Genet Dev. 1994;4:517–522. doi: 10.1016/0959-437x(94)90066-c. [DOI] [PubMed] [Google Scholar]
- 29.Winnier G, Blessing M, Labosky PA, Hogan BLM. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 30.Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]