Abstract
In humans, esotropia of early onset is associated with a profound asymmetry in smooth pursuit eye movements. When viewing is monocular, targets are tracked well only when they are moving nasally with respect to the viewing eye. To determine whether this pursuit abnormality reflects an anomaly in cortical visual motion processing, we recorded eye movements and cortical neural responses in nonamblyopic monkeys made strabismic by surgery at the age of 10–60 d. Eye movement recordings revealed the same asymmetry in the monkeys’ pursuit eye movements as in humans with early-onset esotropia. With monocular viewing, pursuit was much stronger for nasalward motion than for temporalward motion, especially for targets presented in the nasal visual field. However, for targets presented during ongoing pursuit, temporalward and nasalward image motion was equally effective inmodulating eye movement. Single-unit recordings made from the same monkeys, under anesthesia, revealed that MT neurons were rarely driven binocularly, but otherwise had normal response properties. Most were directionally selective, and their direction preferences were uniformly distributed. Our neurophysiological and oculomotor measurements both suggest that the pursuit defect in these monkeys is not due to altered cortical visual motion processing. Rather, the asymmetry in pursuit may be a consequence of imbalances in the two eyes’ inputs to the “downstream” areas responsible for the initiation of pursuit.
Keywords: artificial strabismus, visual cortex, motion processing, smooth pursuit, eye movements, binocular interaction, MT, development
Strabismus of early onset disrupts a number of visual functions in humans, monkeys, and cats. Improper binocular alignment early in life almost invariably leads to a failure of stereoscopic depth perception. Strabismus is often also associated withamblyopia, a loss of visual resolution and sensitivity in the nonpreferred eye. Both of these effects of ocular misalignment early in life have clear correlates in the responses of neurons in the visual cortex. The failure of stereoscopic depth perception has been related, in cats and monkeys, to a loss of binocular interaction in the responses of visual cortical neurons (Hubel and Wiesel, 1965; Crawford and Von Noorden, 1979; Von Noorden, 1980; Wiesel, 1982). The presence of amblyopia is often accompanied by a reduced representation of the amblyopic eye in visual cortex, and reduced resolution and sensitivity in cortical neurons driven by that eye (Eggers and Blakemore, 1978;Eggers et al., 1984; Kiorpes et al., 1987; Movshon et al., 1987).
Early-onset strabismus also has marked effects on oculomotor behavior, but the neural basis for the eye movement defects is not understood.Tychsen and Lisberger (1986a) described an asymmetry in the pursuit eye movements of adult subjects who had been esotropic strabismics as infants. When viewing was monocular, these individuals had stronger pursuit eye movements for target motion in a nasalward direction with respect to the viewing eye. Tychsen and Lisberger proposed that this asymmetry might result from a defect in the representation of motion in the extrastriate visual motion pathways. Their proposal was based on the fact that the nasal–temporal pursuit asymmetry was most evident in the first 100 msec of the pursuit, which is driven directly by visual motion inputs (Lisberger and Westbrook, 1985). In support of this idea, Tychsen and Lisberger showed that their observers systematically misjudged the relative speed of nasally and temporally moving targets in a manner that was consistent with their pursuit deficits.
An alternate view is that the nasal–temporal motion asymmetry in pursuit arises from a defect deeper in the oculomotor system, and not from properties of the visual motion sense per se. Two recent reports raise the possibility that normal visual motion signals can under some conditions fail to gain access to pathways that allow the initiation of pursuit. Schwartz and Lisberger (1994) showed that a brief perturbation of target motion elicited little pursuit response during fixation, even though the same perturbation elicited a strong response during ongoing pursuit. Grasse and Lisberger (1992) described a monkey with an up–down pursuit asymmetry that resembled the nasal–temporal pursuit asymmetry of human infantile strabismics. Although this monkey was unable to initiate upward pursuit eye movements, it was able to use upward image motion to modulate ongoing pursuit as well as to program the amplitude of saccadic eye movements. These results suggest that visuo-motor processing for pursuit must be explicitly enabled to initiate pursuit eye movements, and that the underlying neural machinery can be accessed in a direction-specific manner.
In the present experiments, we have studied pursuit eye movements and the neural representation of direction of target motion in monkeys with experimentally produced early-onset strabismus. Behavioral experiments revealed that a nasal–temporal pursuit asymmetry like that in human strabismics is also seen in these monkeys; as in humans, the asymmetry was more pronounced for targets delivered to the temporal retina. Moreover, as in the monkey reported by Grasse and Lisberger (1992), the asymmetry seen so clearly at the onset of pursuit was absent from the eye movements evoked by image motion presented during pursuit. Single-unit recordings revealed that the strabismus did not change the motion-signaling properties or the distribution of preferred direction of MT neurons. Strabismus did, however, modify the binocularity of MT neurons in a way that could limit the effectiveness of temporalward motion in eliciting pursuit. We conclude that the nasal–temporal motion asymmetry in pursuit is not a simple product of modified visual motion processing, and suggest instead that it is a consequence of a modified binocular balance in the visual inputs to areas responsible for pursuit initiation.
Some of these results have been presented briefly previously (Walton and Lisberger, 1989; Movshon and Kiorpes, 1992; Movshon et al., 1995).
MATERIALS AND METHODS
Table 1 presents information on the subjects of these experiments, 6 pigtailed macaque monkeys (M. nemestrina), made strabismic between the ages of 10 and 60 d. Two of the monkeys (PW and SY) were used for both eye movement and single-unit recording; the other 4 were used only for single-unit recording. Esotropic strabismus (crossed eyes) was induced in 5 of the monkeys by recession of the lateral rectus and resection of the medial rectus muscles of the left eye (hereafter referred to as thedeviated eye); in monkeys PW and SY the lateral rectus muscle of the right eye was also cut to aid in the establishment of the strabismus (Kiorpes et al., 1989). After such surgery, the lateral rectus muscles typically reattach and qualitatively normal ocular motility is maintained. When evaluated with monocular cover testing and quantitative eye movement recording, the magnitude of the esotropia was 20 deg in monkey PW and 25 deg in monkey SY. One monkey (AP) was made esotropic by injection of Botulinum A neurotoxin into the lateral rectus muscle of the left eye; this monkey’s initial esotropia subsequently resolved into an exotropia. Table 1 also lists relative visual acuity data for these monkeys, tested using techniques that we have described previously (Kiorpes et al., 1989, 1993). Monocular testing revealed that 5 of the 6 monkeys had similar visual acuity in the two eyes and were therefore not amblyopic. The one mild amblyope (FS) contributed only 18 cells to the physiological data and was not involved in the pursuit experiments. Control data for the electrophysiological recordings were obtained from 8 cynomolgus monkeys (M. fascicularis) with normal eye alignment. Control data for the oculomotor recordings were taken from 2 rhesus monkeys (M. mulatta) with normal eye alignment. Although the control monkeys were not of the same species as the strabismic monkeys, there is no reason to think that there is any fundamental difference in the visual motion processing or smooth eye movements of these different macaque species.
Table 1.
Neuronal correlates of a directional pursuit asymmetry
| Monkey | Animal number | Born | Recorded | Treatment | Acuity difference |
|---|---|---|---|---|---|
| EX | F83430 | 12/15/83 | 8/7/85 | Eso 57 d surgical type 2 | 0.19 |
| FS | F84005 | 1/5/84 | 8/20/85 | Eso 36 d surgical type 2 | 0.51 |
| CA | F83330 | 9/22/83 | 7/13/91 | Eso 29 d surgical type 2 | 0.28 |
| AP | M87152 | 6/19/87 | 6/24/92 | Eso/exo 60 d toxin | 0.05 |
| PW | T82462 | 11/29/82 | 3/7/94 | Eso 11 d surgical type 1 | 0.00 |
| SY | F83013 | 1/11/83 | 3/14/94 | Eso 10 d surgical type 1 | −0.07 |
Subjects. The treatment column indicates the method of surgery as follows. Surgical type 1: transection of left and right lateral rectus; resection of left medial rectus to limbus. Surgical type 2: transection of the left lateral rectus; resection of left medial rectus to limbus. Toxin: injection of botulinum into left lateral rectus; injection of antitoxin into nasal, superior orbit. The “Acuity difference” column gives the logarithm of the interocular difference in spatial resolution measured with grating targets. The age at test ranged from 16 weeks to 2 years.
Oculomotor recording methods
Behavioral training and surgical preparation. Our general methods for training monkeys and recording their oculomotor behavior have been detailed previously (Lisberger and Westbrook, 1985). Monkeys were trained using a modification of the reaction-time task ofWurtz (1969) to fixate and track moving targets for fluid reinforcements. They were then anesthesized with halothane and a sterile surgical procedure was used to implant scleral search coils on both eyes to monitor eye position. In one monkey (SY), we also placed bolts and dental acrylic on the skull as a head holder to stabilize his head during experiments. The other monkey (PW) was deemed unlikely to adapt to mechanical stabilization of the head. He was trained to use a bite-bar to initiate trials, thereby restraining his head adequately to allow us to monitor eye movements. Eye movements were measured by placing the monkeys in the center of a 6 ft magnetic field coil system that operated on the rotating field principle (Collewijn, 1977). At the end of the oculomotor experiments, the monkeys were again anesthesized with halothane and sterile procedure was used to remove the eye coils, connectors, and head implant and to reclose the skin around the wound.
Target presentation and experimental design. A stationary 0.2 deg red target and a moveable 0.5 deg white target were projected onto the back of a tangent screen 114 cm from the monkey. Targets were several log units above detection threshold, and the screen was dimly illuminated by overhead incandescent lights. The position of the moveable target was controlled by a pair of mirror galvanometers; position feedback from the galvanometers was used to monitor horizontal and vertical target position. All experiments were done with monocular viewing. The eye coils were calibrated by having the monkey fixate targets at different locations with monocular viewing. Thereafter, we measured eye position and target position and issued reinforcements if eye position was maintained within a 3–4 deg window around target position. It was necessary to use a larger window than is typical because the strabismic monkeys exhibited nystagmus during attempted fixation.
In most experiments, different target motions and positions were presented in individual randomly ordered trials. Each trial began when the monkey fixated the red spot at straight ahead gaze for a random duration from 600 to 1000 msec. At least 300 msec before the end of the fixation interval, the white tracking target appeared either at straight ahead gaze or at an eccentric position. The monkey was required to continue fixating at straight ahead gaze until the fixation target was extinguished and then to track the moving target for 400–1000 msec. Because of the poor pursuit of strabismic monkeys for temporalward target motion, it was occasionally necessary to use fixation windows as large as 8 deg and to suspend fixation contingencies for up to 500 msec after the onset of target motion. The fixation requirements were the same for both directions of target motion and therefore did not bias the monkeys’ performance. Suitable controls were used to ensure that the monkeys could not correctly anticipate the direction of target motion before the tracking target started to move (Lisberger and Westbrook, 1985). In a few experiments, data were acquired continuously while the monkey either pursued sinusoidal target motion at a range of frequencies or fixated stationary targets at different locations.
Data acquisition and analysis. Experiments were controlled and the data were acquired and analyzed with the aid of a laboratory computer. The general data analysis procedure was first to edit each eye speed record to remove saccades. Trials were then grouped according to the exact parameters and direction of target motion, aligned on the onset of target motion, and averaged. We typically measured the average eye acceleration in the first 100 msec of pursuit. To obtain standard deviations of eye acceleration, we divided the standard deviation of eye speed 100 msec after the onset of pursuit by 0.1 sec. We would have preferred to measure eye acceleration in the first 100 msec of pursuit in each individual trial and compute means and standard deviations from those individual measurements, but the fixation nystagmus in the strabismic monkeys and the fact that their eye movements were neither as smooth nor as crisp as those in control monkeys made it difficult to point out the onset of pursuit reliably in individual trials. We do not think this introduced artifacts because the two techniques for data analysis provided nearly identical results in control monkeys (Lisberger and Westbrook, 1985).
Electrophysiological recording methods
Surgical preparation and maintenance. The animals (weights: 4–16 kg) were prepared for acute single-unit recording using methods we have described in detail previously (Movshon et al., 1987;Levitt et al., 1994). They were premedicated with atropine (0.25 mg), and acepromazine (0.05 mg/kg) or diazepam (Valium: 0.5 mg/kg). After induction of anesthesia with intramuscular injections of ketamine HCl (Vetalar: 10–30 mg/kg), cannulae were inserted into the trachea and the saphenous veins, the animal’s head was fixed in a stereotaxic frame, and surgery was continued under intravenous anesthesia. In early experiments, we used continuous infusion of sodium thiopental (Pentothal: 1–2 mg/kg/hr) for anesthesia. Later, we used the opiate anesthetic sufentanil citrate (Sufenta: 4–8 μg/kg/hr). Infusion of the surgical anesthetic continued throughout the recordings. We noticed no difference in the properties of MT units under these two anesthetic regimes.
To minimize eye movements, paralysis was maintained with an infusion of pancuronium bromide (Pavulon: 0.1 mg/kg/hr) or vecuronium bromide (Norcuron: 0.1 mg/kg/hr) in lactated Ringer’s solution with dextrose (5–20 ml/hr). Animals were artificially ventilated with room air or a mixture of 50–70% N2O in O2. Peak expired CO2 was maintained near 4% by adjusting the tidal volume of the ventilator. Rectal temperature was kept near 37°C with a thermostatically controlled heating pad. Animals received daily injections of a broad-spectrum antibiotic (Bicillin: 300,000 U) to prevent infection, as well as dexamethasone (Decadron: 0.5 mg/kg) to prevent cerebral edema. EKG, EEG, autonomic signs, and rectal temperature were monitored continuously to ensure the adequacy of anesthesia and the soundness of the animal’s physiological condition.
Tungsten-in-glass microelectrodes (Merrill and Ainsworth, 1972) were introduced by a hydraulic microdrive through a small guide needle into the portions of MT representing the central visual fields. After the electrode was in place in the cortex, the exposed dura was covered with warm agar. Action potentials were conventionally amplified, displayed, and played over an audio-monitor. The recording sessions lasted between 36 and 110 hr.
Physiological optics. The pupils were dilated and accommodation was paralyzed with topical atropine, and the corneas were protected with +2D gas-permeable hard contact lenses. When necessary, supplementary lenses were chosen by direct ophthalmoscopy to make the retinas conjugate with the display screen. The power of the lenses was then adjusted as necessary to optimize the visual responses of recorded units. Contact lenses were removed periodically for cleaning. At this time, the eyes were rinsed with saline and infiltrated with a few drops of ophthalmic antibiotic solution (Gentamicin). At least once a day, the locations of the foveas were recorded using a reversible ophthalmoscope.
Characterization of receptive fields. We initially mapped the receptive fields of single MT neurons by hand on a tangent screen using black and white geometric targets. For each neuron, we recorded the location and size of the neuron’s minimum response fields and determined its selectivity for the orientation, direction of motion, and size of stimuli. Ocular dominance was assessed qualitatively using the 7-point scale of Hubel and Wiesel (1968). Units were classified as ocular dominance group 4 if we could not distinguish any difference between the responses to stimulation of the two eyes. They were classified as groups 3 or 5 if they responded well to both eyes but with a discernible preference for the contralateral or ipsilateral eye, respectively, as groups 2 or 6 if they responded predominantly to the contralateral or ipsilateral eye, respectively, with a weak response to the other eye, and as groups 1 or 7 if they responded only to the contralateral or ipsilateral eye, respectively.
We used a mirror to place the preferred eye’s receptive field on the face of a display oscilloscope that subtended 10 deg at the animal’s eye. Textures consisting of several hundred randomly placed bright dots were generated and moved under computer control; the mean luminance of the random-dot displays was between 5 and 10 cd/m2. For the minority of neurons that were unresponsive to moving textures, we used achromatic sinusoidal gratings or sharp-edged contours with a mean luminance between 40 and 80 cd/m2. For most neurons, we determined the tuning parameters qualitatively by adjusting the speed and direction of movement of the targets while listening to the discharge over the audio-monitor. This allowed us to estimate the preferred direction, the bandwidth of directional selectivity, and the preferred and high-cutoff speeds. For some neurons we verified the accuracy of our qualitative estimates of directional selectivity with quantitative assessment of tuning parameters, using methods described previously (Levitt et al., 1994). However, the importance of sampling large numbers of neurons in each of the strabismic monkeys made it impossible to derive quantitative estimates of parameters such as tuning widths for direction or speed.
Reconstruction of recording sites. During recording, small electrolytic lesions were produced at locations of interest along the electrode tracks by passing DC current (2 μA for 2–5 sec, tip negative) through the electrode. At the end of the experiment, the animals were killed with an overdose of Nembutal and perfused through the heart with 2 l of 0.1 m PBS followed by 2 l of a solution containing 4% paraformaldehyde in 0.1 m PBS. Blocks containing the region of interest were stored overnight in the cold in a postfix solution of 4% paraformaldehyde plus 30% sucrose, after which 40-μm-thick sections were cut on a freezing microtome. Sections were stained for Nissl substance with cresyl violet, or myelin using the methods of Galyas (1969). Most recordings were verified histologically to lie within area MT, as defined by standard histological criteria (Van Essen et al., 1981). In the few cases for which we were unable to recover all the electrode tracks, we took the distinctive concentration of directionally selective neurons and the size of their receptive fields to identify recording sites as lying within MT (Desimone and Ungerleider, 1986).
RESULTS
Nasal–temporal asymmetry in pursuit eye movements
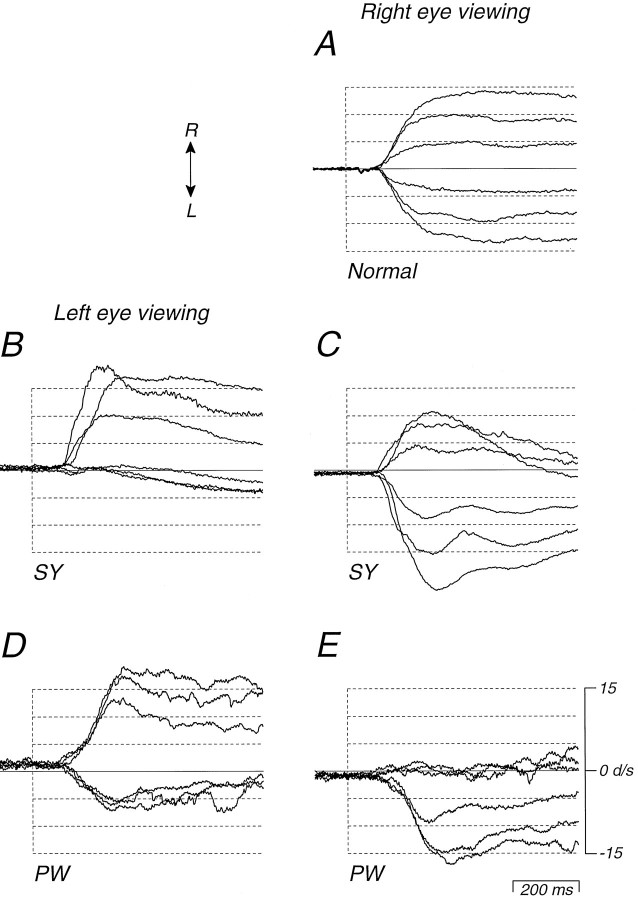
Figure 1 shows examples of the fixation and pursuit eye movements of one of the strabismic monkeys. The data in Figure1A are from monkey PW and show tracking eye movements when target motion was sinusoidal at 0.3 Hz, ±20 deg. When the monkey viewed with the right eye, he tracked smoothly during the leftward phase of sinusoidal target motion (downward deflections of traces), but largely tracked with saccades during the rightward phase. The situation reversed when the monkey viewed with his left eye: he emitted smooth tracking during the rightward phase of target motion and saccadic tracking during the leftward phase. In this and all other experiments, we systematically varied the viewing eye and the eye whose movements were monitored. In each monkey, the direction of the motion asymmetry depended only on which eye was viewing and was expressed equally well in the movements of both the viewing and the nonviewing eye; we saw no sign that the early surgical manipulation of the extraocular muscles had an important effect on ocular motility.
Fig. 1.
Tracking and fixation eye movements of strabismic monkey PW during monocular viewing. A, Dashed traces show target position, and solid tracesshow eye position during tracking of a sinusoidal target oscillation at 0.3 Hz and ±20 deg. B, Eye position during fixation of a stationary target at straight ahead gaze, showing “latent” nystagmus. Upward deflections of the traces indicate rightward target and eye motion (arrows).
Figure 1B illustrates for monkey PW the “latent” nystagmus that was typical of our monkeys and of humans with early-onset esotropia (Tychsen and Lisberger, 1986a). When the monkey attempted fixation of a stationary spot with the left eye, both eyes showed a nystagmus with slow phases drifting to the right and small, quick phases back to the left. When the monkey viewed with the right eye, both eyes had slow phases to the left. Thus, the direction of the slow phase was always nasalward with respect to the viewing eye. We did not investigate the effect of varying viewing conditions on the amplitude of the latent nystagmus, but Tychsen and Lisberger (1986a)found that the amplitude was larger during fixation of a target than in complete darkness and that increasing the illumination of the background relative to the fixation target decreased the amplitude of the nystagmus slightly in humans.
Figure 2 shows typical single-trial records of pursuit eye movements from a control and a strabismic monkey, using a variant of the “step-ramp” paradigm of Rashbass (1961). For example, Figure2A shows target and eye position and speed traces for a trial that presented temporalward target motion to a control monkey viewing through the right eye. About 100 msec after the onset of target motion, the monkey accelerated his eyes smoothly to match the target speed of 15 deg/sec. Because the target started to the left of fixation and moved to the right, it was nearly centered in the visual field as pursuit was initiated. As a result, the trial included only a single small catch-up saccade that occurred well after accurate pursuit had been established. Figure 2, B and C, illustrates the nasal–temporal asymmetry in the initiation of pursuit for monkey SY viewing through his left eye. In these trials, the target started at the point of fixation. For nasalward target motion (rightward), the initiation of pursuit consisted of a brisk eye acceleration that brought eye speed rapidly up to target speed, which was 15 deg/sec (Fig. 2B). For temporalward target motion (leftward), however, the initial eye acceleration was weak and was interrupted by a saccade that allowed the eye to catch up with the position of the target (Fig. 2C). Throughout the trial, eye speed remained much lower than the target speed of 15 deg/sec. These trials also show one strategy that we introduced during experiments on monkey SY to improve the quality of the data. After the target had moved at constant speed for 1 sec, it stopped for 700 msec to allow the monkey to fixate the target and complete the trial successfully even if he had been unable to generate strong smooth pursuit eye movements. This strategy allowed us to relax the fixation requirements during target motion so that the monkey was not punished for his inability to keep up with the target, but at the same time permitted us to retain excellent control over the monkey’s behavior by requiring fixation of a target at the end of the trial.
Fig. 2.

Typical examples of the initiation and maintenance of pursuit for step-ramp target motion in control and strabismic monkeys. In each panel, the dashed traces show target speed and position, and the solid traces show eye speed and position. A, One example of the initiation of pursuit for viewing through the right eye in a monkey with normal eye alignment. Target motion is rightward, which is temporalward with respect to the viewing eye. B, One example of the initiation of pursuit from a strabismic monkey (SY), viewing nasalward (rightward) target motion through his left eye. C, A similar example from the same strabismic monkey (SY), viewing temporalward (leftward) target motion through his left eye.Upward deflections of the traces indicate rightward target and eye motion (arrows).
The character of the nasal–temporal asymmetry in the initiation of pursuit eye movements by the strabismic monkeys is illustrated in more detail in Figure 3. Nasalward and temporalward pursuit in normal monkeys is relatively symmetrical, as shown in Figure3A. The target moved rightward or leftward at 5, 10, or 15 deg/sec, indicated by the dashed traces. The solid traces are averaged eye speed responses for a monkey with normal eye alignment, viewing through the right eye. Approximately 100 msec after the onset of target motion, the eye accelerated rapidly to the left or right depending on the direction of target motion. For both leftward and rightward target motion, eye speed rose to a sustained level close to the target speed, regardless of target direction. The nasal–temporal asymmetry in the initiation of pursuit in the strabismic monkeys is summarized in Figure3B–E, for viewing through each eye in each monkey. Each panel contains 6 averages of eye speed (solid traces) aligned on the onset of target motion for targets that started at 3 deg eccentric and moved to the left or right at speeds of 5, 10, and 15 deg/sec (dashed traces). Although the magnitude of the asymmetry varied in the different panels, pursuit was consistently stronger for rightward target motion when the monkeys viewed through the left eye (Fig. 3B,D) and for leftward target motion when the monkeys viewed through the right eye (Fig. 3C,E). In each case, the asymmetry is evident both in the early eye acceleration at the onset of pursuit and in the sustained eye speed toward the end of each record. In monkey PW, the asymmetry was larger when he viewed through the right eye, and in monkey SY it was larger when he viewed through the left eye. In each monkey, temporalward target motion failed to elicit significant eye acceleration with viewing through the eye with the greater asymmetry.
Fig. 3.
Averaged pursuit responses of control and strabismic monkeys during step-ramp target motion at different speeds. In each panel, the dashed lines show the steps of target speed from 0 to 5, 10, and 15 deg/sec in each direction, and thesolid lines show averages of the evoked eye speed for at least 10 trials. A, Data from a normal monkey viewing through his right eye. B–E, Data from the two strabismic monkeys, viewing through each eye. B, Monkey SY, left eye viewing. C, Monkey SY, right eye viewing.D, Monkey PW, left eye viewing. E, Monkey PW, right eye viewing. Note the small offsets in eye speed at the start of the trace, which were caused by the small nasalward speed associated with the latent nystagmus. Upward deflections of the traces indicate rightward target and eye speed (arrows).
Figure 3 illustrates two additional features of the eye movements of the strabismic monkeys. First, in each panel, the baseline eye speed before the onset of pursuit is offset slightly from zero. This is due to the small nasalward drift caused by the latent nystagmus illustrated in Figure 1B. The nasalward speed of the slow phase of the nystagmus averaged 0.7, 1.0, 2.4, and 1.4, deg/sec in the first 100 msec of the records shown in Figure 3, B, C,D, and E, respectively. Second, the traces for nasalward target motion show an initial overshoot of the target speed followed by a slowing to match target speed. Thus, whereas temporalward target motion elicited weak pursuit, nasalward target motion elicited unusually strong pursuit for a given target speed.
Topographic organization of the nasal–temporal pursuit asymmetry
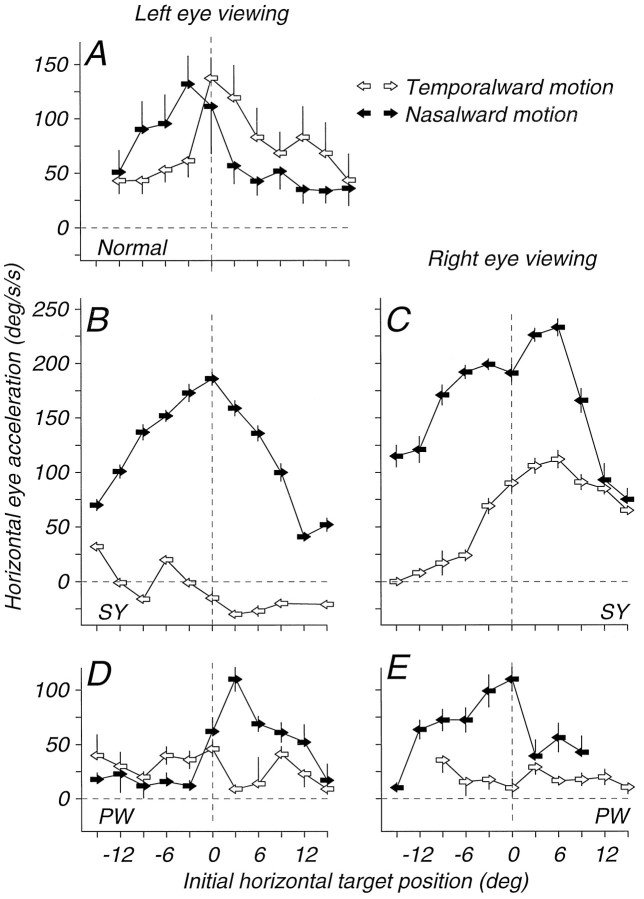
The data in Figure 3 document the pursuit behavior of normal and strabismic monkeys for targets whose motion swept across only the central 3 deg of the visual field. To compare the pursuit of strabismic monkeys with the responses of cortical neurons, we wished to know how the pursuit asymmetry varied over the wider range of visual field locations represented by the receptive fields of the MT neurons whose properties we describe in the second part of the paper. The results are shown in Figure 4.
Fig. 4.
Dependence of the nasal bias in the initiation of pursuit on target direction and visual field location. Eachpoint plots the averaged eye acceleration during the first 80 msec (A, control monkeys) or 100 msec (B–E, strabismic monkeys) of pursuit, for target motion at 15 deg/sec across the visual field position indicated on the abscissa. As shown in the upper right, the arrows used as symbols indicate the direction of target motion; filled arrows indicate nasalward target motion, and open arrows indicate temporalward target motion. A, Data for viewing through the left eye of a normal monkey. B–E, Data from the two strabismic monkeys, plotted separately for viewing through the left and right eyes. B, Monkey SY, left eye viewing. C, Monkey SY, right eye viewing. D, Monkey PW, left eye viewing. E, Monkey PW, right eye viewing. Positive values of eye acceleration indicate eye acceleration in the direction of target motion. Negative values on the ordinate indicate positions in the visual field for which temporalward target motion initiated nasalward pursuit. Error bars show standard deviations as described in Materials and Methods.
To study pursuit for targets across a wider range of visual field positions, we used target motions that consisted of an initial step to a particular position in the visual field followed by a smooth “ramp” of motion. Targets stepped to locations up to 18 deg eccentric along the horizontal meridian and moved toward or away from the position of fixation at 15 deg/sec. Each point in Figure4A plots the eye acceleration in the first 80 msec of pursuit as a function of the initial position of the tracking target. Although we typically used a 100 msec analysis interval, a slightly shorter analysis interval was used for control monkeys because they tended to end the interval of pursuit prematurely by emitting saccades.
As we have reported previously (Lisberger and Westbrook, 1985), eye acceleration for control monkeys was higher for target motion toward the position of fixation (shown by the vertical dashed line) than for target motion away from the position of fixation. In addition, the initiation of pursuit was symmetrical, so that eye acceleration depended on both the initial target position and the direction of motion with respect to the position of fixation, but did not depend on whether the target moved nasally (filled arrows) or temporally (open arrows) with respect to the viewing eye. Thus, the data in Figure 4A show a “toward-away” asymmetry: a nasalward pursuit bias for targets that started in the left visual hemifield and a temporalward pursuit bias for targets that started in the right hemifield. Similar data were obtained on a second monkey with normal eye alignment.
Figure 4B–E summarizes the nasal–temporal asymmetries in the initiation of pursuit along the horizontal meridian in each of the two strabismic monkeys when they were tested using the same paradigm. Each graph plots eye acceleration in the first 100 msec of pursuit as a function of the initial position of the moving target. The initial eye acceleration was generally larger for nasalward target motion (filled arrows) than for temporalward target motion (open arrows). The data from monkey SY provide the clearer picture, partly because he generated much larger eye accelerations than did PW. For viewing through the left eye (Fig. 4B), rightward target motion evoked large values of initial eye acceleration, whereas leftward target motion evoked very small eye accelerations. For some initial target positions, eye acceleration was in the opposite direction to the target motion (“wrong-way pursuit”), and is plotted as negative. For viewing through the right eye (Fig. 4C), there was also a clear nasal–temporal asymmetry, but temporalward (rightward) target motion evoked eye acceleration in the correct direction. In monkey PW, there was a nasal–temporal asymmetry across all initial target positions for viewing through the right eye (Fig. 4E). For viewing with the left eye, the asymmetry was apparent only in the right hemifield (Fig. 4D); in the left hemifield temporalward pursuit acceleration generally exceeded nasalward acceleration. The plots in Figure 4B–Eare strikingly similar to those presented by Tychsen and Lisberger (1986a) for humans with early-onset strabismus. The abnormalities in the shape of the curves relating eye acceleration to initial target position are similar to theirs and in the most extreme cases, both the humans and our monkeys exhibited wrong-way pursuit.
Interpretation of the wrong-way pursuit in animals with latent nystagmus is not necessarily straightforward. It may be that the wrong-way pursuit is merely another manifestation of the latent nystagmus when the pursuit target is rendered less salient by a large eccentric target position. Two observations make this unlikely. First, wrong-way pursuit was seen only for left eye viewing in monkey SY whereas latent nystagmus was evident for each eye of each monkey. Second, the wrong-way pursuit in monkey SY (Fig. 4B) was largest for temporalward target motion toward the position of fixation from 3 deg eccentric, normally the most effective and salient of initial positions for pursuit targets.
Figure 4 suggests that the nasalward pursuit biases were most pronounced in the nasal hemifield of each eye (the right hemifield of the left eye and the left hemifield of the right eye). To analyze this possibility, we combined the data from the one normal and two strabismic monkeys, and separately the data of Tychsen and Lisberger (1986a) from one normal and four strabismic human subjects. The data for each viewing eye were first normalized to the highest eye acceleration observed for targets presented in the central 3 deg of that eye’s visual field. The data were then reordered into a coordinate system based on nasal and temporal position and nasalward versus temporalward motion, and averaged. Figure 5,A and B, shows these normalized averages for nasalward and temporalward target motion for the monkeys; Figure 5,C and D, shows similar averages for the humans. In each case, the control subjects (open symbols) and strabismic subjects (filled symbols) had very similar values of normalized eye acceleration in the temporal visual hemifield (unshaded) and pronounced differences at the fovea and in the nasal visual hemifield (shaded). Moreover, there were two distinct components to the abnormalities: in the nasal hemifield, strabismics’ pursuit of temporalward motion was reduced relative to control subjects (Fig. 5B,D), whereas strabismics’ pursuit of nasalward motion was enhanced (Fig.5A,C).
Fig. 5.
Normalization of the relationship between eye acceleration at the initiation of pursuit and the visual field position of the moving target for monkeys (A, B) from our study and for the humans (C, D) reported by Tychsen and Lisberger (1986a,b). Each panel plots normalized eye acceleration as a function of initial target position in nasal–temporal coordinates. The hatched area of each graph indicates target positions in the nasal visual hemifield (temporal hemiretina). All data from all eyes have been transformed so that the responses to nasalward motion are shown in Aand C and plotted as arrows pointing to the left. Responses to temporalward motion are analyzed in B and D and plotted as arrows pointing to the right. Open arrows show responses from subjects with normal eye alignment, and filled arrows show data from strabismic subjects.
Directional asymmetry in pursuit for target motions in two dimensions
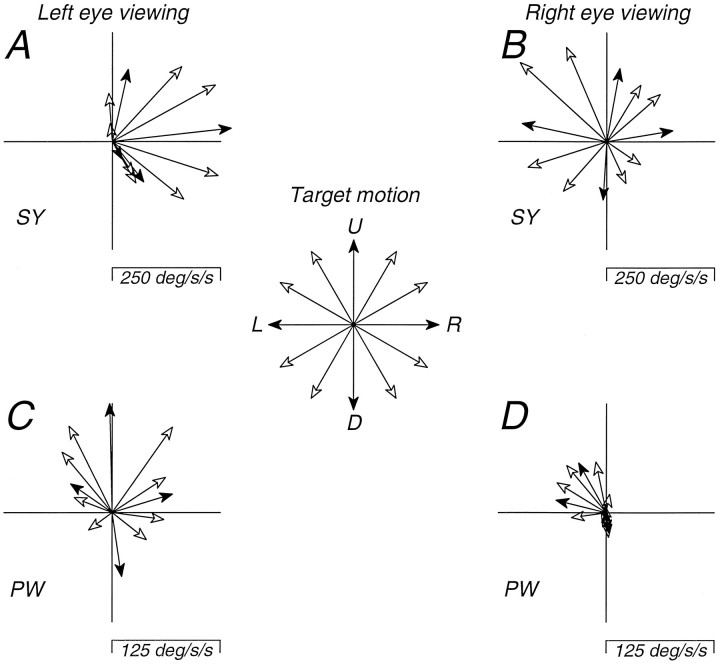
The experiments presented so far concentrated on pursuit along the horizon. To explore the possibility that these monkeys showed pursuit deficits for other directions of motion, we measured the initiation of pursuit for a number of directions. For this experiment, the target started at straight ahead gaze and moved at 15 deg/sec in one of 12 directions corresponding to the 12 hr on the clock (target motion shown at the center of Fig. 6). The data are summarized in Figure 6 as vector plots in polar coordinates, where each vector indicates the direction and amplitude of the first 100 msec of eye acceleration. The direction of target motion is indicated by the letters “R,” “U,” “L,” and “D” indicating the vectors that correspond to rightward, upward, leftward, and downward target motion, respectively. This experiment revealed that the asymmetry in pursuit had a vertical component in both monkeys. For monkey SY, the left eye had the largest asymmetry and target motion that was nasalward with a small upward component evoked the largest eye accelerations (Fig. 6A). When SY viewed with the right eye (Fig.6B), target motion that was upward and nasalward also evoked the largest eye acceleration. When monkey PW viewed with the left eye (Fig. 6C), there was a clear bias favoring targets that had a nasalward (rightward) component of target motion, but upward target motion evoked the largest initial eye acceleration while downward and temporalward (leftward) target motion evoked the smallest initial eye acceleration. When PW viewed with the right eye (Fig.6D), only targets with a nasalward (leftward) component of motion evoked significant initial eye accelerations. These results suggest that the pursuit anomalies in these monkeys involve directions other than horizontal. Thus, the nasal–temporal pursuit asymmetry seen for target motion along the horizon is probably better regarded as a distortion of the normally uniform directional profile for the initiation of pursuit eye movements (Lisberger and Pavelko, 1989).
Fig. 6.
Dependence of pursuit responses in strabismic monkeys on the direction of target motion. Each vectorrepresents the direction and magnitude of the eye acceleration during the first 100 msec of pursuit elicited by a step-ramp pursuit target whose motion (15 deg/sec) began at the center of gaze and proceeded in 1 of the 12 “clock-face” directions indicated by the central rosette. A, Monkey SY, left eye viewing.B, Monkey SY, right eye viewing. C, Monkey PW, left eye viewing. D, Monkey PW, right eye viewing. Filled arrowheads indicate the cardinal directions.
Lack of nasal–temporal asymmetry for image motion presented during pursuit
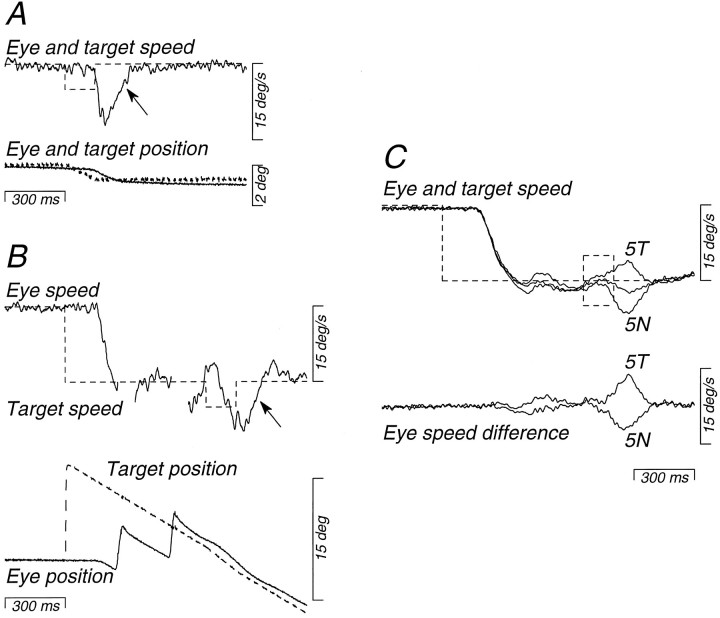
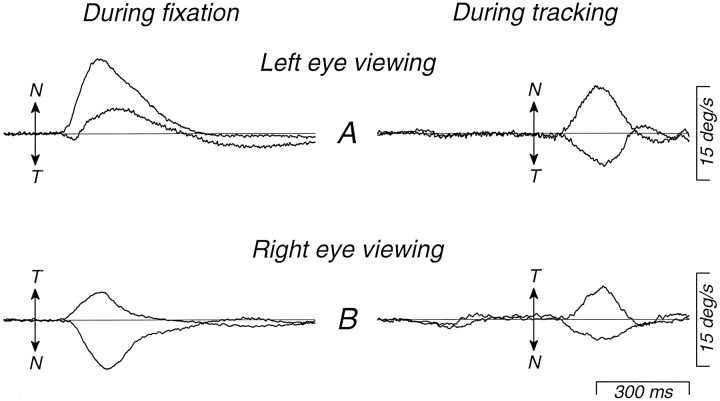
The data presented so far can be explained in two ways. The nasal–temporal asymmetry in pursuit could reflect either a nasal–temporal asymmetry in visual motion processing, or an inability to use visual motion signals to initiate temporalward pursuit. To distinguish these alternatives, we compared responses to brief nasalward or temporalward image motions imposed either duringfixation of a stationary target, when the pursuit system had not yet been activated, or during tracking of nasalward target motion, when the pursuit system had already been engaged. The target motions we used are illustrated in Figure 7 for an experiment in which the right eye was viewing so that leftward target motion (downward deflection of the traces) was nasalward. On half of the trials, the monkey fixated a stationary target and at an unexpected time the target moved nasalward or temporalward at 5 deg/sec for 150 msec; Figure 7A shows a nasalward trial. The perturbation consisted of a brief ramp of target position that provided a brief pulse of target speed (dashed trace). In the other half of the trials, the monkey tracked nasalward target motion at 15 deg/sec and the target speed either increased or decreased by 5 deg/sec for 150 msec, or remained at 15 deg/sec. Figure 7B shows a trial in which nasalward velocity increased. It is difficult to see the perturbation in the target position traces of Figure 7B because the increment from 15 to 20 deg/sec causes only a brief and small increase in slope. However, the perturbations imposed during fixation and pursuit were identical and, because the perturbations were brief, they were over before the monkey could respond to them. The monkey was tracking or fixating the target accurately at the time the perturbations were imposed, so that the perturbations produced nearly the same retinal image motion under the very different initial conditions of fixation and tracking. We did not devise this experiment until after the eye coils had been removed from monkey PW, and it was performed only on monkey SY.
Fig. 7.
Target motions used to demonstrate a difference in the nasal bias for responses to brief perturbations of target motion, depending on whether the perturbations were presented during fixation (A) or ongoing pursuit (B). Data are for viewing with the right eye by strabismic monkey SY. Dashed traces show target speed and position, and solid traces show eye position and speed. Perturbations were provided by brief pulses of target speed with amplitudes of 5 deg/sec and durations of 150 msec. A, Example of the response to a nasalward perturbation of target motion presented during fixation. B, Example of a response to the same nasalward perturbation presented during pursuit of target motion at 15 deg/sec. Interruptions of the eye speed trace occur where saccades were excised from the record. The arrows inA and B indicate the responses to the pulses. C, The upper traces show averaged eye and target speed records from interleaved sets of trials in which the speed pulses were nasalward (5N), temporalward (5T), or absent. The lower traces show eye speed difference records, obtained by subtracting averaged eye speed during the two types of “pulse” trials from averaged eye speed on “no-pulse” trials. Upward deflections of the traces show rightward or, in this case, temporalward eye and target motion.
Figure 7C shows how we analyzed the results. The traces at the top of Figure 7C show the average eye speed evoked by three target motions that all began with a step of target speed from zero to 15 deg/sec nasally. In two cases, the target was perturbed at an unpredictable time after motion onset, as described above. In the third case, control trials, there was no perturbation of target speed. Both temporalward and nasalward perturbations (5T and5N, respectively) caused eye velocity to deviate from the control trials. To isolate the response to the nasalward and temporalward perturbations of target speed, we subtracted the average eye speed without the perturbation from each of the two averages obtained with perturbations. This yielded traces of “eye speed difference” (bottom of Fig. 7C), which reveal brief responses to the perturbations on a baseline that is relatively flat and close to zero. When analyzing the responses to perturbations of target motion during fixation, we similarly subtracted the eye velocity during control fixation trials, which had a small nasalward value because of the latent nystagmus in the strabismic monkeys.
Figure 8 shows averages of the time course of eye speed evoked by perturbations of target motion during fixation (left) and during nasalward pursuit (right) for all the experiments we did on monkey SY. When the left eye was viewing (Fig. 8A), the nasal–temporal motion asymmetry was so large during fixation that the eye speed responses to leftward (temporalward) perturbations had large components in the wrong direction. During pursuit, in contrast, the responses to temporalward perturbations were in the correct direction and the amplitudes of the responses to nasalward and temporalward perturbations were quite similar, although a mild nasal–temporal motion asymmetry persisted. When the right eye was viewing (Fig. 8B), the asymmetry was much milder during fixation, so that the responses to temporalward perturbations were in the correct direction and about half as large as those to nasalward perturbations. During pursuit, however, the responses to temporalward perturbations were at least as large as those to nasalward perturbations. On average, the nasal–temporal motion asymmetry seen in this monkey’s eye movements at the initiation of pursuit was eliminated. To quantify these data, we measured the average eye speed (for fixation trials) or difference eye speed (for tracking trials) for the interval between 100 and 300 msec after the onset of the motion perturbation, and calculated the nasal response bias as (sn − st)/(sn + st), where sn andst were the difference eye speeds for nasalward and temporalward perturbations, respectively. These values are positive for nasalward biases, negative for temporalward biases, and zero for symmetric responses. For left eye viewing, the nasal response bias was 1.68 during fixation and 0.23 for perturbations delivered during tracking. For right eye viewing, the bias was 0.27 during fixation and 0.03 for perturbations delivered during tracking. Thus these data show that monkey SY’s pursuit asymmetry was largely abolished when image motion was presented during tracking, suggesting that the asymmetry was not due to anomalous visual motion processing.
Fig. 8.
Averaged eye speed difference response of strabismic monkey SY to nasalward and temporalward pulses of target speed, presented either during fixation (left traces) or during tracking (right traces). Thearrows indicate the nasalward (N) or temporalward (T) direction and the time of onset of the 150 msec perturbations of target speed. For the records on the left (fixation), eye speed difference was obtained by subtracting the speed of the latent nystagmus, estimated by computing the mean eye speed from the first 100 msec of the record. For the records on the right (tracking), eye speed difference was obtained as in Figure 7. A, Left eye viewing. B, Right eye viewing.
Response properties and eye dominance of neurons in MT
The visual response properties of units in MT of the strabismic monkeys were mostly indistinguishable from those recorded in control animals. Unit and background activity was brisk and directionally selective, and showed evidence of the usual columnar sequence of preferred directions characteristic of MT (Albright, 1984). Of 414 MT units recorded from 6 strabismic monkeys, 359 (87%) were classified as directionally selective (unresponsive to stimuli moving in their nonpreferred direction), 27 (7%) were directionally biased (responsive, but more weakly to stimuli moving in their nonpreferred direction), and 28 (7%) were nondirectional. By comparison, of 218 units recorded from the 8 control monkeys, 180 (83%) were directionally selective, 19 (9%) were directionally biased, and 12 (6%) were nondirectional. In both strabismic and control animals, we encountered a few units that could not be reliably driven by visual stimuli, but these seemed equally rare in both groups of monkeys.
The most striking difference in response properties between neurons in strabismic and control animals was in their binocular interaction. As reported previously (Zeki, 1974b, 1978; Maunsell and Van Essen, 1983a,b), the MT neurons we recorded in control monkeys were almost invariably binocularly driven. Of the 218 cells we recorded from control monkeys, 97% were classified in ocular dominance groups 3, 4, or 5 because they were driven well through either eye (Fig.9A). In contrast, the eye dominance distributions for 416 neurons recorded from the left and right hemispheres of the 6 strabismic monkeys (Fig. 9B) show a strong tendency to monocularity. The proportion of binocularly driven neurons in these animals was sharply reduced so that only 26% (107/416) of the neurons were in eye dominance groups 3–5. Moreover in the left hemispheres, 62% (98/157) of the cells recorded (Fig.9B, left) strongly preferred the contralateral eye (dominance groups 1 and 2). Only 15% (23/157) strongly preferred the ipsilateral eye (dominance groups 6 and 7). In contrast, in the right hemispheres (Fig. 9B, right), nearly equal numbers of units strongly preferred each eye (contralateral eye: 35%, 91/259; ipsilateral eye: 37%, 97/259). The contralateral-eye bias in the left hemisphere is presumably related to the fact that it was ipsilateral to the deviated eye.
Fig. 9.

Distributions of eye dominance for neurons recorded from MT in control and strabismic monkeys. A, Data from both hemispheres of normal monkeys. B, Data from the left and right hemispheres of the 6 strabismic monkeys.C, Data from the left and right hemispheres of monkey AP, whose strabismus was created by toxin injection. D, Data from the left and right hemispheres of the 5 monkeys whose strabismus was created surgically. The eye dominance scale is that ofHubel and Wiesel (1968), with neurons in group 1 receiving input only from the contralateral eye, neurons in group 4 receiving equal input from both eyes, and neurons in group 7 receiving input only from the ipsilateral eye.
In strabismic monkeys, it is also important to note that as a result of the loss of binocular inputs, the strength of visual input fromeither eye and particularly from the ipsilateral eye was markedly reduced. In normally reared monkeys, essentially all MT neurons receive effective input from each eye. In the strabismic animals, the contralateral eye had effective input (dominance groups 1–5) to only 73% of MT neurons (164/226). The ipsilateral eye had substantially less effective input, to only 42% of MT neurons (94/226).
When neurons had binocular inputs, even unequal ones, preferred directions were usually as similar in the two eyes as they were in controls. A few cells had opposite preferred directions in the two eyes, as is occasionally seen in MT in normal animals (Zeki, 1974a).
We noticed that neurons of similar eye preference were clustered together in MT. Neurons often tended to have similar eye dominance for distances between 0.25 and 1 mm as the electrode was driven along a track. We evaluated the regularity of the observed sequences of eye preference in a subset of our electrode penetrations with a runs test. We used the test only on data from tracks or portions of tracks in which more than 12 neurons were recorded, and within which the gap between adjacent recording sites did not exceed 0.15 mm. Ten electrode penetrations from 4 monkeys met these criteria. The runs test showed significant regularity on 9 of the 10 (p < 0.005 for 7 of the 9, p < 0.01 for the other 2). It would go beyond the data to assert that these clusters were truly columnar in structure, but it is perhaps noteworthy that they were of a spatial scale that is similar to that of the eye dominance columns in the primary visual cortex. Because almost all neurons in MT are driven strongly from both eyes in monkeys with normal eye alignment, there is no sign of a regular pattern of eye dominance in our control data.
The eye dominance histogram in monkey AP was different from those of the other monkeys, perhaps because of the differences in his treatment. Instead of surgery on the eye muscles at an early age, monkey AP’s treatment had a relatively late onset and consisted of an injection of botulinum toxin that caused esotropia transiently followed by permanent exotropia. The histograms for monkey AP (Fig. 9C) showed a much higher proportion of units in eye dominance groups 3–5 (49%, 59/121) and did not show the shift in dominance toward the contralateral eye that was evident in the left hemispheres of the other monkeys. Removing AP’s data from the grouped histograms did not alter the general eye dominance findings: the histograms in Figure9D, for the 5 surgically strabismic monkeys alone, are not materially different from those shown in Figure 9B for the entire group.
Direction and speed selectivity of neurons in MT
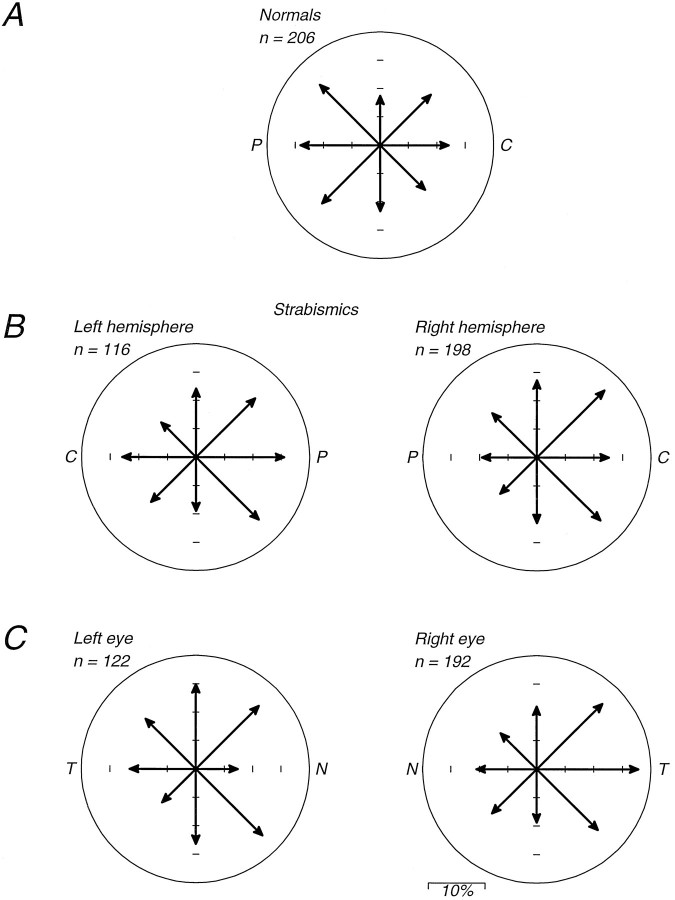
In monkeys reared with normal eye alignment, there is a tendency for MT neurons to prefer movements away from the center of gaze, and this tendency is more pronounced in the representation of the peripheral visual field (Albright, 1984). Figure10A shows that this effect is subtly apparent in the distribution of direction preferences for 206 directionally selective or directionally biased neurons we recorded from control monkeys. In these plots, the data recorded from both hemispheres have been folded together and are drawn as though all were collected from the right hemisphere. The length of each vector indicates the number of cells having a given preferred direction. The arrow pointing to “C” in Figure 10A indicates motion toward the vertical meridian, and the arrow pointing to “P” indicates motion away from the vertical meridian. Because most of the cells in our sample had receptive fields near the horizontal meridian, motion toward “C” or “P” indicates motion toward the center of the visual field or the periphery, respectively. Overall, in normal monkeys, there was a slight preponderance of cells preferring motion toward the periphery. If we neglect the 42 cells preferring directions within ±22.5 deg of vertical in Figure 10A (as we will do throughout this section to quantify motion asymmetries along the horizon), 44% (90/206) preferred motion toward the periphery, and 36% (74/206) preferred motion toward the center of the visual field.
Fig. 10.
Distributions of direction preference for neurons recorded from MT in control and strabismic monkeys. The vectors represent the proportion of neurons preferring directions within ±22.5 deg of the indicated direction. A, Data from normal monkeys. Data from both hemispheres have been combined and plotted as though they had been recorded from the right hemisphere. Theright arrow therefore indicates the number of cells with preferred directions toward the vertical meridian (centralward, labeledC); the left arrow indicates the number of cells with preferred directions away from the vertical meridian (peripheralward, labeled P). B, Data from the 6 strabismic monkeys, plotted separately for the left and right hemispheres. Note that centralward (C) and peripheralward (P) directions are now mirror-reversed for the two hemispheres. C, Distributions of direction preference for neurons with significant responses for stimulation of the right or left eye, plotted separately for each eye; significant responses were taken to be those in eye dominance groups 1–5 for the contralateral eyes and groups 3–7 for the ipsilateral eyes. Nasalward and temporalward directions are indicated for each eye by the labelsN and T, respectively.
Figure 10B shows that strabismus had no large effects on the distributions of direction preference for cells in MT. The data are presented separately for each hemisphere, and each plot is again marked with “C” and “P” to indicate preferred directions toward the center or the periphery of the visual field. However, the distributions suggest some subtle anomalies. Cells in the left hemisphere, as expected, tended to prefer motion toward the periphery (46 vs 33%), whereas cells in the right hemisphere had a preference for motion toward the center of the visual field (45 vs 30%).
To determine whether the nasal–temporal motion asymmetry in the pursuit of strabismic monkeys has a correlate in the direction preferences of MT neurons, we separated our data for strabismics according to the preferred eye for each cell and analyzed direction preference in relation to temporalward versus nasalward motion (indicated by “T” and “N” on the axes of Fig. 10C). Strabismus caused no major nasal–temporal directional asymmetry for the cell population analyzed in this way. All preferred directions were present in substantial numbers in the populations of cells driven by either eye. Cells preferring the left eye had a slight preference for nasalward motion (40 vs 32%), whereas cells preferring the right eye had a more marked preference for temporalward motion (49 vs 30%). Although the precise bias varied from animal to animal, neurons dominated by the right eye had a stronger temporalward bias than neurons dominated by the left eye in 5 of the 6 animals. The absence of a nasalward directional bias in the MT neurons contrasts sharply with presence of such an bias in the pursuit data shown in Figures 3, 4, 5, 6 for monkeys PW and SY. In Figure 6 we documented a pattern of vertical pursuit imbalance in the strabismic monkeys. Like the nasal–temporal asymmetry, this imbalance was not associated with an uneven distribution of neuronal direction preferences. Of the 75 neurons preferring directions within ±22.5 deg of vertical, 41 (13% of the total) preferred upward motion and 34 (11%) preferred downward motion.
It is possible that pursuit anomalies could arise even if all preferred directions of motion were represented in the visual cortex, if neurons preferring some directions had abnormal response properties. We noticed no difference in the vigor of responses for neurons that preferred different directions, so we examined the neurons’ speed preferences to see if neurons having temporalward direction preferences were abnormal in this respect. Figure 11 plots the speed and direction preferences of 276 neurons from 6 strabismic monkeys. Each point plots the data for one cell; the angular coordinate gives the preferred direction, and the radial coordinate gives the preferred speed. We plot data for all cells regardless of eye preference, left–right reversing data for cells preferring the right eye so that the coordinates are nasal–temporal, as if all cells preferred the left eye (a comparable manipulation tagging cells by hemisphere yielded a similar result). There was no discernible inhomogeneity of the representation of direction and speed: all directions of motion were uniformly represented and all preferred speeds were represented for all directions of motion. Data from individual animals were more variable, because the samples in each monkey were smaller, but none showed reliable inhomogeneity. We have similarly analyzed our data to see whether there were differences between groups of neurons preferring different directions or different eyes with respect to speed cutoffs, narrowness of direction tuning, or overall responsiveness. The results were uniformly negative.
Fig. 11.
Polar scatter plot showing the distribution of preferred target speeds and directions for MT neurons recorded in the 6 strabismic monkeys. Each point shows the responses of one cell; the angular coordinate indicates the preferred direction, and the radial coordinate represents the preferred speed (note the logarithmic scale). Data from both hemispheres are combined as if all neurons responded to stimulation of the left eye, so that directions can be defined as nasalward or temporalward.
Direct comparison of MT responses and pursuit behavior in two monkeys
Figures 8, 9, 10 reveal no relationship between pursuit deficits and MT neuronal properties when the data are pooled across animals. However, the pursuit data in Figure 4 reveal substantial variation in the pursuit deficits from monkey to monkey, eye to eye, and hemifield to hemifield. We took advantage of the fact that we made both pursuit and unit recordings from monkeys SY and PW to compare directly the responses of cells in MT and pursuit behavior in these 2 monkeys.
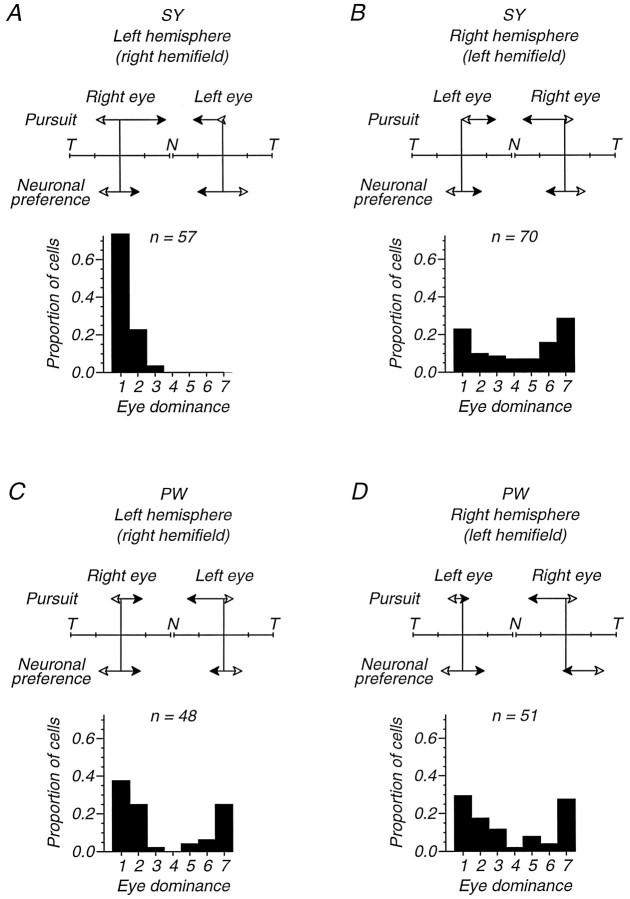
Figure 12 presents this comparison, showing our data on pursuit, neuronal direction preference, and eye dominance for each eye and hemisphere in monkeys SY and PW. Consider Figure12A, which compares the results of MT recordings in the left hemisphere of monkey SY with pursuit experiments that provided visual inputs to that hemisphere by using targets in theright visual hemifield. The pairs of vectors labeled “Pursuit” summarize the nasal–temporal asymmetry in the initiation of pursuit for monocular viewing of these targets through each eye. The length of each vector represents the eye acceleration for nasalward and temporalward target motion (filled and open arrowheads, respectively). These show a mild but clear nasalward bias for target motion in the right hemifield of the right eye, and a more profound nasalward bias—with wrong-way pursuit for temporalward target motion—in the right hemifield of the left eye. The pairs of vectors labeled “Neuronal preference” summarize the direction preferences of neurons in this hemisphere that had effective input from each eye. The length of each vector indicates the proportion of cells that preferred nasalward or temporalward target motion (filled and open arrowheads, respectively). There was no bias for nasalward or temporalward motion for neurons in this hemisphere that responded to the right eye; this conclusion cannot apply to the left eye because only 2 cells had effective input from that eye. Finally, the ocular dominance histogram shows the representation of each eye in SY’s left hemisphere. Strikingly, we found no cells that preferred the left eye in this hemisphere, although we studied 57 cells and a larger number of multiunit sites in 4 microelectrode penetrations. Thus, Figure12A shows that for inputs transmitted through the left MT of monkey SY, the nasal–temporal asymmetry in pursuit was most profound for the eye that contributed less input.
Fig. 12.
A three-way comparison of pursuit strength, neuronal direction preference, and neuronal eye dominance for the two strabismic monkeys that were used for both pursuit and MT recordings. Each panel summarizes the pursuit and MT neuronal responses for visual signals in one visual hemifield (and thus one hemisphere) of one of the monkeys. Within each panel, pursuit and direction preference data are presented separately for each eye. The double-headed vectors at the top of each panel indicate the directional bias in pursuit and in the preferences of MT neurons. Theupper vector pairs (labeled Pursuit) summarize the average eye acceleration data from Figure 4, combined for target eccentricities of 3, 6, and 9 deg in the hemifield appropriate to the indicated hemisphere. The accelerations are normalized so that the ends of the T–N–T scale correspond to the largest eye acceleration value obtained in that monkey (i.e., the peak values on the corresponding plots in Fig. 4). T indicates temporalward eye acceleration, and N indicates nasalward eye acceleration. Open arrowheads indicate responses to temporalward target motion, and filled arrowheadsindicate responses to nasalward target motion. The lower vector pairs (labeled Neuronal preference) show the proportions of neurons that received effective input from the indicated eye and preferred directions with a temporalward (T,open arrowheads) or nasalward (N,filled arrowheads) component. For the contralateral eye, “effective input” was assumed for neurons in eye dominance groups 1–5; for the ipsilateral eye, we used groups 3–7. Neurons preferring directions within ±22.5 deg of vertical are excluded. The ends of theT–N–T scale correspond to 100% of the direction-selective neurons for the indicated eye and hemisphere. At least 20 neurons contribute to each vector pair, except for the left eye/left hemisphere of monkey SY (2 neurons) and the left eye/left hemisphere of monkey PW (11 neurons). The eye dominance distributions are conventional. A, B, Data from the two hemispheres of monkey SY. C, D, Data from the two hemispheres of monkey PW.
The other panels of Figure 12 make similar comparisons of neuronal properties in one hemisphere with pursuit behavior elicited by targets presented to the corresponding visual hemifield. With the exception of targets in the left hemifield of the left eye of monkey PW (Fig.12D), every pair of “Pursuit” arrows shows a nasal bias in the initiation of pursuit. In contrast, none of the “Neuronal preference” arrows show a nasal bias in the direction preferences of neurons in MT. The only clearly biased neuronal preference in the entire dataset was for the MT cells in the right hemisphere that responded to stimulation of the right eye of monkey PW, which favored temporalward motion (Fig.12D). We conclude that even using unpooled data, there was no association of the nasalward bias in pursuit with any neuronal direction preference in MT.
The data in Figure 12 do, however, suggest a different basis for the pursuit biases. Figure 12B–D shows in milder form the relationship evident in Figure 12A: the pursuit asymmetry for targets in a given hemifield tended to be larger for the eye that contributed the weaker input to MT in the corresponding hemisphere. In three cases (Fig.12A,C,D), the pursuit bias was larger for targets presented to the ipsilateral eye, and that eye was more weakly represented in the eye dominance distribution of MT neurons. In the fourth case (Fig. 12B), the pursuit bias was larger for targets presented to the contralateral eye, and in this case that eye was also more weakly represented in the eye dominance distribution. This association suggests that although the pursuit biases are not explained by the motion signaling properties of MT neurons, the biases are associated with abnormalities in the strength of the two eyes’ inputs to these neurons.
DISCUSSION
Our pursuit measurements show that strabismic monkeys, like strabismic humans, exhibit systematic biases in pursuit eye movements that favor responses to targets moving nasalward with respect to the viewing eye. As in humans, the monkeys’ biases were sometimes so severe as to cause “wrong-way” pursuit for targets moving temporalward. In addition, both strabismic monkeys showed the latent nystagmus that is a consistent component of the eye movement syndrome in strabismic humans. The similarity of the eye movement syndromes in naturally strabismic humans and artificially strabismic monkeys appears to resolve the issue of whether the motion processing deficits cause the strabismus or vice versa (Tychsen, 1993). The loss of binocular alignment early in life is by itself sufficient to create replicas of the pursuit and oculomotor symptoms found in strabismic humans. Thus, it seems likely that strabismus causes the motion deficits we andTychsen and Lisberger (1986a) have reported, and correspondingly unlikely that the pursuit or motion processing deficits themselves cause strabismus.
Our measurements of the direction preference of MT units in strabismic monkeys failed to demonstrate a discernible relation between neuronal direction preference and pursuit bias. Indeed, we found a qualitatively normal distribution of direction preferences for the samples of MT cells recorded in 6 strabismic monkeys, and also in the 2 monkeys used for pursuit experiments. Thus, the neural basis for the nasalward direction bias in pursuit does not arise in the direction preferences of MT cells. This conclusion is supported by the fact that in the one strabismic monkey tested, brief nasalward and temporalward perturbations of ongoing target motion evoked symmetricchanges in eye speed, even though the same perturbations evoked a clear nasal bias if presented during fixation. We conclude that the nasal bias in pursuit cannot be understood as a simple defect in visual motion processing, including directionality, either in MT or other parts of the cortical motion system.
Although strabismus did not produce the predicted modifications in directional selectivity in MT, it did sharply reduce the degree of binocular interaction in MT neurons. This implies a substantial plasticity of cortico-cortical connections. The changes in the responses of MT cells are unlikely to be a secondary consequence of changes in the inputs to MT, even though the loss of binocular interaction in MT in the surgically strabismic monkeys is very similar to that reported previously for cells in V1 (see Crawford and Von Noorden, 1979; Wiesel, 1982). Each site in the central field of MT receives convergent input from more than 100 mm2 of V1 cortex (Van Essen et al., 1981; Maunsell and Van Essen, 1983c). If the projection from V1 to a particular portion of MT were not eye-selective, MT neurons would be binocularly driven because of this massive convergence, even though their V1 inputs were monocular. Instead, in strabismic animals, it is clear that local clusters or columns of MT neurons receive eye-specific inputs from neurons in V1 and elsewhere, to acquire their extreme eye dominance values. This implies that the cortico-cortical projections from V1 and V2 to MT can show the same kind of binocular plasticity as the thalamocortical projection from the LGN to V1, and suggests the intriguing possibility that suitable anatomical techniques might reveal a set of eye dominance columns in MT of strabismic monkeys.
There are, of course, other possible explanations of the nasal pursuit bias in strabismic primates. We noticed that the pursuit bias seemed most pronounced for stimuli presented in combinations of eyes and hemifields whose cortical influence had been most weakened by the strabismus. This led us to wonder whether the explanation might lie in the altered pattern of binocular inputs produced by strabismus. If this notion is correct, then the explanation almost certainly lies in parts of the cortical pursuit system that are downstream from the “pure” motion processing in area MT. We note in passing that this kind of explanation suggests that the distortions of speed perception documented by Tychsen and Lisberger (1986a) would best be considered a consequence of a response bias, rather than of a sensory anomaly.
A “downstream” explanation for the pursuit bias
Lesion studies in the cortical pursuit system have suggested a distinction between the visual motion processing for pursuit and other processing that might be more closely related to the direction of the eye movement itself. Lesions of area MT cause deficits in pursuit that can be attributed to a “motion scotoma” in the affected part of the visual field; lesions of area MST or of the “frontal pursuit area” cause deficits that are more closely related to the direction of required pursuit (Newsome et al., 1985; Dürsteler and Wurtz, 1988; MacAvoy et al., 1991). Specifically, lesions of MST cause a reduction in the sustained eye velocity during pursuit toward the side of the lesion, with or without a companion deficit in visual motion processing for pursuit. The “directional deficit” following lesions suggests that MST and the frontal pursuit area in each hemisphere have a special role in generating pursuit toward that hemisphere. MST, like MT, contains a high proportion of directionally selective neurons (Maunsell and Van Essen, 1983c; Tanaka et al., 1986). In an interesting correlation to our findings of a dissociation between direction biases in MT and pursuit of strabismic monkeys, lesions of MST cause a directional deficit even though all directions of motion are represented in the preferences of its neurons. This apparent paradox is resolved by the finding that neurons with ipsiversive direction preferences provide the outputs from MST to subcortical structures (Hoffmann et al., 1992). The frontal pursuit area may be similarly organized: all directions of pursuit are equally represented in unit responses (Gottlieb et al., 1994), but microstimulation preferentially elicits ipsiversive pursuit (Gottlieb et al., 1993).
To evaluate the idea outlined earlier, that the nasalward pursuit bias in strabismic subjects can be understood as a consequence of the abnormal ocular dominance in the outputs from MT, we now present a model of the conceptual (but certainly not anatomically exact) organization of the pursuit system. In the model (Fig.13), we consider MST and the frontal pursuit area together as the “cortical pursuit system” (CPS) and we assume that MT provides the principal visual motion signals for the CPS. Each hemiretina projects to MT in one hemisphere with weightsaxx, and each MT projects to both the right and left CPS with weights bxx. In this notation, thex’s in the subscripts indicate the site of origin and termination of each connection so that aLRindicates the crossed projection from the left eye to the right MT andbRR indicates the uncrossed projection from the right MT to the right CPS. The crossed projection from each MT (bLR and bRL) is needed to allow both hemifields of both eyes access to both the leftward and rightward cortical pursuit systems, and corresponds to the fact that the visual receptive fields of neurons in MST do not respect the vertical meridian and extend far into the ipsilateral visual field (Desimone and Ungerleider, 1986); the responses of neurons in the frontal pursuit area also do not depend on which hemifield is stimulated (MacAvoy et al., 1991).
Fig. 13.

Diagram showing a simplified flow of signals through the cortical pursuit system. The left andright sides of the diagram indicate the left and right eyes and hemispheres, respectively. At the level of the eyes, the signals are divided according to nasal and temporal hemiretina. The values of the aXX coefficients indicate the percentage of cells in each MT that receive inputs from the eye of origin. The values of the bXXcoefficients indicate the strength of connection from each MT to the “higher” parts of the cortical pursuit system (CPS), which may include area MST and the frontal pursuit area. Thearrows inside each CPS indicate that the output signals from each are driven only by elements preferring ipsilaterally directed target motion. P indicates the output of the pursuit system.
Although the inputs to each CPS include a representation of all directions of motion, their outputs are directional because they are selected to include only signals related to ipsilaterally directed target motion. This selection, which could correspond to the bias in the preferred directions of the output neurons from the CPS (demonstrated for MST by Hoffmann et al., 1992), creates a nonlinearity in the model so that the final pursuit command (P) is either CPSL orCPSR, depending on whether target motion is to the left or the right. This simple model can be reduced to equations that predict the strength of pursuit for each direction of target motion in each of the hemifields of the two eyes. For example, for leftward target motion in the left hemifield of the left eye (visual motion inputs go through the right MT), the activity in the left and right cortical pursuit systems are:
Because the target is moving to the left, the output neurons from the CPSR are not active but the output neurons from the CPSL are active and the expected pursuit is:
Similar logic allows computation of P for the seven other combinations of viewing eye, cortical hemisphere that receives the visual inputs from the stimulated hemifield, and direction of target motion:
In normal monkeys, every MT cell receives input from both eyes, so that values of the a weights are all 1.0. Thus, pursuit recordings, by establishing the values of the P’s, also establish the values of the b weights in control subjects. The last two rows of Table 2 show the values of theb weights calculated for one of the control monkeys (AB), demonstrating that the normal “toward–away” asymmetry can be produced by the model in Figure 13 if the b weights on the crossed pathways from MT to the contralateral CPS are ∼0.5 and theb weights on the uncrossed pathways from MT to the ipsilateral CPS are ∼1. For the strabismic monkeys, our MT recordings establish the values of the a weights for the strength of the inputs from each eye to MT in each hemisphere. Our pursuit recordings establish the value of the P’s for each combination of viewing eye, MT in the two hemispheres, and direction of target motion. Thus, the data provide the values of the aweights and the P’s and the equations given above make it possible to compute the b weights. These are shown separately for viewing with each eye of each strabismic monkey (SY and PW) in the first 4 rows of Table 2, and reveal that it is arithmetically possible to use the model in Figure 13 to account for the transformation from the MT responses to the pursuit we recorded in the strabismic monkeys. However, the particular values of theb weights show the assumptions that must be made to make this kind of model work.
Table 2.
Neuronal correlates of a directional pursuit asymmetry
| Monkey/Eye | bLL | bLR | bRL | bRR |
|---|---|---|---|---|
| SY, Right eye | 1.00 | 0.49 | 1.34 | 0.26 |
| SY, Left eye | −3.08 | 15.80 | 0.01 | 1.32 |
| PW, Right eye | 0.82 | 0.37 | 1.89 | 0.53 |
| PW, Left eye | 0.67 | 2.59 | 0.57 | 0.23 |
| AB, Right eye | 0.85 | 0.48 | 0.50 | 1.00 |
| AB, Left eye | 1.00 | 0.48 | 0.72 | 0.98 |
Proposed weights of the connections from MT to the cortical pursuit system (CPS) for inputs from each eye and each hemifield in the 2 strabismic monkeys (PW and SY) and 1 control monkey (AB). The weights are derived on the basis of the model architecture shown in Figure 13. For each monkey, the values of the b weights were calculated according to the equations given in the text after normalizing eye acceleration to have a maximum value of 1.0 for all eight combinations of viewing eye, hemisphere, and direction of pursuit in that monkey. For the control monkey, we assumed that the values of the aweights were all 1.0, because all units in MT of the control monkeys received inputs from both eyes.
First, comparison of the b weights for the two eyes of each monkey reveals that the strength of each of the 4 projections from MT to the CPS must be very different for inputs from the 2 eyes of a given monkey. This assumption is realizable in the strabismic monkey because most of the cells are dominated by one eye or the other and it is plausible to assume different output strengths from the cells dominated by the two eyes. In contrast, the values of the b weights were similar for the control monkey, as expected, because MT cells in control monkeys received nearly equal inputs from the two eyes. Second, to produce “wrong-way” pursuit, the value of the relevant bweight must be negative. A more realistic version of the model could prevent this problem by mutual inhibition between the right and left CPS. With subtractive inhibition, such a model reduces algebraically to the form shown in Figure 13, but can produce wrong-way pursuit without negative values of any of the b weights. Third, to account for the fact that the right hemifield of the left eye of monkey SY supported excellent nasalward pursuit without a large representation in MT, bLL must have a value that is probably too high to be physiologically realistic.
The variation in the values of these coefficients underlines the fact that any explanation for the nasal pursuit bias in terms of “downstream” abnormalities must include changes in cortico-cortical connections. To explain the very weak pursuit of temporalward motion in the nasal hemifield, for example, it is necessary to postulate a large reduction in the strength of the within hemisphere connections from MT to the ipsilateral CPS. To explain the supernormal pursuit of nasalward motion in the nasal hemifield, it is necessary to postulate an enhancement in the strength of collosal connection from MT to the contralateral CPS.
Latent nystagmus and pursuit bias
The latent nystagmus and the pursuit asymmetry both reflect a bias toward nasalward motion with respect to the viewing eye. Thus, the most parsimonious explanation is that they are manifestations of the same underlying defect. We suppose that the latent nystagmus is a secondary effect representing the response of the pursuit system to a small nondirectional increase in the firing rate of cells that receive inputs from the viewing eye. The model of Figure 13 and the values of thea weights, from our MT recordings, and the bweights (Table 2) are consistent with this idea. If we assume that during monocular viewing, there are equal, nondirectional inputs to both hemifields of one eye, then the output of the CPS contralateral to the viewing eye is always larger than the output of the CPS ipsilateral to the viewing eye. Thus, a simple comparison of the outputs of the two sides of the CPS should always drive the eyes smoothly in a nasalward direction with respect to the viewing eye.
Conclusion
Although undeniably speculative, the class of explanation represented by the model we have described is attractive because it accounts for the pursuit bias in strabismic animals without requiring MT to have an abnormal representation of the direction of motion. Instead, it links the nasalward pursuit bias to the previously documented ipsiversive pursuit bias in higher cortical pursuit areas, most notably MST. Clearly the operation of the full cortical pursuit system, as well as the effects of strabismus thereon, remain to be worked out. But we are drawn to this explanation for our results, not least because it unifies our thinking about all the effects of strabismus, by attributing its effects on pursuit eye movements to the same kind of alteration of cortical binocularity that is well known from earlier studies in other parts of visual cortex.
Footnotes
This work was supported by grants from the National Eye Institute (EY02017 to J.A.M., EY05864 to L.K., and EY03878 to S.G.L.), and by The Howard Hughes Medical Institute. We thank Jack Beusmans, Leslie Cameron, Daniel Kiper, and Juliana von Rumohr for their help with some of these experiments.
Correspondence should be addressed to Dr. Lynne Kiorpes, Center for Neural Science, New York University, 4 Washington Place, Room 809, New York, NY 10003.
REFERENCES
- 1.Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- 2.Collewijn H. Eye- and head movements in freely moving rabbits. J Physiol (Lond) 1977;266:471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford MLJ, Von Noorden GK. The effects of short-term experimental strabismus on the visual system in Macaca mulatta. Invest Ophthalmol Vis Sci. 1979;18:496–505. [PubMed] [Google Scholar]
- 4.Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- 6.Eggers HM, Blakemore C. Physiological basis of anisometropic amblyopia. Science. 1978;201:264–267. doi: 10.1126/science.663654. [DOI] [PubMed] [Google Scholar]
- 7.Eggers HM, Gizzi MS, Movshon JA. Spatial properties of striate cortical neurons in esotropic macaques. Invest Ophthalmol Vis Sci [Suppl] 1984;25:278. [Google Scholar]
- 8.Galyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb JP, MacAvoy MG, Bruce CJ. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- 11.Grasse K, Lisberger SG. Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J Neurophysiol. 1992;67:164–179. doi: 10.1152/jn.1992.67.1.164. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann K-P, Distler C, Ilg U. Callosal and superior temporal sulcus contributions to receptive field properties in the macaque monkey’s nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract. J Comp Neurol. 1992;321:150–162. doi: 10.1002/cne.903210113. [DOI] [PubMed] [Google Scholar]
- 13.Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artifical squint. J Neurophysiol. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- 14.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol (Lond) 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiorpes L, Boothe RG, Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS. Effects of early unilateral blur on the macaque’s visual system. I. Behavioral observations. J Neurosci. 1987;7:1318–1326. doi: 10.1523/JNEUROSCI.07-05-01318.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiorpes L, Carlson MR, Alfi D. Development of visual acuity in experimentally strabismic monkeys. Clin Vision Sci. 1989;4:95–106. [Google Scholar]
- 17.Kiorpes L, Kiper DC, Movshon JA. Contrast sensitivity and vernier acuity in amblyopic monkeys. Vision Res. 1993;33:2301–2311. doi: 10.1016/0042-6989(93)90107-8. [DOI] [PubMed] [Google Scholar]
- 18.Levitt JB, Kiper DC, Movshon JA. Receptive fields and functional architecture of macaque V2. J Neurophysiol. 1994;71:2517–2542. doi: 10.1152/jn.1994.71.6.2517. [DOI] [PubMed] [Google Scholar]
- 19.Lisberger SG, Pavelko TA. Directional and topographic organization of the visual inputs for smooth pursuit eye movements in monkeys. J Neurophysiol. 1989;61:173–185. doi: 10.1152/jn.1989.61.1.173. [DOI] [PubMed] [Google Scholar]
- 20.Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Maunsell JHR, Van Essen DC. Functional properties of neurons in the middle temporal visual area (MT) of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983a;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- 23.Maunsell JHR, Van Essen DC. Functional properties of neurons in the middle temporal visual area (MT) of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol. 1983b;49:1148–1167. doi: 10.1152/jn.1983.49.5.1148. [DOI] [PubMed] [Google Scholar]
- 24.Maunsell JHR, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983c;3:2563–2583. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrode. Med Biol Eng. 1972;10:495–504. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- 26.Movshon JA, Kiorpes L. Effects of artificial strabmisus on macaque MT. Soc Neurosci Abstr. 1992;18:1456. [Google Scholar]
- 27.Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque’s visual system. III. Physiological observations. J Neurosci. 1987;7:1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movshon JA, Lisberger SG, Kiorpes L, Walton PD, O’Keefe LP. Effects of strabismus on pursuit eye movements and MT neurons in macaque monkeys. Perception. 1995;24S:6. doi: 10.1523/JNEUROSCI.16-20-06537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newsome WT, Wurtz RH, Dürsteler MR, Mikami A. Deficits in visual motion perception following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol (Lond) 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz JD, Lisberger SG. Modulation of the level of smooth pursuit activation by initial tracking conditions in monkeys. Vis Neurosci. 1994;11:411–424. doi: 10.1017/s0952523800002352. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Hikosaka H, Saito H, Yukie F, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tychsen L. Motion sensitivity and the origins of infantile strabismus. In: Simons K, editor. Early visual development, normal and abnormal. Oxford UP; New York: 1993. pp. 364–390. [Google Scholar]
- 34.Tychsen L, Lisberger SG. Maldevelopment of visual motion processing in humans who had strabismus with onset in infancy. J Neurosci. 1986a;6:2495–2508. doi: 10.1523/JNEUROSCI.06-09-02495.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tychsen L, Lisberger SG. Visual motion processing for the initiation of smooth pursuit eye movements in humans. J Neurophysiol. 1986b;56:953–968. doi: 10.1152/jn.1986.56.4.953. [DOI] [PubMed] [Google Scholar]
- 36.Van Essen DC, Maunsell JHR, Bixby JL. The middle temporal visual area in the macaque: myeloarchitecture, connections, functional properties and topographic representation. J Comp Neurol. 1981;199:293–326. doi: 10.1002/cne.901990302. [DOI] [PubMed] [Google Scholar]
- 37.Von Noorden GK. Mosby; St. Louis: 1980. Burian and Von Noorden’s binocular vision and ocular motility: theory and management of strabismus. . [Google Scholar]
- 38.Walton PJ, Lisberger SG. Binocular misalignment in infancy causes directional asymmetries in pursuit. Invest Ophthalmol Vis Sci [Suppl] 1989;30:304. [Google Scholar]
- 39.Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- 40.Wurtz RH. Visual receptive fields of striate cortex neurons in awake monkeys. J Neurophysiol. 1969;32:727–742. doi: 10.1152/jn.1969.32.5.727. [DOI] [PubMed] [Google Scholar]
- 41.Zeki SM. Cells responding to changing image size and disparity in the cortex of the rhesus monkey. J Physiol (Lond) 1974a;242:827–841. doi: 10.1113/jphysiol.1974.sp010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol (Lond) 1974b;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeki SM. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol (Lond) 1978;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]