Abstract
The weaver (wv) gene (GIRK2) is a member of the G-protein-gated inwardly rectifying potassium (GIRK) channel family, known effectors in the signal transduction pathway of neurotransmitters such as acetylcholine, dopamine, opioid peptides, and substance P in modulation of neurotransmitter release and neuronal excitability. GIRK2 immunoreactivity is found in but not limited to brain regions known to be affected in wv mice, such as the cerebellar granule cells and dopaminergic neurons in the substantia nigra pars compacta. It is also observed in the ventral tegmental area, hippocampus, cerebral cortex, and thalamus. GIRK2 and GIRK1, a related family member, have overlapping yet distinct distributions in rat and mouse brains. In regions where both channel proteins are expressed, such as the cerebral cortex, hippocampus, and cerebellum, they can be co-immunoprecipitated, indicating that they interact to form heteromeric channels in vivo. In the brain of thewv mouse, GIRK2 expression is decreased dramatically. In regions where GIRK1 and GIRK2 distributions overlap, both GIRK1 and GIRK2 expressions are severely disrupted, probably because of their co-assembly. The expression patterns of these GIRK channel subunits provide a basis for consideration of the machinery for neuronal signaling as well as the differential effects of the wvmutation in various neurons.
Keywords: weaver mouse, G-protein, inwardly rectifying potassium channel, dopamine, hippocampus, substantia nigra, cerebellum, heteromultimerization, GIRK
The weaver (wv) mouse exhibits a diverse range of defects, including ataxia (Sidman et al., 1965; Rakic and Sidman, 1973a,b; Hatten et al., 1984), dopamine deficiency (Lane et al., 1977; Schmidt et al., 1982; Roffler-Tarlov and Graybiel, 1984), seizures (Eisenberg and Messer, 1989), and hypospermatogenesis (Vogelweid et al., 1993; Harrison and Roffler-Tarlov, 1994). Recently, a G-protein-activated inwardly rectifying potassium channel (GIRK), Kir3.2 or GIRK2 (Lesage et al., 1994; Bond et al., 1995; Tsaur et al., 1995), has been identified as the wv gene (Patil et al., 1995;Kofuji et al., 1996; Navarro et al., 1996; Slesinger et al., 1996; for reviews, see Goldowitz and Smeyne, 1995; Hess, 1996). GIRK channels are membrane proteins that conduct K+ currents at or near the resting membrane potential, and they are important in controlling cell excitability (Hille, 1992; Jan and Jan, 1994; Kubo, 1994; Doupnik et al., 1995; Wickman and Clapham, 1995a,b). They are regulated by G-proteins and have been shown to mediate the actions of G-protein-coupled receptors for transmitters (Breitwieser and Szabo, 1985; Pfaffinger et al., 1985; North, 1989; Brown, 1990; Brown and Birnbaumer, 1990; Nicoll et al., 1990). GIRK2 mRNA is found in brain regions known to be affected by the wv mutation, such as the cerebellar granule cells and substantia nigra (SN) (Karschin et al., 1996; Kobayashi et al., 1995), and both GIRK1 and GIRK2 proteins are expressed in the cerebellar granule cells and Purkinje cells during development (Patil et al., 1995; Kofuji et al., 1996; Navarro et al., 1996; Slesinger et al., 1996).
Kir channels are tetramers (Yang et al., 1995) and hence could exist as homo- or heteromeric complexes. In heterologous expression systems, GIRK1 (Dascal et al., 1993; Kubo et al., 1993), unlike GIRK2, does not seem to form functional homomeric channels and may require either GIRK2 or GIRK4 to form functional channels (Duprat et al., 1995; Kofuji et al., 1995; Krapivinsky et al., 1995a,b; Lesage et al., 1995; Hedin et al., 1996). Co-expression of GIRKs 1 and 2 (or 1 and 4) in heterologous systems most likely leads to the formation of both homomeric GIRK2 (or GIRK4) channels and heteromeric GIRK1/2 (or 1/4) channels (Duprat et al., 1995; Kofuji et al., 1995; Krapivinsky et al., 1995a,b; Lesage et al., 1995; Slesinger et al., 1996; Spauschus et al., 1996; Velimirovic et al., 1996). Interestingly, the wvmutant form of GIRK2 (G156S) causes the homomeric GIRK2 channels to be nonselective and conduct sodium as well as potassium ions, whereas the function of the heteromeric GIRK1/GIRK2 channels is greatly reduced by the wv mutation (Kofuji et al., 1996; Navarro et al., 1996;Slesinger et al., 1996; but see Surmeier et al., 1996). Thus, the effects of the wv mutation may vary with the type of GIRK channel subunits expressed by the neuron.
To study the GIRK channels in vivo, we used Western blotting, in situ hybridization, and immunohistochemistry to determine the distribution of GIRK1 and GIRK2 in wild-type rat and mouse brains. Co-immunoprecipitation of GIRK1 and GIRK2 from wild-type brain regions and the drastic decrease in expression of both channel proteins in the wv mouse brain indicate that heteromultimers of GIRK1 and GIRK2 exist as a major component of GIRK channels in the mammalian brain.
MATERIALS AND METHODS
In vitro expression of channel proteins. GIRK1, GIRK2, GIRK4, and IRK1 mRNAs were synthesized in vitrofollowing manufacturer’s instructions (Ambion T7 kit) and injected into Xenopus oocytes. Oocytes were processed after 2 d for Western analysis of channel proteins. The oocytes were lysed by pipetting and washed in 50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, and protease inhibitors (see “Brain membrane preparation”). Residual membrane was solubilized in 2% SDS sample buffer (includes 5% β-mercaptoethanol), vortexed with acid-washed glass beads, heated to 75°C for 45 min, and analyzed by Western blotting. The presence of proteins was shown by recording specific inward rectifier K+ currents from oocytes or by probing the blots with channel-specific antibodies. GIRK1, GIRK2, GIRK4, and IRK1 proteins were also synthesized via in vitro translation in the presence of rabbit reticulocyte lysate (Promega, Madison, WI) and analyzed similarly. The presence of proteins was assayed by35S-methionine incorporation and exposure to autoradiographic film as well as by probe of the blots with channel-specific antibodies.
Brain membrane preparation. Adult Sprague Dawley male rats were anesthetized by brief exposure to halothane (Sigma, St. Louis, MO), decapitated, and quickly dissected for cerebral cortex, hippocampus, cerebellum, spinal cord, and liver. For the cortex, care was taken to remove as much white matter as possible. Tissues were chopped in ice-cold 0.32 m sucrose, 5 mm Tris, pH 7.4, 50 μg/ml pA-PMSF, 1 μg/ml leupeptin, 2 μg/ml aprotinin A, and 1 μg/ml pepstatin (Boehringer Mannheim, Mannheim, Germany), and dounce-homogenized. Membrane isolation was carried out via differential centrifugation steps and stored in −80°C as aliquots in 20 mm Tris, pH 7.4, 1 mm EDTA, and protease inhibitors until use. Protein concentration was assayed using the Bio-Rad kit (Bio-Rad, Richmond, CA), with BSA as standard.
Antibody production and purification. The peptides for GIRK1, GIRK2, and IRK1 were synthesized by Dr. C. Turck (Howard Hughes Medical Institute, University of California, San Francisco). The peptide sequences are as follows: GIRK1N (residues 6–42) RKFGDDYQVVTTSSSGSGLQPQGPGQGPQQQLVPKKKC; GIRK1C (residues 346–375) CHATFEVPTPPYSVKEQEEMLLMSSPLIAPA; GIRK2N (residues 20–51) DQDVESPVAIHQPKLPKQARDDLPRHISRDRTC (1 and 9 identical, and 2 and 2 conserved amino acids to GIRK3 and GIRK4, respectively); GIRK2C (residues 403–422) CEKNPEEQTERNGDVANLENE (0 and 3 identical, and 1 and 3 conserved amino acids to GIRK3 and GIRK4, respectively) (based on the sequence of GIRK2 or GIRK2A; Lesage et al., 1995; Tsaur et al., 1995). Peptide coupling and generation of polyclonal antibodies in rabbits were done by Caltag Corporation (South San Francisco, CA). Antibodies were affinity-purified with appropriate peptide columns, which were generated by coupling peptides to Sulfolink coupling gel (Pierce, Rockford, IL) following the manufacturer’s protocol.
Western blotting. Protein samples were prepared in sample buffer (125 mm Tris, pH 6.8, 20% glycerol, 1–2% SDS, 5% β-mercaptoethanol), heated at 75°C for 30 min, and analyzed by 10% SDS-PAGE gels. Western blots were blocked with Superblock (Pierce) or 5% nonfat dried milk in TBST buffer (150 mm NaCl, 10 mm Tris, pH 8.0, 0.1% Tween 20) for 10 min or 1 hr, respectively. Primary antibody (1 μg/ml), primary antibody in the presence of competitive peptides (10 μg/ml), and secondary antibody [donkey anti-rabbit antibody (Amersham, Arlington Heights, IL)] were diluted in 2% normal goat serum and 0.5% BSA in TBST. Blots were incubated in primary antibodies for 1 hr at room temperature or overnight at 4°C, washed in TBST, incubated in secondary antibody for 30 min, developed by the ECL method, and exposed briefly to Hyperfilm-ECL (Amersham).
Immunohistochemistry. The animals used included adult Sprague Dawley rats (5–6 weeks old) and wv as well as wild-type C57BL/6 mice. wv mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred by N. Patil as well as by Y.J.L. Mice were genotyped by sequencing tail genomic DNA for the presence of the G to A mutation. All animals were treated in accordance with the policy on the use of animals in neuroscience research. Animals were exposed briefly to halothane (Sigma), injected intraperitoneally with pentobarbital, and perfused with 4% formaldehyde (Polysciences, Warrington, PA) and 0.1% glutaraldehyde in PBS. The brain and spinal cord were dissected and post-fixed for 30 min to overnight. Fifty micrometer vibratome sections were collected in 0.1 m Tris, pH 7.6; blocked with 1–3% H2O2, 0.1 m Tris, pH 7.6; washed with 50 mm Tris, 100 mm NaCl, and 50 mm Tris, pH 7.6, 100 mm NaCl, 0.1% Triton X-100; and then blocked in 3–10% normal goat serum and 0.1–3% BSA. Rabbit polyclonal antibodies were used at 1 μg/ml. Monoclonal antibodies against calbindin (Sigma), parvalbumin (Sigma), P65 (courtesy of L. F. Reichardt and I. Fariñas), and tyrosine hydroxylase (TH) (courtesy of L. F. Reichardt and I. Fariñas; Pel-Freeze Biologicals, Rogers, AR) were used at 1:1000 or 1:5000 dilution. Biotinylated donkey anti-rabbit or anti-mouse IgG Fab (Jackson Laboratory) were used at 1:200 dilution as secondary antibodies. Sections were developed with Vectastain ABC kit (Vector Labs, Burlingame, CA) and diaminobenzidine and mounted in Permount (Fisher Scientific, Houston, TX). Different brain areas were identified based on comparison with the rat brain atlas of Paxinos and Watson (1986). Antibodies specific for sequences in the N- and C-terminal domains of the same channel subunit yielded the same staining patterns, although antibodies against N-terminal sequences in general gave stronger staining. Little or no staining was detected in the absence of GIRK1 or GIRK2 antibody, in the presence of preimmune sera instead of the primary antibodies, or when the primary antibody was incubated with the antigenic peptide (see Fig. 3C; data not shown).
Fig. 3.
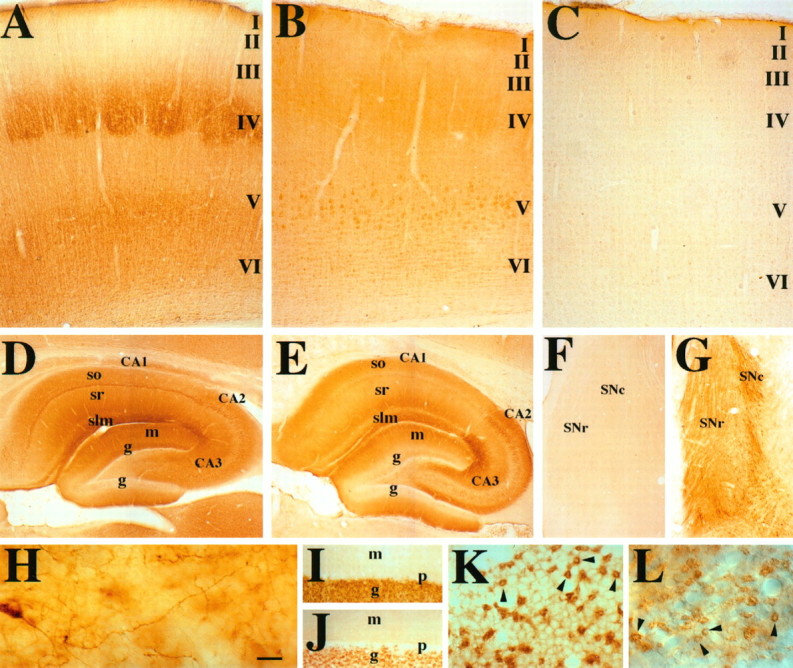
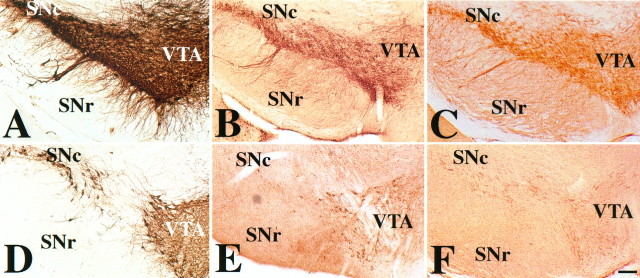
Higher-magnification views of GIRK1 and GIRK2 staining in rat cerebral cortex, hippocampus, substantia nigra, and cerebellum. A, GIRK1 immunoreactivity in the barrel cortex. There is intense staining of layers IV–VI neurons and neurites. See text for details. I–VI correspond to layers of the cerebral cortex. B, GIRK2 immunoreactivity in the hindlimb area of cortex. In the forelimb and hindlimb areas of the cortex, there is more intense staining of all layers. In particular, the layer V large pyramidal cells are the most strongly stained cells in the cortex. C, Negative control for cortex with the omission of the primary antibody. D, GIRK1 immunoreactivity of the hippocampal formation. The entire molecular layer, which includes terminal fields of the perforant pathway and commissural fibers, is strongly stained. Note that the superior blade of the dentate granule cell is much more stained than the inferior blade, despite no differences in mRNA level (DePaoli et al., 1994;Karschin et al., 1994). There is strong staining of the CA3,CA2, and CA1 pyramidal cells (CA1 > CA2 = CA3). Stratum lacunosum moleculare (slm), the terminal field of the perforant pathway, is strongly stained. Other layers that receive commissural fibers [stratum radiatum (sr)] and nonhippocampal fibers, such as those from the thalamus [stratum oriens (so)], are stained moderately. Stratum lucidum, the terminal field for the mossy fibers, has relatively little immunoreactivity. E, GIRK2 immunoreactive pattern in the hippocampus. Strongest staining is in the CA2 pyramidal cells and CA3 pyramidal cells closest to CA2. There is light staining of theCA1 pyramidal cells. The intensity of staining inslm, sr, and so (in strong to weak order, slm > so > sr) corresponds to that of the pyramidal cell layer (CA2 > CA3 > CA1). F, G, GIRK1 and GIRK2 staining, respectively, in the SN. There is little GIRK1 staining, whereas GIRK2 immunoreactivity can be seen in the cell body and dendrites of the SN pars compacta (SNc) neurons. H, Higher-magnification view of a SNc neuron sending dendrite ventrally into the SN pars reticulata (SNr). GIRK1 (I, K) and GIRK2 (J, L) immunoreactivity in the cerebellum is shown. For both GIRK1 and GIRK2, there is strong staining of the granule cell layer, moderate staining of the deep cerebellar nuclei, and light staining of the molecular layer. There is little staining of the Purkinje cells. K, L, Higher-magnification view of GIRK1 and GIRK2 immunoreactivity of the cerebellar granule cell layer, respectively. Both GIRK1 and GIRK2 antibodies stain the glomeruli (arrowheads) very strongly, whereas GIRK1 antibodies also highlight the outline of granule cells. Scale bars: A–C, 0.2 mm; D, E, 0.3 mm; F, G, 0.6 mm; H, 0.02 mm; I, J, 0.04 mm; K, L, 0.02 mm.g, Granule cell layer in the dentate gyrus of the hippocampus (D, E) or granule cell layer of the cerebellum (I, J); CA1–3, regions of hippocampus proper; m, molecular layer of the dentate gyrus of the hippocampus (D, E) or of the cerebellum (I, J); p, Purkinje cell layer. For additional abbreviations, see legend to Figure 2.
In situ hybridization. Antisense and sense oligonucleotides (45-mers) were designed for the hamster GIRK2 sequence and end-labeled with α-33P-dATP or α-35S-dATP (Amersham). The entire in situhybridization protocol was carried out as published (Wisden and Morris, 1994). End-labeling with α-33P-dATP gave better signal-to-noise ratio than with α-35S-dATP. The antisense oligonucleotides contained the sequences gaccaggacgtggaaagcccagtggccattcaccagccaaagttgcct (N terminus), ctggctaacagggcagagctgcccctgagttggtctgtgtccagc (C terminus), and gagaagaacccggaagagcagacggagaggaatggtgacgtggc (C terminus control; with five nucleotides different from the corresponding rat sequence). Complementary sense oligonucleotides to the above sequences were also synthesized and used as controls. The C terminus control and sense oligonucleotide showed background level of signal (data not shown).
Immunoprecipitation. For immunoprecipitation experiments, an equal amount of rat brain membranes from cortex, hippocampus, and cerebellum was lysed in nondenaturing buffer containing 1–2% Triton X-100, 150–500 mm NaCl, 50 mm Tris, pH 7.4, 1 mm EDTA, and protease inhibitor cocktail (see “Brain membrane preparation”). Our previous experience with immunoprecipitation of voltage-gated K+ channels showed that this detergent and salt concentration is strong enough to solubilize rat brain membranes and to prevent nonspecific interactions, but not strong enough to abolish channel subunit interactions (Sheng et al., 1993). Membranes were also solubilized in 1–2% CHAPS or 60 mmn-octyl glucoside, and preliminary evidence indicated that use of different detergents did not alter the results of the immunoprecipitation experiments. The solubilized supernatant was precleared with some or all of the following items: a nonspecific, purified rabbit antibody; purified recombinant protein A-sepharose beads (Pierce); and avidin and biotin beads (Pierce). After preclearing, the solubilized membrane was incubated first with antibodies for 2 hr to overnight in 4°C and then with recombinant protein A-sepharose beads for 2 hr, washed extensively (four to seven times) in 1 ml of lysis buffer/protease inhibitors, and analyzed on 10% SDS-PAGE and by Western blotting with biotinylated antibodies. No specific signals were detected when we omitted the primary antibodies during immunoprecipitation (data not shown).
Because GIRK1 (62 kDa) and GIRK2 (48–50 kDa) are both about the size of the IgH chain (55 kDa), the strong IgH band obscured the relevant portion of the blot, thereby rendering interpretation of immunoprecipitation experiments nearly impossible. Different approaches to covalently couple purified antibodies to beads either abolished the ability of the antibodies to immunoprecipitate or failed to consistently remove all IgH from the blots (data not shown). We therefore chose to avoid the use of secondary antibodies that would mark the IgH band. Instead, we biotinylated antibodies against GIRK1 N or C termini or GIRK2 N terminus after affinity purification and used streptavidin-HRP for detection. Affinity-purified antibodies were biotinylated with NHS-LC-Biotin (Pierce) following the manufacturer’s directions and purified using swift desalting columns (Pierce). Antibodies were washed with PBS and concentrated with Centriprep columns (Amicon, Beverly, MA).
To ensure that the biotinylated antibodies did not react with IgH chain, we denatured 1 μg of immunopure rabbit IgG (Pierce) and analyzed with unlabeled versus biotinylated antibodies (data not shown). Biotinylated antibodies did not react with denatured IgH and Ig light chains, but they did give rise to two kinds of undesirable background bands. On blots probed with biotinylated antibodies, there were strong 85–120 kDa bands, which could not be competed off with 10 μg competitive peptides (Fig. 4C). The other kind of background band was a result of the cross-reactivity of the antibodies with protein A, which ran as 40–50 kDa molecular weight bands. The presence of this kind of background band was confirmed when whole protein A-sepharose beads (Pharmacia, Piscataway, NJ) or recombinant purified protein A-sepharose beads (Pierce) were treated with SDS sample buffer, analyzed on Western blots, and visualized by different biotinylated antibodies (data not shown). As a result, a lysis buffer control (see Fig. 4, BUFFER) was processed in parallel with all immunoprecipitation experiments to distinguish between authentic immunoprecipitated proteins and background protein A bands.
Fig. 4.
GIRK1 and GIRK2 are co-immunoprecipitated from rat cerebral cortex, hippocampus, and cerebellum. A, Immunoprecipitation and co-immunoprecipitation of GIRK1 and GIRK2 from membranes of cerebral cortex (CTX), hippocampus (HP), cerebellum (CB), or buffer control (BUFFER). Membranes were immunoprecipitated with different channel antibodies (labeled at top underIP) and probed with biotinylated antibody against GIRK1 C terminus or GIRK2 N terminus (labeled on the leftunder Western). B, Controls for immunoprecipitation experiments. The antibodies used for immunoprecipitation are listed above each lane (First IP). After the first immunoprecipitation, the “immunodepleted” supernatants were then subjected to a second immunoprecipitation (Second IP), and the lanes in the two blots are matched exactly. G2+pG2,GIRK1 is not co-immunoprecipitated by GIRK2 antibody in the presence of competitive GIRK2 peptide. G2+pG1,GIRK1 is co-immunoprecipitated by GIRK2 antibody in the presence of noncompetitive GIRK1 peptide. G1+pG1,GIRK1 is not immunoprecipitated by GIRK1 antibody in the presence of competitive GIRK1 peptide. G1+pG2,GIRK1 is immunoprecipitated by GIRK1 antibody in the presence of noncompetitive GIRK2 peptide. IRK1,GIRK1 is not co-immunoprecipitated by IRK1 antibody.Kv1.4, GIRK1 is not co-immunoprecipitated by Kv1.4 antibody. See Results and Materials and Methods for details. C, In the presence of competitive peptide, biotinylated antibody against GIRK2 did not stain GIRK2 bands, but the background 85 and 120 kDa bands remained.
RESULTS
Western analysis shows that both GIRK1 and GIRK2 are present in cerebral cortex, hippocampus, and cerebellum
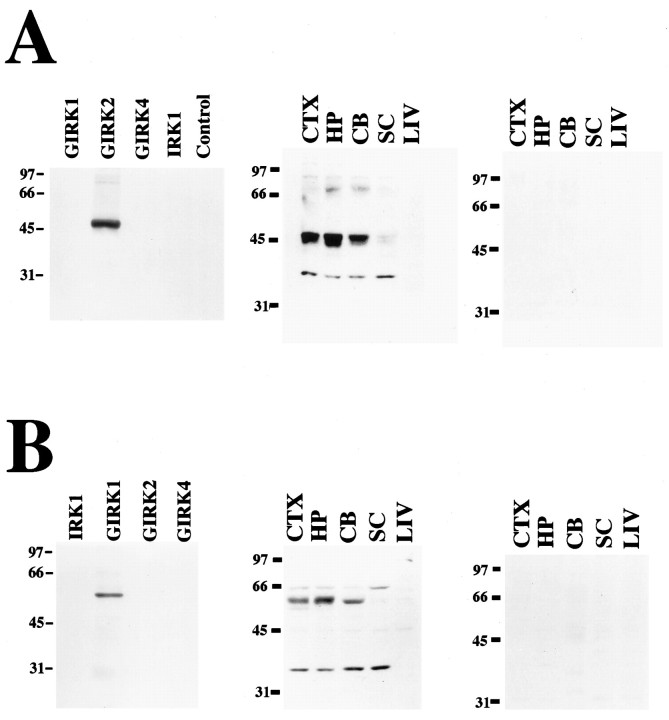
We generated rabbit polyclonal antibodies for analysis of GIRK2 and GIRK1 channel proteins in vivo. To demonstrate antibody specificity, GIRK2 and GIRK1 antibodies were tested on Western blots containing GIRK1, GIRK2, GIRK4, and IRK1 proteins synthesized in vitro. Channel proteins were generated in Xenopusoocytes injected with cRNA and by in vitro translation in rabbit reticulocyte lysate. The presence of different channel proteins was demonstrated by recording specific inwardly rectifying K+ currents from the oocytes, probing with antibodies against each channel, or by autoradiography of proteins containing35S-methionine. Antibody against the N terminus of GIRK2 recognized a specific 48 kDa band in oocytes injected with GIRK2 cRNA but not in oocytes injected with water or other inwardly rectifying K+ channel cRNAs (Fig. 1A,left). Antibody against the N terminus of GIRK1 detected a 55 kDa band in the lane containing GIRK1 protein synthesized in rabbit reticulocyte lysate in the absence of pancreatic microsomal membrane, but it did not detect a band in lanes containing GIRK2, GIRK4, or IRK1 proteins (Fig. 1B, left).
Fig. 1.
GIRK1 and GIRK2 are present on membranes from different rat brain regions. A, B,Left, Western blots probed with antibody against the N terminus of GIRK2 or GIRK1, respectively, to demonstrate antibody specificity. In A, left, Western blot of GIRK1, GIRK2, GIRK4, and IRK1 proteins expressed inXenopus oocytes is probed with antibody against the N terminus of GIRK2. Control represents oocytes injected with water. InB, left, Western blot of in vitro synthesized IRK1, GIRK1, GIRK2, and GIRK4 proteins is probed with antibody against the N terminus of GIRK1. A,B, Middle, membranes from rat cerebral cortex (CTX), hippocampus (HP), and cerebellum (CB) contain GIRK2 (48 kDa) and GIRK1 (58–60 kDa) proteins, respectively. There are also faint GIRK2 bands in the spinal cord. See Results for details. A,B, Right, control Western blots using antibody against the N terminus of GIRK1 or GIRK2 in the presence of antigenic peptide. SC, Spinal cord; LIV, liver.
Affinity-purified rabbit polyclonal antibodies against the N or C terminus of GIRK2 and GIRK1 were used to identify GIRK2- and GIRK1-immunoreactive bands in membranes prepared from rat cerebral cortex and hippocampus as well as from cerebellum. Antibodies against the N terminus of GIRK2 detected a broad double band of ∼48-50 kDa in cerebral cortex, hippocampus, cerebellum, and spinal cord (Fig.1A, middle). The size of the GIRK2-immunoreactive band was consistent with that of the in vitro translated protein, and the doublet may reflect the presence of alternatively spliced forms of GIRK2 (Lesage et al., 1994, 1995;Tsaur et al., 1995; Isomoto et al., 1996) (N. Patil, unpublished observation). The 38 kDa band was a cross-reacting protein detected on Western blots of rat but not mouse brain membranes. Antibody against the N terminus of GIRK2 preadsorbed with competitive peptide gave rise to no immunoreactive bands (Fig. 1A,right). Antibody against the C terminus of GIRK2 could not detect any immunoreactive bands on Western blots of brain membranes.
Antibody against the N or C terminus of GIRK1 stained an immunoreactive band of ∼60-62 kDa in cerebral cortex, hippocampus, and cerebellum (Fig. 1B, middle; data not shown). No GIRK1 protein was detectable in liver, consistent with the observed absence of GIRK1 mRNA based on Northern analysis (Kubo et al., 1993). The 35 kDa band was recognized by antibody against the N terminus but not by antibody against the C terminus of GIRK1. GIRK1 antibodies in the presence of antigenic peptide could not detect any immunoreactive bands (Fig. 1B, right).
Immunohistochemistry and in situ hybridization reveal overlapping yet distinct expression patterns of GIRK1 and GIRK2 channel subunits in rat
We used antibodies against the N- or C-terminal cytoplasmic domains of GIRK1 and GIRK2 to study their distribution in rat brain (for controls, see Materials and Methods). Antibodies against either the N or the C terminus of the same channel subunit gave rise to similar immunoreactive patterns. Immunohistochemical studies using these antibodies revealed that GIRK1 and GIRK2 have overlapping distribution in some brain regions (Figs. 2,3, Table 1). Strong GIRK1 and GIRK2 immunoreactivity was observed in cerebral cortex (CTX) (Figs. 2A,B, 3A,B, Table 1), lateral septal nuclei (data not shown), hippocampal formation (HP) (Figs. 2A,B, 3D,E, Table1), and cerebellum (Figs. 2D,E, 3I–L), whereas certain other regions express predominantly GIRK1 or GIRK2. Strong GIRK1 but low or background level of GIRK2 staining was found in the caudate-putamen (CP) and globus pallidus (Fig.2A,B), thalamus (Th) (Fig.2A,B; for a few exceptions see Table 1 and Fig.2B), oculomotor nucleus (Table 1), red nucleus (data not shown), and mesencephalic nuclei of trigeminal nerve (data not shown). Strong GIRK2 but background level of GIRK1 staining was observed in the SN (Fig. 3F–H) and ventral tegmental area (VTA) (data not shown but see Fig. 6A for GIRK2 staining in mouse brain). Low or background level staining for GIRK1 and GIRK2 was found in the hypothalamus, locus coeruleus, and nucleus basalis of Meynert, regions where electrophysiological studies have revealed regulation of Kir channels by specific neurotransmitters (Table 1).
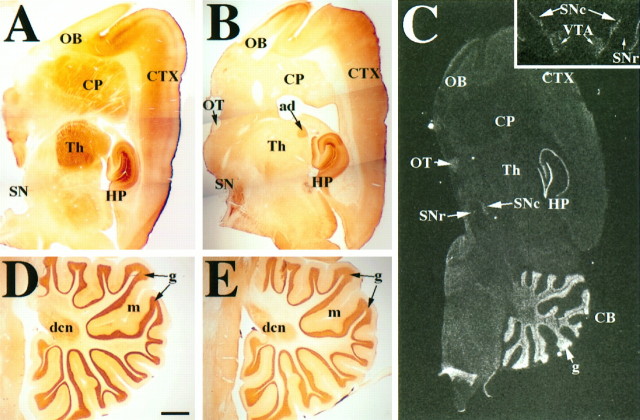
Fig. 2.
Overview of GIRK1 and GIRK2 distribution in the rat brain. A, D, Parasagittal view of GIRK1 antibody staining pattern. There is strong staining in the cerebral cortex (CTX), caudate-putamen (CP), globus pallidus, thalamus (Th), hippocampus (HP), and cerebellum (CB) but not in the substantia nigra (SN) and hypothalamus. B, E, Parasagittal view of GIRK2 immunoreactivity. There is strong staining in the CTX, HP, SN, anterodorsal thalamic nucleus (ad), and CB but not in many thalamic nuclei, CP, and hypothalamus. C, Parasagittal view of GIRK2 mRNA distribution. Inset is a coronal view of GIRK2 mRNA distribution in the ventral midbrain. Signal can be observed in the HP, CB, SN pars compacta (SNc), and ventral tegmental area (VTA) but not in corpus callosum, TH, olfactory bulb (OB), and SN pars reticulata (SNr). Scale bars: A, B, 1 mm;C and inset, 2 mm; D, E, 0.8 mm. dcn, Deep cerebellar nuclei; g, granule cell layer of the cerebellar cortex; m, molecular layer of the cerebellar cortex; OT, optic tract.
Table 1.
Protein distribution of GIRK1 and GIRK2 in rat brain and spinal corda
| GIRK1 | GIRK2 | GIRK1 | GIRK2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal | type | Signal | type | Signal | type | Signal | type | |||
| Telencephalon | ||||||||||
| Olfactory bulb | + | + | a,d | |||||||
| Cerebral cortex | ||||||||||
| Layer I, II, III | + | d,e | + | c | ||||||
| Layer IV | +++ | a,d,e | ++ | c | ||||||
| Layer V | ++ | a,d | +++ | a,b,d | ||||||
| Layer VI | ++ | a,d | + | a,d | ||||||
| Island of Calleja | +/− | ++ | a,d | |||||||
| Caudate-putamen | + | a,d | +/− | d | ||||||
| Globus pallidus | + | a,b,c | +/− | c | ||||||
| Nucleus basalis of Meynert | +/− | +/− | ||||||||
| Nucleus diagonal band | + | a,b,c | ||||||||
| Lateral septal nucleus | +++ | e | +++ | e | ||||||
| Medial septal nucleus | +/− | +/− | ||||||||
| Dentate gyrus | ||||||||||
| Molecular layer | +++ | d | ++ | d | ||||||
| Granule cells | ||||||||||
| Superior blade | ++ | c | + | c | ||||||
| Inferior blade | +/− | c | + | c | ||||||
| Hilus | ++ | a,c | ++ | a,b,c,d | ||||||
| Hippocampus | ||||||||||
| CA3 | ||||||||||
| Stratum oriens | ++ | c | ++ | c | ||||||
| Stratum pyramidal | ++ | a,b,d | ++ | a,b,d | ||||||
| Stratum radiatum | ++ | d | ++ | d | ||||||
| Stratum lucidum | +/− | d | +/− | d | ||||||
| Stratum lacunosum molecular | +++ | d | +++ | d | ||||||
| CA2 | ||||||||||
| Stratum oriens | ++ | c | ++ | c | ||||||
| Stratum pyramidal | ++ | a,b,d | +++ | a,b,d | ||||||
| Stratum radiatum | ++ | d | ++ | d | ||||||
| Stratum lacunosum molecular | +++ | d | +++ | d | ||||||
| CA1 | ||||||||||
| Stratum oriens | ++ | d | + | d | ||||||
| Stratum pyramidal | +++ | a,c | +/++ | a,c | ||||||
| Stratum radiatum | ++ | d | + | d | ||||||
| Stratum lacunosum molecular | +++ | d | ++ | a,d | ||||||
| Subiculum | + | d | ++ | a,d | ||||||
| Presubiculum | + | d | +/− | d | ||||||
| Parasubiculum | + | d | +/− | d | ||||||
| Diencephalon | ||||||||||
| Thalamus | ||||||||||
| Paraventricular | +/− | d | ||||||||
| Lateral dorsal | +++ | a,d | +/− | d | ||||||
| Lateral posterior | ++ | d | +/− | d | ||||||
| Anterior dorsal | ++ | a,d | ||||||||
| Anterior medial | +/- | d | ||||||||
| Medial dorsal | +++ | a,d | ||||||||
| Ventral medial | + | a,d | + | a,d | ||||||
| Ventral lateral | +++ | a,d | + | a,d | ||||||
| Ventral posterior | +++ | a,d | ||||||||
| Central medial | ++ | a,d | ||||||||
| Central lateral | + | a,d | ||||||||
| Gelatinosus | ++ | a,d | ||||||||
| Rhomboid | +/− | d | ||||||||
| Reuniens | +/− | d | ||||||||
| Reticular | ++ | c | + | c | ||||||
| Posterior | +++ | a,d | ||||||||
| Parafascicular | + | a,d | ||||||||
| Hypothalamus | ||||||||||
| Anterior and lateral anterior | +/− | a,d | +/− | |||||||
| Lateral | + | a,b,c,d | ||||||||
| Dorsal medial, ventral medial, tuber cinerium | +/− | |||||||||
| Entopeduncular nucleus | +/− | c | +/− | |||||||
| Anterior pretectal nucleus | +/− | |||||||||
| Deep mesencephalic nucleus | +/− | c | ||||||||
| Mesencephalon | ||||||||||
| Superior colliculus | + | d | + | d | ||||||
| Oculomotor (III) nucleus | +++ | a,d | +/− | |||||||
| Red nucleus | +++ | a,b,c | +/− | |||||||
| Ventral tegmental area | +/− | +++ | a,b,c | |||||||
| Substantia nigra | ||||||||||
| Compacta | +/− | +++ | a,b,c | |||||||
| Reticulata | +/− | +++ | c | |||||||
| Inferior colliculus | + | a,d | ||||||||
| Metencephalon | ||||||||||
| Postdorsal tegmental nucleus | ++ | a,d | ||||||||
| Lateral dorsal tegmental nucleus | + | a,b,d | ||||||||
| Locus coeruleus | +/− | a,d | +/− | |||||||
| Mesencephalic nucleus trigeminal nerve | +++ | a | +/− | |||||||
| Motor, sensory trigeminal nucleus | ++ | a,c,d | ||||||||
| Pontine reticulata nucleus | + | a,b,c,d | ||||||||
| Raphe pontine nucleus | ++ | |||||||||
| Cerebellum | ||||||||||
| Molecular layer | + | d | + | d | ||||||
| Granule cell layer | ||||||||||
| Granule cell | + | a | +/− | |||||||
| Glomeruli | +++ | +++ | ||||||||
| Purkinje cells | +/− | +/− | ||||||||
| Deep cerebellar nuclei | ++ | ++ | ||||||||
| Spinal cord | +/− | |||||||||
Signal: staining intensities of +++ strong, ++ moderate, + light, +/− little to background level; type: staining of a = cell body, b = principal neurite, c = diffuse neurite, d = diffuse, e = fiber.
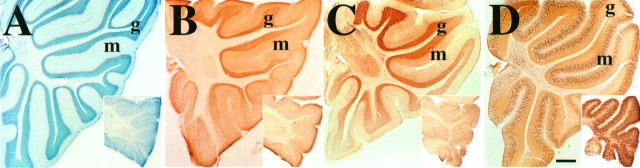
Fig. 6.
Comparison of GIRK1, GIRK2, and IRK1 staining between wild-type and wv mouse cerebellum. Parasagittal views of cerebella from wild-type mice and from PND19 wvmice (inset) counterstained with toluidine blue (A) or stained with antibodies against GIRK2 (B), GIRK1 (C), and IRK1 (D). There is a dramatic loss of the granule cell layer (g) and corresponding reduction in the staining of GIRK2 and GIRK1. The IRK1 staining of Purkinje cell body (p) and dendrites still persists in thewv cerebellum. The magnification is the same in each panel and in the insets. m, Molecular layer. Scale bar, 0.2 mm.
GIRK1 and GIRK2 immunoreactivities were observed mostly in the somatodendritic subcellular compartment and sometimes in the axon-like fibers (e.g., lateral septal nucleus), and in some brain regions they may exist in both compartments (see Table 1 for details). These observations are consistent with a previous study of GIRK1 protein distribution (Ponce et al., 1996). Both GIRK1 and GIRK2 antibodies strongly stained cell bodies and dendrites of cells of the hippocampal formation (Fig. 3D,E). GIRK2 immunoreactivity was prominent in the cell body and apical dendrite of layer V pyramidal cells in both the forelimb and hindlimb somatosensory cortex (Fig. 3B) and in both cell body and dendrites of the dopaminergic neurons in the SN (Fig. 3G,H). Unlike the predominantly somatodendritic localization of GIRK1 and GIRK2 immunoreactivities in the above brain structures, GIRK1 and GIRK2 immunoreactivities in the lateral septal region seemed to exist in axon-like fibers (data not shown).
In some brain regions, GIRK1 and GIRK2 antibodies seemed to stain synaptic regions, implying that these channel subunits may exist in pre- and/or postsynaptic membranes. In the cerebral cortex, GIRK1 immunoreactivity appeared to mark the whisker “barrels” in the primary somatosensory cortex (Fig. 3A). The barrels in layer IV of the somatosensory cortex are composed of postsynaptic stellate neurons as well as ascending presynaptic axon terminals from the ventral posterior thalamus (Waite and Tracey, 1995). Because layer IV of both cerebral cortex and ventral posterior thalamus expresses high levels of GIRK1 mRNA (DePaoli et al., 1994; Karschin et al., 1994,1996), GIRK1 protein may be present in pre- and/or postsynaptic membranes. Both GIRK1 and GIRK2 antibodies strongly stained the terminal fields of the perforant pathway (part of the trisynaptic circuit and serial/parallel sensory information processing system) in the hippocampus (stratum lacunosum moleculare or slm) and dentate gyrus (outer molecular layer), as well as commissural projection fields (stratum radiatum or sr) that contain intrahippocampal connections (Fig. 3D,E, Table 1). The cerebellar glomeruli, synaptic regions containing granule cell dendrites and mossy fiber terminals, also exhibited strong GIRK1 and GIRK2 immunoreactivity (Fig. 3K,L, arrowheads). Both GIRK1 and GIRK2 staining outlined the border of the glomeruli, suggesting that the staining may be on the postsynaptic membrane.
This pattern for GIRK1 protein distribution correlated well with the GIRK1 mRNA distribution determined by in situ hybridization (DePaoli et al., 1994; Karschin et al., 1994, 1996). To test whether the GIRK2 expression revealed by immunohistochemistry was also consistent with the GIRK2 mRNA expression pattern, we carried outin situ hybridization experiments with specific oligonucleotide probes (for sequence and controls, see Materials and Methods). Strong GIRK2 mRNA signal was present in SN pars compacta (SNc, Fig. 2C and inset), VTA (Fig.2C, inset), hippocampus (HP, Fig.2C), and cerebellar granule cell layer (g, Fig. 2C). Light staining can be seen in cerebral cortex (CTX, Fig. 2C). Low to undetectable signal was observed in striatum [e.g., caudate-putamen (CP)], thalamus (Th), hypothalamus, SN pars reticulata (SNr), and cerebellar Purkinje cell layer. The pattern we observed is consistent with that of Kobayashi et al. (1995). Thus, the overall GIRK2 mRNA distribution confirmed the immunoreactive pattern.
Antibodies against GIRK1 or GIRK2 co-immunoprecipitate GIRK1 and GIRK2 channel subunits from rat cerebral cortex, hippocampus, and cerebellum
Antibodies against the N terminus of GIRK1 immunoprecipitated GIRK1 protein of ∼62 kDa from cerebral cortical, hippocampal, and cerebellar membranes, as revealed by Western analysis using biotinylated antibodies against GIRK1 C terminus (Fig.4A) (for an explanation of background bands and rationale for biotinylating primary antibodies, see Materials and Methods). Less immunoreactivity was present in hippocampus, and the band on the Western blot was not evident in the exposure used in Figure4A. The GIRK2 antibodies also immunoprecipitated GIRK2 proteins of 48–50 kDa that stained with biotinylated antibodies against the N terminus of GIRK2 (Fig. 4A). It seemed that more GIRK1 and GIRK2 were immunoprecipitated from the cerebral cortex than from the hippocampus or cerebellum.
We found that antibodies against GIRK1 or GIRK2 co-immunoprecipitated both GIRK1 and GIRK2 channel subunits. When membranes were immunoprecipitated with GIRK1 antibody but probed for the presence of GIRK2, we observed co-immunoprecipitation of the 48–50 kDa GIRK2 doublet (Fig. 4A). Conversely, the GIRK1 protein band was co-immunoprecipitated by GIRK2 antibodies, as indicated by a broad band of 62 kDa GIRK1 immunoreactivity (Fig. 4A). Again, the cortex band was the most intense, although GIRK1 and GIRK2 from all three brain regions were co-immunoprecipitated.
To ensure that the immunoprecipitation and co-immunoprecipitation results were specific, we carried out some control experiments. The addition of respective antigenic peptides to GIRK1 or GIRK2 antibodies resulted in the absence of immunoprecipitation of the GIRK1 band by GIRK1 antibody (Fig. 4B, G1+pG1) and no co-immunoprecipitation of the GIRK1 band by the GIRK2 antibody (G2+pG2) from the cerebral cortex, whereas the addition of nonantigenic peptides had no effects (Fig. 4B,G1+pG2 and G2+pG1). Such results were attributable to the ability of the antigenic peptides to prevent immunoprecipitation rather than a lack of channel subunits in the membrane, as shown by a second immunoprecipitation of proteins from the “immunodepleted” supernatant after the first immunoprecipitation (Fig. 4B, Second IP). For example, although GIRK2 antibody could not co-immunoprecipitate GIRK1 band in the presence of GIRK2 peptide (Fig. 4B,G2+pG2), GIRK1 protein could be immunoprecipitated from the “immunodepleted” supernatant (Fig. 4B, GIRK1 lane below the G2+pG2 lane). In the presence of nonantigenic peptide, GIRK2 antibody could co-immunoprecipitate GIRK1 protein (Fig.4B, G2+pG1), but could not further co-immunoprecipitate GIRK1 from the “immunodepleted” supernatant (Fig. 4B, GIRK2 lane below the G2+pG1 lane). The same was true for GIRK1 antibody immunoprecipitation. GIRK1 could not immunoprecipitate the GIRK1 band in the presence of GIRK1 peptide (Fig. 4B, G1+pG1); from the (non) “immunodepleted” supernatant, GIRK2 antibody could co-immunoprecipitate the leftover GIRK1 protein (Fig.4B, GIRK2 lane below the G1+pG1 lane). In the presence of nonantigenic peptide, GIRK1 antibody could immunoprecipitate GIRK1 protein (Fig. 4B,G1+pG2), and no more GIRK1 protein was present in the immunodepleted supernatant (Fig. 4B, GIRK1 lane below the G1+pG2 lane).
Using antibodies against the Kir channel IRK1 or a voltage-gated K+ channel Kv1.4 (Sheng et al., 1993), we were not able to co-immunoprecipitate GIRK1 (Fig.4B, IRK1 and Kv1.4) or GIRK2 (data not shown) from the cerebral cortex. The IRK1 and Kv1.4 antibodies could immunoprecipitate IRK1 and Kv1.4 channel subunits, respectively, from brain membranes (data not shown; Sheng et al., 1993). Furthermore, we showed that GIRK1 was present in the cortical membrane used for IRK1 and Kv1.4 immunoprecipitations. In the IRK1-immunodepleted supernatant, GIRK1 protein could be co-immunoprecipitated by GIRK2 antibody (Fig. 4B, GIRK2 lane below the IRK1 lane), and in the Kv1.4-immunodepleted supernatant, GIRK1 could be immunoprecipitated by GIRK1 antibody (Fig. 4B, GIRK1 lane below the Kv1.4 lane). Taken together, these results indicated that the interaction between GIRK1 and GIRK2 as revealed by co-immunoprecipitation was specific.
Distribution of GIRK1 and GIRK2 immunoreactivity in mice is similar to that in rats
By using antibody against the N or C terminus of GIRK1 or GIRK2, we found GIRK1 and GIRK2 channel proteins in C57BL/6 mice in similar brain regions, as well as at relative intensities, as those in Sprague Dawley rats, although some species differences of GIRK2 staining in the cortex and thalamus were apparent. Although GIRK1 but not GIRK2 was found in the whisker barrels in the rat, both GIRK1 and GIRK2 immunoreactivity was observed in the whisker barrels in the mouse (data not shown). In the rat thalamus, GIRK2 is found in a few nuclei (Table1), whereas in the mouse GIRK2 staining was observed in many thalamic nuclei (data not shown). We could observe this more broad distribution of GIRK2 in the mouse when using antibodies against either the N or C terminus, although the antibody against the C terminus consistently gave lighter staining (compare Fig. 7B,E withC,F).
Fig. 7.
Reduction in dendritic and cell body staining of GIRK2 in the wv SN pars compacta (SNc) and ventral tegmental area (VTA). Comparison of coronal sections of wild-type and wv midbrain stained with antibodies against TH (A, D), N terminus of GIRK2 (B, E), or C terminus of GIRK2 (C, F). There is a dramatic decrease in both cell number and dendritic staining of GIRK2-positive neurons in the SNc andVTA, whereas the number of TH-positive neurons decreases in the SNc but remains about the same in the VTA. Scale bar, 0.1 mm.
Decrease in GIRK2 and GIRK1 protein levels inwv mice
Western blots of membranes prepared from wv and wild-type littermates showed that the GIRK2 band in wv mice was less intense than that from wild-type littermates (Fig.5A). The decrease in the 48 kDa GIRK2 band was consistent with the decrease in immunostaining (see below). The 58–60 kDa GIRK1 band in wv brain membrane also seemed slightly less intense than that of the wild-type littermate (Fig.5B). The additional 55 kDa GIRK1 band in wv brain may reflect an elevated level of unglycosylated GIRK1 proteins. Antigenic peptide against either GIRK2 or GIRK1 antibody competed off all immunoreactive bands.
Fig. 5.
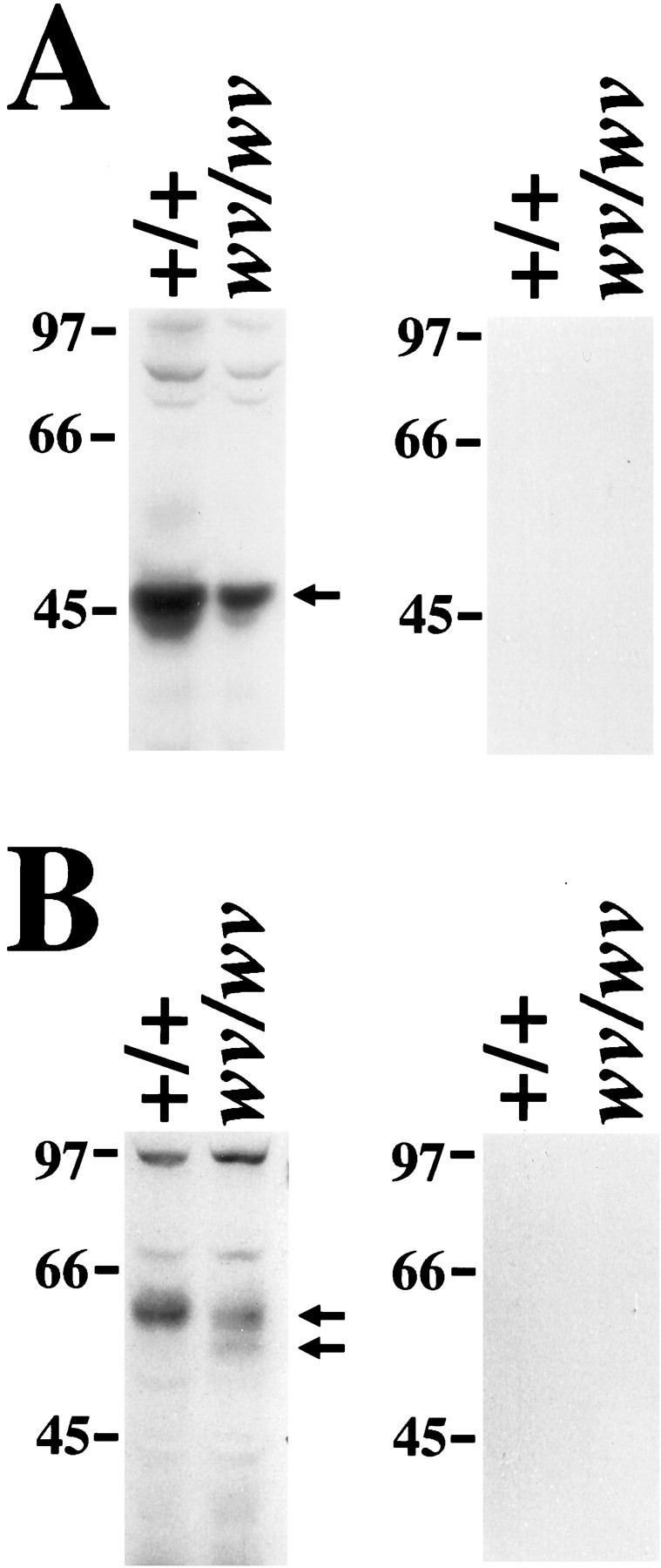
Decreased level of both GIRK2 and GIRK1 proteins in the wv brain. A, Left, Western blotting with antibody against GIRK2 shows that there is less GIRK2 protein in the wv brain than in wild-type brain. Fifty micrograms of membrane from wild-type littermate orwv brain were loaded onto each lane. B,Left, Western blotting with antibody against the N terminus of GIRK1 demonstrates that there is a slight decrease in the 58–60 kDa GIRK1 band, but there is an additional 55 kDa band, which may represent an unglycosylated form of GIRK1 in the wvbrain. A, B, Right, Peptide competition controls for antibodies against the N terminus of GIRK2 or GIRK1, respectively.
Abnormal GIRK2 expression in cerebellum and SN of thewv mice
We looked for changes of protein expression in the wvmouse as a result of the GIRK2 G156S mutation, particularly in the cerebellum and SN, which are known to exhibit cell death. In the cerebellum, we used antibodies against GIRK1, GIRK2, IRK1 (found in Purkinje cell body and dendrites; Y. Joyce Liao, unpublished data), calbindin (calcium-binding protein found in cerebellar molecular layer and Purkinje cells; Baimbridge and Miller, 1982; McRitchie et al., 1996), and P65 synaptotagmin (found in synapses; Matthew et al., 1981) to assess differences between wv mice and wild-type littermates. At postnatal day (PND) 19 (Fig.6B,C) and PND27 (data not shown), antibodies against GIRK2 and GIRK1 gave nearly uniform staining in the cerebellum of wv mice compared with the discrete staining patterns in the wild-type littermates. This difference is consistent with the observation that GIRK1 and GIRK2 are normally expressed in granule cells (Kobayashi et al., 1995; Kofuji et al., 1996; Navarro et al., 1996; Slesinger et al., 1996), and that most granule cells inwv mice have disappeared by PND19 (Fig. 6, compareA with inset). IRK1, calbindin, and P65 synaptotagmin immunoreactivity were observed in both wild-type andwv mice (Fig. 6D; data not shown), suggesting that Purkinje cells in wv mice can still differentiate to some extent. The IRK1-positive Purkinje cell bodies and dendrites in wv cerebellum were disorganized, and they filled up the entire cerebellar cortex (Fig. 6D,inset), consistent with previous observations of Purkinje cell abnormalities in wv mice (Rakic and Sidman, 1973a,b; Smeyene and Goldowitz, 1990).
We examined the SN of wild-type and wv mice for distribution of GIRK1 (absent in SN; this study), GIRK2, TH (present in SNc and VTA dopaminergic neuronal cell body and dendrites; Roffler-Tarlov and Graybiel, 1984), Kv1.4 (found in axons in SNr; Sheng et al., 1992), calbindin (found in SNr; Gaspar et al., 1994; McRitchie et al., 1996), and P65 synaptotagmin (in synapses; Matthew et al., 1981). At PND19, we found reduced cell number and dendritic staining of GIRK2-positive cells in the SNc and VTA. With increasing age, we found even fewer GIRK2-positive cells in SNc and VTA (compare Fig.7B,C with E,F). The number of TH-positive cells in the SNc also decreased with age, but there was no observable difference in the number of TH-positive cells in the VTA (Fig. 7, compare A and D). We found little difference in the staining of Kv1.4, calbindin, and P65 synaptotagmin between wv and wild-type SN (data not shown).
The hippocampal formation of wv mice exhibits abnormal GIRK2 as well as GIRK1 staining
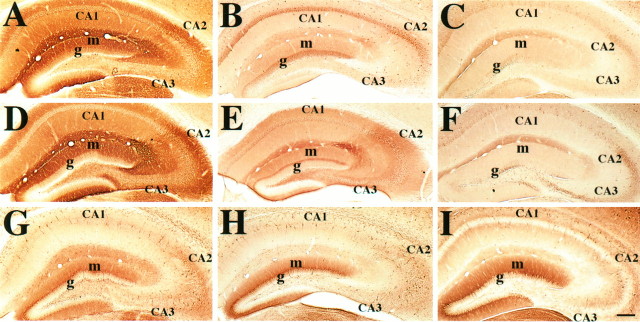
The hippocampi of PND19, PND27, and PND95 wv and wild-type littermate mice were examined for immunoreactivity with antibodies against GIRK1, GIRK2, IRK1 (found in cell bodies as well as dendrites of dentate granule cells and CA3-1 pyramidal cells; Y. Joyce Liao, unpublished observation), Kv1.4 (found in axons and axon terminals; Sheng et al., 1992), calbindin (calcium-binding protein in dentate granule cell bodies and dendrites; Baimbridge and Miller, 1982), parvalbumin (calcium-binding protein in basket pyramidal interneurons), and P65 synaptotagmin (in synapses; Matthew et al., 1981). At PND19, there was no dramatic difference between wvand wild-type hippocampi (data not shown). By PND27 (Fig.8B,E) and also at PND95 (Fig.8C,F), significant change in expression of GIRK2 as well as GIRK1 became apparent. The wv hippocampus no longer showed intense dendritic staining of GIRK2 in the molecular layer of the dentate gyrus and strata oriens and radiatum of CA3-1 areas, although the cell bodies still exhibited light immunoreactivity comparable with that in the wild-type (compare Fig.8D with E,F). Interestingly, there was a similar reduction in GIRK1 immunoreactivity in wvhippocampi (compare Fig. 8A with B,C). We observed less prominent differences between wild-type and wvhippocampi in their staining pattern of IRK1 (Fig.8G–I) and Kv1.4 (data not shown), two channel subunits that were not found to associate with GIRK1 and GIRK2 (this paper). There was also relatively little difference in the staining patterns for calbindin, parvalbumin, and P65 synaptotagmin in these mice (data not shown). No gross reduction in hippocampal cell number or size was observed. Thus, the wv mutation appeared to affect the expression of GIRK1 and GIRK2 specifically.
Fig. 8.
The GIRK2 mutation in wv mice results in defects not only in GIRK2 but also in GIRK1 expression patterns in the hippocampus. Serial sections of hippocampi from PND27 wild-type littermate (A, D, G), PND27 wv(B, E, H), and PND95 wv (C, F, I) mice for GIRK1 (A–C), GIRK2 (D–F), and IRK1 (G–I) staining. There is a dramatic decrease in dendritic staining of both GIRK1 and GIRK2 staining in the wv hippocampi, whereas a light level of immunoreactivity persists in the cell bodies. There is relatively little difference in IRK1 staining and in the number of cell bodies and structure of the hippocampus between wv and wild-type littermates. See Results for additional details. Scale bar, 0.2 mm.
DISCUSSION
Using antibodies specific for GIRK1 or GIRK2, we have determined the expression patterns for these two G-protein-gated Kir channel subunits in mammalian brain and verified that these patterns of protein expression match the mRNA distributions (DiPaoli et al., 1994; Karschin et al., 1994, 1996; Kobayashi et al., 1995; this study) and are consistent with the reported GIRK1 protein distribution (Ponce et al., 1996). We show further that GIRK1 and GIRK2 were specifically co-immunoprecipitated from cerebral cortex, hippocampus, and cerebellum. These findings, together with previous studies suggesting that GIRK1 and GIRK2 in heterologous systems can co-assemble to form heteromeric channels (Duprat et al., 1995; Kofuji et al., 1995, 1996; Lesage et al., 1995; Navarro et al., 1996;Slesinger et al., 1996), indicate that GIRK1/GIRK2 heteromultimeric channels exist in the mammalian brain. The dramatic alteration in GIRK1, as well as GIRK2 protein expressions in the wv mice, suggests that a significant proportion of GIRK1 channel subunit exists in complex with GIRK2 in the mouse brain.
GIRK channels of different composition exist in different parts of mammalian brain
GIRK channels may exist in the brain as homomeric channels composed of one subunit type or as heteromeric complexes of two or more subunit types. GIRK2 can form functional homo- or heteromeric channels in heterologous expression systems. It is therefore conceivable that homomeric GIRK2 channels and heteromeric channels containing GIRK2 may be found in different proportions in different regions of the brain. Given that GIRK1 does not seem to form functional homomeric channels in heterologous systems (Duprat et al., 1995; Kofuji et al., 1995;Krapivinsky et al., 1995a,b; Lesage et al., 1995; Hedin et al., 1996), its association with GIRK2 in vivo may form the molecular basis for some of the GIRK1-containing channels in central neurons. Because GIRK1 and GIRK2 channel proteins overlap in many brain regions in the rat and even more so in the mouse, the GIRK1 subunit may be encountered in heteromeric complexes with GIRK2 in many brain regions. In regions where GIRK1-4 all seem to be expressed, such as the hippocampus, cerebral cortex, and thalamus (Karschin et al., 1994,1996; Kobayashi et al., 1995; Ponce et al., 1996; Spauschus et al., 1996; this study), heteromeric GIRK channels may also consist of more than two different channel subunits, and channels with different subunit stoichiometry could conceivably exist in the same neuron.
Potential transmitter receptors for in vivoregulation of Kir channels composed of GIRK1 and/or GIRK2 subunits
Transmitter receptors that activate Kirchannels via G-proteins include those for acetylcholine (M2), adenosine (A1), ATP (P2), dopamine (D2), GABAB, opioid (μ, δ, κ), serotonin (5-HT1, 5-HT2), norepinephrine (α2), and somatostatin (North et al., 1987; North, 1989; Brown, 1990;Nicoll et al., 1990; Hille, 1992, 1994; Inoue and Yoshi, 1992). Other receptors such as those for substance P, neurotensin, thyrotrophin-releasing hormone, and angiotensin II inhibit Kir channels and cause membrane depolarization (reviews listed above; Stanfield et al., 1985; Yamaguchi et al., 1990; Takano et al., 1995). In vitro co-expression experiments have shown that GIRK channel subunits can form homo- or heteromeric channels that are activated by neurotransmitter receptors such as M2acetylcholine receptor (Dascal et al., 1993; Kubo et al., 1993;Krapivinsky et al., 1995a,b; Slesinger et al., 1996), β2-adrenergic receptor (Lim et al., 1995), δ opioid receptor (Dascal et al., 1993; Lesage et al., 1994), μ opioid receptor (Chen and Yu, 1994; Kovoor et al., 1995), κ opioid receptor (Henry et al., 1995; Ma et al., 1995), and 5-HT1A receptor (Dascal et al., 1993; Kovoor et al., 1995).
Through the use of electrophysiological and distribution studies, many neurotransmitter receptors that regulate Kir via G-proteins have been found in brain regions that also express GIRK1 and/or GIRK2. Although in vitro evidence shows that many neurotransmitter receptors have the potential to regulate Kir channels composed of GIRK1-4 subunits, it is not known just which combination of receptors and GIRK channels mediates the neurotransmitter effects in different brain regions. We provide here a brief listing of potential receptors that may regulate Kirchannels containing GIRK1 and/or GIRK2 channel subunits (for review, see North et al., 1987; North, 1989; Brown, 1990; Nicoll et al., 1990; Inoue et al., 1992; acetylcholine receptor: Levey et al., 1991;Karschin et al., 1994; Butcher, 1995; adenosine A1 receptor: Weber et al., 1990; Schwabe et al., 1991; dopamine D2 receptor:Mansour et al., 1990; opioid receptors: Mansour et al., 1987; Meng et al., 1993; Thompson et al., 1993; Yasuda et al., 1993; Arvidsson et al., 1995; Mestek, 1995; somatostatin receptor: Breder et al., 1992; Gonzalez et al., 1992; Karschin et al., 1994; Reisine and Bell, 1995). In brain regions that express both GIRK1 and GIRK2, the cerebral cortex expresses the adenosine A1 receptors, receptors for opioid peptides, and somatostatin receptors. The hippocampus and dentate gyrus express the A1 receptors, GABABreceptors, D2 dopamine receptors, 5-HT1Areceptors, μ receptors, and somatostatin receptors. The cerebellar granule cells express the A1 receptors and somatostatin receptors (transiently during development). Of regions that express GIRK2 but only low levels of GIRK1, the A1 adenosine receptors, κ1 and μ opioid receptors, and D2 dopamine receptors are found in the SNc and VTA. The present study, combined with previous studies of transmitter receptor distributions, provides a basis for further characterization of the molecular composition of Kir channels that are effectors of various transmitter receptors.
The somatodendritic localization of GIRK1 and GIRK2 channel subunits in some brain regions suggests that these channels may be involved in specific functions
The relatively ubiquitous GIRK2 and GIRK1 immunoreactivity and their regulation by many different transmitter receptors when expressed in heterologous systems indicate that they are likely to contribute to a significant fraction of the GIRK channels in vivo. The presence of mRNA and the somatodendritic localization of GIRK1 and GIRK2 proteins in certain central neurons (e.g., cerebral cortical pyramidal cells, SN dopaminergic neurons, and cerebellar granule cells) suggest that they are on postsynaptic membranes. Many G-protein-coupled receptors are found in dendrites and cell bodies, where they may mediate some of the longer lasting effects of transmitters released from nerve terminals of presynaptic neurons. Similar receptors are also present in nerve terminals, where they may regulate transmitter release. In the SN, the D2 autoreceptors in dopaminergic neurons may regulate the dendritic release of dopamine, a neurotransmitter believed to be important for the self-regulation of dopaminergic neurons (Cheramy et al., 1981). Because GIRK2 is highly expressed in the SNc (Figs. 3G,H, 7B,C) but in low or background level in the striatum (Fig. 2B), it seems more likely that GIRK2 is involved in controlling dendritic dopamine release than in controlling release from nigral dopaminergic axon terminals. In many other regions of the brain, GIRK1 and GIRK2 are localized to sites of synapses, such as the glomeruli of the cerebellar granule cell layer (Fig. 3K,L), perforant pathway terminal fields in the hippocampal formation (Figs. 3D,E,8A,D), and layer IV of the barrel cortex (Fig.3A). To elucidate the function of the GIRK channel subunits in these regions, it will be important to use electron microscopy to determine their pre- and/or postsynaptic localization and to identify transmitter receptors that may regulate them in vivo. Recently, Ponce et al. (1996) showed by electron microscopy that GIRK1 is found in the postsynaptic membrane of the granule cell dendrites, and they also showed that GIRK1 may be present presynaptically in the thalamic projections to the layer IV of the cerebral cortex and stratum lacunosum moleculare of the hippocampus.
Downregulation of GIRK1 as well as GIRK2 protein expression and distribution in the wv mice may be a result of their association in vivo
It is surprising that the wv mutation, a single nucleotide change, could have resulted in the decrease in protein level as well as the dramatic alteration in protein distribution of both GIRK1 and GIRK2 channel subunits. Given that GIRK1 and GIRK2 interactin vivo to form heteromeric complexes, the simplest explanation for the downregulation of both proteins is the removal of GIRK1/GIRK2 channel complexes that contain abnormal GIRK2wvprotein. Central neurons may target the GIRK1/GIRK2wvchannel complex for degradation attributable to either abnormal structure or function. The selective removal of aberrant channel complexes may take place soon after protein synthesis in the endoplasmic reticulum or at the level of protein targeting. Some of the downregulation of expression may also take place after the aberrant GIRK1/GIRK2wv complex has reached the plasma membrane, where its abnormal electrophysiological properties would be evident. Because abnormal current as a result of the wv mutation can be recorded from the cell body of dissociated cerebellar granule cells (Kofuji et al., 1996; Surmeier et al., 1996) (P. A. Slesinger, unpublished observation), some GIRK channel complex containing the GIRK2wv subunit is clearly inserted into the plasma membrane of the cell body. Because more of the unglycosylated form of GIRK1 can be found in the wv mice, it seems likely that some of the GIRK1/GIRK2wv complex is being retained in the endoplasmic reticulum.
Mutation in GIRK2 may directly or indirectly give rise to cerebellar and dopaminergic dysfunctions in wv mice
The G156S mutation in GIRK2 causes a range of abnormalities of channels expressed in Xenopus oocytes, including loss of potassium selectivity of GIRK2 channels and reduction of function of heteromultimers of GIRK1 and GIRK2 (Kofuji et al., 1996; Navarro et al., 1996; Slesinger et al., 1996). It thus appears that thewv mutation could qualitatively exert different effects in different neurons, depending on the level of expression of GIRK2 and other Kir channel subunits such as GIRK1. In neurons that express high levels of GIRK2 but not GIRK1, such as the dopaminergic neurons in the SN, the wv mutation could lead to chronic or inhibitory transmitter-induced depolarization and potentially to cell death. In other neurons that normally produce heteromeric channels containing GIRK1 and GIRK2 subunits, such as those in the cerebral cortex, hippocampus, thalamus, and cerebellum, this mutation may weaken or abolish certain signaling processes. It seems likely that cerebellar granule cells could belong to this latter category. Although no dramatic anatomical defects in hippocampus, cerebral cortex, and thalamus have been reported (but see Sekiguchi et al., 1995), we find that the subcellular distribution of both GIRK1 and GIRK2 channel subunits in these brain regions is severely altered in thewv mice. It is possible that neurons in these brain regions have compensatory mechanisms that prevent them from dying, unlike the cerebellar granule cells and SN dopaminergic neurons. These mechanisms may facilitate the removal of free calcium from the cytoplasm, removal of mutant channel complexes from the plasma membrane, or substitution of GIRK2 functions with other GIRK channel subunits. To understand the roles of G-protein-gated Kir channels in vivoand in the different wv mutant phenotypes, it would be important to determine the channel compositions and transmitter receptors that regulate these channels in different cell types during development and in the adult.
Footnotes
This research was supported by a grant from the National Institute of Mental Health to the Silvio Conte Neuroscience Center at the University of California, San Francisco (UCSF), the UCSF Neuroscience Graduate Program, and the Medical Scientist Training Program. Y.N.J. and L.Y.J. are Howard Hughes Medical Institute investigators. We thank A. Collins and P. A. Slesinger for oocyte injections, J. Yu for technical assistance, E. Reuveny for providing cRNA, N. Patil for providingweaver mice, L. Reichardt and Isabel Fariñas for P65 and TH antibodies, L. Ackerman and William Walantus for assistance with photography, and A. Basbaum for instructions on spinal cord dissection. We also thank C. Bargmann, D. Bredt, S. Gompert, T. Hensch, T. Hwang, P. Slesinger, and M. Vetter for insightful discussions and critical comments on an earlier draft of this manuscript.
Correspondence should be addressed to Lily Yeh Jan, Howard Hughes Medical Institute, University of California, San Francisco, San Francisco, CA 94143-0724.
REFERENCES
- 1.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- 3.Bond CT, Ammala C, Ashfield R, Blair TA, Gribble F, Khan RN, Lee K, Proks P, Rowe ICM, Sakura H, Ashford MJ, Adelman JP, Ashcroft FM. Cloning and functional expression of the cDNA encoding an inwardly-rectifying potassium channel expressed in pancreatic β-cells and in the brain. FEBS Lett. 1995;367:61–66. doi: 10.1016/0014-5793(95)00497-w. [DOI] [PubMed] [Google Scholar]
- 4.Breder CD, Yamada Y, Yasuda K, Seino S, Saper CB, Bell GI. Differential expression of somatostatin receptor subtypes in brain. J Neurosci. 1992;12:3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- 6.Brown D. G-proteins and potassium currents in neurons. Annu Rev Physiol. 1990;52:215–242. doi: 10.1146/annurev.ph.52.030190.001243. [DOI] [PubMed] [Google Scholar]
- 7.Brown AM, Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- 8.Butcher LL. Cholinergic neurons and networks. In: Paxinos G, editor. The rat nervous system. Academic; San Diego, CA: 1995. pp. 1003–1015. [Google Scholar]
- 9.Chen Y, Yu L. Differential regulation by cAMP-dependent protein kinase and protein kinase C of the μ-opioid receptor coupling to a G protein-activated K+ channel. J Biol Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- 10.Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 11.Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D, Lester HA, Davidson N. Atrial G-protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci USA. 1993;90:10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePaoli AM, Bell GI, Stoffel M. G protein-activated inwardly rectifying potassium channel (GIRK1/KGA) mRNA in adult rat heart and brain by in situ hybridization histochemistry. Mol Cell Neurosci. 1994;5:515–522. doi: 10.1006/mcne.1994.1063. [DOI] [PubMed] [Google Scholar]
- 13.Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 14.Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunski M, Romey G, Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun. 1995;212:657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg B, Messer A. Tonic/clonic seizures in a mouse mutant carrying the weaver gene. Neurosci Lett. 1989;96:168–172. doi: 10.1016/0304-3940(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar P, Ben Jelloun N, Febvret A. Sparing of the dopaminergic neurons containing calbindin-D28K and of the dopaminergic mesocortical projections in weaver mutant mice. Neuroscience. 1994;61:293–305. doi: 10.1016/0306-4522(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 17.Goldowitz D, Smeyne RJ. Tune into the weaver channel. Nature Genet. 1995;11:107–109. doi: 10.1038/ng1095-107. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez B, Leroux P, Lamacz M, Bodenant C, Balazs R, Vaudry H. Somatostatin receptors are expressed by immature cerebellar granule cells: evidence for a direct inhibitory effect of somatostatin on neuroblast activity. Proc Natl Acad Sci USA. 1992;89:9627–9631. doi: 10.1073/pnas.89.20.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SMW, Roffler-Tarlov S. Male-sterile phenotype of the neurological mouse mutant weaver. Dev Dynamics. 1994;200:26–38. doi: 10.1002/aja.1002000104. [DOI] [PubMed] [Google Scholar]
- 20.Hatten ME, Liem RK, Mason CA. Defects in specific associations between astroglia and neurons occur in microcultures of weaver mouse cerebellar cells. J Neurosci. 1984;4:1163–1172. doi: 10.1523/JNEUROSCI.04-04-01163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- 22.Henry DJ, Grandy DK, Lester HA, Davidson N, Chavkin C. κ-opioid receptors couple to inwardly rectifying potassium channels when co-expressed by Xenopus oocytes. Mol Pharmacol. 1995;47:551–557. [PubMed] [Google Scholar]
- 23.Hess EJ. Identification of the weaver mouse mutation: the end of the beginning. Neuron. 1996;16:1073–1076. doi: 10.1016/s0896-6273(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 24.Hille B. Ionic channels of excitable membranes, 2nd ed. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 25.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:409–442. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 26.Inoue M, Yoshi M. Modulation of ion channels by somatostatin and acetylcholine. Prog Neurobiol. 1992;38:203–230. doi: 10.1016/0301-0082(92)90040-l. [DOI] [PubMed] [Google Scholar]
- 27.Isomoto S, Kondo C, Takahashi N, Matsumoto S, Yamada M, Takumi T, Horio Y, Kurachi Y. A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun. 1996;218:286–291. doi: 10.1006/bbrc.1996.0050. [DOI] [PubMed] [Google Scholar]
- 28.Jan LY, Jan YN. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- 29.Karschin C, Schrebmayer W, Dascal N, Lester H, Davidson N, Karschin A. Distribution and localization of a G protein-coupled inwardly rectifying K+ channel in the rat. FEBS Lett. 1994;348:139–144. doi: 10.1016/0014-5793(94)00590-7. [DOI] [PubMed] [Google Scholar]
- 30.Karschin C, Dißmann E, Stühmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun. 1995;208:1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- 32.Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 34.Kovoor A, Henry DJ, Chavkin C. Agonist-induced desensitization of the mu opioid receptor-coupled potassium channel (GIRK1). J Biol Chem. 1995;270:589–595. doi: 10.1074/jbc.270.2.589. [DOI] [PubMed] [Google Scholar]
- 35.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995a;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 36.Krapivinsky G, Krapivinsky L, Velimirovic B, Wickman K, Navarro B, Clapham DE. The cardiac inward rectifier K+ channel subunit, CIR, does not comprise the ATP-sensitive K+ channel, IKATP. J Biol Chem. 1995b;270:28777–28779. doi: 10.1074/jbc.270.48.28777. [DOI] [PubMed] [Google Scholar]
- 37.Kubo Y. Towards the elucidation of the structural-functional relationship of inward rectifying K+ channel family. Neurosci Res. 1994;21:109–117. doi: 10.1016/0168-0102(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 38.Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- 39.Lane JD, Nadi NS, McBride WJ, Aprison MH, Kusano K. Contents of serotonin, norepinephrine and dopamine in the cerebrum of the “staggerer,” “weaver” and “nervous” neurologically mutant mice. J Neurochem. 1977;29:349–350. doi: 10.1111/j.1471-4159.1977.tb09629.x. [DOI] [PubMed] [Google Scholar]
- 40.Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot J-P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- 41.Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- 42.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim NF, Dascal N, Labarca C, Davidson N, Lester HA. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβγ subunits in Xenopus oocytes. J Gen Physiol. 1995;105:421–439. doi: 10.1085/jgp.105.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma GH, Miller RJ, Kuznetsov A, Philipson LH. κ-Opioid receptor activates an inwardly rectifying K+ channel by a G protein-linked mechanism: coexpression in Xenopus oocytes. Mol Pharmacol. 1995;47:1035–1040. [PubMed] [Google Scholar]
- 45.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 46.Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthew W, Tsavaler L, Reichardt LF. Identification of a synaptic vesicle specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J Comp Neurol. 1996;364:121–150. doi: 10.1002/(SICI)1096-9861(19960101)364:1<121::AID-CNE11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Meng F, Xie G-X, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat κ opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human μ opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarro B, Kennedy ME, Velimirovic B, Bhat D, Peterson AS, Clapham DE. Nonselective and Gβγ-insensitive weaver K+ channels. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- 52.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 53.North A. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.North RA, Williams JT, Surprenant A, Christie MJ. μ and δ receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nature Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 56.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed. Academic; San Diego: 1986. [Google Scholar]
- 57.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K+ channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 58.Ponce A, Bueno E, Kentros C, Vega-Saenz de Miera E, Chow A, Hillman D, Chen S, Zhu L, Wu MB, Wu X, Rudy B, Thornhill WB. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J Neurosci. 1996;16:1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakic P, Sidman RL. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973a;152:103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- 60.Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973b;152:133–162. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 61.Reisine T, Bell G. Molecular properties of somatostatin receptors. Neuroscience. 1995;67:777–790. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- 62.Roffler-Tarlov S, Graybiel AM. weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature. 1984;307:62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt MJ, Sawyer BD, Perry KW, Fuller RW, Foreman MM, Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci. 1982;2:376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwabe U, Lorenzen A, Grun S. Adenosine receptors in the central nervous system. J Neurotransm [Suppl] 1991;34:149–155. doi: 10.1007/978-3-7091-9175-0_19. [DOI] [PubMed] [Google Scholar]
- 65.Sekiguchi M, Nowakowski RS, Nagato Y, Tanaka O, Guo H, Madoka M, Abe H. Morphological abnormalities in the hippocampus of the weaver mutant mouse. Brain Res. 1995;696:262–267. doi: 10.1016/0006-8993(95)00974-u. [DOI] [PubMed] [Google Scholar]
- 66.Sheng M, Tsaur M-L, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 67.Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- 68. Sidman R, Green M, Appel S. Catalogue of neurological mutants of the mouse. 1965. Harvard; Cambridge, MA: UP. [DOI] [PubMed] [Google Scholar]
- 69.Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 70.Smeyne RJ, Goldowitz D. Purkinje cell loss is due to a direct action of the weaver gene in Purkinje cells: evidence from chimeric mice. Dev Brain Res. 1990;52:211–218. doi: 10.1016/0165-3806(90)90237-s. [DOI] [PubMed] [Google Scholar]
- 71.Spauschus A, Lentes K-U, Wischmeyer E, Dibmann E, Karschin C, Karschin A. A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. J Neurosci. 1996;16:930–938. doi: 10.1523/JNEUROSCI.16-03-00930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- 73.Surmeier DJ, Mermelstein PG, Goldowitz D (1996) Theweaver mutation of GIRK2 results in a loss of inwardly rectifying K+ current in cerebellar granule cells. Proc Natl Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- 74.Takano K, Stanfield PR, Nakajima S, Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995;14:999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 75.Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat μ opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 76.Tsaur M-L, Menzel S, Lai F-P, Espinosa R, III, Concannon P, Spielman RS, Hanis CL, Cox NJ, Le Beau MM, German MS, Jan LY, Bell GI, Stoffel M. Isolation of a cDNA clone encoding a KATP channel-like protein expressed in insulin-secreting cells, localization of the human gene to chromosome band 21q22.1, and linkage studies with NIDDM. Diabetes. 1995;44:592–596. doi: 10.2337/diab.44.5.592. [DOI] [PubMed] [Google Scholar]
- 77.Velimirovic BM, Gordon EA, Lim NF, Navarro B, Clapham DE. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Lett. 1996;379:31–37. doi: 10.1016/0014-5793(95)01465-9. [DOI] [PubMed] [Google Scholar]
- 78.Vogelweid CM, Verina T, Norton J, Harruff R, Ghetti B. Hypospermatogenesis is the cause of infertility in the male weaver mutant mouse. J Neurogenet. 1993;9:89–104. doi: 10.3109/01677069309083452. [DOI] [PubMed] [Google Scholar]
- 79.Waite PME, Tracey DJ. Trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 705–724. [Google Scholar]
- 80.Weber R, Jones CR, Lohse MJ, Palacios JM. Autoradiographic visualization of A1 adenosine receptors in rat brain with [H3]H-cyclopentyl-1,3-dipropylxanthine. J Neurochem. 1990;54:1344–1353. doi: 10.1111/j.1471-4159.1990.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 81.Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995a;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 82.Wickman K, Clapham DE. G-protein regulation of ion channels. Curr Opin Neurobiol. 1995b;5:278–285. doi: 10.1016/0959-4388(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 83.Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ hybridization protocols for the brain. Academic; San Diego: 1994. pp. 9–34. [Google Scholar]
- 84.Yamaguchi K, Nakajima Y, Nakajima S, Stanfield PR. Modulation of inwardly rectifying channels by substance P in cholinergic neurons from rat brain in culture. J Physiol (Lond) 1990;426:499–520. doi: 10.1113/jphysiol.1990.sp018151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J, Jan YN, Jan LY. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995;14:1047–1054. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 86.Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. Cloning and functional comparison of κ and δ opioid receptors from mouse brain. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]