Abstract
Functional and structural abnormalities in the medial prefrontal cortex (MPFC) and overactive dopamine (DA) neurotransmission are thought to be the key pathologies in schizophrenia. To understand the role of MPFC in the pre- and postpubertal development of the subcortical DA system, the effects of neonatal [postnatal day 7 (PD7)] MPFC excitotoxic lesions on locomotor behaviors and the expression of DA receptor subtypes and DA transporter were investigated in Sprague Dawley rats at PD35 and PD56, respectively. No significant differences in the novelty or d-amphetamine-induced locomotion were observed between sham-operated and ibotenic acid-lesioned rats at PD35. Postpubertally (at PD56), however, the locomotor activity of lesioned rats in the novel environment and afterd-amphetamine administration was enhanced significantly compared with controls. The expressions of DA D1, D2, D3, and D4 receptors and DA transporter were then estimated in MPFC-lesioned and sham-operated rats at PD39 and PD60. The levels of DA D2 receptors, measured using [3H]-YM-09151-2 binding, and its mRNA byin situ hybridization, were observed to be significantly increased at PD60 in striatal and limbic areas of lesioned rats. Levels of other DA receptor subtypes were not significantly affected at any time points. Lesioned rats at PD39 show a small increase in DA transporter level in the shell of nucleus accumbens; however, this effect seems to wear off at PD60. The data suggest that neonatal MPFC lesions may alter the functional development and maturation of mesolimbic/nigrostriatal DA systems in that neonatally lesioned rats grow into a behavioral/neurochemical deficit.
Keywords: prefrontal cortex, schizophrenia, animal model, nucleus accumbens, ibotenic acid, dopamine receptors, neurodevelopment
Considerable evidence from clinical, neuropsychological, brain imaging, and postmortem neuroanatomical studies strongly implicates the medial prefrontal cortex (MPFC) in the pathophysiology of schizophrenia (for review, see Weinberger et al., 1994). MPFC neuronal activity exerts an important regulatory control on the subcortical dopamine (DA) system, the overactivity of which is believed to underlie some of the psychotic symptoms of the disease (for review, see Goldstein and Deutch, 1992). The importance of the MPFC in regulating the action of the limbic system, especially the mesolimbic DA system, can be recognized by examining its connectivity. First, the neurons of the MPFC are interconnected with the limbic cortex directly through intracortical projections (Goldman-Rakic et al., 1984; Jay and Witter, 1991). In addition, the MPFC projects to the substantia nigra pars compacta and ventral tegmental area (VTA), the sources of striatal and mesocorticolimbic DAergic projections, respectively (Sesack and Pickel, 1992). Furthermore, MPFC efferents to the VTA control the DA output to the nucleus accumbens (Taber et al., 1995; Karreman and Moghaddam, 1996). Additional MPFC connections include direct glutamatergic excitatory projections to the caudate-putamen and nucleus accumbens (Sesack et al., 1989; Deutch, 1992), which may offer further control of DA release (Grace, 1991; Karreman and Moghaddam, 1996).
Biochemical studies have indicated that lesion of the MPFC or activation of its pathways alters the function of the subcortical DA systems. For example, excitotoxic lesions of the adult rat MPFC have been associated with a transiently enhanced DA turnover in limbic regions (Christie et al., 1986), and 6-hydroxydopamine lesions of MPFC have been reported to increase limbic DA transmission (Pycock et al., 1980; Leccese and Lyness, 1987) as well as enhance the responsiveness of the mesolimbic DA neurons to stress (Deutch et al., 1990; Doherty and Gratton, 1996). Chemical and electrical stimulation of the MPFC have been shown to increase DA release in the striatum (Murase et al., 1993; Taber and Fibiger, 1993). Furthermore, adult rats with MPFC excitotoxic lesions exhibit higher locomotor response to novelty, a transitory increase in amphetamine-induced locomotor activity (Jaskiw et al., 1990), swim-stress-induced locomotion (Jaskiw and Weinberger, 1992), and a decrease of the cataleptogenic effect of haloperidol (Worms et al., 1985), all of which are behaviors related to DA transmission.
In recent years, various evidence has accumulated that implicates cortical neuronal maldevelopment in schizophrenia (for review, seeWeinberger and Lipska, 1995). Although studies on MPFC lesions induced in the adult rat provide important insights into the regulatory functions of this structure on DA systems, they do not address the consequences of lesions to the MPFC on the development of subcortical DAergic activity. Because schizophrenia symptoms typically appear after puberty, i.e., in adolescence or early adulthood, it is important to understand the role of early neurodevelopmental lesions on the maturation of DA systems before and after puberty. Recently, the developmental aspects of disrupting the prefrontal–temporal connectivity were addressed by performing neonatal lesions of the ventral hippocampus (VH) (Lipska et al., 1992, 1993a,b). These studies have shown that neonatal lesions of the VH result in a delayed, postpubertal onset of behavioral hyperactivity (Lipska et al., 1993a,b), which may be associated with decreased DA D3 receptors in the nucleus accumbens (Flores et al., 1996). In the present investigation, we have assessed the developmental consequence of neonatal ibotenic acid-induced lesions of MPFC in postnatal day (PD) 7 rats. At pre- and postpubertal ages, animals were tested in behavioral paradigms commonly used to asses the functioning of the mesolimbic DA system, which demonstrated a postpubertal onset of increased amphetamine-induced locomotion in the neonatally lesioned rats. DA receptor subtypes and the DA transporter were also measured at both ages and revealed an increase in the DA D2 receptors that correlated with the onset of behavioral changes in the MPFC-lesioned animals. The results suggest an important role of developing MPFC in the functional maturation of subcortical DA activity.
MATERIALS AND METHODS
Materials.d-Amphetamine sulfate, ibotenic acid, ketanserine, butaclamol, 8-OH-DPAT, GTP, GBR-12909, and DA were purchased from RBI (Natik, MA); 1,3-di(2-5-tolyl)guanidine (DTG) was from Sigma (St. Louis, MO). [3H]-SCH23390 (70 Ci/mmol), [3H]-YM-09151-2 (86 Ci/mmol), and [3H]-WIN-35428 (84 Ci/mmol) were obtained from DuPont NEN (Boston, MA). [3H]-7-OH-DPAT (139 Ci/mmol), [3H]-Hyperfilm, and microscale tritium standards were purchased from Amersham Canada (Toronto, Ontario). Oligonucleotides probes were synthesized by the Sheldon Biotechnology Facility of McGill University (Montreal, Quebec). 2-Methyl-butane was purchased from BDH (Montreal, Quebec), and EDTA from Boehringer Mannheim (Laval, Quebec). Gelatin and bovine serum albumin were purchased from Fisher Scientific (Montreal, Quebec) and Calbiochem (La Jolla, CA) respectively. All others chemical used were also of analytical reagent quality.
Neonatal MPFC lesions. Pregnant Sprague Dawley rats were obtained at gestational day 14–17 from Charles River Canada (St. Constant, Quebec). Animals were housed individually in a temperature- and humidity-controlled environment on a 12 hr light/dark cycle with free access to food and water. The day after birth, litters of six to eight male pups were formed, and on PD7 each pup (weighing 15–17 gm) was assigned to either a sham (n = 16) or a lesion (n = 20) group. All surgical procedures described in this study have been approved previously by McGill University Animal Care Committee in accordance with the guidelines of the Canadian Council for Animal Care. After the induction of anesthesia by hypothermia, pups were positioned on a platform fixed to a stereotaxic Kopf instrument. An incision was made over the skull and 0.3 μl ibotenic acid (10 μg/μl) or an equal volume of vehicle (0.1 m PBS, pH 7.4) was injected in each side of the MPFC during a 2 min period through a 30 gauge stainless steel cannulae positioned at the following coordinates: anteroposterior, +2.5 mm; mediolateral, ± 0.4 mm to bregma; ventrodorsal −2.2 mm from dura. The cannulae remained in place for 5 min after completion of the infusion. After the procedure, the pups were placed under a warming lamp for recovery and then returned to their mothers. On PD21 animals were weaned and grouped two or three animals per cage.
Behavioral testing. Four (PD35) or 7 weeks (PD56) after neonatal lesions, the locomotor activity of sham-operated and ibotenic acid-lesioned rats was assessed in two-photocell activity boxes connected to an IBM computer equipped with a software (ACTANAL) developed by Concordia University (Montreal, Quebec). The locomotor activity of each animal was assessed under the following testing conditions. (1) Locomotion after exposure to a novel environment: unacclimatized rats were placed in the activity boxes, and the beam crosses were recorded for 60 min. (2) Locomotion afterd-amphetamine injection: 2 d after the first test, rats were again placed in the activity boxes, and basal locomotor activity was recorded for 60 min. Animals were injected first with 0.9% NaCl, and then after 60 min of locomotor assessment withd-amphetamine sulfate (1 mg/kg, s.c., dissolved in 0.9% NaCl); locomotion was monitored further for the next 120 min. Locomotor activity results were analyzed by applying two-way ANOVA followed by Newman–Keuls tests for post hoc comparisons, with lesion and age as independent factors (p < 0.05 was considered significant).
Brain processing. Four sham-operated and five lesioned rats per group were selected at random and killed by rapid decapitation 48 hr after the last testing day (PD39 and PD60, respectively). Brains were removed rapidly, frozen in isopentane (−40°C), and stored at −80°C until use. For assessment of lesion size, coronal sections at the level of the MPFC were stained with 0.5% cresyl violet and examined using a light microscope where lesions and probe placement could be visualized. Frozen brains were sectioned at 15 μm thickness on the coronal plane using a Leitz cryostat. Sections were collected on cleaned, gelatin-coated microscope slides (four sections/slides), thaw-mounted, desiccated under vacuum at 4°C overnight, and then stored at −80°C until the day of the experiment.
Receptor autoradiography. Coronal brain sections taken at the level of the nucleus accumbens and caudate-putamen [plate 10–11 of Paxinos and Watson atlas (1986)] were used in the following protocols. For D1-like receptor binding, sections were first preincubated for 10 min at room temperature in buffer containing 50 mm Tris-HCl, pH 7.4, 154 mm NaCl, 1 mm EDTA, and 0.1% bovine serum albumin. Sections were then incubated for 90 min at room temperature in the same buffer with the addition of 2 nm [3H]-SCH-23390 (74 Ci/mmol) and 30 nm ketanserin (to mask possible binding of ligand to serotonergic 5-HT2 sites). Nonspecific binding was determined on adjacent brain sections by adding 1 μm (+)-butaclamol to the buffer. Incubations were terminated by dipping the slides in ice-cold buffer followed by two consecutive 10 min washes in buffer. After a final dipping in ice-cold distilled water, slides were dried at room temperature and apposed to [3H]-Hyperfilm for 5 d, alongside microscales-calibrated tritium standards.
For D2-like and D4 receptor binding, the slides were first preincubated for 10 min at room temperature in buffer containing 50 mmTris-HCl, pH 7.4, 120 mm NaCl, 1 mm EDTA, 5 mm KCl, 1.5 mm CaCl2, and 4 mm MgCl2. Sections were then incubated for 2 hr at room temperature in the same buffer containing 1 nm[3H]-YM-09151-2 (86 Ci/mmol) with or without 100 nm raclopride, for assessment of D4 or D2 receptor bindings, respectively. 8-OH-DPAT (50 nm) was added in each case to mask possible binding of the ligands to serotonergic 5-HT1a sites. Nonspecific binding was determined on adjacent brain sections by adding 1 μm (+)-butaclamol in the buffer. Incubations were terminated by dipping the slides in ice-cold buffer followed by two consecutive 10 min washes in the same buffer. After a final dipping in ice-cold distilled water, slides were dried at room temperature and apposed to [3H]-Hyperfilm for 12 d, alongside microscales-calibrated tritium standards.
[3H]-7-OH-DPAT binding to D3 receptor was assessed according to the procedure of Lévesque et al. (1992), with minor modifications (Flores et al., 1996). Tissue sections were first preincubated for 30 min in buffer containing 50 mmTris-HCl, pH 7.4, 100 mm NaCl, and 300 μmGTP. Sections were then incubated for 2 hr at room temperature with 2 nm [3H]-7-OH-DPAT, 50 mmTris-HCl, pH 7.4, 100 mm NaCl, 300 μm GTP, and 5 μm DTG (to block binding to ς site). 1 μm DA was used in adjacent sections to determine nonspecific labeling. Incubations were terminated by washing the brain sections twice for 10 min each in ice-cold 50 mm Tris-HCl, pH 7.4. After a brief dipping in ice-cold distilled water, brain sections were dried rapidly and apposed to [3H]-Hyperfilm for 6 weeks, alongside microscales-calibrated tritium standards.
DA transporter binding was assayed essentially according to the method of Kaufman et al. (1991). The sections were first preincubated for 20 min at 4°C in 50 mm Tris-HCl, pH 7.4, containing 100 mm NaCl. Sections were then incubated for 2 hr at 4°C in the same buffer containing 10 nm[3H]-WIN-35428 (84 Ci/mmol). Nonspecific binding was determined on adjacent brain sections by adding 1 μmGBR-12909 in the buffer. Incubations were terminated by washing the brain sections twice for 1 min each in ice-cold 50 mmTris-HCl, pH 7.4. After a brief dipping in ice-cold distilled water, brain sections were dried rapidly and apposed to [3H]-Hyperfilm for 6 week.
In situ hybridization. The probe for D2 receptor was a 39-mer oligonucleotide complementary to nucleotides 937–975 of the coding region of rat D2 receptor cDNA (5′-GGT CCGGGTTTTGCCATTGGGCATGGTCTGGATCTCAAA) (Bunzow et al., 1988) and recognizes both alternatively spliced isoforms (D2L and D2S) of the receptor mRNA. The probe for D1 receptor was a 39-mer oligonucleotide complementary to nucleotides 1240–1278 of the coding region of rat D1 receptor cDNA (5′-ATAGTCCAATATGACCGATAAGGCTGGGGACAGCTTCTC) (Zhou et al., 1990). The oligonucleotides were 3′-end-labeled with [α-35S] dATP and terminal transferase and purified through an NACS column (Bethesda Research Labs, Mississauga, ON).In situ hybridization was performed on 15 μm coronal sections, essentially according to previously described procedure (Srivastava et al., 1992). The sections were fixed in 4% paraformaldehyde, and after acetylation (triethanolamine and acetic anhydride) and defatting the sections were hybridized by placing 80–100 μl of hybridization mixture containing [α-35S]-labeled oligonucleotide probe (3 × 105 cpm), 5× SSC, 50% formamide, 2× Denhardt’s solution, 10% dextran sulfate, 100 μg/ml salmon sperm DNA, and 50 μg/ml yeast tRNA at 42°C for 15–18 hr in a humidified box. They were washed 4 × 30 min in 2× SSC at room temperature, 2 × 30 min in 0.1× SSC at 45°C, and finally 1 × 15 min in 0.1× SSC at room temperature. After a brief rinse in ethanol containing 0.3 m ammonium acetate, pH 7.0, sections were air-dried and apposed to [3H]-Hyperfilm.
Data analysis. Films from autoradiography and in situ assays were analyzed using a computerized image analysis system (MCID-4, Imaging Research, St. Catherine, Ontario). The binding data were analyzed in brain subregions according to Paxinos and Watson (1986), namely, the dorsolateral, dorsomedial, and ventral caudate-putamen, the fundus striatum, the shell and core of the nucleus accumbens, olfactory tubercles, and the islands of Calleja, and are expressed as femtomole per milligram wet tissue. Optical density ofin situ films was analyzed in the same regions, and the results are expressed in random optical density units. Comparison between groups was carried out by applying two-way ANOVA and post hoc Newman–Keuls, with lesion and age as independent factors andp < 0.05 considered significant.
RESULTS
Verification of the lesion
Bilateral reduction in the size of the frontal cortex was seen in the neonatally lesioned rats. Cresyl violet-stained sections obtained from neonatal lesioned animals, at both PD39 and PD60, revealed moderate bilateral damage to the MPFC, with neuronal loss, atrophy, and apparent retraction of the MPFC. Small cavities in the MPFC also were seen occasionally (Figs. 1 and 2).
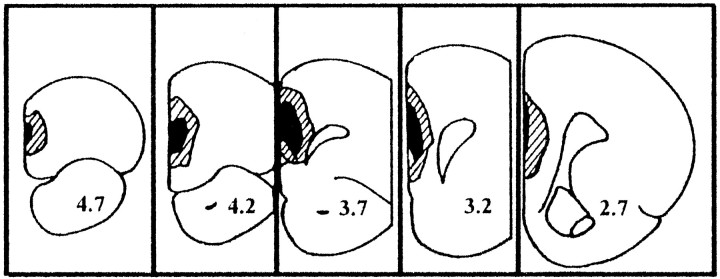
Fig. 1.
Schematic drawing of coronal sections (modified from Paxinos and Watson, 1986) illustrating lesion boundaries and the areas of neural loss and gliosis determined from Nissl-stained coronal sections from PD60 rats with ibotenic acid lesion of the neonatal MPFC. The stippled lines and solid black areas indicate the largest and smallest lesions, respectively. Coordinates refer to distance in millimeters anterior to bregma (Paxinos and Watson, 1986).
Fig. 2.
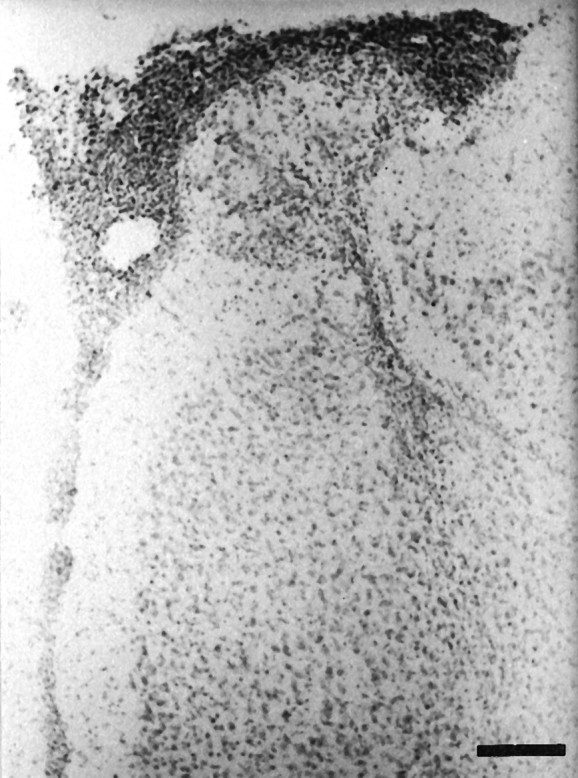
Photomicrograph of a coronal section of MPFC stained with cresyl violet showing retraction of the tissue, gliosis, and neural loss in PD60 neonatally lesioned rats. Scale bar, 160 μm.
Behavioral testing
The prepubertal (PD35) and postpubertal (PD56) effects of neonatal MPFC lesions on locomotor activity in a novel environment are illustrated in Figure 3. In the two age groups, both lesioned and sham-operated animals exhibited active exploratory behavior when placed in a novel environment. Although no potentiation of novelty-induced locomotion was seen in neonatally lesioned rats at PD35, a significant increase in exploratory behavior was observed at PD56 in neonatally MPFC-lesioned rats during the first 10 min period (Fig. 3).
Fig. 3.
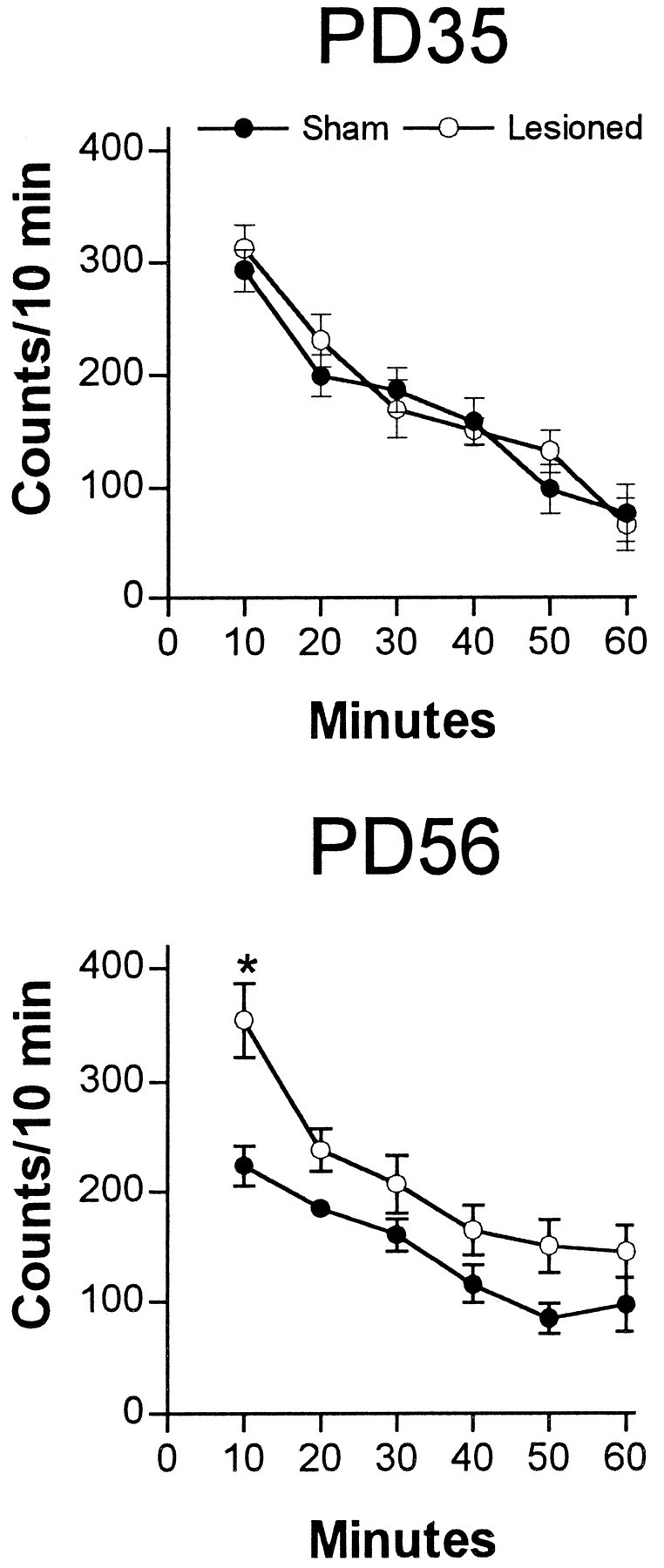
Locomotor activity (mean number of beam interruptions per 10 min ± SEM; n = 8–10 per group) in a novel environment of sham-operated or neonatal MPFC-lesioned animals tested either at PD35 or PD56. Locomotor activity was determined as described in Materials and Methods. At PD35, lesioned animals did not differ from sham-operated animals at any testing interval. In contrast, at PD56, lesioned rats were more active than the sham-operated rats during the first 10 min of exploration (*p < 0.05).
Amphetamine administration induced a marked increase in the locomotor activity at PD35 and PD56 in neonatally lesioned rats (Fig.4). Analysis of the entire period ofd-amphetamine effect revealed no significant differences in the locomotor activity between sham-operated and MPFC-lesioned rats at PD35. Locomotor activity, however, was significantly increased (83%) at PD56 in neonatally MPFC-lesioned animals when compared with sham-operated controls (F(3,32) = 6.035;p = 0.0022) (Fig. 4). No significant effect of saline injection (vehicle) was observed in any of the groups (Fig. 4).
Fig. 4.
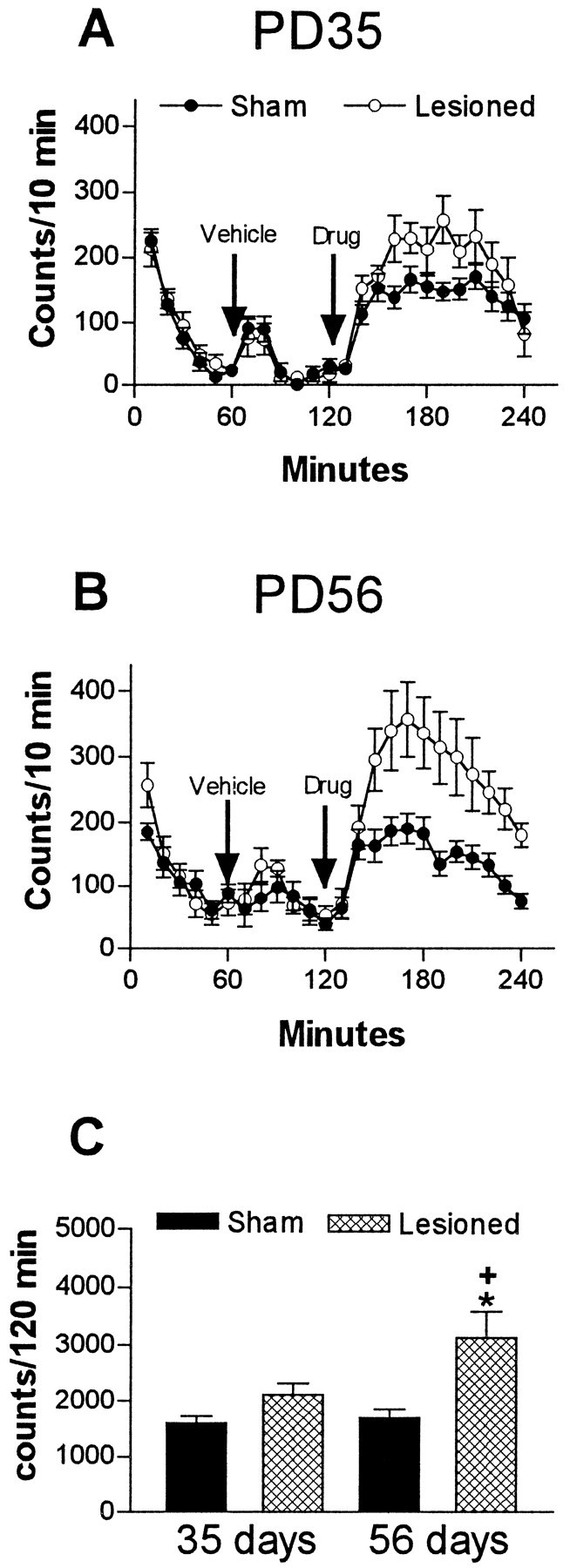
Locomotor activity after vehicle (saline) andd-amphetamine administration (1 mg/kg, s.c) of sham-operated or ibotenic acid-lesioned groups (mean number of beam interruptions per 10 min ± SEM; n = 8–10 per group). A, Temporal profile of locomotor activity at PD35. B, Temporal profile of locomotor activity at PD56. C, Analysis of total activity scores after d-amphetamine reveals that lesioned rats are significantly more active than sham-operated rats only at PD56. *p < 0.01, significantly different from the sham-operated groups; +p < 0.05, significantly different from PD35 lesioned rats.
[3H]-SCH-23390 binding
D1-like receptors, as measured by [3H]-SCH-23390 binding, are distributed throughout the dorsal and ventral regions of the striatum. The distribution and density of [3H]-SCH-23390 binding did not differ significantly between the sham-operated and neonatal MPFC-lesioned animals at either PD39 or PD60 (Table 1).
Table 1.
Quantitative evaluation of D1-like receptors by autoradiography and D1 receptor mRNA by in situhybridization in subregions of the striatum and in the olfactory tubercles
| D1-like [3H]-SCH-23390 | D1 in situ hybridization | |||||
|---|---|---|---|---|---|---|
| Sham (n = 4) | Lesion (n = 5) | % change | Sham (n = 4) | Lesion (n = 5) | % change | |
| PD 39 | ||||||
| CP-dorsolateral | 410 ± 25 | 386 ± 36 | −6.7 | 0.90 ± 0.02 | 0.87 ± 0.07 | −3.7 |
| CP-dorsomedial | 380 ± 23 | 350 ± 38 | −7.9 | 0.84 ± 0.01 | 0.78 ± 0.04 | −7.8 |
| CP-ventral | 404 ± 23 | 387 ± 38 | −4.3 | 0.86 ± 0.02 | 0.87 ± 0.03 | 1.0 |
| Fundus-CP | 423 ± 30 | 399 ± 34 | −5.5 | 0.94 ± 0.04 | 0.90 ± 0.03 | −4.8 |
| Accumbens-core | 379 ± 18 | 312 ± 37 | −17.6 | 0.76 ± 0.05 | 0.8 ± 0.07 | −5.7 |
| Accumbens-shell | 378 ± 20 | 324 ± 36 | −14.2 | 0.90 ± 0.03 | 0.84 ± 0.05 | −6.2 |
| Olfactory tubercle | 339 ± 15 | 296 ± 31 | −12.5 | 1.20 ± 0.04 | 1.13 ± 0.12 | −6.4 |
| PD 60 | ||||||
| CP-dorsolateral | 388 ± 9.2 | 383 ± 6.6 | −1.5 | 0.74 ± 0.09 | 0.87 ± 0.1 | 17.4 |
| CP-dorsomedial | 366 ± 5.5 | 356 ± 10.2 | −2.7 | 0.71 ± 0.06 | 0.73 ± 0.1 | 1.9 |
| CP-ventral | 387 ± 5.2 | 394 ± 11.5 | 1.6 | 0.72 ± 0.08 | 0.82 ± 0.1 | 13.4 |
| Fundus-CP | 387 ± 14 | 405 ± 6.9 | 4.7 | 0.77 ± 0.09 | 0.94 ± 0.06 | 22.0 |
| Accumbens-core | 357 ± 9.1 | 368 ± 16.5 | 3.1 | 0.60 ± 0.06 | 0.65 ± 0.08 | 6.82 |
| Accumbens-shell | 359 ± 6.4 | 376 ± 11.1 | 4.8 | 0.69 ± 0.06 | 0.70 ± 0.12 | 1.7 |
| Olfactory tubercle | 321 ± 13.1 | 347 ± 4.5 | 8.1 | 1.09 ± 0.14 | 1.19 ± 0.1 | 9.2 |
Receptor levels are expressed in fmol/mg wet tissue, and in situ hybridization data are expressed as random optical density and represent the mean ± SEM of pooled values obtained from four sections per animal. Striatal subregions are defined according to Paxinos and Watson atlas (1986). CP, Caudate-putamen.
[3H]-YM-09151-2 binding
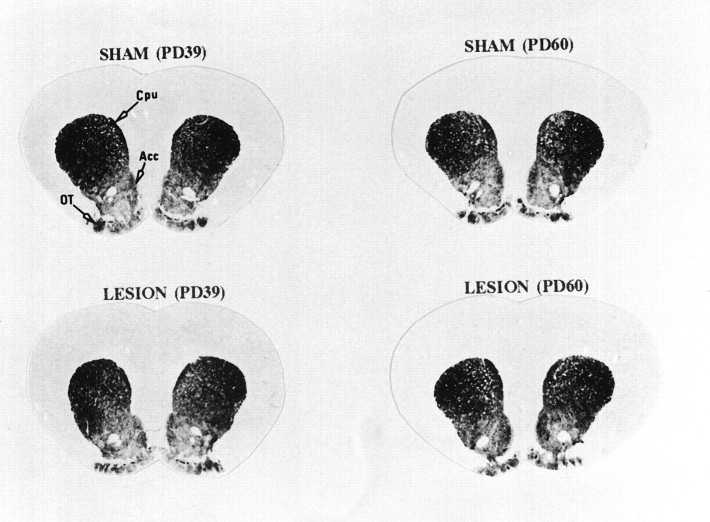
[3H]-YM-09151-2 binds DA receptor subtypes belonging to the D2 family (D2, D3, and D4). Total D2-like receptor binding shows a dorsoventral density gradient maximal in the caudate-putamen region. The distribution and level of D2-like binding sites did not differ significantly between the neonatally sham-operated and MPFC-lesioned animals at PD39 (Table 2, Fig. 5). At PD60, however, the level of D2-like binding sites was increased significantly in the nucleus accumbens shell of the MPFC-lesioned animals compared with sham controls (19%;F(3,14) = 4.923; p = 0.0159) (Table 2, Fig. 5). Putative DA D4 receptor binding, measured by [3H]-YM-01951-2 in the presence of raclopride, did not differ significantly between control and MPFC-lesioned rats (Table 2). The level of D2 and D3 receptors, measured by subtracting [3H]-YM-01951-2 binding in the presence of raclopride from total [3H]-YM-01951-2 binding, was significantly increased in the shell of the nucleus accumbens (35%;F(3,14) = 4.598; p = 0.0193) and the dorsolateral caudate-putamen (21%; F(3,14) = 4.765; p = 0.0172) (Table 3).
Table 2.
Quantitative evaluation of D2-like DA receptors in subregions of the striatum and in the olfactory tubercles
| D2-like [3H]-YM-09151-2 | D4-like [3H]-YM-09151-2 + raclopride | |||||
|---|---|---|---|---|---|---|
| Sham (n = 4) | Lesion (n = 5) | % change | Sham (n = 4) | Lesion (n = 5) | % change | |
| PD 39 | ||||||
| CP-dorsolateral | 190 ± 3.6 | 192 ± 4.0 | 1.0 | 76 ± 2.0 | 73 ± 2.8 | −3.2 |
| CP-dorsomedial | 152 ± 2.9 | 140 ± 4.01 | −2.5 | 59 ± 2.9 | 58 ± 2.5 | −2.9 |
| CP-ventral | 138 ± 3.9 | 140 ± 6.67 | 1.7 | 58 ± 2.2 | 57 ± 2.5 | −2.3 |
| Fundus-CP | 120 ± 3.4 | 126 ± 4.2 | −3.0 | 55 ± 2.0 | 52 ± 1.8 | −6.8 |
| Accumbens-core | 92 ± 3.2 | 89 ± 3.9 | −2.7 | 41 ± 1.7 | 38 ± 0.8 | −6.7 |
| Accumbens-shell | 91 ± 3.4 | 84 ± 3.92 | −8.2 | 40 ± 2.0 | 36 ± 0.7 | −9.3 |
| Olfactory tubercle | 85 ± 6.2 | 85 ± 4.5 | −0.3 | 42 ± 1.6 | 48 ± 3.3 | 11.4 |
| PD 60 | ||||||
| CP-dorsolateral | 173 ± 1.7† | 187 ± 6.7 | 8.0 | 73 ± 1.3 | 67 ± 2.1 | −9.3 |
| CP-dorsomedial | 132 ± 4.0 | 140 ± 2.7 | 6.5 | 56 ± 1.2 | 53 ± 1.8 | −6.3 |
| CP-ventral | 125 ± 2.0 | 138 ± 1.8 | 10.0 | 55 ± 1.0 | 54 ± 3.2 | −2.5 |
| Fundus-CP | 107 ± 3.4 | 117 ± 7.3 | 9.8 | 50 ± 1.2 | 52 ± 4.2 | 4.0 |
| Accumbens-core | 84 ± 3.0 | 94 ± 1.9 | 11.5 | 41 ± 2.7 | 39 ± 2.6 | −5.4 |
| Accumbens-shell | 85 ± 3.7 | 101 ± 3.42_150† | 18.7 | 42 ± 4.2 | 44 ± 4.8 | 2.9 |
| Olfactory tubercle | 89 ± 4.3 | 92 ± 3.5 | 3.5 | 49 ± 1.9 | 53 ± 1.3 | 8.9 |
Receptor levels are expressed in fmol/mg wet tissue and represent the mean ± SEM of pooled values obtained from four sections per animal. Striatal subregions are defined according to Paxinos and Watson atlas (1986). CP, Caudate-putamen.
F2_150: p < 0.05 compared with age-matched sham group;
F2_152: p < 0.05 compared with corresponding PD39 group.
Fig. 5.
Effect of neonatal MPFC lesions on D2 DA receptor binding in the striatum and nucleus accumbens. Coronal sections were incubated, as described in Materials and Methods, in the presence of 1 nm [3H]-YM-09151-2. Results from the quantitative analysis of [3H]-YM-09151-2 binding are presented in Table 2. Cpu, Caudate-putamen;Acc, nucleus accumbens; OT, olfactory tubercles; PD39, postnatal day 39; PD60, postnatal day 60.
Table 3.
Quantitative evaluation of D2–D3 DA receptors by autoradiography and D2 mRNA by in situ hybridization in subregions of the striatum and in the olfactory tubercles
| D2 + D3 [3H]-YM-09151-2 − ([3H]-YM-09151-2 + raclopride) | D2 in situ hybridization | |||||
|---|---|---|---|---|---|---|
| Sham (n = 4) | Lesion (n = 5) | % change | Sham (n = 4) | Lesion (n = 5) | % change | |
| PD 39 | ||||||
| CP-dorsolateral | 114 ± 5.6 | 119 ± 3.1 | 3.8 | 1.47 ± 0.27 | 1.63 ± 0.27 | 11.2 |
| CP-dorsomedial | 92 ± 2.1 | 83 ± 4 | −10.4 | 1.17 ± .2 | 1.32 ± 0.2 | 13.1 |
| CP-ventral | 80 ± 5.8 | 83 ± 5.5 | 4.7 | 1.21 ± 0.22 | 1.36 ± 0.2 | 12.4 |
| Fundus-CP | 65 ± 4.9 | 74 ± 5.0 | 14.5 | 1.24 ± 0.22 | 1.39 ± 0.21 | 12.7 |
| Accumbens-core | 51 ± 4.1 | 52 ± 2.8 | 0.5 | 0.90 ± 0.12 | 0.92 ± 0.13 | 2.8 |
| Accumbens-shell | 51 ± 4.6 | 47 ± 3.2 | −7.4 | 0.78 ± 0.1 | 0.75 ± 0.1 | −3.1 |
| Olfactory tubercle | 43 ± 5.6 | 38 ± 3.2 | −8.8 | 2.08 ± 0.22 | 2.5 ± 0.3 | 20.1 |
| PD 60 | ||||||
| CP-dorsolateral | 99 ± 1.5† | 120 ± 5.43_151 | 21.0 | 2.24 ± 0.08 | 2.74 ± 0.11†† | 22.2 |
| CP-dorsomedial | 75 ± 4.1 | 87 ± 2.78 | 16.3 | 1.71 ± 0.06 | 2.19 ± 0.093_150† | 27.8 |
| CP-ventral | 69 ± 2.2 | 83 ± 2.6 | 20.1 | 1.87 ± 0.04 | 2.28 ± 0.09†† | 22.3 |
| Fundus-CP | 57 ± 2.6 | 65 ± 4.5 | 14.9 | 1.76 ± 0.03 | 2.4 ± 0.13_150†† | 35.8 |
| Accumbens-core | 43 ± 2.9 | 55 ± 1.4 | 27.7 | 1.4 ± 0.06†† | 1.57 ± 0.02†† | 12.3 |
| Accumbens-shell | 43 ± 2.3 | 58 ± 1.63_150 | 34.9 | 1.27 ± 0.07†† | 1.6 ± 0.083_151†† | 24.3 |
| Olfactory tubercle | 40 ± 3.5 | 39 ± 2.5 | −2.8 | 2.94 ± 0.03 | 2.78 ± 0.12 | −5.27 |
Receptor levels are expressed in fmol/mg wet tissue, and in situ hybridization data are expressed as random optical density and represent the mean ± SEM of pooled values obtained from four sections per animal. Striatal subregions are defined according to Paxinos and Watson atlas (1986). CP, Caudate-putamen.
F3_150: p < 0.05;
F3_151: p < 0.01 compared with age-matched sham group;
F3_152: p < 0.05;
F3_153: p < 0.01 compared with corresponding PD39 group.
[3H]-7-OH-DPAT binding
DA D3 receptors, measured by [3H]-7-OH-DPAT binding, were found principally in the islands of Calleja, the olfactory tubercle, and the shell of the nucleus accumbens (Table4). A low level of D3 receptor expression was also detected in the striatum, particularly in its medial portion. D3 receptor levels in the fundus of caudate-putamen and the core of the nucleus accumbens were significantly higher at PD60 than PD39 in both sham-operated and lesioned groups and may indicate an age-related enhancement in D3 receptors. Compared with sham-operated controls, none of the MPFC-lesioned animals showed any significant differences in D3 binding levels.
Table 4.
Quantitative evaluation of D3 receptors and dopamine transporter levels in subregions of the striatum and in the olfactory tubercles
| D3 [3H]-7-OH-DPAT | DA transporter [3H]-WIN-35428 | |||||
|---|---|---|---|---|---|---|
| Sham (n = 4) | Lesion (n = 5) | % change | Sham (n = 4) | Lesion (n = 5) | % change | |
| PD 39 | ||||||
| CP-dorsolateral | 0.93 ± 0.16 | 1.09 ± 0.13 | 16.7 | 18.3 ± 0.7 | 18.5 ± 1.3 | 1.0 |
| CP-dorsomedial | 1.05 ± 0.20 | 1.37 ± 0.14 | 30.4 | 16.7 ± 1.2 | 17.1 ± 0.8 | 2.1 |
| CP-ventral | 2.02 ± 0.26 | 2.05 ± 0.19 | 1.7 | 16.5 ± 0.8 | 16.9 ± 0.4 | 2.2 |
| Fundus-CP | 2.87 ± 0.61 | 2.39 ± 0.57 | −16.9 | 16.9 ± 0.5 | 17.0 ± 0.4 | 0.7 |
| Accumbens-core | 5.28 ± 0.49 | 4.30 ± 0.39 | −18.6 | 15.4 ± 0.4 | 13.6 ± 0.7 | −11.6 |
| Accumbens-shell | 7.74 ± 0.81 | 7.81 ± 0.59 | 0.9 | 15.3 ± 0.4 | 11.9 ± 0.64_150 | −22.2 |
| Olfactory tubercle | 5.10 ± 0.36 | 5.83 ± 0.47 | 14.2 | 17.2 ± 0.2 | 15.2 ± 1.0 | −11.7 |
| Island of Calleja | 32.86 ± 3.42 | 29.69 ± 2.01 | −9.64 | n.d. | n.d. | |
| PD 60 | ||||||
| CP-dorsolateral | 0.92 ± 0.17 | 1.00 ± 0.13 | 8.2 | 19.5 ± 1.1 | 19.6 ± 0.6 | 0.3 |
| CP-dorsomedial | 1.51 ± 0.18 | 1.73 ± 0.15 | 14.5 | 15.9 ± 1.6 | 19.6 ± 0.3 | 23.8 |
| CP-ventral | 2.85 ± 0.15 | 2.86 ± 0.13 | 0.41 | 16.6 ± 1.2 | 18.9 ± 1.1 | 14.1 |
| Fundus-CP | 5.50 ± 0.41 | 5.35 ± 0.56 | −2.7 | 16.9 ± 0.7 | 16 ± 0.7 | −5.8 |
| Accumbens-core | 7.69 ± 0.59†† | 8.24 ± 0.74†† | 7.14 | 12.5 ± 0.9 | 15 ± 1.0 | 19.7 |
| Accumbens-shell | 9.46 ± 0.40†† | 9.84 ± 0.26†† | 4.0 | 10.2 ± 1.0†† | 12.5 ± 0.4 | 21.5 |
| Olfactory tubercle | 5.42 ± 0.22 | 5.14 ± 0.28 | −1.9 | 11.7 ± 1.4 | 13.0 ± 0.4 | 11.6 |
| Island of Calleja | 30.87 ± 1.27 | 33.46 ± 0.61 | 8.4 | n.d. | n.d. | |
Receptor levels are expressed in fmol/mg wet tissue and represent the mean ± SEM of pooled values obtained from four sections per animal. Striatal subregions are defined according to Paxinos and Watson atlas (1986). CP, Caudate-putamen; n.d., not determined.
F4_150: p < 0.05 compared with age-matched sham group;
F4_153: p < 0.01 compared with corresponding PD39 group.
[3H]-WIN-35428 binding
The DA transporters, as measured using [3H]-WIN-35428, are distributed throughout the dorsal and ventral part of the striatum. DA transporter levels in the shell of the nucleus accumbens are lower at PD60 than at PD39 in the sham group. The distribution and level of DA transporter binding sites do not differ significantly between the sham-operated and neonatally MPFC-lesioned animals at PD60 (Table 4). At PD39, however, the level of DA transporter binding sites was significantly decreased (22%) in the shell of the nucleus accumbens in the MPFC-damaged animals (F(3,14) = 10.413; p = 0.0007) (Table 4).
In situ hybridization
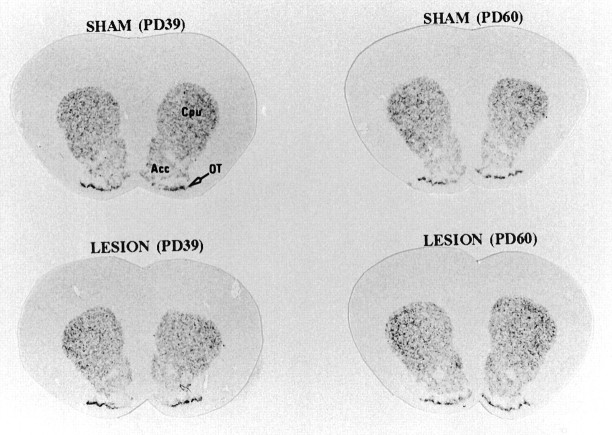
The distribution and level of D1 mRNA did not differ significantly between the sham-operated and MPFC-lesioned animals in any age group (Table 1). At PD60, however, D2 receptor mRNA was significantly increased in the lesioned animals compared with sham controls in the shell of the nucleus accumbens (24%;F(3,14) = 36.065; p < 0.0001), dorsomedial caudate-putamen (28%; F(3,14) = 9.164; p = 0.0013), and fundus caudate-putamen (36%;F(3,14) = 10.553; p = 0.0007) (Table 3, Fig. 6). D2 mRNA levels in the nucleus accumbens were higher at PD60 than at PD39 in both the sham and lesioned group, whereas in the caudate-putamen of the lesioned group there were also higher D2 mRNA levels at PD60 compared with PD39.
Fig. 6.
Effect of neonatal MPFC lesions on the expression of D2 receptor mRNA in the striatum and nucleus accumbens assessed byin situ hybridization. Coronal sections were fixed and hybridized with a [α-35S]-labeled D2R probe. Results from the quantitative analysis of in situ hybridization are presented in Table 3. Cpu, Caudate-putamen;Acc, nucleus accumbens; OT, olfactory tubercles; PD39, postnatal day 39; PD60, postnatal day 60.
DISCUSSION
The present study demonstrates the age-dependent nature of the effects of neonatal MPFC excitotoxic lesion on behaviors linked to the mesolimbic DA system. Neonatally induced MPFC damage by ibotenic acid produced an increase in locomotor behavior at PD56, evident during the exploratory period as well as after d-amphetamine administration. Furthermore, as demonstrated by autoradiography andin situ hybridization, these behavioral changes at PD56 occur concomitantly with an increase in the expression of DA D2 receptors in the shell of the nucleus accumbens and caudate-putamen (dorsomedial and fundus). Similarly lesioned rats tested at PD35, however, did not differ significantly from sham-operated animals either in terms of behavior or DA receptor expression.
In contrast to neonatal lesions, excitotoxic lesions of the adult rat MPFC result in a transient (present at 2 but not 4 weeks postlesion) increase in locomotion in response to a novel environment (Jaskiw and Weinberger, 1990) or d-amphetamine (Jaskiw et al., 1990). We have also observed that novelty andd-amphetamine effects were not significant 4 or 7 weeks after MPFC lesion of adult rats, time periods that correspond to the postoperative intervals of the present study (unpublished results). It is interesting to note that lesions in the adult rat MPFC have been associated with an increase in the swim-stress-induced locomotion (Jaskiw and Weinberger, 1992). Furthermore, 6-hydroxydopamine lesions of the MPFC also enhance responsiveness to stress (Deutch, 1992;Doherty and Gratton, 1996). Thus, it is possible that our observation of an increased locomotor activity in neonatally MPFC-lesioned rats at PD56 present within the first 10 min after placing the animals in the locomotor activity boxes could be a result of both previous handling stress and a novel environment.
Previous reports have suggested that behavioral outcome of frontal lesion depends on the age at which lesions are made as well as the age when behavioral assessments are done. For example, animals receiving early neonatal lesion (PD1-4) show more severe cognitive behavioral deficits than similarly lesioned adult animals. In contrast, PD7-10 lesioned animals show a sparing or recovery of behavioral deficits (related to memory and learning) when tested as adults (Kolb and Whishaw, 1981; de Brabander et al., 1991a; Freeman and Stanton, 1992;Kolb and Gibb, 1993). In the light of evidence of functional recovery after neonatal frontal lesions, our findings support the opposite; i.e., the behavioral deficit (or at least a behavioral effect) is dormant prepubertally and seems to express at approximately postpubertal age. Our results thus are more consistent with early frontal damage in nonhuman primates, which shows sparing of memory functions in infants that later becomes a deficit in adults (Goldman-Rakic et al., 1983). Interestingly, our findings in neonatal MPFC-lesioned rats are similar to previous reports on neonatal excitotoxic lesion of the VH that also demonstrated postpubertal onset of enhanced d-amphetamine- and stress-induced hyperlocomotion (Lipska et al., 1993a,b; Flores et al., 1996). It is not clear whether there are common mechanisms in the postpubertal onset of behavioral changes in neonatal MPFC and VH lesions; however, it is known that the VH sends excitatory projections to regions of the MPFC (Jay and Witter, 1991). Therefore, a neonatal lesion of the VH (deafferenting MPFC) may affect medial prefrontal cortical functions during neonatal development and consequently alter DA-related behaviors, as suggested previously (Lipska et al., 1993a,b, 1994;Weinberger and Lipska, 1995).
It has been suggested that changes in cortical dendritic arborization (dendritic branching or spine density) may be responsible for behavioral sparing or recovery after frontal cortical lesions (Kolb and Whishaw, 1989; Kolb and Gibb, 1993). Integrity of cortical noradrenergic afferents to cortex seems necessary for behavioral sparing, because cortical 6-OHDA blocks the behavioral sparing and alters cortical morphogenesis after neonatal frontal cortex damage in rats (Kolb and Sutherland, 1992). Furthermore, de Brabander et al. (1991b) showed an increase of the DA innervation to the cortex after neonatal MPFC lesions in rats. In view of the complex neuroanatomical and neurochemical reorganization in the frontal cortex after neonatal injury, the delayed behavioral changes seen in the neonatal ibotenic acid-lesioned animals may be attributed to neurodevelopmental disturbances and compensatory changes, such as sprouting and rerouting, that could cause changes in the pattern of neural projections, including changes in mesocortical DAergic projections (Kolb et al., 1994). Consistent with the evidence of neural reorganization after frontal cortical damage, there are reports of alterations in cell adhesion and extracellular matrix molecules in the rat and mouse striatum (Poltorak et al., 1993; Butler et al., 1994) after frontal cortex lesions.
Another aim of the present study was to evaluate the pre- and postpubertal expressions of D1-like, D2, D3, and D4 DAergic receptors and DA transporter in rats that had undergone bilateral ibotenic acid lesions of the MPFC at PD7. Significant increases in the expression of D2 receptors were observed at PD60 in the shell of the nucleus accumbens and dorsomedial and fundus areas of the caudate-putamen. In contrast, the overall expression of the D1-like, D3, and D4 receptors did not change significantly after neonatal MPFC lesioning. The neonatal MPFC lesions also induced a small but significant reduction of the DA transporter in the shell of the nucleus accumbens at PD39; however, there were not significant differences at PD60. The reduction in the prepubertal level of DA transporter seems to be without significant behavioral consequence, insofar as novelty- and amphetamine-induced locomotion are concerned. It may be indicative, however, of the beginning of postlesion compensatory changes in the mesolimbic DA system occurring prepubertally, which eventually results in overt behavioral changes at pubertal age, possibly mediated by increased levels of D2 receptors. Previous studies in MPFC-lesioned adult rats have not shown significant changes in the effects of quinpirole, a D2 agonist, on locomotor behavior (Braun et al., 1993). Our studies on neonatal MPFC lesion thus suggest a critical difference between the consequences of lesions in adult and neonatal animals insofar as D2 receptors are concerned, albeit indirectly. It is also possible that the changes in the D2 receptor levels reported here may not be a consequence of the loss of direct MPFC projection but may relate to neurodevelopmental disturbances secondary to an early MPFC damage.
A key finding of the present study, namely the increase in D2 receptors in the shell of the nucleus accumbens associated with potentiated amphetamine-induced locomotion, is particularly interesting in light of our previous report of a decrease in D3 receptors in the same area of neonatal VH-lesioned rats (Flores et al., 1996). Neonatal VH lesions have been reported to result in postpubertal increase in novelty-, stress-, and amphetamine-induced locomotor activity in rats (Lipska et al., 1993a,b; Flores et al., 1996). Thus, the behavioral consequences of both neonatal VH and MPFC lesions appear to emerge postpubertally, although differential changes in DA receptor subtypes accompany the behavioral changes. The reason for a differential effect on DA receptor subtypes in these two lesion paradigms is not clear. MPFC projects to both substantia nigra pars compacta and VTA (Sesack and Pickel, 1992). Stimulation of MPFC leads to increased DA release within the nucleus accumbens through the VTA (Taber et al., 1995; Karreman and Moghaddam, 1996), whereas stimulation of the VH produces a triphasic DA release via direct connections to the nucleus accumbens (Fibiger and Phillips, 1996). VH lesions, on the other hand, might not affect DA release through VTA but might directly alter the level of D3 receptors in the accumbens.
Previous studies in patients with schizophrenia have suggested an aberration during development of the prefrontal-temporolimbic cortices (for review, see Weinberger and Lipska, 1995). Morphometric studies have reported cytoarchitectural disorganization of the MPFC in schizophrenic brains (Benes et al., 1986, 1991; Raine et al., 1992), and positron emission tomography studies in schizophrenic patients have demonstrated a reduced blood flow and metabolic activity in the prefrontal cortex (popularly termed hypofrontality) (Ingvar and Franzen, 1974, Buchsbaum et al., 1990; Andreasen et al., 1992; Berman et al., 1992). Increased neuronal density without cell loss has been reported in prefrontal area 9 and occipital area 17 in the schizophrenic brain, suggesting neuronal atrophy and loss of dendritic arborization in these areas (Selemon et al., 1995). Along with these anatomical and functional alterations, increments in the levels of D2-like DA receptors in the caudate-putamen and nucleus accumbens of schizophrenics have been reported in many studies, although some of these changes could be attributed to increases in the D4 receptor (Seeman and Niznik, 1990; Seeman et al., 1993). Our findings in neonatal MPFC-lesioned rats agree with some of the features of human schizophrenics: for example, postpubertal increase in mesolimbic DA-related behavior associated with an increment in the level of D2 receptor in the nucleus accumbens.
In summary, increases in spontaneous andd-amphetamine-induced locomotor activity are evident in the PD56 neonatal MPFC-lesioned rats, which may be attributable in part to increased expression of the DA D2 receptor in the nucleus accumbens. These data are also consistent with previous studies implicating MPFC neurons in the modulation of subcortical DA activity in the adult rat.
Footnotes
This study was supported in part by grants from the Fonds de la Recherche en Santé du Québec (FRSQ). G.F. is a postdoctoral fellow of the Consejo Nacional de Ciencia y Tecnologia, Mexico. G.K.W. is supported by the Max Stern studentship from McGill University. L.K.S. and R.Q. are Chercheur-boursier and Chercheur-boursier de mérite exceptionel of the FRSQ. We are grateful to Dr. Joseph Rochford for his help and suggestions in behavioral assessments, Dr. Alain Gratton for a critical reading of this manuscript, and Dr. Jean-Guy Chabot for help in computer analysis of the autoradiographic data.
Correspondence should be addressed to Lalit K. Srivastava, Douglas Hospital Research Center, Neuroscience Division, 6875 Lasalle Boulevard, Verdun, Québec, Canada H4H 1R3.
REFERENCES
- 1.Andreasen NC, Rezai K, Alliger R, Swayze VW, II, Flaum M, Kirchner P, Cohen G, O’Leary DS. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with xenon 133 single photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- 2.Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 3.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vicent SL. Deficit in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 4.Berman KF, Torrey EF, Daniel DG, Weinberger DR. Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Arch Gen Psychiatry. 1992;49:927–934. doi: 10.1001/archpsyc.1992.01820120015004. [DOI] [PubMed] [Google Scholar]
- 5.Braun AR, Jaskiw GE, Vladar K, Sexton RH, Kolachana BS, Weinberger DR. Effects of ibotenic acid lesion of the medial prefrontal cortex on dopamine agonist-related behaviors in the rat. Pharmacol Biochem Behav. 1993;46:51–60. doi: 10.1016/0091-3057(93)90316-l. [DOI] [PubMed] [Google Scholar]
- 6.Buchsbaum MS, Nuechterlein KH, Haier RJ, Wu J, Sicotte N, Hazlett E, Asarnow R, Potkin S, Guich S. Glucose metabolic rate in normals and schizophrenics during the continuous performance test assessed by positron emission tomography. Br J Psychiatry. 1990;156:216–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- 7.Bunzow JR, VanTol HHM, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- 8.Butler AK, Rougon G, Chesselet M-F. PSA-NCAM in the developing striatum: effects of early post-natal lesions of the cerebral cortex. Soc Neurosci Abstr. 1994;20:988. [Google Scholar]
- 9.Christie MJ, Rowe PJ, Beart PM. Effect of excitotoxin lesions in the medial prefrontal cortex on cortical and subcortical catecholamine turnover in the rat. J Neurochem. 1986;47:1593–1597. doi: 10.1111/j.1471-4159.1986.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 10.de Brabander JM, de Bruin JP, van Eden CG. Comparison of the effects of neonatal and adult medial prefrontal cortex lesions on food hoarding and spatial delayed alternation. Behav Brain Res. 1991a;42:67–75. doi: 10.1016/s0166-4328(05)80041-5. [DOI] [PubMed] [Google Scholar]
- 11.de Brabander JM, van Eden CG, de Bruin JP. Neuroanatomical correlates of sparing of function after neonatal medial prefrontal cortex lesion in rats. Brain Res. 1991b;568:24–34. doi: 10.1016/0006-8993(91)91375-b. [DOI] [PubMed] [Google Scholar]
- 12.Deutch AY. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm [Suppl] 1992;36:61–89. doi: 10.1007/978-3-7091-9211-5_5. [DOI] [PubMed] [Google Scholar]
- 13.Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- 14.Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- 15.Fibiger HC, Phillips A (1996) Cortical and subcortical regulation of dopamine release: implications for the pathophysiology of schizophrenia. Can Coll Neuropsychopharmacol Abstr 3.
- 16.Flores G, Barbeau D, Quirion R, Srivastava LK. Decreased binding of dopamine D3 receptors in limbic subregions following neonatal bilateral lesion of rat hippocampus. J Neurosci. 1996;16:2020–2026. doi: 10.1523/JNEUROSCI.16-06-02020.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman HJ, Jr, Stanton ME. Medial prefrontal cortex lesions and spatial delayed alternation in the developing rat: recovery or sparing. Behav Neurosci. 1992;106:924–932. doi: 10.1037//0735-7044.106.6.924. [DOI] [PubMed] [Google Scholar]
- 18.Goldman-Rakic PS, Isseroff A, Schwartz ML, Bugbee NM. The neurobiology of cognitive development. In: Mussen PH, editor. Handbook of child psychology, Vol II, Infancy and developmental psychobiology. Wiley; New York: 1983. pp. 281–344. [Google Scholar]
- 19.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992;6:2413–2421. [PubMed] [Google Scholar]
- 21.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 22.Ingvar DH, Franzen G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaskiw GE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex potentiate FG-7142-induced attenuation of exploratory activity in the rat. Pharmacol Biochem Behav. 1990;36:695–697. doi: 10.1016/0091-3057(90)90276-n. [DOI] [PubMed] [Google Scholar]
- 24.Jaskiw GE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex augment swim-stress-induced locomotion. Pharmacol Biochem Behav. 1992;41:607–609. doi: 10.1016/0091-3057(92)90380-x. [DOI] [PubMed] [Google Scholar]
- 25.Jaskiw GE, Karoum F, Freed WJ, Phillips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Res. 1990;534:263–272. doi: 10.1016/0006-8993(90)90138-2. [DOI] [PubMed] [Google Scholar]
- 26.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 27.Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an affect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman MJ, Spealman RD, Madras BK. Distribution of cocaine recognition sites on monkey brain: I. In vitro autoradiography with [3H]CFT. Synapse. 1991;9:177–187. doi: 10.1002/syn.890090304. [DOI] [PubMed] [Google Scholar]
- 29.Kolb B, Whishaw IQ. Neonatal frontal lesions in the rat: sparing of learned but not species-typical behavior in the presence of reduced brain weight and cortical thickness. J Comp Physiol Psychol. 1981;95:863–879. doi: 10.1037/h0077849. [DOI] [PubMed] [Google Scholar]
- 30.Kolb B, Whishaw IQ. Plasticity in the neocortex: mechanisms underlying recovery from early brain damage. Prog Neurobiol. 1989;32:235–276. doi: 10.1016/0301-0082(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 31.Kolb B, Gibb G. Possible anatomical basis of recovery of function after neonatal frontal lesions in rats. Behav Neurosci. 1993;107:799–811. doi: 10.1037//0735-7044.107.5.799. [DOI] [PubMed] [Google Scholar]
- 32.Kolb B, Sutherland RJ. Noradrenaline depletion blocks behavioral sparing and alters cortical morphogenesis after neonatal frontal cortex damage in rats. J Neurosci. 1992;12:2321–2330. doi: 10.1523/JNEUROSCI.12-06-02321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolb B, Gibb G, van der Kooy D. Neonatal frontal cortical lesions in rats alter cortical structure and connectivity. Brain Res. 1994;645:85–97. doi: 10.1016/0006-8993(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 34.Leccese AP, Lyness WH. Lesions of dopamine neurons in the medial prefrontal cortex: effects on self-administration of amphetamine and dopamine synthesis in the brain of the rat. Neuropharmacology. 1987;26:1303–1308. doi: 10.1016/0028-3908(87)90091-8. [DOI] [PubMed] [Google Scholar]
- 35.Lévesque D, Díaz J, Pilon C, Martres MP, Giros B, Souil B, Schott D, Morgat JL, Schwartz JC. Identification, characterization, and localization of the dopamine D3 receptor in the rat brain using 7-[3H]hydroxy-N-N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipska BK, Jaskiw GE, Chrapusta S, Karoum F, Weinberger DR. Ibotenic acid lesion of the ventral hippocampus differentially effects dopamine and its metabolites in the nucleus accumbens and prefrontal cortex in the rat. Brain Res. 1992;585:1–6. doi: 10.1016/0006-8993(92)91184-g. [DOI] [PubMed] [Google Scholar]
- 37.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993a;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 38.Lipska BK, Jaskiw GE, Weinberger DR. Delayed effects of neonatal hippocampal damage on haloperidol-induced catalepsy and apomorphine-induced stereotypic behaviors in the rat. Dev Brain Res. 1993b;75:213–222. doi: 10.1016/0165-3806(93)90026-7. [DOI] [PubMed] [Google Scholar]
- 39.Lipska BK, Jaskiw GE, Weinberger DR. The effects of combined prefrontal cortical and hippocampal damage on dopamine-related behaviors in rat. Pharmacol Biochem Behav. 1994;48:1050–1057. doi: 10.1016/0091-3057(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 40.Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- 41. Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed. 1986. Academic; San Diego: Press. [Google Scholar]
- 42.Poltorak M, Herranz AS, Williams J, Lauretti L, Freed WJ. Effects of frontal cortical lesions on mouse striatum: reorganization. J Neurosci. 1993;13:2217–2229. doi: 10.1523/JNEUROSCI.13-05-02217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–77. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 44.Raine A, Lencz T, Reynolds GP, Harrison G, Sheard C, Medley I, Reynolds LM, Cooper JE. An evaluation of structural and functional prefrontal deficit in schizophrenia: MRI and neuropsychological measures. Psychiatry Res. 1992;45:123–137. doi: 10.1016/0925-4927(92)90006-p. [DOI] [PubMed] [Google Scholar]
- 45.Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 46.Seeman P, Guan HC, Van Tol HHM. Dopamine D4 receptors are elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 47.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 48.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neural targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 49.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phasseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava LK, Morency MA, Mishra RK. Ontogeny of dopamine D2 receptor mRNA in rat brain. Eur J Pharmacol. 1992;225:143–150. doi: 10.1016/0922-4106(92)90094-c. [DOI] [PubMed] [Google Scholar]
- 51.Taber MT, Fibiger HC. Electrical stimulation of the medial prefrontal cortex increases dopamine release in the striatum. Neuropsychopharmacology. 1993;9:271–275. doi: 10.1038/npp.1993.63. [DOI] [PubMed] [Google Scholar]
- 52.Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 53.Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophrenia Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger DR, Aloia MS, Goldberg TE, Berman KF. The frontal lobes and schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:419–427. doi: 10.1176/jnp.6.4.419. [DOI] [PubMed] [Google Scholar]
- 55.Worms P, Willigens MT, Continsouza-Blanc D, Lloyd KG. The effect of different type of cortical lesions on drug-induced catalepsy in rats: a pharmacological analysis. Eur J Pharmacol. 1985;113:53–59. doi: 10.1016/0014-2999(85)90342-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhou QY, Grandy DK, Thambi L, Kushner JA, VanTol HHM, Cone R, Pribnow D, Salon J, Bunzow JR, Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990;347:76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]