Abstract
Four monkeys (Macaca mulatta) were trained preoperatively in an automated object-in-place memory task in which they learned 20 new scenes in each daily session. In the object-in-place memory task, the correct, rewarded response in each scene is to a particular object of a pair, which always occupies a particular position in a unique background that has been generated using randomly chosen colors and shapes. Each animal then underwent two surgeries, with a period of testing after each. In the first, control surgery, each animal had either a unilateral lesion of the perirhinal cortex or unilateral fornix–fimbria transection, combined with section of the body and splenium of the corpus callosum and the anterior commissure (to prevent interhemispheric transfer of visual information). The disconnection was completed in the second surgery, after which all animals had a unilateral perirhinal cortex ablation in one hemisphere, unilateral fornix–fimbria transection in the contralateral hemisphere, and partial forebrain commissurotomy. The monkeys performance was compared for the learning of 200 scenes, preoperatively and after each surgery. After control surgery, the animals were mildly impaired on the object-in-place task. After disconnection, the animals showed a severe impairment in object-in-place memory. We conclude from this that, in episodic memory, the perirhinal cortex provides input of visual object information to the subiculum, hippocampus, and fornix.
Keywords: episodic memory, primates, perirhinal cortex, fornix, hippocampus, spatial memory, visual object memory, amnesia
Much research attention has been focused on the role of the hippocampus and anatomically related structures in episodic memory. In nonhuman primates, one approach, which stresses the spatial nature of episodic memory, has been proposed by several researchers. This has been described as object–place association (Gaffan and Saunders, 1985), memory for the location of objects (Parkinson et al., 1988), and object-in-place memory (Gaffan, 1994a). Single-cell recording studies in monkey hippocampus have also found strong evidence for a role in visual–spatial encoding (Rolls et al., 1995). Additional evidence from studies of humans with damage to the structures necessary for episodic memory, the Delay–Brion (Delay and Brion, 1969) circuit consisting of hippocampal formation, fornix, mamillary bodies, anterior thalamus, and cingulate gyrus suggests that they are impaired in both episodic and spatial memory (Smith and Milner, 1981; Gaffan et al., 1991; Kapur et al., 1994).
The proposal that the perirhinal cortex is important in knowledge about objects has received support from both monkey lesion and single-cell recording experiments. Both Brown et al. (1987) and Miller et al. (1991) recorded from cells in this area during a visual recognition task and found differences in responses to seen and novel stimuli.Horel et al. (1987) showed that both cooling and aspiration lesions of the inferior temporal gyrus, including perirhinal tissue, caused impairments in recognition memory. Gaffan and Murray (1990) tested object discrimination learning and delayed-matching-to-sample performance in animals with rhinal lesions. They found an impairment in both reacquisition of learned discriminations and delayed-matching-to-sample performance. More recent studies that have tested object memory in monkeys with lesions limited to perirhinal cortex have found severe impairments. Meunier et al. (1993) used a delayed-nonmatching-to-sample task and found large postoperative deficits. Gaffan (1994b) found that perirhinal ablation caused impairments in delayed-nonmatching-to-sample and discrimination learning with complex naturalistic scenes.
Anatomical studies of the connections of the perirhinal cortex show dense reciprocal connections between this area and the hippocampal formation involving highly processed sensory information from temporal association areas (Amaral and Insausti, 1990; Suzuki and Amaral, 1994). The hippocampus also has reciprocal connections with the parahippocampal gyrus which, in turn, receives highly processed sensory information from posterior parietal areas, as well as from caudal TE, TEO, and V4. Suzuki and Amaral (1994) suggest that this pattern of connectivity implicates the parahippocampal gyrus in spatial memory. This anatomical evidence strongly supports the proposal that the hippocampal system integrates information about objects and their positions in space.
Because both the experimental and the anatomical evidence suggests that the perirhinal cortex supplies information about objects to the hippocampus–fornix system, one should expect that disconnection of the perirhinal cortex from the fornix would impair object-in-place memory. The present experiment tests this hypothesis by training animals on an object-in-place memory task known to be sensitive to bilateral fornix transection [Gaffan, 1994a (task 5)] and then making crossed unilateral lesions of the fornix and perirhinal cortex, combined with partial forebrain commissurotomy (involving sectioning the body and splenium of the corpus callosum) to prevent intrahemispheric transfer of visual information between the hemispheres (Noble, 1973).
MATERIALS AND METHODS
Subjects. These were four young adult rhesus monkeys (Macaca mulatta), three of which were male and one female. At the time of their first surgery, they weighed on average 5.8 kg. They had participated as normal animals in other studies before taking part in the present experiment. All of their experimental training before the present experiment had been in memory tasks with complex artificial scenes, similar to those used in the present experiment. Throughout this experiment, except while recovering after surgery, all animals received one session of training every day, at least 5 d/week.
Each animal was operated on twice, with behavioral testing after each operation. Three of the monkeys had unilateral fornix–fimbria transection and partial forebrain commissurotomy in their first surgery and unilateral perirhinal ablation in the contralateral hemisphere in their second surgery. The fourth monkey (S3), had a perirhinal ablation in the first surgery and a contralateral fornix–fimbria transection plus commissurotomy in the second surgery. In all monkeys except one (S4), the perirhinal lesion was in the right hemisphere and the fornix–fimbria transection was in the left hemisphere. S4 had the lesions in the opposite hemispheres.
Surgery. Operations were carried out under aseptic conditions and, after ketamine premedication, barbiturate anesthesia was maintained until the end of the surgery. In all cases, after completion of the intended lesion, the bone flap was replaced and secured with loose silk sutures. Overlying tissue was sutured in anatomical layers. Before the resumption of training, 10–14 d were allowed for postoperative recovery.
Perirhinal cortex lesion. The arch of the zygoma was removed, and the temporal muscle was detached from the cranium and retracted. A bone flap was raised over the frontal and temporal lobe. The medial and posterior limits of the flap were in a crescent shape extending from within 5 mm of the midline at the brow to the posterior insertion of the zygomatic arch. The anterior limit of the flap was the brow and the orbit. Ventrally, the flap extended from the posterior insertion of the zygomatic arch to the level of the superior temporal sulcus in the lateral wall of the temporal fossa anteriorly. The ventral anterior part of this bone opening was then extended with a rongeur ventrally into the wall of the temporal fossa to reach the base of the temporal fossa. The dura mater was cut to expose the dorsolateral frontal and lateral temporal lobes. The most anterior part of the rhinal sulcus was visualized by retracting the frontal lobe from the orbit with a brain spoon. The dorsal limit of the removal on the anterior face of the temporal pole was ∼2 mm ventral to the lateral sulcus. A line of pia mater was cauterized, and the underlying cortex was removed by aspiration in the lateral bank of the anterior part of the rhinal sulcus and in the adjacent 2 mm of cortex on the third temporal convolution. The monkey’s head was then tilted to an angle of 120° from vertical, and the base of the temporal lobe was retracted from the floor of the temporal fossa with a brain spoon. The posterior tip of the first part of the ablation was identified visually and then extended in the lateral bank of the rhinal sulcus to the posterior tip of the sulcus, again removing 2 mm of laterally adjacent tissue. The dura mater was sewn and the wound closed in layers.
Body and splenium of the corpus callosum transection and anterior commissure section. A D-shaped bone flap was cut over the left hemisphere and removed. The dura was cut along the curved side of “D” and was reflected to expose part of the hemisphere and the midline. The hemispheres were separated, and the veins obscuring access to the corpus callosum were cauterized. The hemisphere was retracted from the falx to enable access to the interhemispheric fissure. A glass aspirator was used to section the corpus callosum near the midline (see below, Unilateral fornix transection) from the posterior limit of the splenium to the level of the interventricular foramen. The descending column of the fornix was gently retracted with a narrow brain spoon to enter the third ventricle. The anterior commissure was visualized and sectioned in the midline by electrocautery and aspiration with a 23-gauge metal aspirator that was insulated to the tip.
Unilateral fornix transection. The splenium section (described above) was carried out 1–2 mm lateral from the midline so as to open the lateral ventricle between the fimbria–fornix and the splenium. When the posterior limit of the fimbria and, anteriorly, the lateral limit of the fornix had both been exposed, the fimbria–fornix was cut unilaterally in a line between these two limits. The dura was replaced over the cortex but not sewn.
Histology. At the conclusion of the behavioral experiments, the animals were deeply anesthetized, then perfused through the heart with saline followed by formol/saline solution. The brains were blocked in the coronal stereotaxic plane posterior to the lunate sulcus, removed from the skull, and allowed to sink in sucrose/formalin solution. The brains were cut in 50 μm sections on a freezing microtome. Every fifth section was retained and stained with cresyl violet.
Figure 1 shows representative sections revealing the unilateral perirhinal lesion in each animal. In every case, the ablation was as intended. The ablation included the entire anterior–posterior extent of the lateral bank of the rhinal sulcus. The cortex in the medial bank of the rhinal sulcus and the cortex medial to the sulcus were substantially intact. The lateral limit of the ablation was in every case on the inferior temporal gyrus, lateral to the rhinal sulcus, leaving the cortex in the anterior middle temporal sulcus intact. The ablation extended slightly more laterally in cases S2 and S4 (the bottom two rows in Fig. 1) than in S3 and S1. Degeneration was visible in the white matter adjacent to the cortical removal. Although coronal sections are most appropriate for visualizing the main part of the ablation, as shown in Figure 1, the most anterior part of the ablation is difficult to interpret in coronal sections through the temporal pole. These are shown in the far left section from each animal in Figure 1. However, inspection of the temporal pole before the brains were sectioned confirmed that the ablation extended anteriorly and dorsally on the anterior face of the temporal pole to within 2 mm of the lateral sulcus, in the lateral bank of the most anterior part of the rhinal sulcus and in the laterally adjacent cortex, sparing the tissue medial to the rhinal sulcus.
Fig. 1.
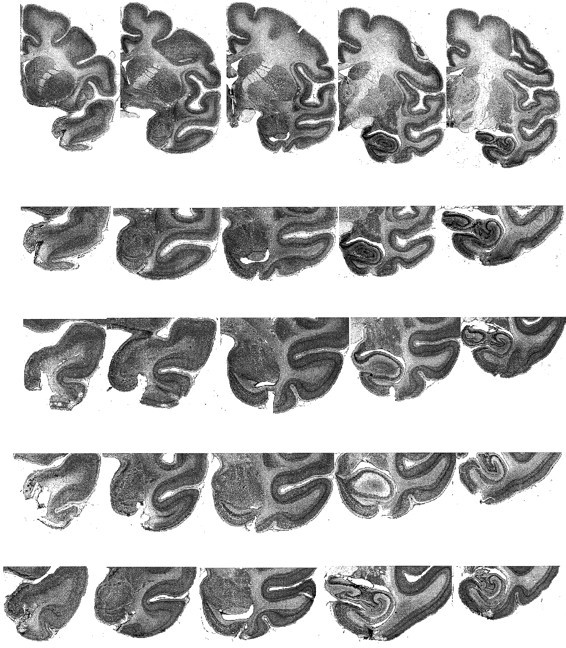
Five sections from each animal to illustrate the unilateral perirhinal cortex lesion. The entire hemisphere is shown in the top row, for orientation, and the temporal lobe is shown at greater magnification in the four lower rows. The sections, running from anterior at the left to posterior at the right, are from S3 (rows 1 and 2), S1 (row 3), S2 (row 4), and S4 (row 5). Sections are ∼2.5 mm apart in the fixed tissue. See text for description of ablations. Sections from animals S1, S2, and S3 are from the right hemisphere. The sections from animal S4 are from the left hemisphere and have been mirror-image-reversed for ease of comparison with the other animals.
Figure 2 shows representative sections revealing the unilateral fimbria–fornix in animal S3. In every case, there was a complete unilateral section of the fornix with no damage outside the fornix except for the incision in the corpus callosum and at most minor unilateral damage to the most inferior part of the cingulate gyrus at the site of the corpus callosum incision.
Fig. 2.
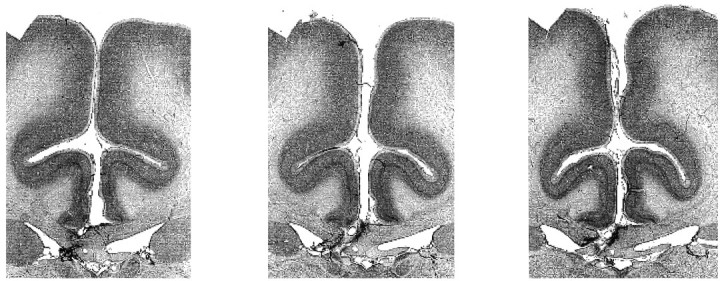
Three sections from one animal (S3) to illustrate the method of unilateral fornix transection. The sections are 1.5 mm apart in the fixed tissue and run from anterior on theleft to posterior on the right. Sections from the other animals were similar to these.
Test apparatus. The monkey was brought to the training apparatus in a wheeled transport cage, which was then fixed to the front of the apparatus. The monkey could reach out through bars at the front of the transport cage to touch a touch-sensitive monitor screen that was 150 mm from the front of the cage. The screen was 380 mm wide and 280 mm high. Each scene in the experiment, as described below (see Stimulus materials), occupied the entire screen. A closed-circuit television system allowed the experimenter in another room to watch the monkey. Small food rewards (pellets, 190 mg) were delivered into a hopper placed centrally underneath the monitor screen. A single large food reward was delivered at the end of each training session by opening a box that was set to one side of the centrally placed hopper. The box contained peanuts, raisins, proprietary monkey food, fruit, and seeds. The amount of this large reward was adjusted for individual animals to avoid obesity. The small and large rewards dispensed in the training apparatus provided the entire daily diet of the monkeys on days with a training session. Opening of the box with the large food reward, like all other aspects of the stimulus display and the experimental contingencies during any session of training, was under computer control.
Stimulus materials. The computer-generated scenes used in this experiment were generated in the same way as those described in Gaffan [1994 (task 5)]. Foreground objects, of which there were two in each scene, consisted of randomly selected small typographic characters, each placed in a constant location in the scene. Backgrounds were generated using an algorithm that drew a random number of ellipses and ellipse segments (between 2 and 7) of random color, position, and size, on a randomly colored initial background. The entire color space was equally available to objects and to backgrounds. Each animal was tested using a different set of scenes. Feedback for a correct response consisted of the correct object flashing on and off while a reward pellet was delivered. Each scene contained one correct and one incorrect object. Each of these objects was always presented in a constant place in a constant unique background.
Experimental procedure. The lists of object-in-place scenes contained 20 scenes. Each new list of 20 scenes was presented for one session of 160 trials (8 trials per scene). Each scene was presented once in each of 8 blocks of 20 trials within a session. The order of presentation of the 20 scenes was the same in each block. Every scene had a correct response area, where the positive (rewarded) foreground object was displayed, and an incorrect response area, which contained the negative (unrewarded) foreground object. On each trial, the display remained on the screen until the animal touched either the positive or the negative object. If the monkey touched the positive object, it flashed on and off in the scene for 2400 msec and a reward pellet was dispatched into the hopper in front of the monkey. An intertrial interval of 10 sec then began, ending with the presentation of the next trial in the session. In blocks 2 through 8 in each session, if the object touched was the negative, the screen blanked, followed after an intertrial interval of 10 sec by the next scheduled trial in the session, without any correction trials in the scene in which an error had been committed. Correction trials were presented only in the first block of 20 trials in each session, that is, the first run through the list. A correction trial consisted in the presentation of the scene with only one object in it, the positive; when a response to the positive was emitted, flashing and food followed as in the main trials of the task. For the final scene in the session, the final reward pellet was followed by a large food reward (see Apparatus) and the animal was given at least 10 min to eat some of this food and take the remainder into the cheek pouches, before being returned to the home cage. For this trial alone among the trials after the first block of 20 trials, a correction trial was presented if an error was made (thus ensuring that the large food reward was always obtained).
Preoperative training and testing. Subjects received one training session per day, at least 5 d/week, until a stable level of performance was reached. Each animal received at least 60 sessions. For establishing the preoperative level of performance, 10 lists of 20 new scenes were then presented, a total of 200 scenes, one list per session for 10 sessions.
Postoperative testing. After each of the two surgeries, the animals were trained on 12 new lists of scenes, over 12 consecutive sessions. Of these 12 lists, the first two were discounted for statistical analysis (this procedure was adopted in previous experiments using this task, and it allows for the often variable performance of monkeys when they restart training after surgery). Each list contained 20 new scenes, which were repeated 8 times. Each new set was presented in one session only.
RESULTS
Table 1 shows the percent error scores for each individual monkey in each phase of the experiment. Each of the animals showed an increase in error score after unilateral perirhinal cortex ablation or after unilateral fornix transection and partial forebrain commissurotomy. In the final stage of the experiment, after the second surgery, each animal showed a much larger increase in error score.
Table 1.
Percent error score in trials 2–8
| Monkey | Preoperation 1 | Postoperation 1 | Postoperation 2 |
|---|---|---|---|
| S1 | 3.79 | 9.00 | 22.14 |
| S2 | 5.00 | 9.14 | 28.57 |
| S3 | 4.79 | 8.00 | 27.79 |
| S4 | 3.29 | 5.43 | 21.50 |
| Mean | 4.22 | 7.89 | 25.00 |
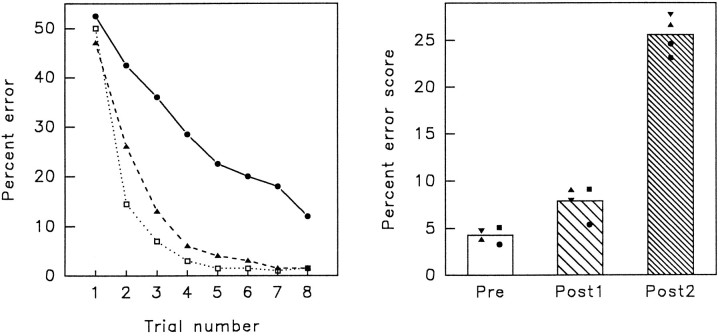
Figure 3 shows the animals’ averaged learning curves for 10 lists of scenes in each phase of the experiment (on theleft) and compares the animals’ total percent error score (on the right). Every list of 20 new scenes was presented for eight trials, one list per session. The final 10 preoperative sessions were compared with 10 sessions after operation one, and 10 sessions after operation two.
Fig. 3.
Results from the three phases of the experiment.Left, Average trial-by-trial learning in 10 lists of 20 scenes. Each list was presented for eight trials. Open square, Preoperative; filled triangle, postoperation 1; filled circle, postoperation 2. Right, Average percentage error score for all four animals in each phase of the experiment. Subject 1, triangle; Subject 2, square; Subject 3, inverted triangle; Subject 4, circle.
To compare efficiency of learning in each phase of the experiment, a one-way ANOVA of the three phases of the experiment showed a significant effect of surgical treatments (F(2,6) = 148.45, p < 0.001). A planned comparison (using the pooled error term) comparing preoperation score and postoperation one score showed a significant effect (t(6) = 2.85, p < 0.05), which reflects the fact that there was an increase in error rate in phase 2, after unilateral perirhinal lesion, or after partial forebrain commissurotomy and unilateral fornix transection. A planned comparison between postoperation one and postoperation two scores revealed a highly significant difference in error score (t(6) = 13.28, p < 0.01), indicating that in phase 3, after complete disconnection, the animals were much more impaired than in phase 2.
A further analysis was made of the post-first-surgery data to test whether errors were related to the side (left or right) of the unilateral lesion. In any scene, there were 15 possible horizontal positions that the S+ or the S− could occupy. In the analysis, these 15 positions were expressed for each scene as ranging from the position most ipsilateral to the side of the unilateral lesion in the animal that learned that scene (position 1), to the position most contralateral to the side of the lesion (position 15). (Each individual monkey learned a different series of scenes.) Then the total of errors was summed, across all scenes for all monkeys, for each of these 15 possible positions, separately for the position of the S+ and the position of the S−. For the S−, there was no evidence of a relationship between horizontal position of the S− and number of errors made (t(13) = 0.44, p = 0.66). Although the data for the S+ suggested that more errors were made when the S+ was contralateral to the lesion, there was no significant relationship (t(13) = 1.16, p = 0.27).
DISCUSSION
In the first stage of surgery, the perirhinal cortex was ablated unilaterally in one monkey, whereas in the other three monkeys the fornix was transected unilaterally in combination with partial forebrain commissurotomy (section of the anterior commissure and of the posterior part of the corpus callosum). Monkeys showed only a mild impairment in the object-in-place memory task after this first stage of surgery. The impairment at this stage (see Table 1, Fig. 3) was quantitatively similar to the impairments in visual learning that have been reported in earlier studies after unilateral amygdalectomy or unilateral ablation of inferior temporal cortex (E. A. Gaffan et al., 1988; Gaffan and Murray, 1990; Gaffan et al., 1993). Thus, it appears that temporal lobe lesions in the monkey produce little effect on visual memory when the lesions are confined to one hemisphere.
In the second stage of surgery, we operated on the hemisphere that had been left intact in the first stage. After the second surgery, all monkeys had unilateral perirhinal cortex ablation in one hemisphere and unilateral fornix transection in the opposite hemisphere, combined with partial forebrain commissurotomy. All of the animals now showed a severe impairment in object-in-place learning. Quantitatively, this impairment was similar in severity to those that have been seen in earlier studies of the object-in-place memory task after bilateral lesions in the fornix (Gaffan, 1994a), the mamillary bodies (Parker and Gaffan, 1996), or the anterior nuclei of the thalamus (A. Parker and D. Gaffan, unpublished observations).
The perirhinal cortex and the subiculum of the hippocampus are reciprocally connected within each hemisphere (Amaral and Insausti, 1990) and, in addition, can exchange information indirectly by projections to and from the entorhinal cortex (Insausti et al., 1987;Witter and Amaral, 1991). A majority of the fibers that project in the fornix to the mamillary bodies and anterior thalamus arise from cells of the subiculum and/or hippocampus (Amaral and Insausti, 1990). Thus, in the normal brain visual information can flow from the perirhinal cortex via the subiculum and fornix into the mamillary bodies and anterior thalamus. This information flow is prevented by the surgical disconnection that was carried out in the present experiment, crossed unilateral lesions in the perirhinal cortex and fornix combined with partial forebrain commissurotomy. The effect of this disconnection shows that normal performance of the object-in-place memory task depends on information flow from the perirhinal cortex into the fornix, and then to the mamillary bodies and anterior thalamic nuclei.
The perirhinal cortex and the fornix can function independently of each other in some memory tasks. This is shown by the dissociated effects of bilateral lesions in these two structures. For example, monkeys with bilateral fornix transection were impaired in a serial spatial discrimination learning task in which one of two locations (left and right) contained food, but monkeys with bilateral perirhinal cortex ablation were not impaired in this task (Gaffan, 1994b). Therefore, the fornix has a function in some spatial memory tasks that does not depend on the perirhinal cortex. On the other hand, the same monkeys with bilateral perirhinal cortex ablations were more severely impaired than the fornix-transected monkeys in visual delayed-matching-to-sample with naturalistic pictures (Gaffan, 1994b). Furthermore, lesions restricted to the perirhinal cortex produced severe impairments in delayed-nonmatching-to-sample with visual objects (Meunier et al., 1993), but fornix transection produced only mild impairments in several experiments using delayed-matching-to-sample or delayed-nonmatching-to-sample with visual objects. [Gaffan (1992)reviewed these experiments and concluded that this mild impairment is itself probably attributable to the spatial cues that differentiate sample presentations from retention tests in delayed-matching-to-sample or delayed-nonmatching-to-sample with visual objects.] Therefore, the perirhinal cortex has a function in some visual delayed-matching-to-sample tasks that does not depend on the fornix. These dissociations show that the perirhinal cortex and the fornix are not parts of a single memory system. In the object-in-place task, however, information needs to flow from perirhinal cortex into the fornix, as the present results show. The present experiment, therefore, presents an instance of cooperation between qualitatively different memory systems (Suzuki, 1996).
The pathway through subiculum, fornix, mamillary nuclei, and anterior thalamus is essential for normal episodic memory in the human brain (Delay and Brion, 1969; Kopelman, 1995). In the monkey, this pathway is essential not only for the object-in-place task but also for spatial memory tasks of various kinds. The object-in-place task does not overtly require the monkey to remember the spatial organization of the scenes in the task, but memory for spatial organization of the scenes enhances performance by reducing interference in memory among scenes (Gaffan, 1994a). All of these tasks that rely on the fornix for normal performance in the monkey, therefore, overtly or covertly require the animal to remember the spatial arrangement of scenes. Memory for spatial organization plays an analogous role in human episodic memory (Gaffan, 1994a), and increased susceptibility to interference among different scenes or contexts, therefore, may underlie the effects of fornix transection on human episodic memory (McMackin et al., 1995).
Ablations restricted to the perirhinal cortex, on the other hand, impair trial-unique matching- or nonmatching-to-sample (Meunier et al., 1993; Gaffan, 1994b), but lesions of both perirhinal and entorhinal cortex did not impair matching-to-sample with a restricted set of stimuli that were repeatedly used within each session (Eacott et al., 1994). Eacott et al. (1994) argued that this pattern of spared and impaired memory performance reflected the role of the perirhinal cortex in identifying multiple individual objects.
In summary, therefore, we draw three conclusions, of which the third is new and is supported by the present study. (1) The fornix, but not the perirhinal cortex, is essential for tasks such as the simple spatial learning task of Gaffan (1994b; described above), in which the monkey needs to remember the spatial arrangement of a scene but not the location of multiple individual objects. (2) The perirhinal cortex, but not the fornix, is essential for tasks such as trial-unique matching-to-sample with objects, in which the monkey needs to remember multiple individual objects but not their spatial arrangement. (3) Information flow from the perirhinal cortex into the subiculum and fornix is essential for tasks such as that of the present experiment, in which the monkey needs to remember spatial arrangements of multiple individual objects.
Footnotes
This research was supported by the Medical Research Council (UK). We thank Judi Wakeley for help in training the monkeys.
Correspondence should be addressed to Dr. Amanda Parker, Department of Experimental Psychology, Oxford University, South Parks Road, Oxford OX1 3UD, UK.
REFERENCES
- 1.Amaral DG, Instausi R. Hippocampal formation. In: Paxinos G, editor. The human nervous system. Academic; London: 1990. pp. 711–755. [Google Scholar]
- 2.Brown MW, Wilson FAW, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- 3.Delay J, Brion S. Masson; Paris: 1969. Le syndrome de Korsakoff. . [Google Scholar]
- 4.Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablation in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 5.Gaffan D. The role of the hippocampus-fornix-mammillary system in episodic memory. In: Squire LR, Butters N, editors. Neuropsychology of memory, 2nd Ed. Guilford; New York: 1992. pp. 336–346. [Google Scholar]
- 6.Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J Cog Neurosci. 1994a;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- 7.Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994b;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- 8.Gaffan D, Gaffan EA. Amnesia in man following transection of the fornix: a review. Brain. 1991;114:2611–2618. doi: 10.1093/brain/114.6.2611. [DOI] [PubMed] [Google Scholar]
- 9.Gaffan D, Murray EA. Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. J Neurosci. 1990;10:3479–3493. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffan D, Murray EA. Monkeys (Macaca fascicularis ) with rhinal cortex ablations succeed in object discrimination learning despite 24 hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- 11.Gaffan D, Saunders RC. Running recognition of configural stimuli by fornix-transected monkeys. Q J Exp Psychol. 1985;37B:61–71. doi: 10.1080/14640748508402087. [DOI] [PubMed] [Google Scholar]
- 12.Gaffan EA, Gaffan D, Harrison S. Disconnection of the amygdala from visual association cortex impairs visual reward-association learning in monkeys. J Neurosci. 1988;8:3144–3150. doi: 10.1523/JNEUROSCI.08-09-03144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci. 1993;5:968–975. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 14.Horel JA, Pytko-Joiner DE, Voytko ML, Salsbury K. The performance of visual tasks while segments of the inferotemporal cortex are suppressed by cold. Behav Brain Res. 1987;23:29–42. doi: 10.1016/0166-4328(87)90240-3. [DOI] [PubMed] [Google Scholar]
- 15.Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey. II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- 16.Kapur N, Scholey K, Barker S, Mayes A, Brice J, Fleming J. The mammillary bodies revisited: their role in human memory functioning. In: Cermak LS, editor. Neuropsychological explorations of memory and cognition: essays in honor of Nelson Butters. Plenum; New York: 1994. pp. 159–189. [Google Scholar]
- 17.Kopelman MD. The Korsakoff syndrome. Br J Psychiatry. 1995;166:154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- 18.McMackin D, Cockburn J, Anslow P, Gaffan D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir. 1995;135:12–18. doi: 10.1007/BF02307408. [DOI] [PubMed] [Google Scholar]
- 19.Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller EK, Lin L, Desmione R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 21.Noble J. Interocular transfer in the monkey: rostral corpus callosum mediates transfer of object learning set but not of single problem learning. Brain Res. 1973;50:147–162. doi: 10.1016/0006-8993(73)90601-x. [DOI] [PubMed] [Google Scholar]
- 22.Parker A, Gaffan D (1996) Mamillary body lesions in monkeys impair object-in-place memory: functional unity of the fornix-mamillary system. J Cog Neurosci, in press. [DOI] [PubMed]
- 23.Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J Neurosci. 1988;8:4159–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolls ET, Robertson RG, Georges-Francois P. The representation of space in the primate hippocampus. Soc Neurosci Abstr. 1995;21:1494. [Google Scholar]
- 25.Smith ML, Milner B. The role of the right hippocampus in recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin Neurosci. 1996;8:3–12. [Google Scholar]
- 27.Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 28.Witter MP, Amaral DG. Entorhinal cortex of the monkey. V. Projections to the dentate gyrus, hippocampus and subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]