Abstract
Midbrain dopaminergic neurons are known to release dopamine from somata and/or dendrites located in the substantia nigra (SN) and the ventral tegmental area (VTA). There is considerable controversy, however, about the subcellular sites for somatodendritic dopamine storage in these regions. In the present study, we used dual-labeling electron microscopic immunocytochemistry to localize the vesicular monoamine transporter-2 (VMAT2), a novel marker for sites of intracellular monoamine storage, within identified dopaminergic (tyrosine hydroxylase-containing) neurons in the rat SN and VTA. In dopaminergic perikarya, immunogold labeling for VMAT2 was localized to the Golgi apparatus, tubulovesicles that resembled smooth endoplasmic reticulum (SER), and the limiting membranes of multivesicular bodies. In dopaminergic dendrites, VMAT2 was extensively localized to tubulovesicles that resembled saccules of SER, and less frequently localized to isolated small synaptic vesicles (SSVs) or large dense-core vesicles (DCVs). In rare cases, VMAT2-immunoreactive SSVs were clustered within the cytoplasm of an SN or a VTA dendrite. Dopaminergic dendrites in the VTA contained a significantly higher number of immunogold particles for VMAT2 per unit area than those in the SN. Together, these observations support the proposal that dopamine is stored in and may be released from dendritic SSVs and DCVs, but suggest that the SER is the major site of dopamine storage within midbrain dopaminergic neurons. In addition, they provide new evidence that dopaminergic dendrites in the VTA may have greater potential for reserpine-sensitive storage and release of dopamine than those in the SN.
Keywords: vesicular transport, ultrastructure, dendritic release, dopamine, monoamine, substantia nigra, ventral tegmental area, smooth endoplasmic reticulum, synaptic vesicle, dense-core vesicle, tubulovesicle, immunogold, neuroprotection
Midbrain dopaminergic neurons in the substantia nigra (SN) and ventral tegmental area (VTA) play a critical role in the central regulation of motor and motivational functions (for review, seeRoth and Elsworth, 1995). These functions are largely mediated by the release of dopamine from axon terminals in the striatum and cortex, but are also modulated through local somatodendritic release of dopamine in the SN and VTA (Björklund and Lindvall, 1975; Groves et al., 1975; Geffen et al., 1976; Korf et al., 1976; Nieoullon et al., 1977a,b; Cuello and Iversen, 1978; Cheramy et al., 1981; Kalivas et al., 1989; Santiago and Westerink, 1991; Pucak and Grace, 1994). Under normal physiological conditions, this somatodendritic dopamine release is largely calcium-dependent, suggesting that it occurs through a stimulus-secretion coupling process such as vesicular exocytosis (Geffen et al., 1976; Cheramy et al., 1981). In addition, the application of reserpine, a specific inhibitor of vesicular monoamine transport, significantly reduces dendritic dopamine stores (Björklund and Lindvall, 1975; Elverfors and Nissbrandt, 1991;Heeringa and Abercrombie, 1995). Together, these observations have led to the proposal that in somata and dendrites, dopamine is stored within a vesicular pool and released through vesicle-mediated exocytosis.
Despite the evidence for vesicle-mediated exocytosis from midbrain dopaminergic neurons in the SN and VTA, ultrastructural studies have called into question whether there is a sufficient number of vesicles to account for the observed somatodendritic dopamine release. Dopaminergic dendrites in the SN contain clusters of electron-lucent vesicles and form dendrodendritic synapses, but these occur infrequently and are almost exclusively observed in the SN pars compacta (SNC) (Wilson et al., 1977; Groves and Linder, 1983). In contrast, in the SN pars reticulata (SNR), where dendritic dopamine release has been demonstrated most clearly, dopaminergic dendrites contain few synaptic vesicles and rarely, if ever, form dendrodendritic synapses (Wassef et al., 1981; Groves and Linder, 1983). Furthermore, after intranigral injection of radiolabeled monoamines, the autoradiographic silver grains have shown no specific association with dendritic vesicles (Sotelo, 1971; Cuello and Iversen, 1978). Studies in which the uptake of false neurotransmitters was used to identify intracellular dopamine storage sites, however, have shown that within nigral somata and dendrites, there is extensive uptake into tubulovesicular organelles that resemble smooth endoplasmic reticulum (SER) (Hattori et al., 1979; Mercer et al., 1979). Together, these observations have suggested that the SER is the major site of somatodendritic dopamine storage in the SN. Thus, it has been proposed that somatodendritic dopamine release may occur primarily from saccules of SER, rather than from vesicles (Mercer et al., 1979; Wassef et al., 1981).
In the present study, we have used electron microscopic immunocytochemistry with a high titer antiserum directed against the vesicular monoamine transporter-2 (VMAT2), the vesicular monoamine carrier that is expressed in neurons (Erickson et al., 1992; Liu et al., 1992a; Nirenberg et al., 1995; Peter et al., 1995), as a novel marker for determining the potential subcellular sites for dopamine storage and release within dopaminergic [tyrosine hydroxylase (TH)-immunoreactive] neurons in the rat SN and VTA. These results clarify the cellular basis for dendritic release of dopamine from midbrain dopaminergic neurons. In addition, they are the first to demonstrate differences in the expression of VMAT2 in these functionally distinct midbrain dopaminergic nuclei.
MATERIALS AND METHODS
Antisera. An affinity-purified rabbit polyclonal antiserum directed against a synthetic peptide [(C)SYPIGDDEESESD] at the C terminus of VMAT2 was raised in rabbits as described previously (Peter et al., 1995). The specificity of this antiserum has been demonstrated by Western blot analysis and immunocytochemistry (Nirenberg et al., 1995; Peter et al., 1995). A well characterized mouse monoclonal antiserum directed against TH was purchased from Incstar (Stillwater, MN).
Tissue preparation. The methods for tissue preparation and immunolabeling were based on those of Leranth and Pickel (1989). Four adult male Sprague–Dawley rats (250–400 gm; Taconic Farms, Germantown, NY) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused through the ascending aorta with 40 ml of heparin (1000 U/ml heparin in 0.15 m NaCl) and 50 ml of 3.75% acrolein, followed by 200 ml of 2% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. The brains were removed and postfixed for 30 min in 2% paraformaldehyde. Sections through the midbrain were cut on a Lancer vibratome at a thickness of 30–40 μm, incubated for 30 min in a solution of 1% sodium borohydride in PB to remove active aldehydes, and rinsed in PB until bubbles no longer emerged from the tissue. The tissue sections were then cryoprotected for 15 min in a solution of 25% sucrose and 3.5% glycerol in 0.05 m PB, rapidly frozen in freon followed by liquid nitrogen, and thawed at room temperature in PB.
Immunocytochemistry. All incubations were carried out at room temperature with agitation and were followed by several washes with PB, Tris-saline (TS) (0.9% NaCl in 0.1 mTris, pH 7.6), or 0.01 m PBS. The sections were incubated overnight at room temperature in either (1) the VMAT2 primary antibody solution [anti-VMAT2 diluted 1:6000–1:15,000 in 0.1% bovine serum albumin-TS (for single labeling)]; (2) anti-TH diluted 1:10,000 in the VMAT2 primary antibody solution (for double labeling); or (3) the anti-VMAT2 primary antibody preadsorbed with an excess of the peptide sequence against which it was raised (as a negative control).
Sections that were prepared for double labeling used the avidin-biotin complex method (Hsu et al., 1981) for immunoperoxidase detection of TH, as follows. The sections were incubated for 30 min in a 1:400 dilution of biotinylated goat anti-rabbit immunoglobulin G (IgG) in 0.1% BSA, for 30 min in a 1:100 dilution of avidin-biotin peroxidase complex, and for 6 min in a solution consisting of 22 mg of 3,3′ diaminobenzidine (DAB) and 10 μl of 30% hydrogen peroxide in 100 ml 0.1 m TS, pH 7.6. They were then processed for immunogold–silver labeling, as described below.
All sections were labeled for VMAT2 using the silver-enhanced immunogold method (Chan et al., 1990), as previously described (Nirenberg et al., 1995). In brief, the sections were incubated for 2 hr in a 1:50 dilution of colloidal gold (1 nm)-labeled anti-rabbit IgG (Amersham, Arlington Heights, IL), fixed for 10 min in 2% glutaraldehyde in PBS, and reacted for 5–8 min with a silver solution using a light-stable intenSEM kit (Amersham).
Electron microscopy. Sections that were prepared for electron microscopy were fixed in 2% osmium tetroxide for 60 min, dehydrated in a series of graded ethanols and propylene oxide, and flat-embedded in Epon 812 between two pieces of Aclar plastic. Ultrathin sections from the medial third of the SNC and SNR and from the parabrachial and paranigral portions of the VTA (Paxinos and Watson, 1986) were collected from the outer surface of the plastic-embedded tissue using a Research and Manufacturing Company (Tucson, AZ) ultramicrotome. These sections were sampled from rostrocaudal levels ranging between −5.2 mm and −5.8 mm from bregma, as illustrated in the atlas of Paxinos and Watson (1986). The ultrathin sections were then counterstained with lead citrate and uranyl acetate and examined with a Philips CM-10 or a Philips 201 electron microscope (Mahwah, NJ).
Identification of labeled profiles. The classification of labeled profiles and subcellular organelles was based on the criteria of Peters et al. (1991). Perikarya were identified by the presence of a nucleus, Golgi apparatus, and endoplasmic reticulum. Dendrites were distinguished from unmyelinated axons by the presence of extensive cisternae of endoplasmic reticulum and a high proportion of uniformly distributed microtubules. Axodendritic synapses were defined by the presence of a junctional complex, a restricted zone of parallel membrane appositions with a slight enlargement of the intercellular space, a cluster of presynaptic vesicles, and an associated postsynaptic thickening. Dendrodendritic synapses were identified by the presence of parallel apposed membranes and an associated presynaptic cluster of synaptic-like vesicles. Nonsynaptic contacts (appositions) were defined by the presence of closely spaced parallel plasma membranes that were not separated by glial processes but lacked recognizable synaptic specializations.
Identification of subcellular organelles. Small synaptic vesicles (SSVs) were identified by their 30–60 nm cross-sectional diameter, round-to-pleomorphic shape, and electron-lucent lumen. Large dense-core vesicles (DCVs) were identified by their size (80–120 nm in cross-sectional diameter) and the presence of a characteristic electron-dense core surrounded by an electron-lucent halo. Tubulovesicles, many of which resembled saccules of SER (Broadwell and Cataldo, 1983; Peters et al., 1991), were defined as tubular or tubulovesicular structures that were irregular in shape and larger than the SSVs (>60 nm in maximal cross-sectional diameter). These tubulovesicles were usually electron-lucent, but in some cases contained an electron-dense precipitate.
Data analysis. For quantitative analysis, the number of immunogold particles for VMAT2 per unit area (in μm2) was tabulated within a total of 1500 TH-immunoreactive dendritic profiles in three regions: the medial third of the SNC, the medial third of the SNR, and the parabrachial and paranigral subnuclei of the VTA. This tabulation was performed on a Dell 466/T computer (Austin, TX) using a Microcomputer Imaging Device system (Imaging Research, Ontario, Canada). To ensure consistency of the immunolabeling, and to eliminate potential concerns about differential tissue fixation or permeabilization, the same number of TH-immunoreactive dendritic profiles per region (50–150) was examined from each vibratome section. These profiles were sampled randomly from four vibratome sections, which were derived from two experimental animals. In each of the three regions, the number of gold particles per square micrometer of cross-sectional dendritic area was determined for 500 TH-immunoreactive dendritic profiles. The results are reported as the mean number of gold particles per unit area ± SEM, as calculated using a Fisher’s Test for analysis of variance. Statistical significance was defined by a p value of <0.05.
RESULTS
Immunogold labeling for VMAT2 was localized to membrane-bound organelles in the cytoplasm of perikarya, dendrites, and axonal processes and terminals in both the SN and the VTA. As in previous studies (Nirenberg et al., 1995; Peter et al., 1995), the pattern of immunolabeling observed with this antiserum was eliminated or markedly reduced by preadsorption of the antiserum with the peptide sequence against which it was generated. By electron microscopy, selective immunogold labeling for VMAT2 was seen in sections that were sampled from the surface of the tissue (Figs. 1, 2, 3, 4, 5, 6, 7), but was not observed in sections that were sampled from deeper within the tissue, where there is known to be limited penetration of immunoreagents (Leranth and Pickel, 1989).
Fig. 1.
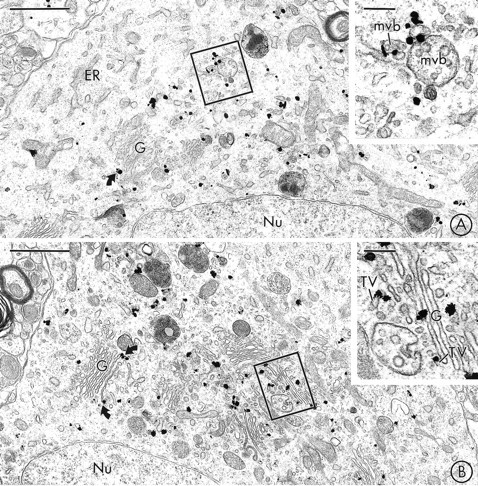
VMAT2 is localized to saccules of Golgi and multivesicular bodies in neuronal perikarya in the SN and VTA.A, Immunogold particles for VMAT2 are seen in the region of the Golgi apparatus (G) of a labeled perikaryon in the SNC, but are not detected in the adjacent rough endoplasmic reticulum (ER). The inset shows the boxed regionat higher magnification. Immunogold labeling for VMAT2 is seen along the limiting membranes of two multivesicular bodies (mvb).B, Immunogold labeling for VMAT2 is localized to the Golgi apparatus (G) of a perikaryon in the VTA. Many of the gold particles contact lateral saccules of Golgi lamellae (curved arrows). The inset shows the boxed region at higher magnification. Gold particles are localized to saccules of Golgi (G) and associated vesicles and tubulovesicles (TV). Nu, Nucleus. Scale bars: A, B, 1 μm; inset in A, B, 0.2 μm.
Fig. 2.
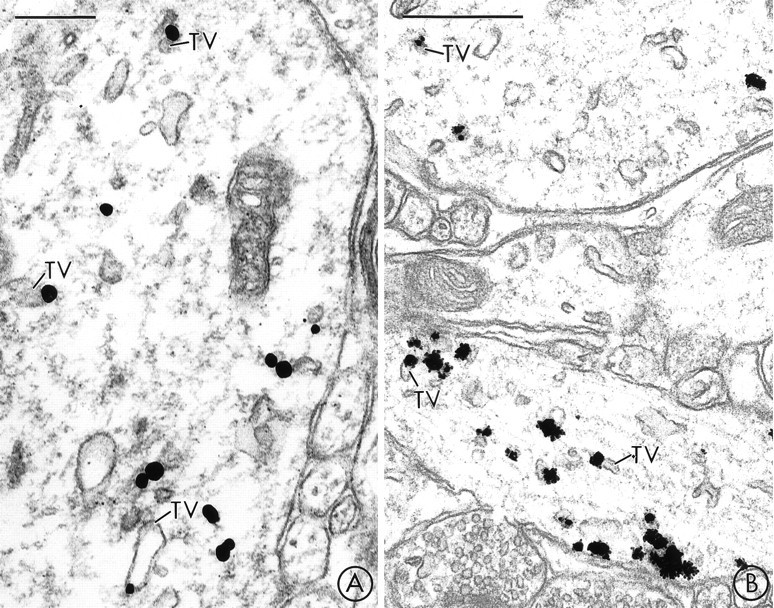
In midbrain dendrites, prominent immunogold labeling for VMAT2 is localized to tubulovesicles that resemble smooth endoplasmic reticulum. A, B, A large dendrite in the SN (A) and two large dendrites in the VTA (B) contain immunogold labeling for VMAT2 that is primarily localized to large, electron-lucent tubulovesicles (TV) that resemble SER. Scale bars: A, 0.25 μm; B, 0.5 μm.
Fig. 3.

In SN dendrites, VMAT2 is localized to tubulovesicles of SER, and more rarely to SSVs or DCVs. A, In dually labeled tissue, gold particles for VMAT2 are seen within the cytoplasm of a medium-sized dendrite that also contains immunoperoxidase labeling for TH. The peroxidase reaction product is seen as an electron-dense precipitate throughout the cytoplasm, which is notably absent in dendrites processed only for VMAT2 (C). The immunogold particles are localized to tubulovesicles (TV) that are larger than the unlabeled synaptic vesicles (uV) seen in adjacent unlabeled axon terminals (UT). One of the unlabeled terminals (UT) forms a synaptic junction with the labeled dendrite (arrow). Scale bars: B, C, 0.5 μm. In larger dendrites, immunogold particles for VMAT2 are also localized to large tubulovesicles (TV), clusters of smaller SSVs, and an occasional DCV. Scale bars: 0.5 μm;inset, 0.2 μm.
Fig. 4.

In the VTA, VMAT2-labeled SSVs are sometimes clustered near dendrodendritic contacts. A, Immunogold labeling for VMAT2 and immunoperoxidase labeling for TH are seen in two large dendrites (De). Some of the immunogold particles are localized to a cluster of small synaptic vesicles (V) and larger electron-lucent tubulovesicles (TV) near a point of contact (straight arrow) between the two dendrites (De). A similar cluster of vesicles and tubulovesicles (curved arrow) contains no detectable immunogold labeling in the observed plane of section, but contained several immunogold particles for VMAT2 in a serial section (not shown). A VMAT2-labeled dense-core vesicle (DCV) is also seen in the “presynaptic” dendrite. B, In a section processed for single labeling, immunogold particles for VMAT2 are localized to a cluster of small synaptic vesicles (V) in a dendrite (De) near its site of contact (arrow) with an unlabeled dendrite (UDe). The labeled vesicles are similar in size to the unlabeled synaptic vesicles (uV) seen in an adjacent unlabeled terminal (UT). Scale bars:A, B, 0.5 μm.
Fig. 5.
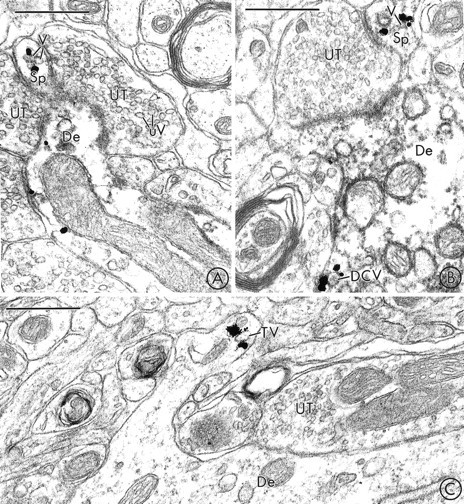
In rare cases, VMAT2-labeled vesicles are localized to dendritic spines in the SNR and the VTA. A, B, Immunogold labeling for VMAT2 and immunoperoxidase labeling for TH are colocalized in two dendrites in the SNR. Immunogold particles are localized to small synaptic vesicles (V) in dendritic spines (Sp). These vesicles are similar in size to the unlabeled small synaptic vesicles (uV) seen in adjacent unlabeled axon terminals (UT).C, Gold particles for VMAT2 are localized to a tubulovesicular organelle (TV) in a dendritic spine in the VTA. The labeled tubulovesicle is larger than the synaptic vesicles seen in an adjacent unlabeled axon terminal (UT). De, Dendrite. Scale bars:A–C, 0.5 μm.
Fig. 6.
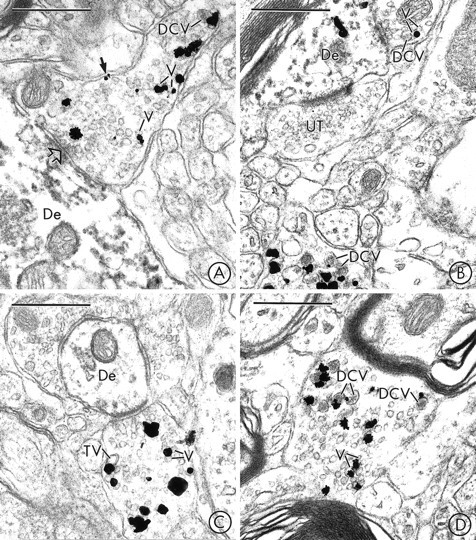
In the SN and VTA, VMAT2 is localized to SSVs and DCVs in axon terminals that usually lack detectable TH-immunoreactivity. A, Immunogold particles for VMAT2 are localized to small synaptic vesicles (V), an isolated DCV, and the plasma membrane (closed arrow) of an axon terminal in the SN that lacks detectable peroxidase reaction product for TH. The VMAT2-containing terminal forms a synaptic contact (open arrow) with a dendrite (De) that contains intense peroxidase reaction product for TH, but no detectable immunogold labeling for VMAT2. B, Immunogold particles for VMAT2 are seen in two axon terminals in the SN. The terminal at thetop contacts a dendrite (De) that is labeled with immunoperoxidase for TH and immunogold for VMAT2. The terminal at thebottom contains numerous VMAT2-labeled large dense-core vesicles (DCV). The VMAT2-immunoreactive terminals contain no detectable peroxidase labeling for TH. UT, Unlabeled terminal. C, Immunogold particles for VMAT2 are localized to small synaptic vesicles (V) and a larger electron-lucent tubulovesicular structure (TV) in an axon terminal in the VTA that lacks detectable peroxidase reaction product for TH. The VMAT2-labeled terminal contacts a dendrite (De) that is lightly immunoperoxidase-labeled for TH. D, An axon terminal in the VTA contains immunogold particles that are localized to the membranes of large dense-core vesicles (DCV) and small synaptic vesicles (V). The VMAT2-labeled terminal contains no detectable peroxidase reaction product for TH. Scale bars:A–D, 0.5 μm.
Perikarya
Neuronal perikarya in both the SN (Fig. 1A) and the VTA (Fig. 1B) contained extensive immunogold labeling for VMAT2. This immunogold labeling was localized most prominently to tubulovesicular organelles, most of which resembled saccules of SER (Peters et al., 1991; Broadwell and Cataldo, 1983) (Fig.1A,B), but some of which resembled SSVs. In both regions, there was also extensive VMAT2-labeling of the Golgi apparatus, particularly along its lateral saccules (Fig.1A,B). VMAT2-immunoreactive DCVs, some of which were located close to the trans-Golgi network, were also detected in dopaminergic perikarya in the SN and VTA. In some of the labeled SN (Fig. 1A) and VTA perikarya, immunogold particles for VMAT2 were seen along the limiting membranes of multivesicular bodies (MVBs). In contrast, there was no detectable immunolabeling for VMAT2 along the nuclear or plasma membranes of perikarya in either region, and only rare immunogold labeling for VMAT2 along saccules of rough endoplasmic reticulum. The subcellular localization of VMAT2 was most clearly resolved in tissue that was prepared for single immunogold labeling of VMAT2 (Fig. 1A,B), but was similar to that seen in double-labeled tissue, where there was also a diffuse electron-dense peroxidase reaction product for TH.
Dendrites and dendritic spines
In the SN, VMAT2-containing dendritic profiles were distributed throughout the pars compacta and the pars reticulata. Within the VMAT2-labeled dendrites, most of the immunogold labeling was associated with large tubulovesicles that had the morphological appearance of dendritic SER (Peters et al., 1991) (Figs.2A, 3A,B). Less frequently, VMAT2-labeling was localized to the membranes of DCVs (Fig.3C) or to smaller electron-lucent vesicles that resembled SSVs (Fig. 3B). In rare cases, VMAT2-labeled SSVs formed a cluster within the cytoplasm of an SN dendrite (Fig.3B).
In tissue that was dual-labeled for both VMAT2 and TH, most of the VMAT2-labeled dendrites also contained detectable peroxidase reaction product for TH (Fig. 3A). Although the VMAT2-labeled dendrites in the SN were sometimes apposed to and/or received synaptic contacts from unlabeled axon terminals (Fig. 3A) or dendrites (not shown), they were only rarely apposed to other VMAT2- or TH-labeled dendrites.
On several occasions, immunogold labeling for VMAT2 was detected in dendritic spines in the SNR (Fig. 5A,B). Within these spines, VMAT2 was associated prominently with electron-lucent vesicles and tubulovesicles (Fig. 5A,B). The labeled spines were frequently apposed to and/or received asymmetric contacts from unlabeled terminals. In some cases, these unlabeled axon terminals contacted both the spine and the shaft of the same VMAT2-containing dendrite. In sections that were double-labeled with immunogold for VMAT2 and immunoperoxidase for TH, most of the VMAT2-labeled spiny dendrites contained detectable reaction product for both markers (Fig.5A,B).
In the VTA, as in the SN, immunogold labeling for VMAT2 was localized most prominently to tubulovesicles that resembled saccules of SER (Fig. 2B). Less frequently, immunogold labeling was localized to smaller electron-lucent vesicles that resembled SSVs (Fig.4A,B) or to DCVs (Fig. 4A). In several cases, clusters of VMAT2-labeled vesicles were observed near segments of the dendritic plasma membrane that were apposed to and/or formed dendrodendritic synapses onto other dendrites. Some of the apposed dendrites contained (Fig. 4A) and some lacked (Fig.4B) detectable VMAT2 immunoreactivity. In sections that were dual-labeled for both VMAT2 and TH, many of the of the pre- and/or postsynaptic dendrites in which there was immunogold labeling for VMAT2 also contained peroxidase reaction product for TH (Fig. 4A). VMAT2 also was detected occasionally in dendritic spines in the VTA (Fig. 5C). Within these spines, VMAT2 was selectively associated with vesicles and tubulovesicles (Fig.5C).
By quantitative analysis, there were significantly more gold particles for VMAT2 per unit area within TH-immunoreactive dendrites in the VTA (1.78 ± 0.10/μm2) than in TH-immunoreactive dendritic profiles in either the SNC (1.30 ± 0.07/μm2; p < 0.0001) or the SNR (1.22 ± 0.07/μm2; p < 0.0001). In contrast, when TH-immunoreactive dendrites in the SNC and SNR were compared, there was no statistical difference in the density of immunogold labeling for VMAT2 (p = 0.47).
Axons and axon terminals
Immunogold labeling for VMAT2 was frequently detected in axon terminals and in small, unmyelinated axons in the SN (Fig.6A,B) and VTA (Fig. 6C,D). Within the labeled axonal processes, gold particles for VMAT2 were localized predominantly to the membranes of SSVs (Fig. 6A,C,D) or DCVs (Fig. 6A,B,D) and only infrequently to larger tubulovesicles (Fig. 6C). In rare cases, one or more immunogold particles seemed to contact the cytoplasmic surface of the plasma membrane of an axon terminal that contained VMAT2-labeled vesicles (Fig.6A).
In sections that were dually labeled with immunogold for VMAT2 and immunoperoxidase for TH, a small number of unmyelinated axons in the SN and the VTA contained immunoreaction product for both markers. The majority of the VMAT2-containing unmyelinated axons in the SN (Fig.6A,B) and the VTA (Fig. 6C,D), however, did not contain detectable immunoreaction product for TH. This was the case even when other TH-immunoreactive structures were clearly observed within adjacent profiles within the same tissue section (Fig.6A–C). The VMAT2-labeled axon terminals in both regions were frequently apposed to, and in some cases formed synaptic junctions with, TH-labeled dendrites, as illustrated in the VTA (Fig.6C) and SN (Fig. 6A).
DISCUSSION
This is the first study to examine the ultrastructural localization of VMAT2 within midbrain dopaminergic neurons. Our results show that within dopaminergic perikarya and dendrites in the SN and VTA, VMAT2 is extensively localized to tubulovesicles that resemble SER, and only rarely localized to SSVs and DCVs. These findings suggest that the SER is the major site of reserpine-sensitive somatodendritic dopamine storage in these regions. We have also shown quantitative differences in the levels of expression of VMAT2 in dopaminergic dendrites in the SN and VTA. These differences may contribute to the differential levels of somatodendritic dopamine release in the SN and VTA and/or to the differential susceptibility of the dopaminergic neurons in these regions to parkinsonism-inducing neurotoxins.
Methodological considerations
The subcellular localization of VMAT2 to saccules of Golgi tubulovesicles of SER and vesicles in the SN and VTA is consistent with electron microscopic studies of uptake of false neurotransmitters and radiolabeled monoamines, which have identified these organelles as potential sites of monoamine storage (Cuello and Iversen, 1978; Mercer et al., 1979; Groves and Linder, 1983). The immunocytochemical methods used in the present study, however, offer several potential advantages over the methods used in previous experiments. First, the immunogold–silver method offers greater spatial resolution than autoradiography, thereby permitting identification of the specific organelles that are potential sites of monoamine storage in the SN and VTA. Second, because dopamine is the only catecholamine that is present in perikarya and dendrites in these regions (Pickel and Sesack, 1995), dual labeling for TH, a specific marker for catecholaminergic neurons, has permitted us to identify the VMAT2-containing cells as dopaminergic neurons. Third, because VMAT2 is an intrinsic membrane protein of vesicles and other subcellular organelles, immunocytochemical localization of this transporter provides a novel approach for identifying potential monoamine storage sites that is not limited by the potential for nonspecific uptake of exogenous radiolabeled or false neurotransmitters. Although the immunocytochemical methods used in the present study cannot discriminate between functional and nonfunctional sites of expression of VMAT2, the interpretation of our findings in light of those of previous uptake and autoradiographic studies provides compelling new information regarding the probable sites of reserpine-sensitive somatodendritic storage of dopamine in midbrain dopaminergic neurons.
Dopaminergic perikarya: potential sites of membrane synthesis and recycling
The pattern of VMAT2-immunolabeling along saccules of Golgi, tubulovesicular organelles, DCVs, and occasional MVBs in TH-immunoreactive (dopaminergic) perikarya in the SN and VTA was similar to the distribution of VMAT2-immunolabeling that we showed previously in the nuclei of the solitary tract (NTS) (Nirenberg et al., 1995). These labeled organelles, which were usually distant from the perikaryal plasma membrane, probably include sites of synthesis, transport, and recycling of VMAT2-containing membranes. The Golgi apparatus, for example, is known to be involved in both the post-translational modification and recycling of vesicular membrane proteins such as VMAT2 (Griffiths and Simons, 1986; Kelly, 1993) and in the synthesis of immature DCVs (Tooze, 1991). Similarly, SSV precursor membranes, which are synthesized in neuronal perikarya, are believed to be tubulovesicular in morphology (Schwarzenbrunner et al., 1990; Jahn and Südhof, 1993; Mundigl and De Camilli, 1994). Furthermore, MVBs have been implicated in the recycling and degradation of vesicular membrane proteins (Jahn and Südhof, 1993; Wittich et al., 1994;Bannon et al., 1995).
Although the VMAT2-labeled perikaryal organelles may include sites of nonfunctional expression of VMAT2 or its C-terminal domain, experiments using the uptake of false neurotransmitters have shown evidence that the trans-Golgi apparatus, DCVs, and tubulovesicles that resemble SER in SN perikarya can sequester monoamines (Wilson et al., 1977; Mercer et al., 1979). Thus, it is likely that at least some of the VMAT2-labeled saccules of Golgi and SER seen in dopaminergic perikarya are also sites of reserpine-sensitive storage of dopamine.
Dopaminergic dendrites: potential sites of dopamine storage and release
We have shown that small numbers of VMAT2-containing SSVs are present in the cytoplasm of dopaminergic dendrites and dendritic spines in both the SN and VTA. These findings are consistent with those of false neurotransmitter uptake studies, which have demonstrated that the dendrites of dopaminergic neurons in the SN contain relatively few synaptic vesicles with a functional monoamine uptake carrier (Sotelo, 1971; Groves and Linder, 1983). We have also seen isolated DCVs in dopaminergic dendrites in the SN and VTA. Previous studies have shown that dendritic DCVs in the SN can specifically take up 5-hydroxydopamine (Sotelo, 1971; Groves and Linder, 1983). Together, these observations suggest that both SSVs and DCVs are involved in the reserpine-sensitive storage and calcium-dependent release of dopamine from midbrain dopaminergic dendrites.
Dopaminergic perikarya and dendrites in the SN and VTA were also filled with extensive networks of VMAT2-labeled tubulovesicles. In fact, some of the organelles that have met our morphological criteria for SSVs may have been tubulovesicular structures, which can be indistinguishable from SSVs when examined in cross-section (Ayala, 1994). The prominent localization of VMAT2 to dendritic tubulovesicles is similar to that which we have demonstrated in the NTS (Nirenberg et al., 1995). Some of these tubulovesicles may represent nonfunctional sites of storage, transport, or recycling of VMAT2-containing membranes; however, there is also convincing evidence that tubulovesicular organelles can store dopamine (Wilson et al., 1977) and in fact may be the major site of somatodendritic dopamine storage (Mercer et al., 1979). The characteristic morphological appearance of these tubulovesicles strongly suggests that they are composed of saccules of SER, as has been presumed in earlier studies (Hattori et al., 1979; Mercer et al., 1979; Wassef et al., 1981). It is also possible, however, that these tubulovesicles represent a novel type of membranous organelle, such as the one that was identified recently in PC12 cells by its expression of the facilitated glucose transporter GLUT4 (Herman et al., 1994).
Given the limited detection of VMAT2-containing vesicles, the VMAT2-containing tubulovesicular organelles are likely to represent the major site of reserpine-sensitive dopamine storage in the SN and VTA. Thus, these tubulovesicular organelles probably contribute more significantly than SSVs or DCVs to somatodendritic dopamine release (Mercer et al., 1979; Wassef et al., 1981). The somatodendritic release of dopamine from these tubulovesicular organelles might potentially involve an exocytosis-like mechanism (Llinás, 1979). Alternatively, dopamine might be released from these organelles into the dendritic cytoplasm, causing an increase in the local dopamine concentration and thereby permitting dendritic release of dopamine through reversal of the plasmalemmal dopamine transporter (Bannon et al., 1995). This possibility is supported by our recent demonstration that the dopamine transporter is localized to the plasma membranes of dopaminergic dendrites and dendritic spines in the SN (Nirenberg et al., 1996). A similar mechanism for dopamine release through reverse dopamine transport has been demonstrated in vitro under pharmacological conditions in which the plasmalemmal dopamine gradient has been reversed (Bernardini et al., 1991; Sulzer et al., 1993).
Nondopaminergic terminals: potential sites of storage and release of other monoamines
In addition to the TH-immunoreactive perikarya and dendrites, we have also observed numerous VMAT2-immunoreactive axon terminals in both the SN and the VTA. These VMAT2-containing axonal processes almost never contained detectable TH-immunolabeling, but were frequently apposed to and/or formed synaptic contacts with dopaminergic (TH-immunoreactive) dendrites in both the SN and the VTA. The presence of VMAT2 immunolabeling and absence of detectable TH-immunolabeling in these axon terminals suggests that they contain monoamines other than catecholamines. In particular, serotonergic neurons in the raphe nuclei are known to make dense projections to both dopaminergic and nondopaminergic dendrites in the SN (Fibiger and Miller, 1977;Nedergaard et al., 1988) and VTA (Herve et al., 1987; Van Bockstaele et al., 1994). Interestingly, there is also evidence that serotonergic inputs to the SN, like those to the striatum and accumbens nucleus, may facilitate the dendritic release of dopamine from midbrain dopaminergic neurons by causing local changes in the dendritic calcium potential (Williams and Davies, 1983; Nedergaard et al., 1988; Jacocks and Cox, 1992). The rare unmyelinated axon terminals that contained reaction product for both VMAT2 and TH may be derived from dopaminergic neurons in the A8 dopamine cell group that have minor projections to the SN and the VTA (Deutch et al., 1988).
Differential localization of VMAT2 in the SN and VTA: functional implications
Dopaminergic neurons in the SN and VTA are known to differ markedly in their roles in motor and motivational functions, their biochemical and pharmacological properties, and their susceptibility to exogenous neurotoxins and neurodegenerative disease (for review, seeRoth and Elsworth, 1995). The present study is the first, however, to show that there are higher levels of VMAT2 per unit area in dopaminergic dendrites in the VTA than in either the SNC or the SNR. These findings suggest that dopaminergic neurons in the VTA contain a larger pool of reserpine-sensitive dopamine storage and release sites than those in the SN. Interestingly, in vitro studies have shown that there are higher levels of somatodendritic dopamine release in the VTA as compared with the SN (Rice et al., 1994). Together, these findings suggest that the greater release of dopamine in the VTA as compared with the SN is at least partially attributable to the presence of higher levels of storage and release of dopamine from individual dendrites in this region.
Recent evidence has also shown that vesicular monoamine transporters can confer neuroprotection by sequestering dopamine neurotoxins such as 1-methyl-4-phenylpyridinium (MPP+) that have been used in experimental models for Parkinson’s disease (Liu et al., 1992a,b; Stern-Bach et al., 1992; Edwards, 1993; Liu et al., 1994). Thus, our observation that there are higher levels of VMAT2 in dopaminergic dendrites in the VTA as compared with those in the SN may also explain the greater susceptibility of nigrostriatal dopaminergic neurons to neurotoxic insult. Additional studies are necessary to determine whether there are comparable differences in the expression of VMAT2 in the human SN and VTA that might potentially contribute to the greater vulnerability of the nigrostriatal dopaminergic neurons to drug-induced parkinsonism and idiopathic Parkinson’s disease (Edwards, 1993).
Footnotes
This research was supported by National Institute of Mental Health (NIMH) Grants MH00078 (V.M.P.) and MH40342 and by the National Institute on Drug Abuse (Grant DA04600). R.H.E. is supported by NIMH Grant MH50712 and by a National Alliance for Research on Schizophrenia and Depression Established Investigator Award. We thank Drs. Carrie T. Drake and Adena L. Svingos for helpful critical commentary, and Ms. Joy Hornung for photographic assistance.
Correspondence should be addressed to Dr. Melissa J. Nirenberg, Department of Neurology and Neuroscience, Cornell University Medical College, 411 East 69th Street, Room KB-410, New York, NY 10021.
REFERENCES
- 1.Ayala J. Transport and internal organization of membranes: vesicles, membrane networks and GTP-binding proteins. J Cell Sci. 1994;107:753–763. doi: 10.1242/jcs.107.4.753. [DOI] [PubMed] [Google Scholar]
- 2.Bannon MJ, Granneman JG, Kapatos G. The dopamine transporter: potential involvement in neuropsychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 179–188. [Google Scholar]
- 3.Bernardini GL, Gu X, Viscardi E, German DC. Amphetamine-induced and spontaneous release of dopamine from A9 and A10 dendrites: an in vitro electrophysiological study in the mouse. J Neural Transm Gen Sect. 1991;84:183–193. doi: 10.1007/BF01244969. [DOI] [PubMed] [Google Scholar]
- 4.Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- 5.Broadwell RD, Cataldo AM. The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. I. Cell bodies and dendrites. J Histochem Cytochem. 1983;31:1077–1088. doi: 10.1177/31.9.6309951. [DOI] [PubMed] [Google Scholar]
- 6.Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 8.Cuello AC, Iversen LL. Interactions of dopamine with other neurotransmitters in the rat substantia nigra: a possible functional role of dendritic dopamine. In: Garattini S, Pujol JF, Samanin R, editors. Interactions between putative neurotransmitters in the brain. Raven; New York: 1978. pp. 127–149. [Google Scholar]
- 9.Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann NY Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RH. Neuronal degeneration and the transport of neurotransmitters. Ann Neurol. 1993;34:638–645. doi: 10.1002/ana.410340504. [DOI] [PubMed] [Google Scholar]
- 11.Elverfors A, Nissbrandt H. Reserpine-insensitive dopamine release in the substantia nigra? Brain Res. 1991;557:5–12. doi: 10.1016/0006-8993(91)90109-9. [DOI] [PubMed] [Google Scholar]
- 12.Erickson JD, Eiden L, Hoffman B. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fibiger HC, Miller JJ. An anatomical and electrophysiological investigation of the serotonergic projection from the dorsal raphe nucleus to the substantia nigra of the rat. Neuroscience. 1977;2:975–987. [Google Scholar]
- 14.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- 16.Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- 17.Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopamine neurons. Science. 1975;190:522–529. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- 18.Hattori T, McGeer PL, McGeer EG. Dendro axonic neurotransmission. II. Morphological sites for the synthesis, binding and release of neurotransmitters in dopaminergic dendrites in the substantia nigra and cholinergic dendrites in the neostriatum. Brain Res. 1979;170:71–83. doi: 10.1016/0006-8993(79)90941-7. [DOI] [PubMed] [Google Scholar]
- 19.Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- 20.Herman GA, Bonzelius F, Cieutat AM, Kelly RB. A distinct class of intracellular storage vesicles, identified by expression of the glucose transporter GLUT4. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 22.Hsu SM, Raine L, Fanger H. The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabeled antibody (peroxidase) procedures. J Histochem Cytochem. 1981;29:577–599. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 23.Jacocks HM, Cox BM., 3d Serotonin-stimulated release of [3H]dopamine via reversal of the dopamine transporter in rat striatum and nucleus accumbens: a comparison with release elicited by potassium, N -methyl-d-aspartic acid, glutamic acid, and d-amphetamine. J Pharmacol Exp Ther. 1992;262:356–364. [PubMed] [Google Scholar]
- 24.Jahn R, Südhof TC. Synaptic vesicle traffic: rush hour in the nerve terminal. J Neurochem. 1993;61:12–21. doi: 10.1111/j.1471-4159.1993.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalivas PW, Bourdelais A, Abhold R, Abbott L. Somatodendritic release of endogenous dopamine: in vivo dialysis in the A10 dopamine region. Neurosci Lett. 1989;100:215–220. doi: 10.1016/0304-3940(89)90687-3. [DOI] [PubMed] [Google Scholar]
- 26.Kelly RB (1993) Storage and release of neurotransmitters. Cell/Neuron 72/10[Suppl]:43–53. [DOI] [PubMed]
- 27.Korf J, Zieleman M, Westerink BHC. Dopamine release in the substantia nigra? Nature. 1976;260:257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- 28.Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborsky L, editors. Neuroanatomical tract-tracing methods II, recent progress. Plenum; New York: 1989. pp. 129–172. [Google Scholar]
- 29.Liu L, Xu W, Harrington KA, Emson PC. The molecular cloning and expression of a human synaptic vesicle amine transporter that suppresses MPP+ toxicity. Brain Res Mol Brain Res. 1994;25:90–96. doi: 10.1016/0169-328x(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Peter D, Roghani A, Schuldiner S, Privé GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992a;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N -methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1992b;89:9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llinás R. The role of calcium in neuronal function. In: Schmitt FO, Worden FG, editors. The neurosciences: fourth study program. MIT; Cambridge, MA: 1979. pp. 555–571. [Google Scholar]
- 33.Mercer L, del Fiacco M, Cuello AC. The smooth endoplasmic reticulum as a possible storage site for dendritic dopamine in substantia nigra neurones. Experientia. 1979;35:101–103. doi: 10.1007/BF01917903. [DOI] [PubMed] [Google Scholar]
- 34.Mundigl O, De Camilli P. Formation of synaptic vesicles. Curr Opin Cell Biol. 1994;6:561–567. doi: 10.1016/0955-0674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 35.Nedergaard S, Hopkins C, Greenfield SA. Do nigro-striatal neurones possess a discrete dendritic modulatory mechanism? Electrophysiological evidence from the actions of amphetamine in brain slices. Exp Brain Res. 1988;69:444–448. doi: 10.1007/BF00247591. [DOI] [PubMed] [Google Scholar]
- 36.Nieoullon A, Cheramy A, Glowinski J. Nigral and striatal dopamine release under sensory stimuli. Nature. 1977a;269:340–342. doi: 10.1038/269340a0. [DOI] [PubMed] [Google Scholar]
- 37.Nieoullon A, Cheramy A, Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977b;266:375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- 38.Nirenberg MJ, Liu Y, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci USA. 1995;92:8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. Academic; Orlando: 1986. The rat brain in stereotaxic coordinates. . [DOI] [PubMed] [Google Scholar]
- 41.Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995;15:6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters A, Palay SL, Webster Hd. Oxford UP; New York: 1991. The fine structure of the nervous system. . [Google Scholar]
- 43.Pickel VM, Sesack SR. Psychopharmacology: the fourth generation of progress (Bloom FE, Kupfer DJ, ed), Raven; New York: 1995. Electron microscopy of central dopamine systems. pp. 257–268. [Google Scholar]
- 44.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- 45.Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Exp Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- 46.Roth RH, Elsworth JD. Psychopharmacology: the fourth generation of progress (Bloom FE, Kupfer DJ, ed), Raven; New York: 1995. Biochemical pharmacology of midbrain dopamine neurons. pp. 227–243. [Google Scholar]
- 47.Santiago M, Westerink BHC. The regulation of dopamine release from nigrostriatal neurons in conscious rats: the role of somatodendritic autoreceptors. Eur J Pharmacol. 1991;204:79–85. doi: 10.1016/0014-2999(91)90838-h. [DOI] [PubMed] [Google Scholar]
- 48.Schwarzenbrunner U, Schmidle T, Obendorf D, Scherman D, Hook V, Fischer-Colbrie R, Winkler H. Sympathetic axons and nerve terminals: the protein composition of small and large dense-core and a third type of vesicles. Neuroscience. 1990;37:819–827. doi: 10.1016/0306-4522(90)90111-g. [DOI] [PubMed] [Google Scholar]
- 49.Sotelo C. The fine structural localization of norepinephrine-3H in the substantia nigra of the rat. An autoradiographic study. J Ultrastruct Res. 1971;36:824–841. doi: 10.1016/s0022-5320(71)90033-5. [DOI] [PubMed] [Google Scholar]
- 50.Stern-Bach Y, Keen J, Bejerano M, Steiner-Mordoch S, Wallach M, Findlay J, Schuldiner S. Homology of a vesicular amine transporter to a gene conferring resistance to 1-methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1992;89:9730–9733. doi: 10.1073/pnas.89.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 52.Tooze SA. Biogenesis of secretory granules: implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS Lett. 1991;285:220–224. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- 53.Van Bockstaele EJ, Cestari DM, Pickel VM. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neurons. Brain Res. 1994;647:307–322. doi: 10.1016/0006-8993(94)91330-7. [DOI] [PubMed] [Google Scholar]
- 54.Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in pars reticulata of substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 55.Williams J, Davies JA. The involvement of 5-hydroxytryptamine in the release of dendritic dopamine from slices of rat substantia nigra. J Pharm Pharmacol. 1983;35:734–737. doi: 10.1111/j.2042-7158.1983.tb02880.x. [DOI] [PubMed] [Google Scholar]
- 56.Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- 57.Wittich B, Volknandt W, Zimmerman H. SV2 and o-rab3 remain associated with recycling synaptic vesicles. J Neurochem. 1994;63:927–937. doi: 10.1046/j.1471-4159.1994.63030927.x. [DOI] [PubMed] [Google Scholar]


