Abstract
We studied the functional anatomy of affect-laden autobiographical memory in normal volunteers. Using H215O positron emission tomography (PET), we measured changes in relative regional cerebral blood flow (rCBF). Four rCBF measurements were obtained during three conditions: REST, i.e., subjects lay at rest (for control); IMPERSONAL, i.e., subjects listened to sentences containing episodic information taken from an autobiography of a person they did not know, but which had been presented to them before PET scanning (nonautobiographical episodic memory ecphory); and PERSONAL, i.e., subjects listened to sentences containing information taken from their own past (autobiographical episodic memory ecphory).
Comparing IMPERSONAL with REST (nonautobiographical episodic memory ecphory) resulted in relative rCBF increases symmetrically in both temporal lobes including the temporal poles and medial and superior temporal gyri. The same loci, however, with a stronger lateralization to the right hemisphere were activated in the comparison PERSONAL to REST (autobiographical episodic memory ecphory). In addition, the right temporomesial, right dorsal prefrontal, right posterior cingulate areas, and the left cerebellum were activated. A comparison of PERSONAL and IMPERSONAL (autobiographical vs nonautobiographical episodic memory ecphory) demonstrated a preponderantly right hemispheric activation including primarily right temporomesial and temporolateral cortex, right posterior cingulate areas, right insula, and right prefrontal areas. The right temporomesial activation included hippocampus, parahippocampus, and amygdala.
These results suggest that a right hemispheric network of temporal, together with posterior, cingulate, and prefrontal, areas is engaged in the ecphory of affect-laden autobiographical information.
Keywords: PET, autobiographical memory, episodic memory, retrieval, cingulate, limbic system
Memory commonly is subdivided into a number of forms (Squire and Knowlton, 1995; Tulving, 1995), and it is assumed that different brain regions contribute differentially to the various forms of memory processing (Squire et al., 1993; Markowitsch, 1995a). Two of the main forms of memory are episodic memory and semantic memory (Tulving, 1995), also subsumed under declarative memory (Squire, 1987). Episodic memory is supposed to deal with individual (“autobiographical”) episodes that are definable with respect to time and locus. Semantic memory contains impersonal facts (e.g., knowledge about the world), which we need for verbal and nonverbal interaction with our environment.
The usefulness of the distinction between episodic and semantic memory sometimes has been argued (Cermak and Craik, 1979), given the fact that semantic memory frequently constitutes a generalization from initially episodically encoded information. However, there are experiments demonstrating both acquisition of semantic in the absence of the ability to acquire episodic information (Tulving et al., 1991) and impairments in acquiring both forms (Gabrieli et al., 1988; Verfaellie and Cermak, 1994). Although both forms most likely engage overlapping limbic system structures for information acquisition (Buckner and Tulving, 1995), memory retrieval seems to depend on different brain regions and, furthermore, seems to rely on different systems for the retrieval of episodic and semantic information.
Results of studies performed with positron emission tomography (PET) suggest a left hemispheric preponderance for the retrieval of semantic information and a right hemispheric preponderance for the retrieval of episodic information (Fletcher et al., 1995a; Tulving et al., 1994a,b,c, 1996).
In most clinical studies on amnesics, it has been shown that the retrieval of episodic (autobiographical) memory is much more affected than that of semantic memory (general knowledge) (Markowitsch, 1995a,b). Reasons for this may be sought in the uniqueness of episodic information (Damasio, 1990), in its higher emotional character (Sarter and Markowitsch, 1985a,b; Markowitsch et al., 1994), or in the brain regions in which damage affects ecphory of episodic versus general knowledge material (Markowitsch, 1995a,b). Tulving (1983) used the term “ecphory” to describe the process by which retrieval cues interact with stored information so that an image or a representation of the information in question appears. It is of interest to investigate whether in the intact human brain, the ecphory of presented autobiographical material will affect different brain regions compared with the retrieval of semantically closely similar material, which is, however, not associated by the subject with his or her own past.
In this study, we wanted to demonstrate the functional anatomy of ecphory of affect-laden autobiographical material. We used PET to image significant changes in relative regional cerebral blood flow (rCBF) as an index of changes in local neuronal activity in the brains of normal human subjects when they listened to sentences containing affect-laden episodic autobiographical information. We then intended to relate those relative rCBF changes to changes obtained when the same subjects listened to sentences containing semantically similar episodic but nonautobiographical material. Based on evidence available from human and nonhuman lesion data (LeDoux, 1995; Markowitsch, 1995a,b; Sarter and Markowitsch, 1985a,b), we hypothesized that the anterolateral and temporomesial parts of the temporal lobes (including the amygdala) as well as the inferolateral prefrontal cortex were involved in the ecphory of past personal experiences.
MATERIALS AND METHODS
Subjects. Seven normal, healthy male volunteers (age 21–37 years) were recruited. All subjects were right-handed and had no history or evidence of any medical, neurological, or psychiatric disease. Informed consent was obtained before participation from all subjects. The study was approved by the local ethics committee of the University of Cologne, and permission to administer radioactivity was obtained from the federal administration authorities.
Paradigm design. The experiment involved 12 sequential measurements of relative rCBF and consisted of one control condition (REST) and two testing conditions (IMPERSONAL, PERSONAL), which were presented in a counterbalanced order (ABCCBAACBBCA). The order of scans was counterbalanced rather than randomized to avoid variable order artifacts attributable to the small number of subjects studied. The conditions were as follows: REST, i.e., subjects were studies in a resting condition with eyes closed and no auditory or visual stimulation (baseline condition, for control); IMPERSONAL, i.e., subjects were studied during auditory presentation of sentences that contained episodic information taken from an autobiography of a person they did not know, but which had been presented to them about 1 hr before PET scanning; PERSONAL, i.e., subjects were studied while they listened to sentences containing episodic information taken from their own past (autobiographical information). The sentences contained brief episodes from significant events of each subject’s past or someone else’s past, respectively. Examples for sentences are: “When you were 15 you took part in a swimming marathon and succeeded to swim 10 miles,” and “He tore off his shirt to demonstrate his scars to the nurse.” Subjects were instructed to imagine what happened to the person in the described situations (IMPERSONAL) or to imagine what happened to themselves in the described situations (PERSONAL). Ten sentences were presented during IMPERSONAL and PERSONAL situations. The information presented during PERSONAL had been obtained in semistandardized interviews weeks before PET scanning. Information was collected from childhood, adolescence, and early adulthood; the subjects were kept unaware of the purpose of the interviews.
PET-scanning techniques. Relative rCBF was measured by recording the regional distribution of cerebral radioactivity after the intravenous injection of 15O-labeled water (15O is a positron emitter with a half-life of 2.1 min) (Mazziotta et al., 1985; Fox and Mintun, 1989). The PET measurements were carried out using a CTI ECAT Exact HR PET scanner (CTI, Knoxville, TN) with a total axial field of view of 15 cm covering the whole brain (Wienhard et al., 1994). Data were acquired in three-dimensional mode (Townsend et al., 1991) with interdetector collimating septa removed, allowing the collection of reliable data of regional brain activation from a single subject (Watson et al., 1993).
For each measurement of relative rCBF, 10 mCi of H215O were given intravenously. Emission data were collected sequentially over 90 sec after tracer arrival in the brain. This process was repeated for each emission scan, with 10 min between scans to allow for adequate decay of radioactivity. This gave an estimate of rCBF. Because no arterial blood samples were taken (Mazziotta et al., 1985), no calibration was possible and the term “relative” rCBF used in this paper implies that there was no absolute quantification of rCBF. The emission scan data then were corrected for effects of radiation attenuation by the skull (by means of a transmission scan taken before the first relative rCBF measurement). After attenuation correction, the data were reconstructed to 47 transverse planes by three-dimension filtered-back projection using a Hanning filter of cutoff frequency 0.4 cycles per pixel. The resolution of the resulting images was 5.5 mm (at full-width half-maximum).
Magnetic resonance imaging. On a separate occasion, a magnetic resonance (MR) image of each subject’s brain was obtained to exclude morphological–pathological abnormalities. This was performed with a 1 Tesla system (Magnetom, Siemens, Germany) using a FLASH sequence (flip angle, 40°; repetition time, 40 msec; echo time, 15 msec) producing 64 transaxial T1-weighted tomograms.
Image processing. All calculations and image manipulations were performed on a Sparc workstation (Sun Computers). ANALYZE and PROMATLAB software (MathWorks) was used to calculate and display images. Statistical parametric mapping (SPM) software (Wellcome Department of Cognitive Neurology, London, UK) was used for image realignment and smoothing and to create statistical maps of significant relative rCBF changes (Friston et al., 1995a,b).
Realignment, transformation, and smoothing of PET images.Using SPM software (Friston et al., 1995a), all PET scans were realigned to the first emission scan to correct for any head movement. The PET images then were transformed into a standard stereotactic anatomical space (Talairach and Tournoux, 1988; Friston et al., 1995a) using linear proportions and a nonlinear sampling algorithm. This allowed accounting for differences in brain size and shape. PET images then were filtered using a low-pass Gaussian filter to reduce the variance attributable to individual anatomical variability and to improve signal-to-noise ratio (Friston et al., 1995a). The resulting pixel size in stereotactic space was 2 × 2 mm with an interplane distance of 4 mm. The group data were expressed in terms of standard stereotactic coordinates in the x-, y-, andz-axes (defined in Table1).
Table 1.
Increases in brain activity associated with autobiographical and nonautobiographical episodic memory retrieval
| Region | Side | Coordinates | Zstatistic | ||
|---|---|---|---|---|---|
| x | y | z | |||
| IMPERSONAL–REST (nonautobiographical episodic memory retrieval) | |||||
| Medial/superior temporal | L | −52 | −20 | −4 | 7.06 |
| Gyrus (BA 21) | R | 56 | −24 | 0 | 8.24 |
| PERSONAL–REST (autobiographical episodic memory retrieval) | |||||
| Medial/superior temporal gyrus (BA 21/38) | R | 44 | 0 | −12 | 12.17 |
| Dorsal/superior frontal gyrus (BA 6) | R | 4 | 8 | 60 | 4.23 |
| Posterior cingulate area (BA 23/30) | R | 4 | −54 | 8 | 4.13 |
| Cerebellum | L | −10 | −82 | −28 | 4.58 |
| PERSONAL–IMPERSONAL (autobiographical episodic memory) | |||||
| Superior temporal gyrus/insula (BA 38) | R | 38 | 6 | −12 | 7.03 |
| Medial temporal gyrus (BA 21)/periamygdaloid | R | 42 | −4 | −16 | 7.03 |
| Medial temporal gyrus (BA 21/37) | R | 50 | −52 | 8 | 4.05 |
| Anterior insula | R | 28 | 18 | −4 | 6.69 |
| Posterior cingulate area (BA 29/30) | R | 12 | −52 | 8 | 3.98 |
Coordinates (in standard stereotactic space) (Talairach and Tournoux, 1988) refer to the local maxima as indicated by the highestZ statistic within an area of activation (p < 0.05, corrected for multiple nonindependent comparisons) associated with (1) nonautobiographical episodic memory retrieval (IMPERSONAL–REST); (2) autobiographical episodic memory retrieval (PERSONAL–REST); and (3) autobiographical episodic memory (PERSONAL–IMPERSONAL). X, Distance (mm) to right (+) or left (−) of the midsagittal line; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; z, distance above (+) or below (−) the intercommissural (AC–PC) line. For each anatomical location, the Talairach and Tournoux’ estimate of the Brodmann area is given in parentheses. R, Right; L, left.
Statistical analysis. After stereotactic normalization, the main effects of the two testing conditions (IMPERSONAL, PERSONAL) were estimated on pixel-by-pixel basis using SPM (Friston et al., 1995b). Global differences in CBF were first covaried out (Friston et al., 1995b), treating global activity as the covariate. This controlled for systematic state-dependent differences in global blood flow associated with the different conditions. Then for each pixel across all subjects and all scans, the mean values were calculated for the control (REST) and the two activation tasks separately, and comparisons of the means thereafter were made using t statistics (Friston et al., 1995b) subsequently transformed into normally distributed Zstatistics. The resulting set of Z values constituted a statistical parametric map (SPM{t} map) (Friston et al., 1995b). For the group, data significance was set top < 0.05, corrected for multiple nonindependent comparisons. In addition, PET data were analyzed for each individual subject in an identical way to the group data to allow single-subject data analysis.
Localization of activations. The stereotactic coordinates of the pixels of local maximum significant changes in relative rCBF within areas of significant relative rCBF change associated with the different tasks were determined. The anatomical localization of these local maxima was assessed by referring to the standard stereotactic atlas ofTalairach and Tournoux (1988) and validated additionally after superimposition of the SPM{t} maps on an arbitrary MR image that had undergone stereotactic transformation into the same standard stereotactic space (Friston et al., 1995a).
Planned comparisons of differences. Three planned comparisons were made. First, a comparison was made of the condition associated with neutral nonautobiographical episodic memory ecphory (IMPERSONAL) and the control (REST). Second, a comparison of autobiographical episodic memory ecphory (PERSONAL) with the control (REST) was performed. Third, autobiographical memory ecphory (PERSONAL) was compared with nonautobiographical memory ecphory (IMPERSONAL). These comparisons were intended to identify those cortical areas concerned with the modalities in question (i.e., autobiographical memory ecphory and episodic memory ecphory in general). For completeness of data analysis and presentation, the respective reverse comparisons also were calculated.
RESULTS
Nonautobiographical episodic memory ecphory versus baseline (IMPERSONAL–REST)
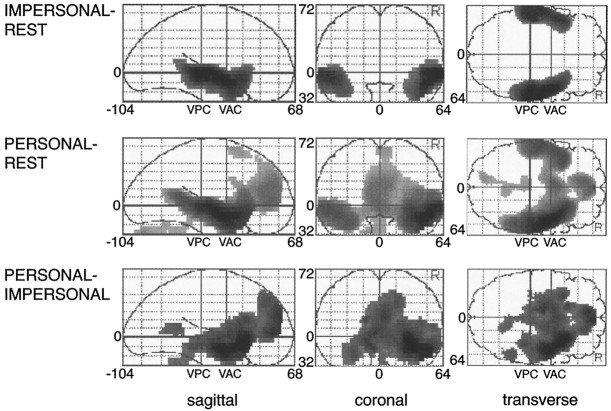
Table 1, IMPERSONAL–REST summarizes the principal areas with increases in relative rCBF associated with the difference between IMPERSONAL and REST. Figure 1 provides a pictorial demonstration of the areas with relative rCBF increase. There are highly symmetrical bilateral increases in the temporal lobes, namely the superior and medial temporal gyri including both temporal poles and Brodmann area 21 (BA 21). Decreases in relative rCBF associated with the task were seen in the medial (BA 7) and the lateral inferior parietal cortex (BA 40) bilaterally, the right fusiform gyrus (BA 19/37), the left caudate nucleus, and the midbrain (Table 2a,IMPERSONAL–REST).
Fig. 1.
Relative rCBF increases during nonautobiographical and autobiographical episodic memory retrieval. Relative rCBF increases for the group of seven subjects associated with nonautobiographical episodic memory retrieval (IMPERSONAL–REST), autobiographical episodic memory retrieval (PERSONAL–REST), and autobiographical memory (PERSONAL–IMPERSONAL). Areas of significant (p < 0.05, corrected for multiple nonindependent comparisons) relative rCBF increases associated with the different tasks are shown as through projections onto representations of the standard stereotactic space as defined by Talairach and Tournoux (1988). The sagittal images (left) view the brain from the side, the coronal images (middle) view the brain from the back, and the transverse images (right) view the brain from the top. R, Right; VAC, vertical plane through the anterior commissure; VPC, vertical plane through the posterior commissure. Numbers at axes refer to coordinates of stereotactic space. The exact coordinates of the local maxima within areas of activation and their Z statistics are given in Table 1.
Table 2.
Decreases in brain activity associated with autobiographical and nonautobiographical episodic memory retrieval
| Region | Side | Coordinates | Zstatistic | ||
|---|---|---|---|---|---|
| x | y | z | |||
| IMPERSONAL–REST (nonautobiographical episodic memory retrieval) | |||||
| Medial parietal cortex (BA 7) | L | −16 | −62 | 48 | 4.96 |
| R | 18 | −64 | 36 | 5.38 | |
| Inferior lateral parietal cortex (BA 40) | L | −44 | −32 | 24 | 4.67 |
| R | 52 | −30 | 36 | 4.68 | |
| Fusiform gyrus (BA 19/37) | R | 50 | −60 | −12 | 5.23 |
| Caudate nucleus | L | −14 | 14 | 16 | 4.86 |
| Midbrain | L | −6 | −14 | −16 | 4.62 |
| PERSONAL–REST (autobiographical episodic memory retrieval) | |||||
| Lateral inferior parietal cortex (BA 40) | L | −46 | −38 | 28 | 7.43 |
| R | 54 | −28 | 32 | 6.82 | |
| Inferior occipital cortex (BA 19) | L | −44 | −62 | −8 | 7.21 |
| R | 38 | −72 | −8 | 6.15 | |
| Dorsolateral prefrontal cortex (BA 9) | R | 42 | 30 | 32 | 4.98 |
| PERSONAL–IMPERSONAL (autobiographical episodic memory) | |||||
| Lateral inferior parietal cortex (BA 40/39) | L | −50 | −56 | 28 | 5.15 |
| R | 46 | −50 | 36 | 4.85 | |
| Inferior frontal cortex (BA 10) | L | −38 | 42 | 12 | 5.57 |
| Inferior occipital cortex (BA 19) | L | −44 | −62 | −8 | 4.59 |
| Fusiform gyrus (BA 18) | R | 22 | −86 | −12 | 4.56 |
Coordinates (in standard stereotactic space) (Talairach and Tournoux, 1988) refer to the local maxima as indicated by the highestZ statistic within an area of deactivation (p < 0.05, corrected for multiple nonindependent comparisons) associated with (1) nonautobiographical episodic memory retrieval (IMPERSONAL–REST); (2) autobiographical episodic memory retrieval (PERSONAL–REST); and (3) autobiographical episodic memory (PERSONAL–IMPERSONAL). X, Distance (mm) to right (+) or left (−) of the midsagittal line; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; z, distance above (+) or below (−) the intercommissural (AC–PC) line. For each anatomical location, the Talairach and Tournoux’ estimate of the Brodmann area is given in parentheses. R, Right; L, left.
Autobiographical episodic memory ecphory versus baseline (PERSONAL–REST)
Comparing the presentation of autobiographical sentences with the resting state (PERSONAL–REST) similarly resulted in relative rCBF increases in both medial and superior temporal gyri, however, with a lateralization toward the right hemisphere (Table 1,PERSONAL–REST, Fig. 1). Again, the temporopolar cortex was activated bilaterally, although more pronounced on the right than on the left, and right dorsal frontal and right posterior cingulate areas were activated together with the cerebellum (primarily left). Relative rCBF decreases associated with the task were seen in the inferior lateral parietal cortex (BA 40) bilaterally, the inferior occipital cortex (BA 19), and the right dorsolateral prefrontal cortex (BA 9) (Table 2PERSONAL–REST).
Autobiographical versus nonautobiographical episodic memory ecphory (PERSONAL–IMPERSONAL)
The comparison of the autobiographical sentences versus the neutral sentences condition (PERSONAL–IMPERSONAL) demonstrated preponderantly right hemispheric relative rCBF increases. These were seen in the lateral and medial aspects of the right temporal lobe including hippocampal, parahippocampal, and amygdaloid areas, the right anterior insula, the right posterior cingulate area, the right temporoparietal junction, and right prefrontal cortex (Table 1PERSONAL–IMPERSONAL, Figs. 1, 2). Relative rCBF decreases associated with this task again were seen in the lateral inferior parietal cortex (BA 40/39) bilaterally, the left inferior occipital cortex (BA 19), the right fusiform gyrus (BA 18), and the left inferior frontal cortex (BA 10) (Table 2PERSONAL–IMPERSONAL).
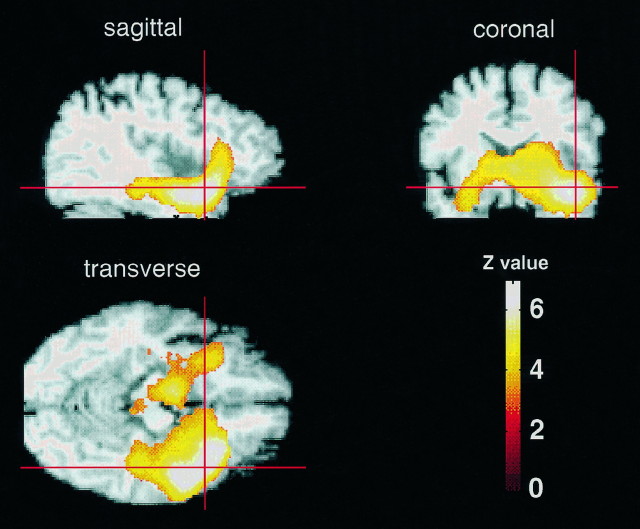
Fig. 2.
Functional anatomy of temporal activations during affect-laden autobiographical memory. Same data as in Figure 1, but here the group SPM{t} map has been sectioned in sagittal, coronal, and transverse planes and is displayed on top of an arbitrary MR image that has been normalized spatially to the same anatomical space. The red cross-hair indicates the local maximum within the area of activation. The color barindicates the Z statistics achieved (Z value). This figure details the functional anatomy of temporal activations associated with autobiographical memory (PERSONAL–IMPERSONAL) and their relationship to underlying anatomy. Note that the activations are predominantly on the right (left image corresponds to subjects’s left) and include temporomedial, temporolateral, and insular areas.
Single-subject data analysis
In five subjects, the pattern of activations was congruent to the group one. One subject failed to show any significant activation; the remaining showed primarily temporo-occipital activations.
DISCUSSION
The present study demonstrates functional neuronal activity associated with the ecphory of autobiographical memory in a network of primarily right hemispheric regions including temporomedial and temporolateral cortex, amygdala and hippocampus–parahippocampus, insula, posterior cingulate cortex, temporoparietal cortex, and prefrontal cortex. Some of these areas are known to be part of or are at least closely associated with retrieval of episodic long-term memory. Furthermore, they are part of the expanded limbic system network (Nauta, 1979) and are involved in affect-based information coding.
Experimental design
A number of recent PET-studies investigated the brain regions engaged in memory processing (see also Heiss et al., 1992; Frackowiak, 1994; Buckner and Tulving, 1995; McCarthy, 1995). Most closely related to our experiment are studies focusing on an anatomical basis for the retention of long-term verbal memory (Andreasen et al., 1995) or on encoding or retrieval processes in verbal and visual memory (Grasby et al., 1994; Kapur et al., 1994; Shallice et al., 1994; Tulving et al., 1994a,b,c, 1996; Buckner et al., 1995; Fletcher et al., 1995a; Kapur et al., 1995; Petrides et al., 1995) .
Whereas in the above studies, subjects actually acquired new information before being scanned, in the present experiment, subjects had to ecphorize (“re-evoke”) own past (autobiographical) events taken from their childhood, adolescence, and early adulthood. While being scanned, they listened to sentences, each describing an important episode of their past. Neuronal activation during this condition was compared with activation during a second condition in which subjects listened to sentences containing semantically very similar material taken, however, from another person that the subjects did not know. For both conditions, subjects were instructed to imagine what had happened during the described situation. By necessity, studying autobiographical memory ecphory (PERSONAL) involves retrieval of episodic memory. Because we were interested in the ecphory of autobiographical old memory rather than of episodic memory per se, the latter was subtracted from the first (IMPERSONAL–PERSONAL).
Areas activated
Medial temporal lobes
In our study, there was no temporomedial activation associated with the ecphory of nonautobiographical memory and, indeed, only few PET studies actually have reported activation of medial temporal lobes with memory tasks (Squire et al., 1992; Grasby et al., 1993; Kapur et al., 1995). By contrast, autobiographical memory ecphory led to strong activation of left and right temporomesial areas including hippocampus and amygdala. Interestingly, this activation was much more pronounced on the right. Our data suggest that autobiographical memory ecphory engages amygdaloid and hippocampal regions. This is in good accordance with the idea that limbic structures of the temporal cortex are of crucial importance for affect-sensitive mnestic processing.
Lateral temporal lobes
In the present study, both the middle and superior temporal gyri were involved in nonautobiographical and autobiographical memory ecphory. Areas activated included BA 21 and previously have been associated with word–sentence recognition and understanding (Mazoyer et al., 1993). However, when comparing PERSONAL with IMPERSONAL, significant activations were seen in all three right temporal gyri, in particular, the middle and superior temporal gyri. This suggests that autobiographical memory ecphory, in addition to sentence recognition and understanding required in the studied task, engages these brain areas. This is concordant with experimental findings stressing the importance of neocortical temporal areas in episodic memory representation (Merzenich and Sameshima, 1993; Sakai and Miyashita, 1994) and supports the notion of a multimodal representation of episodic memory.
Temporal poles
The temporal poles have been shown to be part of the cortical representation of speech. This was demonstrated when subjects listened to stories (Mazoyer et al., 1993) or read stories in silence (Fletcher et al., 1995b). Both activation conditions (IMPERSONAL, PERSONAL) involved the listening and understanding of sentences with narrative character. These regions, anatomically distinct from the medially situated allocortical temporal regions necessary for memory encoding and consolidation (Markowitsch, 1995a), also seem to be involved in the ecphory of autobiographical memory as a comparison of IMPERSONAL and PERSONAL revealed activation of the right temporal pole.
Insula
Insular activation has been reported in many PET experiments. We hypothesize that the insular activation during autobiographical memory ecphory may be attributed to a strong activation of the limbic system caused by the high emotional impact of the task.
Cingulate cortex
Clinical studies have established a posterior cingulate cortex contribution to episodic memory (Rudge and Warrington, 1993). Activation of this region has been described during encoding of verbal memory (Grasby et al., 1993, 1994; Kapur et al., 1994; Fletcher et al., 1995a). In the present study, the posterior cingulate cortex was activated during the ecphory of autobiographical, but not of nonautobiographical, memory. Reasons for this activation remain speculative, although an activation of this important neural link between the prefrontal cortex and the hippocampus (Goldman-Rakic et al., 1984) causes little surprise. Furthermore, PERSONAL versus IMPERSONAL ecphory may require differential attentional mechanisms during the two activation tasks.
Prefrontal cortex
Activation of prefrontal cortex during memory-associated tasks has been observed previously, and it has been proposed that such activation may be related to the degree of monitoring and verification involved in a recall task (Fletcher et al., 1995a). Additional support to this hypothesis is provided by clinical observations of retrograde amnesia in patients with combined damage of anterolateral temporal areas and prefrontal areas (Markowitsch, 1995b), suggesting that the prefrontal cortex acts as a kind of control center for effortful initiation of recall (Jetter et al., 1986) and the sequencing and organizing of information (Milner et al., 1985; Goldman-Rakic and Friedman, 1991;Stuss et al., 1994; Goldman-Rakic, 1995). This, together with the anterolateral temporal cortex which provides a connecting link to posterior cortical centers of integration as the major storage places of engrams (Markowitsch et al., 1985), implies an important role of the prefrontal cortex in the initiation of directed ecphory. The results on prefrontal activation obtained in the present study are in good agreement with the above ideas, because the prefrontal cortex is activated during the ecphory of autobiographical memory retrieval.
Cerebellum
A contralateral cerebellar activation has been observed during numerous PET studies on memory tasks (Barker et al., 1991;Grasby et al., 1994; Molchan et al., 1994; Tulving et al., 1994b;Andreasen et al., 1995; Buckner and Tulving, 1995; Buckner et al., 1995) and was found in the present study during autobiographical memory ecphory. Andreasen et al. (1995) speculated recently that the cerebellum may participate in memory processing apart from procedural and automatic aspects of memory. Evidence for such a role comes from both animal studies and neuropsychological experiments in patients with acquired brain damage (Schmahmann, 1992; Daum et al., 1993; Grafman et al., 1993; Leiner et al., 1993) .
Areas deactivated
The decreases associated with the differences among the conditions seem to reflect reduced visual and spatial imagery (occipital and parietal regions) (Ogden, 1993; Kosslyn et al., 1993), reduced effort in language comprehension (BA 40, Wernicke area) (Démonet et al., 1993), and less effort in general attentive functions (prefrontal regions) (Tulving et al., 1994c).
Laterality
Based on PET activation studies (Shallice et al., 1994; Tulving et al., 1994a,b,c, 1996; Nyberg et al., 1996) and clinical data from neurological patients with selective retrograde amnesia in the episodic (Kapur et al., 1992; Markowitsch et al., 1993a,b) or semantic memory domain (De Renzi et al., 1987; Grossi et al., 1988), it has been hypothesized that the right anterior temporal and right inferolateral prefrontal cortex, structures interconnected via the ventral branch of the uncinate fascicle, are involved in episodic memory retrieval, whereas the same structural combination in the left hemisphere may be responsible for semantic memory retrieval. The present findings, indicating a huge preponderance of activation in the right hemisphere, support this hypothesis for the episodic memory system in general and for autobiographical memory retrieval in particular.
Further observed predominantly right-sided activation in the periamygdaloid, hippocampal–parahippocampal, insular, and posterior cingulate regions also may be explained as a correlate for the frequently found emotional character of autobiographical memories (Sarter and Markowitsch, 1985a,b; Adolphs et al., 1994, 1995;Barbas, 1995; Cahill et al., 1995; Devinsky et al., 1995; LeDoux, 1995). Alternatively, one might speculate that at least in part the right-sided preponderance of activations might be linked to the self-representation involved in autobiographic memory ecphory.
Possible confounds
Sentences from the nonautobiographical and the autobiographical conditions by necessity differed in that during the autobiographical condition, material was presented that was (1) familiar to the subjects for many years, (2) affectively more relevant, and (3) more personal. Any or all of these factors could contribute to the described activations. Furthermore, because subjects had to imagine what had happened to another person during the nonautobiographical condition as opposed to imagining what had happened to themselves during the autobiographical condition, comparing the two conditions also could reflect blood flow changes associated with imagining oneself versus imagining others. These potential confounds will have to be addressed by additional functional imaging studies.
PET (Martin et al., 1995, 1996) and neuropsychological evidence (Shallice and Kartsounis, 1993) argue for the existence of separable cortical regions involved in the storage or activation of different semantic properties. One therefore might speculate that knowledge about oneself could be another class of semantic knowledge. However, if such a restricted kind of “autobiographical knowledge module” existed, it most likely would recruit additional information such as emotional–affective flavor from other regions (e.g., the amygdala), making it altogether a wider network than those suggested presently for singular categories of semantic information.
Clinical implications
The process of autobiographical memory ecphory is of particular importance in patients with retrograde amnesia. It has been assumed that their “lost” episodic information still exists in the brain as engrams that cannot be retrieved consciously (Markowitsch, 1995b). Both in patients with so-called focal retrograde amnesia and with psychogenic amnesia, stored information most likely remains available (Kapur, 1993; Markowitsch, 1995b), although it seems to be inaccessible (Tulving and Pearlstone, 1966), i.e., it cannot be ecphorized. Although it can be only speculated which mechanisms may be responsible for such an inaccessibility, the present results provide some evidence for the brain regions involved in autobiographical memory ecphory under normal conditions.
Our study shows that ecphory of autobiographical memories is dependent on a restricted network of mostly right hemispheric brain areas that are in part different from those activated by retrieval of episodic nonautobiographical memory. Nevertheless, the circuits for the ecphory of active autobiographical and nonautobiographical memory are related. Together with the results from neuropsychological investigations on single cases with selective retrograde amnesia, this study provides strong evidence that right hemispheric temporal cortical areas are the key regions for autobiographical memory ecphory. They are supported by surrounding right hemispheric “satellite” regions such as the amygdala, hippocampus–parahippocampus, posterior cingulate, and insular and prefrontal cortex.
Footnotes
We are grateful to our volunteers who made this study possible. We thank both anonymous reviewers for helpful comments.
Correspondence should be addressed to Dr. Gereon Fink, Wellcome Department of Cognitive Neurology, Institute of Neurology, 12 Queen Square, London WC1N 3BG, UK.
REFERENCES
- 1.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 2.Adolphs R, Tranel D, Damasio H, Damasio A. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LLB, Hichwa RD. Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbas H. Anatomical basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 5.Barker WW, Yoshii F, Loewenstein DA, Chang JY, Apicella A, Pascal S, Boothe TE, Ginsberg MD, Duara R. Cerebrocerebellar relationship during behavioral activation: a PET study. J Cereb Blood Flow Metab. 1991;11:48–54. doi: 10.1038/jcbfm.1991.5. [DOI] [PubMed] [Google Scholar]
- 6.Buckner RL, Tulving E. Neuroimaging studies of memory: theory and recent PET results. In: Boller F, Grafman J, editors. Handbook of neuropsychology, Vol. 10. Elsevier; Amsterdam: 1995. pp. 439–466. [Google Scholar]
- 7.Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. Involvement of the amygdaloid complex in emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 9.Cermak LS, Craik FIM. Lawrence Erlbaum; Hillsdale, NJ: 1979. Levels of processing in human memory. . [Google Scholar]
- 10.Damasio AR. Category-related recognition defects as a clue to the neural substrates of knowledge. Trends Neurosci. 1990;13:95–98. doi: 10.1016/0166-2236(90)90184-c. [DOI] [PubMed] [Google Scholar]
- 11.Daum I, Schugens M, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- 12.Démonet JF, Wise R, Frackowiak RSJ. Language functions explored in normal subjects by positron emission tomography: a critical review. Hum Brain Mapp. 1993;1:39–47. [Google Scholar]
- 13.De Renzi E, Liotti M, Nichelli P. Semantic amnesia with preservation of autobiographic memory: a case report. Cortex. 1987;23:575–597. doi: 10.1016/s0010-9452(87)80050-3. [DOI] [PubMed] [Google Scholar]
- 14.Devinsky O, Morell MJ, Vogt BA. Contributions of anterior cingulate cortex. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RSJ, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory: an in vivo study in humans. Brain. 1995a;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995b;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 17.Fox PT, Mintun MA. Non-invasive functional brain mapping by change distribution analysis of average PET images of H215O tissue activity. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- 18.Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:1–25. [Google Scholar]
- 19.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- 20.Frackowiak RSJ. Functional mapping of verbal memory and language. Trends Neurosci. 1994;17:109–115. doi: 10.1016/0166-2236(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 21.Gabrieli JDE, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 1988;7:157–177. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 22.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS, Friedman HR. The circuitry of working memory revealed by anatomy and metabolic imaging. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. Oxford UP; New York: 1991. pp. 72–91. [Google Scholar]
- 24.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 25.Grafman J, Litvan I, Hallett M. Cerebellar cognition. Neurology. 1993;43:2153–2154. doi: 10.1212/wnl.43.10.2153. [DOI] [PubMed] [Google Scholar]
- 26.Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ. Functional mapping of brain areas implicated in auditory memory function. Brain. 1993;116:1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RSJ, Dolan RJ. A graded-task approach to the functional mapping of brain areas implicated in auditory-verbal memory function. Brain. 1994;117:1271–1282. doi: 10.1093/brain/117.6.1271. [DOI] [PubMed] [Google Scholar]
- 28.Grossi D, Trojano L, Grasso A, Orsini A. Selective “semantic amnesia” after closed-head injury. Cortex. 1988;24:457–464. doi: 10.1016/s0010-9452(88)80009-1. [DOI] [PubMed] [Google Scholar]
- 29.Heiss W-D, Pawlik G, Holthoff V, Kessler J, Szelies B. PET correlates of normal and impaired memory functions. Cerebrovasc Brain Metab Rev. 1992;4:1–27. [PubMed] [Google Scholar]
- 30.Jetter W, Poser U, Freeman RB, Jr, Markowitsch HJ. A verbal long term memory deficit in frontal lobe damaged patients. Cortex. 1986;22:229–242. doi: 10.1016/s0010-9452(86)80047-8. [DOI] [PubMed] [Google Scholar]
- 31.Kapur N. Focal retrograde amnesia in neurological disease: a critical review. Cortex. 1993;29:217–234. doi: 10.1016/s0010-9452(13)80177-3. [DOI] [PubMed] [Google Scholar]
- 32.Kapur N, Ellison D, Smith MP, Mcellan DL, Burrows EH. Focal retrograde amnesia following bilateral temporal lobe pathology. Brain. 1992;115:73–85. doi: 10.1093/brain/115.1.73. [DOI] [PubMed] [Google Scholar]
- 33.Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RSJ. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31:99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, Craik F, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosslyn SM, Alpert NM, Thompson WL, Maljkovic V, Weise SB, Chabris CF, Hamilton SE, Rauch SL, Buonanno FS. Visual mental imagery activates topographically organized visual cortex: PET investigations. J Cognit Neurosci. 1993;5:263–287. doi: 10.1162/jocn.1993.5.3.263. [DOI] [PubMed] [Google Scholar]
- 36.LeDoux JE. Emotion: clues from the brain. Ann Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 37.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- 38.Markowitsch HJ. Anatomical basis of memory disorders. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT; Cambridge, MA: 1995a. pp. 665–679. [Google Scholar]
- 39.Markowitsch HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Res Rev. 1995b;21:117–127. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 40.Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primate’s temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol. 1985;242:425–458. doi: 10.1002/cne.902420310. [DOI] [PubMed] [Google Scholar]
- 41.Markowitsch HJ, Calabrese P, Haupts M, Durwen HF, Liess J, Gehlen W. Searching for the anatomical basis of retrograde amnesia. J Clin Exp Neuropsychol. 1993a;15:947–967. doi: 10.1080/01688639308402610. [DOI] [PubMed] [Google Scholar]
- 42.Markowitsch HJ, Calabrese P, Liess J, Haupts M, Durwen HF, Gehlen W. Retrograde amnesia after traumatic injury of the temporo-frontal cortex. J Neurol Neurosurg Psychiatry. 1993b;56:988–992. doi: 10.1136/jnnp.56.9.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markowitsch HJ, Calabrese P, Würker M, Durwen HF, Kessler J, Babinsky R, Brechtelsbauer D, Heuser L, Gehlen W. The amygdala’s contribution to memory—a PET study on two patients with Urbach-Wiethe disease. NeuroReport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- 44.Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider G. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- 45.Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- 46.Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaenc S, Cohen L, Mehler J. The cortical representation of speech. J Cognit Neurosci. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- 47.Mazziotta JC, Huang SC, Phelps ME, Carson RE, MacDonald NS, Mahoney K. A noninvasive positron computed tomography technique using oxygen-15 labelled water for the evaluation of neurobehavioral task batteries. J Cereb Blood Flow Metab. 1985;5:70–78. doi: 10.1038/jcbfm.1985.10. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy G. Functional neuroimaging of memory. The Neuroscientist. 1995;1:155–163. [Google Scholar]
- 49.Merzenich MM, Sameshima K. Cortical plasticity and memory. Curr Opin Neurobiol. 1993;3:187–196. doi: 10.1016/0959-4388(93)90209-h. [DOI] [PubMed] [Google Scholar]
- 50.Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4:137–142. [PubMed] [Google Scholar]
- 51.Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nauta WJH. Expanding borders of the limbic system concept. In: Rasmussen T, Marino R, editors. Functional neurosurgery. Raven; New York: 1979. pp. 7–23. [Google Scholar]
- 53.Nyberg L, Tulving E, Habib R, Nilsson L-G, Kapur S, Houle S, Cabeza R, McIntosh AR (1996) Functional brain maps of retrieval mode and recovery of episodic information. NeuroReport, in press. [PubMed]
- 54.Ogden JA. Visual object agnosia, prosopagnosia, achromatopsia, loss of visual imagery, and autobiographical amnesia following recovery from cortical blindness: case MH. Neuropsychologia. 1993;31:571–589. doi: 10.1016/0028-3932(93)90053-3. [DOI] [PubMed] [Google Scholar]
- 55.Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudge P, Warrington E. Selective impairment of memory and visual perception in splenial tumours. Brain. 1993;114:349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- 57.Sakai K, Miyashita Y. Visual imagery: an interaction between memory retrieval and focal attention. Trends Neurosci. 1994;17:287–289. doi: 10.1016/0166-2236(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 58.Sarter M, Markowitsch HJ. The amygdala’s role in human mnemonic processing. Cortex. 1985a;21:7–24. doi: 10.1016/s0010-9452(85)80013-7. [DOI] [PubMed] [Google Scholar]
- 59.Sarter M, Markowitsch HJ. The involvement of the amygdala in learning and memory: a critical review with emphasis on anatomical relations. Behav Neurosci. 1985b;99:342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- 60.Schmahmann JD. An emerging concept: the cerebellar contribution to higher function. Arch Neurol. 1992;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 61.Shallice T, Kartsounis LD. Selective impairment of retrieving people’s names: a category specific disorder? Cortex. 1993;29:281–291. doi: 10.1016/s0010-9452(13)80181-5. [DOI] [PubMed] [Google Scholar]
- 62.Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 63.Squire LR. Oxford UP; New York: 1987. Memory and brain. . [Google Scholar]
- 64.Squire LR, Knowlton B. Memory, hippocampus, and brain systems. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT; Cambridge, MA: 1995. pp. 825–837. [Google Scholar]
- 65.Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- 67.Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- 68.Talairach J, Tournoux P. Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain. . [Google Scholar]
- 69.Townsend BW, Geissbuhler A, Defrise M, Hoffman EJ, Spinks TJ, Bailey DL, Gilardi MC, Jones T. Fully three-dimensional reconstruction for a PET-camera with retractable septa. IEEE Trans Biomed Eng. 1991;10:505–512. doi: 10.1109/42.108584. [DOI] [PubMed] [Google Scholar]
- 70.Tulving E. Oxford UP; Oxford: 1983. Elements of episodic memory. . [Google Scholar]
- 71.Tulving E. Organization of memory: quo vadis? In: Gazzaniga MS, editor. The cognitive neurosciences. MIT; Cambridge, MA: 1995. pp. 839–847. [Google Scholar]
- 72.Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. J Verb Learn Verb Behav. 1966;5:381–391. [Google Scholar]
- 73.Tulving E, Hayman CA, Macdonald CA. Long-lasting perceptual priming and semantic learning in amnesia: a case experiment. J Exp Psychol Learn Mem Cogn. 1991;17:595–617. doi: 10.1037//0278-7393.17.4.595. [DOI] [PubMed] [Google Scholar]
- 74.Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994a;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tulving E, Kapur S, Markowitsch HJ, Craik G, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci USA. 1994b;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S. Novelty encoding networks in the human brain: data from positron emission tomography studies. NeuroReport. 1994c;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- 77.Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Functional neuroanatomy of encoding and retrieval of pictorial information in memory. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 78.Verfaellie M, Cermak LS. Acquisition of generic memory in amnesia. Cortex. 1994;30:293–303. doi: 10.1016/s0010-9452(13)80200-6. [DOI] [PubMed] [Google Scholar]
- 79.Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- 80.Wienhard K, Dahlbom M, Erikson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss WD. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;181:110–118. [PubMed] [Google Scholar]