Abstract
Exposure to mild stress is known to activate dopamine (DA), serotonin (5-HT), and norepinephrine (NE) metabolism in the anteromedial prefrontal cortex (m-PFC). Neuroanatomical site(s) providing afferent control of the stress activation of the m-PFC monoaminergic systems is at present unknown. The present study used a conditioned stress model in which rats were trained to fear a substartle-threshold tone paired previously with footshock and assessed for behavioral, neuroendocrine, and neurochemical stress responses. Bilateral NMDA-induced excitotoxic lesioning of the basolateral and central nuclei of the amygdala was performed before or after training. Pretraining amygdala lesions blocked stress-induced freezing behavior, ultrasonic vocalizations, adrenocortical activation, and dopaminergic metabolic activation in the m-PFC. Post-training amygdala lesions blocked stress-induced m-PFC DA, 5-HT, and NE metabolic activation. Post-training amygdala lesions also blocked stress-induced freezing and defecation, and greatly attenuated adrenocortical activation. These data provide evidence of amygdalar control of stress-induced metabolic activation of the monoaminergic systems in the m-PFC, as well as amygdalar integration of behavioral and neuroendocrine components of the rat stress response. These results are discussed in terms of possible relevance to stress-induced exacerbation of schizophrenic symptoms and the pathophysiology of post-traumatic stress disorder.
Keywords: dopamine, norepinephrine, serotonin, prefrontal cortex, nucleus accumbens, corticosterone, freezing, ultrasonic vocalization, amygdala, post-traumatic stress disorder, schizophrenia
Animals, including humans, respond to perceived threat with a coordinated set of psychophysiological reactions known as the stress response. In many animals, the stress response is characterized by cessation of ongoing behavior, increased startle reactivity, alterations in cardiovascular functioning, changes in autonomic tone, and activation of neuroendocrine axes. Within the brain, the dopamine (DA), norepinephrine (NE), and serotonin (5-HT) biogenic amine systems that innervate the cortex, and in particular the medial prefrontal cortex (m-PFC), also are activated in response to threatening stimuli (Bliss et al., 1968; Thierry et al., 1976; Weiss et al., 1981; Deutch and Roth, 1990; Tanaka et al., 1990; Inoue et al., 1993). Early investigations (Bliss, 1968) indicated that exposure of rats to an uncontrollable stressor such as footshock resulted in decreased cortical levels of tissue NE and concomitant increases in the levels of noradrenergic metabolites, whereas the same stressor resulted in accelerated metabolism of DA and 5-HT without changing the absolute level of these amines.
Metabolic activation of the DA system in the rat m-PFC is one of the most intensively studied central neurochemical correlates of the stress response (Deutch and Roth, 1990). Exposure to mild stress preferentially activates this m-PFC DA system while not affecting DA metabolism in other terminal regions, such as the caudatoputamen. The DA cell bodies that are activated by mild stress are located in the ventral tegmental area (VTA) of Tsai in the A10 catecholamine cell group (Lindvall and Bjorklund, 1984) and project to the cortex where they terminate on dendritic shafts of spiny pyramidal cells and/or GABAergic interneurons (Sequela et al., 1988; Goldman-Rakic et al., 1989; Gellman and Aghajanian, 1993; Vincent et al., 1993).
Neuroanatomical tract tracing studies using various tracing techniques have demonstrated that the medial aspect of the central nucleus of the amygdala projects to the VTA, as well as to the locus coeruleus and raphe nuclei (Wallace et al., 1989; Gonzales and Chesselet, 1990;Wallace et al., 1992). Electrophysiological studies have demonstrated that a large majority of presumptive DA cells in the VTA respond to electrical stimulation of the amygdala over both mono- and polysynaptic pathways (Maeda and Mogenson, 1981).
The current study was initiated to investigate the effects of excitotoxic lesion of the amygdala on the behavioral, neuroendocrine, and central neurochemical components of the stress response to determine whether some or all of these responses are coordinated concurrently within the amygdala (LeDoux, 1992). A battery of tests was developed to measure various components of the rat stress reaction elicited in response to a tone paired previously with footshock. Parameters monitored in the present work include freezing behavior, locomotor activity, ultrasonic vocalization, defecation, and serum corticosterone levels. The effects of amygdalectomy on stress-induced alterations in DA, NE, and 5-HT utilization in the m-PFC, as well as on the mesolimbic DA and 5-HT systems innervating the nucleus accumbens septi (NAS), also were assessed.
MATERIALS AND METHODS
Animals and housing conditions. Male albino Sprague–Dawley rats (Camm, Wayne, NJ) weighing 300–400 gm were used. All rats were caged individually and acclimated for 2 weeks before experimentation. Rats were maintained on a 12:12 hr light/dark schedule with lights off at 1500 hr. Food and water were available ad libitum. All studies were conducted in the dark during the active phase of the rat diurnal activity cycle, beginning 1–6 hr after dark onset. The rationale for testing in the dark was to approximate the psychophysiological conditions when these nocturnal animals are most likely to encounter threatening stimuli under natural conditions.
Apparatus. Testing was conducted in a modified standard conditioning chamber isolated in a sound attenuation cubicle. All surfaces of the chamber were washed with 70% ethanol after each animal exposure. An infrared television camera (Sanyo Electronics, Japan) and infrared illuminator were positioned over the test cage and connected to a video recording system located in an adjoining room. Ultrasonic vocalizations were detected using a miniature narrow-bandwidth microphone (Panasonic, Tokyo, Japan) with a center frequency of 21 kHz. The microphone signal was amplified, filtered, digitized, and analyzed by computer. Behavioral events (freezing, grooming, crossings, and rears) were monitored remotely on a television screen and coded by an observer using an electronic push-button/toggle-switch assembly connected to a computer.
Surgical procedures. A delivery cannula was constructed from a 5 cm length of 30 gauge stainless steel tubing and attached to the needle of a 2 μl Hamilton syringe positioned in a manual micrometer. Rats were anesthetized with sodium pentobarbital (65 mg/kg) and atropine bromide (0.3 mg/kg), intraperitoneally, and placed in a stereotaxic frame using blunt ear bars to protect the tympanic membranes. NMDA (Sigma, St. Louis, MO) was dissolved in PBS, final pH 7.4, at a concentration of 20 μg/μl and delivered at an infusion rate of 0.08 μl/min via an injection cannula. An infusion volume of 0.20 μl NMDA was delivered at depths of 8.3 mm and 8.1 mm below the skull surface at 2.5 mm posterior to bregma and 4.5 mm lateral to the midline (Paxinos and Watson, 1986). Sham lesions were conducted using PBS infusions at the same coordinates and delivery volumes. All lesions were bilateral.
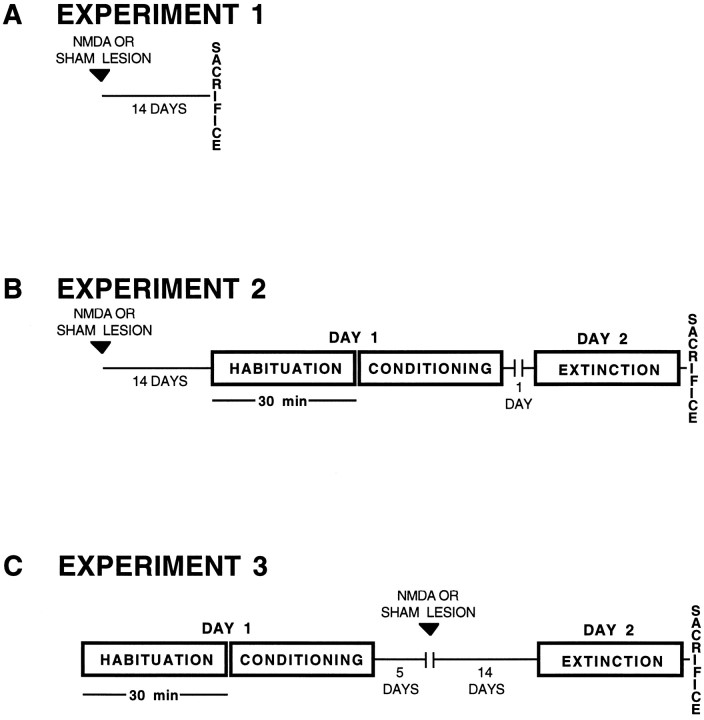
Conditioning and testing procedures. All studies were conducted in the dark during the active phase of the rat diurnal activity cycle. Assignment to treatment group was random. Animals were run at the same time each evening throughout these studies. The experimental design used in this study is described below and presented schematically in Figure 1.
Fig. 1.
Experimental design for testing the effect of amygdala lesions on the behavioral, neuroendocrine, and neurochemical components of the stress response. A shows design of experiment 1 for testing effects of lesions on basal serum corticosterone and basal medial prefrontal cortical biogenic amine metabolism. B shows design of experiment 2 for testing the effect of pretraining amygdala lesions on stress responses.C shows design of experiment 3 for testing the effect ofpost-training amygdala lesions on stress responses. Note that in the post-training design, all animals were surgically naive at the time of conditioning.
Experiment 1. For determination of the effects of amygdalectomy on basal m-PFC biogenic amine metabolism andbasal serum corticosterone, animals received sham or excitotoxic lesions of the amygdala as described above. After a 2 week recovery period, animals were removed from their home cages and killed. These animals were not exposed to the test apparatus.
Experiment 2. To test the effect of pretrainingamygdalectomy on stress responses, animals were prepared with sham or excitotoxic amygdala lesions. These groups were subdivided further into sham lesion/nonstressed (Sh/NS), sham lesion/stressed (Sh/S), amygdalectomy/nonstressed (A-/NS), and amygdalectomy/stressed (A-/S) groups. After a 2 week postlesion recovery period, each animal was introduced into the test chamber and allowed to explore the cage freely for 30 min. In the last 5 min of this habituation period, three 5 sec, 56 dB, substartle-threshold white noise tones were presented randomly by computer. A 30 min conditioning period then was initiated. In the stress groups, animals were presented with ten 5 sec, 56 dB white noise tones that coterminated with a 0.5 sec, 0.4 mA footshock (measured according to the method of Sananes and Davis, 1992). One tone–footshock pair was presented randomly within each of 10 consecutive 3 min intervals. In the nonstressed control groups, animals were treated as above except that they did not receive footshock. At the conclusion of the conditioning period, test subjects were returned to their home cages. The next day, animals were reintroduced into the testing chamber for 30 min. During this extinction trial, all animals were presented randomly with ten 56 dB white noise toneswithout footshock. At the conclusion of the extinction trial, animals were decapitated rapidly and trunk blood was collected for determination of serum corticosterone and brain tissue obtained for neurochemical analyses.
Experiment 3. To test the effect of post-trainingamygdalectomy on stress responses, stressed and nonstressed control groups were habituated and conditioned in the testing chamber as described above. The animals were divided into three groups: Sh/NS, Sh/S, and A-/S. Animals then were subjected to either sham or excitotoxic lesions of the amygdala 5 d afterconditioning. After lesioning, the animals were allowed to recover for 2 weeks before extinction testing was conducted as detailed above. Note that these post-training lesioned animals were surgically naive at the time of conditioning.
Behavioral measurements. Ultrasonic vocalizations had to meet the following criteria: (1) Remote behavioral observation of the animal confirmed deep, prolonged respiration at ∼0.5 Hz preceded by short, shallow rapid panting at ∼3–4 Hz (Frysztak and Neafsey, 1991); (2) Call duration was >20 msec as visualized on a digital oscilloscope; and (3) Call tracings appeared on both the oscilloscope and computer monitor screen. Freezing was defined strictly as absence of visible movement of the animal, including vibrissae, except that related to respiration. This behavior has been used as an index of fear in the rat (Blanchard and Blanchard, 1969; Fanselow, 1980; Conti et al., 1990). Crossings were coded each time the base of the tail crossed gridlines drawn on the video monitoring screen. Rears were counted when an animal raised both forepaws off the cage bars and was not otherwise engaged in grooming behavior. Defecation was quantitated by counting the number of fecal boli present in the test chamber at the end of the conditioning or extinction trial. Animals were coded initially by an observer not blind to the experimental conditions. Video records of 11 randomly selected animals were recoded by an observer blind to the experimental conditions. Statistical analysis showed a very high correlation between results generated by unblind and blind behavioral observations (crossings and rearings, r2 = 0.98; freezing, r2 = 0.99). Therefore, the initial unblinded behavioral results were used for all statistical analyses.
Neurochemical and neuroendocrine analyses.Neurochemical analyses were performed on ex vivo tissue samples harvested from the same animals used for behavioral and neuroendocrine measurements. Schematic depictions of the regions dissected for neurochemical analysis are presented in Figure2. Tissue DA and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), and 5-HT and its metabolite 5-hydroxy-indoleacetic acid (5-HIAA) were isolated according to a modification of the procedure of Reinhard et al. (1982). These compounds were assayed using reversed-phase HPLC coupled with amperometric electrochemical detection. Regional levels of DA and 5-HT system metabolic activity were assessed by calculating the ratio of the acid metabolite to the parent neurotransmitter in postmortem tissue. We did not observe any significant changes in the absolute tissue level values of DA or 5-HT in response to stress, a finding consistent with a large body of previous work dating back to the 1960s (Bliss et al., 1968) and with previous work from this laboratory (Goldstein et al., 1994). Therefore, as in our previous work, we present DA and 5-HT metabolic activity as the ratio of metabolite to parent neurotransmitter. Measurement of cortical NE metabolism was determined in a separate assay from tissue measurements of the major rat noradrenergic metabolite 3-methoxy-4-hydroxyphenylglycol sulfate (MHPG), by a modification of the method of Elsworth et al. (1983). Results are expressed as total MHPG (i.e., free plus sulfate conjugated MHPG). We did not measure endogenous levels of NE in the current study. Serum corticosterone was measured by a magnetic radioimmunoassay developed in this laboratory as detailed in a previous report (Goldstein et al., 1994). Tissue samples from some animals were excluded from analysis because of technical difficulties with individual dissections or sample preparation.
Fig. 2.
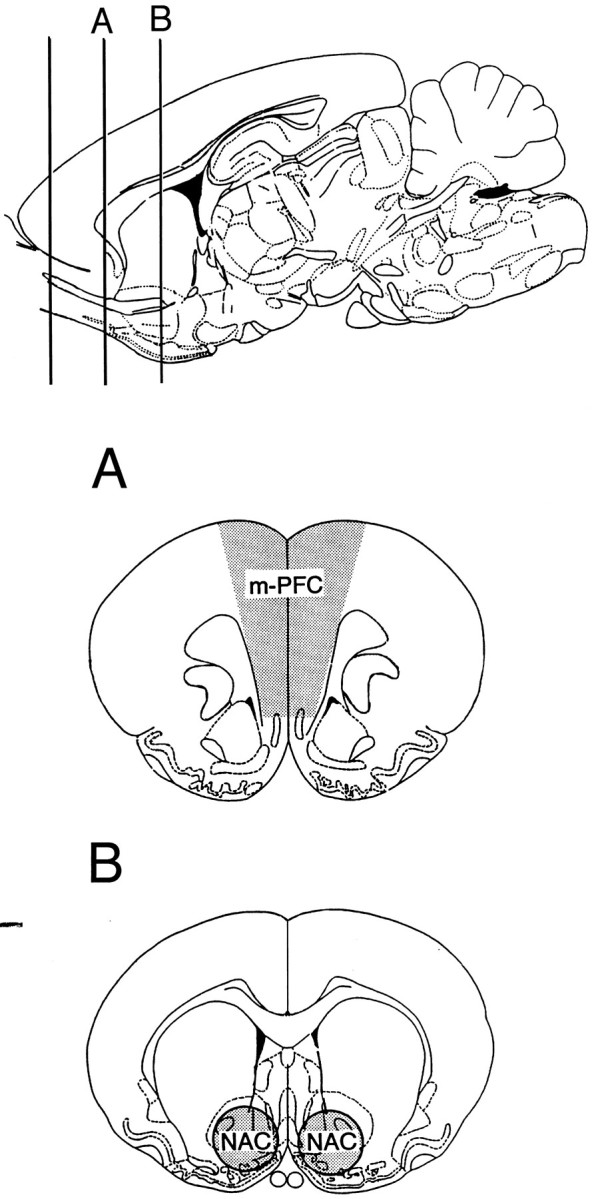
Atlas diagram detailing tissue regions harvested for postmortem monoamine neurochemical analyses. Tissue sections (2 mm) were obtained after killing and dissected as indicated.m-PFC, Medial prefrontal cortex; NAC, nucleus accumbens.
Histological procedures. Formalin-fixed brains were sectioned (40 μm), mounted, and stained with cresyl violet. The extent of neuronal cell loss and reactive gliosis was assessed microscopically and transcribed onto atlas sections from Paxinos and Watson (1986). Histological criteria for inclusion in the amygdalectomy group required evidence of bilateral damage (i.e., heavy gliosis, neuronal degeneration and dropout, and/or cavitation) within the rostrocaudal extent of the basolateral and central amygdala nuclei.
Statistics. Data from experiments 1 and 3 were analyzed by one-way ANOVA for significant effect of treatment. A two-way ANOVA was used in experiment 2 to test for significant effects of lesion, treatment, and lesion-by-treatment interaction. The Student–Newman–Keuls post hoc multiple comparison procedure was used for comparison among multiple means when appropriate. Temporal analyses of behavioral data were made using repeated-measures ANOVA. All analyses were conducted using the SuperANOVA computer program (Abacus Concepts, Berkeley, CA). Values are expressed as means ± SEM. Statistical significance was set at p < 0.05.
RESULTS
Verification of lesions
Representative photomicrographs of amygdala lesions are shown in Figure 3. A schematic depiction of the range of amygdala lesions is shown in Figure 4. The saline-injected sham control animals did not sustain damage to any part of the amygdala, adjacent perirhinal cortex, or caudatoputamen. Examination of brain sections from animals sustaining excitotoxic lesion of the amygdala revealed marked bilateral gliosis and bilateral neuronal degeneration throughout the rostral–caudal extent of this structure, typically extending between 2.00 mm and 3.30 mm posterior to bregma (Paxinos and Watson, 1986). The lesion area included the lateral and basolateral nuclei as well as the central nucleus. The largest lesions spread medially to include the basomedial and medial nuclear complexes. Some animals in the pretraining and post-training experiments also sustained damage unilaterally or bilaterally in the dorsal and ventral endopiriform nuclei. These histological findings are consistent with other descriptions of large NMDA lesions in these areas (Cahill and McGaugh, 1990; Gallagher et al., 1990; Sananes and Davis, 1992). In 8 of the original 26 lesioned animals in the pretraining experiment, the lesions were judged incomplete by the criteria above and were excluded from the data analyses. Only 1 of 10 lesioned animals in the post-training experiment failed to meet lesion criteria.
Fig. 3.
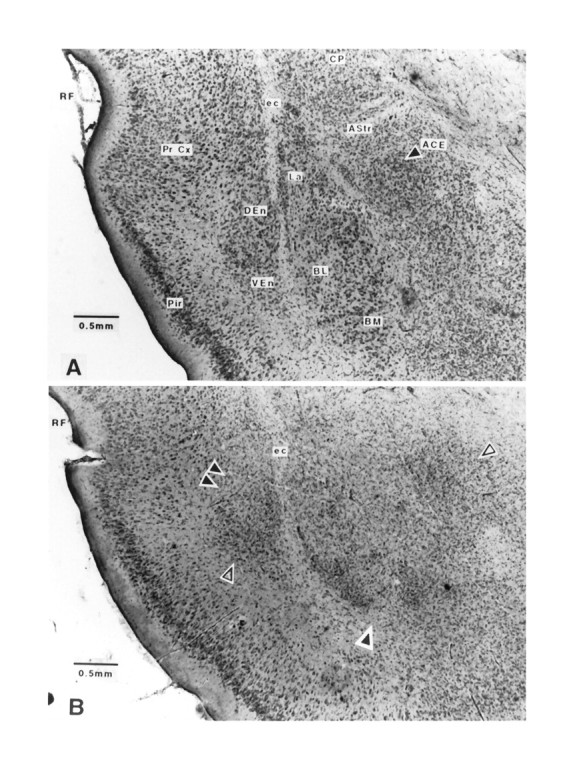
Sham and excitotoxic lesions of the amygdala.A, Sham lesions of the amygdala. ACE, Central nucleus of the amygdala; AStr, amygdalostriatal transition zone; BL, basolateral nucleus of the amygdala;BM, basomedial nucleus of the amygdala; CP, caudatoputamen; DEn, dorsal endopiriform nucleus;ec, external capsule; La, lateral nucleus of the amygdala; Pir, piriform cortex; Pr Cx, perirhinal cortex; VEn, ventral endopiriform nucleus. B, Excitotoxic lesions of the amygdala. RF, Rhinal fissure;ec, external capsule. Note heavy gliosis and neuronal dropout in the lateral, basolateral, and central nuclei of the amygdala. Double black arrows and single shaded arrow indicate gliosis and neuronal dropout in dorsal and ventral endopiriform nuclear regions, respectively. Single black arrow points to neuronal dropout in the ventral basolateral and basomedial nuclei of the amygdala. Single open arrow points to heavy gliosis and neuronal dropout in the central nucleus of the amygdala. Photomicrographs at 4.5× after formalin fix and cresyl violet stain. Scale bars are as noted in photomicrographs.
Fig. 4.
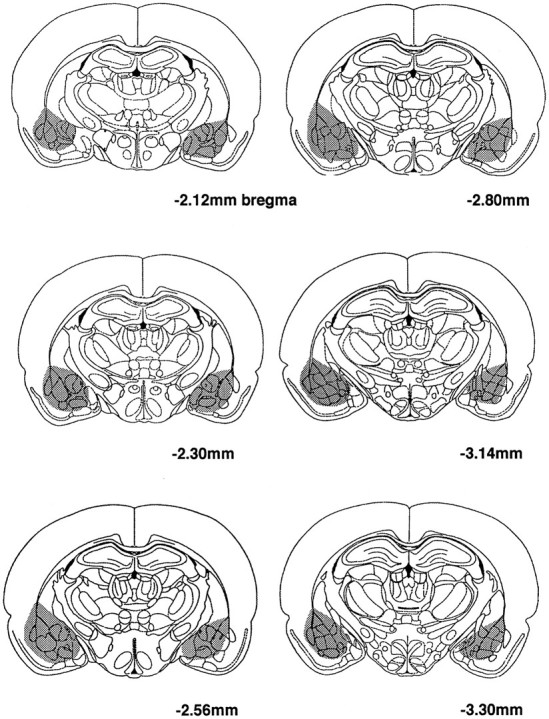
Depiction of the range of amygdala lesions in this study (superimposed on schematic diagram reproduced from Paxinos and Watson, 1986).
Experiment 1: effect of amygdala lesions on basal corticosterone, DA, and 5-HT metabolism in the m-PFC
To examine the effect of amygdalectomy on basal serum corticosterone levels and m-PFC biogenic amine utilization, animals were prepared with either sham or NMDA-induced amygdala lesions. Two weeks after lesioning, animals were killed. Note that these animals were not exposed to the conditioning apparatus. Mean m-PFC DOPAC/DA and 5-HIAA/5-HT levels for sham- and amygdala-lesioned animals were statistically indistinguishable as shown in Table 1. Thus, excitotoxic lesions of the amygdala do not alter basal m-PFC DA or 5-HT metabolism when compared with sham lesions. Likewise, no significant difference was noted in serum corticosterone levels between sham- and amygdala-lesioned animals, 99 ± 20 and 68 ± 14 ng/ml, respectively; note the trend toward lower serum corticosterone levels in the lesioned group. Measurement of MHPG was not made in this set of animals.
Table 1.
Effect of amygdala lesion on basal neurochemistry and serum corticosterone
| m-PFC | SHAM n = 8 | AMYGn = 9 | ||
|---|---|---|---|---|
| DA | (ng/mg protein) | 1.80 ± 0.22 | 1.93 ± 0.22 | ND |
| DOPAC | (ng/mg protein) | 0.25 ± 0.03 | 0.26 ± 0.03 | ND |
| DOPAC/DA | 0.14 ± 0.01 | 0.13 ± 0.01 | ND | |
| 5-HT | (ng/mg protein) | 10.8 ± 0.87 | 11.52 ± 0.83 | ND |
| 5-HIAA | (ng/mg protein) | 2.32 ± 0.18 | 2.49 ± 0.19 | ND |
| 5-HIAA/5-HT | 0.21 ± 0.01 | 0.22 ± 0.02 | ND | |
| Corticosterone | (ng/ml serum) | 98.8 ± 20.4 | 67.7 ± 13.8 | ND |
Effect of amygdala lesion on basal cortical DA and 5-HT utilization in the m-PFC and basal serum corticosterone. Animals were subjected to bilateral sham or excitotoxic lesioning of the amygdala 2 weeks before being killed. Animals were never exposed to the conditioning chamber and were simply removed from the home cage before being killed. ND, No statistically significant effect of lesion,p > 0.05.
Experiment 2a: effect of pretraining amygdala and sham lesions on conditioned stress-induced behavioral, neuroendocrine, and m-PFC DA responses
In experiment 2, the effects of pretraining NMDA lesions of the amygdala on behavioral, neuroendocrine, and neurochemical indices of aversive conditioning were assessed. Two weeks after sham or excitotoxic lesion of the amygdala, animals were subjected to aversive conditioning with extinction trial testing on the following day. Note that data presented in this section are derived from responses during the extinction trial on day 2 when animals are presented only with the tone (i.e., without footshock).
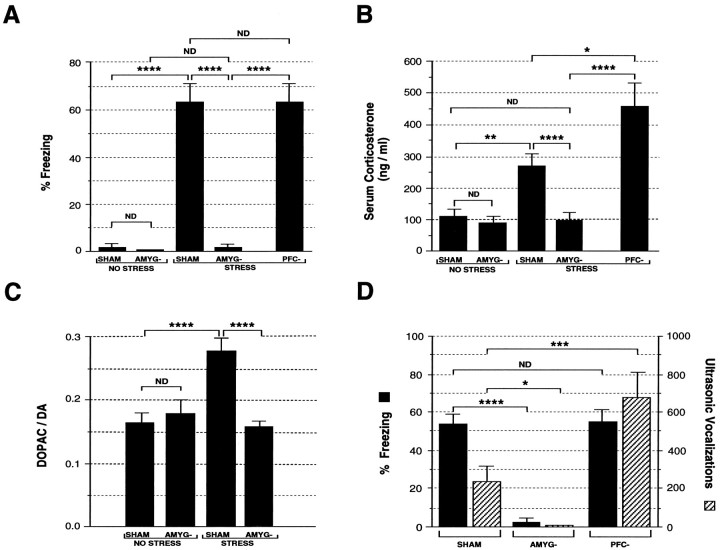
Behavioral results are presented in Figure5A. Significant effects on freezing behavior were observed for lesion, F(1,32) = 59, p < 0.0001; treatment,F(1,32) = 58, p < 0.0001; and lesion by treatment interaction,F(1,32) = 54, p < 0.0001. Both the Sh/NS and A-/NS groups demonstrated low levels of freezing, with mean percent of time freezing: 2 ± 2 and 0.3 ± 0.3%, respectively. These two groups were statistically indistinguishable. In contrast, the Sh/S group exhibited marked freezing behavior, 64 ± 8%, which was statistically different from the Sh/NS and A-/NS groups,p < 0.0001. The A-/S group showed block of this stress-induced freezing response with a mean freezing response of 1 ± 1%, p < 0.0001 versus the Sh/S group. The A-/S freezing response was statistically indistinguishable from either of the two nonstressed groups.
Fig. 5.
Effect of pretraining amygdala lesions on behavioral, neuroendocrine, and neurochemical indices of the stress response. A shows effect of pretraining amygdala lesions on stress-induced freezing behavior. B shows effect of pretraining amygdala lesions on stress-induced serum corticosterone levels. C shows effect of pretraining amygdala lesions on stress-induced DA metabolism (expressed as the DOPAC/DA ratio) in the m-PFC. D shows effect of amygdala lesions on stress-induced freezing and ultrasonic vocalization during the conditioning period. Animals were subjected to bilateral sham (SHAM) or excitotoxic lesions of the amygdala (AMYG-) 14 d before conditioning. STRESS and NO STRESS indicate experimental conditions in which animals were or were not exposed to shock during conditioning, respectively. Number of animals per group are (A–C) SHAM/NO STRESS, 10; AMYG-/NO STRESS, 7; SHAM/STRESS, 9; AMYG-/STRESS, 10 (A) and 11 (B, C); and (D) SHAM, 9; AMYG-, 10. ****, Statistically significant difference, p < 0.0001; ***, statistically significant difference, p < 0.001; **, statistically significant difference, p < 0.01; *, statistically significant difference, p < 0.05;ND, no statistically significant difference.
The effects of pretraining amygdalectomy on serum corticosterone are depicted in Figure 5B. Significant effects on adrenocortical activation were detected for lesion,F(1,33) = 14, p < 0.001; treatment, F(1,33) = 5, p < 0.05; and lesion by treatment interaction,F(1,33) = 4, p < 0.05. Both the Sh/NS and A-/NS groups demonstrated low, statistically indistinguishable corticosterone levels: 137 ± 30 and 87 ± 14 ng/ml, respectively. In contrast, the Sh/S group exhibited a marked stress-induced elevation in serum corticosterone to 277 ± 46 ng/ml, a 102% increase over the Sh/NS group, p < 0.01. The A-/S group showed complete block of this stress-induced serum corticosterone elevation with a mean level of 83 ± 25 ng/ml; this was significantly different from the Sh/S group, p < 0.001, and statistically indistinguishable from either the Sh/NS or A-/NS groups. This result cannot be attributed to amygdala lesion-induced suppression of baseline serum corticosterone levels (see above, Experiment 1).
The effect of pretraining amygdalectomy on m-PFC DA utilization is shown in Figure 5C. Significant effects on m-PFC DA activation were detected for lesion,F(1,33) = 9, p < 0.005; treatment, F(1,33) = 7, p < 0.02; and lesion by treatment interaction,F(1,33) = 15, p < 0.001. Both the Sh/NS and A-/NS groups demonstrated low DOPAC/DA ratios, 0.16 ± 0.2 and 0.18 ± 0.02, respectively. In contrast, the Sh/S group exhibited a marked stress-induced elevation in m-PFC DA utilization to 0.28 ± 0.02, a 71% increase in the DOPAC/DA ratio versus the Sh/NS group, p < 0.001. This effect was blocked in the A-/S group, which exhibited a mean DOPAC/DA ratio of 0.16 ± 0.01; this was significantly different from the Sh/S group, p < 0.001, and statistically indistinguishable from the Sh/NS and A-/NS groups. This result cannot be attributed to amygdala lesion-induced suppression of the baseline m-PFC DOPAC/DA ratio (see above, Experiment 1).
Experiment 2c: effect of pretraining amygdala lesions on behavioral indices of unconditioned stress
Behavior during the conditioning period on day 1 also was altered by amygdalectomy (Fig. 5D). Note that the data to be presented in this section refer to behavior observed during the conditioning period (second half-hour on the training day, day 1) when animals were receiving either tone–shock pairs or tones alone without shock. Animals that were subjected to the tone alone during conditioning did not freeze, vocalize, or defecate during the second half-hour of training on day 1.
A significant effect of amygdalectomy was noted on freezing behaviorduring conditioning, F(1,17) = 68, p < 0.0001. The mean percentages of time engaged in freezing during the conditioning session for the Sh/S and A-/S groups were 53 ± 6 and 3 ± 1%, respectively. A significant effect of lesion also was noted for ultrasonic vocalizations during conditioning, F(1,18) = 11, p < 0.005. The mean number of vocalizations during the conditioning session for the Sh/S and A-/S groups were 239 ± 80 and 0 ± 0 calls, respectively. A significant effect of lesion also was noted for stress-induced defecation during conditioning,F(1,18) = 24, p < 0.0001. The mean number of fecal boli produced during the second half-hour of the conditioning session for the Sh/S and A-/S groups were 14 ± 1 and 4 ± 1 fecal boli, respectively. Thus, the amygectomy group showed blockade of freezing, ultrasonic vocalization, and defecation during presentation of tone–shock pairs when compared with the sham-lesioned animals.
Experiment 3: effect of post-training amygdala lesions on behavioral and neuroendocrine indices of conditioned stress
Because the pretraining amygdala lesions appeared to alter several indices of the behavioral stress response during aversive conditioning, additional studies were carried out to assess the effect of post-training amygdalectomy on the behavioral, neuroendocrine, and neurochemical conditioned stress responses. In this series of experiments, aversive conditioning was conducted before lesioning (Fig. 1), thus avoiding the potentially confounding effect of the lesion on learning. Note that nearly 3 weeks (19 d) intervened between conditioning and testing.
ANOVA revealed a significant effect of treatment on freezing behavior,F(2,19) = 29, p < 0.0001 (Fig. 6A). The mean percentages of time engaged in freezing during the extinction period were 0.5 ±.5, 48 ± 9, and 0.3 ± 0.2% for the Sh/NS, Sh/S, and A-/S groups, respectively. Post-training amygdalectomy thus resulted in blockade of the stress-induced freezing response.
Fig. 6.
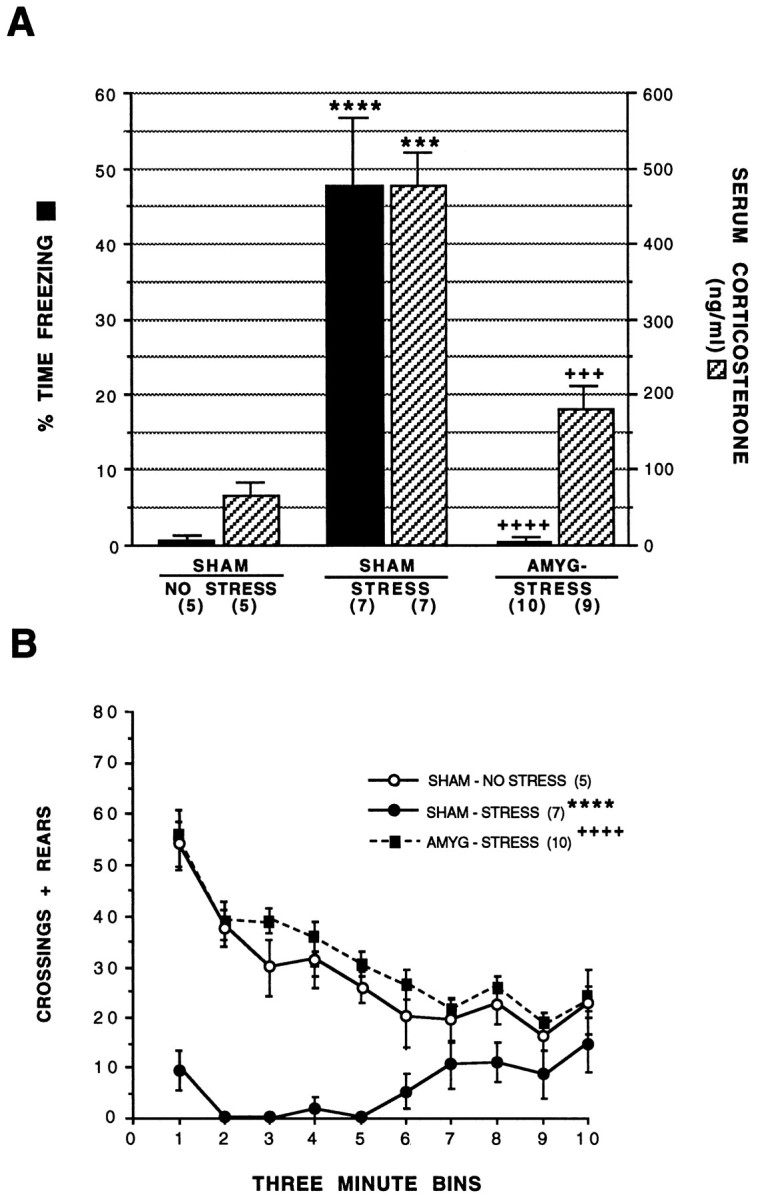
Effect of post-training amygdala lesions on behavioral and neuroendocrine indices of the stress response. A shows effect of post-training amygdala lesions on stress-induced freezing behavior and serum corticosterone levels.B shows effect of post-training amygdala lesions on the temporal course of locomotor activity (Crossings + Rears) during the 10 successive 3 min time bins that make up the 30 min extinction period. Note that the amygdala lesion group exposed to stress behaves similarly to the sham lesion control group, which was not exposed to stress. Animals were subjected to bilateral sham (SHAM) or excitotoxic lesions of the amygdala (AMYG-) 5 d after conditioning. STRESS andNO STRESS indicate experimental conditions in which animals were or were not exposed to shock during conditioning, respectively. ****, Statistically significant difference versus the sham/no stress group, p < 0.0001; ***, statistically significant difference versus the sham/no stress group, p < 0.001; ++++, statistically significant difference versus the sham/stress group, p < 0.0001; +++, statistically significant difference versus the sham/stress group, p < 0.001. Numbers in parenthesis indicate number of animals in each group.
Changes in locomotor behavior, measured as the combined score for crossing and rears, also were noted in the post-training amygdalectomy group. ANOVA revealed a significant effect of treatment on total locomotor activity, F(2,18) = 46.10, p < 0.0001. The mean locomotor scores during the extinction period were 282 ± 32, 61 ± 18, and 317 ± 17 for the Sh/NS, Sh/S, and A-/S groups, respectively. Note that within the sham lesion groups, the stress condition resulted in a 81% decrease in locomotor activity; post-training amygdalectomy blocked this stress-induced suppression of locomotion. The temporal course of this effect is presented in Figure 6B.
ANOVA revealed a significant effect of treatment on the number of fecal boli produced during the extinction trials,F(2,18) = 16, p < 0.02. Mean number of fecal boli in the Sh/NS, Sh/S, and A-/S groups were 2 ± 1, 6 ± 1, and 1 ± 1, respectively.
Adrenocortical activation also was affected by post-training amygdalectomy (Fig. 6A). ANOVA revealed a significant effect of treatment on serum corticosterone levels,F(2,18) = 17, p < 0.0001. The mean serum corticosterone levels after the extinction period were 65 ± 17, 465 ± 76, and 157 ± 25 ng/ml for the Sh/NS, Sh/S, and A-/S groups, respectively. Post-training amygdalectomy thus resulted in a 66% attenuation of the stress-induced adrenocortical response.
Experiment 3: effect of post-training amygdala lesions on neurochemical indices of stress
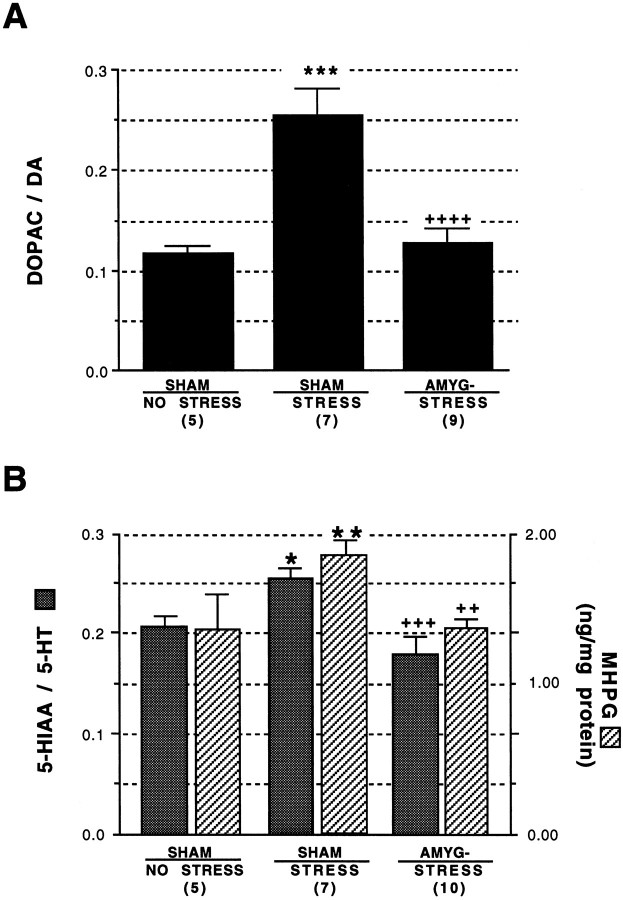
The results of neurochemical analysis of m-PFC DA utilization are shown in Figure 7A. ANOVA revealed a significant effect of treatment on m-PFC DA utilization,F(2,18) = 21, p < 0.0001. The mean DOPAC/DA ratios after the extinction period were 0.12 ± 0.01, 0.25 ± 0.03, and 0.13 ± 0.01 for the Sh/S, Sh/NS, and A-/S groups, respectively. Note that within the sham lesion groups, conditioned stress resulted in a 124% increase in m-PFC DA utilization, compared with the 71% increase when analyses were done on tissue obtained immediately after aversive conditioning. Post-training amygdalectomy resulted in blockade of this stress-induced elevation in m-PFC DA utilization. Also note that the mean m-PFC DOPAC/DA ratio for the A-/S group was statistically indistinguishable from the Sh/NS control group, paralleling the results from the pretraining lesion study.
Fig. 7.
Effect of post-training amygdala lesions on neurochemical indices of the stress response in the m-PFC. A shows effect of post-training amygdala lesions on stress-induced DA utilization in the m-PFC. ***, Statistically significant difference versus the sham/no stress group,p < 0.001; ++++, statistically significant difference versus the sham/stress group, p < 0.0001. Bshows effect of post-training amygdala lesions on stress-induced 5-HT utilization and NE metabolism in the m-PFC. Animals were subjected to bilateral sham (SHAM) or excitotoxic lesions of the amygdala (AMYG-) 5 d after training. STRESS andNO STRESS indicate experimental conditions in which animals were or were not exposed to shock during conditioning, respectively. **, Statistically significant difference versus the sham/no stress group, p < 0.01; *, statistically significant difference versus the sham/no stress group, p < 0.05; +++, statistically significant difference versus the sham/stress group,p < 0.001; ++, statistically significant difference versus the sham/stress group, p < 0.01. Numbers inparenthesis indicate number of animals in each group.
To answer the question of whether the effect of post-training amygdalectomy on DA utilization is specific for this neurotransmitter system, neurochemical measurements of 5-HT and NE utilization were performed on the same m-PFC tissue samples on which the DA analyses were conducted. These data are shown in Figure 7B. ANOVA revealed a significant effect of treatment on m-PFC 5-HT utilization,F(2,19) = 11, p < 0.001. The mean 5-HIAA/5-HT ratios after the extinction period were 0.21 ± 0.01, 0.26 ± 0.01, and 0.18 ± 0.001 for the Sh/NS, Sh/S, and A-/S groups, respectively. Note that within the sham lesion groups, the stress condition resulted in a statistically significant 22% increase in m-PFC 5-HT utilization, p < 0.05, whereas post-training amygdalectomy resulted in blockade of this stress-induced elevation, p < 0.001. Also note that the mean m-PFC 5-HIAA/5-HT ratio for the A-/S group was statistically indistinguishable from the Sh/NS control group. ANOVA also revealed a significant effect of treatment on m-PFC MHPG concentration,F(1,19) = 7, p < 0.01. The mean m-PFC MHPG concentrations after the extinction period were 1.4 ± 0.2, 1.8 ± 0.1, and 1.4 ± 0.04 ng/mg protein for the Sh/S, Sh/NS, and A-/S groups, respectively. Note that within the sham lesion groups, the conditioned stress resulted in a statistically significant 27% increase in m-PFC MHPG concentration, whereas post-training amygdalectomy resulted in blockade of this stress-induced elevation of MHPG. Also note that the mean m-PFC MHPG concentration for the A-/S group was statistically indistinguishable from that of the Sh/NS group.
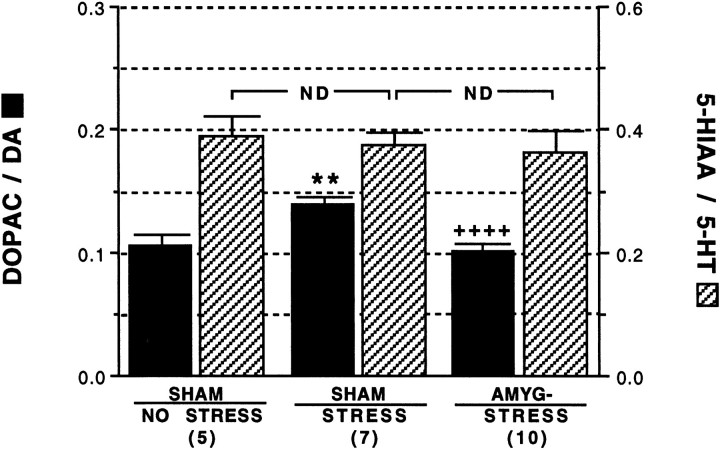
The regional specificity of the effect of amygdalectomy on m-PFC DA utilization was assessed by examining the DOPAC/DA ratio in the NAS. These data are shown in Figure 8. ANOVA revealed a significant effect of treatment on NAS DA utilization,F(2,19) = 22, p < 0.0001. The mean DOPAC/DA ratios after the extinction period were 0.11 ± 0.01, 0.14 ± 0.005, and 0.11 ± 0.002 for the Sh/NS, Sh/S, and A-/S groups, respectively. Note that within the sham lesion groups, the conditioned stress resulted in a statistically significant 23% increase in NAS DA utilization, whereas post-training amygdalectomy resulted in blockade of this stress-induced elevation, p < 0.0001. Also note that the mean NAS DOPAC/DA ratio for the A-/S group was statistically indistinguishable from the Sh/NS control group. Note that the 5-HT system in the NAS was not activated by stress (Fig. 8). Mean 5-HT utilization values for the Sh/NS, Sh/S, and A-/S groups were 0.39 ± 0.04, 0.38 ± 0.02, and 0.37 ± 0.04, respectively. Noradrenergic metabolism in the NAS was not assessed.
Fig. 8.
Effect of post-training amygdala lesions on stress-induced DA and 5-HT utilization in the NAS. Animals were subjected to bilateral sham (SHAM) or excitotoxic lesions of the amygdala (AMYG-) 5 d after conditioning.STRESS and NO STRESS indicate experimental conditions in which animals were or were not exposed to shock during conditioning, respectively. **, Statistically significant difference versus the sham/no stress group, p < 0.01; ++++, statistically significant difference versus the sham/stress group,p < 0.0001; ND, no statistically significant difference between groups, p > 0.05. Numbers in parenthesis indicate number of animals in each group.
DISCUSSION
The principal finding of this study is that bilateral excitotoxic amygdala lesions result in blockade of the mesocortical monoaminergic responses to stress induced by reexposure to stimuli paired previously with an unconditioned stressor. The amygdala lesions also attenuated associated adrenocortical activation, freezing, ultrasonic vocalization, and defecation. These findings suggest that the amygdala is important in linking aversive stimuli to the normally contingent behavioral, neuroendocrine, and cortical monoamine responses to psychological stress.
Pretraining versus post-training lesions of the amygdala
Pretraining amygdalectomy blocked behavioral responses to footshock and to stress induced by reexposure to stimuli paired previously with footshock. It is noteworthy that amygdala lesions have not been found to alter flinch amplitude or the flinch threshold to footshock (Cahill and McGaugh, 1990; Sananes and Davis, 1992). However, the finding that pretraining amygdalectomy blocked ultrasonic vocalization, freezing, and defecation during conditioning suggests that this lesion may have interfered with the perception and/or expression of fear during conditioning and, thus, may have altered aversive memory formation.
This possibility prompted a study using post-training amygdalectomy; i.e., subjects were conditioned before lesioning, thus obviating the potentially confounding effect of the lesion on efficacy of conditioning. As with the pretraining lesions, post-training lesions greatly attenuated adrenocortical activation and blocked freezing, defecation, and conditioned stress-induced activation of m-PFC monoamine metabolism.
The amygdala and afferent control of the m-PFC DA, NE, and 5-HT systems
Increased DA utilization in the m-PFC is produced by a variety of unconditioned aversive stimuli (Thierry et al., 1976; Fadda et al., 1978; Reinhard et al., 1982; Claustre et al., 1986; Roth et al., 1988;Pei et al., 1990), unconditioned psychological stress (Kaneyuki et al., 1991), and conditioned stress (Herman et al., 1982; Deutch et al., 1985, Goldstein et al., 1994). Exposure to more substantial or prolonged stress results in recruitment of the mesolimbic DA system innervating the NAS (Roth et al., 1988). Consistent with these studies, we demonstrated a robust increase in m-PFC DA utilization in response to stimuli paired previously with footshock; a more modest increase was noted in the NAS.
The finding that both pretraining and post-training amygdalectomy block the increase in m-PFC DA utilization implicates the amygdala in afferent control of the mesocortical DA system response to psychological stress. This extends work by Davis et al. (1994) that demonstrated that amygdala lesions block activation of the m-PFC DA system in response to footshock and novelty. The central nucleus of the amygdala projects to the VTA (Gonzales and Chesselet, 1990), site of the DA perikarya that innervate the m-PFC. In addition, the central nucleus communicates indirectly with the VTA through projections to the bed nucleus of the stria terminalis, periaqueductal gray (PAG), lateral hypothalamus, and nucleus parabrachialis (Phillipson, 1979).
Alternatively, amygdala influence on m-PFC DA metabolism could involve control of DA release and/or metabolism locally within the m-PFC. This hypothesis is based on the presence of reciprocating connections between the cortex-like basolateral amygdala nucleus and m-PFC (Krettek and Price, 1977; Sesack et al., 1989; McDonald, 1991). Electrophysiological studies have demonstrated that stimulation of the basolateral nucleus alters neuronal firing in the m-PFC with latencies consistent with mono- and polysynaptic pathways (Perez-Jaranay and Vives, 1991). The m-PFC in turn sends efferent projections that synapse on DA neurons in the VTA (Sesack and Pickel, 1992).
Exposure to stress also results in regional increases in NE metabolism in rodents, (Dunn, 1988; Tanaka et al., 1990) (for review, see Glavin, 1985), primates (Mason et al., 1968; Redmond, 1987), and humans (Rubin et al., 1970; Maas et al., 1971; Sweeney et al., 1978). Recent studies using in vivo microdialysis have shown that m-PFC NE release is enhanced by unconditioned stress (Cenci et al., 1992; Nakane et al., 1994; Finlay et al., 1995). These findings are consistent with observed stress-induced increases in electrophysiological activity in locus coeruleus neurons (Jacobs et al., 1991).
Consistent with these observations, we have demonstrated that NE metabolism in the m-PFC is activated by the stress of reexposure to stimuli paired previously with footshock. We did not measure endogenous levels of NE in the current study. It is very likely, however, that the tissue levels of NE decline after exposure to our conditioned stress model, similar to findings documented in a wealth of previous work from many other laboratories investigating the effect of stress on the noradrenergic system (Tsuda et al., 1986). The noradrenergic metabolic activation noted in the present study was blocked by post-training amygdalectomy, suggesting that the amygdala may provide functional afferent regulatory input to the NE system during psychological stress. The central nucleus of the amygdala is known to send indirect (Aston-Jones et al., 1986) and direct (Cedarbaum and Aghajanian, 1978;Wallace et al., 1989) projections to the locus coeruleus, site of the NE cell bodies that innervate the cortex (Lindvall and Bjorklund, 1984). Immunohistochemical studies also have demonstrated that afferents from the central nucleus of the amygdala impinge on tyrosine hydroxylase-positive neurons in the rostral portion of the locus coeruleus (Wallace et al., 1989, 1992).
Stress-induced increases in brain 5-HT metabolism also have been observed previously (Thierry et al., 1968; Dunn, 1988), although not in all studies (Kaneyuki et al., 1991). Inoue et al. (1993) demonstrated an increase in m-PFC 5-HIAA levels in rats reexposed to a context paired previously with footshock, a finding consistent with the present as well as a previous study from our laboratory (Goldstein et al., 1994).
The demonstration that postconditioning amygdalectomy blocks activation of the m-PFC 5-HT system resulting from reexposure to stimuli paired previously with footshock suggests that the amygdala also provides afferent regulatory input to the 5-HT system during psychological stress. The central nucleus of the amygdala sends projections to the dorsal raphe nucleus (Wallace et al., 1992); serotonergic neurons from both the dorsal and median raphe nuclei in turn project to the m-PFC (Azmitia and Segal, 1978; Moore et al., 1978).
The amygdala and the adrenocortical response
Studies in nonprimate mammals (Redgate, 1970; Redgate and Fahringer, 1973) have confirmed Mason’s early pioneering work in awake primates demonstrating activation of the hypothalamic–pituitary–adrenocortical axis by electrical stimulation of the amygdala (Mason, 1959). Recent work in rodents indicates that the amygdala, and more specifically, the central nucleus of the amygdala, is involved in adrenocortical stress activation (Beaulieu et al., 1986; Roozendaal et al., 1991; Van de Kar et al., 1991). The present findings corroborate these studies and are consistent with known neuroanatomical connections of the central nucleus to distal structures controlling the adrenocortical response (Swanson et al., 1983; van der Kooy et al., 1984; Gray et al., 1989).
Effects of amygdalectomy on ultrasonic vocalization, freezing, and defecation
Ultrasonic vocalizations in the 22 kHz range are elicited under a variety of situations that evoke defense responses in laboratory rats and are thought to be antipredator signals to conspecifics (Blanchard et al., 1991; Miczek et al., 1991). The present study demonstrates that amygdalectomy blocks stress-induced ultrasonic vocalization. It is known that both auditory and somatosensory information reach the lateral and basolateral amygdala directly from the thalamus, as well as via indirect connections through the cortex (LeDoux, 1992). The lateral and basolateral amygdala send projections to the central nucleus, which projects to the ventral aspect of the PAG (Hopkins and Holstege, 1978), an area that in turn projects to laryngeal, pharyngeal, respiratory, and perioral motoneurons in the nucleus ambiguus and retroambiguus (Jurgens and Pratt, 1979; Yajima et al., 1982; Holstege, 1989). Electrical stimulation within the PAG elicits ultrasonic vocalization in anesthetized rats (Yajima et al., 1980). Thus, amygdalectomy may disrupt the relay of incoming sensory information to output neurons activating ultrasonic vocalization.
Both pretraining and post-training amygdalectomy also blocked stress-induced freezing, findings consistent with other reports (Blanchard and Blanchard, 1972; Roozendaal, 1991). The PAG appears to be an important effector nucleus for organizing stress-induced freezing, because lesioning of either the PAG or the central nucleus of the amygdala results in complete abolition of this response (LeDoux et al., 1988). Stress-induced defecation also was blocked by amygdalectomy, possibly by disrupting the connection of the central nucleus to the dorsal motor nucleus of the vagus (Schwaber et al., 1982; Veening et al., 1984).
General considerations
The present study extends the growing literature implicating the amygdala in the coordination of behavioral, neurohumoral, and central neurochemical responses to psychological stress. However, our data do not allow us to make claims concerning the precise mechanism(s) responsible for this effect. It is possible that the lesioned animals can no longer integrate incoming sensory information. It is also possible that the effector nuclei for the various arms of the stress response have been disconnected so that the stress responses simply cannot be expressed. A third possibility is that the lesioned animals are expressing certain stress responses, but not those measured in this study. Finally, amygdalectomy may have a direct effect on one or a limited number of stress responsive systems, which interact secondarily with other systems to give rise to the observed results. Future research will be necessary to clarify these issues.
Nevertheless, the findings do suggest a role for the amygdala in afferent control of the m-PFC DA system response to stress. This may be relevant clinically, because cortical DA dysfunction is proposed to play a role in schizophrenia (Weinberger et al., 1986; Davis et al., 1991; Goldstein and Deutch, 1992; Reynolds, 1992) and symptoms of schizophrenia are thought to be stress responsive (Rabkin, 1980;Bebbington et al., 1993). Amygdala input to the VTA, thus, may be an important neurobiological substrate for the mediation of symptom exacerbation in this disorder. Psychological stress, dysregulation of central monoamine systems, and the amygdala also have been proposed to play a role in the development of post-traumatic stress disorder (PTSD) (Charney et al., 1993; Southwick et al., 1993; Goldstein et al., 1994). The present findings integrate these factors and, thus, may contribute to our understanding of PTSD, as well as other anxiety and affective disorders (Chrousos and Gold, 1992; Post, 1992).
Footnotes
This work was supported by U.S. Public Health Service Grants MH14092 and MH28849. We thank Drs. Ariel Deutch, Michael Davis, and Jane Taylor for helpful discussions during the course of this investigation. We also thank L. Chasney for her help in the preparation of this manuscript.
Correspondence should be addressed to Robert H. Roth, Department of Pharmacology, B-254 SHM, Yale University School of Medicine, New Haven, CT 06520.
REFERENCES
- 1.Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- 2.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 3.Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, Lewis S. Life events and psychosis: initial results from the Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu S, DiPaolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implications of the serotonergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in a visible burrow system. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 8.Bliss EL, Ailion J, Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J Pharmacol Exp Ther. 1968;164:122–134. [PubMed] [Google Scholar]
- 9.Cahill L, McGaugh JL. Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Behav Neurosci. 1990;104:532–543. doi: 10.1037//0735-7044.104.4.532. [DOI] [PubMed] [Google Scholar]
- 10.Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- 11.Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- 12.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:294–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 13.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 14.Claustre Y, Rivy JP, Dennis T, Scatton B. Pharmacological studies on stress-induced increase in frontal cortical dopamine metabolism in the rat. J Pharmacol Exp Ther. 1986;238:693–700. [PubMed] [Google Scholar]
- 15.Conti LH, Naeiver CR, Ferkany JW, Abreau ME. Footshock-induced freezing behavior in rats as a model for assessing anxiolytics. Psychopharmacology. 1990;102:492–497. doi: 10.1007/BF02247130. [DOI] [PubMed] [Google Scholar]
- 16.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 17.Davis M, Hitchcock JM, Bowers MB, Berridge CW, Melia KR, Roth RH. Stress-induced activation of prefrontal cortex dopamine turnover: blockade by lesions of the amygdala. Brain Res. 1994;664:207–210. doi: 10.1016/0006-8993(94)91972-0. [DOI] [PubMed] [Google Scholar]
- 18.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 19.Deutch AY, Tam S-Y, Roth RH. Footshock and conditioned stress increase in 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985;333:143–146. doi: 10.1016/0006-8993(85)90134-9. [DOI] [PubMed] [Google Scholar]
- 20.Dunn A. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51:406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 21.Elsworth JD, Roth RH, Redmond DE. Relative importance of 3-methoxy-4-hydroxyphenylglycol as norepinephrine metabolites in rat, monkey, and humans. J Neurochem. 1983;417:786–793. doi: 10.1111/j.1471-4159.1983.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 22.Fadda F, Argiolas A, Melis MR, Tissari AH, Onali PL, Gessa G. Stress-induced increase in 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and N. accumbens: reversal by diazepam. Life Sci. 1978;23:2219–2224. doi: 10.1016/0024-3205(78)90207-2. [DOI] [PubMed] [Google Scholar]
- 23.Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 24.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 25.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1:418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gellman RL, Aghajanian GK. Pyramidal cells in the piriform cortex receive a convergence of inputs from monoamine activated GABA-ergic interneurons. Brain Res. 1993;600:63–73. doi: 10.1016/0006-8993(93)90402-9. [DOI] [PubMed] [Google Scholar]
- 28.Glavin GB. Stress and brain noradrenaline: a review. Neurosci Biobehav Rev. 1985;9:233–243. doi: 10.1016/0149-7634(85)90048-x. [DOI] [PubMed] [Google Scholar]
- 29.Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;89:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. The NMDA glycine site antagonist (+)-HA-966 selectively regulates conditioned stress-induced metabolic activation of the mesoprefrontal cortical dopamine but not serotonin systems: a behavioral, neuroendocrine, and neurochemical study in the rat. J Neurosci. 1994;14:4937–4950. doi: 10.1523/JNEUROSCI.14-08-04937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992;6:2413–2421. [PubMed] [Google Scholar]
- 32.Gonzales C, Chesselet MF. Amygdalonigral pathway: an anterograde study in the rat with phaseolus vulgaris leucoagglutinin (PHA-L). J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- 33.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 34.Herman JP, Guillonneau D, Dantzer R, Scatton B, Semerdjian-Rouguier L, LeMoal M. Differential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the rat. Life Sci. 1982;30:2207–2214. doi: 10.1016/0024-3205(82)90295-8. [DOI] [PubMed] [Google Scholar]
- 35.Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Koyama T, Yamashita I. Effect of conditioned fear stress on serotonin metabolism in the rat brain. Pharmacol Biochem Behav. 1993;44:371–374. doi: 10.1016/0091-3057(93)90476-a. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog Brain Res. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- 39.Jurgens U, Pratt R. Role of the periaqueductal gray in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- 40.Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M, Tanaka M. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Res. 1991;557:154–161. doi: 10.1016/0006-8993(91)90129-j. [DOI] [PubMed] [Google Scholar]
- 41.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 42.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 43.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindvall O, Bjorklund A. General organization of cortical monoamine systems. In: Descarries L, Reader TR, Jasper HH, editors. Monoamine innervation of the cerebral cortex. Liss; New York: 1984. pp. 9–40. [Google Scholar]
- 45.Maas JW, Dekirmenjian H, Fawcett J. Catecholamine metabolism: depression and stress. Nature. 1971;230:330–331. doi: 10.1038/230330a0. [DOI] [PubMed] [Google Scholar]
- 46.Maeda H, Mogenson GJ. Electrophysiological responses of the ventral tegmental area to electrical stimulation of amygdala and septum. Neuroscience. 1981;6:367–376. doi: 10.1016/0306-4522(81)90130-5. [DOI] [PubMed] [Google Scholar]
- 47.Mason JW. Plasma 17-hydroxycorticosteroid levels during electrical stimulation of the amygdaloid complex in conscious monkeys. Am J Physiol. 1959;196:44–48. doi: 10.1152/ajplegacy.1958.196.1.44. [DOI] [PubMed] [Google Scholar]
- 48.Mason JW. Organization of psychoendocrine mechanisms. Psychosom Med. 1968;30:565–808. [Google Scholar]
- 49.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 50.Miczek KA, Tournatzky W, Vivian J. Ethnology and neuropharmacology: rodent ultrasounds. In: Olivier B, Mos J, Slangen JI, editors. Animal models in psychopharmacology. Birkhauser; Basel: 1991. pp. 409–427. [Google Scholar]
- 51.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 52.Nakane H, Shimizu N, Hori T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am J Physiol. 1994;267:R1559–R1566. doi: 10.1152/ajpregu.1994.267.6.R1559. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. Academic; San Diego: 1986. The rat brain in stereotaxic coordinates (2nd ed). . [Google Scholar]
- 54.Pei Q, Zetterstrom T, Fillenz M. Tail pinch-induced changes in the turnover and release of dopamine and 5-hydroxytryptamine in different brain regions of the rat. Neuroscience. 1990;35:133–138. doi: 10.1016/0306-4522(90)90127-p. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res. 1991;564:97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- 56.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–144. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 57.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 58.Rabkin JG. Stressful life events and schizophrenia: a review of the research literature. Psychol Bull. 1980;87:408–425. [PubMed] [Google Scholar]
- 59.Redgate ES. ACTH release evoked by electrical stimulation of brain stem and limbic system sites in the cat: the absence of ACTH release upon infundibular area stimulation. Endocrinology. 1970;86:806–823. doi: 10.1210/endo-86-4-806. [DOI] [PubMed] [Google Scholar]
- 60.Redgate ES, Fahringer EE. A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology. 1973;12:334–343. doi: 10.1159/000122182. [DOI] [PubMed] [Google Scholar]
- 61.Redmond DE. Studies of the nucleus locus coeruleus in monkeys and hypotheses for neuropsychopharmacology. In: Melzer HY, editor. Psychopharmacology: the third generation of progress. Raven; New York: 1987. pp. 967–976. [Google Scholar]
- 62.Reinhard JF, Bannon MJ, Roth RH. Activation by stress of dopamine synthesis and metabolism in the prefrontal cortex: antagonism by diazepam. Naunyn Schmiedebergs Arch Pharmacol. 1982;318:374–377. doi: 10.1007/BF00501182. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds GP. The amygdala and the neurochemistry of schizophrenia. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. Wiley; New York: 1992. pp. 561–574. [Google Scholar]
- 64.Roozendaal B, Koolhaas JM, Bohus B. Attenuated cardiovascular, endocrine and behavioral response after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 1991;50:771–775. doi: 10.1016/0031-9384(91)90016-h. [DOI] [PubMed] [Google Scholar]
- 65.Roth RH, Tam S-Y, Ida Y, Yang JX, Deutch AY. Stress and mesocorticolimbic dopamine systems. Ann N Y Acad Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- 66.Rubin RT, Miller RG, Clark BR, Poland RE, Arthur RJ. The stress of aircraft carrier landings. II. 3-methoxy-4-hydroxyphenylglycol excretion in naval aviators. Psychosom Med. 1970;32:589–597. doi: 10.1097/00006842-197011000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Sananes CB, Davis M. N -methyl-d-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- 68.Scwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sequela P, Watkins KC, Descarries L. Ultrastructural features of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 1988;442:11–22. doi: 10.1016/0006-8993(88)91427-8. [DOI] [PubMed] [Google Scholar]
- 70.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminal in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 71.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leukoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 72.Southwick SM, Krystal JH, Morgan A, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 73.Swanson LW, Sawchenko PE, Rivier J, Vale W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 74.Sweeney DR, Maas JW, Heninger GR. State anxiety, physical activity, and urinary 3-methoxy-4-hydroxyphenethylene glycol excretion. Arch Gen Psychiatry. 1978;35:1418–1424. doi: 10.1001/archpsyc.1978.01770360022002. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka M, Tsuda A, Yokoo H, Yoshida M, Ida Y, Nishimura H. Involvement of the brain noradrenaline system in emotional changes caused by stress in rats. Ann NY Acad Sci. 1990;597:159–174. doi: 10.1111/j.1749-6632.1990.tb16165.x. [DOI] [PubMed] [Google Scholar]
- 76.Thierry AM, Fekete M, Glowinski J. Effects of stress on the metabolism of noradrenaline, dopamine and serotonin (5-HT) in the central nervous system of the rat. II. Modifications of serotonin metabolism. Eur J Pharmacol. 1968;4:384–389. doi: 10.1016/0014-2999(68)90023-x. [DOI] [PubMed] [Google Scholar]
- 77.Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesocortical dopamine system by stress. Nature. 1976;263:242–243. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 78.Tsuda A, Tanaka M, Ida Y, Tsujimaru S, Nagasaki N. Effects of shock controllability on rat brain noradrenaline turnover under FR-1 and FR-3 Sidman avoidance schedule. Physiol Behav. 1986;37:945–950. [PubMed] [Google Scholar]
- 79.van de Kar LD, Piechowski RA, Rittenhouse PA, Grey TS. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 80.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;244:349–359. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 81.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brain stem sites involved in central autonomic regulation: a combined retrograde transport immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- 82.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989;97:252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- 84.Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 85.Weinberger DR, Berman KF, Zee RF. Physiological dysfunction of dorsolateral prefrontal cortex. I. Regional cerebral blood flow (rCBF) evidence. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 86.Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine and serotonin levels in various regions of the rat brain. Brain Res Rev. 1981;3:167–205. [Google Scholar]
- 87.Yajima Y, Hayashi Y, Yoshii N. The midbrain central gray substance as a highly sensitive neural structure for the production of ultrasonic vocalization in the rat. Brain Res. 1980;198:446–452. doi: 10.1016/0006-8993(80)90759-3. [DOI] [PubMed] [Google Scholar]
- 88.Yajima Y, Hayashi Y, Yoshii N. Ambiguus motoneurons discharging closely associated with ultrasonic vocalization in rats. Brain Res. 1982;238:445–450. doi: 10.1016/0006-8993(82)90121-4. [DOI] [PubMed] [Google Scholar]