Abstract
Previous studies in animals and humans suggest that monoamines enhance behavior-evoked neural activity relative to nonspecific background activity (i.e., increase signal-to-noise ratio). We studied the effects of dextroamphetamine, an indirect monoaminergic agonist, on cognitively evoked neural activity in eight healthy subjects using positron-emission tomography and the O15 water intravenous bolus method to measure regional cerebral blood flow (rCBF). Dextroamphetamine (0.25 mg/kg) or placebo was administered in a double-blind, counterbalanced design 2 hr before the rCBF study in sessions separated by 1–2 weeks. rCBF was measured while subjects performed four different tasks: two abstract reasoning tasks—the Wisconsin Card Sorting Task (WCST), a neuropsychological test linked to a cortical network involving dorsolateral prefrontal cortex and other association cortices, and Ravens Progressive Matrices (RPM), a nonverbal intelligence test linked to posterior cortical systems—and two corresponding sensorimotor control tasks. There were no significant drug or task effects on pCO2 or on global blood flow. However, the effect of dextroamphetamine (i.e., dextroamphetamine vs placebo) on task-dependent rCBF activation (i.e., task − control task) showed double dissociations with respect to task and region in the very brain areas that most distinctly differentiate the tasks. In the superior portion of the left inferior frontal gyrus, dextroamphetamine increased rCBF during WCST but decreased it during RPM (ANOVA F(1,7) = 16.72, p < 0.0046). In right hippocampus, blood flow decreased during WCST but increased during RPM (ANOVAF(1,7) = 18.7, p < 0.0035). These findings illustrate that dextroamphetamine tends to “focus” neural activity, to highlight the neural network that is specific for a particular cognitive task. This capacity of dextroamphetamine to induce cognitively specific signal augmentation may provide a neurobiological explanation for improved cognitive efficiency with dextroamphetamine.
Keywords: dextroamphetamine, rCBF, PET, monoamines, dopamine, working memory, hippocampus, dorsolateral prefrontal cortex
The modulatory effects of monoaminergic neurotransmitters on neurophysiological function in the cortex have been shown previously in animal and human studies. Early studies of sensory stimulation in monkeys demonstrated that monoamines suppress spontaneous background neural firing while specifically enhancing cortical neural responses to a sensory stimulus (Foote et al., 1975).Segal and Bloom (1976b) demonstrated that norepinephrine exaggerated inhibition of hippocampal response to an unconditioned tone, and then enhanced the excitatory response to that same tone once it became a conditioned stimulus for reward. Norepinephrine also has been shown (Woodard et al., 1979) to enhance signal-to-noise (STN) responses in many other brain areas including the somatosensory cortex, cerebellum, lateral geniculate nucleus, and spinal trigeminal nucleus. Brozoski et al. (1979) reported that depletion of dopamine in prefrontal cortex impaired the ability of monkeys to perform delayed-response tasks, similar to ablation of the dorsolateral prefrontal cortex (DLPFC).Sawaguchi and Goldman-Rakic (1991) found that local pharmacological blockade of D1 receptors in the DLPFC impaired performance on an oculomotor delayed-response task. Weinberger et al. (1988) demonstrated in schizophrenic patients that central dopamine levels, as evidenced by CSF concentration of the dopamine metabolite homovanillic acid, predicted prefrontal regional cerebral blood flow (rCBF) during performance of a prefrontally linked task, the Wisconsin Card Sorting Task (WCST). Recently, an inverted U-type dose–response relationship has been shown between D1 receptor stimulation in the prefrontal cortex and delay-related prefrontal cortex neuronal firing (Williams and Goldman-Rakic, 1995) or delayed-response performance (Murphy et al., 1996). Their report confirms earlier studies by Bauer and Fuster (1978)and Arnsten and Goldman-Rakic (1990) that showed that there appears to be an optimal range of dopamine stimulation in the prefrontal cortex and that either too little or too much dopamine results in diminished prefrontal cortex function.
The STN effects of monoamines on neurophysiological function are further supported by studies using monoamine agonists. Segal and Bloom (1976a) reported that dextroamphetamine could facilitate self-stimulating behavior and reduce spontaneous cell discharges in the hippocampus, actions that are seen with locus ceruleus stimulation and are thought to be mediated by norepinephrine. More recently, in a study on the effects of amphetamine on cognitively related rCBF patterns in schizophrenic patients, Daniel et al. (1991), using xenon −133 dynamic single photon emission computer tomography to measure rCBF while subjects performed the WCST, demonstrated a striking effect of dextroamphetamine on task-dependent activation of rCBF. In contrast to placebo, dextroamphetamine produced specific and selective activation of the DLPFC, and this correlated with improved performance on the task. However, this earlier study was undertaken in an illness-specific population with a limited resolution rCBF technique.
Dextroamphetamine is a nonspecific indirect monoamine agonist (Weiner, 1972). Under certain circumstances, it has been shown to improve cognitive efficiency and measures of attention (Robins and Everitt, 1987). The preliminary rCBF data from studies of patients with schizophrenia (Daniel et al., 1991) suggest that its cognitive effects may be related to its capacity to modulate cognitively related cortical STN. We undertook the present study to explore this possibility further. In particular, we sought to examine the following: (1) Does the effect of dextroamphetamine on enhancing cortical STN extend to normal subjects, to cognitive tasks other than the WCST, and to cortical regions other than the frontal lobes? (2) If so, does the regional pattern of the effect differ according to different regional demands of the task? To date, there is no study of normal subjects that has examined monoamine-related task-specific rCBF changes with these goals.
MATERIALS AND METHODS
Subjects. Eight healthy subjects (four males and four females, mean age 25 years, range 22–32 years) were studied in a double-blind placebo-controlled manner. Each subject signed informed consent to participate in this study, which had the approval of the National Institutes of Mental Health institutional review board and the National Institutes of Health radiation safety committee. The subjects were screened for past and present history of neurological, psychiatric, or substance abuse problems, and had no history of other medical problems or medical treatment relevant to cerebral metabolism and blood flow. Subjects were instructed to refrain from nicotine and caffeine for 4 hr and from over-the-counter medications for 24 hr before the positron-emission tomography (PET) scan.
PET scans. rCBF measurements were made using the O15 water intravenous bolus PET technique. A total of eight measurements, four each on two separate days, were carried out in each subject. For each rCBF measurement, subjects received an intravenous bolus of 37.5 mCi of O15water 1 min after initiation of the cognitive or sensorimotor control task. The PET scans were performed on a Scanditronix PC2048–15B brain tomograph (15 contiguous slices; reconstructed in-plane resolution 6–6.5 mm; axial resolution 6.5 mm). During the scan procedure, the subjects lay supine with their heads immobilized in a thermoplastic mask. The time course of regional cerebral radiation concentration was determined simultaneously for the 15 slices by collecting a total of 16 scans (12 × 10 sec, 4 × 30 sec) during the 4 min after arrival of the tracer in the brain. The slices were obtained parallel to the canthomeatal line, and the lowest slice was collected 16 mm above the canthomeatal line. Transmission scans obtained in the same planes as the PET scans were used to correct for count attenuation by tissue and skull. Scan data were reconstructed with corrections for attenuation, scatter, random coincidences, and deadtime.
An arterial input function was measured via automated arterial blood sampling, with blood withdrawn continuously at a rate of 3.8 ml/min, and coincident events were counted by paired sodium iodide detectors and corrected for random coincidences and dispersion (Daube-Witherspoon et al., 1992). The arterial time–activity curve was fitted with a least-squares method (Koeppe et al., 1985) on a pixel-by-pixel basis to the real time–activity curves to produce quantitative images of rCBF. For regional analyses, rCBF values for each pixel then were expressed as a percentage of the mean rCBF value for the entire brain (i.e., the data were “normalized” to the global mean).
Cognitive tasks. rCBF was measured while subjects performed two cognitive tasks, the Wisconsin Card Sorting Task (WCST) and Ravens Progressive Matrices (RPM), and two matching sensorimotor control tasks, Wisconsin Card Sorting Control (WCSC) and Ravens Progressive Matrices Control (RPMC).
The WCST has for many years been a standard of neuropsychological testing of the prefrontal cortex in man. The WCST increases rCBF in DLPFC in normal subjects (Weinberger et al., 1986; Berman et al., 1995) and is particularly sensitive to dysfunction of DLPFC (Milner, 1963;Milner and Petrides, 1984). For this study, a computerized version of the WCST was used. Subjects viewed a computer screen that displayed five boxes. Subjects were asked to match the contents of the center box to one of the four outside boxes. Subjects were not informed of how to make the match, but had to determine from trial and error whether to match on the basis of color, shape, or number using feedback displayed on the screen after each response. After the subject has made a series of correct responses, the “rule” changes and subjects must determine a new rule for matching. The sensorimotor control task for the WCST was a no-delay, matching-to-sample task designed to be similar to the WCST in visual stimulation and motor response requirements, but without the abstract reasoning and working memory components of the WCST. Subjects simply matched the central target stimulus to one of four unchanging surrounding answer boxes. Stimuli were presented on a computer monitor mounted above the subject. For both tasks, subjects responded to each trial with a minimum of motor activity by pushing one of the four buttons arranged in a cross-shaped array corresponding to the arrangement of answers on the screen. The buttons were mounted on a 2 × 3 × 0.5 inch response box that was held in the right hand. Before the PET scans, subjects were trained in this mode of response until the association between the answer on the screen and the corresponding button became automatic.
RPM was first published in 1938 as a nonverbal “test of a person’s present capacity to form comparisons, reason by analogy, and develop a logical method of thinking, and of innate inductive ability” (Raven, 1938). It is generally accepted as a measure of general intelligence and has been shown to correlate with a number of standardized intelligence tests. Previous studies have shown that posterior cortical regions and hippocampus are especially activated (Berman et al., 1988;Haier et al., 1992; Ostrem et al., 1993) in association with this task. For this test, subjects are shown pictures of matrices (i.e., related patterns), each of which is a figural design with a part removed. The subject must choose the correct missing part from six to eight alternatives shown in the same visual frame. The matrices increase in difficulty as the test continues. The control task was a simple no-delay “match-to-sample” task. Subjects were asked to report verbally their choices for both of the RPM conditions.
Because each subject underwent the study on two separate occasions (drug or placebo), to minimize learning effect, subjects were given instructions on the performance of the tasks and were allowed to practice the tasks before they were taken into the scanning room. For RPM, the matrices on which the subjects practiced were not repeated and a fresh set of matrices were used during each scanning session. Tasks were begun 1 min before the injection of O15water and were continued throughout the ensuing 4 min of the scan period. Performance on the WCST was scored as per Heaton et al. (1993), whereas performance of RPM was analyzed as percent of correct trials.
Test conditions and drug administration. Subjects were studied in a double-blind cross-over design during two PET sessions separated by 1 to 2 weeks. Each session consisted of an initial sham resting procedure performed to acclimatize the subject to the procedure, followed by four separate rCBF measurements made during performance of the two cognitive tasks (WCST and RPM) and their matching sensorimotor control tasks (WCSC and RPMC). The order of the tasks was counterbalanced across subjects, but kept constant for the two visits of each subject. Approximately 120 min before each PET session, subjects received an oral dose of either placebo or dextroamphetamine (0.25 mg/kg). Timing of administration of dextroamphetamine was based on pharmacokinetic data indicating that plasma levels of dextroamphetamine administered orally peak 2–3 hr after administration (Angrist et al., 1987). The order of the drug and placebo administration also was counterbalanced across subjects. A simple mood rating scale (Goldberg et al., 1991) was administered before and 2 hr after administration of the drug. Profile of Mood States (POMS) (McNair et al., 1992) and Speilberger anxiety scales (Speilberger, 1983) also were administered after the PET scans on each test day. Arterial pCO2 levels were determined at the end of each scan. Blood pressure and heart rate were obtained at baseline and every half hour for 2 hr after administration of the drug. Serum drug levels were obtained at the beginning of each PET session and at the end of each rCBF measurement. Serum dextroamphetamine levels were measured using gas chromatography analysis (National Psychopharm Laboratories, Knoxville, TN) with a sensitivity of 5 ng/ml. Because of technical difficulties, only seven of the eight subjects had serum amphetamine levels measured.
Image processing and statistical analysis. Coplanar magnetic resonance image (MRI) scans were obtained for each subject using the same external landmarks as for the PET scan (i.e., the canthomeatal line). As for the PET studies, a set of fifteen 6.5-mm-thick T2-weighted MRI slices were obtained. Each of the PET scans of a given subject were coregistered to his or her MRI scan using ANALYZE (Biomedical Imaging Resource, Mayo Foundation), a three-dimensional contour matching algorithm (Jaing et al., 1992). Individualized regions of interest (ROIs) for each subject were drawn on the MRIs for a variety of cortical and subcortical regions. Cortical ROIs included inferior, middle, and superior frontal gyri and superior temporal, parietal, and occipital cortices. Anterior cingulate, thalamus, caudate, putamen, and hippocampal ROIs also were drawn (Fig.1). These individualized ROI templates then were applied to the coregistered PET rCBF scans of each subject, and the mean normalized rCBF value for each ROI for each of the task conditions was determined. To reduce the number of comparisons, area-weighted averages for like structures were combined across several slices as reported previously (Berman et al., 1995). This ROI approach was chosen because of previous experience with it in the analysis of rCBF data associated with these tasks. For global CBF and each region separately, a 2 × 2 ANOVA with two repeated measures (task and drug) was performed to assess the interaction between drug and task. Post hoc matched-pairt tests between drug and/or task conditions, pCO2, global CBF, and task performance scores also were performed. Because each subject had serum amphetamine levels measured five times at ∼12 min intervals, one-way ANOVA was performed to determine whether there was a significant change in amphetamine levels over time.
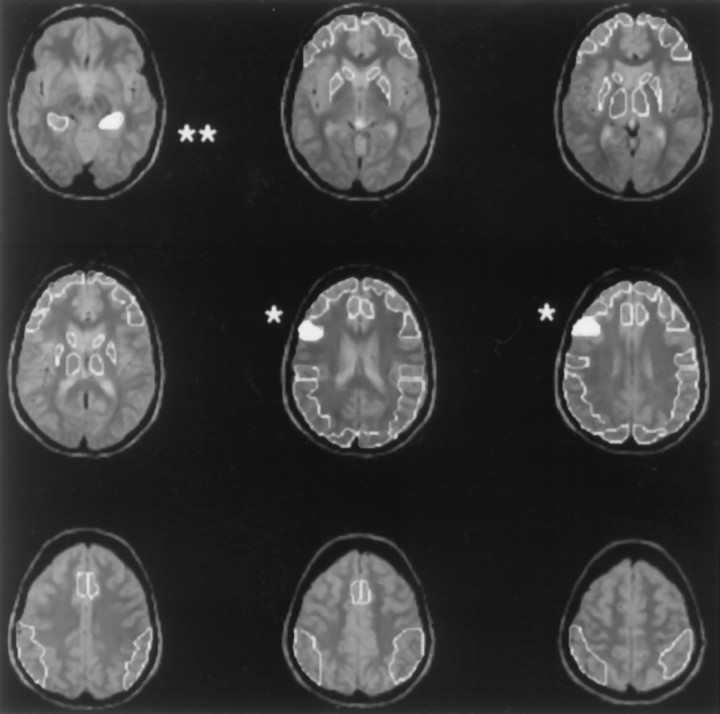
Fig. 1.
An example of the individualized ROI templates that were drawn on the MRI of each subject. Locations of regions in which overall repeated-measures ANOVA (Drug × Task interaction) reached statistical significance are shown in solid whiteand are indicated by asterisks. For statistical analysis, area-weighted averages were calculated across several slices for like structures including (1) superior portion of the inferior frontal gyrus in slices indicated by a single asterisk, and (2) hippocampal area in the slice indicated by a double asteriskand in the next most inferior slice (data not shown).
RESULTS
Serum dextroamphetamine concentrations
Serum dextroamphetamine levels ∼2 hr after drug administration ranged from 24 to 51 ng/ml (mean 36.14 ng/ml). There was no significant difference in the five dextroamphetamine levels that were obtained at 12 min intervals (one-way ANOVA F(4,30) = 0.26, p = 0.90), suggesting that serum concentrations were stable across the four rCBF measurements.
Autonomic variables
There were no significant drug or task effects on pCO2. Dextroamphetamine caused a modest, nonsignificant increase in mean systolic blood pressure (from 104 mm/Hg at baseline to 114 mm/Hg ∼2 hr after administration). No significant alteration in pulse was observed (Table 1).
Table 1.
Autonomic variables
| At baseline | At 2 hr | Pairedt | p (two-tailed) | ||
|---|---|---|---|---|---|
| Systolic blood pressure mm/Hg (SD) | Placebo | 108.4 (11.7) | 107.5 (14.8) | 0.42 | 0.68 |
| Amphetamine | 103.4 (14.2) | 114.4 (16.3) | 2.00 | 0.09 | |
| Pulse (rate/min) (SD) | Placebo | 70 (13) | 67 (7) | 1.00 | 0.35 |
| Amphetamine | 66 (5) | 68 (11) | 0.57 | 0.60 |
Mood scales
Subjects reported significantly higher anxiety ratings on the Speilberger anxiety scale after administration of dextroamphetamine. On the POMS and Amphetamine Mood Rating scales, subjects reported feeling more happy and friendly while on dextroamphetamine (Table2).
Table 2.
Effect of amphetamine on mood scales
| Placebo | Amphetamine | Pairedt | p (two- tailed) | |
|---|---|---|---|---|
| Speilberger anxiety scalea | 42.00 | 45.90 | 3.11 | 0.02* |
| POMSb | ||||
| Tension–anxiety | 0.55 | 0.30 | 0.91 | 0.39 |
| Anxiety–hostility | 0.18 | 0.12 | 1.65 | 0.14 |
| Vigor | 1.48 | 2.00 | 1.46 | 0.18 |
| Depression | 0.16 | 0.16 | 0.00 | 1.00 |
| Fatigue | 1.23 | 0.78 | 1.01 | 0.34 |
| Confusion | 0.36 | 0.12 | 1.09 | 0.31 |
| Friendly | 2.18 | 2.60 | 2.43 | 0.05 |
| Amphetamine mood rating scale2_c | ||||
| Happy | 15.63 | 7.50 | 3.33 | 0.01* |
| Sad | 0.00 | −2.50 | 0.37 | 0.72 |
| Irritated | 15.00 | −1.25 | 1.76 | 0.12 |
| Friendly | −6.87 | 11.25 | 2.31 | 0.05* |
| Anxious | −6.87 | −7.50 | 1.45 | 0.19 |
| Energy | −4.37 | 0.62 | 0.44 | 0.67 |
| Focused | −3.37 | 4.37 | 0.78 | 0.46 |
| Sexual | −5.62 | −14.63 | 1.36 | 0.21 |
a,bScales obtained at 90–120 min after drug administration.
Means of difference scores of mood rating scales before and after drug administration (postdrug administration scales were obtained 90–120 min after drug administration).
Task performance
Dextroamphetamine produced an improvement in RPM performance (i.e., percent of correct responses). No significant improvement was seen in WCST performance (Table 3).
Table 3.
Effect of amphetamine on task performance
| Placebo mean (SD) | Amphetamine mean (SD) | Paired t | p value | |
|---|---|---|---|---|
| WCST | ||||
| Percent conceptual level3_a | 93.0 (6.7) | 93.4 (3.8) | 0.39 | 0.71 |
| Percent correct responses | 93.9 (5.4) | 94.3 (4.0) | 0.18 | 0.86 |
| Number of categories | 8.4 (1.4) | 8.6 (1.5) | 0.34 | 0.74 |
| Percent perseverative error | 4.0 (3.4) | 3.7 (3.2) | 0.66 | 0.53 |
| RPM | ||||
| Number completed | 27.5 (2.6) | 29.3 (2.8) | 1.51 | 0.18 |
| Percent correct responses | 78.5 (10.1) | 87.4 (7.2) | 2.61 | *0.035 |
Percent conceptual level: number of “conceptual level responses” divided by the total number of trials. “Conceptual level responses” are correct responses that occur consecutively in runs of three or more and probably reflect some insight into the correct sorting principle.
Global CBF
The two-way ANOVA examining Task × Drug interaction as well as main effects of drug and task on global rCBF was not significant. Similarly, the more liberal matched paired t tests revealed no significant task- or drug-dependent changes in global rCBF (Table 4).
Table 4.
Global rCBF* (ml/min/100 gm ± SEM) by task and by drug conditions*
| Placebo | Amphetamine | Paired t across drug | p across drug | |
|---|---|---|---|---|
| WCST | 42.8 (1.6) | 41.0 (1.6) | −1.48 | 0.18 |
| WCSC | 42.7 (1.4) | 41.3 (1.5) | −1.03 | 0.34 |
| RPM | 43.2 (1.3) | 43.4 (1.6) | 0.63 | 0.55 |
| RPMC | 41.2 (1.5) | 42.0 (1.3) | 0.49 | 0.64 |
*Two-way repeated-measures ANOVA (Drug × Task) was nonsignificant, F(3,18) = 1.16, p = 0.35; main effect of drug was nonsignificant,F(1,6) = 0.006, p = 0.94; main effect of task was nonsignificant, F(3,18) = 0.97, p = 0.43.
Regional CBF effects
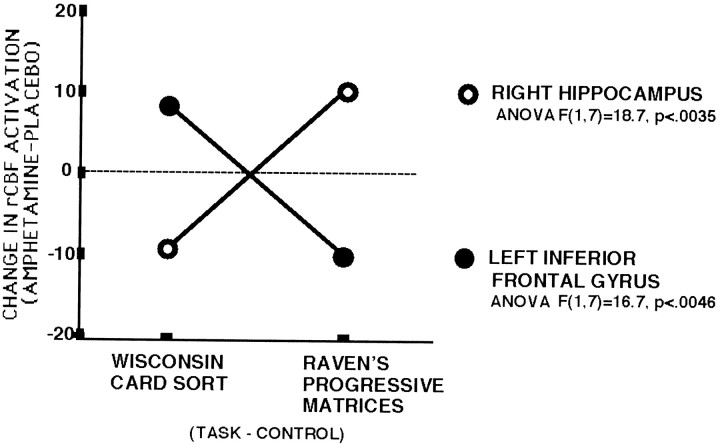
ANOVAs of the ROIs revealed that of the 32 brain regions tested, only 2 showed significant task by drug interactions. Consistent with previous studies, both tasks tended to increase rCBF in DLPFC during placebo. With dextroamphetamine, the rCBF increase in the DLPFC during the WCST was augmented, whereas during the RPM it was blunted (ANOVAF(1,7) = 16.7, p < 0.0046). In contrast, right hippocampal activation decreased during the WCST but increased during RPM (ANOVA F(1,7) = 18.71, p < 0.0035) after dextroamphetamine. A borderline significant Drug × Task interaction occurred in the right superior frontal gyrus. During placebo, this region, compared with its control task, was relatively deactivated during both WCST and RPM. With dextroamphetamine, activation in this area was increased during both tasks, but the magnitude of the change was greater during WCST (ANOVA F(1,7) = 5.42, p = 0.053) (Fig. 2).
Fig. 2.
Region-by-task interaction effect of dextroamphetamine. Double dissociations were seen (1) in the superior portion of the left inferior frontal gyrus, where rCBF increased during WCST but decreased during RPM, and (2) in the right hippocampal area, where rCBF increased during RPM but decreased during WCST.
DISCUSSION
The results of this study show that dextroamphetamine, although having no clear global CBF effects, induced cognitive-specific signal changes in selected cortical areas. During the WCST, there was increased “signal” relative to the control task (i.e., activation) in the superior portion of the left inferior frontal gyrus, a region shown in several other studies to be consistently activated by performance of this task (Berman et al., 1986, 1995; Weinberger et al., 1986, 1988; Rubin et al., 1991; Marenco et al., 1993; Catafau et al., 1994), and relatively decreased activation of the right hippocampus, an area not normally activated (Berman et al., 1995). In contrast, during RPM, amphetamine evoked an opposite pattern of regional changes, i.e., increased right hippocampal “signal” and decreased activation in the left inferior frontal gyrus. We will discuss these findings as they relate to four issues: (1) the regional specificity of the tasks; (2) the effects of monoamines on cortical activity; (3) the regionally specific neuromodulatory effect of monoamines; and (4) the regional and “task-specific” neuromodulatory effect of dextroamphetamine.
Regional specificity of the tasks
Although both WCST and RPM are thought to involve abstract reasoning and problem solving, they differ along a number of important dimensions that may explain the differences in the neural systems most crucial for the performance of each task. RPM, although generally accepted as a good indicator of general intelligence, involves considerably more visuospatial processing and computational problem solving than does the WCST. Additionally, although WCST trial processing occurs over very short periods of time (2–3 sec), RPM trials occur over considerably longer durations, suggesting that RPM might also differ from the WCST by requiring more long-term mnemonic processes. Animal studies suggest that working memory tasks with short delays analogous to the WCST may be mediated by DLPFC (Goldman-Rakic and Rosvold, 1970), whereas tasks with longer delays also may require mediation by the hippocampus (Zola-Morgan and Squire, 1985). Milner (1963, 1964) has shown that the WCST is a sensitive indicator of the integrity of the DLPFC, and patients with frontal lobe pathology do poorly on this task. In contrast, although patients with postrolandic lesions do poorly on the RPM (Basso et al., 1973), there is no evidence that patients with prefrontal lesions have particular difficulty with it.
In a study on normal subjects and patients with schizophrenia, a disease process wherein dysfunction of the prefrontal cortex has been implicated (Weinberger et al., 1986), Berman et al. (1988) demonstrated that normal subjects did not activate DLPFC while performing RPM to the degree that they did during the WCST (Weinberger et al., 1986). In addition, they demonstrated that schizophrenic patients, like normal subjects, had maximal rCBF elevations posteriorly with no significant DLPFC deficit while performing the RPM. Recently, Ostrem et al. (1993), while demonstrating common areas of activation for the two tasks, i.e., the superior portion of the inferior frontal gyrus (Brodman areas 9 and 46), the occipital lobe, and inferior parietal lobule (areas 7 and 40), found that DLPFC activation was greater during the WCST. They also demonstrated differential activation of the hippocampus. Hippocampal activation increased during RPM but showed a relative decrease during WCST. From these studies, it can be inferred that WCST and RPM have different evoked neural patterns and that DLPFC probably is more critical for WCST performance and hippocampus for RPM performance. Our present data suggest that amphetamine exaggerates these specific neurofunctional differences between the tasks by enhancing the neural activation signals in the regions that are differentially most critical for the various cognitive operations involved.
Effects of monoamines on cortical activity
A diverse range of findings have been reported on the effects of administration of amphetamine, a nonspecific monoamine agonist, on cortical activity in animals and humans. Although some animal studies (Nahorski and Rogers, 1973; Carlsson et al., 1975; Berntman et al., 1976, 1978; McCulloch and Harper, 1977; Neuser and Hoffmeister, 1977;Wechsler et al., 1979; Porrino et al., 1983) reveal diffuse increases in cerebral blood flow and glucose metabolism after acute parenteral administration in the resting state, other reports using sensory and/or behavioral activation paradigms suggest that dopamine and norepinephrine suppress spontaneous neural firing while specifically enhancing the capacity of neural systems to increase activity focally in response to a specific stimulus or task (Foote et al., 1975; Johnson et al., 1983; Sawaguchi, 1987). These studies along with other animal studies (Bunney et al., 1987; Robins and Everitt, 1987) suggest that catecholamines modulate the ratio of neurofunctional STN. Our present data are consistent with these observations: during each of the two task paradigms, cortical activity increased in those areas most critical for the task (i.e., increased physiological “signal”), but decreased in areas that may be less critical (i.e., decreased “noise”).
Regionally specific neuromodulatory effects of monoamines
There is evidence supporting the regionally specific neuromodulatory role of the different monoamines. Selective dopaminergic neurotransmission in the DLPFC has been shown to be important in carrying out tasks involving working memory (Sawaguchi and Goldman-Rakic, 1991; Williams and Goldman-Rakic, 1995). In a like manner, there are electrophysiological studies (Glowinski et al., 1984;Mantz et al., 1988; Mogenson and Yim, 1991) and imaging studies that support the neuromodulatory role of dopamine at this site. Daniel et al. (1989) demonstrated that the dopamine receptor agonist apomorphine augmented relative rCBF in schizophrenic patients while they performed the WCST. Friston et al. (1992) reported that in normal subjects during performance of a verbal memory task, apomorphine attenuated rCBF increases in DLPFC and augmented rCBF in the posterior cingulate. Kapur et al. (1994) described similar findings with apomorphine. More recently, Dolan et al. (1995) demonstrated that cognitive task-related activation of the anterior cingulate cortex in schizophrenic patients can be modulated by apomorphine. Despite the focus on dopamine, norepinephrine appears to have as important an influence on prefrontal cortex function. Li and Mei (1994) have shown that infusion of yohimbine, an α2 antagonist, into the DLPFC markedly impairs delayed-response performance in a delay-dependent manner, paralleling the effects seen by Sawaguchi and Goldman-Rakic with dopamine (1991).
Similarly, monoamine agonists have been shown to have neuromodulatory effects in the hippocampal region. Segal and Bloom (1976a) demonstrated the facilitating action of dextroamphetamine in inhibiting spontaneous cellular discharges in the hippocampus, presumably attributable to norepinephrine agonism. Using buspirone, an anxiolytic with predominantly 5-HT1A agonistic effects, Coop and McNaugghton (1991)demonstrated that reduction in hippocampal rhythmical slow activity in rats was mediated by 5-HT1A receptors and not by D2 receptors. Similarly, 5-HT agonists modulate CA1 pyramidal cell firing patterns (Sprouse and Aghajanian, 1988). Likewise, Friston et al. (1992)reported that in normal subjects during performance of a verbal memory task, buspirone attenuated blood flow increases in the retrosplenial region. Monoaminergic neuromodulatory effects thus can be differentiated at two discrete brain areas (DLPFC and hippocampus) that are implicated in the functional anatomy of memory, and also highlighted in the results of this study. Our data are consistent with the interpretation that the effects of dopamine on prefrontal working memory play a major role during the WCST, whereas 5-HT affects hippocampal function during RPM, but we cannot exclude the possibility that both regional findings may result from dopaminergic and/or noradrenergic effects.
Anatomic and “task-specific” neuromodulatory effect of dextroamphetamine
The effect of amphetamine on monoaminergic activity is nonspecific, including release of dopamine, norepinephrine, and 5-HT from storage sites in nerve terminals (Weiner, 1972, 1980; Moore, 1978;Creese, 1983; Kuczenski, 1983, 1989; Glennon et al., 1987). Although the d-isomer of amphetamine, dextroamphetamine, is relatively more selective in enhancing dopaminergic activity, effects on other monoamines such as 5-HT and norepinephrine also have been observed (Bonhomme et al., 1995; West et al., 1995). Thus, the rCBF effects in this study, although likely related primarily to dopaminergic activity, also may involve an interplay of different monoamines.
Our results, although supporting the notion that performance of the two abstract reasoning neuropsychological tasks used in this study is dependent on different neural systems subserving different aspects of memory, also support the notion that dextroamphetamine has the capacity to differentially modulate these neural systems. The hippocampal region has been implicated in tasks requiring spatial memory (Parkinson et al., 1988; Rolls, 1991; O’Keefe, 1993) and working memory that involve long processing times (Goldman-Rakic and Friedman, 1988) and is activated by RPM. Similarly, there is converging evidence supporting the role of DLPFC for the performance of the WCST (Milner, 1963, 1964;Berman et al., 1995). Dextroamphetamine, presumably through its effects on monoaminergic neurotransmission, appears to selectively enhance the signal in the hippocampal region during performance of RPM and in the DLPFC during WCST.
It should be noted that these task-based, regionally specific effects of dextroamphetamine on rCBF were accompanied by a statistically significant improvement in the performance of RPM (increase in percent of correct responses). In contrast, no significant difference was noted in performance of the WCST. This lack of improvement in performance during the WCST may be because (1) the subjects were made to practice the tasks before scanning sessions to preclude any learning effect, and (2) it is likely that there was a ceiling effect on the performance of the WCST, the simpler task. In contrast, for RPM, because separate sets of matrices were used for the practice and scanning sessions, there was no ceiling effect, leaving room for improvement.
Our data suggest that dextroamphetamine, rather than having a fixed pharmacological effect on rCBF patterns, enhances the specific neural systems called on for optimal performance of a certain task. During cognitive tasks that have regionally different activation patterns, dextroamphetamine enhances the distinctiveness of the individual patterns and accentuates their differences. These neurophysiological effects of dextroamphetamine may explain its positive impact on cognitive efficiency.
Footnotes
We gratefully acknowledge Dr. Terry Goldberg for helpful suggestions on the study design and mood scales.
Correspondence should be addressed to Dr. Daniel R. Weinberger, Clinical Brain Disorders Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health Neuroscience Center at Saint Elizabeth’s, 2700 Martin Luther King Avenue, SE, Washington, DC 20032.
REFERENCES
- 1.Angrist B, Corwin J, Bartlett B, Cooper F. Early pharmacokinetics and clinical effects of oral d -amphetamine in normal subjects. Biol Psychiatry. 1987;22:1357–1368. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten A, Goldman-Rakic P. Stress impairs prefrontal cortex cognitive function in monkeys: role of dopamine. Soc Neurosci Abstr. 1990;16:164. [Google Scholar]
- 3.Basso A, DeRenzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain. 1973;96:715–728. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- 4.Bauer RH, Fuster JM. Effects of d -amphetamine and prefrontal cortical cooling on delayed matching-to-sample behavior. Pharmacol Biochem Behav. 1978;8:243–249. doi: 10.1016/0091-3057(78)90311-8. [DOI] [PubMed] [Google Scholar]
- 5.Berman KF, Zec RF, Weinberger DR. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- 6.Berman KF, Illowsky BP, Weinberger DR. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 1988;45:616–622. doi: 10.1001/archpsyc.1988.01800310020002. [DOI] [PubMed] [Google Scholar]
- 7.Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 8.Berntman L, Carlsson C, Hagerdal N, Siesjo BK. Excessive increase in oxygen uptake and blood flow in the brain during amphetamine intoxication. Acta Physiol Scand. 1976;97:264–266. doi: 10.1111/j.1748-1716.1976.tb10261.x. [DOI] [PubMed] [Google Scholar]
- 9.Berntman L, Carlsson C, Hagerdal N, Siesjo BK. Circulatory and metabolic effects in the brain induced by amphetamine sulfate. Acta Physiol Scand. 1978;102:310–323. doi: 10.1111/j.1748-1716.1978.tb06078.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonhomme N, Cador M, Stinus L, Moal M, Spampinato U. Short- and long-term changes in dopamine and serotonin receptor binding sites in amphetamine-sensitized rats: a quantitative autoradiographic study. Brain Res. 1995;675:215–223. doi: 10.1016/0006-8993(95)00067-z. [DOI] [PubMed] [Google Scholar]
- 11.Brozoski TJ, Brown RN, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex in rhesus monkey. Science. 1979;31:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 12.Bunney BS, Sesak SR, Silver NL. Mid brain dopaminergic systems: neurophysiology and electrophysiological pharmacology. In: Meltzer HY, editor. Psycho- pharmacology: the third generation of progress. Raven; New York: 1987. pp. 113–126. [Google Scholar]
- 13.Carlsson C, Hagerdal N, Siesjo BK. Influence of amphetamine sulfate on cerebral blood flow and metabolism. Acta Physiol Scand. 1975;94:128–129. doi: 10.1111/j.1748-1716.1975.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 14.Catafau AM, Parellada E, Lomena FJ, Bernardo M, Pavia J, Ros D, Setoain J, Gonzalez-Monclus E. Prefrontal and temporal blood flow in schizophrenia: resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. J Nuclear Med. 1994;35:935–941. [PubMed] [Google Scholar]
- 15.Coop CF, McNaugghton N. Buspirone affects hippocampal rhythmical slow activity through serotonin 1A rather than D2 receptors. Neuroscience. 1991;40:169–174. doi: 10.1016/0306-4522(91)90182-n. [DOI] [PubMed] [Google Scholar]
- 16.Creese I. Raven; New York: 1983. Stimulants: neurochemical, behavioral, and clinical perspectives. . [Google Scholar]
- 17.Daniel DG, Berman KF, Weinberger DR. The effect of apomorphine on regional cerebral blood flow in schizophrenia. J Neuropsychiatry Clin Neurosci. 1989;1:377–384. doi: 10.1176/jnp.1.4.377. [DOI] [PubMed] [Google Scholar]
- 18.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daube-Witherspoon ME, Chon KS, Green SL, Carson RE, Herscovitch P. Factors affecting dispersion correction for continuous blood withdrawal and counting systems. J Nuclear Med. 1992;33:1010. [Google Scholar]
- 20.Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RSJ, Grasby PM. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180–182. doi: 10.1038/378180a0. [DOI] [PubMed] [Google Scholar]
- 21.Foote SL, Freedman R, Oliver PA. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- 22.Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Grasby PM, Bench CJ, Frith CD, Cowen PJ, Liddle PF, Frackowiak RSJ, Dolan RJ. Measuring the neuromodulatory effects of drugs in man with positron emission tomography. Neurosci Lett. 1992;141:106–110. doi: 10.1016/0304-3940(92)90345-8. [DOI] [PubMed] [Google Scholar]
- 24.Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- 25.Glowinski J, Tassin JP, Thierry AM. The mesocortico-prefrontal dopaminergic neurons. Trends Neurosci. 1984;7:415–418. [Google Scholar]
- 26.Goldberg TE, Bigelow LB, Kleinman JE, Daniel DG, Weinberger DR. Effects of the coadministration of amphetamine and haloperidol on the affect, motor behavior, and cognition of patients with schizophrenia. Am J Psychiatry. 1991;148:78–84. doi: 10.1176/ajp.148.1.78. [DOI] [PubMed] [Google Scholar]
- 27.Goldman-Rakic PS, Friedman HR. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8:4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman-Rakic PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- 29.Haier RJ, Siegel B, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–426. [Google Scholar]
- 30.Heaton RK, Cheloune GJ, Tally JL, Kay GG, Curtiss G (1993) Wisconsin Card Sorting Test manual revised and expanded. Odessa, FL: Psychological Assessment Resources.
- 31.Jaing H, Holten K, Robb R. Image registration of multimodality 3D images by Chamfer matching, biomedical image processing and 3 dimensional microscopy. SPIE. 1992;1660:356–366. [Google Scholar]
- 32.Johnson SW, Palmer MR, Freedman R. Effects of dopamine on spontaneous and evoked activity of caudate neurons. Neuropharmacology. 1983;22:843–851. doi: 10.1016/0028-3908(83)90130-2. [DOI] [PubMed] [Google Scholar]
- 33.Kapur S, Meyer J, Wilson A, Houle S, Brown GM. Modulation of cortical neuronal activity by a serotonergic agent: a PET study in humans. Brain Res. 1994;646:292–294. doi: 10.1016/0006-8993(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 34.Koeppe RA, Holden JE, Ip WR. Performance comparison of parameter estimation techniques for the quantitation of local cerebral blood flow by dynamic positron computed tomography. J Cereb Blood Flow Metab. 1985;5:224–234. doi: 10.1038/jcbfm.1985.29. [DOI] [PubMed] [Google Scholar]
- 35.Kuczenski R. Biochemical actions of amphetamine and other stimulants. In: Creese I, editor. Stimulants: neurochemical, behavioral, and clinical perspectives. Raven; New York: 1983. pp. 31–61. [Google Scholar]
- 36.Kuczenski R. Concomitant characterization of behavioral and striatal monoamine response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 38.Mantz J, Milla C, Glowinski J, Thierry AM. Differential effects of ascending neurons containing dopamine and noradrenaline in the control of spontaneous activity and of evoked responses in the rat prefrontal cortex. Neuroscience. 1988;27:517–526. doi: 10.1016/0306-4522(88)90285-0. [DOI] [PubMed] [Google Scholar]
- 39.Marenco SR, Coppola R, Daniel DG, Zigun JR, Weinberger DR. Regional cerebral blood flow during the Wisconsin Card Sorting Test in normal subjects studied by xenon-133 dynamic SPECT: comparison of absolute values, percent distribution values, and covariance analysis. Psychiatry Res Neuroimag. 1993;50:177–192. doi: 10.1016/0925-4927(93)90029-h. [DOI] [PubMed] [Google Scholar]
- 40.McCulloch J, Harper AN. Cerebral circulatory and metabolism changes following amphetamine administration. Brain Res. 1977;121:196–199. doi: 10.1016/0006-8993(77)90452-8. [DOI] [PubMed] [Google Scholar]
- 41.McNair DM, Lorr M, Droppleman LF (1992) Profile of mood states. San Diego: Educational and Industrial Testing Service.
- 42.Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:100–110. [Google Scholar]
- 43.Milner B. McGraw-Hill; New York: 1964. Some effects of frontal lobotomy in man. . [Google Scholar]
- 44.Milner B, Petrides M. Behavioral effects of frontal lobe lesions in man. Trends Neurosci. 1984;7:403–407. [Google Scholar]
- 45.Mogenson GJ, Yim CC. Neuromodulatory functions of the mesolimbic dopamine system: electrophysiological and behavioral studies. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system. Wiley; New York: 1991. pp. 105–130. [Google Scholar]
- 46.Moore KE (1978) Amphetamines: biochemical and behavioral actions in animals. In: Handbook of psychopharmacology, Vol 11, Stimulants (Iversen LL, Iversen SD, Synder SH, eds). New York: Plenum.
- 47.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahorski SR, Rogers KF. In vivo effects of amphetamine metabolites in metabolic rate in the brain. J Neurochem. 1973;21:667–686. doi: 10.1111/j.1471-4159.1973.tb06012.x. [DOI] [PubMed] [Google Scholar]
- 49.Neuser V, Hoffmeister F. The influence of psychotropic drugs on the local cerebral glucose utilization of the rat brain. In: Ingvar DH, Lason NA, editors. CBF VIII—cerebral function, metabolism and circulation. Munksgaard; Copenhagen: 1977. pp. 102–103. [PubMed] [Google Scholar]
- 50.O’Keefe J. Hippocampus, theta, and spatial memory. Curr Opin Neurobiol. 1993;3:917–924. doi: 10.1016/0959-4388(93)90163-s. [DOI] [PubMed] [Google Scholar]
- 51.Ostrem JL, Berman KF, Mattay VS, Weinberger DR. Cerebral activation during problem solving and abstract reasoning as demonstrated with PET: a neural network subserving intelligence. Soc Neurosci Abstr. 1993;19:792. [Google Scholar]
- 52.Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J Neurosci. 1988;8:4059–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porrino LJ, Lucignani G, Dow-Edwards D, Sokoloff L. Different anatomical substrates for amphetamine-induced stereotype and locomotion demonstration by measurements of local rates of glucose utilization. J Cereb Blood Flow Metab. 1983;3:S210–S211. [Google Scholar]
- 54.Raven JC. HK Lewis; London: 1938. Progressive matrices. . [Google Scholar]
- 55.Robins TW, Everitt VJ. Psychopharmacologic studies of arousal and attention. In: Stahl SM, Iversen SD, Goodman EC, editors. Cognitive neurochemistry. Oxford UP; Oxford: 1987. pp. 135–170. [Google Scholar]
- 56.Rolls ET. Functions of the primate hippocampus in spatial and nonspatial memory. Hippocampus. 1991;1:258–261. doi: 10.1002/hipo.450010310. [DOI] [PubMed] [Google Scholar]
- 57.Rubin P, Holm S, Friberg L, Videbech P, Andersen HS, Bendsen BB, Stromso N, Larsen JK, Lassen NA, Hemmingsen R. Altered modulation of prefrontal and subcortical brain activity in novel diagnosed schizophrenia and schizophreniform disorder: a regional cerebral blood flow study. Arch Gen Psychiatry. 1991;48:987–995. doi: 10.1001/archpsyc.1991.01810350027004. [DOI] [PubMed] [Google Scholar]
- 58.Sawaguchi T. Catecholamine sensitivities of neurons related to a visual reaction time task in the monkey prefrontal cortex. J Neurophysiol. 1987;58:1100–1122. doi: 10.1152/jn.1987.58.5.1100. [DOI] [PubMed] [Google Scholar]
- 59.Sawaguchi T, Goldman-Rakic PS. D dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 60.Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. III. Hippocampal cellular responses to locus coeruleus stimulation in the awake rat. Brain Res. 1976a;107:499–511. doi: 10.1016/0006-8993(76)90140-2. [DOI] [PubMed] [Google Scholar]
- 61.Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. IV. The effects of locus coeruleus stimulation on evoked hippocampal unit activity. Brain Res. 1976b;107:513–525. doi: 10.1016/0006-8993(76)90141-4. [DOI] [PubMed] [Google Scholar]
- 62.Speilberger CD. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the state-trait anxiety. [Google Scholar]
- 63.Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- 64.Wechsler LR, Savaki HE, Sokoloff L. Effects of d - and l -amphetamine on local cerebral glucose utilization in the conscious rat. J Neurochem. 1979;32:15–22. doi: 10.1111/j.1471-4159.1979.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 65.Weinberger DR, Berman KF, Illowsky B. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 66.Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow (rCBF) evidence. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 67.Weiner N. Pharmacology of central nervous system stimulants. In: Zarafonetis CJD, editor. Drug abuse: proceedings of the international conference. Lea and Febiger; Philadelphia: 1972. pp. 243–251. [Google Scholar]
- 68.Weiner N. Norepinephrine, epinephrine, and the sympathomimetic amines. In: Goodman AG, Gilman A, editors. The pharmacological basis of therapeutics. Macmillan; New York: 1980. p. 161. [Google Scholar]
- 69.West WB, Van Groll BJ, Appel JB. Stimulus effects of d -amphetamine. II. DA, NE, and 5-HT mechanisms. Pharmacol Biochem Behav. 1995;51:69–76. doi: 10.1016/0091-3057(94)00361-l. [DOI] [PubMed] [Google Scholar]
- 70.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 71.Woodard DJ, Moises HC, Waterhouse BD, Hoffer BJ, Freedman R. Modulatory actions of norepinephrine in the central nervous system. Fed Proc. 1979;38:2109–2116. [PubMed] [Google Scholar]
- 72.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]