Abstract
The effects of serotonin (5-HT) and GABA on two Ca2+ currents, a transient low-voltage-activated current (tLVA) and a sustained high-voltage-activated current (sHVA) were examined in isolated photoreceptors of Hermissenda. The sHVA current was blocked by 5-HT and reduced by activation of protein kinase C (PKC) with phorbol 12-myristate 13-acetate. The effects of 5-HT were transiently reversed by staurosporine and partially blocked by the PKC inhibitor peptide [PKC(19–36)]. GABA enhanced both the tLVA and sHVA currents at low concentrations (5 nm to 5 μm) and reduced the sHVA current at high concentrations (>10 μm). The GABA-mediated enhancement of the Ca2+ current at low concentrations was sensitive to block by picrotoxin. The protein kinase A (PKA) inhibitor peptide [PKI(6–22)amide] blocked enhancement of both Ca2+ currents produced by cAMP analogs and GABA, suggesting that the effects at low concentrations may be PKA mediated. Caged GTP-γ-S released by flash photolysis reduced the sHVA current, and pretreatment of the photoreceptors with pertussis toxin blocked the effects of higher concentrations of GABA, indicating that at higher concentrations, the effects may be G-protein mediated.
Keywords: calcium current, γ-aminobutyric acid, neuromodulation, cellular plasticity, Hermissenda, serotonin
Neural networks can perform diverse functions by the combined actions of classical transmitters and neuromodulators (for review, see Harris-Warrick and Marder, 1991). Modulatory inputs may produce diverse activity patterns of neural networks by activating different second messengers that affect several membrane conductances. The photoreceptors of Hermissenda crassicornis are sites not only for the process of phototransduction but also for Ca2+-dependent neuronal plasticity (Alkon and Rasmusson, 1987; Crow, 1988). Numerous studies have provided evidence that Ca2+, neurotransmitters/neuromodulators and second messengers contribute to enhanced excitability of identified type B photoreceptors observed after classical conditioning (Alkon, 1984; Farley and Auerbach, 1986; Crow et al., 1991; Falk-Vairant and Crow, 1992; Matzel and Alkon, 1991; Matzel and Rogers, 1993). Voltage-clamp studies of the B photoreceptors in conditioned animals have revealed a reduction in the amplitude of two K+ currents, the transient (IA) and Ca2+-activated K+(IK,Ca) currents (Alkon et al., 1985;Farley, 1988). Serotonin (5-HT) produces reductions ofIA and IK,Cathat are similar to changes found in conditioned animals (Farley and Wu, 1989; Acosta-Urquidi and Crow, 1993). The reduction ofIA and IK,Ca by 5-HT may be mediated by the activation of protein kinase C (PKC) (Farley and Auerbach, 1986). In addition, GABA paired with depolarization of the photoreceptors produces enhanced excitability of the B photoreceptors (Matzel and Alkon, 1991; Alkon et al., 1993). Although the mechanism for the contribution of GABA to enhanced excitability in type B photoreceptors is unknown, recent evidence suggests that GABA increases intracellular Ca2+at the synaptic terminals of the photoreceptors (Alkon et al., 1993).
To further examine modulation of membrane currents inHermissenda, we studied the effects of GABA and 5-HT on two recently characterized Ca2+ currents in the type A and type B photoreceptors, a transient low-voltage-activated (tLVA) current and a sustained high-voltage-activated (sHVA) current (Yamoah and Crow, 1994a), and provide evidence for the contribution of second messengers to the modulation. At low concentrations (≤5 μm), GABA increased the tLVA and sHVA Ca2+ currents, whereas at high concentrations (>10 μm), GABA blocked the sHVA current. The sHVA Ca2+ current was reduced by 5-HT. cAMP analogs enhanced both the tLVA and sHVA Ca2+currents. The effects of GABA and cAMP were blocked with inhibitors of protein kinase A (PKA). High concentrations of GABA may produce effects that are mediated by a G-protein, as shown by the reduction in the sHVA current by release of caged GTP-γ-S and pretreatment with pertussis toxin (PTX). Activators of PKC reduced the sHVA Ca2+ current; however, the time course of the effect was delayed (>10 min) compared with the effects of 5-HT (<5 min) on Ca2+ currents. These results suggest that the effect of 5-HT on Ca2+ currents may involve PKC, a direct action on the channels, or an as yet unidentified second messenger. A preliminary report of these results has been presented (Yamoah and Crow, 1994b).
MATERIALS AND METHODS
Cell preparation. Adult Hermissenda were obtained from Sea Life Supply (Sand City, CA). The photoreceptors were isolated using the protocol outlined in Yamoah and Crow (1994a). Briefly, the nervous systems were dissected from the animal and incubated for 10 min at 4°C in 1.0 mg/ml protease type VIII (Sigma, St. Louis, MO) and 7 mg/ml dispase grade II (Boehringer Mannheim, Mannheim, Germany) in artificial seawater (ASW) composed of (in mm): 420 NaCl, 10 KCl, 10 CaCl2, 22.9 MgCl2, 25.5 MgSO4, 15 HEPES (free acid) at pH of 7.8 (NaOH). The initial enzyme treatment allows slow diffusion into the interstitial space of the nervous system. The nervous systems were transferred to room temperature (21°C) for 10 min and then incubated at 4°C for 20–30 min in fresh ASW. This procedure not only digested the connective tissue and glia around the photoreceptors, but also loosened the capsule surrounding them. The eyes were pinched off from the nervous system, desheathed in 35 mm sterile culture dishes, and placed under an inverted microscope. Photoreceptors were identified as type A or type B on the basis of their position relative to the lens and the optic nerve. Isolated eyes without a lens and the stump of the optic nerve were therefore discarded. Desheathed photoreceptors were isolated from the eyes using mechanical agitation and a fire-polished pipette. The average yield for this procedure was two photoreceptors per eye. Isolated cells were incubated in ASW with 10 mm glucose and 50 mg/ml gentamicin sulfate (Sigma) at 4°C before electrophysiological experiments were performed.
Unless indicated, all chemicals were obtained from Sigma. A stock solution of 5 mm GABA was made with bath solution and stored at −20°C. Aliquots of the stock solution were added to bath solutions to achieve a desired concentration (5 nm–1000 μm) and perfused into the experimental chamber (0.8–1.0 ml) at a rate of 1.0–1.5 ml/min. A solution of 10 mm of 5-hydroxytryptamine creatinine sulfate complex (5-HT) was prepared and applied as that described for GABA. Stocks solutions of 1 mm staurosporine (Calbiochem, La Jolla, CA), 5 mm isobutylmethylxanthine (IBMX) (Calbiochem), membrane-permeable analogs of 5 mm cAMP, 8-(4-chlorophenylthio)-cAMP, and 5 mmcAMP-N6,O2′-dibutyryl (Calbiochem) were made in 100% dimethylsulfoxide (DMSO, Fisher Scientific, Fair Lawn, NJ). These solutions were applied to the bath to give final concentrations from the nanomolar to micromolar range, depending on the agent. The final concentration of DMSO in the bath was less than 0.2%. Stock solutions of synthetic peptide inhibitors of PKA [PKAI(6–22)amide] and PKC [PKC(19–36)] (Gibco, Grand Island, NY) were reconstituted in distilled water (500 μm) and stored at −20°C. Aliquots of these solutions were added to the pipette solution to make a final concentration of 5–20 μm in the peptide. All experiments with peptide inhibitors were performed within 48 hr of reconstitution of the stock solution. PTX and guanosine 5′-O-(2-thiodiphosphate) trilithium salt (GDP-β-S) and guanosine 5′-O-(3-thiophosphate) tetralithium salt, (GTP-γ-S) were obtained from Sigma and Calbiochem. “Caged” GTP-γ-S was obtained from Molecular Probes (Eugene, OR).
Recording solutions. Bath solutions were made from (in mm): 400 tetraethylammonium (TEA) acetate, 10 CaCl2, 50 MgS04, 5 4-aminopyridine (4-AP), 15 HEPES (free acid), pH 7.7, with TEA-hydroxide and CsOH (TEA/OH). The pipette solution was made of (in mm): 20 NaCl, 2 MgCl2, 10 EGTA, 20 TEACl, 300 CsCl, 300 N-methyl-d-glucamine (NMG), 10 glutathione (reduced), 5 Mg(ATP), 1 Na2(GTP), 40–50 HEPES, pH 7.4 (TEA-OH). Nystatin (0.1 μm) was prepared in 100% methanol. Osmolarity of all solutions ranged from 0.96 to 1.00 Osm. The pipette solution was stored in 15 ml aliquots at −20°C, and fresh pipette solutions were used daily.
Voltage clamp. Ca2+ currents were recorded with a standard patch-clamp technique at the whole-cell configuration (Hamill et al., 1981) using the Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA). Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) with a Flaming Brown micropipette puller, model P80/PC (Sutter Instruments, San Rafael, CA). The tips of the pipettes were fire polished and coated with SYLGARD #184 (Dow Corning, Midland, MI). Pipettes had a final resistance of 0.7–1.6 MΩ. Nystatin patches were established by filling the tips of the pipettes with the nystatin solution and backfilling with the pipette solution. Seals were established by the application of negative pressure at the end of the tubing connected to the pipette holder. Seal resistance ranged from 0.8 to 1.2 GΩ. Series resistance was 3.3 ± 1.2 MΩ (n = 29) and 7.8 ± 2.6 MΩ (n = 6) for nystatin patches. Series resistance was minimized with a compensatory circuit. Current records were filtered at 5 kHz and digitized at a frequency of 10 kHz with a Digidata interface (Axon Instruments). Ca2+ currents were evoked with command pulses from a personal computer (Gateway, Sioux City, SD) at a sample rate of 250 μsec/point using PCLAMP software version 5.7.1 and an A/D converter (Digidata, Axon Instruments). Passive leakage current was subtracted on-line with a mean of four hyperpolarizing pulses one fourth the size of the test potential. Photolysis was achieved by a Chadwick Helmuth Strobex Model 278 xenon arc flash lamp (230 J maximum output). The light was collected with an ellipsoidal mirror and the spectrum reflected by a dichroic mirror (Acton Research, Acton, MA) onto the aperture of a liquid light guide (Oriel, Stratford, CT). Aperture output of the liquid light guide was focused onto the cell using a plano-convex condenser lens and the microscope objective (Nikon Fluor 20×, M.A. 0.4). Wavelength selection was enhanced between the condenser and the preparation by reflecting the UV light off a second dichroic mirror centered at ∼350 mm. Electromagnetic impulse-dependent current artifact lasted a few milliseconds.
Data analysis. Analysis of the current traces was made with CLAMPFIT software (Axon Instruments). The criteria used for acceptance of current records were similar to that outlined in Yamoah and Crow (1994a). (1) Series resistance (except for nystatin-containing electrodes) and seal resistance were <4.5 MΩ and >0.6 GΩ, respectively. (2) Active current traces did not exhibit “pumps.” (3) The current–voltage relation had a negative peak as the step potentials approached the apparent reversal potential of Ca2+. Statistics were computed with SigmaPlot software (Jandel Scientific, San Rafael, CA). Descriptive data are presented as means ± SD. Statistical differences within groups were determined using t tests for correlated means, and between-group differences consisted of t tests for independent groups. Two-tailed tests were used in the statistical analysis unless otherwise indicated.
RESULTS
Effect of 5-HT on ICa
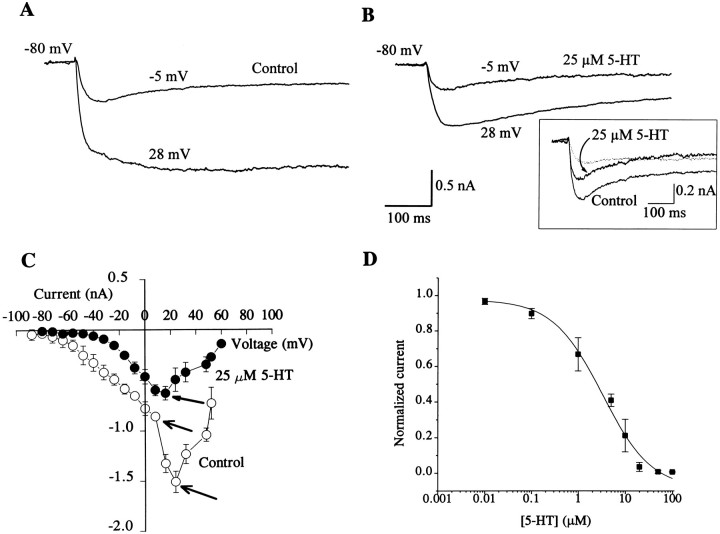
Calcium currents and their modulation by 5-HT and GABA were studied in both type A and type B photoreceptors by blocking the outward currents IA,IK,V, and IK,Cawith 4-AP, TEA, and Cs+. Inward Na+ current was blocked by choline substitution of Na+, and the inward rectifier current was blocked by Cs+. Two components of Ca2+ currents, a tLVA and an sHVA, have been identified previously in the photoreceptors (Yamoah and Crow, 1994a,b). Type A and type B photoreceptors were identified on the basis of their anatomical positions in the eyes. Isolated cells near the lens were classified as type A, and cells in the posterior part of the eye near the stump of the optic nerve were classified as type B. Further classification of A and B photoreceptors as medial or lateral was not possible with these isolation procedures. The mean peak Ca2+ current recorded from type A and type B photoreceptors was not statistically different (type A: = −1.1 ± 0.41 nA, n = 16; type B: = −1.2 ± 0.41 nA, n = 15; t29 = 0.66, NS). The sHVA Ca2+ current recorded from both type A and type B photoreceptors was reduced by the application of 5-HT. An example of the tLVA and sHVA Ca2+ currents recorded from a type A cell is shown in Figure 1A. The whole-cell currents were elicited from a holding potential of −80 mV. With EGTA, ATP, and GTP in the patch pipette, the Ca2+ currents remained stable for at least 60 min after establishing the whole-cell configuration. As shown in Figure1B, the application of 25 μm 5-HT blocked the sHVA current and had little, if any, effect on the tLVA current (see Fig. 1C). The magnitude of the reduction in the sHVA current in both type A and type B cells by 5-HT was similar (type A control: = −1.1 ± 0.4 nA; 5-HT: = −0.59 ± 0.3 nA; n = 15;t14 = 8.8; p < 0.0001) (type B control: = −1.2 ± 0.4 nA; 5-HT: = 0.65 ± 0.3 nA; n = 15;t14 = 10.9; p < 0.0001). The time course of the effect of 5-HT on the sHVA current (<5 min) was faster than the time course of current rundown (>60 min). The current–voltage relationship shown in Figure 1C revealed two apparent peaks (∼5 and 30 mV) measured under control conditions (open circles) before the application of 5-HT. However, in the presence of 25 μm 5-HT, only one peak was expressed at 5–10 mV (filled circles, Fig. 1C). At holding potentials greater than or equal to −30 mV, at which the sHVA Ca2+ current was predominantly activated, 5-HT reduced the magnitude of the current without altering the inactivation kinetics. The effects of 5-HT were reversible within 5–7 min after washout (see Fig. 2C). Figure 1Dillustrates the dose–response relationship for the effects of 5-HT on the sHVA Ca2+ current. The half-blocking concentration of 5-HT on the sHVA Ca2+ current was 2.4 μm. The concentrations of 5-HT applied to isolated photoreceptors were lower than that reported previously for isolated nervous systems (Farley and Wu, 1989). Presumably higher concentrations are required for intact isolated nervous systems to overcome diffusional barriers and possible 5-HT uptake by both neurons and glial cells.
Fig. 1.
Effect of 5-HT on the sHVA Ca2+ current. Traces were recorded from a type A cell. Superimposed Ca2+ currents in Aand B were elicited from a holding potential of −80 mV to step depolarizations of −5 and 28 mV. A, Two Ca2+ currents were recorded: a tLVA current and an sHVA current. B, The sHVA Ca2+ current was blocked by 5-HT (25 μm). The inset consists of superimposed current traces elicited at −5 mV for control and after the application of 5-HT (25 μm). The difference current that represents the current blocked by 5-HT is shown by thedotted line. The current that was blocked by 5-HT was predominantly the sustained component of the Ca2+current. C, Current–voltage plot of Ca2+ current before (open circles) (n = 7; data were from 3 type A and 4 type B cells) and after the application of 5-HT (filled circles) (n = 7). Currents were corrected for passive leakage current, and the peak current was plotted for each step potential. The control plot had two apparent peaks (open arrows). The application of 5-HT (25 μm) reduced the current, resulting in anI–V plot showing a single peak (closed arrow). D, Dose–response relationship. Normalized current was plotted for different 5-HT concentrations, and a curve through the points was fitted with a logistic function (n = 5). Normalized current used to generate the dose–response curve followed the equation (I − Imin)/(Imax − Imin), where I is the current magnitude at a given concentration of 5-HT andImin is the maximum current at which the effect of the 5-HT had saturated. The saturation concentration for 5-HT, GABA, and baclofen was 1 mm, and 2 μm for phorbol esters (see Figs. 2, 3, 4).Imax is the maximum control current before the application of 5-HT. The estimated half-blocking concentration of 5-HT was 2.4 μm.
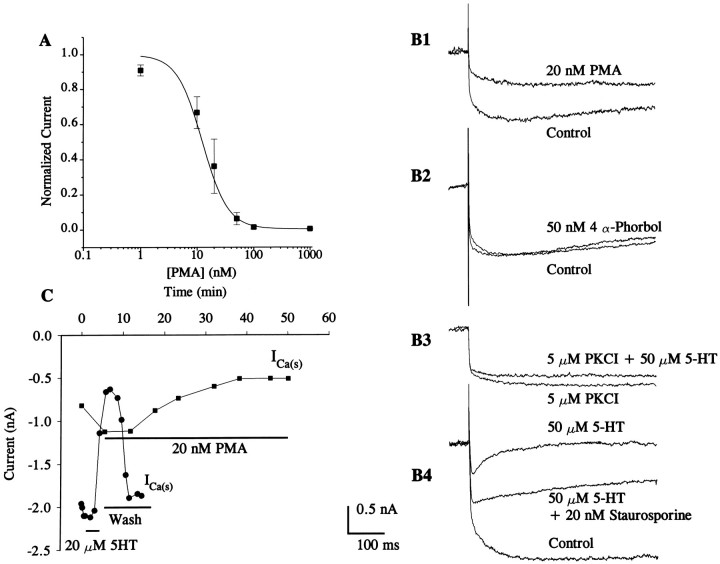
Fig. 2.
Effects of PMA and inhibitors of PKC on Ca2+ currents. A, Mean dose–response curve showing the relationship between PMA concentration and reduced sHVA Ca2+ current. The estimated half-blocking concentration of PMA was 12.3 nm with a power of 2 from the logistic function used to fit the data points. Data points were generated from four different experiments from two type A and two type B cells. B, Control traces (type A cell) were elicited from a holding potential of −80 mV to a step potential of 20 mV in normal bath solutions and in a solution containing PMA (20 nm) (B1) or 4α-phorbol (50 nm) (B2) (type A cell). As shown inB1, PMA (20 nm) reduced, but did not eliminate, the sHVA Ca2+ current, and 4α-phorbol did not affect the sHVA current (B2).B3, Ca2+ currents, from a type B cell, were recorded in the presence of the PKC inhibitor (5 μm) [PKC(19–36)] (PKCI). In the presence of PKC(19–36), 5-HT (50 μm) had a small effect on the Ca2+ current.B4, The broad-spectrum kinase inhibitor staurosporine (20 nm) transiently (3–5 min) reversed the effects of 5-HT (traces were recorded from a type B cell). However, 10 min after the application of staurosporine in the presence of 5-HT, the sHVA Ca2+ current was reduced to the original 5-HT-induced magnitude. C, Time course of 5-HT and PMA effects on Ca2+ currents. The photoreceptor was held at −80 mV and stepped to 20 mV to activate the sHVA component of the Ca2+ current. The magnitude of the current after application of 5-HT (20 μm) and after washout is shown. 5-HT blocked the sustained current elicited at 20 mV (filled circles). PMA (20 nm) reduced the sHVA Ca2+current (filled boxes); however, the time course of action was delayed compared with the effects of 5-HT.
Effects of PKC activators and inhibitors on Ca2+ current
Previous research has shown that the effects of 5-HT on diverse K+ currents may be mediated by PKC (Farley and Auerbach, 1986; Crow et al., 1991). A potential role of PKC in mediating the effects of 5-HT on the sHVA Ca2+current was examined by measuring currents after the application of the PKC activator phorbol 12-myristate 13-acetate (PMA). The dose–response curve is shown in Figure 2A. The phorbol ester PMA is an effective activator of PKC inHermissenda photoreceptors (Crow et al., 1991). Whereas 5-HT completely blocked the sHVA current, PMA partially reduced the current as shown in Figure 2B1. The statistical analysis of the difference scores for the 5-HT and PMA group data showed that 5-HT resulted in a significantly larger reduction in the sHVA current compared with PMA (5-HT: = 0.78 ± 0.05 nA;n = 9; PMA: = 0.40 ± 0.08 nA;n = 9, t16 = 4.11, p < 0.005). The inactive analog of phorbol ester, 4 α-phorbol, did not affect either component of the Ca2+ current as shown in Figure 2B2. It is possible that PMA not only reduced sHVA but also removed the inactivation of the transient component of the Ca2+ current. However, this is unlikely, because PMA did not affect currents from cells that predominantly expressed the tLVA current (data not shown). Thus, the effects of PMA cannot be attributed to its secondary effect on either the changes in the magnitude or the kinetics of the tLVA current.
The phorbol ester produced different kinetic effects on the sHVA current compared with 5-HT. However, this finding may be the result of the effect of phorbol esters on other cellular processes in addition to activation of PKC. A specific inhibitor of PKC (PKCI), [PKC(19–36)], reduced but did not completely block the effects of 5-HT on the sHVA current (Fig. 2B3). In addition, staurosporine, a broad spectrum kinase inhibitor (Tamaoki et al., 1986; Yanagihara et al., 1991) transiently (3–5 min) reversed the effects of 5-HT (note that 5-HT was applied 3 min before staurosporine was perfused into the bath) (see Fig. 2B4). However, 10 min after application of both 5-HT and staurosporine, the Ca2+ current was reduced. It is conceivable that activation of PKC by 5-HT might increase over time and that prolonged 5-HT exposure may overcome the inhibitory effects of staurosporine.
We observed that the reduction in the Ca2+current produced by 20 nm PMA was delayed relative to the effects of 20 μm 5-HT, as shown in Figure 2C. The effects of 5-HT on sHVA currents were observed within 3 to 5 min of application; however, the effects of PMA were not observed until 10–15 min after bath application. A summary of the group data and statistical results for the effects of 5-HT, active and inactive phorbol esters, and PKC inhibitors is presented in Table1.
Table 1.
Effects of 5-HT, active and inactive phorbol esters, and PKC inhibitors on calcium currents
| Control | Experimental | df | t | p | ||
|---|---|---|---|---|---|---|
| Treatment | nA | Treatment | nA | |||
| — | −1.29 ± 0.14 (9) | 5-HT | −0.50 ± 0.03 (9) | 8 | 15.1 | <0.005 |
| — | −1.02 ± 0.22 (9) | PMA | −0.61 ± 0.14 (9) | 8 | 5.4 | <0.005 |
| — | −1.10 ± 0.07 (6) | 4-α-Phorbol | −1.09 ± 0.07 (6) | 5 | 0.6 | NS |
| PKCI | −0.99 ± 0.22 (7) | PKCI + 5-HT | −0.82 ± 0.21 (7) | 6 | 2.8 | <0.05 |
| 5-HT | −0.16 ± 0.09 (7) | 5-HT + Staura | −0.90 ± 0.21 (7) | 6 | 14.6 | <0.005 |
| 5-HT | −0.20 ± 0.09 (7) | 5-HT + Staurb | −0.24 ± 0.12 (7) | 6 | 1.0 | NS |
Vh was −80 mV, and the step voltage was +20 mV. Data are expressed as the mean peak current ± SD (number of cells). Computed t statistics are for correlated means. NS, Not significant; Staur,a 3–5 min after staurosporine application; Staurb, 7 or more min after staurosporine application.
Effect of GABA on Ca2+ current
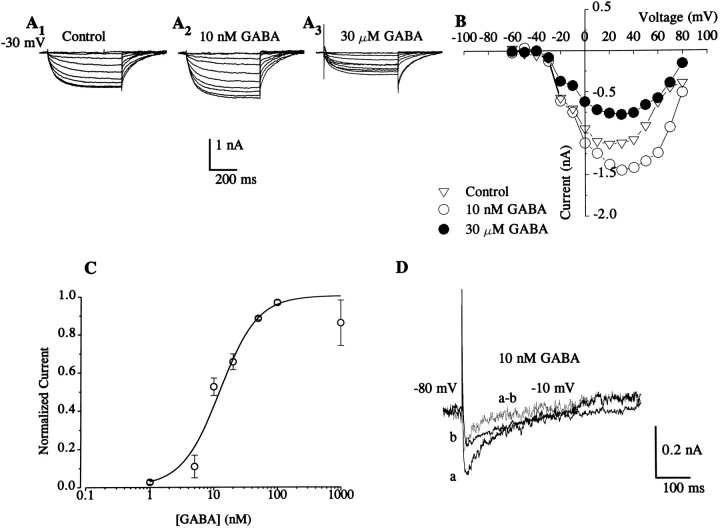
We investigated the effects of GABA on the two voltage-activated Ca2+ currents by using known analogs and inhibitors of GABA receptors. The effect of GABA on the sHVA current was studied from a holding potential greater than −35 mV at which the tLVA current is predominantly inactivated. At low concentrations (5–1000 nm), GABA enhanced the sHVA current in both type A and B photoreceptors (type A control: = −1.18 ± 0.4 nA, 15 nm; GABA: = −1.43 ± 0.38 nA; n = 6, t5 = 6.08, p < 0.005) (type B control: = −1.13 ± 0.47 nA, 15 nm; GABA: = −1.43 ± 0.45 nA; n = 7, t6 = 7.66, p < 0.005). The data were obtained from the peak current elicited at 20 mV. An example from a type B cell of enhancement of sHVA by 10 nm GABA is shown in Figure3, A and A2. In contrast to the enhancement of sHVA by l0 nm GABA, 30 μm GABA reduced the sHVA current as shown by the example in Figure 3A3. At higher concentrations (∼10–1000 μm), GABA reduced the sHVA current compared with the controls in both type A and B cells (type A control: = −1.17 ± 0.32 nA, 25 μm; GABA: = 0.66 ± 0.20 nA; n = 6, t5 = 8.98, p < 0.005) (type B control: = −1.12 ± 0.31 nA, 25 μm; GABA: = 0.72 ± 0.21 nA; n = 7, t6 = 5.52, p < 0.005). However, we did not qualitatively detect a reduction of the tLVA current in the presence of high concentrations of GABA at step potentials at which we assumed that the tLVA current was activated. A current–voltage plot for a control and two concentrations of GABA (10 nm and 30 μm) is shown in Figure 3B. A dose–response curve illustrating the effects of low concentrations of GABA on sHVA is shown in Figure 3C. At holding potentials at which the tLVA Ca2+current can be activated (less than or equal to −50 mV), enhancement of the current by low concentrations of GABA was also observed (see difference current from a type A cell in Fig. 3D). The effects of low concentrations of GABA at holding potentials at which the tLVA Ca2+ can be activated was similar for both type A and type B cells (type A control: = −0.19 ± 0.08 nA, 15 nm; GABA: = −0.24 ± 0.08 nA; n = 7, t6 = 9.95, p < 0.005) (type B control: = −0.17 ± 0.07 nA, 15 nm; GABA: = −0.22 ± 0.07 nA; n = 9, t8 = 9.05, p < 0.005).
Fig. 3.
Effects of GABA on Ca2+current. A, A family of superimposed Ca2+ currents elicited from a type B cell at a holding potential of −30 mV. A1, Control; A2, after the application of 10 nm GABA;A3, after the application of GABA (30 μm). B, Current–voltage plot of control and GABA-induced effects on the Ca2+current. Graph was generated from current traces shown inA1–A3. From a holding potential of −30 mV, the sHVA Ca2+ current was the predominant Ca2+ current activated by the command steps. A low concentration of GABA (10 nm) enhanced the current. Higher concentrations of GABA (30 μm) reduced the sHVA Ca2+ current. C, Dose–response curve was generated using the same equation as described in Figure 1D. The half-activation concentration for GABA at low concentrations as estimated from four experimental data points with a logistic function was 12.5 nm using a power of 1.5 as the best fit. Imax was equal to the current recorded at the saturation concentration of GABA between 500 and 1000 nm. For each cell, a profile of the effect of GABA from a lower to higher concentration was plotted as the example shown in Figure 4E. Imaxwas the peak of the profile of the biphasic concentration curve.Imin is the current measured before GABA application. D, At low concentrations, GABA enhanced the tLVA Ca2+ current (see A). Traces from a type A cell were generated from a holding potential of −80 mV to a step depolarization of −10 mV. The trace in a is in the presence of 10 nm GABA, and the trace inb is the control. The difference current (dotted line) corresponds to the increase in the tLVA Ca2+ current attributable to GABA.
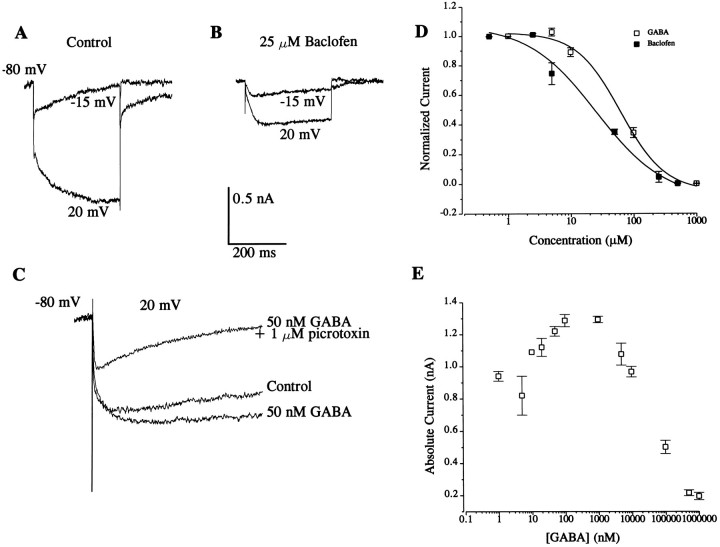
The two effects of GABA were studied further with baclofen, a GABAB receptor agonist. In preparations perfused with 25 μm baclofen, there was a steady-state reduction in the Ca2+ currents compared with control current measurements (see Table 2). Figure4B shows an example of the steady-state effects of baclofen compared with control conditions in Figure4A. These results suggest that activation of GABAB receptors is sufficient to produce inhibition of Ca2+ currents, an observation similar to that reported from studies of dorsal root ganglion (DRG) neurons (Scott et al., 1990). It has been postulated that two subtypes of GABAB receptors are present in the photoreceptors, one gating K+ channels and another regulating closure of K+ channels (Alkon et al., 1993). However, after enhancement of the Ca2+ current with GABA (50 nm), the application of picrotoxin (1 μm), a GABAA receptor antagonist, inhibited the current (Fig. 4C). These results suggest the possible involvement of either GABAAreceptors or a subclass of as yet unidentified GABA receptors in the enhancement of Ca2+ currents. Dose–response curves for baclofen and a high concentration of GABA are shown in Figure 4D. The concentration profile of the effects of GABA on the sHVA Ca2+ current is plotted in Figure4E.
Table 2.
Effects of GABA, baclofen, picrotoxin, cAMP analog, IBMX, PKAI, and PKCI on calcium currents
| Control | Experimental | df | t | p | ||
|---|---|---|---|---|---|---|
| Treatment | nA | Treatment | nA | |||
| — | −0.95 ± 0.29 (7) | GABA* (10 nm) | −1.09 ± 0.38 (7) | 6 | 2.5 | <0.05 |
| — | −0.17 ± 0.06 (7) | GABAI,t (10 nm) | −0.23 ± 0.04 (7) | 6 | 3.1 | <0.025 |
| — | −0.94 ± 0.30 (7) | GABA* (30 μm) | −0.67 ± 0.20 (7) | 6 | 3.0 | <0.025 |
| — | −1.01 ± 0.11 (9) | Baclofen (25 μm) | −0.66 ± 0.27 (9) | 8 | 3.4 | <0.01 |
| GABA (50 nm) | −0.78 ± 0.21 (6) | GABA (50 nm) + Picro (1 μm) | −0.19 ± 0.16 (6) | 5 | 9.5 | <0.005 |
| — | −1.28 ± 0.16 (7) | Dibu.cAMP (2.5 nm) | −2.17 ± 0.33 (7) | 6 | 7.5 | <0.005 |
| — | −0.90 ± 0.18 (5) | IBMX (200 μm) | −1.16 ± 0.21 (5) | 4 | 4.3 | <0.025 |
| PKAI (5 μm) | −1.00 ± 0.21 (5) | PKAI (5 μm) + GABA (50 nm) | −0.43 ± 0.13 (5) | 4 | 4.8 | <0.01 |
| PKCI (5 μm) | −1.08 ± 0.26 (6) | PKCI (5 μm) + GABA (25 μm) | −0.57 ± 0.12 (6) | 5 | 3.7 | <0.025 |
Asterisk indicates that the Vh was −30 mV and step potential was +20 mV. Vh for other experiments was −80 mV, and the step voltage was −20 mV. The transient component of the calcium current (It) was elicited from a holding potential of −80 mV to step potentials of −10 mV. Data are expressed as the mean peak current ± SD (number of cells). Computed t statistics are for tests of correlated means. Picro, Picrotoxin; dibu.cAMP, cAMP-N6,O6-dibutyril.
Fig. 4.
Effects of a GABAB agonist and a GABAA antagonist on Ca2+ currents. A, Control traces generated from a holding voltage of −80 mV to step voltages of −15 and 20 mV. B, Baclofen (25 μm) reduced the currents compared with the control. C, In a different cell, a control current trace was elicited by stepping from −80 to 20 mV followed by the bath application of GABA (50 nm). The current was enhanced in the presence of GABA. The application of the GABAA antagonist picrotoxin (1 μm) reduced the current.D, Dose–response curve for high concentrations of GABA and baclofen. Curves were generated as described in the legends for Figures1, 2, 3. Because GABA elicited a dual effect, a concentration profile of the effects of GABA on each cell was generated as shown inE. Normalized current used to generate the dose–response curve followed the equation (I − Imin)/(Imax − Imin), where I is the current magnitude at a given concentration of GABA andImin is the minimum current at which the effect of GABA had saturated (1 mm).Imax is the plateau current extrapolated from E. The half-blocking concentrations for GABA and baclofen estimated from these curves were 62.5 and 25.5 μm, respectively. E, Graph of the concentration profile of GABA on the sustained Ca2+ current. The effect of GABA on the Ca2+ current shows an initial rise in the current magnitude at low concentrations and drop in the size of the current as the concentration of GABA increases. The peak current was observed at ∼100 nm GABA.
Effects of cAMP on calcium current
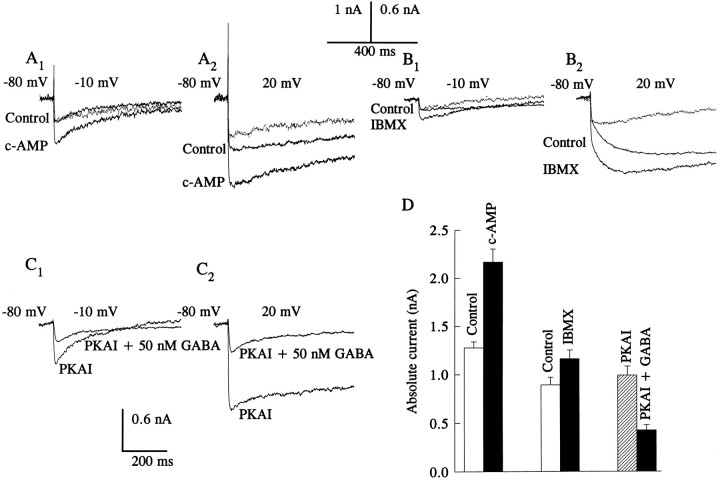
Administration of dibutyryl-cAMP or chlorophenylthio-cAMP (2.5 mm) produced an increase in the amplitude of the Ca2+ current at both low- and high-step voltages from negative holding potentials (less than or equal to −50 mV) (see Fig. 5A1, A2). The profile of the cAMP analog-induced current at −10 and 20 mV step depolarizations suggests that the current consisted of both the tLVA and sHVA Ca2+ currents. The analysis of the group data elicited by a voltage step to −10 mV from a holding potential of −80 mV revealed that the cAMP analogs resulted in a significant enhancement of the tLVA current (control: = 0.27 ± 0.05 nA; cAMP: = 0.44 ± 0.09 nA; n = 4, t3 = 3.79, p < 0.05). The cAMP analog-induced current exhibited inactivation. At holding potentials at which the sHVA Ca2+ current was predominantly activated (greater than or equal to −30 mV), a similar enhancement of the Ca2+ current was observed in the presence of the cAMP analogs. The effects of IBMX, a phosphodiesterase inhibitor known to enhance the actions of cAMP-dependent phosphorylation, suggest that the increase in the Ca2+ current was mediated by PKA (see Fig.5B1, B2, Table 2). In the presence of IBMX, the tLVA Ca2+ current elicited by a voltage step to −10 from −80 mV was enhanced (control: = 0.25 ± 0.08 nA, IMBX: = 0.41 ± 0.13 nA;n = 3, t2 = 4.56, p < 0.05).
Fig. 5.
Effects of a cAMP analog, IBMX, and PKAI on Ca2+ currents. Current traces were generated before and after the bath application of cAMP analogs (2.5 mm); both the dibutyryl and the chlorophenylthio analogs of cAMP had similar effects. The difference current traces after the treatment are represented by the dotted lines. The cAMP analogs increased both tLVA (A1) and sHVA (A2) Ca2+ currents. Similar results were obtained after the application of IBMX (200 μm), as shown in B1 andB2. After dialysis of cells with PKAI (5 μm), GABA (50 nm) reduced the magnitude of the Ca2+ currents as shown inC1 and C2. Traces in A andB were generated from type A cells, and traces inC were from a type B cell. D, Group data showing the effects of both cAMP analogs (n = 7) and IBMX (n = 5) on the sHVA Ca2+ current and the effect of GABA on the sHVA Ca2+ current in the presence of PKAI (n = 6).
To test whether the effects of low concentrations of GABA (see Fig.3A) were mediated by PKA, cells were first perfused internally with the PKA inhibitor (PKAI), [PKI(6–22)amide] (5 μm) before the bath application of GABA. After dialysis of cells with PKAI, application of GABA (50 nm) reduced the Ca2+current as shown in the example in Figure 5C. The group data for the effects of the cAMP analogs, IBMX, and PKAI on the sHVA current are shown in Figure 5D, and the statistical analysis of the group data is included in Table 2.
Possible [PKC(19–36)] effects on the actions of GABA
As shown in Figures 2B and 3A, PMA and GABA, at micromolar concentrations, reduced the magnitude of the sHVA Ca2+ current. The effects of PMA on the current waveform and time course exhibited different kinetics from the effects of GABA. To further investigate a possible relationship between GABA and PKC we examined the effects of GABA on the Ca2+ currents in the presence of the PKC inhibitor PKCI [PKC(19–36)]. In the presence of PKCI (5 μm) in the patch pipette solution, micromolar concentrations of bath-applied GABA (25 μm) reduced the sHVA current. Thus, PKC does not appear to contribute to the modulation of Ca2+ currents by GABA. The results of the statistical tests of data generated from various treatments are summarized in Table 2.
G-protein-mediated reduction of Ca2+ current
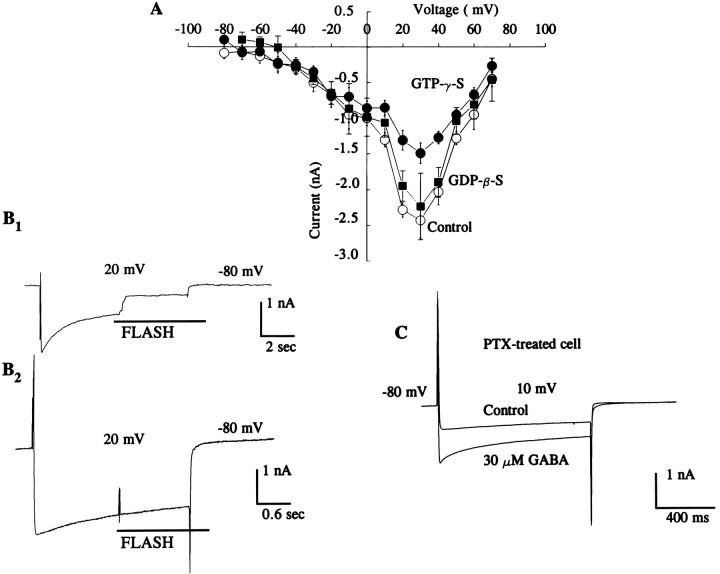
Previous research has implicated G-proteins as a target for Ca2+- mediated plasticity in the photoreceptors (Matzel and Alkon, 1991). We examined this further by studying the effects of G-proteins on Ca2+ currents to determine which second messengers are responsible for the GABA-induced effects. As shown in the current–voltage plot in Figure6A, the nonhydrolyzable form of GTP (GTP-γ-S) reduced the sHVA Ca2+ current compared with normal controls or controls that were dialyzed with GDP-β-S. The analysis of the group data revealed a statistically significant reduction in Ca2+ currents after the application of GTP-γ-S (control: = −2.5 ± 0.24 nA; GTP-γ-S: = −1.5 ± 0.11 nA;n = 3, t2 = 3.15, p < 0.05, one-tailed test). In contrast, GDP-β-S did not produce a significant reduction in the Ca2+current (control: = −2.7 ± 0.18 nA; GDP-β-S: = −2.23 ± 0.33 nA;n = 2, t1 = 2.9, NS, one-tailed test). We further confirmed the results by using a photolabile form of GTP-γ-S. Release of caged GTP-γ-S caused a decline in the magnitude of the Ca2+ current as shown in Figure 6B1. UV light alone did not affect the currents as shown in Figure 6B2. In cells treated with the G-protein inhibitor PTX, the differential effects of GABA on Ca2+ currents were abolished. After treatment with PTX, high concentrations of GABA enhanced the Ca2+ current as shown in Figure 6C. The analysis of group data revealed that GABA (30 μm) enhanced Ca2+currents if cells were pretreated with PTX (PTX control: = −8 ± 0.08 nA, 30 μm; GABA: = −1.44 ± 0.09 nA; t2 = −43.9, p < 0.005). These results suggest that GABA may be acting through at least two second messengers, G-proteins and PKA.
Fig. 6.
G-protein mediates GABA-induced reduction of Ca2+ current. A, Current–voltage relationship for data obtained from control experiments (open circles), controls dialyzed with GDP-β-S (filled boxes), and experiments performed in the presence of GTP-γ-S (5 mm) (filled circles) in the pipette. The magnitude of the sHVA Ca2+ current was significantly reduced in the presence of GTP-γ-S. Currents were generated from −80 mV to a step potential of 30 mV. In contrast to the effects of GTP-γ-S, GDP-β-S (5 mm) failed to reduce the current. Currents were generated from −80 mV to a step potential of 30 mV. When GTP-γ-S was released by flash photolysis from caged GTP-γ-S, the current was reduced as shown in a representative trace in B1. Control recording inB2 shows that the light flash does not reduce the Ca2+ current. C, The effect of PTX on Ca2+ currents. Photoreceptors were incubated in PTX (1 μg/ml) for 6 hr followed by Ca2+ current measurement, before (Control) and after applying GABA (30 μm). The typical GABA-induced reduction of the Ca2+ current was abolished by pretreatment with PTX. In the presence of PTX, GABA only enhanced the Ca2+ current. Currents were generated from −80 mV to a 10 mV step potential. All experiments in this section were conducted in the presence of bath Ca2+ (20 mm).
DISCUSSION
These results show that both 5-HT and GABA modulate Ca2+ currents in the photoreceptors ofHermissenda. 5-HT blocks the sHVA Ca2+current; GABA increases both the tLVA and sHVA currents at low concentrations, and blocks only the sHVA current at higher concentrations. Studies of the photoreceptors in Hermissendahave suggested that the mechanism of plasticity is Ca2+-dependent (Falk-Vairant and Crow, 1992;Matzel and Rogers, 1993). However, the mechanism of the induction and maintenance of Ca2+-dependent neuronal plasticity is not understood. Previous studies have implicated GABA in the mediation of increases in intracellular Ca2+ at the synaptic terminals of the photoreceptors (Alkon et al., 1992). In addition, 5-HT produces enhanced excitability of type B photoreceptors and synaptic facilitation of inhibitory synaptic connections between type B and type A photoreceptors (Farley and Wu, 1989; Crow and Forrester, 1991; Schuman and Clark, 1994). Recently, it was reported that GABA, which under normal conditions is inhibitory, may transform a synapse into an excitatory one by an unknown mechanism (Alkon et al., 1992).
5-HT modulation of Ca2+ current
Modulation of Ca2+ currents by 5-HT has been demonstrated in several neural systems. 5-HT reduces a high-voltage-activated Ca2+ current in dorsal raphe neurons (Penington and Kelly, 1990; Penington et al., 1992), embryonic chick sensory neurons (Dunlap and Fischbach, 1981), and spinal cord neurons (Sah, 1990). As a result of Ca2+ current inhibition, neuronal excitability is reduced (Penington et al., 1992) and action potential durations are shortened (Dunlap and Fischbach, 1981; Penington et al., 1992). In contrast, a low-voltage-activated Ca2+ current in spinal motor neurons is enhanced by 5-HT (Berger and Takahashi, 1990). Calcium currents in Helix aspera neurons (Paupardin-Tritsch et al., 1986) and Aplysia sensory neurons are enhanced by 5-HT through activation of PKC (Edmonds et al., 1990; Braha et al., 1993). However, in neuron R15 of Aplysia, potentiation of Ca2+ currents by 5-HT is mediated by activation of PKA (Levitan and Levitan, 1988). Previous work inHermissenda suggested that 5-HT increased Ca2+ currents in type B photoreceptors (Farley and Wu, 1989) and pedal neurons (Jacklet and Acousta-Urquidi, 1985). The initial studies of Ba2+ currents in the type B photoreceptors did not identify the two components of Ca2+ currents that were recently characterized byYamoah and Crow (1994a). The failure to isolate these currents in earlier studies may be attributable to differences in the experimental procedures and potential current contamination in the voltage-clamp data. Previous studies have reported that in type B photoreceptors, 5-HT reduced IA andIK,Ca (Farley and Wu, 1989), slowed the rate of inactivation of IK,V, and in some cases reduced IK,V (Acosta-Urquidi and Crow, 1993). Moreover, recent evidence suggests that the reduction ofIK,Ca by 5-HT is a consequence of modulation of ICa by 5-HT (Yamoah and Crow, 1995). In addition, 5-HT enhances an inward rectifier current (IIR) (Acosta-Urquidi and Crow, 1993), which is conducted by Na+ and K+ (Matzel et al., 1995). Voltage-clamp protocols used in earlier studies to measure Ba2+ currents in the photoreceptors may not have eliminated other potential ionic current contamination (Farley and Wu, 1989). Because some of these currents are not exclusively carried by K+, shifting EK to 0 mV does not necessarily indicate that a null potential for these conductances will be attained. If inward Ca2+currents are recorded in the presence of any 5-HT-sensitive inward or outward currents, the reduction of outward K+currents or enhancement of the inward rectifier current by 5-HT may be manifested in an apparent increase in a putative Ca2+ current.
Previously, it was reported that 5-HT increased the amplitude of the plateau phase of the generator potential (Farley and Wu, 1989; Crow and Forrester, 1991) by reducing IK,Ca andIA (Farley and Wu, 1989; Acosta-Urquidi and Crow, 1993). In addition, 5-HT enhances the inward rectifier and shifts the current’s apparent reversal potential to more positive potentials (Acosta-Urquidi and Crow, 1993). Such effects would be expected to enhance the amplitude of the generator potential and potentially prolong the afterdepolarization phase of light-elicited generator potentials. Under such conditions, Ca2+ influx should increase. However, a reduction of the sHVA Ca2+ current by 5-HT may be a feedback mechanism to prevent intracellular Ca2+ overload.
The reduction of IK,Ca andIA by 5-HT has been attributed to activation of PKC, because application of phorbol esters and microinjection of PKC into the B photoreceptors produced similar effects (Farley and Auerbach, 1986). The following evidence implicates PKC as a possible candidate for 5-HT-mediated reduction of the sHVA Ca2+ current. PMA, but not 4α-phorbol, reduced the Ca2+ current, and staurosporine transiently (3–5 min) reversed the effects of 5-HT. PKCI reduced the effect of 5-HT on the sHVA Ca2+ current. However, the time course of PMA reduction of the Ca2+ current in the photoreceptors was delayed relative to the effects of 5-HT. Similar delayed effects of phorbol esters have been demonstrated for changes of action potential amplitudes in Aplysia bag cells (Conn et al., 1989). In Aplysia sensory neurons, it has been reported that the time course of the effect of 5-HT lags behind the time course of the effects of phorbol ester (Braha et al., 1993). In this study, we found that effects of 5-HT on Ca2+ current was expressed earlier than the effect of PMA on the Ca2+ current. However, the sequences of activation of PKC by phorbol ester and transmitters are quite different, because phorbol esters must permeate through the cell membrane to activate PKC and, thus, the time courses of activation of their effects are not expected to be identical. Taken together, these results suggest that the reduction of the Ca2+currents in the photoreceptors by 5-HT may be PKC mediated. In addition to PKC modulation, a possible role of CAM kinase II in the modulation of the Ca2+ currents has been suggested and requires further investigation (Matzel and Alkon, 1991).
GABA modulation of Ca2+ currents
GABA has two effects on the amplitude of Ca2+ currents in the photoreceptors that are similar to effects reported for DRG neurons (Scott et al., 1990, 1991). At nanomolar and micromolar concentrations, GABA increases and decreases the Ca2+ currents, respectively.
GABA increases intracellular Ca2+ at the axonal terminal of the photoreceptors as measured with Ca2+ indicator dyes (Alkon et al., 1993). Recently, it was proposed that the GABA-induced rise in intracellular Ca2+ may be the trigger for the induction of cellular plasticity (Alkon et al., 1993). In an independent study,Matzel and Rogers (1993) observed that Ca2+ is required for the induction of plasticity in the photoreceptors. Although the intracellular Ca2+ release hypothesis is intriguing, we propose that GABA-induced increases in Ca2+ influx through voltage-activated Ca2+ channels may augment the intracellular release process, such as a Ca2+-induced Ca2+ release. The contribution of these two processes acting together may exceed a threshold for the induction of Ca2+-dependent changes in the photoreceptors. It is conceivable that beyond the threshold and the capacity of intracellular Ca2+ buffers, reduction of the Ca2+ currents by GABA may serve an important physiological function by preventing possible cell death as a result of intracellular Ca2+ overload.
What GABA receptors are responsible for the modulation of Ca2+ current?
The finding that baclofen reduced the Ca2+ currents suggests that a GABAB receptor is involved in the modulation of Ca2+ channels. Muzzio et al. (1994) have reported similar reduction of Ca2+ currents by baclofen in the photoreceptors. This is similar to the GABABreceptor-mediated inhibition of Ca2+ currents in hippocampal neurons (Pfrieger et al., 1994), cerebellar Purkinje neurons (Mintz and Bean, 1993), and sensory neurons (Scott et al., 1991). However, blockage of current enhancement by picrotoxin, a known GABAA receptor antagonist (Puia et al., 1990), suggests that there may be different subtypes of GABA receptors that are sensitive to picrotoxin, but not similar to the known GABAA anion receptor. The GABA receptor mediating Ca2+ current enhancement may be a subtype of a GABA receptor with some homologies to the GABAAreceptor, because it is known that different subunits of GABA receptors can form receptors with different pharmacologies (Verdoorn et al., 1990; Shimada et al., 1992). The possibility that a novel GABA receptor (GABAC), which has been characterized in bipolar neurons in salamander retina (Lukasiewicz and Werblin, 1994), is also present in Hermissenda photoreceptors requires further investigation. Alternatively, Alkon et al. (1992) have suggested the presence of two subtypes of GABAB receptors that have differential effects on membrane conductances in the photoreceptors. In either case, the GABA enhancement of the Ca2+ currents appears to be mediated through activation of PKA, because cAMP and IBMX essentially mimic the GABA effects, and PKAI blocked the GABA-induced enhancement of the Ca2+ currents.
G-protein and GABA reduction of Ca2+ current
GABA-mediated reduction of the sHVA Ca2+current may result from activation of a G-protein. The evidence for this is as follows: GTP-γ-S, but not GDP-β-S, reduced the sHVA Ca2+ current. Micromolar concentrations of GABA and baclofen also reduced the current. PKCI had no effect on the actions of GABA. An inhibitor of some G-proteins (PTX) blocked the reduction in the sHVA current typically produced by GABA at micromolar concentrations. In PTX-treated cells, GABA enhanced the sHVA Ca2+ current both at nanomolar and micromolar concentrations. Thus, the dual effects of GABA on the Ca2+ current is mediated through activation of PKA and a G-protein.
Several examples of G-protein modulation of Ca2+ currents have been reported from different systems, e.g., G-protein augmentation of agonist and antagonist actions of the dihydropyridine-sensitive high-voltage-activated Ca2+ currents in sensory and sympathetic neurons (Dolphin and Scott, 1989). In sympathetic neurons, it has been suggested that G-proteins regulate Ca2+ channels tonically (Scott and Dolphin, 1990). Interestingly, in DRG neurons, GABABreceptors have been found to be coupled to Ca2+channels via a G-protein (Scott et al., 1990). Moreover, GABA has a dual effect on a low-voltage-activated Ca2+channel in DRG neurons. In contrast, the dual effects of GABA seen inHermissenda photoreceptors were observed on the sHVA Ca2+ current, but not the tLVA Ca2+ current.
Footnotes
This work was supported by National Institutes of Mental Health Grant MH40860 to T.C. E.N.Y. was supported by a Grass Foundation Fellowship. We thank Dr. J. Byrne for the use of his osmometer.
Correspondence should be addressed to Ebenezer N. Yamoah at his present address: Department of Physiology, Johns Hopkins University School of Medicine, 725 North Wolfe Street, Baltimore, MD 21205-2185.
REFERENCES
- 1.Acosta-Urquidi J, Crow T. Differential modulation of voltage-dependent currents in Hermissenda Type B photoreceptors by serotonin. J Neurophysiol. 1993;70:541–548. doi: 10.1152/jn.1993.70.2.541. [DOI] [PubMed] [Google Scholar]
- 2.Alkon DL. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984;266:1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- 3.Alkon DL, Rasmussen H. A spatial-temporal model of cell activation. Science. 1988;215:693–695. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- 4.Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol. 1985;44:278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- 5.Alkon DL, Sanchez-Andres JV, Ito E, Oka K, Yoshioka T, Collin C. Long-term transformation of an inhibitory into excitatory GABAergic synaptic response. Proc Natl Acad Sci USA. 1992;89:11862–11866. doi: 10.1073/pnas.89.24.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkon DL, Anderson MJ, Kuzirian AM, Rogers DF, Fass DM, Collin C, Nelson TJ, Kapetanovic IM, Matzel LD. GABA-mediated synaptic interaction between the visual and vestibular pathways of Hermissenda . J Neurochem. 1993;61:556–566. doi: 10.1111/j.1471-4159.1993.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 7.Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motorneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braha O, Edmonds B, Sacktor T, Kandel ER, Klein M. The contribution of Protein Kinase A and Protein Kinase C to the action of 5-HT on the L-type Ca2+-current of the sensory neurons in Aplysia . J Neurosci. 1993;13:1839–1851. doi: 10.1523/JNEUROSCI.13-05-01839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conn JP, Strong JA, Kaczmarek KL. Inhibitors of protein kinase C prevent enhancement of calcium current and action potentials in peptidergic neurons of Aplysia . J Neurosci. 1989;9:480–487. doi: 10.1523/JNEUROSCI.09-02-00480.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow T. Cellular and molecular analysis of associative learning and memory in Hermissenda. Trends Neurosci. 1988;11:136–142. doi: 10.1016/0166-2236(88)90138-5. [DOI] [PubMed] [Google Scholar]
- 11.Crow T, Forrester J. Light paired with serotonin in vivo produces both short- and long-term enhancement of generator potentials of identified B photoreceptors in Hermissenda . J Neurosci. 1991;11:608–617. doi: 10.1523/JNEUROSCI.11-03-00608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crow T, Forrester J, Williams M, Waxham MN, Neary JT. Down-regulation of protein kinase C blocks 5-HT-induced enhancement in Hermissenda B-photoreceptors. Neurosci Lett. 1991;121:107–110. doi: 10.1016/0304-3940(91)90660-l. [DOI] [PubMed] [Google Scholar]
- 13.Dolphin AC, Scott RH. Interaction between calcium channel ligands and guanine nucleotides in cultured rat sensory and sympathetic neurons. J Physiol (Lond) 1989;413:271–288. doi: 10.1113/jphysiol.1989.sp017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlap K, Fischbach G. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurons. J Physiol (Lond) 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edmonds B, Klein M, Dale N, Kandel ER. Contribution of two types of calcium channels to synaptic transmission and plasticity. Science. 1990;250:1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- 16.Falk-Vairant J, Crow T. Intracellular injections of BAPTA block induction of enhancement in Hermissenda type B-photoreceptors. Neurosci Lett. 1992;147:45–48. doi: 10.1016/0304-3940(92)90771-x. [DOI] [PubMed] [Google Scholar]
- 17.Farley J. Associative training results in persistent reductions in a calcium-activated potassium current in Hermissenda type B photoreceptors. Behav Neurosci. 1988;102:784–802. [Google Scholar]
- 18.Farley J, Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature. 1986;319:220–223. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- 19.Farley J, Wu R. Serotonin-modulation of Hermissenda B photoreceptor ionic currents: implications for mechanisms underlying associative learning. Brain Res Bull. 1989;22:335–351. doi: 10.1016/0361-9230(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 20.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 21.Harris-Warrick RM, Marder EC. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- 22.Jacklet JW, Acosta-Urquidi JA. Serotonin decreases a background current and increases calcium and calcium activated current in pedal neurons of Hermissenda . Cell Mol Neurobiol. 1985;5:407–412. doi: 10.1007/BF00755404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitan ES, Levitan IB. Serotonin acting via cyclic AMP enhances both hyperpolarizing and depolarizing phases of bursting pacemaker activity in the Aplysia neuron R15. J Neurosci. 1988;8:1152–1161. doi: 10.1523/JNEUROSCI.08-04-01152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1212–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matzel LD, Alkon DL. GABA-induced potentiation of neuronal excitability occurs during contiguous pairings with intracellular calcium elevation. Brain Res. 1991;554:77–84. doi: 10.1016/0006-8993(91)90174-t. [DOI] [PubMed] [Google Scholar]
- 26.Matzel DL, Rogers RF. Postsynaptic calcium, but not cumulative depolarization is necessary for the induction of associative plasticity in Hermissenda . J Neurosci. 1993;13:5029–5040. doi: 10.1523/JNEUROSCI.13-12-05029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matzel DL, Yamoah EN, Crow T. The contribution of inward rectification and transient Ca2+ currents to membrane oscillations in Hermissenda photoreceptors. Biophys J. 1995;68:A386. [Google Scholar]
- 28.Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channel in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 29.Muzzio IA, Matzel DL, Rogers RF. Characterization of responses to baclofen in the Hermissenda’s B-photoreceptor. Soc Neurosci Abstr. 1994;20:89. [Google Scholar]
- 30.Paupardin-Tritsch D, Hammond C, Gerschenfeld HM, Nairn A, Greengard P. cGMP-dependent protein kinase enhances Ca2+ current and potentiates the serotonin-induced Ca2+ current increase in snail neurons. Nature. 1986;323:812–814. doi: 10.1038/323812a0. [DOI] [PubMed] [Google Scholar]
- 31.Penington NJ, Kelly JS, Fox AP. Action potential waveforms reveal simultaneous changes in ICa and IKproduced by 5-HT in rat dorsal raphe neurons. Proc R Soc Lond [Biol] 1992;248:171–179. doi: 10.1098/rspb.1992.0059. [DOI] [PubMed] [Google Scholar]
- 32.Penington NJ, Kelly JS. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron. 1990;4:751–758. doi: 10.1016/0896-6273(90)90201-p. [DOI] [PubMed] [Google Scholar]
- 33.Pfrieger FW, Gottmann K, Lux HD. Kinetics of GABAB receptor-mediated inhibition of calcium currents and excitatory synaptic transmission in hippocampal neurons in vitro . Neuron. 1994;12:97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 34.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 35.Sah DW. Neurotransmitter modulation of calcium current in rat spinal cord neurons. J Neurosci. 1990;10:136–141. doi: 10.1523/JNEUROSCI.10-01-00136.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuman EM, Clark GA. Synaptic facilitation at connections of Hermissenda type-B photoreceptors. J Neurosci. 1994;14:1613–1622. doi: 10.1523/JNEUROSCI.14-03-01613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott RH, Dolphin AC. Voltage-dependent modulation of rat sensory neuron calcium channel currents in G-protein activation: effect of a dihydropyridine antagonist. Br J Pharmacol. 1990;99:629–630. doi: 10.1111/j.1476-5381.1990.tb12981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott RH, Wootton JF, Dolphin AC. Modulation of neuronal T-type calcium channel currents by photoactivation of intracellular guanosine 5′-O(3-thio)triphosphate. Neuroscience. 1990;38:285–294. doi: 10.1016/0306-4522(90)90028-3. [DOI] [PubMed] [Google Scholar]
- 39.Scott RH, Pearson HA, Dolphin AC. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36:485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- 40.Shimada S, Cutting G, Uhl GR. γ-Aminobutyric acid A or C receptors? γ-Aminobutyric acid ρ1receptor RNA induces bicuculline-barbiturate- and benzodiazepine-insensitive γ-aminobutyric acid response in Xenopus oocytes. Mol Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- 41.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 42.Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 43.Yamoah EN, Crow T. Two components of calcium currents in the soma of photoreceptors of Hermissenda . J Neurophysiol. 1994a;72:1327–1336. doi: 10.1152/jn.1994.72.3.1327. [DOI] [PubMed] [Google Scholar]
- 44.Yamoah EN, Crow T. Modulation of calcium currents in Hermissenda photoreceptors by 5-HT and GABA. Biophys J. 1994b;66:A49. [Google Scholar]
- 45.Yamoah EN, Crow T. Evidence for a contribution of ICa to serotonergic modulation of IK,Ca in Hermissenda photoreceptors. J Neurophysiol. 1995;74:1349–1354. doi: 10.1152/jn.1995.74.3.1349. [DOI] [PubMed] [Google Scholar]
- 46.Yanagihara N, Tachikawa E, Izumi F, Yasugawa S, Yamamoto H, Miyamoto E. Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II. J Neurochem. 1991;56:294–298. doi: 10.1111/j.1471-4159.1991.tb02595.x. [DOI] [PubMed] [Google Scholar]