Abstract
α2-Adrenoceptors regulate the efficacy at the sympatho-effector junction by means of a feedback inhibition of transmitter release. In chick sympathetic neurons, the mechanism involves an inhibition of N-type calcium channels, and we now present evidence that this effect involves an atypical, phorbol ester-insensitive protein kinase C (PKC). The inhibition of voltage-gated Ca2+ currents by the specific α2-adrenergic agonist UK 14,304 was significantly attenuated when the PKC inhibitors PKCI(19–36), staurosporine, or calphostin C were included in the internal solution used to fill the patch pipettes, or if staurosporine or calphostin C were applied extracellularly; however, phorbol esters as classical activators of PKC or oleoylacetylglycerol did not mimic the effect of UK 14,304, and chronic exposure to 4-β-phorbol dibutyrate (PDBu) did not attenuate it, even though PKCα and -ε isozymes were translocated to plasma membranes by PDBu. The atypical isozyme PKCζ was translocated by 100 μm arachidonic acid (AA), but not by PDBu; 100 μm AA and linoleic acid inhibited voltage-activated Ca2+ currents, and this effect was attenuated when PKCI(19–36) was added to the patch pipette solution. Our observations indicate that classical, new, and atypical PKC isozymes are present in chick sympathetic neurons and that an atypical, phorbol ester-insensitive PKC is involved in the inhibition of voltage-activated calcium currents by α2-adrenoceptor activation.
Keywords: α2-adrenoceptor, calcium current, chick sympathetic ganglion, protein kinase C isozymes, phorbol ester, arachidonic acid
Norepinephrine reversibly inhibits voltage-gated calcium channel currents in sympathetic neurons (Horn and McAfee, 1980;Galvan and Adams, 1982; Bley and Tsien, 1990; Plummer et al., 1991; Elmslie at al., 1992) by an activation of α2-adrenoceptors (Schofield, 1990; Boehm and Huck, 1991; Song et al., 1991). This phenomenon has attracted considerable interest not only because it served as a paradigm for second messenger-dependent signal transduction (Beech et al., 1992;Mathie et al., 1992; Delcour and Tsien, 1993; Golard and Siegelbaum, 1993; Shapiro et al., 1994; Ehrlich and Elmslie, 1995; Ikeda, 1996), but also because a modulation of transmembrane calcium influx subsequently affects the concentration of free intracellular calcium, which is crucial for various biological processes such as transmitter release (Lipscombe et al., 1989; Miller, 1990).
Nevertheless, the signaling cascade that links α2-adrenoceptors and calcium channel inhibition in chick sympathetic neurons has yet to be elucidated. The effects of α2-adrenoceptor activation on calcium currents and on transmitter release are abolished by a pretreatment of cultures for 24 hr with pertussis toxin (Boehm et al., 1992), indicating that inhibitory Go/Gi G-proteins are involved. We do not know, however, whether an (α2-adrenoceptor) activated G-protein interacts directly with calcium channels (Hescheler and Schultz, 1993) or whether second messengers are involved. In line with experiments in rat sympathetic cells (Schwartz and Malik, 1993), we have reported recently that cAMP modulates but does not mediate the inhibition of calcium channels and transmitter release by α2-adrenoceptor activation in chick sympathetic neurons (Boehm et al., 1994). Our experiments also indicate an intricate interaction of Gsα and the α2-adrenoceptor-mediated inhibition of transmitter release, because downregulation of Gsα by cholera toxin caused sensitization of α2-adrenergic effects in chick sympathetic neurons (Boehm et al., 1996).
Another obvious pathway that might convey the α2-adrenergic effect includes activation of protein kinase C (PKC), possibly secondary to an α2-adrenoceptor-mediated regulation of phospholipase C (PLC) or phospholipase A2(PLA2). Numerous reports have implicated PKC in neurotransmitter-induced modulation of Ca2+channels, because both phorbol esters and diacylglycerol analogs mimicked receptor-mediated effects, and PKC inhibitors or chronic exposure to phorbol esters attenuated the action of neurotransmitters (Rane and Dunlap, 1986; Werz and Macdonald, 1987; Ewald et al., 1988;Marchetti and Brown, 1988; Mochida and Kobayashi, 1988; Rane et al., 1989; Boland et al., 1991; Diverse-Pierluissi and Dunlap, 1993).
The present experiments were initiated to investigate further the signaling cascade of the α2-adrenoceptor-mediated inhibition of transmitter release in cultured chick sympathetic neurons. We focused our attention on the effects of α2-adrenoceptor agonists on voltage-gated calcium currents and on the modulation of these effects by various substances known to activate or block the PKC system, because our previous experiments indicated that the N-type calcium channel represents the final effector of the α2-adrenoceptor-mediated autoinhibition of transmitter release in these neurons (Boehm and Huck, in press).
MATERIALS AND METHODS
Cell cultures. The procedures for dissociation and culture of chick sympathetic neurons have been described previously (Boehm et al., 1991, 1994). Briefly, paravertebral sympathetic ganglia were dissected from 12-d-old chick embryos, trypsinized (0.1% for 30 min at 36°C), triturated, resuspended in DMEM (Gibco 041-01885M; Life Technologies, Gaithersburg, MD) containing 2.2 g/l glucose, 10 mg/l insulin, 25,000 IU/l penicillin, and 25 mg/l streptomycin (Gibco 043-05140D), 10 μg/l nerve growth factor (Gibco 0436050), and 5% fetal calf serum (Gibco 011-0620H), and plated on poly-d-lysine-coated (Sigma 1149; Sigma, St. Louis, MO) tissue culture dishes (Nunc 153066; Nunc, Naperville, IL) (∼5 × 105 neurons per dish). Non-neural cells were reduced to <5% by differential plating, as described elsewhere (Boehm et al., 1994), when the cellular distribution of PKC isoforms was investigated.
Electrophysiology. Whole-cell Ca2+currents were recorded at room temperature (20–24°C) from cell bodies of sympathetic neurons after 24–48 hr in vitro, as described elsewhere (Boehm and Huck, 1991). The internal (pipette) solution contained (in mm): 115 N-methyl-d-glucamine, 20 tetraethylammonium chloride, 1.6 CaCl2, 2 Mg-ATP, 2 Li-GTP, 10 EGTA, 10 glucose, and 20 HEPES, adjusted to pH 7.3 with HCl, which results in a nominal calcium concentration of 0.011 μm. For applications of arachidonic acid (AA) or linoleic acid (LinA), cells were dialyzed with 200 U/ml superoxide dismutase (SOD) via the recording pipette to prevent the formation of free radicals (Chan et al., 1988; Keyser and Alger, 1990). The external (bathing) solution consisted of (in mm): 120 choline chloride, 5 CaCl2, 20 glucose, and 10 HEPES, adjusted to pH 7.3 with KOH. Ca2+ currents were elicited by depolarizations from a holding potential of −80 to 0 mV at a frequency of 2–4 min−1. To account for the time-dependent rundown of Ca2+ currents (see Fig. 3), drug effects were evaluated by measuring currents in the presence of test drugs (B) and by comparing them with control currents recorded before (A) and after (washout,C) the application of the drugs (Boehm and Huck, 1991), according to 200 × B/(A + C) = % of control current.
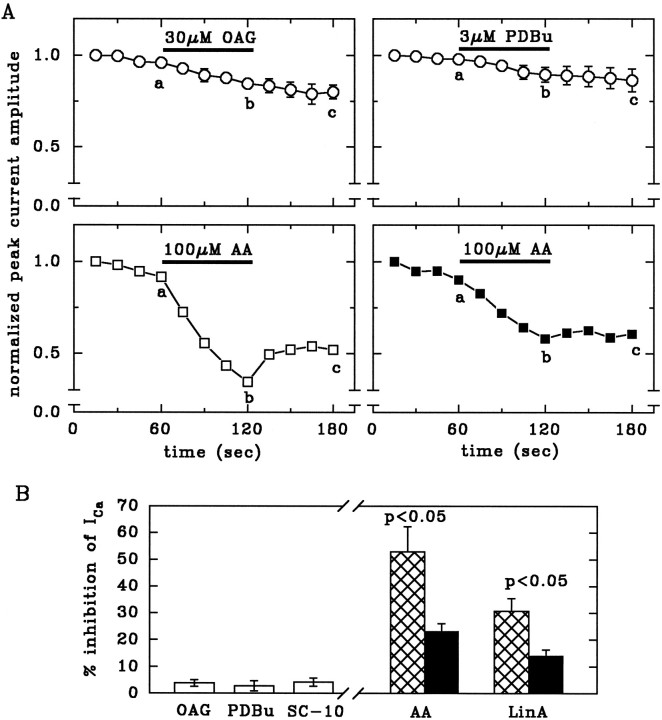
Fig. 3.
AA and LinA, but not phorbol esters or OAG, inhibit Ca2+ currents. A, Time course of peak current amplitudes (normalized to the first amplitude) and effects of OAG, PDBu, and AA. These drugs were applied to cells dialyzed for at least 10 min with standard pipette solution (open circles), with 200 U/ml SOD (open squares), or with SOD plus 10 μm of the peptide inhibitor PKCI(19–36) (filled squares). n = 5–6.B, Inhibition of Ca2+ currents by 30 μm OAG, 3 μm PDBu, 10 μm SC-10, 100 μm AA, or 100 μm LinA, calculated as % inhibition = 100 − (200 b/a + c), wherea, b, and c are the current amplitudes measured after 60, 120, and 180 sec, respectively, as indicated inA. Neurons were dialyzed for at least 10 min with standard pipette solution (open bars), with SOD (hatched bars), or with SOD plus 10 μm of the peptide inhibitor PKCI(19–36) (filled bars), which significantly attenuates the effects of AA and LinA. Levels of significance for the difference between corresponding bars are indicated. n = 5–6.
Determination of the cellular distribution of PKC isoforms.Pure (>95%) neuronal cell cultures were subjected to long-term (24 hr) or short-term (10 min) treatment of a number of known PKC activators, including 4-β-phorbol dibutyrate (PDBu), oleoylacetylglycerol (OAG), AA, and LinA. As a control for PKC-independent effects of phorbol esters, the inactive isomer 4-α-phorbol dibutyrate (αPDBu) was used. Thereafter, cultures were rinsed twice with the bathing solution described above, incubated with 50 mm β-glycerophosphate, 6 mm EGTA, 5 μm leupeptin, and 1 mm phenylmethylsulfonylfluoride in bathing solution, and rapidly frozen by adding liquid nitrogen. After they were thawed, cells were scraped off the dishes and subjected to a second freeze-thaw cycle. Cytosolic and membrane fractions were separated by centrifugation (50,000 × g for 30 min at 2°C). Cytosolic proteins were precipitated with trichloroacetic acid (14% final concentration) and subsequently dissolved in Laemmli sample buffer supplemented with 2% SDS and 40 mmdithiothreitol, whereas pellets were dissolved directly in supplemented Laemmli sample buffer. Samples corresponding to 1–2.5 × 105 cells were applied to SDS-polyacrylamide gels (running gel: 8% acrylamide, 0.21% N,N-methylene bisacrylamide). Proteins were subsequently transferred to nitrocellulose and stained with Ponceau S to verify that comparable amounts had been loaded. The nitrocellulose blots were probed with commercially available peptide antisera specific for PKCα (Santa Cruz Biotechnology, Tebu, France, 1:200 dilution), PKCε (Santa Cruz, 1:200 dilution), PKCζ (Life Technologies, 1:500 dilution, or Santa Cruz, 1:200). Additional isoforms were probed with antisera specific for PKC βI/II, δ, η, and θ (Santa Cruz, 1:200). The specificity of antigen–antibody reactions was checked by probing nitrocellulose blots with antibodies that had been preincubated with their corresponding immunogenic peptides. Immunostaining was carried out with a second antibody conjugated to horseradish-peroxidase using Amersham ECL-reagents (Amersham, Madison, WI). The immunoreactive bands from chick sympathetic cultures comigrated with immunoreactive bands detected in rat cerebral cortical homogenates.
Statistics. Data are given as arithmetic mean ± SEM, unless indicated otherwise; n = number of individual cells in whole-cell recordings. Significance of differences between single data points was evaluated by the unpaired Student’st test, unless indicated otherwise.
Drugs and reagents. AA, LinA, OAG, and SOD were from Sigma (Vienna, Austria);N-(n-heptyl)-5-chloro-1-naphthalenesulfonamide (SC-10), 5-bromo-N-(4,5-dihydro-1-H-imidazol-2-yl)-6-quinoxalinamine (UK 14,304), staurosporine, and calphostin C were from Research Biochemicals (Natick, MA); αPDBu and PDBu, the pseudosubstrate peptide inhibitor of PKC [PKCI(19–36)], a noninhibitory analog peptide [glu27-PKCI(19–36)], and a peptide inhibitor of PKA [PKI(6–22)amide] were from Life Technologies.
SC-10 (10 mm), staurosporine (1 mm), calphostin C (1 mm), and OAG (30 mm) were dissolved initially in dimethyl sulfoxide (DMSO); AA (100 mm) and LinA (100 mm) were dissolved in ethanol. Drugs were then diluted to the final concentrations (1:1000) in buffer, and appropriate controls verified that neither DMSO nor ethanol at 0.1% affected the parameters tested.
RESULTS
Effects of protein kinase inhibitors on the α2-adrenergic inhibition of Ca2+currents
In line with our previous observations (Boehm and Huck, 1991), the specific α2-adrenoceptor agonist UK 14,304 reversibly inhibited voltage-activated calcium currents (Figs.1, 2). The effect of UK 14,304 was reduced progressively within minutes when the PKC pseudosubstrate inhibitor PKCI(19–36) (10 μm) (House and Kemp, 1987) was included in the pipette solution (Figs. 1A,B,2B). Peak calcium current amplitudes in the absence of UK 14,304 were not affected significantly by PKCI(19–36) [control: 756 ± 90 pA, n = 18; PKCI(19–36): 633 ± 69 pA, n = 17;p > 0.25]. Likewise, the dialysis of neurons with calphostin C and staurosporine (both at 1 μm) for 10 min significantly diminished the α2-adrenergic inhibition (Fig.2A,B). A noninhibitory analogous peptide [glu27-PKCI(19–36)] and a selective peptide inhibitor of PKA [PKAI(6–22)amide] (Glass et al., 1989) had no effect on the UK 14,304-induced inhibition (Figs.1A,B, 2B). Even under conditions in which these substances effectively blocked the action of UK 14,304, neither PKCI(19–36) nor calphostin C or staurosporine had any noticeable effect on the somatostatin-induced inhibition of calcium currents (Figs. 1, 2A). In chick sympathetic neurons, staurosporine has been shown previously not to block the calcium current inhibition by somatostatin, although activation of PKC (by pretreatment with OAG or phorbol-12-myristate-13-acetate) significantly reduced the effects of somatostatin (Golard et al., 1993). Because both substances are membrane-permeable, we could apply staurosporine and calphostin C extracellularly after the effects of UK 14,304 had initially been established. Hence, in neurons exposed to 1 μm staurosporine or calphostin C, the subsequent inhibition by UK 14,304 in the continuing presence of either PKC inhibitor was reduced significantly (inhibition before staurosporine or calphostin C: 34.6 ± 2.2%, n = 8; after >3 min staurosporine: 11.6 ± 3.0%, n = 4; p < 0.001; after >3 min calphostin C: 18.8 ± 3.0%, n = 4; p < 0.01).
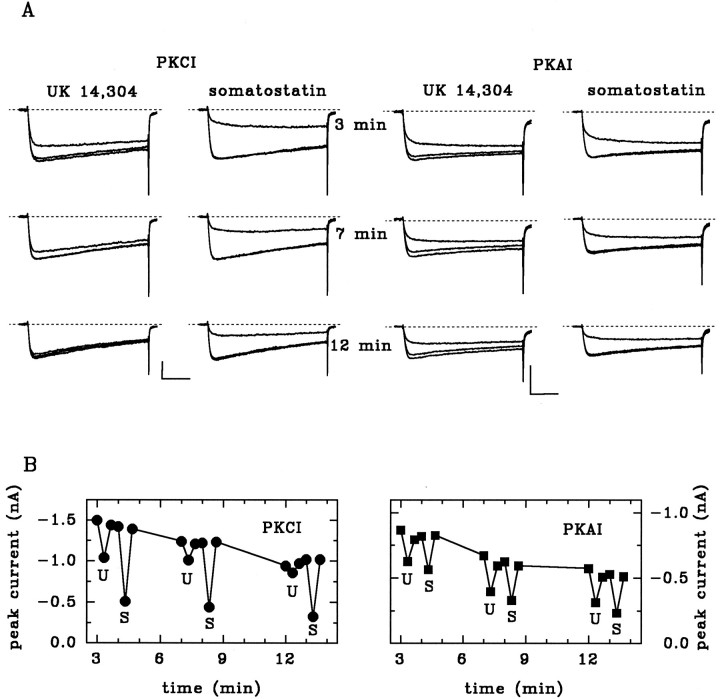
Fig. 1.
The PKC inhibitor PKCI(19–36) prevents the α2-adrenoceptor-mediated but not the somatostatin-mediated inhibition of Ca2+currents. A, Calcium currents were induced from a holding potential of −80 mV by depolarizing pulses to 0 mV. UK (10 μm) or somatostatin (1 μm) were applied to the same cells 3, 7, and 12 min after establishing the whole-cell condition. The recording pipette contained 10 μm peptide inhibitor against protein kinase C [PKCI(19–36)] (left panel) or 10 μm peptide inhibitor against protein kinase A [PKI(6–22)amide] (right panel). Traces show currents before, during, and after application of agonists. Calibration: 0.5 nA, 30 msec. B, Time-plots of recordings from the cells shown above in A. Filled symbols indicate test pulses in the absence or presence of 10 μm UK 14,304 (U) or 1 μm somatostatin (S).
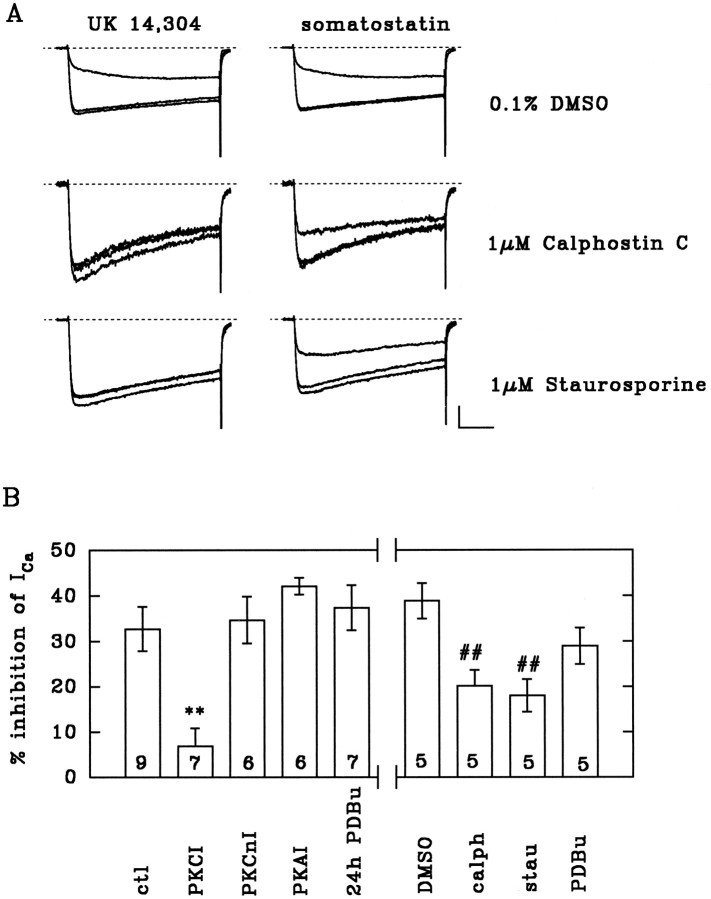
Fig. 2.
α2-Adrenoceptor-mediated inhibition of Ca2+ currents: summary of effects of PKC inhibitors. A, Inhibition of Ca2+ currents by 10 μm UK 14,304 (left) or 1 μm somatostatin (right) in cells dialyzed for at least 10 min with 0.1% DMSO, 1 μm calphostin C, or 1 μm staurosporine. Traces show currents before, during, and after the application of agonists. Calibration: 0.5 nA for DMSO, 0.25 nA for staurosporine, 0.1 nA for calphostin C; 30 msec. B, Inhibition of peak Ca2+ current amplitudes (ICa) by 10 μm UK 14,304 under control conditions (ctl) or with 10 μm PKCI(19–36), a peptide inhibitor of protein kinase C (PKCI); 10 μmglu27-PKCI(19–36), a noninhibitory analog peptide (PKCnI); 10 μmPKAI(6–22)amide, a peptide inhibitor of PKA (PKAI); 0.1% DMSO (DMSO); 1 μm calphostin C (calph); 1 μm staurosporine (stau); or 1 μm PDBu added to the pipette solution. Alternatively, cells pretreated for 24 hr with 10 μm β-phorbol-12,13-dibutyrate (24 hr PDBu) were tested with 10 μm UK 14,304 using regular pipette solution. Currents were recorded 10 min or later after breaking the cell membrane. **, p < 0.01 vs control; ##, p < 0.01 vs DMSO; n indicated in the bars.
To investigate further the role of PKC, a subset of cultures was treated with 10 μm PDBu for 24 hr. Long-lasting exposure of neurons to active phorbol esters downregulates PKC and results, for example, in a marked reduction of stimulated transmitter release (Matthies et al., 1987) or an attenuation of neuropeptide Y-mediated inhibition of voltage-activated calcium currents (Ewald et al., 1988). This treatment, however, failed to alter the α2-adrenergic inhibition of Ca2+ currents (Fig. 2B).
Effects of PKC activators on Ca2+ currents and on the α2-adrenergic inhibition
Our observation that the effects of UK 14,304 on voltage-activated calcium currents were attenuated by inhibitors of PKC, but were independent of a phorbol ester-induced PKC downregulation, indicate the involvement of phorbol ester-insensitive PKC isoforms. In line with this hypothesis, we found no effect of extracellularly applied 3 μm PDBu, 30 μm OAG, or 10 μm SC-10 (an activator of Ca2+-dependent PKC isoforms) (Ito et al., 1986) on voltage-gated calcium currents (Fig. 3). In addition, neither intracellular (Fig. 2) nor extracellular (not shown) applications of 1 μm PDBu significantly reduced the α2-adrenergic inhibition.
By contrast, the application of 100 μm AA (Fig.3A,B) or LinA (Fig. 3B) for 1 min reduced Ca2+ currents in a partially reversible manner. Both AA and LinA have been shown previously to activate not only classical but also atypical, phorbol ester-insensitive PKCs (Asaoka et al., 1992; Nishizuka, 1992; Nakanishi and Exton, 1992). Lower concentrations of AA (30 μm) were also effective (not shown) but less well distinguishable from the rundown of current that occurs in this and other preparations to a variable extent (Kostyuk et al., 1981). The effects of AA and LinA were reduced significantly when 10 μm PKCI was added to the patch pipette solution (Fig. 3A,B).
Pharmacological sensitivities of PKC isoforms in chick sympathetic neurons
At least 12 distinct subtypes of PKC have been characterized and categorized further into three groups (Nishizuka, 1992; Dekker and Parker, 1994): groups A and B comprise “classical” and “new” enzymes, all of which can be activated by active phorbol esters or diacylglycerol analogs. By contrast, group C consists of “atypical” PKC enzymes, which are insensitive to diacylglycerol and phorbol esters (Nishizuka, 1992). In view of the inhibitory action of PKC inhibitors and the lack of effects of phorbol esters on the α2-adrenoceptor-mediated inhibition of calcium currents, we investigated which of the various isoforms of PKC were present in chick sympathetic neurons. Immunoblot analysis with peptide antisera specific for PKC isoforms revealed the presence of α, βI/II, δ, ε, and ζ, but not η and θ, in both the cytosolic and membrane fractions obtained from pure neuronal cell cultures (not shown). Subsequently, PKCα, ε, and ζ as representatives of classical, new, and atypical PKC subtypes, respectively (Nishizuka, 1992), were investigated further.
PKCα and ε migrated with an apparent molecular mass of ∼80 and ∼90–95 kDa, respectively. Immunoblots with antisera specific for PKCζ from two different sources (Life Technologies and Santa Cruz) detected two bands migrating, with an estimated molecular mass of 72 and 78 kDa (Figs. 4, 5). These bands were also detected in homogenates (Fig. 4) and in membrane fractions from rat neocortex (not shown). The predicted molecular mass of PKCζ is 68 kDa; the enzyme purified from renal tissue migrated at ∼78 kDa (Nakanishi and Exton, 1992), and expression of the cDNA directed the synthesis of a ∼64 kDa protein in COS-7 cells (Ono et al., 1989) and of an ∼80 kDa protein in insect and mammalian cells (Liyanage et al., 1992). Thus, it is not clear whether the 72 kDa band observed in sympathetic neurons corresponds to a post-translationally modified PKCζ (e.g., by phosphorylation and/or proteolytic cleavage, known to occur in vivo) (Hug and Sarre, 1993), or to a different atypical PKC-isoform. PKC, for instance, shows not only a 72% identity with PKCζ, but an antibody raised against PKCζ also recognizes PKCι (Selbie et al., 1993). For practical purposes, we subsequently refer to both bands as PKCζ.
Fig. 4.
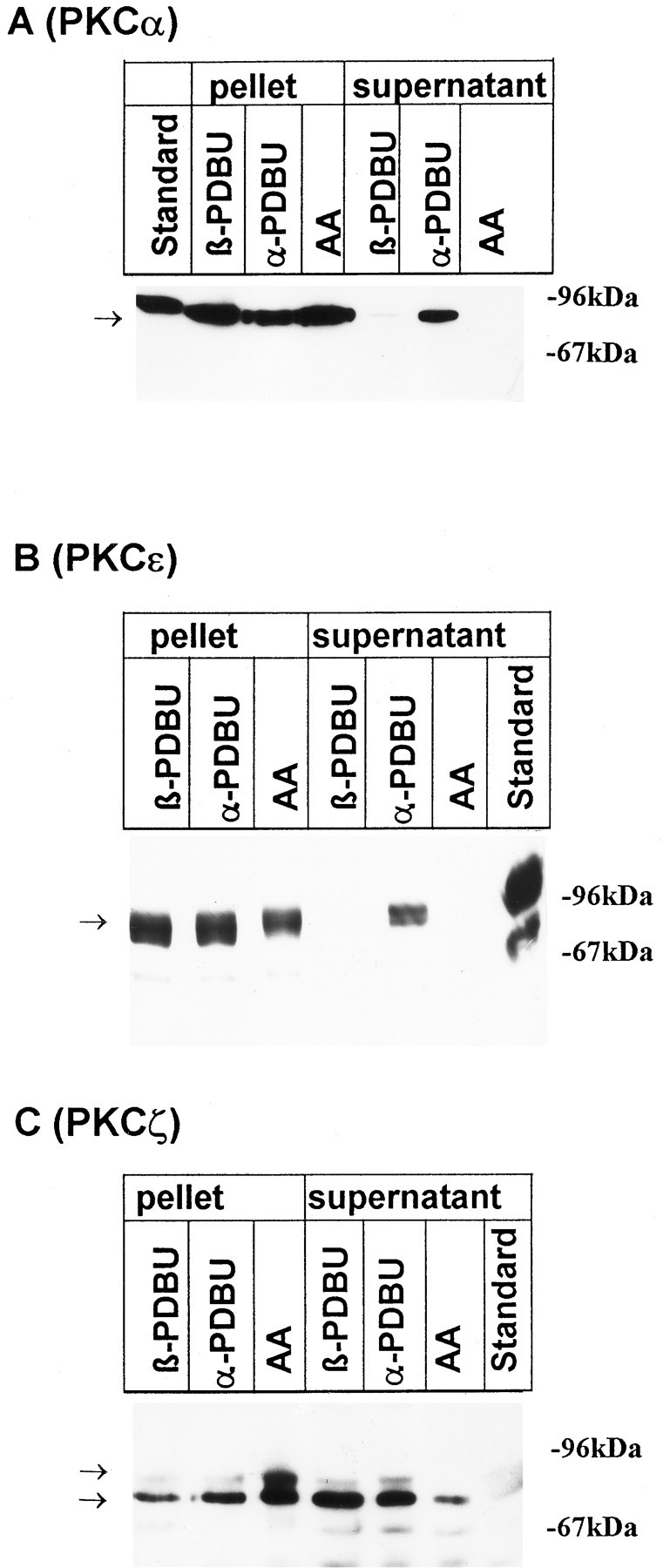
Translocation of PKCα, ε, and ζ isozymes by phorbol esters and AA. The distribution of protein kinase C isoforms was investigated by immunoblots after a 10 min incubation of cultures with 100 μmAA, with the inactive phorbol ester αPDBU, or with the active phorbol ester βPDBU, both at 1 μm. Cells were lysed as described in Materials and Methods; aliquots of the pellet and supernatant corresponding to 2 × 105 cells were applied to SDS-polyacrylamide gels. Nitrocellulose blots were probed with peptide antisera specific for PKCα (A), PKCε (B), and PKCζ (C). Standard: 10 μg protein from rat neocortical homogenate. Arrows indicate the position of bands that were suppressed when antibodies had been preincubated with the corresponding immunogenic peptide (not shown). Data are representative of three additional experiments performed on different preparations.
Fig. 5.
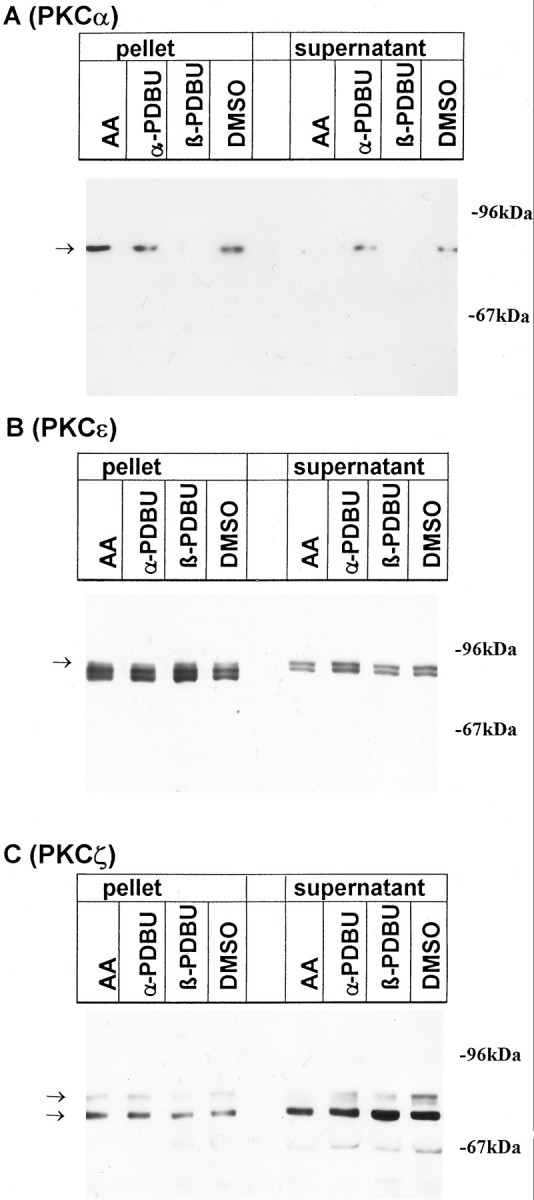
Downregulation of classical, but not atypical, PKC isozymes by phorbol esters. The distribution of protein kinase C isoforms was analyzed by immunoblots after a 24 hr treatment of cultures with 100 μmAA, with 1 μm of the inactive and active phorbol esters αPDBU and βPBDU, respectively, or with the vehicle 0.1% DMSO. Cells were lysed as described in Materials and Methods; aliquots of the pellet and supernatant corresponding to 2 × 105 cells were applied to SDS-polyacrylamide gels. Nitrocellulose blots were probed with peptide antisera specific for PKCα (A), PKCε (B), and PKCζ (C). Arrows indicate the position of bands that were blocked when antibodies had been preincubated with the corresponding immunogenic peptide (not shown). Data are representative of three additional experiments performed on different preparations.
A 10 min incubation of neurons with 1 μm of the active phorbol ester PDBu induced an almost complete translocation of PKCα and PKCε from the cytoplasm to the membrane, whereas the distribution of the PKCζ-bands was unaffected (Fig. 4). Exposure of the neurons to 30 μm OAG mimicked the effects of PDBu (not shown). αPDBu, the inactive analog of PDBu, did not affect the distribution of any of the PKC subtypes (Fig. 4). AA (100 μm), which also activates PKCζ (Nakanishi and Exton, 1992), trans- located all three isoforms from the cytosol to the membrane (Fig. 4) .
Long-term exposure (24 hr) of cultures to PDBu (1 μm), but not αPDBu, eliminated PKCα from both the cytoplasmic and the membrane fraction (Fig.5A). The response of PKC was variable: in two of four neuronal cultures, the levels of PKCε were essentially unaffected (Fig. 5B), whereas a clear-cut decline was observed in the two remaining experiments (not shown). The reason for this variability is not clear but is indicative of a relative refractoriness of PKCε to phorbol ester-dependent downregulation. The extent to which PKCε can be downregulated by phorbol esters is known to vary in different cell lines (Hug and Sarre, 1993). Unlike PKCα and ε, PKCζ-bands were affected in no instance by long-term incubation with the phorbol ester (Fig. 5C).
By contrast to the downregulation of PKCα and PKCε by PDBu, a 24 hr exposure to 100 μm AA consistentlyincreased the immunoreactivity for all three PKC isoforms (α, ε, and ζ) in the particulate fraction. This indicates that AA induced a persistent translocation of the three different PKC subtypes to the membranes but failed to downregulate any of the enzymes (Fig.5).
DISCUSSION
Inhibitors of PKC antagonize the effect of UK 14,304, but phorbol esters have no effect on calcium current and on the α2-adrenoceptor-mediated modulation
In line with our previous observations, the activation of α2-adrenoceptors by UK 14,304 caused an inhibition of voltage-activated calcium current in chick sympathetic neurons (Boehm and Huck, 1991). This inhibition was reduced significantly when the pseudosubstrate peptide inhibitor of PKC [PKCI(19–36), 10 μm] (House and Kemp, 1987) was included in the pipette solution. A noninhibitory analogous peptide [glu27-PKCI(19–36)] had no effect. Staurosporine and calphostin C, two membrane-permeant inhibitors of PKC, exerted effects similar to those of PKCI(19–36), regardless of whether they were applied through the recording pipette or extracellularly.
These results imply that PKC is part of the signaling cascade that leads to an inhibition of voltage-gated calcium channels by UK 14,304. In fact, numerous reports have provided evidence that effects of neurotransmitter receptor activation on calcium currents involve PKC (Diverse-Pierluissi and Dunlap, 1993). Neither the phorbol ester PDBu nor the synthetic diacylglycerol analog OAG, however, were able to mimic the effects of UK 14,304, and downregulation of PKC by a 24 hr incubation of cultures with PDBu did not attenuate the inhibition of calcium currents by UK 14,304. In rat sympathetic neurons, OAG (50 μm) was reported to inhibit calcium currents, but because L-type currents were affected along with N-type currents, and because PKC inhibitors like PKCI(19–31) did not affect transmitter-mediated inhibitions of calcium currents, a direct involvement of PKC on both transmitter- and OAG-mediated effects was ruled out (Plummer et al., 1991). Bley and Tsien (1990) also found no evidence for a PKC-mediated pathway of a peptidergic inhibition in frog sympathetic neurons, because phorbol esters did not mimic the inhibition of calcium currents and the PKC inhibitor staurosporine did not block the peptidergic effects. By contrast, the summary of our observations implies a phorbol ester-insensitive (atypical) PKC as part of the signaling cascade of α2-adrenoceptor-mediated effects in chick sympathetic neurons.
At present, we cannot exclude the possibility that additional signaling pathways independent of PKC may be involved, particularly because a small inhibition of calcium currents by UK 14,304 persisted in the presence of PKCI(19–36). In chick sensory neurons, α2-adrenoceptors couple to two types of pertussis toxin-sensitive G-proteins and use two separate pathways to regulate N-channel function. One includes G-protein βγ-subunits as well as PKC, whereas the second signal cascade seems to be independent of PKC but involves Goα (Diverse-Pierluissi et al., 1995).
Phorbol ester-sensitive and -insensitive PKC isoforms are expressed in chick sympathetic neurons
To date, three major groups of PKC isozymes have been characterized (Asaoka et al., 1992: Nishizuka, 1992; Dekker and Parker, 1994): groups A and B comprise “classical” and “new” enzymes, all of which can be activated by active phorbol esters or diacylglycerol analogs. Group C, by contrast, consists of “atypical” PKC enzymes that are insensitive to diacylglycerol and phorbol esters (Nishizuka, 1992).
PKCα and PKCε, as representatives of classical and new PKC isoforms, responded to short-term incubations of cultures with PDBu and OAG by a translocation from the cytosol to the membrane, indicating that phorbol ester-sensitive PKC isoforms are present in the chick sympathetic neurons. Our data also indicate the presence of “atypical” PKCζ, which was translocated by AA but not by PDBu or OAG. All subtypes of PKC are likely to be inhibited by PKCI (House and Kemp, 1987) and staurosporine (Nakadate et al., 1988), both of which act at the catalytic domain highly conserved between all known PKC subtypes (Nishizuka, 1992; Hug and Sarre, 1993). Likewise, calphostin C, although acting at the regulatory domain of PKCs, seems to inhibit a broad range of PKC isoforms, because it reduced the activity of Ca2+-independent PKC isozymes at least as potently as the classical Ca2+-dependent isoforms (Ozawa et al., 1993). Hence, we may expect that PKCI, staurosporine, and calphostin C all inhibit not only classical isoforms of PKC but also atypical forms unresponsive to phorbol esters. Our experiments indicate that such isoforms are present in chick sympathetic neurons and may therefore mediate the α2-adrenergic inhibition of calcium currents in a phorbol ester-insensitive manner. PKCζ has been demonstrated in nervous tissue and has been suggested as playing a role in the maintenance of long-term potentiation in the hippocampal CA1 region (Sacktor et al., 1993).
AA induces translocation of all PKC isozymes and inhibits voltage-activated calcium channel currents
AA and related fatty acids are known, but not exclusive, activators of most PKC isozymes (Asaoka et al., 1992; Nishizuka, 1992). Our experiments indicate that both short- and long-term incubations of cultures with 100 μm AA induce translocation of PKCα and PKCε as well as PKCζ from the cytosol to the membrane fraction in chick sympathetic cultures. Extracellular applications of 100 μm AA or 100 μmLinA also inhibited voltage-activated calcium currents, and these effects were attenuated by >50% when PKCI(19–36) was included in the pipette solution, indicating a PKC-specific component of the phenomenon. Residual effects in the presence of the PKC inhibitor might be attributable to a straight action on calcium channels, because AA also affects transmembrane ion channels directly (Ordway et al., 1991;Fraser et al., 1993; Meves, 1994).
The link between α2-adrenoceptors and the activation of atypical PKC remains to be identified
Free AA, which may be generated by receptor-mediated activation of PLA2 (Axelrod, 1990), exerts a plethora of effects in the nervous system by the activation of PKC (Axelrod et al., 1988; Keyser and Alger, 1990; Meves, 1994). We therefore tested whether UK 14,304 would enhance cellular levels of AA, thus making AA not only a pharmacological tool but also a physiological candidate in the signal transduction pathway between α2-adrenoceptors and the activation of atypical PKC. Our experiments were inconclusive, however, because short-term incubations with UK 14,304 enhanced the generation of free AA in only 7 of 12 experiments (S. Boehm, S. Huck, and M. Freissmuth, unpublished observations). In addition, the high concentrations required to inhibit calcium currents render AA an unlikely candidate to mediate the effect of UK 14,304. Hence, in the signaling cascade α2-adrenoceptors→activation of an atypical PKC→inhibition of calcium currents, as delineated in this description for chick sympathetic neurons, the missing link or links between α2-adrenoceptors and atypical PKC remain to be identified.
Footnotes
This study was supported by grants from the Austrian Science Foundation (FWF) to M.F. (P10675) and from the Anton-Dreher-Gedächtnisschenkung to S.B. (202/92 and 229/93). We thank D. S. McGehee and V. O’Connor for valuable comments on this manuscript. The perfect technical assistance of G. Koth, A. Motejlek, and K. Schwarz and the skillful artwork of E. Tuisl are gratefully acknowledged.
Correspondence should be addressed to Sigismund Huck, Department of Neuropharmacology, University of Vienna, Waehringerstrasse 13A, A-1090 Vienna, Austria.
Dr. Boehm’s present address: Department of Neurochemistry, Max Planck Institute for Brain Research, Deutschordenstrasse 46, D-60528 Frankfurt, Germany.
REFERENCES
- 1.Asaoka Y, Nakamura S-I, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17:414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990;18:503–507. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod J, Burch RM, Jelsema CL. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988;11:117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- 4.Beech DJ, Bernheim L, Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992;8:97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
- 5.Bley KR, Tsien RW. Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron. 1990;2:379–391. doi: 10.1016/0896-6273(90)90050-p. [DOI] [PubMed] [Google Scholar]
- 6.Boehm S, Huck S. Modulation of calcium currents via α2-adrenoreceptors in embryonic chick sympathetic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:382–385. doi: 10.1007/BF00183015. [DOI] [PubMed] [Google Scholar]
- 7.Boehm S, Huck S (1996) Inhibition of N-type calcium channels: the only mechanism by which presynaptic α2-autoreceptors control sympathetic transmitter release. Eur J Neurosci, in press. [DOI] [PubMed]
- 8.Boehm S, Huck S, Drobny H, Singer EA. Electrically evoked noradrenaline release from cultured chick sympathetic neurons: modulation via presynaptic α2-adrenoceptors and lack of autoinhibition. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:130–132. doi: 10.1007/BF00167393. [DOI] [PubMed] [Google Scholar]
- 9.Boehm S, Huck S, Drobny H, Singer EA. Pertussis toxin abolishes the inhibition of Ca2+ currents and of noradrenaline release via α2-adrenoreceptors in chick sympathetic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:606–609. doi: 10.1007/BF00168956. [DOI] [PubMed] [Google Scholar]
- 10.Boehm S, Huck S, Koth G, Agneter E, Drobny H, Singer EA. α2-Adrenoceptor-mediated inhibition of electrically evoked [3H]noradrenaline release from chick sympathetic neurons: role of cyclic AMP. J Neurochem. 1994;63:146–154. doi: 10.1046/j.1471-4159.1994.63010146.x. [DOI] [PubMed] [Google Scholar]
- 11.Boehm S, Huck S, Motejlek A, Drobny H, Singer EA, Freissmuth M. Cholera toxin induces cyclic AMP-independent downregulation of Gsα-autoreceptors in chick sympathetic neurons. J Neurochem. 1996;66:1019–1026. doi: 10.1046/j.1471-4159.1996.66031019.x. [DOI] [PubMed] [Google Scholar]
- 12.Boland LM, Allen AC, Dingledine R. Inhibition by bradykinin of voltage-activated barium current in a rat dorsal root ganglion cell line: role of protein kinase C. J Neurosci. 1991;11:1140–1149. doi: 10.1523/JNEUROSCI.11-04-01140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan PH, Chen SF, Yu ACH. Induction of intracellular superoxide radical formation by arachidonic acid and by polyunsaturated fatty acids in primary astrocytic cultures. J Neurochem. 1988;50:1185–1193. doi: 10.1111/j.1471-4159.1988.tb10591.x. [DOI] [PubMed] [Google Scholar]
- 14.Dekker LV, Parker PJ. Protein kinase C: a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 15.Delcour AH, Tsien RW. Altered prevalence of gating modes in neurotransmitter inhibition of N-type calcium channels. Science. 1993;259:980–984. doi: 10.1126/science.8094902. [DOI] [PubMed] [Google Scholar]
- 16.Diverse-Pierluissi M, Dunlap K. Distinct, convergent second messenger pathways modulate neuronal calcium currents. Neuron. 1993;10:753–760. doi: 10.1016/0896-6273(93)90175-q. [DOI] [PubMed] [Google Scholar]
- 17.Diverse-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich I, Elmslie KS. Neurotransmitters acting via different G proteins inhibit N-type calcium current by an identical mechanism in rat sympathetic neurons. J Neurophysiol. 1995;74:2251–2257. doi: 10.1152/jn.1995.74.6.2251. [DOI] [PubMed] [Google Scholar]
- 19.Elmslie KS, Kammermeier PJ, Jones SW. Calcium current modulation in frog sympathetic neurones: L-current is relatively insensitive to neurotransmitters. J Physiol (Lond) 1992;456:107–123. doi: 10.1113/jphysiol.1992.sp019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewald DA, Matthies HJG, Perney TM, Walker MW, Miller RJ. The effect of downregulation of protein kinase C on the inhibitory modulation of dorsal root ganglion neuron Ca2+ currents by neuropeptide Y. J Neurosci. 1988;8:2447–2451. doi: 10.1523/JNEUROSCI.08-07-02447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser DD, Hoehn K, Weiss S, MacVicar BA. Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron. 1993;11:633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- 22.Galvan M, Adams PR. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 1982;244:135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- 23.Glass DB, Cheng H-C, Mende-Mueller L, Reed J, Walsh DA. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J Biol Chem. 1989;264:8802–8810. [PubMed] [Google Scholar]
- 24.Golard A, Siegelbaum SA. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993;13:3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golard A, Role LW, Siegelbaum SA. Protein kinase C blocks somatostatin-induced modulation of calcium current in chick sympathetic neurons. J Neurophysiol. 1993;70:1639–1643. doi: 10.1152/jn.1993.70.4.1639. [DOI] [PubMed] [Google Scholar]
- 26.Hescheler J, Schultz G. G-proteins involved in the calcium signalling system. Curr Opinion Neurobiol. 1993;3:360–367. doi: 10.1016/0959-4388(93)90129-m. [DOI] [PubMed] [Google Scholar]
- 27.Horn JP, McAfee DA. Alpha-adrenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol (Lond) 1980;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototype in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 29.Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Tanaka T, Inagaki M, Nakanishi K, Hidaka H. N -(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide, a novel activator of protein kinase C. Biochemistry. 1986;25:4179–4184. doi: 10.1021/bi00363a002. [DOI] [PubMed] [Google Scholar]
- 32.Keyser DO, Alger BE. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990;5:545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- 33.Kostyuk PG, Veselovsky NS, Fedulova SA. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6:2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- 34.Lipscombe D, Kongsamut S, Tsien RW. α-Adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium channel gating. Nature. 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- 35.Liyanage M, Frith D, Livneh E, Stabel S. Protein kinase C group B members PKC-δ, -ε, -ζ and PKC-L(η). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992;283:781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchetti C, Brown AM. Protein kinase C activator 1-oleoyl-2-acetyl-sn -glycerol inhibits two types of calcium currents in GH3 cells. Am J Physiol. 1988;254:C206–C210. doi: 10.1152/ajpcell.1988.254.1.C206. [DOI] [PubMed] [Google Scholar]
- 37.Mathie A, Bernheim L, Hille B. Inhibition of N- and L-type calcium channels by muscarinic receptor activation in rat sympathetic neurons. Neuron. 1992;8:907–914. doi: 10.1016/0896-6273(92)90205-r. [DOI] [PubMed] [Google Scholar]
- 38.Matthies HJG, Palfrey HC, Hirning LD, Miller RJ. Downregulation of protein kinase C in neuronal cells: effects on neurotransmitter release. J Neurosci. 1987;7:1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994;43:175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 40.Miller RJ. Receptor-mediated regulation of calcium channels and neurotransmitter release. FASEB J. 1990;4:3291–3299. [PubMed] [Google Scholar]
- 41.Mochida S, Kobayashi H. Protein kinase C activators mimic the m2-muscarinic receptor-mediated effects on the action potential in isolated sympathetic neurons of rabbits. Neurosci Lett. 1988;86:201–206. doi: 10.1016/0304-3940(88)90571-x. [DOI] [PubMed] [Google Scholar]
- 42.Nakadate T, Jeng AY, Blumberg PM. Comparison of protein kinase C functional assays to clarify mechanisms of inhibitor action. Biochem Pharmacol. 1988;37:1541–1545. doi: 10.1016/0006-2952(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi H, Exton JH. Purification and characterization of the ζ isoform of protein kinase C from bovine kidney. J Biol Chem. 1992;267:16347–16354. [PubMed] [Google Scholar]
- 44.Nishizuka Y. Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 45.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase C ζ subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ordway RW, Singer JJ, Walsh JV. Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991;14:96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- 47.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 48.Plummer MR, Rittenhouse A, Kanevsky M, Hess P. Neurotransmitter modulation of calcium channels in rat sympathetic neurons. J Neurosci. 1991;11:2339–2348. doi: 10.1523/JNEUROSCI.11-08-02339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rane SG, Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci USA. 1986;83:184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rane SG, Walsh MP, McDonald JR, Dunlap K. Specific inhibitors of protein kinase C block transmitter-induced modulation of sensory neuron calcium current. Neuron. 1989;3:239–245. doi: 10.1016/0896-6273(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 51.Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schofield GG. Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an α2-adrenoceptor. Eur J Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz DD, Malik KU. Cyclic AMP modulates but does not mediate the inhibition of [3H]norepinephrine release by activation of α2-adrenergic receptors in cultured rat ganglion cells. Neuroscience. 1993;52:107–113. doi: 10.1016/0306-4522(93)90186-j. [DOI] [PubMed] [Google Scholar]
- 54.Selbie LA, Schmitz-Pfeiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC 105, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 55.Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N -ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song SY, Saito K, Noguchi K, Konishi S. Adrenergic and cholinergic inhibition of Ca2+ channels mediated by different GTP-binding proteins in rat sympathetic neurones. Pflügers Arch. 1991;418:592–600. doi: 10.1007/BF00370576. [DOI] [PubMed] [Google Scholar]
- 57.Werz MA, Macdonald RL. Phorbol esters: voltage-dependent effects on calcium-dependent action potentials of mouse central and peripheral neurons in cell culture. J Neurosci. 1987;7:1639–1647. doi: 10.1523/JNEUROSCI.07-06-01639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]