Abstract
The purpose of this study was to determine the roles of the presynaptic 5-hydroxytryptamine1A(5-HT1A) receptors in the median raphénucleus (MRN) and of the postsynaptic 5-HT1Areceptors in its projection area of the dorsal hippocampus in the social interaction and elevated plus-maze tests of anxiety. Direct administration of the 5-HT1A receptor agonist (±)-8-hydroxy-dipropylaminotetralin (8-OH-DPAT, 200 ng) into the MRN had significant anxiolytic effects in all three test situations examined (social interaction, plus-maze trials 1 and 2). These anxiolytic effects were antagonized by a silent dose (200 ng) of the 5-HT1A receptor antagonist WAY 100635, confirming that they were mediated by 5-HT1A receptors. In contrast, after bilateral administration to the dorsal hippocampus, 8-OH-DPAT (100 ng) had significant anxiogenic effects in the social interaction test and in plus-maze trial 2. These anxiogenic effects were antagonized by silent doses of 5-HT1Areceptor antagonists [(+)WAY 100135, 10 mg/kg s.c., and intrahippocampal (±)tertatolol, 3 μg, respectively], confirming mediation by 5-HT1A receptors. In rats naive to the plus-maze, neither 8-OH-DPAT (50, 100, or 200 ng) nor the 5-HT1A receptor antagonist (±)tertatolol (3 μg) had any significant effect when administered to the dorsal hippocampus. This demonstrates that previous experience of a rat in the plus-maze has a major effect on the sensitivity of dorsal hippocampal 5-HT1A receptors, as we have demonstrated previously for the 5-HT1A receptors in the dorsal raphé nucleus. Overall, our results provide evidence that stimulation of the presynaptic 5-HT1A receptors in the MRN results in an anxiolytic action, whereas stimulation of the post-synaptic 5-HT1A receptors in its projection area results in an anxiogenic effect. These results are consistent with an overall reduction in 5-HT neurotransmission in the dorsal hippocampus having an anxiolytic effect, and they explain the relatively weak anxiolytic profile detected when 5-HT1A receptor agonists are given systemically.
Keywords: 5-HT1A, hippocampus, median raphé nucleus, elevated plus-maze, social interaction, anxiety
It is generally believed that the anxiolytic effects of 5-hydroxytryptamine1A(5-HT1A) receptor agonists are mediated through stimulation of the somatodendritic autoreceptors of the 5-HT neurones in the raphé nuclei, leading to a reduction in 5-HT neuronal firing rate (Sprouse and Aghajanian, 1987) and a subsequent decrease in 5-HT release in terminal areas of the limbic system, such as the hippocampus (Hutson et al., 1989; Sharp et al., 1990). In support of this hypothesis, administration of low doses of the 5-HT1A receptor agonist (±)-8-hydroxy-dipropylaminotetralin (8-OH-DPAT) directly into the dorsal or median raphé nucleus produces anxiolytic effects in the social interaction test (Higgins et al., 1988; Andrews et al., 1994;Hogg et al., 1994) and in foot-shock-induced ultrasonic vocalizations (Schreiber and De Vry, 1993). In the black-white crossing test and conditioned suppression of drinking, anxiolytic effects were found after administration to the median, but not to the dorsal, raphénucleus (Carli and Samanin, 1988).
The effects in the elevated plus-maze of 8-OH-DPAT administration to the dorsal raphé nucleus were found to depend on the experience of the animal; thus, there was no anxiolytic effect of doses up to 200 ng in rats naive to the maze, whereas 100 ng was anxiolytic in rats with one previous 5 min trial in the maze (File and Gonzalez, 1996). In both the social interaction test and the plus-maze, the anxiolytic effects of 8-OH-DPAT administration to the dorsal raphé nucleus were shown to be attributable to actions on 5-HT1A receptors, because they were antagonized by the 5-HT1A receptor antagonist tertatolol (Hogg et al., 1994; File and Gonzalez, 1996). In the social interaction test, the anxiolytic effect of 8-OH-DPAT in the median raphénucleus has not yet been firmly identified as being mediated by 5-HT1A receptors, because it has not been reversed by a 5-HT1A receptor antagonist. The effects in the plus-maze of administration of 5-HT1A receptor ligands to the median raphénucleus have not yet been reported, and it is clear that the relative importance of dorsal and median raphé nuclei differs among different animal tests of anxiety. In the first series of experiments, therefore, we examined the effects of 8-OH-DPAT, of the 5-HT1A receptor antagonist WAY 100635 (Forster et al., 1995), and of their combination in the social interaction test and in rats naive to, or with one previous 5 min trial on, the elevated plus-maze.
In contrast to the anxiolytic effects of 5-HT1Areceptor agonist administration to raphé nuclei, administration of low doses to postsynaptic sites has been reported to have anxiogenic effects. Thus, anxiogenic effects of 5-HT and 8-OH-DPAT have been reported after administration to the amygdala (Hodges et al., 1987;Higgins et al., 1991; Gonzalez et al., in press), and an anxiogenic effect of a low dose of 8-OH-DPAT (100 ng) was found after administration to the dorsal hippocampus (Andrews et al., 1994). Although high doses of 5-HT1A receptor agonists and partial agonists have been reported to have anxiolytic effects after administration to the dorsal hippocampus, this has been suggested as resulting from a spread of the compounds back to the raphénuclei (Jolas et al., 1995). An important question is whether the observed anxiogenic effects of 8-OH-DPAT (as well as the presynaptically mediated anxiolytic effects) are attributable to an action at 5-HT1A receptors. We therefore investigated the effects of 8-OH-DPAT administration to the dorsal hippocampus in the social interaction test and in rats naive to, and experienced with, the plus-maze, and we determined whether the observed effects could be antagonized by 5-HT1A receptor antagonists. Because of the complete unavailability of WAY 100635 for this part of the study, we used two other compounds. In the social interaction study we used (+)WAY 100135, a specific 5-HT1A receptor antagonist (Fletcher et al., 1993), but one that is unsuitable for central administration and therefore was administered subcutaneously. In the plus-maze studies, we used (±)tertatolol (Jolas et al., 1993; Prisco et al., 1993), which is soluble at neutral pH in artificial cerebrospinal fluid (aCSF), and which has been used in previous studies (Andrews et al., 1994; Hogg et al., 1994; File and Gonzalez, 1996). In the social interaction test, the doses of 8-OH-DPAT were determined on the basis of those that were effective in our previous studies; in the elevated plus-maze, the dose of tertatolol was that used in our previous studies. In all other cases, dose–response studies were conducted first to select the doses to be used in the antagonism studies.
MATERIALS AND METHODS
Animals
Male hooded Lister rats weighing 200–300 gm (Olac, Bicester, UK) were housed singly after surgery and allowed to recover for 7 d before behavioral testing. Food and water were freely available, and the room in which they were housed was lit with dim light and maintained at 22°C. Lights were on from 7 A.M. until 7 P.M. To accustom the animals to handling and to keep the cannulae patent, each day after surgery the rats were wrapped gently in a cloth and the stylets were replaced.
Apparatus
The social interaction test arena was a wooden box 60 × 60 cm with 35 cm high walls, and it was lit by high light (300 lux) for the median raphé nucleus studies (because this condition maximizes sensitivity to anxiolytic effects) and by low light (30 lux) for the dorsal hippocampus studies (to maximize sensitivity to anxiogenic effects). A camera was mounted vertically above the arena, and the rats were observed on a monitor in an adjacent room by an observer who was blind to the drug treatment. The time spent in social interaction (sniffing, following and grooming the partner, boxing, and wrestling) provides the measure of anxiety. Infrared photocells were mounted in the walls 4.5 and 12 cm from the floor, and the interruption of these beams provided automated measures of locomotor activity and rearing, respectively (for details, see File, 1980).
The plus-maze was made of wood and consisted of two opposite open arms 50 × 10 cm and two opposite arms enclosed by 40 cm high walls. The arms were connected by a central 10 × 10 cm square, and thus the maze formed a “plus” shape. The maze was elevated 50 cm from the floor and lit by dim light. A closed-circuit TV camera was mounted vertically over the maze, and the behavior was scored from a monitor in an adjacent room. All scores were entered directly into an IBM computer. The percentage of time spent on the open arms of the maze provides the measure of anxiety, and the number of closed arm entries provides the best measure of locomotor activity in this test (Pellow et al., 1985; File, 1991).
Drugs and chemicals
8-OH-DPAT hydrobromide (Research Biochemicals, St. Albans, UK), WAY 100635 (Wyeth, Taplow, UK), and (±)tertatolol hydrochloride (I.R.I.S., Paris, France) were dissolved in aCSF of the following composition (in mm): 126.6 NaCl, 27.4 NaHCO3, 2.4 KCl, 0.5 KH2PO4, 0.89 CaCl2, 0.8 MgCl2, 0.48 Na2HPO4, and 7.1 glucose, pH 7.4. When agonist and antagonist compounds were administered together, they were coadministered in a single injection. Injections were 0.5 μl, except those involving 200 ng 8-OH-DPAT, which were 0.6 μl. They were made over a period of 30 sec, and the needles were left in position an additional 30 sec to allow drug diffusion. (+)WAY 100135 (Wyeth) was dissolved in physiological saline at a concentration of 10 mg/ml and injected subcutaneously 30 min before testing. All drug concentrations reflect base weight.
Procedure
Surgery. Stereotaxic coordinates were verified histologically before each set of cannulations. Rats were anesthetized by inhalation of 3% halothane (May and Baker, Dagenham, UK) in oxygen and positioned in a stereotaxic frame (Kopf Instruments). The skull was exposed, and the incisor bar was adjusted such that bregma and lambda were at the same height. Three indentations were made in the skull to accommodate screws, which together with the application of dental cement held the cannulae in place. For cannulation of the median raphé nucleus, a 14-mm-long steel guide cannula (23 gauge; Cooper’s Needle Works, Birmingham, UK) was positioned at 7.6 mm posterior to bregma, lateral +3.0 mm, and vertical −6.5 mm at an angle of 21°, siting it 2 mm above the median raphé nucleus, using the medial-lateral approach. For bilateral cannulation of the dorsal hippocampus, 7-mm-long steel guide cannulae were positioned at 3.3 mm posterior to bregma, lateral ±2.4, and vertical −1.2 mm, thus siting them 2 mm above the target area. Cannulae were kept patent using either 14- or 7-mm-long stainless steel stylets (30 gauge, Cooper’s Needle Works).
Behavioral testing. On each test day the rats were held gently by wrapping in a cloth, and they were injected using needles constructed from 30 gauge steel tubing that extended 2 mm below the tip of the indwelling cannula. Three minutes after central injection, the rat was placed together with an unoperated partner in the social interaction test arena or alone in the central square of the plus-maze, and its behavior was observed for 7.5 and 5 min, respectively. The time spent by the operated rat in active social interaction was scored by an observer blind to the drug treatment, and locomotor activity and rears were automatically recorded from infrared beam breaks. In the elevated plus-maze, the times spent in open and closed arms were scored, and the number of entries into closed arms was scored by an observer blind to drug treatment. On each test day the animals were tested between 8 A.M. and noon in an order randomized for drug treatment, and the maze was wiped thoroughly after each trial. The effects in rats naive to (trial 1) or experienced with (trial 2) the plus-maze were tested in separate experiments. The rats tested on their second trial in the plus-maze had received a previous, 5 min undrugged exposure to the maze 3 d earlier.
Median raphé nucleus
Rats were randomly allocated to treatment groups. The numbers in parentheses indicate those with verified cannula placements.
Social interaction. Experiment 1: vehicle (n = 10); WAY 100635, 100 ng (n = 11), 200 ng (n = 12), 400 ng (n = 12). Experiment 2: vehicle (n = 8); 8-OH-DPAT, 200 ng (n = 9); WAY 100635, 200 ng (n = 8); 8-OH-DPAT, 200 ng, + WAY 100635, 200 ng (n = 9).
Plus-maze trial 1. Experiment 1: vehicle (n = 8); WAY 100635, 100 ng (n = 10), 200 ng (n = 9), 400 ng (n = 9). Experiment 2: vehicle (n = 9); 8-OH-DPAT 100 ng (n = 9); 200 ng (n = 10). Experiment 3: vehicle (n = 10); 8-OH-DPAT, 200 ng (n = 10); WAY 100635, 200 ng (n = 9); 8-OH-DPAT, 200 ng, + WAY 100635, 200 ng (n = 10).
Plus-maze trial 2. Vehicle (n = 11); 8-OH-DPAT, 200 ng (n = 12); WAY 100635, 200 ng (n = 11); 8-OH-DPAT, 200 ng, + WAY 100635, 200 ng (n = 12).
Dorsal hippocampus
Rats were randomly allocated to treatment groups. The numbers in parentheses indicate those with verified cannula placements.
Social interaction. Vehicle (n = 5); 8-OH-DPAT, 100 ng (n = 8); (+)WAY 100135, 10 mg/kg (n = 8); 8-OH-DPAT, 100 ng, + (+)WAY 100135, 10 mg/kg (n = 5). In this experiment, all rats received both intracerebral injections of aCSF or 8-OH-DPAT (100 ng) and subcutaneous injections of saline or (+)WAY 100135.
Plus-maze trial 1. Vehicle (n = 14); 8-OH-DPAT, 50 ng (n = 10), 100 ng (n = 10), or 200 ng (n = 9); (±)tertatolol, 3 μg (n = 9).
Plus-maze trial 2. Vehicle (n = 9); 8-OH-DPAT, 100 ng (n = 11); (±)tertatolol, 3 μg (n = 6); (±)tertatolol, 3 μg, + 8-OH-DPAT, 100 ng (n = 8).
Histology
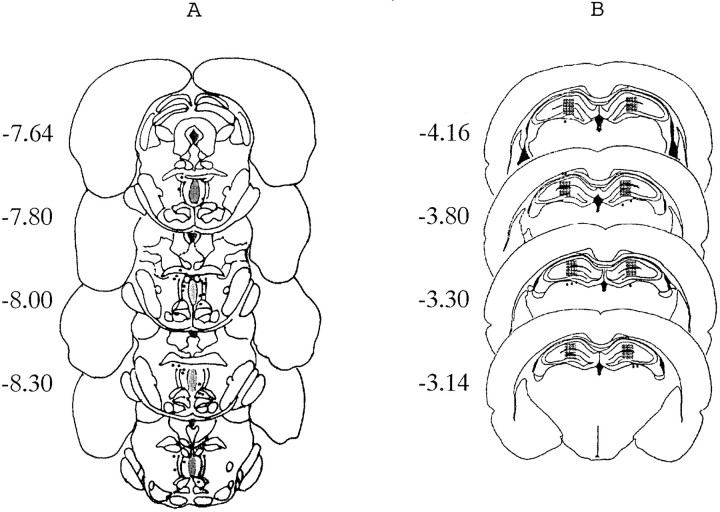
At the end of behavioral testing all animals were killed, the brains were removed, and the injection site was verified histologically (according to the atlas of Paxinos and Watson, 1986) by a person blind to the drug treatment. Figure 1, depicting coronal slices through the median raphé nuclei and dorsal hippocampus, shows the target sites as shaded. The positions of the tips of the injection needles for the animals that were excluded (not in the target area) from statistical analysis are also shown.
Fig. 1.
Diagrammatic representation of coronal sections (mm posterior to bregma) through the rat brain showing the target areas (shaded) of the median raphé nucleus (A) and dorsal hippocampus (B). Placements falling outside the target areas are shown by filled circles marking the site of the tip of the injection needle.
Statistics
The scores from the dose–response studies were analyzed by one-way ANOVA, and those from the antagonism studies were analyzed by two-way ANOVA. Comparisons between individual groups were then made with Duncan’s tests, and the significance of these is shown in the figures.
RESULTS
Median raphé nucleus
Social interaction
WAY 100635 (100–400 ng) was without significant effect in the social interaction test (Table 1); the middle dose (200 ng) was selected for the antagonism study. As can be seen from Figure 2, 8-OH-DPAT significantly increased the time spent in social investigation, and this effect was significantly reversed by WAY 100635. Because 8-OH-DPAT was without significant effect on either locomotor activity (mean ± SEM; vehicle = 664 ± 32.7, DPAT = 690 ± 75) or rears (vehicle = 34.8 ± 2.1, DPAT = 27.9 ± 1.8), the increase in social interaction indicates an anxiolytic effect. The reversal by a silent dose of WAY 100635 provides evidence that this anxiolytic action is 5-HT1A receptor-mediated.
Table 1.
Mean (±SEM) time spent in social interaction, locomotor activity (beam breaks), and rears made in the social interaction test, and % of time spent on open arms and number of closed arm entries on trial 1 in the elevated plus-maze by rats injected in the median raphé nucleus with aCSF (vehicle) or WAY 100635 (100–400 ng)
| Behavioral parameters | Vehicle | WAY 100635 (ng) | ||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Social | 31.7 ± 5.5 | 28.8 ± 4.4 | 38.4 ± 4.3 | 33.6 ± 4.5 |
| Locomotor | 697.7 ± 57.2 | 663.5 ± 41.2 | 696.8 ± 31.2 | 708.3 ± 48.3 |
| Rears | 35.4 ± 1.1 | 38.5 ± 1.3 | 37.4 ± 1.3 | 34.6 ± 1.9 |
| % time on open arms | 36.25 ± 8.4 | 48.6 ± 7.3 | 36.4 ± 5.3 | 31.4 ± 7.0 |
| Number of closed arm entries | 10.9 ± 2.2 | 11.1 ± 1.4 | 12.2 ± 1.1 | 11.4 ± 1.6 |
Fig. 2.
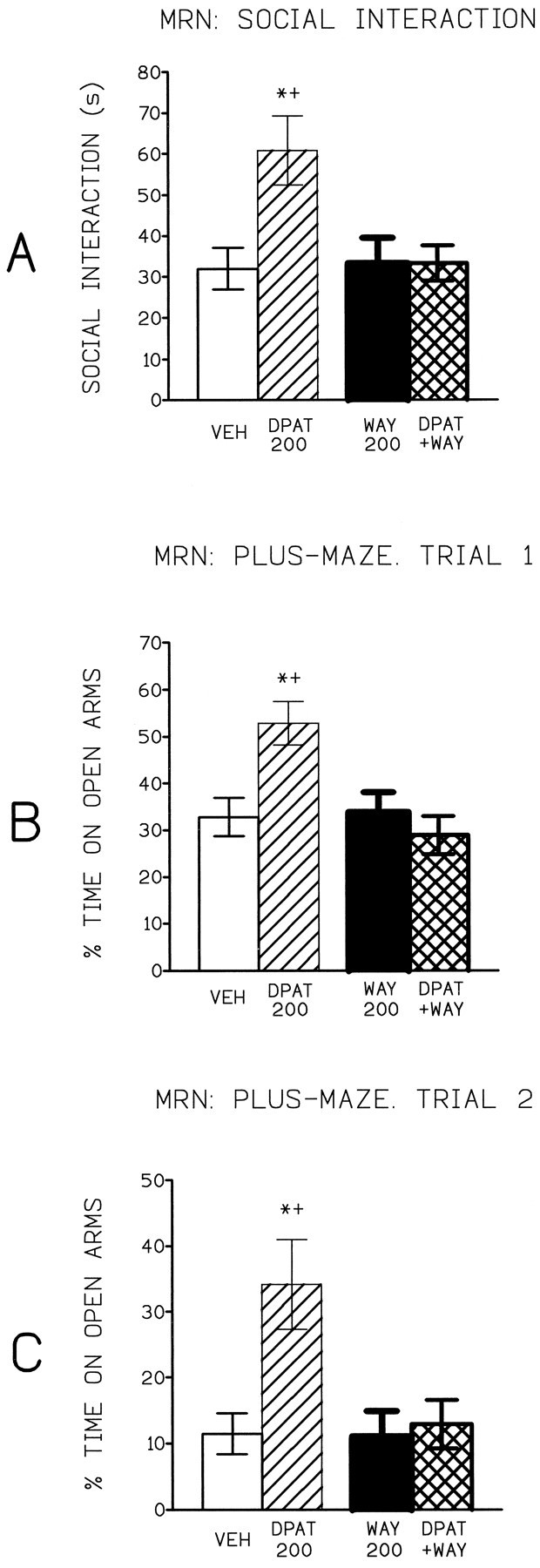
Mean (± SEM) time spent in (A) social interaction or percentage of time on open arms of plus-maze, trials 1 (B) and 2 (C), by rats tested 3 min after injection of aCSF (vehicle), 8-OH-DPAT (200 ng), WAY 100635 (200 ng), and 8-OH DPAT (200 ng) + WAY 100635 (200 ng) into the median raphé nucleus. * p < 0.05 compared with control;+ p < 0.05 compared with DPAT + WAY.
Plus-maze trial 1
WAY 100635 was without effect in the plus-maze (Table 1). In rats naive to the plus-maze, 8-OH-DPAT produced a dose-related increase in the percentage of time spent on the open arms (Table 2). WAY 100635 (200 ng) significantly antagonized this effect of 8-OH-DPAT (200 ng) (Fig. 2), demonstrating the role of 5-HT1A receptors. There were no changes in the number of closed arm entries (Table 2), and thus the increased percentage of time on open arms indicates a specific anxiolytic effect.
Table 2.
Mean (±SEM) of % of time on open arms, and number of closed arm entries made on trial 1 on the elevated plus-maze
| Median raphé | n | % time on open arms | Number of closed arm entries |
|---|---|---|---|
| Vehicle | 9 | 22.8 ± 1.5 | 10.7 ± 1.1 |
| 8-OH-DPAT | |||
| 100 ng | 9 | 43.0* ± 8.4 | 10.0 ± 0.9 |
| 200 ng | 10 | 47.4** ± 4.7 | 8.4 ± 1.2 |
Rats were tested 3 min after administration of vehicle (aCSF), 8-OH-DPAT (50, 100, and 200 ng), or (±)tertatolol (3 μg) to the median raphé nucleus or bilaterally to the dorsal hippocampus.
* p < 0.05; **p < 0.01 compared with vehicle.
Plus-maze trial 2
Once again, 8-OH-DPAT (200 ng) significantly increased the percentage of time spent on the open arms, and this effect was significantly antagonized by WAY 100635 (200 ng), demonstrating mediation by 5-HT1A receptors (Fig. 2). 8-OH-DPAT did not change the number of closed arm entries (mean ± SEM; vehicle = 13.5 ± 1.4, DPAT = 13.2 ± 1.5), and thus, again, 8-OH-DPAT had a specific anxiolytic effect.
Dorsal hippocampus
Social interaction
8-OH-DPAT (100 ng) significantly reduced the time spent in social interaction, and this effect was significantly antagonized by (+)WAY 100135 (10 mg/kg), indicating that it was 5-HT1Areceptor-mediated (Fig. 3). 8-OH-DPAT did not change locomotor activity (mean ± SEM; vehicle = 120.0 ± 11.6, DPAT = 134.4 ± 7.7) or rears (vehicle = 78.4 ± 6.2, DPAT = 76.9 ± 6.9), and thus the decrease in social interaction indicates a specific anxiogenic effect.
Fig. 3.
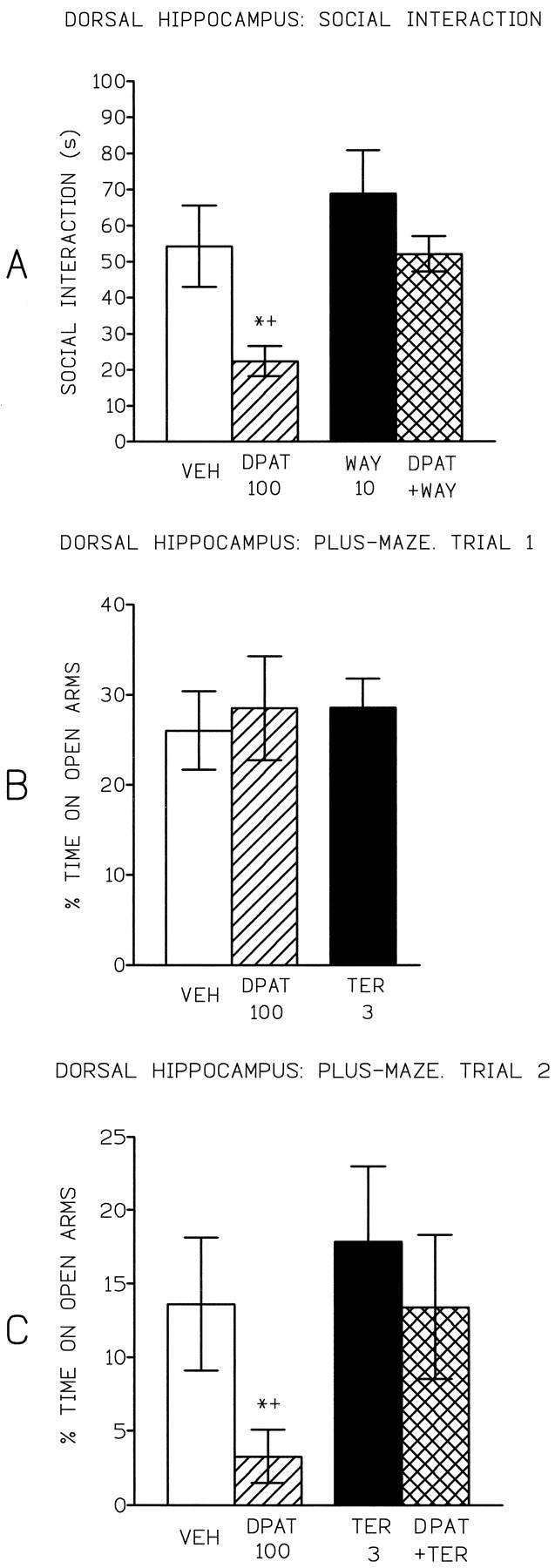
Mean (± SEM) time spent in (A) social interaction by rats tested 30 min after subcutaneous injection of either saline or (+)WAY 100135 (10 mg/kg) and 3 min after intrahippocampal injection of aCSF (vehicle) or 8-OH-DPAT (100 ng), or percentage of time spent on open arms of the plus-maze on trial 1 (B) or trial 2 (C), of rats tested 3 min after intrahippocampal injection of aCSF (vehicle) or 8-OH-DPAT (100 ng), (±)tertatolol (3 μg) or (±)tertatolol (3 μg) + 8-OH-DPAT (100 ng). * p < 0.05 compared with control;+ p < 0.05 compared with DPAT + antagonist.
Plus-maze trial 1
On trial 1, neither of the drugs had any significant effect on any of the measures scored. For comparative purposes, the data for the vehicle, 8-OH-DPAT (100 ng), and (±)tertatolol (3 μg) groups are shown in Figure 3. (The percentage of time spent on open arms for the 8-OH-DPAT 50 and 200 ng groups was 29.7 ± 2.7 and 36.3 ± 4.5, respectively.)
Plus-maze trial 2
On trial 2, 8-OH-DPAT (100 ng) significantly reduced the percentage of time spent on the open arms, and this was significantly reversed by (±)tertatolol (3 μg), which alone was without significant effect (Fig. 3). 8-OH-DPAT had no significant effect on closed arm entries (mean ± SEM; vehicle = 11.0 ± 1.9, DPAT = 10.3 ± 1.3), and thus 8-OH-DPAT seemed to be having a specific anxiogenic effect.
DISCUSSION
The present study has demonstrated the importance of the median raphé nucleus in all three of the test situations investigated. 8-OH-DPAT (200 ng) had anxiolytic actions in all cases, and these were reversed by a silent dose of WAY 100635. In contrast, stimulation of the postsynaptic 5-HT1A receptors by 8-OH-DPAT (100 ng) resulted in an anxiogenic effect in the social interaction test and on trial 2 in the plus-maze. These results therefore provide further evidence for contrasting mediation of anxiety by the pre- and postsynaptic 5-HT1A receptors, which would explain the rather weak and inconsistent effects of peripherally administered 5-HT1A receptor agonists in animal tests of anxiety (Griebel, 1995). The results also provide clear evidence that a reduction in dorsal hippocampal 5-HT activity has anxiolytic effects.
The fact that we were able to detect an anxiogenic effect after agonist administration to the dorsal hippocampus suggests that in the present experiment, 5-HT release in this area was not maximal. Under conditions of maximal 5-HT release, it could be envisaged that the control scores might be so low that further anxiogenic effects after agonist administration would be undetectable. In that case, however, the role of the postsynaptic 5-HT1A receptors could be revealed by the effects of a 5-HT1A receptor antagonist, which would then be expected to have an anxiolytic effect. Indeed, although not significant, this was the direction of the effect of tertatolol on trial 2. The issue of 5-HT tone is crucial to whether a specific 5-HT receptor antagonist would be expected to be silent in a particular brain area and under particular test conditions. It is therefore difficult to draw general conclusions, and for this reason, we established silent doses of each antagonist within each test and brain region involved in the present study.
In rats naive to the plus-maze, there was no evidence at all for an anxiogenic effect of 8-OH-DPAT when administered to the dorsal hippocampus. Indeed, the trend was toward an anxiolytic effect with the highest dose we tested. Anxiolytic effects in the plus-maze have been reported after administration into the dorsal hippocampus of a high (2.5 μg) dose of the 5-HT1A receptor agonist buspirone (Kostowski et al., 1989), and in the social interaction test we have found that a high dose of 8-OH-DPAT (1 μg) has anxiolytic effects (Andrews et al., 1995). It has been elegantly shown by Jolas et al. (1995), however, that the anxiolytic effects of high doses of these compounds is attributable to diffusion back to the raphé nucleus. The issue of anatomical specificity of behavioral effects after central injections is crucial and underlines the importance of verifying each individual cannula placement. For example, we have reported that whereas 8-OH-DPAT injected into the dorsal raphé nucleus has anxiolytic effects on trial 2 in the plus-maze, when the placements fell outside this area the trend was for an anxiogenic effect (File and Gonzalez, 1996).
Our results provide further evidence regarding the relative roles of the dorsal and median raphé in different animal tests. As shown in the present experiment and previously (Higgins et al., 1988; Andrews et al., 1994; Hogg et al., 1994), both of the nuclei play important roles in the social interaction test. In plus-maze naive rats (i.e., on trial 1), however, the present experiment has shown that the median raphé nucleus plays an important role, whereas the dorsal raphé 5-HT pathways are less important. Thus, 8-OH-DPAT (50–200 ng) was without effect when it was injected into the dorsal raphéor into its projection sites in the ventral hippocampus (File and Gonzalez, 1996) or basolateral amygdala (Gonzalez et al., in press). Furthermore, injections of benzodiazepines into the amygdala or lesions of the amygdala are without effect on trial 1 in the plus-maze, whereas they have effects in the defensive burying and social interaction tests (Treit al., 1993; Pesold and Treit, 1994; Gonzalez et al., in press).
There is considerable evidence for major differences between trials 1 and 2 in the plus-maze. A previous 5 min trial in the maze renders rats and mice insensitive to the anxiolytic effects of benzodiazepines (File, 1990; Rodgers and Shepherd, 1993), and it has been shown that crucial to this change is the learning that occurs in the open arms during trial 1 (File et al., 1990; File, 1993). Principal component analysis has shown that the measures of anxiety on trial 1 load on an independent factor from those on trial 2 (File, 1993), which is unlike social interaction where measures from trials 1 and 2 load on the same factor (File, 1991). Thus, previous experience of the maze has radically changed the nature of the anxiety generated by this test, and we have suggested that it changes from a fear of open spaces on trial 1 to an acquired fear of heights on trial 2 (File and Zangrossi, 1993;File et al., 1993). Evidence is accumulating showing that previous experience of the plus-maze changes the sensitivity of the 5-HT system. The present experiment provides evidence for increased sensitivity of the postsynaptic 5-HT1A receptors in the dorsal hippocampus on trial 2. We have shown previously that experience in the plus-maze changes the sensitivity of the 5-HT1Areceptors in the dorsal raphé nucleus to 8-OH-DPAT. Thus, 8-OH-DPAT was without effect on trial 1, whereas it had clear anxiolytic effects on trial 2 (File and Gonzalez, 1996).
In conclusion, we have demonstrated that the 5-HT1A receptor agonist 8-OH-DPAT can mediate anxiolytic and anxiogenic effects on behavior in two tests of rodent anxiety. In both cases, these effects can be antagonized by silent doses of selective 5-HT1A receptor antagonists. This bimodal effect of 5-HT1A receptor activation is dependent on the neuroanatomical location of the receptors and adds further support for a role of 5-HT in the modulation of anxiety states. Furthermore, our results emphasize that the particular 5-HT pathways that are activated can depend crucially on the behavioral test conditions.
Footnotes
L.E.G. is supported by a grant from Consejo Nacional de Investigaciones Científicas y Tecnológicas, University of Los Andes, Mérida, Venezuela. We thank Mr. P. S. Mabbutt for expert technical assistance and Dr. A. Kent, Division of Anatomy, for use of his cryomat.
Correspondence should be addressed to Professor S. E. File, Psychopharmacology Research Unit, UMDS, Guy’s Hospital, London SE1 9RT, UK.
REFERENCES
- 1.Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphé nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharmacol. 1994;264:259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N, Gonzalez LE, File SE. 5-HT1A receptor mediation of the anxiogenic effect of hippocampal injection of 8-OH-DPAT. J Psychopharmacol. 1995;9:A29. [Google Scholar]
- 3.Carli M, Samanin R. Potential anxiolytic properties of 8-hydroxy-2-(Di-N -propylamino)tetralin, a selective serotonin1A receptor agonist. Psychopharmacology. 1988;94:84–91. doi: 10.1007/BF00735886. [DOI] [PubMed] [Google Scholar]
- 4.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 5.File SE. One-trial tolerance to the anxiolytic effects of chlordiazepoxide in the plus-maze. Psychopharmacology. 1990;100:281–282. doi: 10.1007/BF02244419. [DOI] [PubMed] [Google Scholar]
- 6.File SE (1991) The biological basis of anxiety. In: Current practices and future developments in the pharmacotherapy of mental disorders (Meltzer HY, Nerozzi D, eds), pp 159–166. Amsterdam: Excerpta Medica.
- 7.File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- 8.File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A receptor ligands in dorsal raphé and central hippocampus. Pharmacol Biochem Behav. 1996;54:123–128. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- 9.File SE, Zangrossi H., Jr “One-trial tolerance” to the anxiolytic actions of benzodiazepines in the elevated plus-maze, or the development of a phobic state? Psychopharmacology. 1993;110:240–244. doi: 10.1007/BF02246980. [DOI] [PubMed] [Google Scholar]
- 10.File SE, Mabbut PS, Hitchcott P. Characterization of the phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology. 1990;102:98–101. doi: 10.1007/BF02245751. [DOI] [PubMed] [Google Scholar]
- 11.File SE, Zangrossi H, Jr, Viana M, Graeff FG. Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology. 1993;111:491–494. doi: 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, Haskins JT, Jones D, Mansell HL, Reilly Y. WAY 100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur J Pharmacol. 1993;237:283–291. doi: 10.1016/0014-2999(93)90280-u. [DOI] [PubMed] [Google Scholar]
- 13.Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fetcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez LE, Andrews N, File SE (1996) 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res, in press. [DOI] [PubMed]
- 15.Griebel G. 5-hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- 16.Higgins GA, Bradbury AJ, Jones BJ, Oakley NR. Behavioural and biochemical consequences following activation of 5-HT1-like and GABA receptors in the dorsal raphé nucleus of the rat. Neuropharmacology. 1988;27:993–1001. doi: 10.1016/0028-3908(88)90058-5. [DOI] [PubMed] [Google Scholar]
- 17.Higgins GA, Jones BJ, Oakley NR, Tyers MB. Evidence that the amygdala is involved in the disinhibitory effects of a 5-HT3 receptor antagonists. Psychopharmacology. 1991;104:545–551. doi: 10.1007/BF02245664. [DOI] [PubMed] [Google Scholar]
- 18.Hodges H, Green S, Glenn B. Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding but not on discrimination. Psychopharmacology. 1987;92:491–504. doi: 10.1007/BF00176484. [DOI] [PubMed] [Google Scholar]
- 19.Hogg S, Andrews NA, File SE. Contrasting behavioural effects of 8-OH-DPAT in the dorsal raphé nucleus and ventral hippocampus. Neuropharmacology. 1994;33:343–348. doi: 10.1016/0028-3908(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 20.Hutson PH, Sarna GS, O’Connell MT, Curzon G. Hippocampal 5-HT synthesis and release in vivo is decreased by infusion of 8-OHDPAT into the nucleus raphe dorsalis. Neurosci Lett. 1989;100:276–280. doi: 10.1016/0304-3940(89)90698-8. [DOI] [PubMed] [Google Scholar]
- 21.Jolas T, Haj Dahmane S, Lanfumey L, Fattaccini CM, Kidd EJ, Adrien J, Gozlan H, Guardiola-Lemaitre B, Hamon M. (-)Tertatolol is a potent antagonist at pre- and post-synaptic serotonin 5-HT1A receptors in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:453–463. doi: 10.1007/BF00166735. [DOI] [PubMed] [Google Scholar]
- 22.Jolas T, Schreiber R, Laporte AM, Chastanet M, De Vry J, Glaser T, Adrien J, Hamon M. Are postsynaptic 5-HT1A receptors involved in the anxiolytic effects of 5-HT1A receptor agonists and in their inhibitory effects on the firing of serotonergic neurons in the rat? J Pharmacol Exp Ther. 1995;272:920–929. [PubMed] [Google Scholar]
- 23.Kostowski W, Plaznik A, Stefanski R. Intra-hippocampal buspirone in animal models of anxiety. Eur J Pharmacol. 1989;168:393–396. doi: 10.1016/0014-2999(89)90803-0. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. Academic; London: 1986. The rat brain in stereotaxic coordinates. . [DOI] [PubMed] [Google Scholar]
- 25.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 26.Pesold C, Treit D. The septum and amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1994;638:295–301. doi: 10.1016/0006-8993(94)90662-9. [DOI] [PubMed] [Google Scholar]
- 27.Prisco S, Cagnotto A, Talone D, DeBlasi A, Mennini T, Esposito E. Tertatolol, a new β-blocker, is a serotonin (5-hydroxytryptamine1A) receptor antagonist in rat brain. J Pharmacol Exp Ther. 1993;265:739–744. [PubMed] [Google Scholar]
- 28.Rodgers RJ, Shepherd JK. Influence of prior maze experience on behaviour and response to diazepam in the elevated plus-maze and light/dark tests of anxiety in mice. Psychopharmacology. 1993;113:237–242. doi: 10.1007/BF02245704. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber R, De Vry J. Neuronal circuits involved in the anxiolytic effects of the 5-HT1A receptor agonists 8-OH-DPAT, ipsapirone and buspirone in the rat. Eur J Pharmacol. 1993;249:341–351. doi: 10.1016/0014-2999(93)90531-l. [DOI] [PubMed] [Google Scholar]
- 30.Sharp T, Bramwell SR, Clark D, Grahame-Smith DG. In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetized rat using microdialysis: changes in relation to 5-hydroxytryptaminergic neuronal activity. J Neurochem. 1990;53:234–240. doi: 10.1111/j.1471-4159.1989.tb07319.x. [DOI] [PubMed] [Google Scholar]
- 31.Sprouse JS, Aghajanian GK. Electrophysiological response of serotonergic dorsal raphé neurones to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- 32.Treit D, Pesold C, Rotzinger S. Noninteractive effects of diazepam and amygdala lesions in two animal models of anxiety. Behav Neurosci. 1993;107:1099–1105. doi: 10.1037//0735-7044.107.6.1099. [DOI] [PubMed] [Google Scholar]