Abstract
Local infusion of brain-derived neurotrophic factor (BDNF) into the ventral tegmental area (VTA) can prevent and reverse the ability of chronic morphine or cocaine exposure to induce tyrosine hydroxylase (TH) in this brain region. The present study examined a possible role for extracellular signal regulated kinases (ERKs), the major effector for BDNF and related neurotrophins, in morphine and cocaine action in the VTA. Chronic, but not acute, administration of morphine or cocaine increased ERK catalytic activity specifically in the VTA. This increase in ERK activity reflected an increase in the state of phosphorylation of ERK, with no change in levels of total ERK immunoreactivity. Chronic infusions of BDNF into the VTA reduced total ERK immunoreactivity with no change in ERK activity, and also blocked the morphine-induced increase in ERK activity. These results suggest that chronic BDNF elicits a compensatory increase in the phosphorylation of the remaining ERK molecules and thereby prevents any additional increase in response to drug exposure. Such a role for ERK in morphine action was demonstrated directly by chronically infusing antisense oligonucleotides to ERK1 into the VTA. This treatment selectively reduced levels of ERK1 immunoreactivity in a sequence-specific manner without detectable toxicity. Intra-VTA infusion of ERK1 antisense oligonucleotides mimicked the effects of chronic BDNF infusions on ERK immunoreactivity, ERK activity, and TH immunoreactivity in the VTA under both control and morphine-treated conditions. The chronic morphine-induced increases in ERK activity and TH expression in the VTA also were blocked by local infusion of NMDA glutamate receptor antagonists, suggesting a role for glutamate in mediating these drug effects. Together, these findings support a scheme whereby chronic, systemic administration of morphine or cocaine leads to a sustained increase in ERK phosphorylation state and activity in the VTA, which, in turn, contributes to drug-induced increases in TH, and perhaps other drug-induced adaptations, elicited selectively in this brain region.
Keywords: morphine, cocaine, antisense oligonucleotides, ERK, ventral tegmental area, tyrosine hydroxylase, BDNF, NMDA glutamate receptors
The mesolimbic dopamine system, which comprises dopaminergic neurons in the ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc) and other forebrain structures, is implicated in the rewarding properties of several drugs of abuse (Bozarth and Wise, 1986; Kuhar et al., 1991; Koob, 1992). Moreover, adaptations that drugs of abuse induce in the mesolimbic dopamine system after repeated exposures are believed to underlie motivational aspects of drug addiction (Koob, 1992; Nestler, 1992; Self and Nestler, 1995).
One of the most consistent adaptations elicited by several drugs of abuse, including morphine, cocaine, amphetamine, and ethanol, is induction of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis, in the VTA (Beitner-Johnson and Nestler, 1991;Hurd et al., 1992; Sorg et al., 1993; Vrana et al., 1993; Ortiz et al., 1995a). In a recent study, we demonstrated that infusion of the neurotrophins brain-derived neurotrophic factor (BDNF) or neurotrophin-4 directly into the VTA can both prevent and reverse the ability of morphine and cocaine to increase TH levels in this brain region (Berhow et al., 1995). The neurotrophin infusions also attenuated several other drug-induced biochemical adaptations in the mesolimbic dopamine system. These findings suggested that some of the effects of morphine and cocaine in the VTA–NAc conceivably could be mediated via perturbation of neurotrophin signaling pathways.
The neurotrophin signal transduction cascade involves activation of a series of protein kinases, which leads ultimately to a myriad of effects on cell function (Davis, 1993). Binding of neurotrophin to its receptor Trk activates the protein tyrosine kinase intrinsic to the receptor. This leads to autophosphorylation of the receptor, which allows for recognition of the receptor by several intracellular signaling proteins that contain src homology (SH2) domains. Such receptor–protein interactions lead to the activation of Ras (a low molecular weight G-protein) and of a Raf kinase, which in turn phosphorylates and activates a form of MEK ( MAP-kinase and ERK Kinase). Various forms of MEK then phosphorylate two members of the MAP kinase family: extracellular signal-regulated kinases (ERKs) and Jun kinase (JNK). ERKs are a family of protein serine/threonine kinases of which the best characterized members are ERK1 (p44) and ERK2 (p42) (Robbins et al., 1993). JNK is related to the ERKs, but has been shown to be differentially regulated (Minden et al., 1994a,b). Whereas ERK activity is regulated in response to growth factors or phorbol esters (Boulton et al., 1990; Hu and Wieloch, 1994), JNK activity is regulated in response to cytokines or cell stress (Derijard et al., 1994; Su et al., 1994; Westwick et al., 1994). Once activated, ERK can phosphorylate and activate other protein kinases as well as an array of effector proteins, which include TH and certain cytoskeletal proteins and transcription factors (Haycock et al., 1992; Seger and Krebs, 1995).
The objective of the present study was to investigate possible cross-talk between the neurotrophin signal transduction cascade and the biochemical actions of drugs of abuse after chronic exposure. ERK was selected as the primary focus of these studies based on its central role in neurotrophin signaling cascades, the established ability of these cascades to regulate TH expression in cultured cells (Gizang-Ginsberg and Ziff, 1990; Haycock et al., 1992; Lewis et al., 1994; Rabinovsky et al., 1995), and the known modulation of these cascades by second messenger pathways (Gardner et al., 1993). Our studies support a role for ERK in the regulation of TH expression in the VTA in response to chronic morphine and cocaine exposure.
MATERIALS AND METHODS
Neurotrophic factor infusions and drug treatments.Male Sprague–Dawley rats (initial weight 260–275 gm) (CAMM, Wayne, NJ) were used in these studies. Neurotrophic factor infusions involved implantation of osmotic minipumps (Alzet Model 2002) that provide a constant infusion of 0.5 μl/hr for 14 d. BDNF and ciliary neurotrophic factor (CNTF), human recombinant growth factors expressed in E. Coli, were provided by Regeneron Pharmaceuticals (Tarrytown, NY). The growth factors were delivered in a solution containing 10 mm sodium phosphate, pH 7.4, 0.9% NaCl, and 1% bovine serum albumin. The doses of BDNF (2.5 μg/d) and CNTF (1.5 μg/d) were based on previous research (Berhow et al., 1995). Animals were anesthetized with 3 mg/kg Equithesin and implanted with an osmotic minipump connector cannula (28 gauge cannula, 22 gauge connector, Plastic Products). Midline VTA coordinates of −5.3 mm anterior–posterior and 8.4 mm dorsal–ventral were used. The osmotic pump was placed subcutaneously between the scapulae and connected to the cannula via PE60 tubing cut to 2.5 cm in length. Each end was sealed with LocTite glue. The cannula was secured in place with dental cement. Control rats were implanted with osmotic pumps containing vehicle solution.
Acute neurotrophin administration involved similar preoperative techniques as described above. The tip of a Hamilton syringe needle (25 gauge) containing vehicle or BDNF solution was lowered to −8.4 mm dorsal–ventral at the coordinate of −5.3 mm anterior–posterior. A dose of BDNF (5 μg) was delivered in 1 μl volume over a 2 min period. The syringe needle remained inserted for 5 min before removal. Animals then were killed 2 hr later.
Chronic morphine was administered via implantation of one morphine pellet (containing 75 mg of morphine base) [National Institute on Drug Abuse (NIDA)] subcutaneously while rats were under light halothane anesthesia daily for 5 d. Animals were killed on the morning of day 6 via decapitation, and tissue samples were obtained. Control rats underwent sham surgery or no treatment; the two controls yielded equivalent results. Acute morphine was administered 2 hr before the animal was killed. The one-time intraperitoneal injection contained 20 mg/kg morphine sulfate (NIDA) prepared in a saline solution. Concomitant morphine/naltrexone treatments involved administering naltrexone before each morphine implantation as described by Guitart and Nestler (1989), conditions known to block behavioral, electrophysiological, and biochemical aspects of morphine tolerance and dependence. Chronic cocaine was administered twice daily via intraperitoneal injections of cocaine/HCl (15 mg/kg) (NIDA) in 0.9% NaCl for 10 d. Control rats received saline injections. Brains were removed from decapitated rats 12–14 hr after the last cocaine injection. Acute cocaine (20 mg/kg) was administered by intraperitoneal injection 2 hr before the animal was killed.
Immunolabeling of proteins. Brains were removed from decapitated rats and cooled in ice-cold physiological buffer. The VTA, substantia nigra, NAc, and frontal cortex were obtained as 12–15 gauge punches of coronal cross-sections of brain as described previously (for review, see Beitner-Johnson et al., 1992). Brain samples were homogenized in 125 μl of 1% SDS and were adjusted to contain final concentrations of 50 mm Tris, pH 6.7, 4% glycerol, 2% 2-mercaptoethanol, and bromophenol blue as a marker. Samples were then boiled, and aliquots containing given amounts of protein were subjected to SDS/polyacrylamide gel electrophoresis. Immunolabeling was conducted for TH, ERK, phospho-ERK, JAK2, a cytoplasmic kinase associated with the CNTF cascade, glial fibrillary acidic protein (GFAP), neurofilament proteins (NF 200, NF 160, NF 68), phosphatidylinositol-3 kinase (PI3K), and phospholipase C-γ (PLC-γ) as described previously (Beitner-Johnson and Nestler, 1991;Beitner-Johnson et al., 1992; Ortiz et al., 1995b; Widnell et al., 1996). Protein amount used was 5 μg (ERK, TH, GFAP, NF 200), 10 μg (PI3K, PLC-γ, NF 160, NF 68), 20 μg (JAK2), or 30 μg (phospho-ERK). Resolving gels contained 6% (NF 200, NF 160, PLC-γ, JAK2), 7.5% (TH, NF 68, GFAP, PI3K), or 8% (ERK, phospho-ERK) acrylamide with an acrylamide/ bisacrylamide ratio of 30:1.2. Proteins were transferred electrophoretically to nitrocellulose or PVDF (ERK, phospho-ERK) membranes, which were then blocked with 2% nonfat dry milk (all proteins except TH) or 0.01% polyvinylpyrrolidone (TH) in buffer containing 10 mm sodium phosphate, pH 7.2, 140 mm NaCl, and 0.05% Tween 20 (Sigma, St. Louis, MO).
Proteins were then immunolabeled with the following antibodies: anti-TH (diluted 1:5000, John Haycock, Louisiana State University), ERK (diluted 1:5000, Transduction Laboratories, Lexington, KY; diluted 1:2000, Santa Cruz), phospho-ERK (diluted 1:500, New England Biolabs, Beverly, MA), anti-GFAP and anti-NF200 (diluted 1:10000, Sigma), anti-NF160 and anti-NF68 (diluted 1:5000, Sigma), anti-JAK2, anti-PLC-γ, and anti-PI3K (diluted 1:2000, Upstate Biotechnology, Lake Placid, NY). Primary antibodies were detected with peroxidase-linked secondary antibodies (Vector, Burlingame, CA) and with enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL) and autoradiography. The resulting autoradiograms were quantified with a Macintosh-based image analysis system with National Institutes of Health image 1.57 software. Levels of protein immunoreactivity were linear over at least a threefold range of tissue concentration for each of the proteins analyzed. Equal loading and transfer of proteins were confirmed by amido black staining.
ERK activity assay. Samples were prepared as described above with 50 μg of protein loaded per lane for VTA, NAc, substantia nigra, and frontal cortex. Proteins were resolved by SDS/polyacrylamide gel electrophoresis on 8% resolving gels containing 0.1 mg/ml of myelin basic protein (MBP), added before separation. Protein kinases in the gels were assayed by the method of Kameshita and Fujisawa (1989) and modified by Ortiz et al. (1995b). Briefly, after electrophoresis, slab gels were processed with 20% propanol to remove SDS, 6 m guanidine to denature proteins, and 2-mercaptoethanol to renature proteins. Phosphorylation of MBP involves incubating the gels for 1 hr at 30°C in buffer containing 10 mm [γ32-P] ATP. The gels were washed with 5% trichloroacetic acid containing 10 mm sodium pyrophosphate until the radioactivity of the solution became negligible. Phosphorylation of MBP was visualized by autoradiography. Statistical significance was calculated by use of the χ2 test.
In vivo antisense oligonucleotide infusions.Oligonucleotides were infused via osmotic minipumps as described above for BDNF. Oligonucleotides were obtained from Midland Certified Reagent Company (Midland, TX). All sequences were partially modified; that is, modified with phosphorothioate moieties on the 5′ and 3′ ends only. The following sequences were used: 5′-GCC GCC ATC TGG ACT GCT GC-3′ antisense (corresponding to 13 to 33 of the ERK1 sequence); 5′-GCA GCA GTG GAG ATG GCG GC-3′ sense; 5′-CGA AGT CCA GTC GGA CGA CC-3′ scrambled. Oligonucleotides were purified via a high-salt precipitation and ethanol wash as described (Widnell et al., 1996). Oligonucleotides were brought to final concentrations to deliver 5, 10, or 20 μg/d with sterile PBS. Vehicle infusions were given to control animals.
In most studies, animals received intra-VTA infusions of antisense, sense, or scrambled oligonucleotides or vehicle solutions for a 7 d period, at which time the animals were killed. In some cases, on the seventh day of oligonucleotide infusion, animals were anesthetized and the osmotic pump containing the antisense oligonucleotide was replaced with a pump containing vehicle solution. After an additional 5 d, animals were killed and brains removed. In some experiments, animals were treated concomitantly with chronic morphine, as described above, with morphine pellet implantations beginning on day 3 of oligonucleotide infusion. To confirm cannula placement, as well as lack of damage after the antisense oligonucleotide infusions, Nissl staining was performed on a set of animals (see below).
Infusion of NMDA receptor antagonists. NMDA receptor antagonists were infused into the VTA as described above for BDNF.d-AP-5, a potent and selective NMDA receptor antagonist, was obtained from RBI (Natick, MA).l-AP-5, which exhibits close to 100-fold lower potency as an NMDA antagonist (Olverman et al., 1988), was obtained from Alexis. The dose of d-AP-5 used (50 μm infused at a rate of 0.5 μl/d) was based on the concentration of d-AP-5 required to antagonize NMDA receptors in brain slices in vitro (Kogan and Aghajanian, 1995) and in the brain in vivo (Whitton et al., 1994; Jay et al., 1995; Taber et al., 1995).l-AP-5 was used at the same dose as a control.d-AP-7 was used at a dose of 150 μm based on its Kifor the NMDA receptor, which is approximately threefold higher compared with d-AP-5 (Olverman et al., 1988).
Histological analysis. Rats that received chronic intra-VTA infusions of ERK sense or antisense oligonucleotide were anesthetized with 120 mg/kg sodium pentobarbital and perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were removed and kept for 1–2 hr in 4% paraformaldehyde and then in 20% glycerol overnight. Sections were cut at 30 μm. We used Nissl staining and TH immunohistochemistry to study the effect of chronic oligonucleotide infusions on the cytoarchitecture of the midbrain. For Nissl staining, a standard protocol with 0.25% cresyl violet was used. For TH immunohistochemistry, sections were treated with 5% H2O2/5% normal goat serum and incubated in PBS containing a rabbit polyclonal anti-TH antibody (1:2000) (Eugene Tech, Allendale, NJ) overnight at 4°C. Sections were then incubated for 1 hr in biotinylated goat anti-rabbit IgG (1:500) (Vector Labs) and for 1 hr in avidin–biotin complex (Vector Labs). Immunoreactivity was detected with DAB. The histological analyses were performed “blind” with respect to treatment conditions.
RESULTS
Regulation of ERK activity by morphine, cocaine, and BDNF treatments in the VTA
An in-gel assay of ERK catalytic activity was used to study the effect of morphine and cocaine on the enzyme in the VTA. In this assay, MBP is phosphorylated in the presence of [γ-32P]ATP by protein kinases active within the resolving gel. As shown in Figure 1, this assay results in a prominent band at ∼44 kDa, which corresponds to the approximate Mr of ERK1 and ERK2 (the two subtypes were not resolved in this assay). Because MBP is known to be a substrate for several protein kinases in addition to the ERKs, including protein kinase A and protein kinase C, several controls were performed to confirm the identity of the 44 kDa band as the ERKs. First, the 44 kDa band comigrated with purified, activated ERK2 analyzed in separate gel lanes. Second, the 44 kDa band was stimulated dramatically in hippocampal extracts after an acute electroconvulsive seizure, conditions known to activate ERK. Third, analysis of ERK immunoprecipitates prepared from VTA extracts yielded the same 44 kDa band (see Ortiz et al., 1995b).
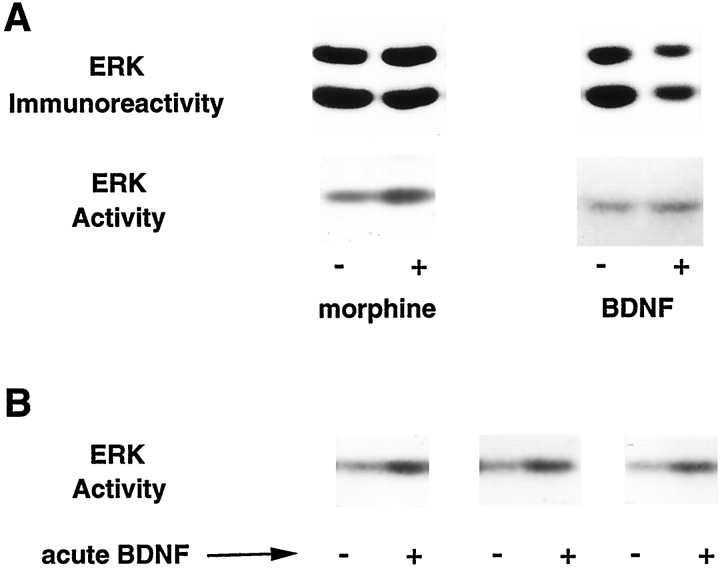
Fig. 1.
A, Effect of chronic morphine treatment and chronic intra-VTA BDNF infusions on ERK immunoreactivity and ERK activity in the VTA. B, Effect of acute intra-VTA BDNF infusions on ERK activity in the VTA. After the various treatments, VTA extracts were analyzed for total ERK immunoreactivity by immunoblotting and for ERK activity measured with an in-gel ERK activity assay. Note that the relative levels of ERK1 and ERK2 shown do not represent an accurate measure of the absolute amounts of these proteins present in the VTA, because the antibody used (Santa Cruz) (see Materials and Methods) shows greater relative reactivity for ERK2 than ERK1. Indeed, ERK1 is the predominant form of the enzyme present in the VTA (for review, see Ortiz et al., 1995b).
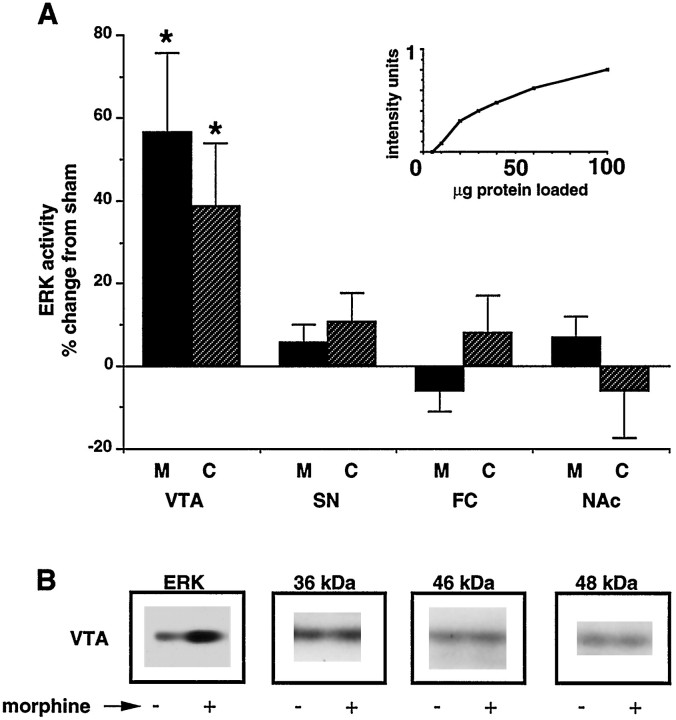
Animals treated with chronic morphine or cocaine demonstrated a 54 and 37% increase in ERK activity, respectively, compared with control (Figs. 1A, 2). This increase was seen only in the VTA and not in other brain regions examined, which included the substantia nigra, frontal cortex, and NAc (Fig. 2). In contrast, chronic morphine and cocaine treatments did not affect the activity of other protein kinases detected by this in-gel assay (see Fig. 2).
Fig. 2.
Effect of chronic morphine and cocaine treatments on ERK activity in selected brain regions. A, Graph quantifying effect of chronic morphine (M) and cocaine (C) treatments on ERK activity as measured with an in-gel ERK activity assay. Regions analyzed included VTA, substantia nigra (SN), frontal cortex (FC), and nucleus accumbens (NAc). Data are expressed as mean ± SEM (n = 8 in each treatment group) (*p < 0.05 vs sham by χ2 test). Inset, Graph illustrating linearity of ERK activity with sample protein content.B, Representative autoradiograms illustrating regulation of ERK (44 kDa) activity by chronic morphine treatment, but not of other unidentified bands of different molecular weights from the same gel.
Acute intra-VTA infusions of BDNF, as expected, also produced a significant increase in ERK activity (153 ± 10% of control,n = 4, p < 0.05) (Fig. 1B). In contrast, chronic infusion of BDNF into the VTA did not result in any change in ERK activity in this brain region (105 ± 9% of control, n = 5) (Fig. 1A). Interestingly, though, when morphine was administered concomitantly with chronic intra-VTA BDNF infusions, the expected increase in VTA ERK activity associated with morphine did not occur (99 ± 15% of control,n = 4).
To ascertain whether drug regulation of ERK activity in the VTA required chronic drug exposure, acute morphine and cocaine studies were undertaken. There was no increase in ERK activity in the VTA in response to a single acute exposure to morphine (112 ± 15% of control, n = 4) or cocaine (105 ± 7% of control,n = 4). Additionally, concomitant administration of naltrexone, an opioid receptor antagonist, along with morphine, under conditions that block morphine tolerance and dependence (see Materials and Methods), prevented the chronic morphine-induced increase in ERK activity (101 ± 5% of control, n = 4); this treatment also prevented the characteristic morphine-induced increase in TH (data not shown).
Regulation of ERK immunoreactivity and phosphorylation state by morphine, cocaine, and BDNF treatments in the VTA
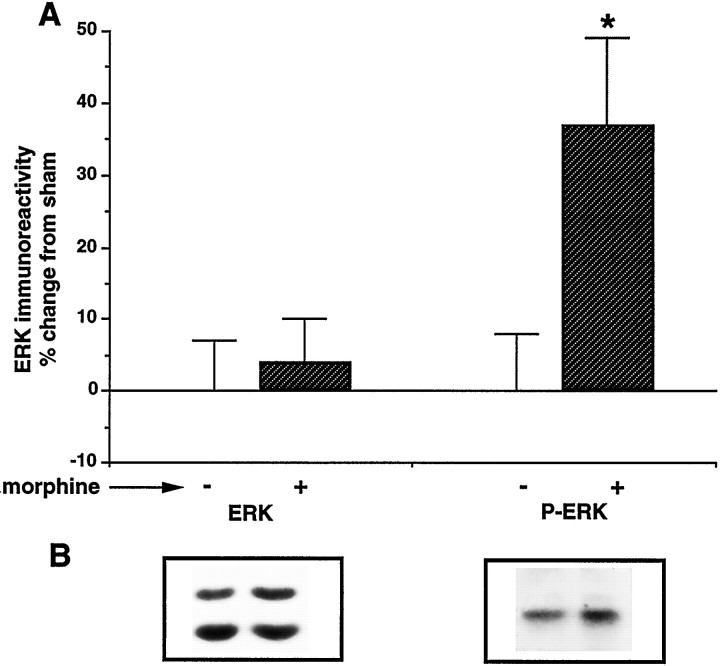
The morphine- and cocaine-induced increases in ERK activity in the VTA could be attributable to increases in levels of ERK protein, increases in the phosphorylation state of ERK, or a combination of the two. To study these possibilities, we measured levels of total ERK and phospho-ERK immunoreactivity by immunoblotting. We did not detect any difference in total ERK immunoreactivity after chronic morphine or chronic cocaine treatment (see Table 1, Figs.1A, 3). This lack of effect of morphine on total ERK immunoreactivity in the VTA is consistent with our previous findings (Ortiz et al., 1995b). The lack of change in ERK protein levels coupled with a significant increase in ERK activity suggests a higher phosphorylation state of the enzyme. To directly verify this, we used an antibody specific for a phosphorylated tyrosine residue (tyr 204) required for ERK activation. In rats treated chronically with morphine, there was a significant increase in levels of phospho-ERK immunoreactivity in the VTA (135 ± 12% of control,n = 6, p < 0.05) (Fig.3).
Table 1.
Effect of ERK1 antisense and sense oligonucleotide infusions (10 μg/d) on levels of ERK1, ERK2, and TH immunoactivity, and of ERK activity, with and without concomitant chronic morphine treatment
| Morphine | ERK1 immunoreactivity | ERK2 immunoreactivity | ERK activity | TH immunoreactivity | |
|---|---|---|---|---|---|
| Vehicle | − | 100 ± 9 (15) | 100 ± 11 (15) | 100 ± 8 (10) | 100 ± 7 (7) |
| + | 106 ± 14 (6) | 95 ± 11 (6) | 154 ± 12* (10) | 158*1_a (8) | |
| Sense (10 μg/d) | − | 96 ± 14 (6) | 109 ± 12 (6) | n.a. | 106 ± 10 (6) |
| + | 95 ± 6 (5) | 92 ± 8 (5) | n.a. | 130 ± 11* (6) | |
| Antisense (10 μg/d) | − | 65 ± 16* (15) | 93 ± 13 (15) | 96 ± 6 (9) | 98 ± 10 (6) |
| + | 59 ± 18* (6) | 104 ± 8 (6) | 98 ± 9 (9) | 103 ± 8 (5) |
Originally published in Berhow et al. (1995).
*p < 0.05 versus vehicle (− morphine treatment) by χ2 test.
Fig. 3.
Effect of chronic morphine treatment on total ERK immunoreactivity and phospho-ERK immunoreactivity in the VTA.A, Graph quantifying effect of chronic morphine treatment on total ERK immunoreactivity (ERK) and phospho-ERK immunoreactivity (P-ERK). Data are expressed as mean ± SEM (*p < 0.05 vs sham by χ2 test). The number of animals used for total ERK immunoreactivity was 15 and for phospho-ERK immunoreactivity was 6. B, Representative autoradiograms of ERK and P-ERK immunoblots are shown. The specificity of the anti-P-ERK antibody for phospho-ERK was demonstrated by analysis of purified dephospho- and phospho-ERK (data not shown).
In contrast to morphine and cocaine, chronic BDNF infusions significantly decreased ERK immunoreactivity in the VTA (78 ± 11 of control, n = 8, p < 0.05) (Fig.1A). The finding of a decrease in total ERK protein with no change in ERK activity suggests that a higher fraction of the ERK protein present is phosphorylated after chronic BDNF infusions. Intra-VTA infusion of CNTF, a member of a distinct neurotrophic factor family that acts via different signaling pathways and that does not attenuate morphine and cocaine actions in the VTA (Berhow et al., 1995), failed to alter ERK immunoreactivity or catalytic activity in the VTA (data not shown).
Effect of ERK1 antisense oligonucleotides on ERK immunoreactivity in the VTA
To study the functional significance of the regulation of ERK activity by chronic morphine and cocaine treatments, we used antisense oligonucleotides to ERK1 to directly alter ERK levels in the VTA. The targeting of ERK1, as opposed to ERK2, is based on the finding that ERK1 is the more abundant form of the enzyme in the VTA (Ortiz et al., 1995b). It has been reported that the use of fully phosphorothioate-modified antisense oligonucleotides is associated with a dose-dependent toxicity around the site of infusion (for review, seeWidnell et al., 1996). To minimize any potential toxicity while maintaining efficacy of the oligonucleotides, partially modified phosphorothioate oligonucleotides were used (see Materials and Methods).
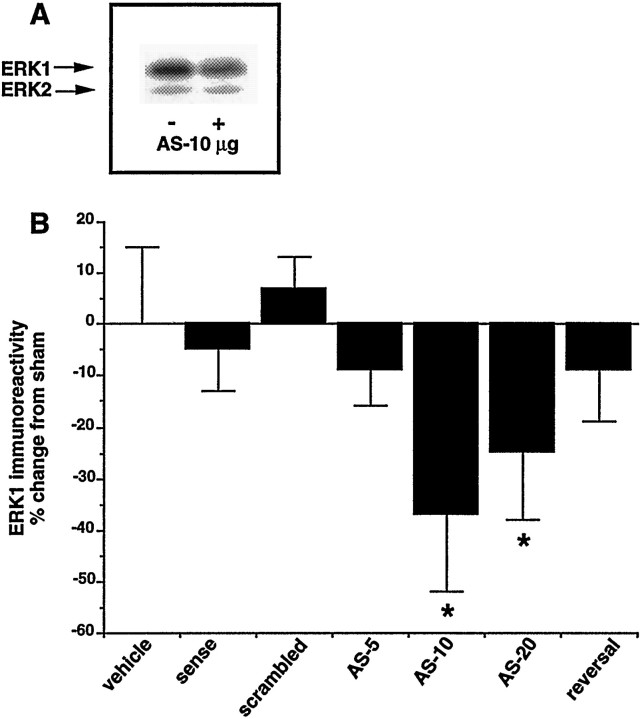
ERK1 antisense oligonucleotides were infused into the VTA for 7 days at a dose of 5, 10, or 20 μg/d. Levels of ERK1 immunoreactivity were significantly reduced at both the 10 and 20 μg/d doses but not at the 5 μg/d dose (Fig. 4). To assess the degree of diffusion of the antisense oligonucleotides, we analyzed the substantia nigra, a region that is in close anatomical proximity to the VTA. After a chronic intra-VTA infusion of 10 μg/d of ERK1 antisense oligonucleotides, there was no change in levels of ERK1 immunoreactivity in the substantia nigra (89 ± 9% of control). The decrease in ERK1 immunoreactivity in the VTA after infusion of ERK1 antisense oligonucleotides at a 10 μg/d dose was not apparent after infusion of sense or scrambled oligonucleotides (Fig. 4).
Fig. 4.
Effect of intra-VTA infusions of ERK1 antisense oligonucleotides on ERK1 immunoreactivity in the VTA. Represented are infusions of vehicle, 10 μg/d sense oligonucleotides, 10 μg/d scrambled oligonucleotides, and 5, 10, and 20 μg/d antisense (AS) oligonucleotide as well as 10 μg/d antisense infusions followed by 5 d of vehicle infusions (reversal). Data are expressed as mean ± SEM (*p < 0.05 vs vehicle by χ2 test). The number of animals used was 6 (vehicle), 7 (sense), 8 (scrambled), 7 (AS-5), 15 (AS-10), 7 (AS-20), and 8 (reversal). Inset, Representative autoradiogram of ERK immunoreactivity without (−) and with (+) ERK1 antisense oligonucleotide (10 μg/d) infusion. Note that the ratio of ERK1 to ERK2 is much greater than that shown in Figures 1 and 3. This is because the anti-ERK antibody used in this experiment (from Transduction Labs) exhibits greater relative reactivity for ERK1 than ERK2 compared with the antibody (from Santa Cruz) used in other experiments (for review, see Ortiz et al., 1995b).
To assess further the specificity of the ERK1 antisense oligonucleotide effects, several other proteins were examined. ERK2 has the overall closest homology to ERK1 in terms of nucleotide sequence. The region of ERK1 selected for the antisense probe has the least homology (<59%) to ERK2 of any region that also includes the AUG translation start site. There was no change in ERK2 immunoreactivity at any of the doses of ERK1 antisense used (Fig. 4, inset, Table 1). Levels in the VTA of other signaling proteins (phosphotidylinositol 3-kinase, phospholipase C-γ, and JAK2), cytoskeletal proteins (neurofilaments, actin), and a marker of gliosis (GFAP) also were not affected by intra-VTA infusion of ERK1 antisense oligonucleotides at a 10 μg/d dose (Table 2).
Table 2.
Proteins unchanged by ERK1 antisense oligonucleotide intra-VTA infusions
| % of sham | |
|---|---|
| GFAP | 102 ± 12 |
| NF 68 | 92 ± 11 |
| NF 160 | 112 ± 9 |
| NF 200 | 106 ± 8 |
| PI3-K | 94 ± 11 |
| PLC-γ | 103 ± 8 |
| JAK2 | 110 ± 12 |
Lack of effect of ERK1 antisense oligonucleotide infusions (10 μg/d) on cellular signaling proteins. Proteins analyzed include glial fibrillary acidic protein (GFAP); neurofilament proteins (NF 68, 160, and 200); phosphatidylinositol 3-kinase (PI3-K); phospholipase C-γ (PLC-γ); and Janus kinase-2 (JAK2).
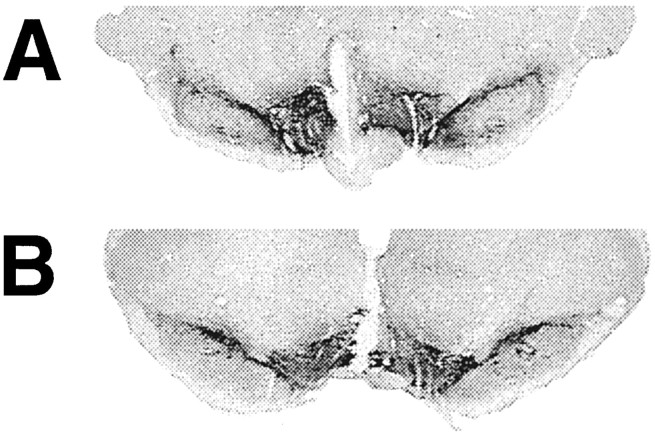
To provide still further support for the interpretation that the antisense oligonucleotide-induced decrease in ERK1 is attributable to a selective action on ERK1 and not to generalized neurotoxicity, we examined the reversibility of this effect as well as the histological integrity of the VTA. To study the reversibility of the antisense oligonucleotide effects, osmotic minipumps containing the oligonucleotides were replaced with pumps containing vehicle solution for an additional 5 d (for review, see Widnell et al., 1996). As shown in Figure 4, levels of ERK1 recovered to control levels within this 5 d period. Histological integrity of the VTA was examined by TH immunohistochemistry (Fig. 5). Equivalent midline cannulae tracts, with their tips in the midline VTA area, were apparent under both the sense and antisense oligonucleotide-treated conditions. Outside these cannulae tracts, TH immunoreactivity was present at equivalent levels, and with equivalent patterns, after treatment with sense and antisense oligonucleotides, as shown in Figure 5. On Nissl staining, areas around the tips of the cannulae showed some gliosis, with no difference between the sense- and antisense-treated groups (data not shown). Outside the immediate vicinity of the cannulae, including the bulk of the VTA, there was no detectable effect of the oligonucleotide infusions on the integrity of the tissue. Moreover, amido black staining of immunoblots demonstrated no effect of antisense oligonucleotide infusions on overall protein patterns of VTA extracts (data not shown).
Fig. 5.
Sections of midbrain from rats after intra-VTA infusion of ERK1 sense (A) or antisense (B) oligonucleotide analyzed by TH immunohistochemistry. Rats received intra-VTA infusions of oligonucleotides for 7 d at a rate of 10 μg/d, after which 30-μm-thick coronal sections of brain were subjected to TH immunohistochemistry as described in Materials and Methods.
Effect of ERK1 antisense oligonucleotides on morphine regulation of ERK activity and TH immunoreactivity in the VTA
Next, we combined the intra-VTA ERK1 antisense oligonucleotide infusions with systemic chronic morphine treatments. In this paradigm, animals received 7 d of antisense oligonucleotide infusions (10 μg/d) with morphine treatments beginning on day 3 and extending until day 7, at which time the animals were used. There was no difference in ERK immunoreactivity between the antisense oligonucleotide-treated animals that received morphine and those that had not; both showed a significant decrease in total ERK1 immunoreactivity relative to controls (Table 1). In the same VTA samples, however, antisense oligonucleotide infusions alone did not alter levels of ERK activity, but completely blocked the ability of morphine to increase ERK activity. The finding that ERK antisense oligonucleotide infusions did not alter ERK activity even though they reduced total ERK immunoreactivity suggests that, like chronic BDNF infusions, ERK antisense oligonucleotide infusions increased the fraction of the remaining ERK molecules that are in the phosphorylated–activated state (see Discussion).
Regulation of TH immunoreactivity generally paralleled that of ERK activity (Table 1). Animals that received the antisense oligonucleotide infusions alone showed no difference in levels of TH immunoreactivity in the VTA compared with control. Animals that were treated concomitantly with the antisense oligonucleotides plus morphine also showed no significant change in TH levels. Thus, the ERK1 antisense oligonucleotide infusions blocked the ability of morphine to increase TH expression.
Effect of NMDA glutamate receptor antagonists on morphine regulation of ERK activity and TH immunoreactivity in the VTA
As a first step in studying the mechanism by which chronic morphine administration might result in a sustained increase in ERK activity, we tested the effect of intra-VTA infusions of NMDA glutamate receptor antagonists. This is based on the reported ability of such antagonists to oppose many of the effects of morphine and other drugs of abuse on mesolimbic dopamine function and on recent evidence that glutamatergic function is increased in the VTA under chronic drug-treated conditions (see Discussion). As shown in Table3, chronic infusion of d-AP-5, a specific and potent antagonist of NMDA receptors, into the VTA blocked the ability of morphine to increase ERK activity in this brain region.d-AP-5 infusions alone failed to alter ERK activity. Intra-VTA infusion of d-AP-7, another potent NMDA receptor antagonist, also prevented the morphine-induced increase in ERK activity, whereas infusion of thel-stereoisomer of AP-5, which exhibits close to a 100-fold lower potency as an NMDA receptor antagonist compared withd-AP-5, failed to produce this effect.
Table 3.
Effect of glutamate receptor antagonist infusions on levels of ERK activity, ERK immunoreactivity, and TH immunoreactivity, with and without concomitant chronic morphine treatment
| Morphine | ERK activity | ERK immunoreactivity | TH immunoreactivity | |
|---|---|---|---|---|
| Vehicle3_a | − | 100 | 100 | 100 |
| + | 154 | 101 | 158 | |
| d-AP-5 | − | 109 ± 13 | 97 ± 9 | 98 ± 6 |
| + | 114 ± 16 | 102 ± 8 | 97 ± 8 | |
| l-AP-5 | + | 142 ± 17 | 104 ± 11 | 137 ±12 |
| d-AP-7 | + | 108 ± 9 | 96 ± 13 | 104 ± 14 |
Data from Table 1.
*p < 0.05 versus vehicle (− morphine treatment) by χ2 test. Data for ERK1 and ERK2, although analyzed separately, were summed to one value; no effect was observed on the individual subtypes (data not shown).
These NMDA receptor antagonists produced parallel effects on TH expression (Table 3). Intra-VTA infusions ofd-AP-5 or d-AP-7 prevented the morphine-induced increase in TH levels in this brain region, without altering TH levels when given alone. In contrast, infusion ofl-AP-5 failed to influence morphine regulation of TH levels.
DISCUSSION
Previous research has shown a pharmacological interaction between neurotrophins and the biochemical adaptations in the VTA that are seen with chronic morphine and cocaine exposure. Specifically, intra-VTA infusions of BDNF or NT4 have been shown to prevent as well as reverse the morphine- and cocaine-induced increase in TH and certain other biochemical adaptations in this brain region (Berhow et al., 1995). These findings led to the current study, in which the neurotrophin signal transduction cascade was examined as one possible site of convergence between the neurotrophins and drugs of abuse. Using an in-gel ERK activity assay, we found that both chronic morphine and chronic cocaine treatments produced a significant increase in ERK activity in the VTA. This increase required chronic administration of the drugs and was not observed in several other brain regions studied. Moreover, the drug-induced increase in ERK activity was shown to be attributable to an increase in the phosphorylation state of the enzyme without a change in total ERK immunoreactivity.
We also found that acute injection of BDNF into the VTA increased levels of ERK activity, without a change in total ERK levels, in this brain region. This increase in ERK activity would be expected based on the known ability of BDNF and other neurotrophins to activate the ERK signaling cascade in cultured cells (for review, see Davis, 1993), although this is the first report of this effect in the brain in vivo. After chronic administration, however, BDNF infusions led to a decrease in total ERK levels, with no net change in ERK activity. These findings suggest that after chronic BDNF infusions, a greater percentage of the remaining ERK molecules are in the phosphorylated–activated form. The chronic BDNF-induced reduction in total ERK levels, with no change in ERK phosphorylation state, can be viewed as a homeostatic response of VTA cells: persistent activation of ERK by BDNF leads to a compensatory decrease in ERK expression, which returns ERK activity to control levels. Chronic BDNF infusions were also found to block the ability of chronic morphine to increase ERK activity in the VTA.
Together, these findings support a hypothesis whereby chronic BDNF infusions attenuate morphine and cocaine regulation of TH and other biochemical endpoints in the VTA by reducing the adaptive capacity of the VTA to respond to drug exposure with increased levels of ERK activity. That is, BDNF, by leading to lower levels of more highly phosphorylated–activated ERK, prevents the ability of morphine or cocaine to further activate the enzyme by phosphorylation. An attractive feature of this model is that it can account for the lack of effect of BDNF infusions alone, as well as the ability of the neurotrophin to completely obliterate morphine and cocaine regulation of TH and other target proteins.
This hypothesis was tested by use of antisense oligonucleotides directed against ERK1 to directly reduce ERK levels selectively in the VTA in vivo. The use of antisense oligonucleotides was necessitated by the lack of specific ERK inhibitors. We found that the infusion of partially phosphorothioate-modified ERK1 antisense oligonucleotides into the VTA resulted in a selective and dose-dependent reduction in ERK1 levels in this brain region. Although the use of intracerebrally administered antisense oligonucleotides should be viewed with caution, the specificity of the ERK1 antisense oligonucleotides was demonstrated by several control experiments (for review, see Stein and Cheng, 1994; Widnell et al., 1996). First, ERK1 antisense oligonucleotides resulted in a selective reduction in levels of ERK1, with no effect on ERK2 (despite its high degree of homology with ERK1) or on several other signaling proteins in the VTA. Second, sense and scrambled oligonucleotides failed to alter ERK1 levels. Third, the antisense oligonucleotide-induced decrease in ERK1 levels was fully reversible within 5 d of discontinuing oligonucleotide infusion. Fourth, histological examination of tissue sections revealed no differences in the integrity of the VTA between antisense and sense oligonucleotide infusions, indicating that the biochemical differences observed under the two treatment conditions cannot be attributed to toxicity. The results confirm the utility of antisense oligonucleotides, under carefully controlled conditions, to study the functioning of proteins in the brain for which no traditional pharmacological antagonists are available (for review, see Widnell et al., 1996).
Chronic intra-VTA infusion of ERK1 antisense oligonucleotides mimicked the effects of chronic intra-VTA infusion of BDNF in several ways. The antisense oligonucleotides decreased levels of total ERK1, with no net effect on ERK activity. It would appear that a larger percentage of the remaining ERK molecules are phosphorylated to compensate for the oligonucleotide-induced decrease in total ERK levels. Interestingly, ERK1 antisense oligonucleotide infusions also blocked the ability of morphine to increase ERK activity and to increase TH levels in the VTA. These data support the hypothesis stated above that morphine regulation of TH may be mediated in part by drug regulation of ERK activity, and that prolonged BDNF treatment prevents these effects by reducing the adaptive capacity of the ERK system. More specifically, the antisense oligonucleotide experiments demonstrate that primary changes in ERKper se are sufficient to mimic BDNF interactions with morphine. Whether such changes in ERK are also necessary for these interactions will require additional investigations.
We also provide evidence in this study that chronic morphine and cocaine treatments result in a sustained increase in ERK activity via a glutamate-dependent mechanism. Intra-VTA infusion of specific NMDA glutamate receptor antagonists prevented the ability of chronic morphine to increase both ERK activity and TH expression. This finding is consistent with the reported ability of NMDA and other glutamate receptor antagonists, given systemically as well as locally within the VTA, to block many of the effects of opiates and cocaine on mesolimbic dopamine function (for review, see Karler et al., 1991; Schenk et al., 1993; Wolf et al., 1994; Fitzgerald et al., 1996). This finding also provides a further association between drug regulation of ERK activity and TH expression, and supports a cellular scheme (shown in Fig.6) by which chronic drug exposure could regulate ERK activity. Recent work has demonstrated that chronic exposure to morphine or cocaine increases levels of expression of specific glutamate receptor subunits in the VTA (Fitzgerald et al., 1996). This upregulation of glutamate receptors could account for the increased electrical excitability of VTA dopamine neurons and their increased sensitivity to glutamate, both of which have been demonstrated directly in electrophysiological investigations (Henry et al., 1989; White et al., 1995). Increased firing of these neurons, in turn, would be expected to lead to increases in intracellular levels of calcium, which has been shown to activate the ERK cascade in several cell culture systems (for review, see Ghosh and Green- berg, 1995) .
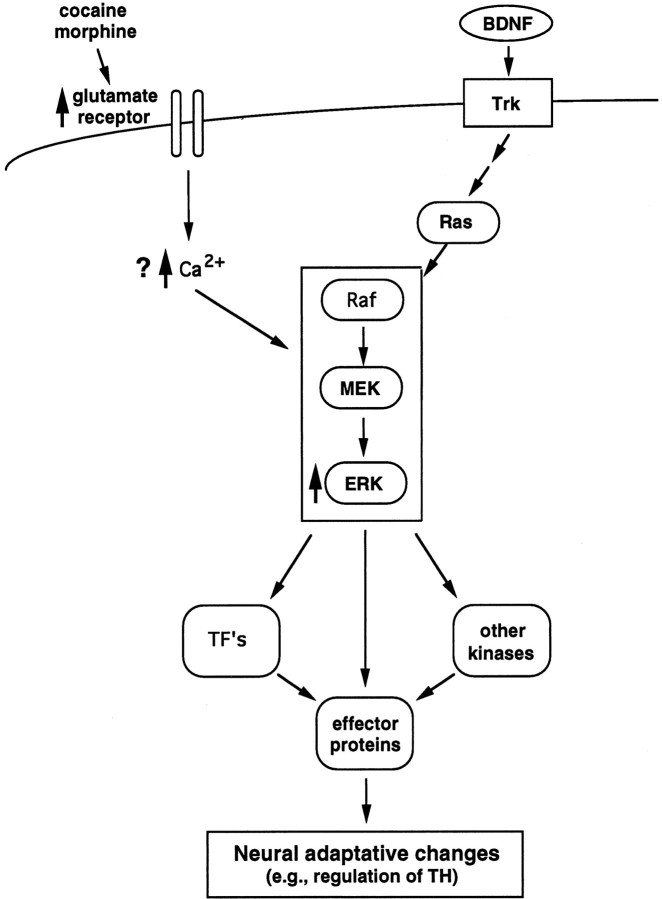
Fig. 6.
Scheme illustrating a possible mechanism by which chronic morphine or cocaine exposure increases ERK activity in the VTA. The neurotrophins, e.g., BDNF, regulate neuronal function via activation of Trk receptors, which leads to the activation of Ras and a protein kinase cascade involving Raf, MEK, and ERK. Activation of ERK then leads to the direct phosphorylation of effector proteins (one example of which is TH), as well as of transcription factors and other protein kinases, which results in the regulation of many additional effector proteins. Chronic morphine and cocaine treatments have been shown to increase levels of specific glutamate receptor subunits (NMDAR1 and GluR1) selectively in the VTA. This increase could account for the increased firing rate of VTA dopamine neurons demonstrated under drug-treated conditions which, in turn, would be expected to increase intracellular Ca2+ levels. Increased Ca2+ levels would then lead to activation of the ERK cascade, as has been demonstrated in cultured cells, although the exact mechanisms remain unknown. The resulting increase in ERK activity would then result in a multitude of downstream effects, including increases in TH expression, as has also been observed in cultured cells.
An alternative mechanism by which chronic morphine or cocaine exposure could conceivably increase ERK activity in the VTA is through regulation of other steps in the neurotrophin signal transduction cascade. However, attempts to demonstrate chronic morphine- or chronic cocaine-induced adaptations at points in these cascades proximal to ERK have to date yielded negative results. For example, preliminary studies indicate that chronic morphine or cocaine exposure does not alter levels of BDNF or TrkB (the predominant form of Trk in the VTA) in the VTA (Russell et al., 1994; Ortiz et al., 1995b).
Clearly, additional work is needed to study the mechanisms by which chronic morphine and cocaine exposure lead to changes in ERK activity selectively in the VTA. In addition, it will be essential in future studies to further evaluate the role played by drug regulation of ERK on TH expression and other biochemical actions of the drugs in this brain region as well as on the profound behavioral effects that these drugs elicit via the mesolimbic dopamine system. It should also be emphasized that morphine and cocaine regulation of ERK is likely just one of several mechanisms by which chronic drug exposure leads to long-term biochemical adaptations in the VTA. As just one example, we have found recently that chronic cocaine treatment increases levels of JAK2, a cytoplasmic protein tyrosine kinase regulated by CNTF, specifically in the VTA (Berhow et al., 1996). Nevertheless, the results of the present study demonstrate novel actions of drugs of abuse, namely, effects on intracellular signaling pathways outside of traditional second messenger cascades in the mesolimbic dopamine system. In this manner, the results highlight the complex array of regulatory mechanisms that are likely to mediate the long-lasting effects of drugs of abuse on brain function.
Footnotes
This work was supported by U.S. Public Health Service Grants DA07359, DA08227, DA10160, and DA00203; the Abraham Ribicoff Research Facilities; and the Yale University MD/PhD Program. We thank John Haycock, Ronald Duman, Moses Chao, Melanie Cobb, David Russell, and Ronald Lindsay for their input regarding these studies and manuscript preparation.
Correspondence should be addressed to Dr. Eric J. Nestler, Laboratory of Molecular Psychiatry, Departments of Psychiatry and Pharmacology, Yale University School of Medicine, Connecticut Mental Health Center, 34 Park Street, New Haven, CT 06508.
REFERENCES
- 1.Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 2.Beitner-Johnson D, Guitart X, Nestler EJ. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci. 1992;12:2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DS, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- 4.Berhow MT, Hiroi N, Kobierski LA, Hyman SE, Nestler EJ (1996) Influence of cocaine on the JAK-STAT pathway in the mesolimbic dopamine system. Soc Neurosci Abstr, in press. [DOI] [PMC free article] [PubMed]
- 5.Boulton TG, Yancopoulos GD, Gregory JS, Slaughter C, Moomaw C, Hsu J, Cobb MH. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 6.Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. In: Harris LS, editor. Problems of drug dependence. US Government Printing Office; Washington, D.C.: 1986. pp. 190–196. [PubMed] [Google Scholar]
- 7.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 8.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Regulation of glutamate receptor subunit expression by drugs of abuse and stress: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner AM, Vaillancourt RR, Johnson GL. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993;268:17896–17901. [PubMed] [Google Scholar]
- 11.Ghosh A, Greenberg ME. Calcium signalling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 12.Gizang-Ginsberg E, Ziff EB. Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev. 1990;4:477–491. doi: 10.1101/gad.4.4.477. [DOI] [PubMed] [Google Scholar]
- 13.Guitart X, Nestler EJ. Identification of morphine- and cyclic AMP-regulated phosphoproteins (MARPPs) in the locus coeruleus and other regions of rat brain: regulation by acute and chronic morphine. J Neurosci. 1989;9:2649–2659. doi: 10.1523/JNEUROSCI.09-12-04371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci USA. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- 16.Hu BR, Wieloch T. Tyrosine phosphorylation and activation of mitogen-activated protein kinase in the rat brain following transient cerebral ischemia. J Neurochem. 1994;62:1357–1367. doi: 10.1046/j.1471-4159.1994.62041357.x. [DOI] [PubMed] [Google Scholar]
- 17.Hurd YL, Linefors N, Brodin E, Brené S, Persson H, Ungerstedt U, Hökfelt T. Amphetamine regulation of mesolimbic dopamine/cholecystokinin neurotransmission. Brain Res. 1992;578:317–326. doi: 10.1016/0006-8993(92)90264-a. [DOI] [PubMed] [Google Scholar]
- 18.Jay TM, Burette F, Laroche S. NMDA receptor-dependent long term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur J Neurosci. 1995;7:247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 19.Kameshita I, Fujiscua H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 20.Karler R, Chaudhry IA, Calder LD, Turkanis SA. DNQX blockade of amphetamine behavioral sensitization. Brain Res. 1991;552:295–300. doi: 10.1016/0006-8993(91)90095-d. [DOI] [PubMed] [Google Scholar]
- 21.Kogan JH, Aghajanian GK. Long-term desensitization in locus coeruleus neurons and its role in opiate withdrawal. Brain Res. 1995;689:111–121. doi: 10.1016/0006-8993(95)00545-2. [DOI] [PubMed] [Google Scholar]
- 22.Koob GF. Drugs of abuse: anatomy, pharmacology, and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 23.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SE, Rao MS, Symes AJ, Daurer WT, Fink JS, Landis SC, Hyman SE. Coordinated regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- 25.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by raf-1 and MEKK. Science. 1994a;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 26.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994b;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olverman HJ, Jones AW, Mewett KN, Watkins JC. Structure/activity relations of N -methyl-d-aspartate receptor ligands as studied by their inhibition of [3H]d-2-amino-5-phosphonopentanoic acid binding in rat brain membranes. Neuroscience. 1988;26:17–31. doi: 10.1016/0306-4522(88)90124-8. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995a;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinase (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995b;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovsky ED, Ramchatesingh J, McManaman JL. Regulation of tyrosine hydroxylase gene expression in IMR-32 neuroblastoma cells by basic fibroblast growth factor and ciliary neurotrophic factor. J Neurochem. 1995;64:2404–2412. doi: 10.1046/j.1471-4159.1995.64062404.x. [DOI] [PubMed] [Google Scholar]
- 32.Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 33.Russell DS, Berhow MT, Widnell KL, Self DW, Nestler EJ. Modulation of neurotrophin receptor tyrosine kinase (RTK) pathways in the mesolimbic dopamine system by drugs of abuse. Soc Neurosci Abstr. 1994;20:1102. [Google Scholar]
- 34.Schenk S, Valadez A, McNamara C, House DT, Higley D, Bankson MG, Giggs S, Horger BA. Development and expression of sensitization to cocaine’s reinforcing properties: role of NMDA receptors. Psychopharmacology. 1993;11:332–338. doi: 10.1007/BF02244949. [DOI] [PubMed] [Google Scholar]
- 35.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 36.Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 37.Sorg BA, Chen S-Y, Kalivas PW. Time of tyrosine hydroxylase expression after behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;266:424–430. [PubMed] [Google Scholar]
- 38.Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents—Is the bullet really magical? Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 39.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 40.Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 41.Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity. J Neurochem. 1993;61:2262–2268. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 42.Westwick JK, Weitzel C, Minden A, Karin M, Brenner DA. Tumor necrosis factor a stimulates AP-1 through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 43.White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administra- tion of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- 44.Whitton PS, Richards DA, Biggs CS, Fowler LJ. N -methyl-d-aspartate receptors modulate extracellular 5-hydroxytryptamine concentration in rat hippocampus and striatum in vivo. Neurosci Lett. 1994;169:215–218. doi: 10.1016/0304-3940(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 45.Widnell KL, Self DW, Russell DS, Lane S, Vaidya V, Nestler EJ. Regulation of expression of cAMP response element binding protein (CREB) in the nucleus accumbens: a functional role for CREB down-regulation by morphine. J Pharmacol Exp Ther. 1996;276:306–315. [PubMed] [Google Scholar]
- 46.Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]