Abstract
Background
Sexually transmitted diseases (STDs) including chlamydia and gonorrhea, cause pelvic inflammatory disease and infertility. We estimated the prevalence of infertility and infertility healthcare seeking.
Methods
We analyzed self-reported lifetime infertility and infertility healthcare-seeking in women aged 18–49 years in the 2013 and 2015 National Health and Nutrition Examination Surveys. Weighted prevalence of infertility and infertility healthcare seeking, prevalence ratios (PRs), and 95% confidence intervals (CIs) were calculated.
Results
Among 2,626 eligible women, 13.8% had self-reported infertility [95% CI 12.3–15.3] with higher prevalence by age: 6.4% [95% CI 4.8–8.0], n=960 18–29 year olds; 14.8% [95 % CI 12.2–17.3], n=799 30–39 year olds; and 20.8% [95% CI 17.2–24.4], n=867 40–49 year olds. Non-Hispanic white women (15.4% [95% CI 13.0–17.8]; n=904) and non-Hispanic black women (12.9% [95% CI 10.3–15.5]; n=575) had the highest infertility prevalences. Women reporting PID treatment (n=122) had higher infertility prevalence (24.2% [95% CI 16.2–32.2]) than women without PID treatment (13.3% [95% CI 11.6–15.0], n=2,485), especially among 18–29 year old women (PR 3.8 [95% CI 1.8–8.0)]. Of 327 women with infertility, 60.9% (95% CI 56.1–65.8) sought healthcare. Women without healthcare insurance sought care less frequently than women with insurance.
Conclusions
In a nationally-representative sample, 13.8% of reproductive-age women reported a history of infertility, of whom 40% did not access healthcare. Self-reported PID was associated with infertility, especially in young women. Annual chlamydia and gonorrhea screening to avert PID may reduce the burden of infertility in the US.
Keywords: sexually transmitted infections, chlamydia, gonorrhea, reproductive health, epidemiology
Summary
Of the 13.8% of reproductive-age women reporting any lifetime infertility, 40% did not access healthcare. Self-reported pelvic inflammatory disease was associated with infertility, especially among young women.
Introduction
Infertility, or the inability to conceive a child in a 12-month period, affected an estimated 6.7% of women in the United States during 2011–2015(1) and can be associated with psychosocial morbidity, including depression and anxiety(2, 3). Healthcare costs associated with treatment for infertility can be considerable, given that a cycle of in vitro fertilization (IVF) is estimated to cost $12,400(4). Female factors in infertility include oocyte aging, ovulatory disorders, tubal and uterine factors (e.g. tubal damage, pelvic adhesions, and endometriosis), and other factors(5).
Tubal factor infertility (TFI) is often caused by sexually transmitted diseases (STDs), notably chlamydia and gonorrhea. These infections may lead to symptomatic or asymptomatic pelvic inflammatory disease (PID) characterized by tubal inflammation and scarring. In the 1980s chlamydia surpassed gonorrhea as the organism most commonly isolated from women with PID(6). Historically, up to 5% of untreated chlamydial infections cause PID in the first few weeks after infection(7). In the year after untreated chlamydia infection, 9.5% of women developed PID(8); however, as many as 30% of women have developed PID after concurrent gonococcal and chlamydial infection(9). Once women have PID, up to 15–20% subsequently develop infertility(7) with a large proportion of this infertility being TFI(10).
Infertility due to STDs is preventable. To this end, public health agencies have supported programs to improve STD prevention and early detection(11). Current data on the epidemiology of infertility may help to guide public health efforts. Questions about infertility were first included in the National Health and Nutrition Examination Survey (NHANES) in the 2013–2014 cycle. We estimate the prevalence of self-reported lifetime infertility and infertility healthcare seeking in a nationally representative sample from the 2013–2016 cycles of NHANES to describe the current epidemiology of infertility in the United States.
Materials and Methods
NHANES is a cross-sectional, nationally-representative, complex, multistage survey to assess the general health status of the non-institutionalized U.S. population(12). Consenting participants complete an interview questionnaire, undergo a physical exam, and submit biological specimens for laboratory tests.
We analyzed data from the 2013–2014 and 2015–2016 NHANES cycles from women of reproductive age (18–49 years of age) who were sexually-experienced (defined as reporting ever having had vaginal sexual intercourse with a man) to determine the weighted lifetime prevalence of self-reported infertility. A lifetime history of infertility was defined as a ‘yes’ answer to the question: ‘Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?’. We also assessed healthcare seeking behavior among women who reported a history of infertility, by the question: ‘Have you ever been to a doctor or other medical provider because you have been unable to become pregnant?’. Hispanic ethnicity was defined for women who reported being Mexican American or those who reported being ‘other Hispanic’.
We analyzed demographic characteristics and characteristics obtained from interview (including (A) self-reported history of chlamydia or gonorrhea in the past 12 months, (B) history of an STD diagnosis [chlamydia or gonorrhea in the past 12 months or having been told of a herpes, genital warts, or human papillomavirus diagnosis], (C) self-reported history of PID treatment), and (D) laboratory factors, including results of Chlamydia trachomatis or Trichomonas vaginalis nucleic acid amplification testing (NAAT)(13) from a urine specimen collected at the time of exam.
To account for the complex survey design, we used provided sampling weights and estimated the weighted prevalence of infertility and infertility healthcare seeking with 95% confidence intervals (CI) for the combined 2013–2014 and 2015–2016 cycles. Furthermore, we estimated the prevalence ratios (PR) with 95% CI to compare the prevalence of infertility among subgroups of interest (e.g., age group, race/ethnicity, and women with STDs or PID).
P-values were calculated using the Rao-Scott chi-square test. We set a statistical significance level at a P-value of less than 0.05. Relative standard errors (RSE) were calculated. A calculated RSE of greater than 30% was highlighted because this value is potentially unreliable and should be interpreted with caution per NHANES guidance(14).
The primary NHANES protocol has National Center for Health Statistics Ethics Review Board approval. Informed consent is sought from participants, and data from NHANES are publically available, thus no additional review was required before obtaining the data and conducting the analysis(12).
Results
Study sample
Overall, 10,251 women were included from the 2013–2014 and 2015–2016 NHANES cycles (mobile exam response rate ranged from: 58.9–77.1%(15)). Of these, 3,423 (33.4%) were of reproductive age (18–49 years of age), 3,304 (96.5%) of these women completed an interview and physical exam, and 2,631 (79.6%) reported being sexually-experienced. Our analytic sample included the 2,626 (99.8%) sexually-experienced women 18–49 years of age who provided an answer to the question on lifetime history of infertility.
Prevalence of lifetime infertility
The weighted prevalence of self-reported lifetime infertility was 13.8% (95% CI 12.3–15.3) (Table 1) among 2,626 women in the 2013–2016 cycles of NHANES and was similar in each of the two cycles (14.7% [95% CI 12.3–17.0] in 2013–2014 and 12.9% [95% CI 11.1–14.8] in 2015–2016). Infertility prevalence increased by age group: the prevalence among women 40–49 years of age was 3.3 times the prevalence (95% CI 2.3–4.5) among women 18–29 years of age (Table 1). By race/ethnicity, non-Hispanic white women (15.4% [95% CI 13.0–17.8]) and non- Hispanic black women (12.9% [95% CI 10.3–15.5]) had the highest infertility prevalences, followed by Hispanic women (10.9% [95% CI 8.7–13.1]). Compared to non-Hispanic white women, Hispanic women had a statistically significantly lower infertility prevalence (PR 0.7 [95% CI 0.5–1.0], p=0.03), whereas for non-Hispanic Asian women this comparison approached statistical significance (PR 0.7 [95% CI 0.5–1.0], p=0.06) (Table 1). Women with higher incomes and greater educational attainment reported infertility more often than women with lower incomes and less education (Table 1).
Table 1:
Prevalence of a self-reported lifetime episode of infertility1 among sexually-experienced women aged 18–49 years, by selected characteristics, National Health and Nutrition Examination Survey, 2013–2016 (N=2,6262)
| Characteristic | Category | Sample size | Weighted prevalence3 (%) | Weighted prevalence3 (%) 95% CI4 | Weighted prevalence ratio3,5 | Weighted prevalence ratio3,5 95% CI4 |
|---|---|---|---|---|---|---|
| TOTAL | 2,626 | 13.8 | 12.3–15.3 | |||
| Age (years) | 18–29 | 960 | 6.4 | 4.8–8.0 | REF | REF |
| 30–39 | 799 | 14.8 | 12.2–17.3 | 2.3 | 1.7–3.2 | |
| 40–49 | 867 | 20.8 | 17.2–24.4 | 3.3 | 2.3–4.5 | |
| Race and Hispanic ethnicity | Non-Hispanic white | 904 | 15.4 | 13.0–17.8 | REF | REF |
| Non-Hispanic black | 575 | 12.9 | 10.3–15.5 | 0.8 | 0.7–1.1 | |
| Hispanic6 | 756 | 10.9 | 8.7–13.1 | 0.7 | 0.5–1.0 | |
| Non-Hispanic Asian | 274 | 10.4 | 6.7–14.1 | 0.7 | 0.5–1.0 | |
| Other or Multi-racial | 117 | 11.4 | 5.3–17.6 | 0.7 | 0.4–1.3 | |
| Ratio of family income to poverty level7 | <1.5 | 1012 | 11.3 | 8.8–13.8 | REF | REF |
| 1.5–3 | 618 | 13.9 | 10.7–17.1 | 1.2 | 0.9–1.7 | |
| >=3 | 820 | 16.3 | 13.7–18.8 | 1.4 | 1.1–2.0 | |
| Marital status | Never married | 641 | 6.1 | 4.6–7.7 | REF | REF |
| Divorced, widowed, separated | 331 | 14.1 | 8.6–19.5 | 2.3 | 1.4–3.8 | |
| Married | 1454 | 17.4 | 15.0–19.8 | 2.8 | 2.1–3.9 | |
| Education | < High school | 459 | 9.5 | 6.4–12.6 | 0.6 | 0.4–0.9 |
| High school graduate/General education diploma | 535 | 12.6 | 9.6–15.5 | 0.8 | 0.6–1.1 | |
| Some college/Associate’s degree | 926 | 14.9 | 12.7–17.1 | 1.0 | 0.8–1.3 | |
| > College graduate | 706 | 15.0 | 12.0–18.0 | REF | REF | |
| Ever had PID8 treatment | Yes | 122 | 24.2 | 16.2–32.2 | 1.8 | 1.2–2.7 |
| No | 2485 | 13.3 | 11.6–15.0 | REF | REF | |
| Previous STD9 diagnosis | Yes | 441 | 17.1 | 13.1–21.1 | 1.3 | 1.0–1.8 |
| No | 2182 | 13.0 | 11.2–14.7 | REF | REF | |
| Told had chlamydia in past 12 months10 | Yes | 53 | 17.0 | 1.0–33.0 | 1.2 | 0.5–3.1 |
| No | 2568 | 13.8 | 12.3–15.2 | REF | REF | |
| Told had gonorrhea in past 12 months | Yes | 7 | NA | NA | NA | NA |
| No | 2615 | NA | NA | NA | NA | |
| Urine Chlamydia NAAT11 result10 | Positive | 45 | 7.9 | 0.4–15.5 | 0.8 | 0.3–2.0 |
| Negative | 1689 | 10.4 | 9.0–11.8 | REF | REF | |
| Urine Trichomonas NAAT11 result | Positive | 96 | 19.9 | 9.6–30.1 | 1.5 | 0.8–2.6 |
| Negative | 2494 | 13.7 | 12.1–15.4 | REF | REF | |
| Ever pregnant | Yes | 1902 | 16.7 | 14.6–18.8 | 2.4 | 1.7–3.3 |
| No | 522 | 7.0 | 5.1–9.0 | REF | REF | |
| Ever used birth control pills | Yes | 1804 | 14.9 | 13.3–16.6 | 1.4 | 1.1–2.0 |
| No | 821 | 10.3 | 7.2–13.4 | REF | REF | |
Prevalence estimates based on response to the question “Have you … ever been to a doctor or other medical provider because you have … been unable to become pregnant?”
Variables with missing data include: Ratio of family income to poverty level (n=176); Marital status (n=200); ever had PID treatment (n=19); previous STD diagnosis (n=3); told had chlamydia in past 12 months (n=5); told had gonorrhea in past 12 months (n=4); urine chlamydia NAAT (n=892); urine trichomonas NAAT (n=36); ever pregnant (n=202); ever used birth control (n=1)
Estimates were weighted to be nationally representative of the U.S. population, accounting for unequal probabilities of selection and nonresponse
CI: Confidence interval
Respondents with missing or unknown values were excluded from prevalence ratio calculations
Hispanic ethnicity includes Mexican American and other Hispanic ethnicity
Poverty level as defined by the Department of Health and Human Services
PID: Pelvic inflammatory disease
Previous STD (Sexually transmitted disease) includes chlamydia or gonorrhea in the last 12 months or ever being told of a herpes, genital warts, or human papillomavirus diagnosis
Relative standard error is > 30% and <50%
NAAT: Nucleic acid amplification test
Overall, the prevalence of infertility did not significantly differ by having had a chlamydia diagnosis in the past 12 months, or Chlamydia trachomatis or Trichomonas vaginalis NAAT positivity at the time of exam (Table 1). The prevalence of infertility among women who reported a prior STD diagnosis was 17.1% (95% CI 13.1–21.1) and 13.0% (95% CI 11.2–14.7) for those without a prior STD. Overall, women who reported a history of PID treatment had an infertility prevalence of 24.2% (95% CI 16.2–32.2), which was 1.8 times the prevalence among women with no history of PID treatment (13.3% [95% CI 11.6–15.0]) (Table 1).
Among women 18–29 years of age, Hispanic and non-Hispanic black women had the highest prevalences of infertility (10.0% [95% CI 6.2–13.8%] and 10.6% [95% CI 6.7–14.5%], respectively). Among women 40–49 years of age, non-Hispanic white and non-Hispanic Asian women had the highest infertility prevalences (24.6% [95% CI 19.3–29.8] and 16.0% [95% CI 10.2–21.9], respectively. (Figure 1). In looking at history of PID treatment among age groups, the difference in infertility prevalence between women with and without reported histories of PID treatment was most pronounced among 18–29 year old women (PR 3.8 [95% CI 1.8–8.0]; 22.1% [95% CI 6.9–37.3] versus 5.8% [95% CI 4.3–7.2] respectively) (Figure 2).
Figure 1. Prevalence of self-reported infertility among sexually-experienced women aged 18–49 years by age and race-ethnicity, National Health and Nutrition Examination Survey, 2013–20161.
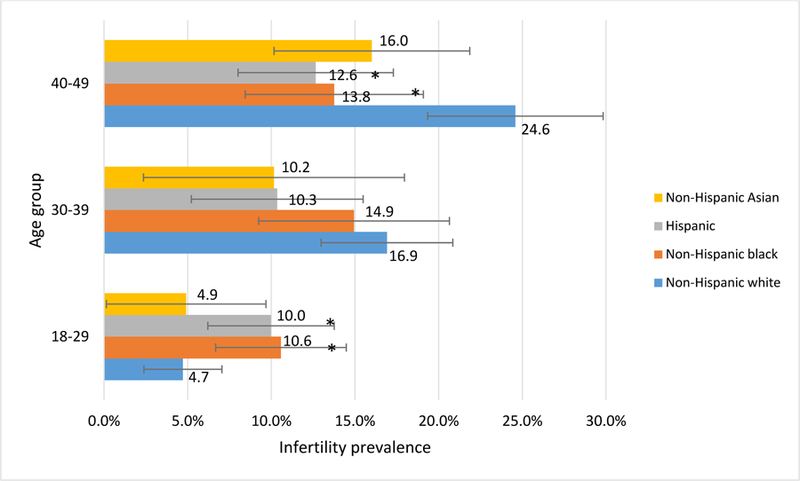
1Based on initial sample size of N=2,509. Women of other or multirace categories (n=117) are excluded because of small sample size.
* Statistically significant difference from non-Hispanic white women (p<0.05, Rao-Scott chi-square)
Figure 2. Prevalence of self-reported infertility among sexually-experienced women aged 18–49 years by age and PID1 treatment history, National Health and Nutrition Examination Survey, 2013–20162.
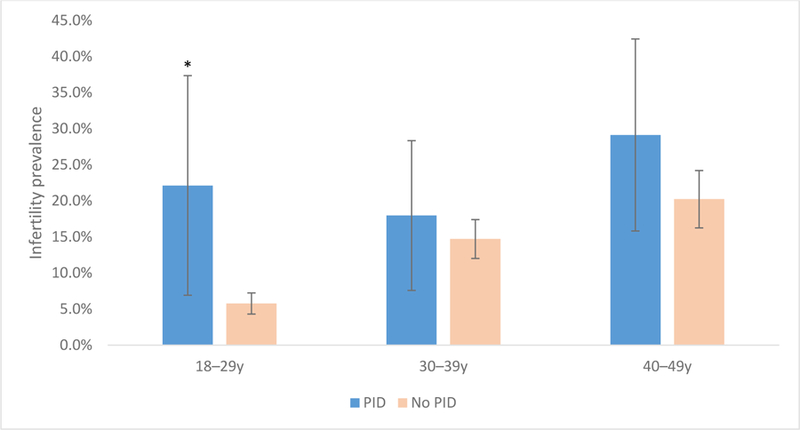
1PID: Pelvic inflammatory disease
2Based on initial sample size of N=2,607. Women without available data for PID treatment are excluded (n=19).
* Infertility prevalence ratio for PID to no PID is 3.8 (95% CI 1.8–8.0), p<0.01. Relative standard error > 30% for infertility prevalence among 18–29 year old women with PID
Prevalence of infertility healthcare seeking
Among the 327 women who self-reported infertility, the weighted prevalence of reporting ever having sought healthcare for infertility was 60.9% (95% CI 56.1–65.8). There was no difference in the prevalence of healthcare seeking between the 2013–2014 and 2015–2016 cycles.
By age group, infertile women 18–29 years of age had the lowest prevalence of seeking care for infertility (33.9%) and infertile women 30–39 years of age had the highest prevalence (70.8%) (PR 2.1 [95% CI 1.4–3.0]) (Table 2). Non-Hispanic black women were less likely to seek infertility-related healthcare than non-Hispanic white women (PR 0.7 [95% CI 0.5–0.8]). Women with the lowest family incomes were less likely to seek care than women with the highest incomes (41.8% ([95% CI 29.6–54.1]) vs. 71.5% [95% CI 64.5–78.6], respectively). Women with health insurance were more likely to have sought care than women without insurance (64.3% [95% CI 58.9–69.7] vs. 45.4% [95% CI 35.5–55.3]) (Table 2).
Table 2:
Prevalence of seeking care for infertility1 among sexually-experienced women aged 18–49 years with reported history of infertility, by selected characteristics, National Health and Nutrition Examination Survey, 2013–2016 (N=3272)
| Characteristic | Category | Sample size | Weighted prevalence3 (%) |
Weighted prevalence3 95% CI4 | Weighted prevalence ratio5 |
Weighted prevalence ratio5 95% CI4 |
|---|---|---|---|---|---|---|
| TOTAL | 327 | 60.9 | 56.1–65.8 | |||
| Age (years) | 18–29 | 69 | 33.9 | 23.1–44.8 | REF | REF |
| 30–39 | 106 | 70.8 | 60.8–80.8 | 2.1 | 1.4–3.0 | |
| 40–49 | 152 | 63.3 | 57.2–69.4 | 1.9 | 1.3–2.6 | |
| Race and Hispanic origin | Non-Hispanic White | 132 | 64.8 | 58.4–71.1 | REF | REF |
| Non-Hispanic Black | 71 | 42.7 | 34.2–51.2 | 0.7 | 0.5–0.8 | |
| Hispanic6 | 79 | 50.0 | 37.5–62.5 | 0.8 | 0.6–1.0 | |
| Non-Hispanic Asian | 30 | 87.0 | 79.1–94.9 | 1.3 | 1.2–1.5 | |
| Other or Multi-Racial | 15 | 70.5 | 50.8–90.3 | 1.1 | 0.8–1.4 | |
| Ratio of family income to poverty level7 | <1.5 | 113 | 41.8 | 29.6–54.1 | REF | REF |
| 1.5–3 | 74 | 61.5 | 52.4–70.6 | 1.5 | 1.0–2.2 | |
| >=3 | 124 | 71.5 | 64.5–78.6 | 1.7 | 1.3–2.3 | |
| Marital status | Never married | 47 | 45.9 | 32.0–59.8 | REF | REF |
| Divorced, widowed, separated | 43 | 56.8 | 42.3–71.3 | 1.2 | 0.8–1.9 | |
| Married | 233 | 63.9 | 57.8–70.0 | 1.4 | 1.0–2.0 | |
| Education | <High school | 43 | 33.8 | 18.6–48.9 | 0.5 | 0.3–0.7 |
| High school graduate/General education diploma | 61 | 43.8 | 28.4–59.1 | 0.6 | 0.4–0.9 | |
| Some college/Associate’s degree | 130 | 62.5 | 54.3–70.7 | 0.8 | 0.7–1.0 | |
| > College graduate | 93 | 74.1 | 65.8–82.4 | REF | REF | |
| Ever had PID8 treatment | Yes | 28 | 51.6 | 29.8–73.4 | 0.8 | 0.5–1.3 |
| No | 296 | 61.5 | 56.2–66.8 | REF | REF | |
| Previous STD9 diagnosis | Yes | 67 | 63.4 | 51.3–75.4 | 1.1 | 0.8–1.3 |
| No | 260 | 60.1 | 54.1–66.1 | REF | REF | |
| Told had chlamydia in past 12 months10 | Yes | 8 | 31.2 | 11.4–51.1 | 0.5 | 0.3–1.0 |
| No | 319 | 61.6 | 56.8–66.5 | REF | REF | |
| Told had gonorrhea in past 12 months | Yes | 0 | NA | NA | NA | NA |
| No | 327 | NA | NA | NA | NA | |
| Urine Chlamydia NAAT11 result12 | Positive | 4 | 25.5 | (0.0–68.5) | 0.4 | (0.08–2.4) |
| Negative | 169 | 59.5 | (51.7–67.3) | REF | REF | |
| Urine Trichomonas NAAT result10 | Positive | 20 | 21.1 | (0.9–41.2) | 0.3 | (0.1–0.9) |
| Negative | 304 | 62.9 | (58.1–67.6) | REF | REF | |
| Ever pregnant | Yes | 277 | 63.5 | (58.0–69.1) | 1.4 | (1.0–2.1) |
| No | 45 | 44.5 | (28.8–60.2) | REF | REF | |
| Ever used birth control pills | Yes | 254 | 63.5 | (57.9–69.2) | 1.3 | (1.0–1.7) |
| No | 73 | 49.2 | (37.6–60.7) | REF | REF | |
| Health insurance coverage | Yes | 252 | 64.3 | 58.9–69.7 | 1.4 | 1.1–1.8 |
| No | 74 | 45.4 | 35.5–55.3 | REF | REF | |
| Routine place for healthcare | Yes | 287 | 63.9 | 57.9–70.0 | REF | REF |
| No | 40 | 37.8 | 18.8–56.8 | 0.6 | 0.3–1.0 | |
Prevalence estimates based on response to the question “Have you … ever been to a doctor or other medical provider because you have … been unable to become pregnant?”
Variables with missing data include: Ratio of family income to poverty level (n=16); Marital status (n=4)
Estimates were weighted to be nationally representative of the U.S. population, accounting for unequal probabilities of selection and nonresponse.
CI: Confidence interval
Respondents with missing or unknown values were excluded from prevalence ratio calculations
Hispanic ethnicity includes Mexican American and other Hispanic ethnicity
Poverty level as defined by the Department of Health and Human Services
PID: Pelvic inflammatory disease
Previous STD (Sexually transmitted disease) includes chlamydia or gonorrhea in the last 12 months or ever being told of a herpes, genital warts, or human papillomavirus diagnosis
Relative standard error is > 30% and <50%
NAAT: Nucleic acid amplification test
Relative standard error is > 30% and <50%
Discussion
In this first assessment of the population-based prevalence of infertility using nationally-representative data from NHANES, we found that nearly 14% of U.S. women aged 18–49 years during 2013–2016 had a self-reported lifetime history of infertility. We found the prevalence of infertility was highest among women who were older, were non-Hispanic white, and who reported higher incomes or educational attainment. The prevalence of infertility was also higher in women with a history of PID treatment compared to those without PID treatment. Among young women, Hispanic and non-Hispanic black women had the highest prevalence of infertility whereas among the oldest women, non-Hispanic white women had the highest prevalence of infertility.
We found that self-reported PID treatment was associated with self-reported infertility, and that this association was most pronounced among young women. Among 18–29 year old women, a history of PID was associated with a fourfold higher prevalence of reported infertility. Chlamydia and gonorrhea, the most common known causes of PID, are reported most frequently in young women 15–24 years of age in the U.S.(16). PID has also been found more frequently in young women 20–24 years old in select populations(17, 18). Although we did not observe an association between self-reported infertility and a self-reported history of chlamydia or gonorrhea, it is possible that this lack of association may be due to underreporting of these often asymptomatic and potentially undiagnosed STDs. Additionally, other sexually transmitted microorganisms besides chlamydia and gonorrhea, including Mycoplasma genitalium, Ureaplasmas, T. vaginalis, and proliferated organisms in the vaginal microbiome have been implicated in the development of PID(6, 19). Trichomonas has specifically been shown to be related to PID development among women with HIV(20). However, more research into the etiology of PID and the potential role of various pathogens and conditions, such as T. vaginalis and bacterial vaginosis, in causing PID and infertility is needed. The high incidence of STDs and PID in young women along with the observed significant relationship between PID and prevalence of infertility suggest that STDs and subsequent PID could be a major reason for infertility in young women. These data serve as reminders that PID-associated infertility can affect young women.
It is possible that we did not see an association between PID and infertility in older women because of the occurrence of non-PID related infertility in addition to TFI in this age group. Another challenge that may preclude seeing an association between STDs, lifetime PID, and lifetime infertility in older women in particular in this cross-sectional analysis is that each of these factors may not have occurred in the expected temporal sequence such that exposure to STDs and subsequent PID occurred prior to experiencing a period of infertility.
The epidemiology of lifetime infertility by race/ethnicity in our sample differs somewhat from what has previously been described, hinting at a changing epidemiology. Whereas we found that prevalence of infertility was not significantly different between non-Hispanic white women and non-Hispanic black women, prior work demonstrated that non-Hispanic black women had a higher prevalence of current infertility compared to non-Hispanic white women(21, 22). In 2006–2010 data from the National Survey of Family Growth (NSFG) among married and cohabiting women aged 22–44 years, non-Hispanic black women were more likely (adjusted odds ratio 1.8 [95% CI 1.1–3.1]) to report current infertility (lack of pregnancy in prior 12 months despite condomless intercourse with a man each month) compared to non-Hispanic white women(23). However, methodological differences may account for the differing results given that Chandra et al. assessed current infertility versus lifetime infertility and used multivariable regression modeling to adjust for age, parity, marital or cohabiting status, education, and poverty.
In addition to the overall difference in infertility prevalence comparing Non-Hispanic white to Hispanic women, we found differing infertility prevalences across age groups. Our observation that the epidemiology of infertility by race/ethnicity differs among age groups and that PID seems to be a contributing factor among the youngest women suggests that there may be differing factors contributing to infertility among different age groups. In the youngest women, where we see that PID is related to lifetime infertility, differing epidemiology of PID among race/ethnicity groups may contribute more to differing prevalences of infertility by race. Among older women where PID does not appear to be a main factor in infertility, other explanations for differing infertility prevalences among race/ethnicity groups, such as deferred child bearing because of education or employment opportunities, might play a role given reported differences in delayed child bearing by race/ethnicity(24). Further epidemiological investigations to understand differences in the epidemiology of infertility by age and race are warranted.
Nearly 40% of women who self-reported infertility did not seek healthcare services for infertility. Notably, women without health insurance were significantly less likely to seek infertility services than women with insurance. While we could not explore specific reasons that women did not seek infertility-related healthcare, it may be that lower socioeconomic status and lack of insurance establish sufficient barriers to care, particularly in light of the considerable economic costs of infertility treatment(4).
Our analysis had limitations. Owing to the cross-sectional and self-reported nature of the data, we cannot determine the temporal relationship between PID and infertility. We are thus limited in our ability to draw causal inferences. Our sample size prevented additional analyses requiring further stratifications, such as by age group, race/ethnicity, and history of PID treatment thus we did not perform a multivariable analysis or report on additional multilevel stratifications that would be ideal to account for confounding factors. In our analysis, we did not find overall differences in infertility prevalence among women with a history of selected STDs or with chlamydia or gonorrhea diagnosis in the last 12 months. The absence of the inclusion of any laboratory test for past infection (e.g. serology) or reported history of chlamydia or gonorrhea exposure before the preceding 12 months within NHANES limits our ability to assess for a relationship with these major contributors to PID and a lifetime self-reported episode of infertility.
Using nationally-representative data collected during 2013–2016, we found that 13.8% or nearly 1 in 7 U.S. women reported a lifetime history of infertility and almost two-thirds sought infertility treatment. Among women who reported a history of PID treatment, nearly 25% experienced infertility. To prevent PID and PID-associated infertility, healthcare providers are encouraged to follow Centers for Disease Control and Prevention and United States Preventive Services Task Force recommendations for annual chlamydia and gonorrhea screening for all women younger than 25 years old, and women with high-risk behaviors older than 25 years(19, 25). Ensuring access to screening and following recommendations for annual chlamydia and gonorrhea screening may reduce PID-related infertility. These data serve as reminders of the important reproductive health sequelae of bacterial STDs. We hope that these findings reinvigorate efforts to better understand the epidemiology and etiology of PID and infertility, and to reinforce approaches to avert preventable causes of infertility.
Footnotes
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. There was no funding source for this project.
References
- 1.National Center for Health Statistics;Pages Accessed at https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertility. Accessed December 7 2018.
- 2.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol 2007;21(2):293–308. [DOI] [PubMed] [Google Scholar]
- 3.Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet 2017;34(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society for Reproductive Medicine 2017;Pages Accessed at https://www.reproductivefacts.org/faqs/frequently-asked-questions-about-infertility/. Accessed May 24 2018.
- 5.Hoffman BL, Schorge JO, Bradshaw KD, Halvorson LM, Schaffer JI, Corton MM. Evaluation of the Infertile Couple. Williams Gynecology, 3e New York, NY: McGraw-Hill Education; 2016. [Google Scholar]
- 6.Holmes K, Sparling PF, Walter S, et al. Sexually Transmitted Diseases, Fourth Edition: McGraw-Hill Professional; 2007. [Google Scholar]
- 7.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010;201 Suppl 2:S134–55. [DOI] [PubMed] [Google Scholar]
- 8.Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. Bmj 2010;340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamm WE, Guinan ME, Johnson C, Starcher T, Holmes KK, McCormack WM. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med 1984;310(9):545–9. [DOI] [PubMed] [Google Scholar]
- 10.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992;19(4):185–92. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention;Pages Accessed at https://www.cdc.gov/std/infertility/ipa.htm. Accessed August 30 2018.
- 12.Centers for Disease Control and Prevention - National Center for Health Statistics 2017;Pages Accessed at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed May 11 2018.
- 13.Centers for Disease Control and Prevention 2018;Pages Accessed at https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2015. Accessed October 25 2018.
- 14.Johnson C, Paulose-Ram R, Ogden C, al. e. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. Vital Health Statistics 2013;2(161). [PubMed] [Google Scholar]
- 15.National Center for Health Statistics 2018;Pages Accessed at https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. Accessed December 6 2018.
- 16.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016 Atlanta: U.S: Department of Health and Human Services; 2017. [Google Scholar]
- 17.Kreisel K, Flagg EW, Torrone E. Trends in pelvic inflammatory disease emergency department visits, United States, 2006–2013. Am J Obstet Gynecol 2018;218(1):117.e1-e10. [DOI] [PubMed] [Google Scholar]
- 18.Apostolou A, Chapman C, Person M, Kreisel K, McCollum J. Trends in Pelvic Inflammatory Disease Among American Indian and Alaska Native Women, Indian Health Service, 2001–2015. Am J Public Health 2018;108(11):1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Morbidity Mortality Weekly Report 2015;64(3). [PMC free article] [PubMed] [Google Scholar]
- 20.Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis 2002;34(4):519–22. [DOI] [PubMed] [Google Scholar]
- 21.Mosher WD. Infertility trends among U.S. couples: 1965–1976. Fam Plann Perspect 1982;14(1):22–7. [PubMed] [Google Scholar]
- 22.Aral SO, Cates W Jr. The increasing concern with infertility. Why now? Jama 1983;250(17):2327–31. [PubMed] [Google Scholar]
- 23.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report 2013(67):1–18, 1 p following 9. [PubMed] [Google Scholar]
- 24.Martin SP. Diverging fertility among U.S. women who delay childbearing past age 30. Demography 2000;37(4):523–33. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force. The guide to clinical preventive services - 2014 U.S. Department of Health and Human Services; 2014. [Google Scholar]


