Abstract
The dense expression of glucocorticoid receptors (GR) within the amygdala, medial prefrontal cortex (mPFC) and paraventricular nucleus of hypothalamus (PVN) mediates many aspect of emotional and stress regulation. Importantly, both prenatal alcohol exposure (PAE) and adolescent stress are known to induce emotional and stress dysregulation. Little is known, however, about how PAE and/or adolescent stress may alter the expression of GR in the amygdala, mPFC, and PVN. To fill this gap, we exposed PAE and control adolescent male and female rats to chronic mild stress (CMS) and assessed GR mRNA expression in the amygdala, mPFC, and PVN immediately following stress or in adulthood. We found that the effects of PAE on GR expression were more prevalent in the amygdala, while effects of adolescent stress on GR expression were more prevalent in the mPFC. Moreover, PAE effects in the amygdala were more pronounced during adolescence and adolescent stress effects in the mPFC were more pronounced in adulthood. GR expression in the PVN was affected by both PAE and adolescent stress. Finally, PAE and/or adolescent stress effects were distinct between males and females. Together, these results suggest that PAE and adolescent CMS induce dynamic alterations in GR expression in the amygdala, mPFC, and PVN, which manifest differently depending on the brain area, age, and sex of the animal. Additionally, these data indicate that PAE-induced hyperresponsiveness to stress and increased vulnerability to mental health problems may be mediated by different neural mechanisms depending on the sex and age of the animal.
Keywords: prenatal alcohol exposure, adolescent stress, glucocorticoid receptor, amygdala, medial prefrontal cortex, rat
1. Introduction
Clinical and preclinical studies indicate that the bidirectional interconnections between the amygdala and medial prefrontal cortex (mPFC) support fundamental aspects of emotional and stress regulation as well as cognitive processing (Cardinal et al., 2002; Gee, 2016; Myers et al., 2012; Tottenham, 2015; Yan et al., 2017; Yizhar and Klavir, 2018). Despite its critical role, the amygdala-PFC circuitry does not structurally and functionally mature until late adolescence/young adulthood (Cressman et al., 2010; Cunningham et al., 2002; Gabard-Durnam et al., 2014; Gee et al., 2013; Lebel et al., 2012; Perlman and Pelphrey, 2011; Swartz et al., 2014; Verwer et al., 1996; Vink et al., 2014). Importantly, rodent studies indicate that amygdala projections to the mPFC emerge earlier than mPFC projections to the amygdala (Bouwmeester et al., 2002a,b), presumably due to the earlier development of the amygdala relative to the mPFC. Indeed, amygdala development peaks during the first two postnatal weeks (Bayer, 1980; Berdel et al., 1997; Berdel and Morys, 2000; Bouwmeester et al., 2002a,b; Cunningham et al., 2002; Ryan et al., 2016; Thompson et al., 2008), while mPFC development is more protracted and extends until late adolescence/young adulthood (van Eden and Uylings, 1985a,b). Consequently, any insults that disturb the typical maturational process of the amygdala and/or mPFC may have negative consequences for the establishment of appropriate connections between these two areas that could lead to emotional and stress dysregulation as well as altered cognitive processing.
The paraventricular nucleus of the hypothalamus (PVN) is a critical part of the stress limbic circuitry, and works closely in conjunction with the amygdala and mPFC in emotional and stress regulation. More specifically, the medial parvocellular dorsal division of the PVN (mpdPVN) contains a set of neurons that integrates all direct and indirect inputs from the amygdala and mPFC (Myers et al., 2012; Ulrich-Lai and Herman, 2009). In rodents, PVN development is well underway during the second half of gestation, with the PVN achieving secretory capability by embryonic day18 (Karim and Sloper, 1980; Shimada and Nakamura, 1973). Similar to the amygdala and mPFC, any insults that disturb the typical maturational process of the PVN could disrupt this critical stress-limbic circuitry and lead to emotional and stress dysregulation.
Alcohol is a teratogen that disrupts fetal brain development and leads to a wide range of cognitive, neurobehavioral, and physiological deficits (Drew and Kane, 2014; Hellemans et al., 2010a; Riley et al., 2011; Valenzuela et al., 2012; Weinberg et al., 2008). The amygdala, the mPFC, and the PVN are among the many brain regions that are negatively affected by prenatal alcohol exposure (PAE). Children exposed to alcohol in utero show long-lasting structural and functional alterations in the amygdala (Donald et al., 2016; Nardelli et al., 2011; Zhou et al., 2018) and PFC (Fryer et al., 2007; Gross et al., 2018; Malisza et al., 2005; O’Hare et al., 2009; Sowell et al., 2007; Wozniak et al., 2013; Zhou et al., 2011). Animal models have extended these findings, demonstrating that PAE induces deleterious structural and functional effects in the amygdala, mPFC, and PVN (Burke et al., 2009; Cullen et al., 2013; Kozanian et al., 2018; Lam et al., 2018a,b; Raineki et al., 2014, 2016; Weinberg et al., 2008; Wilcoxon et al., 2005). Not surprisingly, functions mediated by these areas are impaired in individuals that are exposed to alcohol during pregnancy. Particularly, individuals exposed to alcohol prenatally are hyperresponsive to wide range of stressful stimuli (Jacobson et al., 1999; Lee and Rivier, 1996; Weinberg et al., 2008), and many go on to develop mental health problems including anxiety, depression, and substance use disorders at a rate disproportionate to that of the general population (Famy et al., 1998; O’Connor et al., 2002; Pei et al., 2011). Importantly, animal models have demonstrated that stress exposure can modulate the link between PAE-related alterations in the brain and increased depressive-and anxiety-like behaviors (Cullen et al., 2013; Hellemans et al., 2008, 2010a,b; Lam et al., 2018a,b; Raineki et al., 2014, 2016; Wilcoxon et al., 2005).
Similar to PAE, exposure to stressors during critical developmental periods can disturb the typical maturational process of the amygdala, mPFC and PVN (Fujioka et al., 1999; Tottenham and Galván, 2016; Wulsin et al., 2016). This is particularly relevant for individuals exposed to alcohol during gestation as, besides being hyperresponsive to stress, they are, in general, at a higher risk of exposure to stressful environments throughout the lifespan (O’Connor and Paley, 2006; Streissguth et al., 2004). The negative effects of stress on brain development are heightened during adolescence, as this period is characterized by higher activity of the stress systems in response to both acute and chronic stressors (Doremus-Fitzwater et al., 2009; Hollis et al., 2013; McCormick and Mathews, 2010; Romeo et al., 2004, 2006; Tottenham and Galván, 2016). Studies in both the clinical and preclinical literature provide evidence that exposure to stress during adolescence can result in structural and functional changes in the amygdala, mPFC, and PVN (Márquez et al., 2013; Raineki et al., 2016; Romeo, 2017; Swartz et al., 2015; Wulsin et al., 2016), which may exacerbate the increased vulnerability to mental health problems observed during adolescence (Andersen and Teicher, 2008; Costello et al., 2002; Paus et al., 2008).
Consistent with the critical roles of the amygdala, mPFC, and PVN in regulating the stress response, there is dense expression of the glucocorticoid receptors (GR) in these areas (Cintra et al., 1994; Honkaniemi et al., 1992; Morimoto et al., 1996; Wang et al., 2014). Notably, both PAE and adolescent stress alter GR expression. For example, male and female PAE rats show decreased amygdala GR expression in adulthood (Wilcoxon et al., 2005). In adolescent mice, PAE reduces levels of GR in the nuclear – but not cytosolic – fraction of mPFC neurons (Allan et al., 2014). Our data indicate that PAE reduces GR expression in the infralimbic region (a subregion of the mPFC) of PAE rats (Lam et al., 2018a). The effects of PAE on GR expression in the PVN have not been specifically assessed. Furthermore, analyses of PAE effects on GR in the hypothalamus are not conclusive, as a previous study indicated that PAE increases GR levels in male, but not female rats (Redei et al., 1993), while other studies have indicated no effect of PAE on hypothalamic GR (Halasz et al., 1993; Kim et al., 1999b).
Studies evaluating the effects of adolescent chronic stress on GR expression have focused their attention primarily on the hippocampus (Isgor et al., 2004; Raineki et al., 2018; Sterlemann et al., 2008). The few studies that have assessed amygdala GR expression following adolescent chronic stress indicate that the effects are not straightforward, as amygdala GR expression is reduced in the short term (Xu et al., 2017), but increased in the long term (Papilloud et al., 2018). In the PVN, the effects of adolescent chronic stress have only been investigated in female rats and the results indicate that stress reduced GR mRNA expression in the PVN of adult females (Wulsin et al., 2016).
To fill this gap in the literature, here we evaluate the short-and long-term effects of adolescent stress on GR mRNA expression in the amygdala, mPFC and PVN of control and PAE animals. We used a well-established rat model of PAE in which pregnant rats received either liquid ethanol diet or control diet throughout pregnancy. After weaning, male and female PAE and control animals were exposed either to adolescent chronic mild stress (CMS; PN31–41 for females, PN 37–47 for males) or left undisturbed (non-CMS). Half the animals were tested in the open field and forced swim test (FST) immediately following CMS exposure (short-term effects of CMS), and the remaining animals were tested on these same tasks in adulthood (long-term effects of CMS). Brains were collected 30-min after the FST and processed for GR mRNA by in situ hybridization. Behavioral data were published previously (Raineki et al., 2016). Here, we assessed GR mRNA expression in the amygdala (basolateral, central, medial, and cortical nuclei), the mPFC [anterior cingulate (ACC), prelimbic (PrL), and infralimbic (IL) cortices], and the mpdPVN (Figure 1). As structural and functional maturation of the amygdala occurs earlier than the mPFC, we hypothesize that PAE will have more marked effects on amygdala GR expression while adolescent stress will have more marked effects on mPFC GR expression. Moreover, because PVN development is ongoing during the period of alcohol exposure and because the PVN is an integral part of the stress response, we hypothesize that both PAE and adolescent stress will affect GR expression in the PVN.
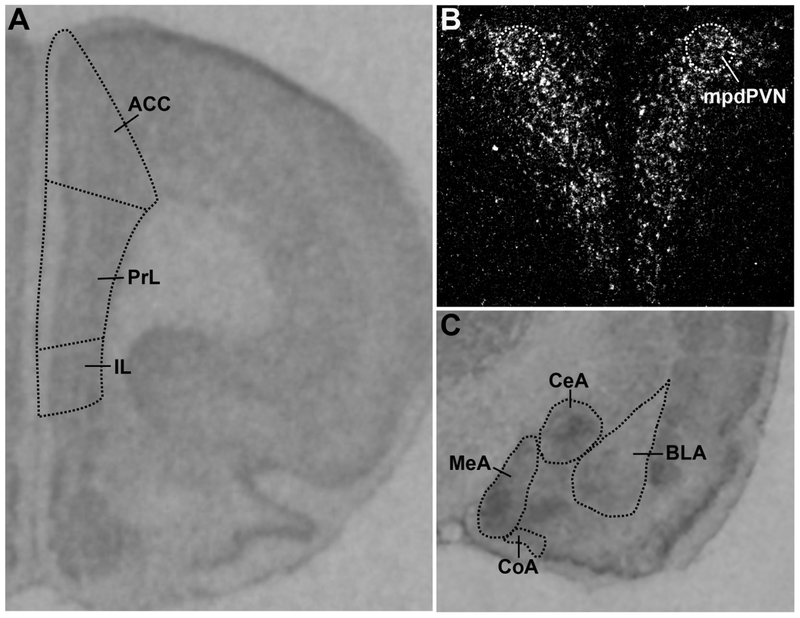
Figure 1.
Representative images of GR mRNA in-situ hybridization in the (A) mPFC, (B) mpdPVN, and (C) amygdala.
2. Results
2.1. Amygdala
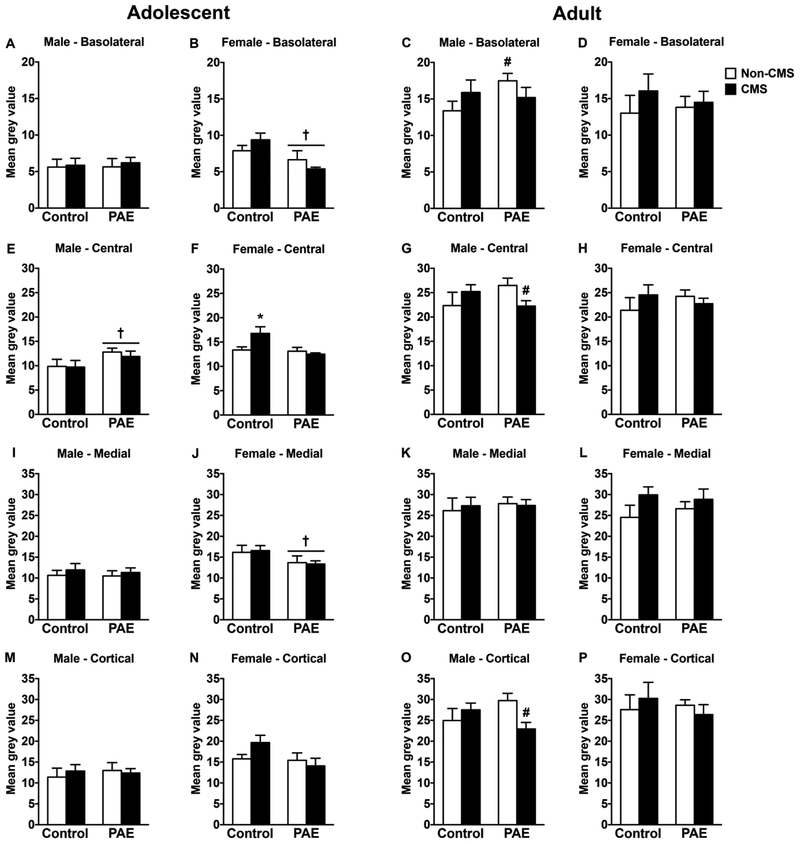
In adolescent males, PAE increased GR mRNA expression in the central amygdala independently of CMS exposure [Figure 2E; significant main effect of prenatal treatment for central amygdala (F(1,28)=4.31, p=0.047)]. However, neither PAE nor CMS affected GR mRNA expression in the other amygdala nuclei (Figure 2A,I,M). In adolescent females, PAE decreased GR mRNA expression in the basolateral and medial amygdala independently of CMS exposure [Figure 2B,J; significant main effect of prenatal treatment for basolateral (F(1,27)=8.74, p=0.006) and medial (F(1,28)=4.34, p=0.046) amygdala nuclei]. Moreover, CMS increased GR mRNA expression in the central amygdala of control but not PAE females. [Figure 2F; significant interaction between prenatal treatment and CMS for central amygdala (F(1,28)=5.44, p=0.027)]. Neither PAE nor CMS affected GR mRNA expression in the cortical amygdala of adolescent females (Figure 2N).
Figure 2.
Short-and long-term effects of adolescent CMS on amygdala GR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of GR mRNA expression in the basolateral (A-D), central (E-H), medial (I-L), and cortical (M-P) amygdala nuclei. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; * indicates that control CMS is different from all other groups; for C, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons; for G and O, # indicates that PAE non-CMS is different from PAE CMS based on a priori comparisons (n = 6–10 for all groups).
In adulthood, PAE non-CMS males showed increased GR mRNA expression in the basolateral amygdala compared to their control counterparts [Figure 2C; a priori analysis for basolateral amygdala comparing PAE non-CMS to control non-CMS (p=0.03)]. Moreover, CMS decreased GR mRNA expression in the central and cortical amygdala nuclei of PAE males, while no changes were observed in the medial amygdala [Figure 2G,K,O; a priori analysis for central (p=0.036) and cortical (p=0.01) amygdala nuclei comparing PAE non-CMS to PAE CMS]. In adult females, neither PAE nor adolescent CMS affected GR mRNA expression in any of the amygdala nuclei analyzed (Figure 2D,H,L,P).
2.2. Medial Prefrontal Cortex
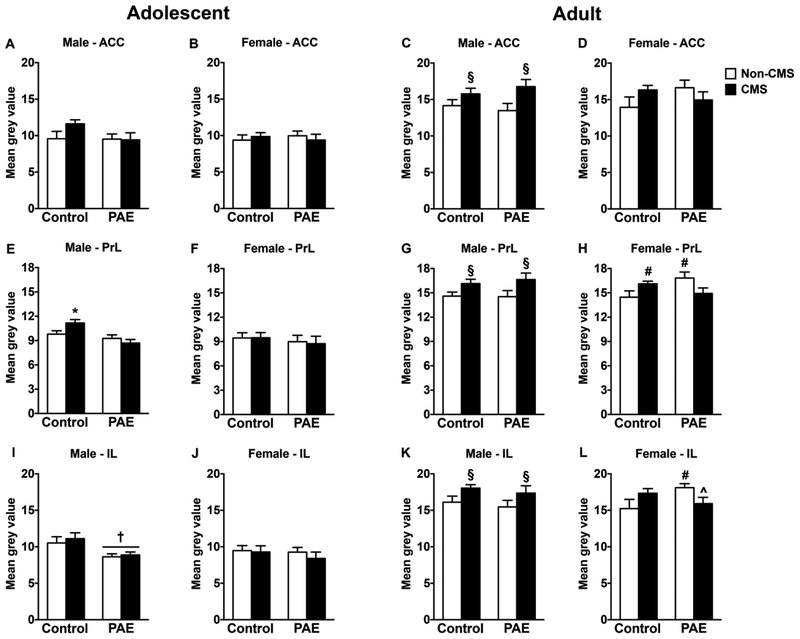
In adolescent males, PAE decreased GR mRNA expression in the IL independently of CMS exposure [Figure 3I; significant main effect of prenatal treatment for IL (F(1,28)=9.92, p=0.002)]. By contrast, CMS increased GR mRNA expression in the PrL of control but not PAE males, while no changes were observed in the ACC [Figure 3A,E; significant interaction between prenatal treatment and CMS for PrL (F(1,28)=4.97, p=0.033)]. In adolescent females, neither PAE nor CMS affected GR mRNA expression in any mPFC subregion (Figure 3B,F,J).
Figure 3.
Short-and long-term effects of adolescent CMS on mPFC GR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of GR mRNA expression in the anterior cingulate (A-D), prelimbic (E-H), and infralimbic (I-L) cortices. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; * indicates that control CMS is different from all other groups; for H, # indicates that control CMS and PAE non-CMS are different from control non-CMS based on a priori comparisons; for L, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons; for H, ^ indicates that PAE CMS is different from PAE non-CMS based on a priori comparisons (n = 6–10 for all groups).
In adult males, adolescent CMS increased GR mRNA expression in all mPFC subregions independently of prenatal treatment [Figure 3C,G,K; significant main effect of CMS for ACC (F(1,30)=7.09, p=0.01), PrL (F(1,30)=7.17, p=0.01), and IL (F(1,30)=4.87, p=0.035)]. In adult females, PAE non-CMS animals showed increased GR mRNA expression in the PrL and IL compared to their control counterparts [Figure 3H,L; a priori analysis for PrL (p=0.05) and IL (p=0.042) comparing PAE non-CMS to control non-CMS]. Moreover, adolescent CMS decreased GR mRNA expression only in the IL of PAE females [Figure 3L; a priori analysis for IL comparing PAE non-CMS to PAE CMS (p=0.05)]. Conversely, adolescent CMS increased GR mRNA expression only in the PrL of control females [Figure 3H; a priori analysis for PrL comparing control non-CMS to control CMS (p=0.046)]. Neither PAE nor CMS affected GR mRNA expression in the ACC of adult females (Figure 3D).
2.3. Paraventricular Nucleus of Hypothalamus
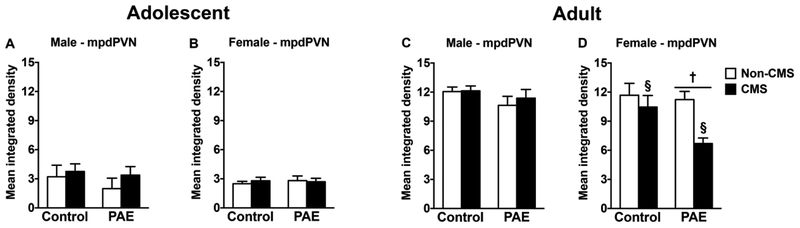
In adolescent males and females and adult males, neither PAE nor adolescent CMS affected GR mRNA expression in the mpdPVN (Figure 4A–C). However, in adult females, PAE decreased GR mRNA expression in the mpdPVN independently of CMS exposure. Moreover, adolescent CMS decreased GR mRNA expression in the mpdPVN independently of prenatal treatment [Figure 4D; significant main effects of prenatal treatment (F(1,27)=4.00, p=0.05) and CMS (F(1,27)=7.47, p=0.01) for mpdPVN].
Figure 4.
Short-and long-term effects of adolescent CMS on mpdPVN GR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean integrated density) of GR mRNA expression in mpdPVN. † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS (n = 5–10 for all groups).
3. Discussion
Our data support our hypothesis and demonstrate that the amygdala is more sensitive to the effects of PAE, the mPFC is more sensitive to the effects of adolescent stress, and the PVN is sensitive to both PAE and adolescent stress. Interestingly, PAE effects on GR mRNA expression in the amygdala were more prominent during adolescence, as PAE females showed reduced GR expression in the basolateral and medial amygdala nuclei while PAE males showed increased GR expression in the central amygdala nuclei during adolescence but not in adulthood. The one PAE effect in adulthood was an increase in GR expression in the basolateral amygdala nuclei. In contrast to the PAE effects in the amygdala, the effects of adolescent stress on the mPFC were more prominent in adulthood. Adolescent stress increased GR mRNA expression in all mPFC subregions of adult males independently of prenatal treatment, and increased GR expression in the PrL of adult females. The only short-term effect of adolescent stress was an increase in GR expression in the PrL of adolescent males. Besides confirming our hypothesis of differential vulnerability of the amygdala and mPFC to PAE and adolescent stress, the current data also revealed that PAE effects on the amygdala are more pronounced during adolescence and that adolescent stress effects on the mPFC are more pronounced in adulthood. Finally, we found that both PAE and adolescent stress affected GR mRNA expression in the PVN. PAE decreased GR expression in the mdpPVN of adult females independent of stress while adolescent stress decreased GR expression in the mdpPVN of adult females independent of prenatal treatment.
The dense expression of GR within the amygdala, mPFC, and PVN is essential for emotional and stress regulation (Cardinal et al., 2002; Cintra et al., 1994; Gee, 2016; Honkaniemi et al., 1992; Morimoto et al., 1996; Tottenham, 2015; Wang et al., 2014; Yan et a., 2017). To this end, the observed alterations in GR expression following PAE and/or adolescent stress could be associated with the altered stress response and increased vulnerability to emotional dysregulation observed in these animals (Cullen et al., 2013; Hellemans et al., 2008, 2010a,b; Lam et al., 2018a,b; Raineki et al., 2016; Rouzer et al., 2017; Wieczorek et al., 2015; Wilcoxon et al., 2005). Of note, the current data evaluate the effect of PAE and adolescent stress on GR mRNA expression in response to an acute stressor – exposure to the FST. It has been shown that while acute exposure to forced swim stress in adulthood can alter the expression of GR mRNA, effects are dependent on sex and area of the brain being analyzed. Specifically, forced swim stress increases GR mRNA in the hypothalamus and mPFC of males; however, in females GR mRNA does not change in the hypothalamus and decreases in the mPFC (Karandrea et al., 2002).
3.1. Amygdala GR mRNA expression during adolescence is modulated by PAE
Our ontogenetic approach revealed that PAE effects on GR mRNA expression are more prominent in the amygdala and perhaps more transient, as differential GR expression is apparent during adolescence but declines by adulthood. We have previously demonstrated a similar transient pattern of PAE effects on stress-related receptor expression in the hippocampus (Raineki et al., 2018). Indeed, PAE resulted in widespread alterations in GR, mineralocorticoid (MR) and type 1 CRH (CRHR1) receptor mRNA expression in the dorsal and/or ventral hippocampus during adolescence but not in adulthood (Raineki et al., 2018). Altered expression of these stress-related receptors in key areas regulating stress and emotion during adolescence suggests that this developmental period may represent a possible window of vulnerability for individuals prenatally exposed to alcohol. Assessments of PAE-induced behavioral alterations in several previous studies by our group and others support this notion. Our evaluation of depressive-like behavior across the lifespan in PAE animals indicates that PAE males exhibit depressive-like behavior in the FST in adolescence, but not in adulthood (Lam et al., 2018b; Raineki et al., 2016). Additionally, moderate and acute alcohol exposure on gestational day 12 results in anxiety-like behaviors during adolescence but not in adulthood (Rouzer et al., 2017). However, PAE effects on neurobehavioral development are not as straightforward: our previous analysis of anxiety-like behavior in the open field indicates that adolescent PAE males and females are hyperactive and spend more time in the center of the field (Raineki et al., 2016). As rodents typically stay primarily in the periphery and avoid the center (Gould et al., 2009), this behavior in adolescent PAE animals represents an inappropriate behavioral response. However, classical features of anxiety-like behavior were observed in adult PAE females, as they spent less time in the center of the open field compared to controls (Raineki et al., 2016).
The present data also demonstrate that GR mRNA expression in the amygdala and mPFC of male and female rats was differentially affected by PAE. PAE reduced GR expression in the basolateral and medial amygdala of adolescent females and increased GR expression in the PrL and IL subregions of adult females. Conversely, PAE increased GR expression in the central amygdala and decreased GR expression in the IL of adolescent males, but increased GR expression in the basolateral amygdala of adult males. These differential PAE-related effects on GR expression in the amygdala-mPFC circuit between males and females suggest that the altered stress response and increased vulnerability to emotional dysregulation may be mediated by different underlying mechanisms in males and females (Cullen et al., 2013; Hellemans et al., 2008, 2010a,b; Lam et al., 2018a,b; Raineki et al., 2016; Rouzer et al., 2017; Wieczorek et al., 2015; Wilcoxon et al., 2005).
Decreases in amygdala GR induced by early-life adversity have been shown to mediate the reduction in anxiety-like behaviors and overall increase in risk-taking behavior (Arnett et al., 2015). This adversity-related behavioral profile is similar to what we previously observed in PAE animals during early adolescence (Raineki et al., 2016). Adolescent PAE animals were hyperactive and spent more time in the center of the open field, a behavioral profile that might reflect a pathological increase in novelty-seeking and risk-taking behaviors. Accordingly, we suggest that, similar to early-life adversity (Arnett et al., 2015), the reduction in GR mRNA expression observed in the basolateral and medial amygdala nuclei of PAE adolescent females may mediate their increased risk-taking behaviors (Raineki et al., 2016).
The clinical and preclinical literature have consistently provided evidence demonstrating that PAE individuals exhibit hypothalamic-pituitary-adrenal (HPA) hyperresponsiveness to a wide range of stressful stimuli (Jacobson et al., 1999; Lee and Rivier, 1996; Nelson et al., 1986; Taylor et al., 1982; Weinberg et al., 2008). Activation of GR in the amygdala, including the central nuclei, has been implicated in mediating the positive feedback loop that potentiates the activity of the HPA axis (Myers et al., 2012). Our data indicate that PAE increased GR mRNA expression in the central amygdala of adolescent males, which potentially contributes to the stress hyperresponsivity observed following PAE.
Similar to the amygdala, activation of GR in the IL subregion of the mPFC stimulates the HPA axis (Myers et al., 2012). Specifically, projections from the IL target the central nucleus of the amygdala (Hurley et al., 1991; Vertes 2004), and this pathway appears to transmit glucocorticoid feedforward information that activates the HPA axis. Nevertheless, our data indicate that PAE reduced GR expression in the IL of adolescent males, a paradoxical effect that suggests either that IL GR during adolescence is not a critical component mediating PAE-related stress hyperresponsivity or that the reduction may be a compensatory response to counterbalance stress hyperresponsivity.
3.2. mPFC GR mRNA expression in adulthood is modulated by adolescent stress
Our data demonstrate that the effects adolescent stress on GR mRNA expression are more prominent in the mPFC and mdpPVN than the amygdala, and that these effects are observed primarily in adulthood. Taken together, these results suggest a possible incubation period for some of the effects of adolescent stress on GR expression. This interpretation is supported by previous work showing that the effects of adolescent chronic stress on the neurocircuitry that underlies stress and emotional regulation can manifest differently depending on the timing of assessment. Indeed, some effects are only observed immediately after stress, some effects only manifest long after the termination of stress, and some effects are enduring and can be observed any time following stress exposure (Bourke and Neigh, 2011; Leussis and Andersen, 2008; Leussis et al., 2008; Raineki et al., 2016, 2018; Romeo, 2017; Tsoory et al., 2008).
The few studies that have evaluated how adolescent stress may affect the structure of the mPFC found that adolescent stress induces dendritic atrophy in adolescent male and female rats (Eiland et al., 2012). Moreover, examination of synaptic plasticity markers, such as spinophilin and synaptophysin reveals that adolescent stress produces persistent decreases in synaptic plasticity in several subregions of the mPFC (Leussis and Andersen, 2008; Leussis et al., 2008). More is known about how adolescent stress affects the long-term function of the mPFC. It has been shown that adolescent stress does not change basal or stress-induced (resident-intruder test) neural activity of the mPFC in adulthood (Márquez et al., 2013). Despite having no effect on neural activity, exposure to adolescent stress promoted long-term alterations in the mPFC serotonergic and GABAergic systems (Márquez et al., 2013; Tzanoulinou et al., 2016). The current data extend these findings by demonstrating that exposure to adolescent stress alters glucocorticoid function in the mPFC. Indeed, adolescent stress increased GR mRNA expression in all subregions of the mPFC of adult males and in the PrL of adult females. Together, these data suggest that adolescence may represent a developmental window of vulnerability for the mPFC, as exposure to stress during this period promoted long-term alterations in the function of several neuroregulatory systems (current data; Márquez et al., 2013; Tzanoulinou et al., 2016). It is not unreasonable to speculate that stress-induced changes in serotonin, GABA, and glucocorticoid function in the mPFC during adolescence may mediate the behavioral dysregulation observed following stress. Indeed, adolescent stress exposure has been shown to induce higher rates of aggression, impair attention, and disrupt emotional regulation (Márquez et al., 2013; Raineki et al., 2016; Tzanoulinou et al., 2016).
3.3. PVN GR mRNA expression in adulthood is modulated by both PAE and adolescent stress
While PAE-induced hyperresponsiveness to stress has been reported in both males and females, sexually dimorphic effects are often observed. Indeed, some studies evaluating the responses of PAE male and female rodents to acute stressors, including swim stress, show heightened corticosterone and/or ACTH responses in females, but not in males (Halasz et al., 1993; Kelly et al., 1991; Lee and Rivier, 1996; Nelson et al., 1986; Taylor et al., 1981, 1982; Weinberg, 1988, 1992; Weinberg et al., 2008). By contrast, in studies using more prolonged and/or repeated stressors, HPA hyperresponsiveness may be observed in PAE males or in both male and female offspring (Kim et al., 1999a; Lee et al., 2000; Taylor et al., 1982; Weinberg, 1992; Weinberg et al., 1996). The current data demonstrate that PAE differentially reduced GR mRNA expression in the mpdPVN only in adult females. As GR within the PVN is a critical regulator of HPA negative feedback (Myers et al., 2012), the reduction in PVN GR expression observed in PAE female, may play a critical role in the increased vulnerability to hyperresponsiveness to acute stressors and impaired negative feedback observed in PAE females.
Similar to PAE, our results indicate that exposure to adolescent stress also reduced mpdPVN GR mRNA expression only in adult females, data that corroborates previous research showing that adolescent chronic stress reduces PVN GR expression in adult females (Wulsin et al., 2016). Exposure to adolescent stress has been shown to induce sex-specific effects in several domains including stress and emotional regulation, cognitive function, and neuroplasticity (Barha et al., 2011; McCormick et al., 2008; Raineki et al., 2016, 2018; Toledo-Rodriguez et al., 2012; Toledo-Rodriguez and Sandi, 2007, 2011). Moreover, some reports suggest that females exposed to adolescent stress show greater and longer-lasting changes in emotional-related behavior compared to males (Bourke and Neigh, 2011; Raineki et al., 2016). Indeed, our previous results indicate that adult females, but not males, exposed to adolescent stress show anxiety-like behavior, as they spent less time in the center of the open field compared to controls (Raineki et al., 2016). Together, these data suggests that females may be more vulnerable than males to the long-term neurobehavioral effects of adolescent stress.
3.4. Unique responses of PAE animals to adolescent stress
A goal of the current study was to evaluate if PAE animals are more vulnerable than controls to the effects of adolescent stress. Overall, our data revealed two patterns in which PAE animals responded to adolescent stress differently from controls. First, PAE animals failed to respond to adolescent stress as compared to control animals. That is, immediately following exposure to adolescent stress, control but not PAE females showed increased GR expression in the central amygdala and control but not PAE males showed increased GR expression in the PrL. This lack of response to adolescent stress in PAE animals may suggest that the mechanisms necessary for a short-term adaptation to chronic stressors may be altered in PAE animals (Allan et al., 2014; Caldwell et al., 2014; McCormick and Mathews, 2010; Romeo, 2017; Romeo et al., 2004, 2006). Second, PAE animals were more susceptible to adolescent stress, as PAE but not control animals showed evidence of adolescent stress effects in adulthood. Adult PAE males exposed to adolescent stress showed reduced GR expression in the central and cortical amygdala nuclei and adult PAE females exposed to adolescent stress showed reduced GR expression in the IL. This increased susceptibility to adolescent stress may further exacerbate the PAE-induced HPA axis dysregulation (Weinberg et al., 2008). These findings could have critical implications for individuals exposed to alcohol in utero, as the clinical literature has shown that individuals prenatally exposed to alcohol are at a higher risk of encountering a more stressful environment throughout the lifespan, including adolescence (O’Connor and Paley, 2006; Streissguth et al., 1991, 2004).
3.5. Conclusions
Here we investigate how exposure to stress during adolescence alters GR mRNA expression in the amygdala, mPFC, and PVN, potentially contributing to the stress hyperresponsivity and increased vulnerability to mental health problems observed following PAE. Our data demonstrated that PAE effects on GR expression were more pervasive in the amygdala and adolescent stress effects on GR expression were more pervasive in the mPFC, while both PAE and adolescent stress affected GR expression in the PVN. Moreover, PAE effects on amygdala GR expression were more pronounced during adolescence, while the adolescent stress effects in the mPFC were more pronounced in adulthood. Finally, males and females were differentially affected by PAE and/or adolescent stress, underscoring the importance of including both sexes. Together, these results suggests that PAE and adolescent CMS induce dynamic alterations in GR expression in the amygdala, mPFC, and PVN, and that these alterations manifest differently depending on brain area, age, and sex of the animal. These results further suggest that the PAE-induced hyperresponsiveness to stress and increased vulnerability to mental health problems may be mediated by different neural mechanisms depending on the sex and age of the animal.
4. Experimental Procedure
4.1. Animals and Breeding
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories (St. Constant, Canada). Rats were pair-housed by sex and maintained at a constant temperature (21 ± 1°C) and on a 12 h light-dark cycle (lights on at 0700 h) with ad libitum access to water and standard lab chow (Harlan, Canada). After a 10-day acclimation period, male and female pairs were placed together for breeding. Vaginal smears were taken each morning, and the presence of sperm was used as an indicator of pregnancy (gestation day 1; G1). All experiments were performed in accordance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals, Canadian Council on Animal Care guidelines, and were approved by the University of British Columbia Animal Care Committee.
4.2. Prenatal Alcohol Exposure
On G1, females were single-housed and randomly assigned to one of three treatment groups: Prenatal Alcohol Exposure (PAE), pair-feeding, or control. Dams in the PAE group were offered ad libitum liquid ethanol diet with 36% ethanol-derived calories (Weinberg-Keiver High Protein Control Diet #710109, Experimental Diet # 710324, Dyets Inc., Bethlehem, PA). The liquid ethanol diet was introduced gradually over the first 3 days with bottles containing: G1–66% control diet, 34% ethanol diet; G2–34% control diet, 66% ethanol diet; G3-21-100% ethanol diet. This diet is formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake (Lan et al., 2006). Blood alcohol levels were measured as previously reported (Workman et al., 2015) and alcohol-consuming dams showed an average of 118.20 ± 8.11 mg/dl. Pair-fed dams were offered a liquid control diet with maltose-dextrin isocalorically substituted for ethanol, in an amount matched to the consumption of an alcohol-fed partner (g/Kg body weight/day of gestation). The control dams were offered ad libitum access to a pelleted form of the liquid control diet. All animals had ad libitum access to water, and those in the PAE and pair-fed groups were provided with fresh diet daily within 1 h prior to lights off to maintain the normal HPA circadian rhythm (Gallo and Weinberg, 1981; Krieger, 1974). Experimental diets were continued through G21. Beginning on G22, all animals were offered ad libitum access to standard laboratory chow and water, which they received throughout lactation. Pregnant dams were left undisturbed except for cage changing (G1, G7, and G14) and weighing (G1, G7, G14, and G21). On the day of birth (postnatal day 1, PN1) the litters were culled to 12 pups with an attempt to preserve an equal number of males and females per litter. Dams and pups were cage changed and weighed on PN1, PN8, PN15, and PN22. Dam and pup body weight data were published in Raineki et al., 2016. On PN22 pups were weaned and group-housed by litter and sex. Effects of adolescent CMS on GR expression in PAE and ad libitum-fed control offspring were presented in the Results section above, whereas specific pair-feeding effects are analyzed and presented separately in the Supplementary Materials.
4.3. Adolescent Chronic Mild Stress (CMS)
One male and one female from each litter were randomly assigned to either the CMS or the non-CMS condition and were pair-housed with another animal of the same sex and prenatal group. Animals in the CMS condition were subjected to 10 consecutive days of chronic, unpredictable, mild stressors. To account for the sexually dimorphic time of pubertal onset (McCormick and Mathews, 2010; Vetter-O’Hagen and Spear, 2012), males and females were exposed to CMS at ages consistent with puberty onset – PN31–41 for females, PN 37–47 for males. On each CMS day, animals received two different stressors at random times: one in the morning (between 0800 and 1200 h) and one in the afternoon (between 1300 and 1800 h), with a minimum of 2 h between stressors. On day 1 of CMS and the day immediately following the end of CMS, within 2 h of lights on, basal blood samples were obtained from all animals (including those in the non-CMS group) via tail nick. After tail nick, all animals were weighed and placed in a new home cage. Pre-and post-CMS blood sample and body weight data were published in Raineki et al., 2016. Except for blood sampling and routine husbandry, animals in the non-CMS condition were left undisturbed during this period. CMS and non-CMS animals were housed in different colony rooms so that non-CMS animals were not exposed to the disturbance and stress odors of the CMS animals (Mackay-Sim and Laing, 1980). The order and type of stressor was randomized, but all animals received the same number of exposures to each stressor over the 10-day period. Stressors included: 1) Platform: exposure to an elevated Plexiglas platform (20 × 20 cm) mounted on 90 cm high post for 10 min; 2) Cage tilt: the home cage was tilted at a 30° angle for 2 h; 3) Novel cage: exposure to a novel cage without food and water, with a small amount of novel bedding for 1 h; 4) Soiled cage: exposure to a soiled cage of another animal of the same sex for 1 h; 5) Restraint: restraint in PVC tubes (tube size varied to ensure proper restraint/immobility of each animal) for 30 min; 6) Social isolation without food and water: overnight isolation in a smaller cage (28 × 17 cm with 12.5 cm high) for 12 h; and 7) Empty water bottle: animals given their empty water bottles for 1 h following the social isolation/food and water deprivation period.
4.4. Behavioral Exposure and Brain Extraction
All animals were tested on the open field before and after CMS exposure. For the post-CMS test, half the animals were tested in adolescence (short-term effects of CMS) and the remaining animals were tested in adulthood (long-term effects of CMS). Following post-CMS open field tests, all animals were exposed to the FST (habituated for 15 min and then tested for 5 min the following day). Pre-and post-CMS behavioral data were published in Raineki et al., 2016. Animals were decapitated 30-min after the end of day 2 FST testing and brains were collected, quickly frozen on dry ice and stored at −80°C.
4.5. Neural assessment of GR by in situ hybridization
4.5.1. Probe
Rat GR probe (456bp fragment in pGem4), provided by Dr. James Herman, was transcribed using 35S-UTP (Perkin-Elmer, Waltham, MA) and the Promega Riboprobe System (Promega Corp., Madison, WI) with polymerase T7 antisense probe. GR probe was purified using Micro Bio-Spin 30 Columns (Bio-Rad, CA, USA) and 0.1 M DTT was added to prevent oxidation.
4.5.2. In situ hybridization
Brains were sectioned coronally (20 μm) using a cryostat (−16°C) and stored at −80°C. Thawed sections were fixed in formalin for 30 min and pre-hybridized as follows: 1 × PBS for 10 min twice, proteinase K (0.1μg/L; 37°C) for 9 min, 0.1 M triethanolamine-hydrochloride (TEA) for 10 min, 0.1 M TEA with 0.25% acetic anhydride for 10 min, 2 × SSC for 10 min twice, dehydrated by a graded series of ethanol, chloroform, and 100% ethanol and air-dried. Probe was applied at 1 × 106 cpm/slide in 75% Hybridization buffer (75% formamide, 3 × SSC, 1× Denhardt’s solution, 200 μg/mL yeast tRNA, 50 mM sodium phosphate buffer (pH 7.4), 10% dextran sulphate, 10 mM DTT) and covered with HybriSlips (Sigma-Aldrich, ON). Sections were incubated overnight at 55°C in chambers humidified with 75% formamide. HybriSlips were removed and slides were rinsed as follows: 2 × SSC twice for 20 min, 2 × SSC for 30 min, 50 μg/L RNAse A solution (37°C) for 60 min, 2 × SSC with 0.01 M DTT for 10 min, 1 × SSC for 10 min, 0.5 × SSC with 0.01 M DTT for 10 min, 0.1 × SSC with 0.01 M DTT (60°C) for 60 min, 0.1 × SSC for 5 min. Sections were dehydrated by a graded series of ethanol and air dried overnight. Amygdala and mPFC hybridized slides were exposed to Kodak BioMax MR film, and developed using Kodak GBX developer and fixer. mpdPVN hybridized slides were dipped in Kodak NTB2 autoradiography emulsion (diluted 50:50 with distilled water), exposed in desiccated sealed, light tight boxes (4°C), developed using Kodak D19 developer and standard Kodak fixer, counterstained with Toluidine Blue, and coverslipped with Permount (Fisher Scientific Ltd.).
4.5.3. Densitometric analysis
The autoradiograph films for the amygdala and mPFC were scanned and analyzed with ImageJ 1.48v (National Institutes of Health, USA). The left and right subregions of the amygdala (basolateral, medial, central, and cortical; bregma −2.16 to −3.00) and mPFC (ACC, PrL, and IL; bregma 3.73 to 2.52) were traced free-hand according to a stereotaxic rat brain atlas (Paxinos and Watson, 2005) in two sections per animal to determine mean grey density levels. Corrected grey levels were obtained by subtracting the background level (corpus callosum) from each of the four measurements. For the emulsion-dipped slides (mpdPVN; bregma −1.72 to −1.92), in situ signals were visualized with a Q-imaging monochrome 12-bit camera attached to a Zeiss Axiokop 2 motorized plus microscope. Images were captured under dark field illumination using Northern Elite 6.0v (Empix Imaging Inc., Mississauga, ON, Canada) and analyzed with ImageJ 1.48v software (National Institutes of Health, USA). The left and right mpdPVN were traced by outlining a fixed circle (0.75 in diameter; scale 300 pixels ⁄ inch) in two sections per animal to determine mean integrated density levels. Corrected integrated densities were obtained by subtracting the background level (corpus callosum) from each of the four measurements. For all the areas, the left and right levels in each measured area were averaged together for analysis.
4.6. Statistical analysis
All data are expressed as mean ± SEM and were analyzed by two-way ANOVA (prenatal treatment and adolescent CMS as factors). When significant, ANOVAs were followed by Newman-Keuls post hoc tests. Sex was not used as a factor in the ANOVAs because females and males were exposed to CMS during different ages (PN31–41 and PN 37–47 respectively). Age was also not used as factor because in situ hybridizations for brains collected during adolescence and adulthood were run separately. Further analyses utilized planned comparisons to test the a priori hypothesis that: 1) PAE will alter animals’ GR mRNA expression compared to controls; and 2) CMS will differentially alter animals’ GR mRNA expression. In all cases, differences were considered significant when p≤0.05. Outliers were identified and removed using the Robust regression and Outlier removal (ROUT) method with Q=0.05.
Supplementary Material
glucocorticoid receptor (GR) expression in the amygdala is altered by prenatal alcohol exposure (PAE)
PAE effects on amygdala GR expression were more pronounced during adolescence
GR expression in the mPFC is altered by adolescent stress
adolescent stress effects on mPFC GR expression were more pronounced in adulthood
Acknowledgments
This research was supported by NIH/NIAAA grants R37 AA007789 and R01 AA022460, Kids Brain Health Network (Canadian Networks of Centers of Excellence) grant 20R64153 to JW. We thank all members of the Weinberg laboratory for their assistance with the animal work. We also thank Parker J. Holman for help in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan AM, Goggin SL, Caldwell KK, 2014. Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning. PLoS One 9, e96200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescence depression. Trends Neurosci 31, 183–191. [DOI] [PubMed] [Google Scholar]
- Arnett MG, Pan MS, Doak W, Cyr PEP, Muglia LM, Muglia IJ, 2015. The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior. Transl. Psychiatry 5, e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LAM, 2011. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus 21, 1216–1227. [DOI] [PubMed] [Google Scholar]
- Bayer SA, 1980. Quantitative 3H-thymidine radioactive analyses if neurogenesis in the rat amygdala. J. Comp. Neurol 194, 845–875. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, 2000. Expression of calbindin-D28k and parvalbumin during development of rat’s basolateral amygdala complex. Int. J. Dev. Neurosci 18, 501–513. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B, 1997. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int. J. Dev. Neurosci 15, 755–765. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN, 2011. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav 60, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, van Ree JM, 2002a. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J. Comp. Neurol 450, 245–255. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM, 2002b. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J. Comp. Neurol 442, 239–249. [DOI] [PubMed] [Google Scholar]
- Burke MW, Palmour RM, Ervin FR, Ptito M, 2009. Neuronal reduction in frontal cortex of primates after prenatal alcohol exposure. NeuroReport 20, 13–17. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Tyler CR, Allan AM, 2014. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcohol. Clin. Exp. Res 38, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosen L, Agnati LF, Okret S, Wikstrom A-C, Gustafsson J-A, Fuxe K, 1994. Mapping and computer densitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience 62, 843–897. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Beiderman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF, 2002. Development and natural history of mood disorders. Biol. Psychiatry 52, 529–542. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H, 2010. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol 518, 2693–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen CL, Brune THJ, Lavidis NA, Mortiz KM, 2013. Low dose prenatal ethanol exposure induced anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS One 8, e54924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM, 2002. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol 453, 116–130. [DOI] [PubMed] [Google Scholar]
- Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ, 2016. Alcohol exposure in utero is associated with decreased gray matter volume in neonates. Metab. Brain Dis 31, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EL, Spear LP, 2009. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol. Behav 97, 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJ, 2014. Fetal alcohol spectrum disorders and neuroimmune changes. Int. Rev. Neurobiol 118, 41–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS, 2012. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS, 1998. Mental health illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry 155, 552–554. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP, 2007. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol. Clin. Exp. Res 31, 1415–1424. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S, 1999. The effects of prenatal stress on the development of hypothalamic paraventricular nucleus in fetal rats. Neuroscience 92, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N, 2014. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage 95, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J, 1981. Corticosterone rhythmicity in the rat: Interactive effects of dietary restriction and schedule feeding. J. Nutr 111, 208–218. [DOI] [PubMed] [Google Scholar]
- Gee DG, 2016. Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity, in: Rutherford HJV, Mayes LC (Eds.), Maternal brain plasticity: Preclinical and human research and implications for intervention. New Directions for Child and Adolescent Development Wiley Periodicals, Inc., San Francisco, no. 153, pp. 87–110. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013. A developmental shift form positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE, 2009. The open field test, in: Gould TD (Ed.), Mood and anxiety related phenotypes in mice: Characterization using behavioral tests Humana Press, New York, pp. 1–20. [Google Scholar]
- Gross LA, Moore EM, Wozniak JR, Coles CD, Kable JA, Sowell ER, Jones KL, Riley EP, Mattson SN, the CIFASD, 2018. Neural correlates of verbal memory in youth with heavy prenatal alcohol exposure. Brain Imaging Behav 12, 806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz I, Aird F, Li L, Prystowsky MB, Redei E, 1993. Sexually dimorphic effects of alcohol exposure in utero on neuroendocrine and immune functions in chronic alcohol exposed adult rats. Mol. Cell. Neurosci 4, 343–353. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J, 2010a. Prenatal alcohol exposure: Foetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rev 34, 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KGC, Verma P, Yoon E, Yu W, Young AH, Weinberg J, 2010b. Prenatal alcohol exposure and chronic mild stress differentially alter depressive-and anxiety-like disorders in male and female offspring. Alcohol. Clin. Exp. Res 34, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KGC, Verma P, Yoon E, Yu W, Weinberg J, 2008, Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann. N. Y. Acad. Sci 1144, 154–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F, Isgor C, Kabbaj M, 2013. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience 249, 232–241. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Pelto-Huikko M, Rechardt L, Isola J, Lammi A, Fuxe K, Gustafsson JA, Wikström AC, Hökfelt T, 1992. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology 55, 451–459. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB, 1991. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol 308, 249–276. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ, 2004. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14, 636–648. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihum JT, Chido LM, 1999. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev. Psychopathol 11, 195–208. [DOI] [PubMed] [Google Scholar]
- Karandrea D, Kittas C, Kitraki E, 2002. Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinology 75, 217–226. [DOI] [PubMed] [Google Scholar]
- Karim MA, Sloper JC, 1980. Histogenesis of the supraoptic and paraventricular neurosecretory cells of the mouse hypothalamus. J. Anat 130, 341–347. [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Mahoney JC, Randich A, West JR, 1991. Indices of stress in rats: Effects of sex, perinatal alcohol and artificial rearing. Physiol. Behav 49, 751–756. [DOI] [PubMed] [Google Scholar]
- Kim CK, Giberson PK, Yu W, Zoeller RT, Weinberg J, 1999a. Effects of prenatal ethanol exposure on hypothalamic–pituitary–adrenal responses to chronic cold stress in rats. Alcohol. Clin. Exp. Res 23, 301–310. [PubMed] [Google Scholar]
- Kim CK, Yu W, Edin G, Ellis L, Osborn JA, Weinberg J, 1999b. Chronic intermittent stress does not differentially alter brain corticosteroid receptor densities in rats prenatally exposed to ethanol. Psychoneuroendocrinology 24, 585–611. [DOI] [PubMed] [Google Scholar]
- Kozanian OO, Rohac DJ, Bavadian N, Corches A, Korzus E, Huffman KJ, 2018. Long-lasting effects of prenatal ethanol exposure on fear learning and development of the amygdala. Front. Behav. Neurosci 12, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT, 1974. Food and water restriction shifts corticosterone, temperature, activity, and brain amine periodically. Endocrinology 95, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Lam VYY, Raineki C, Ellis L, Yu W, Weinberg J, 2018a. Interactive effects of prenatal alcohol exposure and chronic stress in adulthood on anxiety-like behavior and central stress-related receptor mRNA expression: Sex-and time-dependent effects. Psychoneuroendocrinology 97, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam VYY, Raineki C, Takeuchi LE, Ellis L, Woodward TS, Weinberg J, 2018b. Chronic stress alters behavior in the forced swim test and underlying neural activity in animals exposed to alcohol prenatally: Sex-and time-dependent effects. Front. Behav. Neurosci 12, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J, 2006. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J. Neuroendocrinol 18, 672–784. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60, 340–352. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C, 1996. Gender differences in the effects prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis responses to immune signals. Psychoneuroendocrinology 21, 145–155. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C, 2000. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: Role of altered pituitary and hypothalamic function. Mol. Cell. Neurosci 16, 515–528. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Anderson SL, 2008. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 62, 22–30. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Lawson K, Stone K, Anderson SL, 2008. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse 62, 185–192. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Laing DG, 1980. Discrimination of odors from stressed rats by non-stressed rats. Physiol. Behav 24, 699–704. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman A-A, Shiloff D, Jakobson L, Longstaffe S, Chudley AE, 2005. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatr. Res 58, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Márquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C, 2013. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry 3, e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, 2010. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 756–765. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ, 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res 187, 229–238. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M, 1996. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci. Res 26, 235–269. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP, 2012. Neural regulation of the stress response: The many faces of feedback. Cell. Mol. Neurobiol 32, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C, 2011. Extensive deep gray matter volume reductions in children and adolescent with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 35, 1404–1417. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ, 1986. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol. Clin. Exp. Res 10, 397–402. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B, 2006. The relationship of prenatal alcohol exposure and postnatal environment to child depressive symptoms. J. Pediatr. Psychol 31, 50–64. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J, 2002. Psychiatric illness in clinical sample of children with prenatal alcohol exposure. Am. J. Drug Alcohol Abuse 28, 743–754. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O’Connor MJ, Sowell ER, 2009. Altered frontal-parietal functioning during verbal working memory in children and adolescent with heavy prenatal alcohol exposure. Hum. Brain Mapp 30, 3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papilloud A, Veenit V, Tzanoulinou S, Riccio O, Zaneletti O, de Suduiraut IG, Grosse J, Sandi C, 2018. Peripubertal stress-induced heightened aggression: Modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN, 2008. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2005. The Rat Brain in Stereotaxic Coordinates Academic Press, San Diego. [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C, 2011. Mental health issues in fetal alcohol spectrum disorders. J. Ment. Health 220, 473–483. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA, 2011. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J. Exp. Child Psychol 108, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Chew L, Mok P, Ellis L, Weinberg J, 2016. Short-and long-term effects of stress during adolescence on emotionality and HPA function of animals exposed to alcohol prenatally. Psychoneuroendocrinology 74, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Ellis L, Weinberg J, 2018. Impact of adolescence stress on the expression of stress-related receptors in the hippocampus of animals exposed to alcohol prenatally. Hippocampus 28, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Hellemans KGC, Bodnar T, Lavigne KM, Ellis L, Woodward TS, Weinberg J, 2014. Neurocircuitry underlying stress and emotional regulation in animals prenatally exposed to alcohol and subjected to chronic mild stress in adulthood. Front. Endocrinol 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Halasz I, Li LF, Prystowsky MB, Aird F, 1993. Maternal adrenalectomy alters the immune and endocrine functions of fetal alcohol-exposed male offspring. Endocrinology 133, 452–460. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR, 2011. Fetal alcohol spectrum disorders: An overview. Neuropsychol. Rev 21, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, 2017. The impact of stress on structure of the adolescent brain: Implications for adolescent mental health. Brain Res 1654, 185–191. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsores IN, Chhua N, Vernov M, Conrad CD, McEwen BS, 2006. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 147, 1664–1674. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS 2004. Stress and the adolescent brain. Ann. N. Y. Acad. Sci 1094, 202–214. [DOI] [PubMed] [Google Scholar]
- Rouzer AK, Cole JM, Johnson JM, Varlinskaya EI, Diaz MR, 2017. Moderate maternal alcohol exposure on gestational day 12 impacts anxiety-like behavior in offspring. Front. Behav. Neurosci 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Ehrlich DE, Rainnie DG, 2016. Morphology and dendritic maturation of developing principal neurons in the rat basolateral amygdala. Brain Struct. Funct 221, 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nakamura T, 1973. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol 41, 163–173. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY, 2007. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport 18, 635–639. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF, 1991. Fetal alcohol syndrome in adolescent and adults. JAMA 265, 1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK, 2004. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr 25, 228–238. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV, 2008. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Horm. Behav 53, 386–394. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS, 2014. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage 86, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR, 2015. Developmental change in amygdala reactivity during adolescence: Effects of family history of depression and stressful life events. Am. J. Psychiatry 172, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N, 1982. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol. Biochem. Behav 16, 585–589. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Wiechmann AF, Hill MA, Kokka N, 1981. Fetal exposure to ethanol enhances pituitary-adrenal and temperature responses to ethanol in adult rats. Alcohol. Clin. Exp. Res 5, 237–246. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA, 2008. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res, 1200, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C, 2007. Stress before puberty exerts a sex-and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast 2007, 71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C, 2011. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front. Behav. Neurosci 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Pitiot A, Paus T, Sandi C, 2012. Stress during puberty boots metabolic activation associated with fear extinction learning in hippocampus, basal amygdala and cingulate cortex. Neurobiol. Learn. Mem 98, 93–101. [DOI] [PubMed] [Google Scholar]
- Tottenham N, 2015. Social scaffolding of human amygdala-mPFC circuit development. Soc. Neurosci 10, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Galván A, 2016. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav, Rev 70, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Guterman A, Richter-Levin G, 2008. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders. Neuropsychopharmacology 33, 378–393. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S, Garcia-Mompó C, Riccio O, Grosse J, Zanoletti O, Dedousis P, Nacher J, Sandi C, 2016. Neuroligin-2 expression in the prefrontal cortex is involved in attention deficits induced by peripubertal stress. Neuropsychopharmacology 41, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress response. Nat. Rev. Neurosci 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L, 2012. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci 35, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden CG, Uylings HB, 1985a. Cytoarchitectonic development of the prefrontal cortex in the rat. J. Comp. Neurol 241, 253–267. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Uylings HB, 1985b. Postnatal volumetric development of the prefrontal cortex in the rat. J. Comp. Neurol 241, 268–274. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Verwer RW, van Vulpen EH, van Uum JF, 1996. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J. Comp. Neurol 376, 75–96. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP, 2012. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol 54, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS, 2014. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage 91, 70–76. [DOI] [PubMed] [Google Scholar]
- Wang Q, Verweij EW, Krugers HJ, Jöels M, Swaab DF, Lucassen PJ, 2014. Distribution of the glucocorticoid receptor in the human amygdala; change in mood disorder patients. Brain Struct. Funct 219, 1615–1626. [DOI] [PubMed] [Google Scholar]
- Weinberg J, 1988. Hyperresponsiveness to stress: Differential effects of prenatal ethanol on males and females. Alcohol. Clin. Exp. Res 12, 647–652. [DOI] [PubMed] [Google Scholar]
- Weinberg J, 1992. Prenatal ethanol effects: Sex differences in offspring stress responsiveness. Alcohol 9, 219–223. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KGC, 2008. Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol 20, 470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C, 1996. Fetal ethanol exposure: Hypothalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol. Clin. Exp. Res 20, 122–131. [DOI] [PubMed] [Google Scholar]
- Wieczorek L, Fish EW, O’Leary-Moore SK, Parnell SE, Sulik KK, 2015. Hypothalamic-pituitary-adrenal axis and behavioral dysfunction following early binge-like alcohol exposure in mice. Alcohol 49, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE, 2005. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: A role for maternal thyroid hormone deficiency in FAE. Mol. Psychiatry 10, 961–971. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LAM, 2015. Alcohol and pregnancy: Effects in maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology 57, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, Lim KO, 2013. Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res 37, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin A, Wick-Carlson D, Packard BA, Morano R, Herman JP, 2016. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology 65, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang R, Liu Y, Liu D, Jiang H, Pan F, 2017. FKBP5 and specific microRNAs via glucocorticoid receptor in the basolateral amygdala involved in the susceptibility to depressive disorder in early adolescent stressed rat. J. Psychiatr. Res 95, 102–113. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, Yang Z, Gerum S, Biswal BB, Milham MP, Sullivan RM, Castellanos FX, 2017. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl. Psychiatry 7, e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Klavir O, 2018. Reciprocal amygdala-prefrontal interactions in learning. Curr. Opin. Neurobiol 53, 149–155. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, Pei J, Andrew G, Massey A, Massey D, Beaulieu C, 2011. Developmental cortical thinning in fetal alcohol spectrum disorders. NeuroImage 58, 16–25. [DOI] [PubMed] [Google Scholar]
- Zhou D, Rasmussen C, Pei J, Andrew G, Reynolds JN, Beaulieu C, 2018. Preserved cortical asymmetry despite thinner cortex in children and adolescent with prenatal alcohol exposure and associated conditions. Hum. Brain Mapp 39, 72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.