Abstract
The responsiveness of spinal cord nociceptive neurons to innocuous mechanical stimuli can be increased by the release of excitatory amino acids (EAAs) and peptides attributable to an injury-induced barrage of impulses. This sensitization of spinal dorsal horn neurons can also result from administration of phorbol ester by microdialysis, presumably by direct activation of protein kinase C (PKC). This study was designed to examine the effects of central sensitization of spinothalamic tract (STT) neurons produced by intradermal injection of capsaicin on the descending inhibition driven from the periaqueductal gray (PAG) and the possible role of PKC in this process in anesthetized monkeys. Sensitization of responses of STT cells to mechanical stimuli was induced by intradermal injection of capsaicin. PAG inhibition was significantly attenuated when sensitization of responses to mechanical stimuli occurred. However, perfusion of the spinal cord with NPC15437 (a selective PKC inhibitor) by microdialysis could prevent the sensitization of the responses to mechanical stimuli and the reduction in PAG inhibition of these responses induced by capsaicin injection. Results similar to those produced by capsaicin injection were observed when a PKC activator, phorbol ester (12-O-tetradecanoylphorbol-13-acetate), was infused within the dorsal horn by microdialysis. An inactive phorbol ester (4α-phorbol 12,13-didecanoate) had no effect. These results provide evidence that the activation of PKC contributes to the development of central sensitization in dorsal horn neurons produced by chemical stimulation with capsaicin. Attenuation of the effectiveness of PAG inhibition takes place when the sensitization of dorsal horn cells develops, and PKC may play a significant role in this process.
Keywords: protein kinase C, periaqueductal gray, spinothalamic tract neurons, capsaicin, antinociception, spinal cord, monkey
It has been well established that damage to peripheral tissues induces a state of sensory hypersensitivity that includes primary hyperalgesia to noxious heat and mechanical stimuli applied in the damaged region, as well as secondary mechanical hyperalgesia (an increased response to noxious stimuli) and mechanical allodynia (a painful response to innocuous stimuli) in an area surrounding the site of primary hyperalgesia (LaMotte et al., 1982,1983; Mersky, 1986; Campbell et al., 1988). It has been suggested that secondary mechanical hyperalgesia and allodynia are attributable to sensitization of spinal dorsal horn neurons because nociceptors in the area of secondary hyperalgesia and allodynia do not show a lowered threshold (Baumann et al., 1991; LaMotte et al., 1992). Experimentally, sensitization of primate spinothalamic tract (STT) neurons can be produced by intradermal injection of capsaicin (Simone et al., 1991;Dougherty et al., 1992b), which is known to activate selectively primary afferent C-fibers (Baumann et al., 1991). Activation of these fibers triggers release of excitatory amino acids (EAAs) and peptides (Gamse et al., 1979; Sorkin and McAdoo, 1993), which are associated with the sensitization of spinal dorsal horn neurons (Dougherty et al., 1991, 1993). Conversely, this central sensitization can neither be induced nor maintained when NMDA or non-NMDA glutamate receptors or neurokinin 1 receptors are blocked (Dougherty et al., 1992b, 1994).
Protein kinase C (PKC) is believed to be involved in the process of sensitization of dorsal horn neurons (Coderre, 1992; Mao et al., 1992; Paleček et al., 1994). One mechanism for activation of PKC is binding of a ligand to G-protein linked membrane receptors, such as metabotropic glutamate and NK1 receptors (Watling, 1992; Schoepp and Conn, 1993). Released EAAs can stimulate metabotropic receptors, resulting in the activation of phospholipase C and the production of diacylglycerol. This process activates PKC (Manzoni et al., 1990), which in turn potentiates the effectiveness of cellular responses to NMDA by increasing the probability of NMDA channel openings and by reducing the voltage-dependent Mg2+ block of the NMDA receptor–channel complex (Chen and Huang, 1992). On the other hand, activation of PKC inhibits GABA receptors (Leidenheimer et al., 1992). All of these changes would enhance the sensitivity of dorsal horn neuronal responses to excitatory inputs. It has been reported that activation of PKC also suppresses opioid analgesia in the spinal cord (Zhang et al., 1990). We hypothesize that activation of PKC in the spinal cord contributes to the process of sensitization of dorsal horn sensory neurons in part by reducing the effectiveness of central descending inhibitory pathways.
In this study, we examined the changes in the descending inhibition of primate STT neurons produced by stimulation of the periaqueductal gray (PAG) that occur when central sensitization in STT neurons is induced by intradermal injection of capsaicin and the possible role of PKC in this process.
Preliminary results of this work have been reported in abstract form (Lin et al., 1995).
MATERIALS AND METHODS
Young adult monkeys (Macaca fascicularis) of either sex weighing between 1.8 and 2.9 kg were tranquilized by ketamine (10.0 mg/kg, i.m.), then anesthetized with a mixture of nitrous oxide, oxygen, and halothane, followed by an intravenous dose of α-chloralose (60.0 mg/kg). Anesthesia was later maintained by intravenous infusion of pentobarbital sodium (5.0 mg/kg/hr). The level of anesthesia was checked periodically by examining pupillary size and reflexes and by observing vital signs. Once anesthesia was adequate, the animals were paralyzed with gallamine triethiodide (20.0 mg/hr) and artificially ventilated. End-tidal CO2 was kept between 3.5 and 4.5% and core temperature between 37 and 38°C by a servo-controlled heating blanket. Many of the methods used for this study were described in detail in previous papers (Dougherty et al., 1992b; Lin et al., 1994a), and so they will be only briefly described here.
Placement of microdialysis fibers and drug administration. A microdialysis fiber was prepared and the dialysis zone positioned in the spinal dorsal horn as described previously (Dougherty et al., 1992b). Artificial CSF (ACSF) was infused through the dialysis fiber at a rate of 5 μl/min for delivery of drugs. The fiber was placed at a site in spinal cord segments L5–L7 and within laminae III–VI. The drugs delivered by microdialysis were expected to diffuse through at least one spinal segment without significant leak into the blood or CSF (Sluka and Westlund, 1993). NPC15437 [(2,6-diamino-N-([1-oxotridecyl)-2-piperidinyl]methyl)hex an-amide], a highly selective PKC inhibitor (Sullivan et al., 1991, 1992), dissolved in ACSF was administered at a concentration of 10 mm. In some STT cells, a dose–response curve was done with two doses of NPC15437 (1.0 and 10.0 mm). The phorbol esters are potent activators of PKC, although it is possible that they act through other mechanisms as well (Blumberg et al., 1984). In this study, a phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA), was infused into the spinal dorsal horn by microdialysis. For control purposes, an inactive phorbol ester isomer, α-TPA (4α-phorbol 12,13-didecanoate), was also used. Both were diluted in ACSF to the concentration of 1.0 mm and administered into the dorsal horn. Final concentrations of these drugs in the spinal dorsal horn are decreased by the efficiency of diffusion across the fiber wall to around 4–10% (Dougherty et al., 1992b; Sluka et al., 1993) and then by at least another order of magnitude through the tissue (Benveniste et al., 1989). We estimate that the concentrations at the STT cells were in the micromolar range, similar to those used inin vitro experiments (Gerber et al., 1989). It has been demonstrated that PKC activity and binding of phorbol ester to the enzyme are inhibited by NPC15437 with IC50 values of ∼19 and ∼23 μm, respectively, and no inhibition of cAMP-dependent or calcium/calmodulin-dependent protein kinases was observed at concentrations of NPC15437 up to 300 μm (Sullivan et al., 1992). Therefore, we believe that the concentration of NPC15437 used in this study may have a selective action on PKC.
Experimental protocol. Procedures for locating the ventral posterior lateral (VPL) nucleus of the thalamus and the PAG were as described previously (Lin et al., 1994a). A low-impedance (3–5 MΩ) carbon filament electrode was used to record the extracellular activity of STT neurons. STT cells were searched for within 250–750 μm of the edge of a dialysis fiber and isolated using antidromic search stimuli (0.75 mA, 200 μsec, at 0.3 Hz) applied through the VPL electrode (Dougherty et al., 1992b; Lin et al., 1994a). The experiment on each cell began with mapping of the receptive field and determination of control mechanically evoked activity using innocuous and noxious mechanical stimuli. Background activity was recorded for 2 min before application of mechanical stimuli. A set of five points on the receptive field was marked with ink. STT cell responses were evoked consecutively at these points by a battery of mechanical stimuli that included BRUSH, PRESS, and PINCH stimuli. Each stimulus was always applied for 10 sec followed by a 10 sec pause before the next test point was stimulated. Innocuous BRUSH stimuli were delivered by repeated brushing in a stereotyped manner with a camel’s hair brush. PRESS and PINCH stimuli were applied with arterial clips of different sizes. The PRESS stimulus produces firm pressure (144 gm/mm2) near pain threshold when applied on human skin, whereas the PINCH is distinctly painful with a force of 583 gm/mm2. STT neurons were classified as low threshold (LT), wide dynamic range (WDR), or high threshold (HT), according to their responses to graded intensities of mechanical stimulation (Chung et al., 1986). LT cells responded best to innocuous mechanical stimuli, HT cells almost exclusively to noxious mechanical stimuli, and WDR cells to both innocuous and noxious mechanical stimuli. Care was taken to ensure that the BRUSH responses on each occasion were maximal and that each stimulus was applied to the same point. Responses to repeated application of BRUSH and PINCH stimuli in our previous work showed that there is very little variation (<4% for BRUSH and <20% for PINCH) among the responses to mechanical stimuli applied to the same receptive field and repeated every 5 min (Owens, 1991; Dougherty et al., 1992a). A point within the receptive field from which the maximal mechanical response was evoked was chosen for tests of the inhibition induced by PAG stimulation of responses to the three kinds of mechanical stimuli. The PAG was stimulated electrically with trains of 333 Hz, 200 μsec square pulses (1 sec train duration with 2 sec intervals between trains) at an intensity of 100–400 μA (Lin et al., 1994a), while cutaneous mechanical stimuli were applied to the receptive field. Stimulation sites in the PAG were located as described previously and have been verified histologically to be distributed mostly in the lateral or ventrolateral PAG at the level of the oculomotor or trochlear nuclei (Lin et al., 1994).
Once the control responses were recorded, capsaicin (0.1 ml, 3%) was injected intradermally at a site in the receptive field in the same way as used by Dougherty et al. (1992b). Briefly, capsaicin was injected intradermally into the center of the receptive field, which was several centimeters from the nearest site chosen for application of the mechanical stimuli. After 15 min the responses evoked by mechanical stimulation and the PAG-induced inhibition of these responses were retested. Recordings were made again 1–1.5 hr after the first capsaicin injection, at which time all responses had returned toward control level. The solution in the dialysis fiber was then switched from ACSF to ACSF containing NPC15437 for 30–60 min of infusion. All tests were again performed during the drug infusion period and a second intradermal capsaicin injection was then made well away from the first injection site. Finally, 15 min after the second capsaicin injection, responses to all stimulus sets were once more recorded. For two cells, the spinal dorsal horn was pretreated with NPC15437 by microdialysis before the first capsaicin was injected to block sensitization. NPC15437 was then washed out with ACSF for 2–2.5 hr, and then a second capsaicin injection was given to determine whether sensitization now occurred. In another group of STT cells, the effects of the PKC activator, TPA, on mechanical stimulation-evoked responses and the PAG-induced inhibition of them were observed. The spinal cord was perfused with TPA for 15–20 min. In addition, α-TPA was also administered intraspinally as a control in some cells.
Data analysis. The mechanical stimulation-evoked responses were analyzed off line from frequency histograms after subtraction of background activity. Responses to the mechanical stimuli applied to the five points across the receptive field were added to yield a total discharge rate for each type of stimulus. Total discharge rates were calculated for each cell under control conditions, during drug infusion, and after drug washout periods. The inhibitory effects of PAG stimulation on cutaneous mechanical stimulation-evoked responses were evaluated by calculating the percentage of inhibition of evoked activity. Statistical significance was tested using analysis of variance with repeated measures and post hoc paired t tests for differences from the control levels. Values are presented as mean ± SEM. All experiments were approved by the local Animal Care and Use Committee and were consistent with the guidelines of the National Institutes of Health.
RESULTS
The activity of a total 39 STT neurons was recorded from the lumbar spinal cord dorsal horn in 14 experiments. The STT neurons included 37 WDR cells and 2 HT cells. Recordings were obtained from 648 to 2084 μm below the surface of the spinal cord, suggesting that the cell bodies were located in laminae I–VI (Owens, 1991). The effects of capsaicin and NPC15437 were examined on 25 cells. Thirteen of these were used for dose–response curves at two concentrations of NPC15437. Fourteen cells were used to examine the effects of phorbol esters.
Effects of PKC inhibitor on the capsaicin-induced sensitization of STT cells
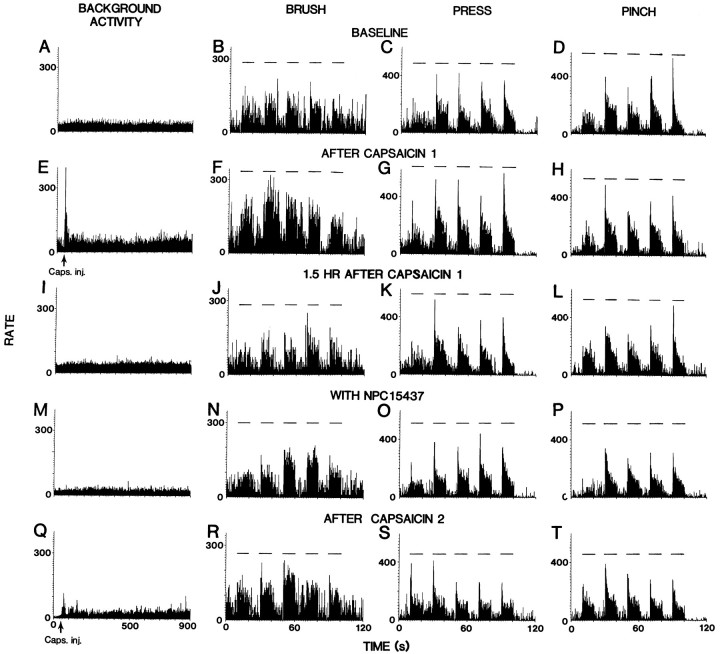
Figure 1 shows rate histograms for a representative STT cell that illustrate the effects of intradermal capsaicin injection before and during intraspinal infusion of NPC15437. The top row (Fig.1A–D) shows the baseline recordings of the background activity and the responses of the cell to BRUSH, PRESS, and PINCH stimuli. The cell responded to the initial injection of capsaicin in a manner typical of most STT cells (Simone et al., 1991; Dougherty et al., 1992b, 1994). There was a very large initial increase in background activity after injection (Fig. 1E). This very high firing rate declined slowly and then remained at an elevated level. The responses to BRUSH and PRESS stimuli, when applied 15 min after injection, were markedly increased (cf. Fig. 1B,C, with Fig. 1F,G). The PINCH response of this cell did not show an obvious increase (Fig. 1H). The background activity and responses to mechanical stimuli returned gradually toward the baseline levels over the next 1.5 hr (Fig. 1I–L), at which time NPC15437 was infused into the dorsal horn for 1 hr. A decrease in background activity was observed after infusion of NPC15437 (Fig. 1M). The responses to BRUSH and PRESS were very near their original levels (Fig. 1N,O), whereas the responses to PINCH were slightly less than the control values (Fig. 1P). A second injection of capsaicin produced a smaller initial increase in cell firing as compared with that induced by the first injection (Fig.1Q). There were no obvious changes in the responses to BRUSH, and the PRESS and PINCH responses decreased slightly (Fig.1R–T).
Fig. 1.
Rate histograms represent changes in the responses of an STT neuron produced by intradermal capsaicin injection and the effects of intraspinal administration of NPC15437. A–D,Baseline background activity and responses to mechanical stimuli (BRUSH, PRESS, and PINCH). Horizontal lines above histograms represent times of application of mechanical stimuli.E–H, Effects produced by the first capsaicin injection.I–L, At 1.5 hr after first capsaicin injection.M–P, Effects of infusion of NPC15437 (10.0 mm) within the dorsal horn. Q–T,Effects of the second capsaicin injection during NPC15437 administration. Bin widths in left column are 1 sec and in others 100 msec.
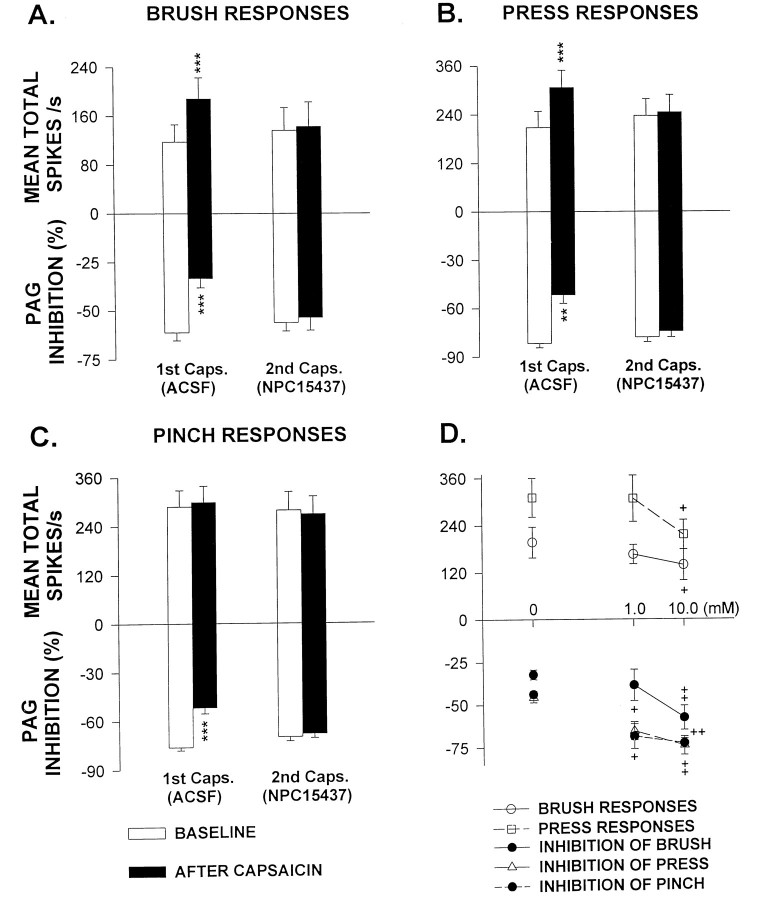
All STT neurons examined showed changes similar to those demonstrated in Figure 1. The upper part of each graph in Figure2A–C summarizes the effects of the PKC inhibitor on changes in the responses of STT cells (n = 10) to mechanical stimuli evoked by capsaicin injection. The results for the cells tested before infusion of NPC15437 are shown by the left pair of bars (ACSF) in each panel and the results during NPC15437 administration by the right pair of the bars. Open bars show the baseline responses before capsaicin injection and solid bars responses 15 min after capsaicin injection. A significant increase in the responses to BRUSH and PRESS (but not PINCH) was observed after the first injection of capsaicin. However, when cells were treated with NPC15437 (10.0 mm), the second capsaicin injection failed to evoke a significant increase in any of the responses to mechanical stimuli. In addition, comparison of the open bars between the left and right pairs shows that NPC15437 had no statistically significant effect on the responses to the mechanical stimuli, although NPC15437 may produce a slight decrease in them in some cells, such as shown in Figure 1. In previous experiments, we have also shown that two successive injections of capsaicin given several hours apart produce comparable effects on the same cell (Dougherty et al., 1994). Recently, we further observed that pretreatment before injection of capsaicin with NPC15437 also prevented the development of sensitization of primate STT cells to BRUSH stimuli without affecting background activity (Sluka et al., 1995). Therefore, attenuation of the responses of cells to the second capsaicin injection is not attributable to adaptation to the effects of capsaicin and suggests that the PKC inhibitor interferes with the responses of STT cells to capsaicin by preventing the development of sensitization that is usually observed.
Fig. 2.
A–C, Bar graphs summarize the effects of NPC15437 infusion on the responses of STT cells to capsaicin injection. The mechanical stimulation-evoked responses are shown in theupper part of each graph and PAG inhibition inlower part of each graph. Pairs of bars at the left of each graph show the mean responses of cells before and after the first capsaicin injection [1st Caps. (ACSF)]. Pairs of bars at the right of each graph show the mean responses of cells before and after the second capsaicin injection during infusion of NPC15437 [2nd Caps. (NPC15437)]. **p < 0.01; ***p < 0.001 compared with the precapsaicin baseline. D, Dose–response curves show the differences in enhanced responses to BRUSH and PRESS stimuli (top) and in blockade of PAG inhibition of peripheral stimulation-evoked responses (bottom) induced by intradermal injection of capsaicin when cells were exposed to two concentrations of NPC15437 in the dialysis fluid (1.0 mm, n = 6; 10.0 mm, n = 7). The concentrations of NPC15437 are plotted on the x-axis.+p < 0.05; ++p < 0.01 compared with the grouped values without infusion of NPC15437 (0 mm, n = 6).
Two concentrations of NPC15437 (1.0 mm,n = 6; 10.0 mm, n = 7) were chosen to make the dose–response observations. NPC15437 was infused into the spinal dorsal horn for 30–60 min before the first injection of capsaicin was made. It was shown that NPC15437 prevents the capsaicin-induced sensitization of STT cells to BRUSH and PRESS stimuli in a dose-related manner (the upper part of Fig.2D). Similar results were seen in the effects on the blockade of PAG inhibition produced by capsaicin injection (thelower part of Fig. 2D, described below).
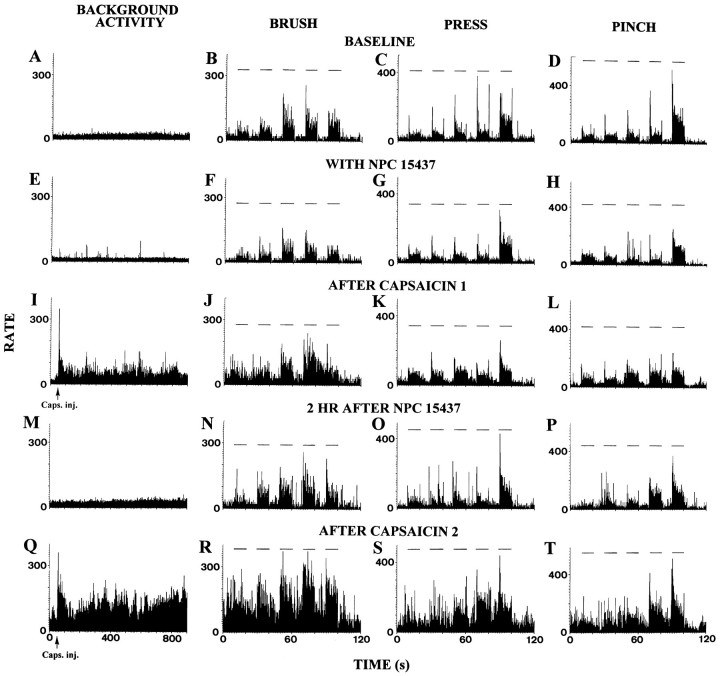
To exclude the possibility that NPC15437 may produce an irreversible effect that could result from a nonspecific action on synaptic interactions, the spinal dorsal horn was pretreated with NPC15437 for 30–60 min before the first capsaicin was injected intradermally while we recorded from two additional STT cells. The results from one of these cells shown in Figure 3 are consistent with the observations described above. NPC15437 itself did not have obvious effects on the responses of the cells (Fig.3E,H), but it largely prevented the development of sensitization of cell to BRUSH and PRESS stimuli (Fig.3J,K). The spinal dorsal horn was then washed out with ACSF for ∼2 hr at which time the responses were similar to the control responses (Fig. 3M–P). Increased responses to BRUSH and PRESS stimuli were observed after the second capsaicin injection was made, indicating that the neurons could now be sensitized (Fig.3R,S).
Fig. 3.
Rate histograms show the responses of an STT cell to mechanical cutaneous stimuli produced by intradermal injection of capsaicin with and then without pretreatment of spinal dorsal horn with NPC15437, respectively. A–D, Baseline background activity and responses to mechanical stimuli (BRUSH, PRESS, and PINCH).Horizontal lines above histograms represent times of application of mechanical stimuli. E–H, Effects of infusion of NPC15437 (10.0 mm) within the dorsal horn.I–L, Effects of the first capsaicin injection during NPC15437 administration. M–P, Two hours after the end of NPC15437 infusion. Q–T, Effects produced by the second capsaicin injection. Bin widths in left column are 1 sec and in others 100 msec.
Effects of intradermal injection of capsaicin on PAG inhibition
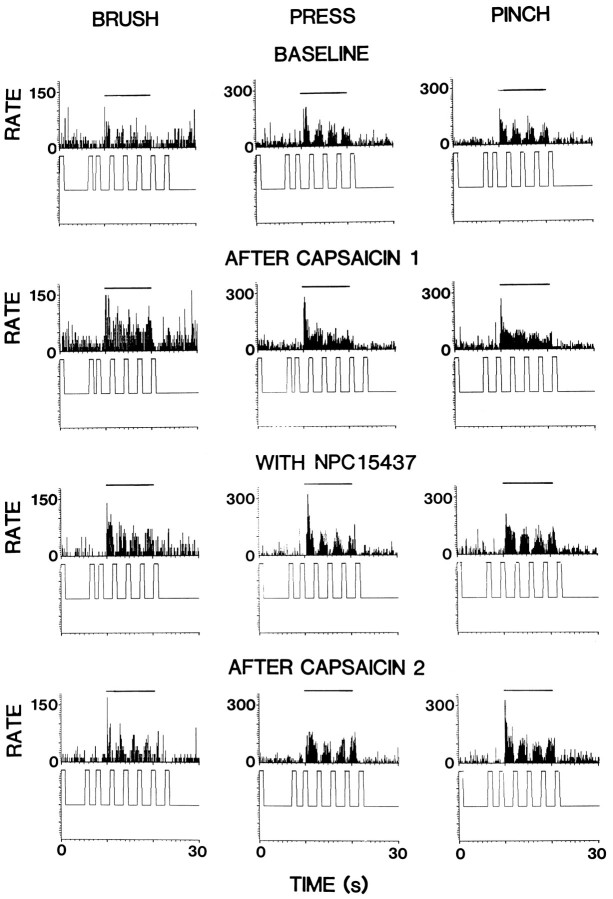
As shown in the top row of Figure 4, stimulation in the PAG produced a profound inhibition of the responses of an STT cell to all three mechanical stimuli. This is consistent with our previous work (Gerhart et al., 1984; Lin et al., 1994a). The percentage of inhibition of the evoked responses for this cell was −68.4% for inhibition of BRUSH, −83.1% for PRESS, and −78.6% for PINCH, respectively. The tests for the effects of capsaicin on PAG inhibition were performed 15 min after capsaicin injection, and it was observed that the inhibition induced by PAG stimulation was significantly attenuated (second row). The percentage of inhibition decreased to −34.6% for BRUSH, −44.0% for PRESS, and −38.9% for PINCH. One and one-half hours after capsaicin injection, inhibition recovered to near the baseline values (data not shown). Similar observations were made in a total of 10 STT cells. The PAG inhibition of BRUSH responses was partially blocked in eight cells, of PRESS responses in seven cells, and of PINCH responses in eight cells. The grouped effects of capsaicin on PAG inhibition reached statistical significance when compared with the baseline values (the left pairs of bars in the lower parts of Fig.2A–C).
Fig. 4.
Blocking effect of intradermal capsaicin injection on PAG inhibition of the responses of an STT cell to mechanical stimuli before and during perfusion of the spinal cord with NPC15437 (10.0 mm). Top row, Control effects of PAG stimulation. Second row, Attenuation of PAG inhibition 15 min after first capsaicin injection. Third row, PAG inhibition during NPC15437 infusion. Bottom row, PAG inhibition 15 min after second capsaicin injection while NPC15437 was being infused. Trains of stimuli were applied in the PAG at times indicated by upward-directed square waves below each histogram. Horizontal lines above histograms represent times of application of mechanical stimuli. Bin widths are 100 msec.
Effects of PKC inhibitor on the blockade of PAG inhibition induced by capsaicin
In the same cell that was described above, the PAG inhibition was comparable to the baseline level 1.5 hr after the first injection of capsaicin. NPC15437 (10.0 mm) was then infused into the dorsal horn for 40 min. There were no obvious changes in inhibitory responses during drug infusion (the third row of Fig. 4). However, the second injection of capsaicin, given with NPC15437 present, did not cause a reduction in PAG inhibition of any of the mechanical stimulation-evoked responses, in contrast to the reduction observed after the first intradermal capsaicin injection (compare second row with bottom row). The grouped data (n = 10) show results similar to those obtained from the individual cell. Comparison of baseline values for PAG inhibition between the left and right sets of bars (open bars in thelower part of each graph in Fig. 2A–C) reveals that NPC15437 had no significant effect on PAG inhibition, but it prevented the blockade of PAG inhibition induced by intradermal injection of capsaicin (right pairs of bars in thelower part of each graph in Fig. 2A–C).
Dose–response curves were made by testing the effects of two concentrations of NPC15437 on the blockade of PAG inhibition of all three mechanical stimulation-evoked responses produced by the first capsaicin injection. The blocking effects were partially prevented when the spinal cord was perfused with 1.0 mm NPC15437 and almost completely prevented when the concentration of NPC15437 reached 10.0 mm (the lower part of Fig. 2D).
Effects of PKC activator on PAG inhibition
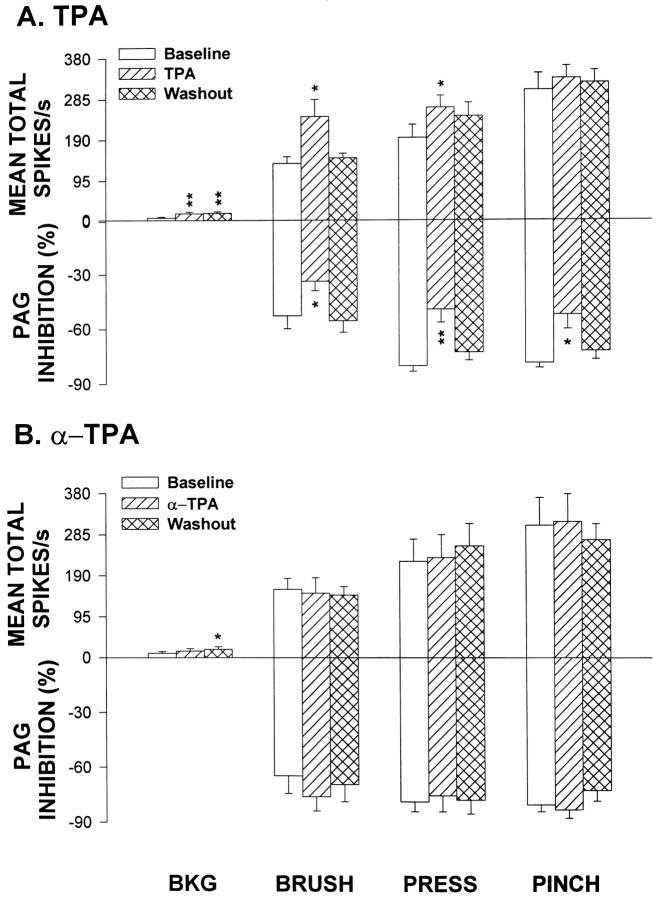
Observations were made on another group of cells separate from that used for capsaicin and NPC15437. Previous work by our group has shown that a PKC activator, TPA, administered into the spinal dorsal horn by microdialysis in the concentration of 0.1 mm produces a substantial increase both in background activity and in the responses evoked by BRUSH (Paleček et al., 1994). In this study, we confirmed the above observations and found that the PRESS responses also increased when the dose of TPA was adjusted to 1.0 mm. Figure 5,A and B, summarizes the grouped changes in background activity and responses evoked by mechanical stimuli (upper part of each graph) produced by infusion of TPA or α-TPA. Figure 5A shows that there was a significant increase in the background activity and responses to BRUSH and PRESS when TPA was infused into the dorsal horn. However, the grouped responses of the cells to PINCH did not show a statistically significant increase. In seven cells, α-TPA was administered and no significant changes were observed except for an increase in background activity that occurred 1 hr after the end of α-TPA infusion (Fig.5B).
Fig. 5.
Bar graphs summarize, respectively, the effects of infusion of TPA (A; n = 9) or α-TPA (B;n = 7) on the averaged background activity, responses of cells to mechanical stimuli (upper part of each graph) and on PAG inhibition (lower part of each graph).Baseline, Before drug infusion; TPA andα-TPA, during drug infusion; Washout, 1 hr after termination of drug infusion. BKG, Background activity. *p < 0.05; **p < 0.01 compared with baseline.
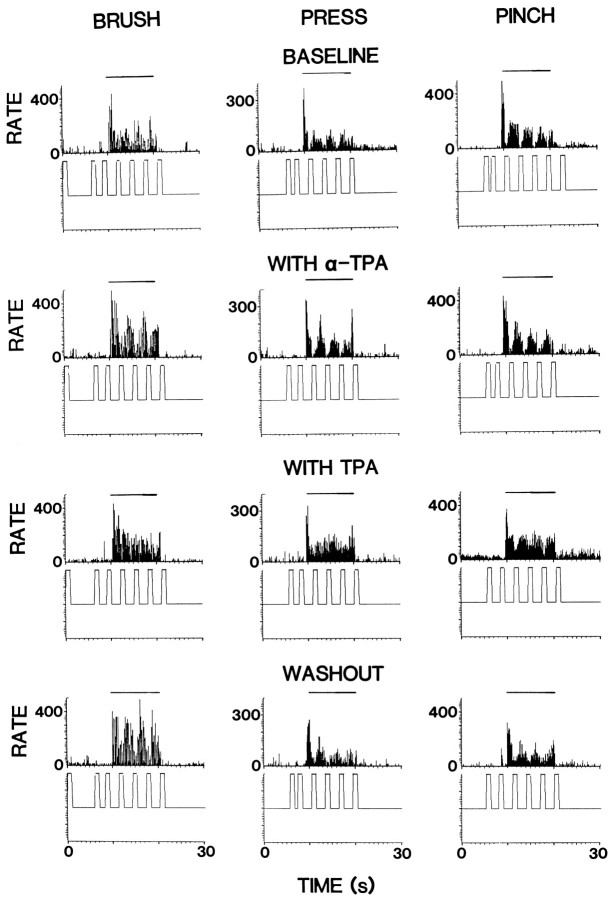
Figure 6 shows the effects of α-TPA and TPA on PAG inhibition of responses evoked by mechanical stimuli in an STT cell. The baseline tests showed PAG inhibition of −55.7, −61.8, and −76.9% for responses to BRUSH, PRESS, and PINCH, respectively (top row). α-TPA was administered into the dorsal horn by microdialysis for 30 min. No obvious changes were found in PAG inhibition during α-TPA infusion (second row). TPA was then infused for 30 min, and PAG inhibition of all three responses to mechanical stimuli was observed to be greatly attenuated (third row). The percentage of inhibition decreased to −6.6% for BRUSH, −15.9% for PRESS, and −38.2% for PINCH. A partial recovery was seen 1 hr after the end of drug infusion (bottom row). PAG-induced inhibition was tested in nine cells. The responses of BRUSH, PRESS, and PINCH were reduced in seven, eight, and seven of the cells tested, respectively. The grouped effects for TPA were significantly different from the control values (lower partof Fig. 5A). These results were similar to those resulting from intradermal injection of capsaicin. α-TPA administration did not produce significant changes in the PAG inhibition (Fig.5B).
Fig. 6.
Changes in the inhibition of the responses of a representative STT cell to mechanical stimuli produced by PAG stimulation when the spinal dorsal horn was perfused with α-TPA (second row) and TPA (third row) for 20 min, respectively. Drug effects recovered partially 1 hr after washout with ACSF (bottom row). Trains of stimuli were applied in the PAG at times indicated by upward-directed square waves below each histogram. Horizontal lines above histograms represent times of application of mechanical stimuli. Bin widths are 100 msec.
DISCUSSION
Changes in the responsiveness of STT neurons to stimulation in the area of secondary hyperalgesia after skin damage have been examined in experiments in which the sensitization was evoked by intradermal injection of capsaicin. It was shown that capsaicin produces an immediate, short-lasting, and robust discharge followed by a period of increased responsiveness to mechanical stimuli and to iontophoretic application of EAAs (Simone et al., 1991; Dougherty and Willis, 1992; Dougherty et al., 1992b). Using this experimental approach, we observed that the inhibition of responses to mechanical stimuli produced by stimulation in PAG is partially blocked when sensitization of STT cells evoked by capsaicin develops. Furthermore, spinal infusion of a PKC inhibitor, NPC15437, can prevent the blocking effects of capsaicin on PAG inhibition in a dose-related manner. On the other hand, perfusion of the spinal dorsal horn with TPA, a phorbol ester that activates PKC, causes an increased responsiveness to mechanical stimuli (mainly to innocuous mechanical stimuli) that is similar to that induced by capsaicin. Sensitization of mechanical stimulation-evoked responses was accompanied by a reduction in PAG inhibition. However, α-TPA, an inactive phorbol ester, does not have significant effects on any of the responses to mechanical stimuli or on PAG inhibition. Thus, it appears that mechanical allodynia and mechanical hyperalgesia may reflect in part a reduction in PAG inhibition and that PKC in the spinal cord dorsal horn may be involved in this process.
Several lines of evidence suggest an involvement of spinal cord PKC in the cellular processing of sensory information. High concentrations of PKC have been found in the substantia gelatinosa of the dorsal horn (Worley et al., 1986; Saito et al., 1988). Administration of an active phorbol ester increases the responses of STT cells to innocuous stimuli (Paleček et al., 1994). In behavioral experiments, the thermal hyperalgesia in a model of peripheral neuropathy is accompanied by an increase in membrane-bound PKC in the dorsal horn (Mao et al., 1992). It has been well documented that EAAs and peptides can act on receptors linked to G-proteins, such as metabotropic glutamate receptors and neurokinin 1 receptors, to cause activation of phospholipase C, production of diacylglycerol, and elevation of PKC activity (Manzoni et al., 1990; Otsuka and Yoshioka, 1993; Schoepp and Conn, 1993). Additionally, activation of PKC can also be mediated by influx of extracellular Ca2+ (Castagna et al., 1982) through channels opened after activation of NMDA and non-NMDA receptors (MacDermott et al., 1986; MacDermott and Dale, 1987; Westenbroek et al., 1990; Lerea et al., 1992). Intradermal injection of capsaicin leads to an increased release of EAAs and peptides in the dorsal horn by activating C-fiber input (Gamse et al., 1979; Brodin et al., 1987;Sorkin and McAdoo, 1993). Therefore, stimulation of C-fibers presumably turns on the PKC system through an action on G-proteins and EAA channels, thus increasing the excitability of nociceptive dorsal horn neurons (Dougherty et al., 1992b, 1994).
PKC activation is known to affect spinal neurotransmission of nociceptive signals. For example, NMDA and non-NMDA-mediated currents in dorsal horn neurons are enhanced by applying phorbol esters or injecting PKC intracellularly (Gerber et al., 1989; Chen and Huang, 1991, 1992). This positive feedback mechanism could account for long-term changes in neuronal excitability associated with the persistent nociception induced by tissue injury (Coderre, 1992). In addition, it has been reported that the functional role of GABA receptors is inhibited by activation of PKC. Therefore, disinhibition may play a role in the increased responsiveness of dorsal horn cells to peripheral stimulation (Leidenheimer et al., 1992).
In the present study, TPA administered into the spinal dorsal horn produced increased responses of STT cells to mechanical stimuli, presumably by activating PKC. An inactive phorbol ester (α-TPA) was ineffective. The action of TPA was similar to that of intradermal injection of capsaicin, implying that TPA, which activates PKC directly without involvement of the initial stages used in the normal signal transduction sequence, could cause much more robust and longer-lasting PKC activation in neuronal tissue than under physiological conditions (Kaczmarek, 1987), and this could be one of the mechanisms by which capsaicin produces sensitization of STT cells. On the other hand, a selective PKC inhibitor, NPC15437, prevented the capsaicin-evoked sensitization of responses to mechanical stimuli in a dose-related manner, although NPC15437 itself did not obviously affect the responses to mechanical stimuli or PAG inhibition. These results suggest strongly that PKC activation is involved in the sensitization of sensory responses of STT cells to peripheral inputs after capsaicin injection.Yashpal et al. (1995) recently showed that noxious thermal stimulation and formalin-induced persistent pain result in an increase in [3H]phorbol 12,13-dibutyrate binding in the spinal cord. Intrathecal application of PKC inhibitors produces significant reductions in nociceptive responses to formalin and in the mechanical hyperalgesia in the hindpaw contralateral to a thermal injury. Thus, it appears that continuous C-fiber input because of tissue damage can potentiate PKC activity via the release of EAAs and neuropeptides and cause secondary mechanical hyperalgesia and allodynia. However, we cannot rule out the possibility that the effects of capsaicin seen in these experiments was in part through mechanisms other than the PKC system. Recent behavioral and physiological studies by our group in rats and monkeys have demonstrated that the allodynia and sensitization of STT cells to BRUSH and PRESS stimuli induced by intradermal injection of capsaicin can be reversed by intraspinal administration of specific inhibitors of PKC, protein kinase A, and protein kinase G, respectively (Sluka et al., 1995; Willis and Sluka, 1995)
An important finding from this study was that the descending inhibitory action on peripheral stimulation-evoked responses of STT cells produced by PAG stimulation is attenuated when these cells are sensitized either by capsaicin or a phorbol ester. As was discussed above, an increased release of EAAs and enhanced activity of EAA receptors within the dorsal horn when PKC is activated would contribute to the hyperexcitability of STT cells. This presumably could reduce the effectiveness of inhibition of peripheral stimulation-evoked responses of STT cells produced by PAG stimulation. On the other hand, spinal inhibitory amino acid receptors, such as glycine and GABA receptors, have been demonstrated to be involved in inhibitory modulation of the responses of STT cell to peripheral inputs (Lin et al., 1996). GABAA and glycine receptors are known to be modulated by phosphorylation by PKC. This process takes place when PKC is activated and catalyzes phosphorylation of certain subunits of the receptors (Kellenberger et al., 1992; Krishek et al., 1994; Vaello et al., 1994). One of the modulatory effects of PKC-dependent phosphorylation of GABAA and glycine receptors is to desensitize the receptors, thus decreasing currents through the inhibitory channels (Leidenheimer et al., 1992; Ragozzino and Eusebi, 1993; Rapallino et al., 1993; Vaello et al., 1994). However, there is also evidence for an upregulation of GABAAreceptors by PKC-induced phosphorylation (Lin et al., 1994b). Recently, we have obtained preliminary data showing that inhibition of STT cells elicited by iontophoretic release of GABA and glycine agonists is reduced either by intradermal injection of capsaicin or intraspinal infusion of TPA (Lin et al., 1995). Another explanation for reduction in the PAG inhibition is that the inhibition is blocked because of desensitization of GABA and glycine receptors, which have been demonstrated to mediate in part the inhibitory modulation of antinociceptive and analgesic actions at the spinal level after stimulation of PAG or nucleus raphe magnus (Sorkin et al., 1993; Lin et al., 1994a). However, no direct evidence is available in this study to link the functional changes in these receptors with the effectiveness of PAG inhibition. In ongoing work, we are examining the effects of capsaicin on the functional activity of spinal GABA and glycine receptors, as well as on other inhibitory receptors and the possible role of PKC in this process to help elucidate the mechanisms by which central sensitization affects descending inhibition (Lin et al., 1995).
Footnotes
This work was supported by National Institutes of Health Grants NS09743 and NS11255. We thank Kelli Gondesen for technical assistance and Griselda Gonzalez for artwork.
Correspondence should be addressed to Dr. William D. Willis, Department of Anatomy and Neurosciences, Marine Biomedical Institute, The University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-1069.
REFERENCES
- 1.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste H, Hansen AJ, Ottosen NS. Determination of brain interstitial concentrations by microdialysis. J Neurochem. 1989;52:1741–1750. doi: 10.1111/j.1471-4159.1989.tb07252.x. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg PM, Jaken S, König B, Sharkey NA, Leach KL, Jeng AY, Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984;33:933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- 4.Brodin E, Linderoth B, Gazelius B, Ungerestedt U. In vivo release of substance P in cat dorsal horn studied with microdialysis. Neurosci Lett. 1987;76:357–362. doi: 10.1016/0304-3940(87)90429-0. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JN, Raja SN, Meyer RA. Painful sequelae of nerve injury. In: Dubner R, Gebhart GF, Bond MR, editors. Proceedings of the Vth World Congress on Pain. Elsevier; Amsterdam: 1988. pp. 135–143. [Google Scholar]
- 6.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 7.Chen L, Huang L-YM. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a μ opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Huang L-YM. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 9.Chung JM, Surmeier DJ, Lee KH, Sorkin LS, Honda CN, Tsong Y, Willis WD. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol. 1986;56:308–327. doi: 10.1152/jn.1986.56.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Coderre TJ. Contribution of protein kinase C to persistent pain following tissue injury. Neurosci Lett. 1992;140:181–184. doi: 10.1016/0304-3940(92)90097-q. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty PM, Willis WD. Enhancement of spinothalamic neuron responses to chemical and mechanical stimuli following combined micro-iontophoretic application of N -methyl-d-aspartic acid and substance P. Pain. 1991;47:85–93. doi: 10.1016/0304-3959(91)90015-P. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty PM, Sluka KA, Sorkin LS, Westlund KN, Willis WD. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res Rev. 1992a;17:1–13. doi: 10.1016/0165-0173(92)90002-4. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty PM, Paleček J, Palečková V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci. 1992b;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty PM, Paleček J, Zorn S, Willis WD. Combined application of excitatory amino acids and substance P produces long-lasting changes in responses of primate spinothalamic tract neurons. Brain Res Rev. 1993;18:227–246. doi: 10.1016/0165-0173(93)90003-i. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty PM, Paleček J, Palečková V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- 17.Gamse R, Molnar A, Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979;25:629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- 18.Gerber G, Kangrga I, Ryu PD, Larew JSA, Randić M. Multiple effects of phorbol esters in the rat spinal dorsal horn. J Neurosci. 1989;9:3606–3617. doi: 10.1523/JNEUROSCI.09-10-03606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhart KD, Yezierski RP, Wilcox TK, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray or adjacent midbrain reticular formation. J Neurophysiol. 1984;51:450–466. doi: 10.1152/jn.1984.51.3.450. [DOI] [PubMed] [Google Scholar]
- 20.Kaczmarek LK. The role of protein kinase C in the regulation of ion channels and neurotransmitter release. Trends Neurosci. 1987;10:30–34. [Google Scholar]
- 21.Kellenberger S, Malherbe P, Sigel E. Function of α1 β2 γ2S GABA type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- 22.Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 23.LaMotte RH, Thalhammer JG, Torebjörk HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2:765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMotte RH, Thalhammer JG, Robinson CJ. Peripheral correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol. 1983;50:1–26. doi: 10.1152/jn.1983.50.1.1. [DOI] [PubMed] [Google Scholar]
- 25.LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol (Lond) 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leidenheimer NJ, McQuilkin SJ, Hahner LD, Whiting P, Harris RA. Activation of protein kinase C selectively inhibits the γ-aminobutyric acidA receptor: role of desensitization. Mol Pharmacol. 1992;41:1116–1123. [PubMed] [Google Scholar]
- 27.Lerea LS, Butler LS, McNamara JO. NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J Neurosci. 1992;12:2973–2981. doi: 10.1523/JNEUROSCI.12-08-02973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Q, Peng YB, Willis WD. Glycine and GABAA antagonists reduce the inhibition of primate spinothalamic tract neurons produced by stimulation in periaqueductal gray. Brain Res. 1994a;654:286–302. doi: 10.1016/0006-8993(94)90491-x. [DOI] [PubMed] [Google Scholar]
- 29.Lin Q, Peng YB, Willis WD. Protein kinase C influences the effectiveness of periaqueductal gray-induced inhibition of primate spinothalamic tract neurons by desensitizing spinal glycine and GABA receptors. Soc Neurosci Abstr. 1995;21:1172. [Google Scholar]
- 30.Lin Q, Peng YB, Willis WD. Role of GABA receptor subtypes in inhibition of primate spinothalamic tract neurons: difference between spinal and periaqueductal gray inhibition. J Neurophysiol. 1996;75:109–123. doi: 10.1152/jn.1996.75.1.109. [DOI] [PubMed] [Google Scholar]
- 31.Lin YF, Browning MD, Dudek EM, Macdonald RL. Protein kinase C enhances recombinant bovine α1β1γ2L GABAA receptor whole-cell currents expressed in L929 fibroblasts. Neuron. 1994b;13:1421–1431. doi: 10.1016/0896-6273(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 32.MacDermott AB, Dale N. Receptors, ion channels, and synaptic potentials underlying the integrative actions of excitatory amino acids. Trends Neurosci. 1987;10:280–284. [Google Scholar]
- 33.MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 34.Manzoni OJJ, Finiels-Marlier F, Sassetti I, Blockaert J, Le Peuch C, Sladeczek FAJ. The glutamate receptor of the Qp-type activates protein kinase C and is regulated by protein kinase C. Neurosci Lett. 1990;109:146–151. doi: 10.1016/0304-3940(90)90553-l. [DOI] [PubMed] [Google Scholar]
- 35.Mao J, Price DD, Mayer DJ, Hayes RL. Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain Res. 1992;588:144–149. doi: 10.1016/0006-8993(92)91354-h. [DOI] [PubMed] [Google Scholar]
- 36.Mersky H. Classification of chronic pain: description of chronic pain syndromes and definition of pain terms. Pain [Suppl] 1986;3:S1. [PubMed] [Google Scholar]
- 37.Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 38.Owens CM (1991) Plastic changes in the responses of primate spinothalamic neurons. PhD thesis, University of Texas Medical Branch.
- 39.Paleček J, Palečková V, Dougherty PM, Willis WD. The effect of phorbol esters on the responses of primate spinothalamic neurons to mechanical and thermal stimuli. J Neurophysiol. 1994;71:529–537. doi: 10.1152/jn.1994.71.2.529. [DOI] [PubMed] [Google Scholar]
- 40.Ragozzino D, Eusebi F. Inhibition of GABA and glycine responses by glutamate in rat hippocampal neurons. Brain Res. 1993;628:115–120. doi: 10.1016/0006-8993(93)90945-j. [DOI] [PubMed] [Google Scholar]
- 41.Rapallino MV, Cupello A, Hyd’en H. The increase in Cl− permeation across the Deiteis’ neuron membrane by GABA on its cytoplasmic side is abolished by protein kinase C activators. Cell Mol Neurobiol. 1993;13:547–558. doi: 10.1007/BF00711463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito N, Kikkawa U, Nishizuka Y, Tanaka C. Distribution of protein kinase C-like immunoreactive neurons in rat brain. J Neurosci. 1988;8:369–382. doi: 10.1523/JNEUROSCI.08-02-00369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- 44.Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- 45.Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993;55:217–225. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- 46.Sluka KA, Willis WD, Westlund KN. Joint inflammation and hyperalgesia are reduced by spinal bicuculline. NeuroReport. 1993;5:109–112. doi: 10.1097/00001756-199311180-00003. [DOI] [PubMed] [Google Scholar]
- 47.Sluka KA, Rees H, Tsuruoka M, Chen PS, Willis WD. The role of G-protein and protein kinases in the sensitization of spinothalamic neurons induced by intradermal injection of capsaicin in the primate. Soc Neurosci Abstr. 1995;21:1408. [Google Scholar]
- 48.Sorkin LS, McAdoo DJ. Amino acids and serotonin are released into the lumbar spinal cord of the anesthetized cat following intradermal capsaicin injections. Brain Res. 1993;607:89–98. doi: 10.1016/0006-8993(93)91492-b. [DOI] [PubMed] [Google Scholar]
- 49.Sorkin LS, McAdoo DJ, Willis WD. Raphe magnus stimulation-induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res. 1993;618:95–108. doi: 10.1016/0006-8993(93)90433-n. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JP, Connor JR, Shearer BG, Burch RM. 2,6-Diamino-N -([1-(1-oxotridecyl)-2-piperidinyl]methyl)hexanamide (NPC15437): a selective inhibitor of protein kinase C. Agents Actions. 1991;34:142–144. doi: 10.1007/BF01993261. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan JP, Connor JR, Shearer BG, Burch RM. 2,6-Diamino-N -([1-(1-oxotridecyl)-2-piperidinyl]methyl)hexanamide (NPC15437): a novel inhibitor of protein kinase C interacting at the regulatory domain. Mol Pharmacol. 1992;41:38–44. [PubMed] [Google Scholar]
- 52.Vaello ML, Ruiz-G’omez A, Lerma J, Mayor FJ. Modulation of inhibitory glycine receptors by phosphorylation by protein kinase C and cAMP-dependent protein kinase. J Biol Chem. 1994;269:2002–2008. [PubMed] [Google Scholar]
- 53.Watling KJ. Nonpeptide antagonists herald new era in tachykinin. Trends Pharmacol Sci. 1992;13:266–269. doi: 10.1016/0165-6147(92)90082-h. [DOI] [PubMed] [Google Scholar]
- 54.Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- 55.Willis WD, Sluka KA. The role of G-protein and protein kinases in allodynia induced by intradermal injection of capsaicin in the rats. Soc Neurosci Abstr. 1995;21:1408. [Google Scholar]
- 56.Worley PF, Baraban JM, Snyder SH. Heterogeneous localization of protein kinase C in rat brain: autoradiographic analysis of phorbol ester receptor binding. J Neurosci. 1986;6:199–207. doi: 10.1523/JNEUROSCI.06-01-00199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yashpal K, Pitcher GM, Parent A, Quirion R, Coderre TJ. Noxious thermal and chemical stimulation induce increases in 3H-phorbol 12,13-dibutyrate binding in spinal cord dorsal horn as well as persistent pain and hyperalgesia, which is reduced by inhibition of protein kinase C. J Neurosci. 1995;15:3263–3272. doi: 10.1523/JNEUROSCI.15-05-03263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang LJ, Wang XJ, Han JS. Phorbol ester suppression of opioid analgesia in rats. Life Sci. 1990;47:1775–1782. doi: 10.1016/0024-3205(90)90352-r. [DOI] [PubMed] [Google Scholar]