Abstract
We developed new biochemical approaches to demonstrate the presence of inositol 1,4,5-trisphosphate (InsP3)-gated calcium channels in presynaptic plasma membranes (SPM) and their involvement in the presynaptic receptor-mediated Ca2+ influx into nerve terminals. In perfusion experiments using SPM vesicles preloaded with45Ca2+, InsP3 elicited the release of45Ca2+ into perfusates in a saturable manner. The InsP3-evoked45Ca2+ release from resealed SPM vesicles was more potent than that from resealed vesicles using any other subcellular fractions. Here we also report the involvement of InsP3-gated mechanisms in the presynaptic receptor-mediated Ca2+ influx into synaptosomes (nerve terminals) by use of such resealed vesicles reconstituted with purified Gi1.
Keywords: InsP3 receptor, presynaptic receptor, Gi1, reconstitution, resealed vesicles, Ca2+
A wide variety of stimulation of receptors by hormones and neurotransmitters results in increased phosphoinositide turnover and mobilization of Ca2+ from intracellular stores (Berridge, 1993; Berridge and Irvine, 1984). Such post-receptor mechanisms involve the stimulation (or inhibition) (seeMisawa et al., 1995) of phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate giving rise to diacylglycerol and inositol 1,4,5-trisphosphate (InsP3). It is well known that InsP3 mobilizes Ca2+ from microsomal organelles, such as rough (Henne et al., 1987) and smooth endoplasmic reticulum (Payne and Fein, 1987) and calciosome (Volpe et al., 1988) in various secretory cells. Thus, it is likely that InsP3-induced calcium mobilization from intracellular organelles is involved in hormone secretion with receptor stimulation.
On the other hand, it is also considered that neurotransmitter release occurs predominantly in nerve terminals in a calcium-dependent manner. Although PLC is reported to be present in nerve terminals (Gerfen et al., 1988) and is assumed to play an important role in presynaptic receptor-mediated regulation of neurotransmitter release, details on the InsP3-mediated calcium mobilization in nerve terminals remain to be determined.
We have reported that kyotorphin (tyrosine–arginine), a neuropeptide that is characterized as a releaser of met-enkephalin from brain slices (Takagi et al., 1979), increased intracellular concentrations of Ca2+, measured by Quin-II fluorometry, and stimulated the uptake of45Ca2+ extracellularly added into brain synaptosomes (Ueda et al., 1986). However, because this 45Ca2+ uptake was not affected by calcium channel blockers and kyotorphin had no effect on the membrane potential in synaptosomes (Ueda et al., 1986), it is unlikely that the voltage-dependent calcium channel is involved in this presynaptic mechanism. Most recently we have reported that kyotorphin stimulated PLC in synaptosomal membranes via Gi1by reconstitution experiments (Ueda et al., 1989). These findings suggest that kyotorphin elicits calcium entry into synaptosomes via an action of InsP3 at the plasma membranes rather than by means of calcium mobilization from intrasynaptosomal organelles. Taking into account the reports that InsP3-specific binding sites are also found in plasma membranes of hepatocytes (Guillemette et al., 1988), lymphocytes (Khan et al., 1992), and neurons (Worley et al., 1987) and that InsP3 receptors are found immunohistochemically in plasma membranes of olfactory cilia (Cunningham et al., 1993) and in nerve terminals of deep cerebellar nuclei (Sharp et al., 1992), we speculated that InsP3-gated calcium channels other than voltage-operated ones are involved in the receptor-operated calcium transport through plasma membranes in nerve terminals. Indeed, there are reports that InsP3-gated calcium channels function in plasma membranes of human lymphocytes, mast cells, and liver (Kuno and Gardner, 1987; Guillemette et al., 1988; Penner et al., 1988). Here we attempted to obtain biochemical evidence for the presynaptic InsP3-gated calcium channels in nerve terminals and clarify the molecular basis of mechanisms in kyotorphin receptor-mediated calcium incorporation into synaptosomes through experiments using resealed presynaptic plasma membrane (SPM) vesicles.
MATERIALS AND METHODS
Materials. InsP3, inositol 1,3,4,5-tetrakisphosphate (InsP4), inositol 1,4-bisphosphate (InsP2), inositol 4-monophosphate (InsP), and inositol (Ins) were purchased from Sigma (St. Louis, MO), and45CaCl2 was purchased from DuPont NEN (Boston, MA). Kyotorphin was a gift from Dr. M. Kubota (Daiichi Pharmaceuticals, Tokyo, Japan) or purchased from Sigma. Other reagents were of analytical grade and were purchased from Sigma or Wako Pure Chemicals (Osaka, Japan).
Preparation of subcellular fractions. Male Sprague–Dawley rats weighing 200–250 gm were decapitated and the whole brains were homogenized in 10 vol of 0.32 m sucrose. The homogenates were centrifuged at 1000 × g for 10 min, and the supernatant was further centrifuged at 12,000 × g for 20 min. Resulting pellets were used for preparation of myelin, synaptosomes, and mitochondria, and the supernatant was used for preparation of microsomes by further centrifugation at 100,000 × g for 60 min, according to Gray and Whittaker (1962). Further subsynaptosomal fractions were prepared by discontinuous density gradient centrifugation of lysed synaptosomes, composed of 0.4, 0.6, 0.8, 1.0, and 1.2 m sucrose (Whittaker et al., 1964). Synaptic vesicles were obtained from the interface between 0.4 and 0.6 m sucrose, SPM from that between 0.6 and 0.8 m and between 0.8 and 1.0 m sucrose, and presynaptic mitochondria from the pellet. [Na+/K+]ATPase and NADPH cytochrome c reductase activities in each subfraction were measured according to Verity (1972) and Kasper (1971), respectively.
Incorporation of 45Ca2+ into and45Ca2+ release from subcellular preparations.For preparation of resealed vesicles, each subcellular preparation per rat brain was hypo-osmotically lysed with 10 ml of 5 mm Tris-HCl buffer, pH 7.5, containing 1 mm MgCl2, 0.574 mm CaCl2, and 1 mmethylene glycol bis (β-aminoethylether)N,N,N′N′-tetraacetic acid/EGTA (TMC buffer) by a Potter–Elvehjem homogenizer and centrifuged at 10,000 × g for 5 min. The free [Ca2+] in the TMC buffer was calculated to be 0.1 μm (Fabiato and Fabiato, 1979). The obtained pellets were resuspended in TMC buffer. Aliquots (10 mg of protein) were incubated in 10 ml of TMC buffer with45Ca2+ (0.5 μCi) at 37°C. At various periods of incubation (0.5–35 min), an aliquot (100 μl) was removed and passed through a GF/C filter (Whatman, Maidstone, UK), followed by three washes with 3 ml of TMC buffer. For preparation of “previously resealed vesicles,” the incubation with45Ca2+ was preceded by prior incubation at 37°C for 30 min in its absence. In some experiments, to examine the ATP- and calmodulin-dependent45Ca2+ incorporation, the free [Ca2+] was adjusted to 10 μm, a concentration required for activation of Ca2+-activated ATPase (calcium pump) by calmodulin using 0.109 mmCaCl2 and 0.1 mm EGTA. Furthermore, in such experiments using unlysed microsomes, the preparation was preloaded with45Ca2+ in the iso-osmotic buffer containing (in mm) KCl 145, NaCl 5, MgCl2 1, CaCl2 0.574, EGTA 1.0, HEPES 10, pH 7.4, in the presence or absence of 1 mm ATP under the condition of 37°C for 30 min. In the experiments for45Ca2+ incorporation into unlysed synaptosomes or saponin-treated permeabilized synaptosomes, another iso-osmotic buffer containing (in mm) NaCl 145, KCl 5, MgCl2 1, CaCl2 0.574, EGTA 1.0, HEPES 10, pH 7.4, in the presence or absence of 1 mm ATP was used. In the latter experiments, saponin (30 μg/ml) was added to the synaptosomes just before 45Ca2+incorporation. The accumulation of45Ca2+ was determined by measuring radioactivity on the filter.
The experiments of 45Ca2+release were performed essentially as described (Ueda et al., 1987). Briefly, aliquots (300–500 μg protein) of lysed preparations were incubated with 45CaCl2 (0.5 μCi) at 37°C for 30 min and centrifuged at 5000 × g for 10 min. The pellets were resuspended in a small volume of TMC buffer, loaded on GF/C filters (diameter 6 mm), fixed in the chamber, and superfused in TMC buffer at a flow rate of 1 ml/min. The45Ca2+ release from resealed vesicles was determined as “fractional release (%)” by measurement of the ratio of the45Ca2+ release (cpm) to the total 45Ca2+ (cpm) in the preparation at the real time, as reported previously (Ueda et al., 1987). The total 45Ca2+ was calculated by summation of45Ca2+ released into perfusates and remained in the preparation after the perfusion experiment. Other details in collection of perfusates, addition of drugs, and estimation of evoked release were also as described (Ueda et al., 1987).
Reconstitution of pertussis toxin-treated membranes with purified Gi1. The pretreatment of SPM with preactivated pertussis toxin (PTX) and reconstitution of PTX-treated membranes with purified Gi1 or Go was performed as reported previously (Ueda et al., 1989). Briefly, freshly prepared SPM (2 mg protein) was pretreated with preactivated 50 μg/ml of PTX in a volume of 100 μl, followed by addition with purified Gi1 or Go (20 pmol/assay).
45Ca2+ influx into intact synaptosomes in membranes prepared from various regions of the rat brain.Procedures of 45Ca2+influx into synaptosomes from various brain regions have been reported previously (Ueda et al., 1986). Briefly, synaptosomes from various brain regions of the rat were prepared as described by Whittaker (1964). After the brain synaptosomes had been preincubated in HEPES-buffered medium (HBM) at 37°C for 10 min, 100 μm kyotorphin and45CaCl2 (0.1 μCi) were added, the incubation extended for another 5 min, then terminated by adding 5 ml of cold HBM, incubating 5 mm EGTA instead of CaCl2. The preparation was then passed through a GF/C glass fiber filter (Whatman). This filter was washed three times with Ca2+-free HMB–EGTA (5 mm), and the radioactivity was counted. Kyotorphin-evoked 45Ca2+influx was represented as percentage of control without kyotorphin.
RESULTS
Accumulation of 45Ca2+ into resealed vesicles derived from SPM
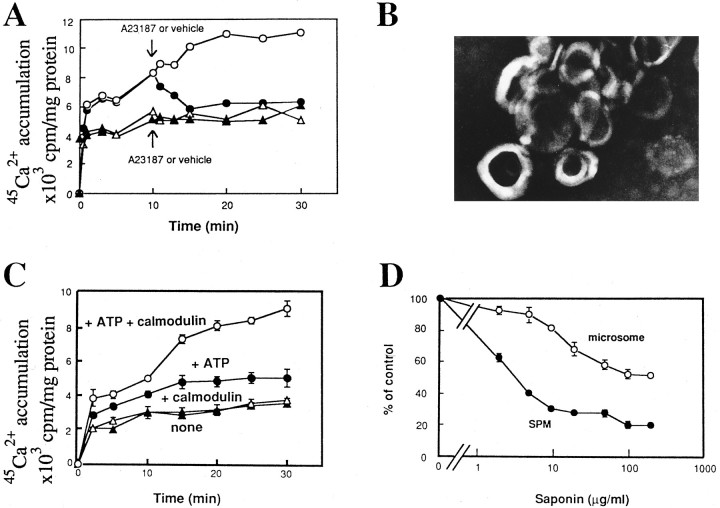
The first step in experiments of45Ca2+ accumulation into resealed vesicles was to incubate the freshly prepared (lysed) SPM with45Ca2+ in TMC buffer at 37°C. Aliquots were periodically removed and passed through GF/C filters to measure 45Ca2+accumulation. As shown in Figure 1A,45Ca2+ accumulation increased as the incubation time increased. There was a rapid increase in 45Ca2+ accumulation within 1 min, then a slow but linear increase within 20 min. The45Ca2+ accumulation reached a plateau at 20–30 min. When 5 μm A-23187, a calcium ionophore, was added to the incubation medium at 10 min after the beginning of incubation, the level of45Ca2+ accumulation decreased with further incubation (Fig. 1A). The45Ca2+ level at 15–30 min after the start of the incubation was 6000 cpm/mg of protein in the presence of 5 μm A-23187, and it was 55–57% of vehicle control without A-23187 at 20–30 min. Because the45Ca2+ level was the same with a higher concentration (10 μm) of A-23187 (data not shown), it is likely that such a decrease by 43–45% is attributed to the incorporation of45Ca2+ inside during formation of resealed SPM vesicles.
Fig. 1.
Accumulation of45Ca2+ into resealed vesicles derived from SPM. A, Freshly prepared SPM (○, •) or previously resealed SPM vesicles (▵, ▴) was incubated with45Ca2+, and aliquot (100 μl) at each incubation time was used for determination of45Ca2+ incorporation, as described in Experimental Procedures. Results in the figure are representative profiles of the time course of45Ca2+ accumulation. Vehicle (○, ▵) or 5 μm A-23187 (•, ▴) was added to the assay tube at 10 min. B, An electron microscopic (negative-staining) image of the resealed SPM vesicles.C, Time course of ATP- and calmodulin-dependent45Ca2+ accumulation into previously resealed SPM vesicles, which was prepared in Experimental Procedures. Vehicle (▴), 5 μg/ml of calmodulin (▵), 1 mm ATP (•), or 5 μg/ml calmodulin plus 1 mm ATP (○) was added simultaneously with45Ca2+ to the tube containing previously resealed SPM vesicles. Experiments were performed under the condition of free [Ca2+] at 10 μm using 0.109 mmCaCl2 and 0.1 mm EGTA. Each point of data represents the mean ± SEM from three separate experiments. D, Blockade of45Ca2+ accumulation into resealed SPM vesicles and unlysed microsomes by pretreatment with various concentrations of saponin. In both experiments using resealed SPM vesicles and unlysed microsomes, incubation was carried out at 37°C for 30 min under the condition of free [Ca2+] at 100 nm in the presence or absence of 1 mm ATP. In resealed SPM vesicles, the ATP-dependent45Ca2+ incorporation in control (without saponin) resealed SPM vesicles (0.25 mg of protein/fraction) was 699 ± 20 cpm/fraction. Experiments using intact microsomes were performed as described in Experimental Procedures. The ATP-dependent 45Ca2+incorporation in control microsomes (0.25 mg of protein/fraction) was 209 ± 9.3 cpm/fraction. Data represent the mean ± SEM from three separate experiments.
In another set of experiments, the SPM was preincubated in the absence of 45Ca2+ at 37°C for 30 min, followed by further incubation with45Ca2+ under the same condition, as mentioned above. In such preparations, the45Ca2+ accumulation was markedly reduced, compared with the previous set of experiments. The45Ca2+ accumulation reached a plateau at the level of 5000–5900 cpm/mg of protein at 10–30 min after the start of incubation with45Ca2+. Such a plateau level was as much as that observed in the previous set of experiments using A-23187. In addition, when 5 μm A-23187 was added to incubation medium at 10 min, there was no more decrease in the level of 45Ca2+accumulation. Thus, it is suggested that45Ca2+ was not actively incorporated into previously resealed vesicles, but just bound to SPM vesicles or aggregates. The formation of resealed vesicles (mostly unilamellar type) during the incubation of lysed SPM was confirmed in electron microscope studies with a negative staining method (Fig. 1B).
Characterization of ATP-dependent 45Ca2+incorporation into previously resealed SPM vesicles
When the previously resealed vesicles were incubated with 45Ca2+ in the presence of 1 mm ATP, there was an active incorporation of 45Ca2+(Fig. 1C). Further addition of calmodulin at 5 μg/ml showed a marked potentiation of ATP-induced45Ca2+ incorporation, whereas calmodulin alone had no significant effect (Fig.1C).
To characterize the SPM vesicles, the effect of saponin on ATP-dependent incorporation was studied. Saponin is known to form micelles with cholesterol mainly found in plasma membranes, and to form small pores in such membranes (Inamitsu and Ohtsuki, 1984). In such experiments, previously resealed SPM vesicles were incubated at 37°C for 30 min with free [Ca2+] at 100 nm containing45Ca2+ in the presence or absence of 1 mm ATP. The ATP-dependent45Ca2+ incorporation (mean ± SEM) defined to be the difference between45Ca2+ incorporations in the presence and absence of ATP was 699 ± 20 cpm/fraction (0.25 mg of protein) from three separate experiments. When various concentrations of saponin were added to resealed vesicles at 37°C for 5 min before incorporation of 45Ca2+ in the presence of ATP, the ATP-dependent incorporation of45Ca2+ was inhibited by saponin in a concentration-dependent manner (Fig. 1D). The IC50 of saponin was 3.5 μg/ml. On the other hand, the ATP-dependent incorporation of45Ca2+ into unlysed microsomes was 2090 ± 93 cpm/fraction (0.25 mg of protein) from three separate experiments. As shown in Figure 1D, however, the ATP-dependent 45Ca2+incorporation into microsomes was less sensitive to saponin treatment than that into SPM vesicles. The IC50 of saponin in microsomal preparations was >100 μg/ml.
InsP3-evoked 45Ca2+ release from resealed SPM vesicles and effects of A-23187 pretreatment on it
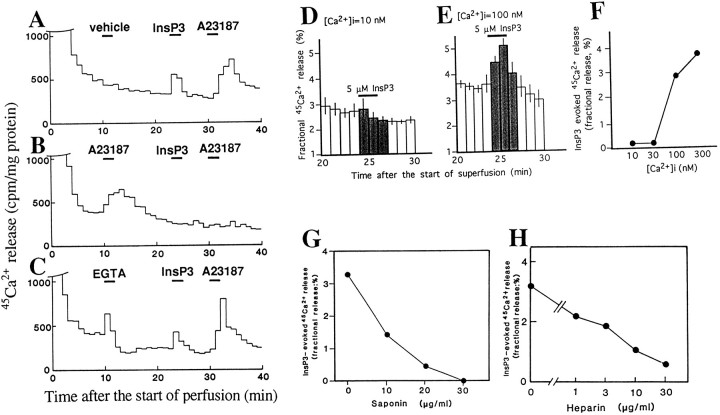
We examined the InsP3-mediated45Ca2+ release from resealed SPM vesicles, prepared as follows: the freshly prepared SPM was incubated with 45Ca2+in TMC buffer at 37°C for 30 min, placed on GF/C filters, and perfused in the TMC buffer. As shown in Figure2A, the basal release of45Ca2+ rapidly decreased and reached a plateau 20 min after the onset of perfusion. The45Ca2+ release was increased by the addition to the medium of InsP3at 5 μm at the 25th and 26th minute, and resting levels were restored by its omission. A-23187, a calcium ionophore added at the 31st and 32nd minute, showed a similar but greater increase in 45Ca2+release, even after treatment with InsP3. However, when A-23187 was pretreated at the 11th and 12th minute, there was no longer any increase in45Ca2+ release by following InsP3 challenge (Fig. 2B). The addition of EGTA, a calcium chelating agent, caused a similar45Ca2+ release, and there was no effect on the 45Ca2+release by following InsP3 and A-23187 challenges (Fig. 2C). These findings suggest that EGTA releases45Ca2+ into perfusates by taking off 45Ca2+, which is adsorbed to vesicles, whereas both challenges with InsP3 and A-23187 release45Ca2+ from the inside of vesicles.
Fig. 2.
Characterization of InsP3-evoked45Ca2+ release from resealed SPM vesicles. A, InsP3- or A-23187-evoked 45Ca2+release. Data in the figure are representative results. Resealed SPM vesicles preloaded with45Ca2+ were perfused at a flow of 1 ml/min in TMC buffer. Each 1 min perfusate was collected for measurement of radioactivity. Results represent45Ca2+ (cpm) released/mg protein of SPM. Vehicle, InsP3 (5 μm), or A-23187 (5 μm) was added to the perfusion medium at the indicated time. B, Blockade of InsP3-evoked45Ca2+ release by pretreatment with A-23187. C, Lack of effect on InsP3-evoked45Ca2+ release by pretreatment with EGTA. D, No significant InsP3-evoked45Ca2+ release in the case with 10 nm[45Ca2+]i(n = 3). Results represent the fractional release (%) as described in Results. E, InsP3-evoked45Ca2+ release (fractional release/%) in the case with 100 nm[45Ca2+]i (n = 3). F, [45Ca2+]i dependency of InsP3-evoked45Ca2+ release (n = 3). InsP3-evoked45Ca2+ release was described in Results. G, Concentration-dependent inhibition of InsP3-evoked45Ca2+ release by saponin.H, Concentration-dependent inhibition of InsP3-evoked45Ca2+ release by heparin.
Characterization of InsP3-mediated45Ca2+ release from resealed SPM vesicles
To further characterize the InsP3-evoked45Ca2+ release, the concentration of 45Ca2+ to be preloaded into newly resealed vesicles was varied. To normalize the variations among separate experiments, we evaluated the InsP3 (or related compounds)-evoked45Ca2+ release as a fractional release, a ratio (%) of the amount (cpm) of45Ca2+ in each fraction to the total amounts (cpm) at real time (Ueda et al., 1987). The basal45Ca2+ release (%) was represented as the sum of six fractional releases from the 22nd to the 24th minute and from the 28th to the 30th minute/2, and the InsP3-evoked increase (%) in the45Ca2+ release was then represented as the sum of three fractional releases from the 25th to the 27th minute—the basal45Ca2+ release. As shown in Figure 2D, there was no significant InsP3 (5 μm)-evoked45Ca2+ release in the case with [45Ca2+]i = 10 nm. When the [45Ca2+]iwas increased to 100 nm, an identical concentration to free [Ca2+]o in perfusion medium (TMC), there was a marked45Ca2+ release (Fig.2E). As expected, the InsP3-evoked45Ca2+ release was further increased at [45Ca2+]i = 300 nm (Fig. 2F).
When resealed SPM vesicles were pretreated (5 min at 37°C) with saponin, the InsP3 (5 μm)-evoked45Ca2+ release was decreased (Fig. 2G). The IC50 of saponin for InsP3-evoked45Ca2+ release was 9 μg/ml, a value equivalent to data obtained with45Ca2+ incorporation, as mentioned above. On the other hand, when 1–30 μg/ml of heparin, known to be a putative InsP3 antagonist (Worley et al., 1987; Ehrlich and Watras, 1988; Kobayashi et al., 1988), was added to the perfusion medium from the 10th minute to the end of perfusion, the InsP3-evoked45Ca2+ release was markedly inhibited (Fig. 2H). The IC50 of heparin was 4.8 μg/ml, a value in good accord with its IC50 in InsP3 binding in cerebellar membranes (Worley et al., 1987).
Kinetics of 45Ca2+ release evoked by InsP3 and related compounds from resealed SPM vesicles
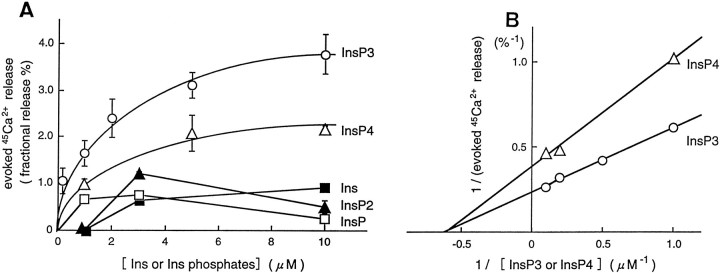
The InsP3-evoked increase in45Ca2+ release from resealed SPM vesicles was concentration-dependent in ranges of 0.5–10 μm InsP3 and InsP4, and these effects appeared to be saturable (Fig. 3A). The double-reciprocal plot showed that apparent Km and maximal response were 1.5 μm and 4.16% for InsP3, whereas they were 1.5 μm and 2.54% for InsP4(Fig. 3B). Ins, InsP, and InsP2 evoked less marked releases compared with InsP3 and InsP4. In addition, the concentration–response curves with InsP and InsP2 were bell-shaped, and thereby kinetic analyses could not be performed. On the other hand, Ins evoked a weak but concentration-dependent45Ca2+ release. It remains unclear whether this effect is attributed to the action on InsP3, InsP4, or other receptors. Details of these weak actions must be further characterized in subsequent studies.
Fig. 3.
Kinetics of45Ca2+ release evoked by InsP3 and related compounds from resealed vesicles of lysed synaptosomes preloaded with45Ca2+. A, Concentration-dependent curve of evoked45Ca2+ release (% fractional release) by various concentrations of InsP3 (○), InsP4 (▵), InsP2 (▴), InsP (□), and inositol (▪). Each experiment was performed in the same preparation so that the kinetics of test compounds can be compared. The data represent the mean ± SEM from three separate experiments. B, Double-reciprocal plots of InsP3- or InsP4-evoked45Ca2+ release.
45Ca2+ release evoked by InsP3from various resealed vesicles composed of different subcellular fractions
To examine the subcellular specificity of InsP3-evoked45Ca2+ release, the effects of InsP3 on the45Ca2+ release were studied in various subcellular preparations (Table 1). As expected, the highest Na+/K+ ATPase activity (a marker enzyme for plasma membranes) was observed in the fractions of microsomes and myelins. A modest level of activity was detected in the synaptosomal fraction. When the synaptosomal fraction was further separated into synaptic vesicles, SPM, and presynaptic mitochondria, highest activity was found in the SPM.
Table 1.
Na+–K+ ATPase, NADPH cytochrome c reductase, and InsP3-evoked 45Ca2+release in subcellular fractions of the rat brain
| Subcellular fractions | Na+–K+aATPase | NADPH cytochrome c reductaseb | Basal releasec(%) | InsP3-evokedd45Ca2+ release (%) |
|---|---|---|---|---|
| Microsomes (P3) | 2.27 | 1.33 | 2.30 ± 0.14 | 1.80 ± 0.18 |
| Myelins (P2A) | 2.22 | 0.83 | 1.86 ± 0.16 | 1.21 ± 0.15 |
| Synaptosomes (P2B) | 1.65 | 0.54 | 1.92 ± 0.11 | 3.61 ± 0.34 |
| Mitochondria (P2C) | 1.55 | 0.93 | 2.16 ± 0.25 | 1.54 ± 0.33 |
| Synaptic vesicles (P2B1) | <0.01 | 0.35 | 2.51 ± 0.36 | 1.58 ± 0.33 |
| Synaptic plasma membranes (P2B2/SPM) | 1.67 | 0.33 | 2.02 ± 0.11 | 5.87 ± 1.24 |
| Presynaptic mitochondria (P2B3) | 0.97 | 0.33 | 1.80 ± 0.16 | 2.56 ± 0.44 |
a,bRatios of activities of Na+–K+ ATPase (a) and NADPH cytochrome c reductase (b) in each subcellular fraction to that of starting brain homogenates. The Na+–K+ATPase activity and NADPH-cytochrome c reductase in starting homogenates were 0.112 mmol/mg of protein/min and 5.98 nmol/mg of protein/min, respectively.
c,dResults represent basal (c) and InsP3-evoked (d) 45Ca2+release (%), represented as described in the text under Results. Data obtained with 5 μm InsP3 (n = 3–6 separate experiments) in various subcellular preparations (300–500 μg/assay) lysed and preloaded with45Ca2+.
In these experiments, preparations were divided into two groups [(P3, P2A, P2B, and P2C) and (P2B1, P2B2, and P2B3)], and experiments using each group were performed at the same time. Total45Ca2+ amounts taken up into resealed vesicle preparations were 1.5–3 × 104 cpm/assay for the first group and 2–4 × 104 cpm/assay for the second group. Marked variations were not observed among subfractions in each group.
On the other hand, NADPH cytochrome c reductase is known to be a marker enzyme for endoplasmic reticulum. This activity was highly found in the microsomal fraction and there was less marked activity in the synaptosomal fraction and its subfractions (Table 1). All subcellular fractions prepared here were hypo-osmotically lysed in TMC and immediately preloaded with45Ca2+, as mentioned above in the case with SPM. As shown in Table 1, the basal fractional45Ca2+ release after InsP3 challenges was similar among all these preparations. However, the InsP3-evoked45Ca2+ release was bigger in the resealed SPM vesicles than in the other resealed vesicles. In this experiment, we measured only total amounts of45Ca2+ uptake in each subcellular preparation for evaluating basal percentage release or InsP3-evoked percentage release, but such total amounts do not represent intravesicular45Ca2+ concentrations. Because the incorporation of45Ca2+ into such resealed vesicles is expected to have occurred in a passive manner, however, the fractional percentage release obtained in the present study should be closely related to this intravesicular concentration. Indeed, the basal percentage release was quite similar among these preparations (Table1). Therefore, it is likely that the difference of InsP3-evoked release is not attributed to the variation of 45Ca2+ uptake among these subfractional preparations, but to specific mechanisms for InsP3 localized in synaptosomes or SPM.
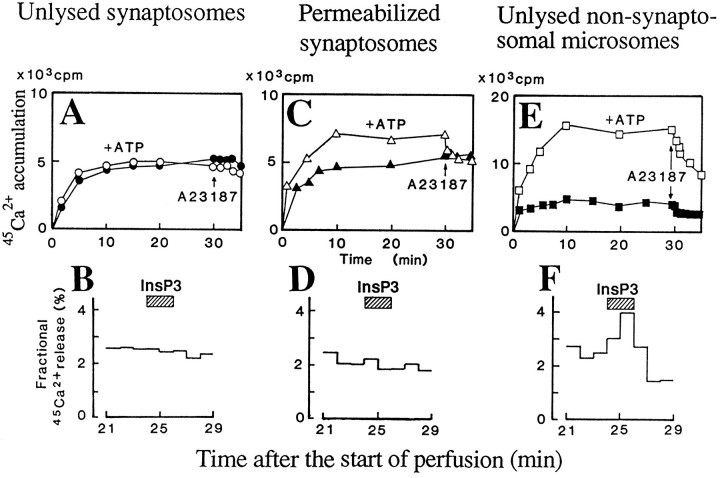
Here we studied the InsP3-evoked45Ca2+ release from intrasynaptosomal organelles. As shown in Figure4A, neither ATP-dependent nor A-23187-sensitive 45Ca2+accumulation was observed in unlysed synaptosomes. In such unlysed synaptosomes that had been incubated with45Ca2+, 5 μm InsP3 had no effect on45Ca2+ release (Fig.4B). On the other hand, in saponin-permeabilized synaptosomes, there was a significant ATP-dependent and A-23187-sensitive 45Ca2+accumulation (Fig. 4C), whereas 5 μmInsP3 had no significant effect on45Ca2+ release from the permeabilized synaptosomes loaded with45Ca2+ in the presence of ATP (Fig. 4D). These findings suggest that some intrasynaptosomal micro-organelles are storage sites for45Ca2+, but they are unlikely targets for InsP3-evoked calcium mobilization. As mentioned before, a marked ATP-dependent and A-23187-sensitive 45Ca2+accumulation was observed in unlysed microsomes that had been prepared from nonsynaptosomal microsomes, as shown in Figure 4E. As expected, InsP3 evoked a significant45Ca2+ release from such unlysed microsomes (Fig. 4F).
Fig. 4.
Lack of InsP3-evoked45Ca2+ release from permeabilized synaptosomes. A, Lack of A-23187-sensitive45Ca2+ incorporation into unlysed synaptosomes. B, Lack of InsP3-evoked45Ca2+ release from unlysed synaptosomes. C, ATP-dependent and A-23187-sensitive45Ca2+ incorporation into saponin-permeabilized synaptosomes. D, Lack of InsP3-evoked45Ca2+ release from saponin-permeabilized synaptosomes. E, Potent ATP-dependent and A-23187-sensitive45Ca2+ incorporation into microsomes. F, InsP3-evoked45Ca2+ release from microsomes. Other details are given in the legends of Figures 1 and2.
Kyotorphin-evoked 45Ca2+ release from resealed SPM vesicles and its guanine nucleotide dependency
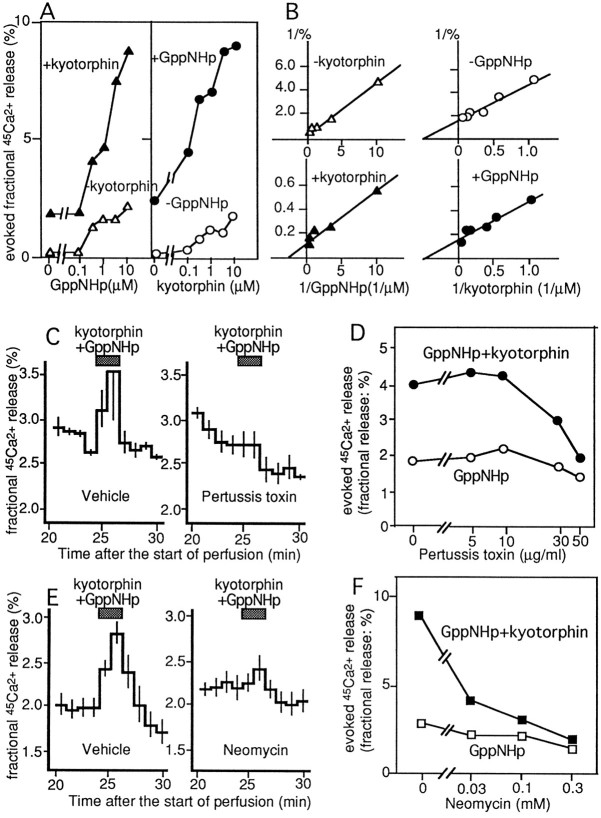
Here we studied the receptor-mediated45Ca2+ release from resealed SPM vesicles of the whole brain, as described above in the case with InsP3. Previously we have reported that kyotorphin evoked 45Ca2+release in such resealed vesicles using SPM, and it was antagonized by leucine–arginine (Ueda et al., 1987), a kyotorphin receptor antagonist (Ueda et al., 1989). In the present experiments, we added GppNHp (an unhydrolyzable analog of GTP) together with kyotorphin in this system to study the involvement of G-proteins in such a receptor-mediated45Ca2+ release in resealed SPM vesicles. Kyotorphin and GppNHp had potentiating effects to each other in evoking 45Ca2+release from such preparations in a concentration-dependent manner, as shown in Figure 5, A and B. TheKm value and maximal response by GppNHp alone were 3.0 μm and 5.0%, respectively. The addition of 100 μm kyotorphin decreased theKm value to 0.4 μmand slightly increased the maximal response to 8.3%. On the other hand, the Km value and maximal response by kyotorphin alone was 2.5 μm and 1.6%, respectively. The addition of 10 μm GppNHp resulted in no change in Km value (2.5 μm), but it did increase the maximal response to 7.8%. The 45Ca2+release by 100 μm kyotorphin plus 10 μm GppNHp was completely blocked in the presence of 100 μm leucine–arginine (data not shown), as reported previously in experiments without GppNHp (Ueda et al., 1987). Thus, it is suggested that the kyotorphin receptor-mediated45Ca2+ release is possibly mediated through G-proteins.
Fig. 5.
Kinetics of kyotorphin- and GppNHp-evoked45Ca2+ release from resealed SPM vesicles and involvements of PTX substrate G-proteins and PLC. A, Kyotorphin- and/or GppNHp-evoked45Ca2+ release were represented as with InsP3-evoked increase (%) in the 45Ca2+ release (see Results). B, Double-reciprocal plots of evoked45Ca2+ release by various combinations of kyotorphin and GppNHp. C, Effects of PTX (50 μg/ml) pretreatments of SPM on 100 μmkyotorphin (plus 10 μm GppNHp)-evoked45Ca2+ release.D, Blockade of kyotorphin (plus 10 μm GppNHp)-evoked45Ca2+ release by pretreatments of SPM with various concentrations of PTX. E, Effects of neomycin (0.3 mm) on 100 μm kyotorphin (plus 10 μm GppNHp)-evoked45Ca2+ release.F, Concentration-dependent inhibition of 100 μm kyotorphin (plus 10 μm GppNHp)-evoked45Ca2+ release by neomycin.
Blockade of kyotorphin-evoked 45Ca2+release by PTX treatment and by addition with neomycin
To study the involvement of G-proteins in the kyotorphin-evoked45Ca2+ release, SPM was pretreated with preactivated PTX. In such treatments, we used highly densed SPM (20 mg protein/ml) so as not to form resealed vesicles before 45Ca2+incorporation. As shown in Figure 5, C and D, a marked reduction of 45Ca2+release by 100 μm kyotorphin plus 10 μm GppNHp was observed at 30–50 μg/ml PTX, concentrations in good accordance with our previous experiments including PTX-catalyzed ADP ribosylation (Ueda et al., 1989).
On the other hand, the45Ca2+ release evoked by 100 μm kyotorphin plus 10 μm GppNHp or by 10 μmGppNHp was concentration-dependently inhibited by 300 μm neomycin, which was added to the perfusion medium from the beginning of experiments (Fig. 5E). The IC50 of neomycin was 30 μm (Fig. 5F), a comparable concentration in inhibiting PLC activity (Cockcroft and Gomperts, 1985).
Recovery of kyotorphin-evoked release of45Ca2+ from resealed SPM vesicles that had been treated with PTX by reconstitution with purified Gi1 but not with Go
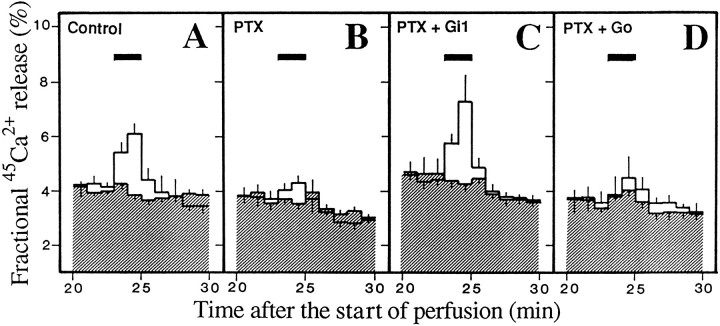
The PTX (50 μg/ml)-treated SPM was reconstituted with Gi1 or Go, which had been purified (>95% purity) from porcine brains (Katada et al., 1987) by incubation at 4°C for 60 min in the presence of 0.01% of CHAPS (a detergent), as described previously (Ueda et al., 1989). As shown in Figure 6, A and B, the45Ca2+ release evoked by 10 μm kyotorphin and 100 μm GppNHp, but not by 100 μm GppNHp alone, was significantly blocked by PTX pretreatments. However, there was no marked reduction in the GppNHp-evoked release by PTX pretreatments. This finding might be explained by the data that PTX treatments block the functional coupling to receptors, but do not affect the intrinsic G-protein activity (Ueda et al., 1990). When PTX-pretreated SPM was reconstituted with purified Gi1, diluted in TMC, incubated with45Ca2+, and used for perfusion experiments, there was a complete recovery of kyotorphin-evoked 45Ca2+release (Fig. 6C). However, there was no significant recovery by reconstitution with purified Go (Fig.6D). All these findings are consistent with our previous report that kyotorphin receptor is coupled to Gi1in rat brain membranes (Ueda et al., 1989).
Fig. 6.
Recovery of kyotorphin-evoked45Ca2+ release from PTX-pretreated and resealed SPM vesicles by reconstitution with Gi1. SPM was treated without (A) or with 50 μg/ml PTX (B,C,D). PTX-treated synaptosomes were reconstituted without (B) or with 20 pmol/assay Gi1 (C) or Go(D). Test drugs were added to the medium at the time indicated by the bar. Open or shadedcolumn represents the data in separate experiments with 10 μm GppNHp alone or with 10 μm GppNHp plus 100 μmkyotorphin, respectively. Results represent the mean ± SEM from three separate experiments. Other details are given in the legend of Figure2.
Relationship between kyotorphin-evoked45Ca2+ release from resealed SPM vesicles and kyotorphin-evoked 45Ca2+ influx into unlysed synaptosomes in various regions of the brain
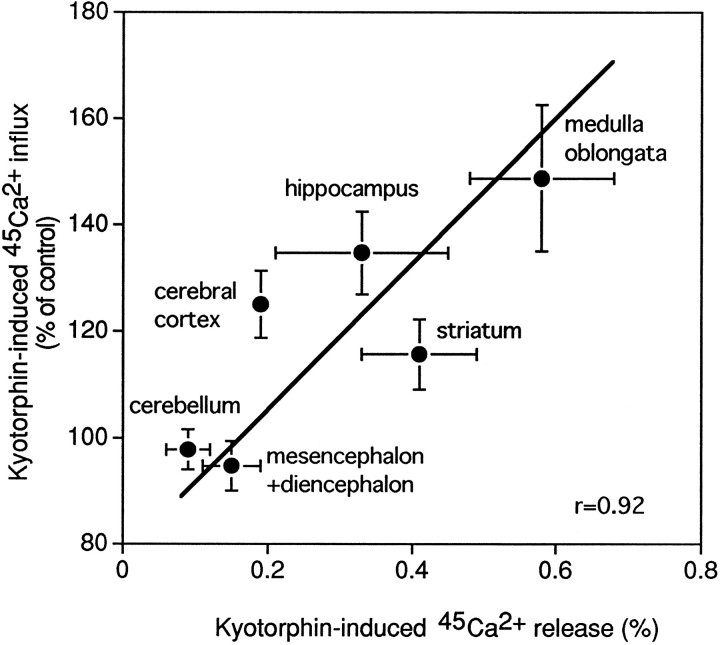
The kyotorphin-evoked45Ca2+ release from resealed SPM vesicles was high in the hippocampus and pons plus medulla, but low in the cerebellum. On the other hand, kyotorphin-evoked influx of45Ca2+ into unlysed synaptosomes from various brain regions was also high in the hippocampus and pons plus medulla, but low in the cerebellum. Accordingly, there was a significant positive correlationship between regional distributions of45Ca2+ release and45Ca2+ uptake (r = 0.92; Fig. 7).
Fig. 7.
Correlationship between regional distributions of kyotorphin-induced45Ca2+ release from resealed SPM vesicles and kyotorphin-induced45Ca2+ influx into unlysed in the rat brain. Kyotorphin-induced45Ca2+ release from resealed SPM vesicles was measured as in the legend of Figure5A. Kyotorphin-induced45Ca2+ influx into unlysed synaptosomes was measured in Experimental Procedures. In both experiments of 45Ca2+influx and release, 0.25 mg of protein was used for each assay. Each point of data represents the mean ± SEM from three to six separate experiments.
DISCUSSION
In addition to the accepted view that InsP3 mobilizes Ca2+ from microsomal organelles, such as rough (Henne et al., 1987) and smooth endoplasmic reticulum (Payne and Fein, 1987) and calciosome (Volpe et al., 1988), in various secretory cells (including neurons), there is growing evidence that InsP3 may have direct effects on calcium channels within the plasma membrane (for review, seeBerridge, 1993; Fasolato et al., 1994; Clapham, 1995). A family of InsP3 receptors has been identified with molecular diversity arising from both alternative splicing and separate genes (Furuichi et al., 1989; Sudhof et al., 1991; Ross et al., 1992). The immunoelectron microscopic analysis also revealed that InsP3 receptors are also found in the plasma membrane as well as in endoplasmic reticulum (ER) (Cunningham et al., 1993; Sharp et al., 1992). However, it remains unclear whether these InsP3 receptors found in different subcellular compartments are identical to one another. Most recently, findings suggest that different species of InsP3 receptors are involved in such different actions through plasma membranes or ER membranes. For example, the InsP3-induced entry of calcium in lymphocytes may be mediated by a new InsP3 receptor, which contains sialic acid and is localized in the plasma membrane (Khan et al., 1992). On the other hand, the binding protein at the plasma membrane of olfactory cells was equally sensitive to InsP3 and InsP4. By contrast, the InsP4-sensitive calcium channel in the plasma membrane of endothelial cells was insensitive to InsP3. Thus, it may be true that multiple forms of InsP3 receptor exist in various cells and that some species of such receptors are involved in calcium transport through the plasma membrane.
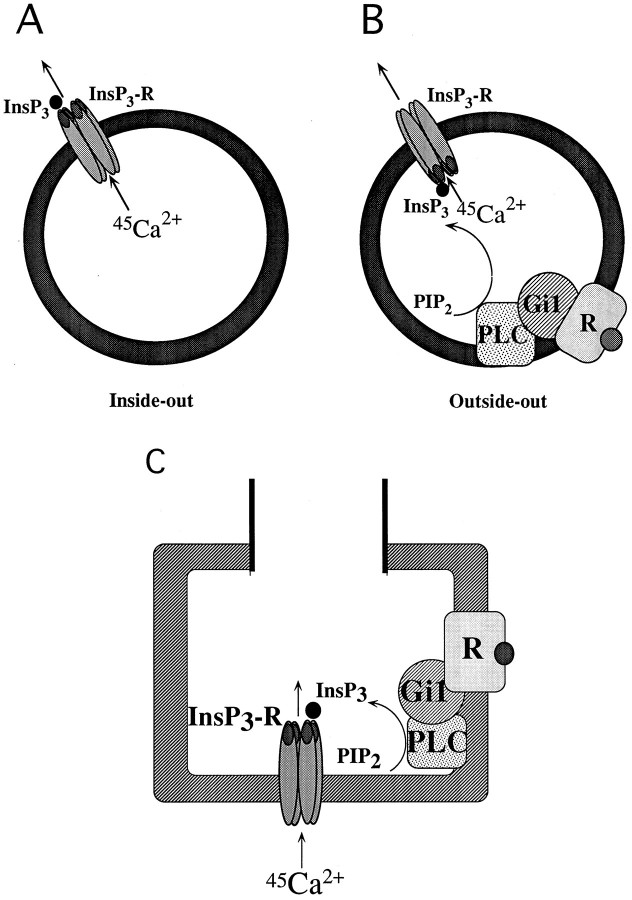
Here we demonstrated the InsP3-evoked Ca2+ transport system in the plasma membrane of nerve terminals in the brain using unique experiments with resealed vesicles. Such preparations likely have both inside-out and outside-out types of vesicles, as shown in Figure 8, Aand B. In the present experiments, the ATP-dependent incorporation of 45Ca2+ was potentiated by calmodulin (Fig. 1C). Because there is a report that Ca2+-dependent ATPase (Ca2+ pump) in plasma membranes is activated by calmodulin (Verma et al., 1989), the present finding may provide important evidence, suggesting that the resealed SPM preparations have inside-out type of vesicles. However, we have no other evidence for the existence of inside-out vesicles independent of the biochemical assays. To our knowledge, the best evidence might be obtained from the immunoelectron microscopical study using specific antibodies against membrane-associated proteins (or their peptide motives), which are intra- and extracellularly located. This should be the subject of future experiments.
Fig. 8.
Proposed model of inside-out and outside-out types of resealed vesicles and working hypothesis of presynaptic InsP3 receptor channel in plasma membranes of nerve terminals. A, Inside-out type of resealed SPM vesicles. B, Outside-out type of resealed SPM vesicles. C, In this model, there is a major calcium channel, a voltage-operated calcium channel (VOC), and a relatively minor calcium channel, InsP3 receptor, in nerve terminals involved in the Ca2+ influx. When agonist (kyotorphin) binds to the presynaptic receptor, Gi1 and PLC are activated. Produced InsP3 activates the InsP3receptor located in plasma membranes of nerve terminals, followed by gating of the calcium channel. Organelles in nerve terminals may not play important roles in the InsP3-evoked Ca2+ mobilization.
As shown in Figure 1D, the ATP-dependent incorporation of45Ca2+ in resealed SPM vesicles was much more efficiently inhibited by saponin than such an ATP-dependent incorporation into unlysed microsomes. Because saponin is well known to form micelles with cholesterol highly located in plasma membranes but not in ER (Inamitsu and Ohtsuki, 1984), it is evident that such an ATP-dependent45Ca2+ incorporation into SPM preparations is mostly attributed to that into inside-out vesicles made of plasma membranes.
One of the major findings in this report is that InsP3 plays a role in Ca2+transport through such plasma membranes. Because such effects in preparations of resealed SPM vesicles were relatively specific for InsP3, and the InsP3-evoked45Ca2+ release was saturable in kinetic analysis, it is evident that the InsP3 receptor is involved in such mechanisms. The 45Ca2+ release by InsP4 was partial in potency, whereas it shows a saturability in kinetic analysis. From the finding that theKm value for InsP4 is similar to that of InsP3, and the maximal response by InsP4 is lower than that by InsP3, it is very likely that different InsP3 and InsP4 sites exist. This view is consistent with the report using olfactory cells (Kalinoski et al., 1992), although further characterizations of InsP4-evoked45Ca2+ transport remain to be done. In Figure 3A, we showed weak effects by Ins, InsP, and InsP2 compared with those by InsP3 and InsP4. Because the concentration–response curves of InsP and InsP2 were bell-shaped, kinetic analyses of these actions could not be performed. On the other hand, Ins evoked a weak but concentration-dependent45Ca2+ release. The maximal response was 25% of InsP3 action. But it remains unclear whether this effect is attributed to the action on InsP3, InsP4, or other receptors. Further studies must be done to fully characterize these weak responses.
Throughout various subcellular fractions, the InsP3-evoked45Ca2+ release was most potent in resealed preparations using SPM. Although fractions of microsomes and myelins are expected to contain plasma membranes of neurons and glia, the InsP3 actions in such preparations were much lower than that in the synaptosomal fraction (Table 1), which is expected to contain presynaptic nerve terminals and nerve ending particles (Whittaker et al., 1964). It is evident that SPM, but not other organelles in nerve terminals (including ER), is responsible for such InsP3 actions because the InsP3-action was most potent in SPM preparations among synaptosomal subfractions (Table 1), and there was no significant InsP3-evoked45Ca2+ release in saponin-permeabilized synaptosomes where45Ca2+ had been previously taken up into intrasynaptosomal organelles through calcium pump (Fig.4C,D). The finding that the InsP3-evoked45Ca2+ release in SPM preparations was abolished by saponin treatment (Fig. 2G) also supports the view that presynaptic plasma membranes are responsible for InsP3 actions. Most recently, several mechanisms via InsP3 actions are reported to be involved in the calcium transport through plasma membranes (Fasolato et al., 1994). They are divided into two mechanisms via second messenger-operated channels (SMOC) and calcium release-activated channels (CRAC). The former mechanism is related to calcium channels directly gated by InsP3 and to those gated by InsP3 plus Ca2+, which is mobilized from ER by InsP3. The latter mechanism, on the other hand, includes the involvement of Ca2+ influx factor (CIF). However, it is unlikely that both SMOC coupled to Ca2+ mobilization from ER and CIF-regulated CRAC are involved in the present experiments, because resealed SPM vesicles are made of subfractionated membranes, where ER and CIF are expected to be absent. Thus, it is strongly suggested that InsP3 mediates Ca2+ transport via SPM in nerve terminals.
Another major finding is that such InsP3-mediated Ca2+ transport mechanisms through presynaptic plasma membranes are linked to the presynaptic receptor, which is coupled to PLC via an activation of Gi1. The present strategy using resealed vesicles has advantages in that the membrane is able to be treated with PTX and reconstituted with purified G-proteins before 45Ca2+uptake and that outside-out-type vesicles possibly exist as well as inside-out ones. Previously, we have reported that kyotorphin (tyrosine–arginine) releases methionine–enkephalin from brain slices (Takagi et al., 1979) by possible mechanisms through an increase in [Ca2+]i in brain slices or through a 45Ca2+ influx into synaptosomes (Ueda et al., 1986). After this report, we have demonstrated that kyotorphin releases45Ca2+ from such resealed vesicles of lysed synaptosomes as presented here (Ueda et al., 1987). Recently, it was revealed that kyotorphin receptor is coupled to PLC through an activation of Gi1 in reconstitution experiments (Ueda et al., 1989). From such findings, we decided to clarify the possible involvement of InsP3 in kyotorphin receptor-mediated45Ca2+ transport through SPM by reconstitution experiments. The kyotorphin-evoked45Ca2+ release was abolished in the presence of neomycin, an inhibitor of PLC (Fig.5E,F). The evidence for the G-protein involvement in kyotorphin actions was demonstrated here, as follows. (1) Kyotorphin potentiated the 45Ca2+release evoked by GppNHp, an unhydrolyzable analog of GTP (Fig.5A,B). The change was observed in the decrease ofKm value for GppNHp, which is consistent with the functional coupling between many receptors and G-proteins (Gilman, 1987). (2) The kyotorphin-evoked45Ca2+ release was abolished by PTX treatment of SPM membranes (Fig. 5C,D). (3) Such kyotorphin actions were recovered by reconstitution of PTX-treated SPM with purified Gi1, but not with Go (Fig. 6), in good accord with our previous reconstitution experiments measuring GTPase and PLC activities (Ueda et al., 1989). Here we also measured45Ca2+ influx into unlysed synaptosomes and 45Ca2+release from resealed SPM vesicles. As shown in Figure 7, the distribution of kyotorphin-evoked45Ca2+ release from resealed SPM vesicles was closely related to those of kyotorphin-mediated 45Ca2+influx. Thus, it is evident that kyotorphin receptors mediate an activation of PLC through Gi1 in such reconstitution experiments, followed by an opening of InsP3-gated calcium channels located in the plasma membrane of nerve terminals.
There are many reports suggesting that InsP3-sensitive calcium stores are present in ER and related to the hormone release in endocrine cells. In the CNS, the nerve terminal is a functional component related to neurotransmitter release. The concentration of Ca2+ in nerve terminals is closely related to the regulation of neurotransmitter release, and, hence, the receptor mechanism mediating calcium mobilization by InsP3 in nerve terminals might play an important role in the presynaptic regulation (Fig.8C).
The present study provides evidence that the receptor operation of calcium ion channel activity is mediated by InsP3 through an activation of G-protein and PLC in neuronal systems, particularly in preparations closely related to presynaptic nerve terminals.
Footnotes
Parts of this study were supported by Grants-in-Aid from the Ministry of Education, Science, and Culture of Japan, and grants from Kato Memorial Research Foundation and Pharmaceutical Research Foundation. The present study has been performed in the Department of Pharmacology, Faculty of Pharmaceutical Sciences, Kyoto University.
Correspondence should be addressed to Hiroshi Ueda, Department of Pharmacology, Faculty of Pharmaceutical Sciences, Nagasaki University, 1–14, Bunkyo-cho, Nagasaki 852, Japan.
REFERENCES
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1984;312:315–321. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 3.Clapham DE. Replenishing the stores. Nature. 1995;375:634–635. doi: 10.1038/375634a0. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft S, Gomperts BD. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985;314:534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham AM, Ryugo DK, Sharp AH, Reed RR, Snyder SH, Ronnett GV. Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience. 1993;57:339–352. doi: 10.1016/0306-4522(93)90067-p. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- 7.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 8.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels. Trends Pharmacol. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 9.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 10.Gerfen CR, Choi WC, Suh PG, Rhee SG. Phospholipase C I and II brain isozymes: immunohistochemical localization in neuronal systems in rat brain. Proc Natl Acad Sci USA. 1988;85:3208–3212. doi: 10.1073/pnas.85.9.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilman AG. G protein: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 12.Guillemette G, Balla T, Baukal AJ, Catt KJ. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988;263:4541–4548. [PubMed] [Google Scholar]
- 13.Henne V, Piiper A, Soling H-D. Inositol 1,4,5-trisphosphate and 5′-GTP induced calcium release from different intracellular pools. FEBS Lett. 1987;218:153–158. doi: 10.1016/0014-5793(87)81037-2. [DOI] [PubMed] [Google Scholar]
- 14.Inamitsu T, Ohtsuki I. Characterization of ATP-dependent Ca2+ uptake by canine brain microsomes with saponin. Eur J Biochem. 1984;145:115–121. doi: 10.1111/j.1432-1033.1984.tb08529.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalinoski DL, Aldinger SB, Boyle AG, Huque T, Marecek JF, Prostwich GD, Restrepo D. Characterization of a novel inositol 1,4,5-trisphosphate receptor in isolated olfactory cilia. Biochem J. 1992;281:449–456. doi: 10.1042/bj2810449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper CB. Biochemical distinctions between the nuclear and microsomal membranes from rat hepatocytes. J Biol Chem. 1971;246:577–581. [PubMed] [Google Scholar]
- 17.Katada T, Oinuma M, Kusakabe K, Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987;213:353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Steiner JP, Snyder SH. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci USA. 1992;89:2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi S, Somlyo AV, Somlyo AP. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988;153:625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- 20.Kuno M, Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- 21.Misawa H, Ueda H, Katada T, Ui M, Satoh M. A subtype of opioid k-receptor is coupled to inhibition of Gi1-mediated phospholipase C activity in the guinea pig cerebellum. FEBS Lett. 1995;361:106–110. doi: 10.1016/0014-5793(95)00162-3. [DOI] [PubMed] [Google Scholar]
- 22.Payne R, Fein A. Inositol 1,4,5 trisphosphate releases calcium from specialized sites within Limulus photoreceptors. J Cell Biol. 1987;104:933–937. doi: 10.1083/jcb.104.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 24.Ross CA, Danoff SK, Schell MJ, Snyder SH, Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci USA. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp AH, Snyder SH, Nigam SK. Inositol 1,4,5-trisphosphate receptors, localization in epithelial tissue. J Biol Chem. 1992;267:7444–7449. [PubMed] [Google Scholar]
- 26.Sudhof TC, Newton CL, Archer BT, Ushkaryov YA, Mignery GA. Structure of a novel InsP3receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi H, Shiomi H, Ueda H, Amano H. A novel analgesic dipeptide from bovine brain is a possible met-enkephalin releaser. Nature. 1979;282:410–412. doi: 10.1038/282410a0. [DOI] [PubMed] [Google Scholar]
- 28.Ueda H, Yoshihara Y, Takagi H. A putative met-enkephalin releaser, kyotorphin enhances intracellular Ca2+ in the synaptosomes. Biochem Biophys Res Comm. 1986;137:897–902. doi: 10.1016/0006-291x(86)91164-2. [DOI] [PubMed] [Google Scholar]
- 29.Ueda H, Fukushima N, Yoshihara Y, Takagi H. A met-enkephalin releaser, kyotorphin-induced release of plasma membrane-bound Ca2+ from rat brain synaptosomes. Brain Res. 1987;419:197–200. doi: 10.1016/0006-8993(87)90583-x. [DOI] [PubMed] [Google Scholar]
- 30.Ueda H, Yoshihara Y, Misawa H, Fukushima N, Katada T, Ui M, Takagi H, Satoh M. The kyotorphin (tyrosine-arginine) receptor and a selective reconstitution with purified Gi, measured with GTPase and phospholipase C assays. J Biol Chem. 1989;264:3732–3741. [PubMed] [Google Scholar]
- 31.Ueda H, Uno S, Harada J, Kobayashi I, Katada T, Ui M, Satoh M. Evidence for receptor-mediated inhibition of intrinsic activity of GTP-binding protein, Gi1 and Gi2, but not Go reconstitution experiments. FEBS Lett. 1990;266:178–182. doi: 10.1016/0014-5793(90)81534-u. [DOI] [PubMed] [Google Scholar]
- 32.Verity MA. Cation modulation of synaptosomal respiration. J Neurochem. 1972;19:1305–1317. doi: 10.1111/j.1471-4159.1972.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 33.Verma AK, Filoteo AG, Stanford DR, Wieben ED, Penniston JT, Strehler EE, Fischer R, Heim R, Vogel G, Mathews S, Strehler-Page M-A, James P, Vorherr T, Krebs J, Carafoli E. Complete primary structure of a human plasma membrane Ca2+pump. J Biol Chem. 1989;263:14152–14159. [PubMed] [Google Scholar]
- 34.Volpe P, Krause K-H, Hashimoto S, Zorzato F, Pozzan T, Meldolesi J, Lew DP. “Calciosome,” a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci USA. 1988;85:1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittaker VP, Michaelson IA, Kirkland RJ. The separation of synaptic vesicles from nerve-ending particles (synaptosomes). Biochem J. 1964;90:293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worley PF, Baraban JM, Supattapone S, Wilson VS, Snyder SH. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1987;263:4541–4548. [PubMed] [Google Scholar]