Abstract
The effects of emotional stressors on the release of arginine vasopressin (AVP) and oxytocin (OXT) within the rat hypothalamus and the origin and physiological significance of AVP released within the hypothalamic paraventricular nucleus (PVN) were investigated. First, adult male Wistar rats with a microdialysis probe aimed at the PVN or the supraoptic nucleus were exposed to either a dominant male rat (social defeat) or a novel cage. Release of AVP within the PVN was significantly increased in response to social defeat but not to novelty. In contrast to an activation of the hypothalamic–pituitary–adrenal (HPA) system, neither stressor stimulated the hypothalamic–neurohypophysial system (unchanged plasma AVP and OXT and unchanged release within the supraoptic nucleus [AVP] and the PVN [OXT]). Next, we demonstrated by simultaneous microdialysis of the suprachiasmatic nucleus and the PVN that AVP measured in PVN dialysates during social defeat was probably of intranuclear origin. Finally, a mixture of a V1 AVP and the α-helical corticotropin-releasing hormone (CRH) receptor antagonists administered via inverse microdialysis into the PVN caused a significant increase in the plasma adrenocorticotropic hormone (ACTH) concentration compared with vehicle-treated controls both under basal conditions and during social defeat, indicating inhibitory effects of intra-PVN-released AVP and/or CRH on HPA system activity. The antagonists failed to affect anxiety-related behavior of the animals as assessed with the elevated plus-maze. Taken together, our results show for the first time that AVP is released within the PVN in response to an emotional stressor. We hypothesize that this intranuclear release provides a negative tonus on ACTH secretion.
Keywords: microdialysis, social defeat, novelty, stress, oxytocin, HPA system, ACTH, paraventricular nucleus, supraoptic nucleus, suprachiasmatic nucleus, anxiety
Within the mammalian brain, the nonapeptides arginine vasopressin (AVP) and oxytocin (OXT) are synthesized in, transported by, and secreted from two distinct classes of neurons (Sofroniew, 1983). Magnocellular vasopressinergic and oxytocinergic neurons of the hypothalamic paraventricular (PVN), supraoptic (SON), and accessory nuclei constitute the hypothalamic–neurohypophysial system (HNS) (Hatton, 1990), whereas parvocellular vasopressinergic neurons are found in the hypothalamus within the suprachiasmatic nucleus (SCN) and the parvocellular part of the PVN (de Vries et al., 1985). The latter represents the origin of the hypothalamic–pituitary–adrenal (HPA) system as its neurons project predominantly to the external layer of the median eminence (Alonso and Assenmacher, 1981), where AVP and corticotropin-releasing hormone (CRH) are released into the portal blood to act synergistically as secretagogues of the adrenocorticotropic hormone (ACTH) at the adenohypophysis (Plotsky, 1991; Antoni, 1993; Whitnall, 1993).
In the past 30 years, evidence had been accumulated that AVP and OXT act not only as hormones in the blood circulation (Cunningham and Sawchenko, 1991), but also as neuromodulators/transmitters within the CNS (de Wied et al., 1993; Landgraf, 1995). In fact, endogenous, centrally released AVP seems to be critically involved in a variety of brain functions, including learning, memory, and emotionality (de Wied et al., 1993; Landgraf et al., 1995a; Engelmann et al., 1996). However, central effects of AVP have been revealed mainly by pharmacological approaches, which presuppose the intracerebral release of the neuropeptide. Thus, the measurement of dynamic changes in the concentration of extracellular AVP in distinct brain areas under defined experimental conditions would provide a missing link for the physiological involvement of the endogenous neuropeptide in the regulation of autonomic, endocrine, and behavioral parameters. In this context, the introduction of push-pull perfusion and microdialysis as microinvasive perfusion techniques has allowed monitoring of the release of AVP and OXT not only from nerve terminals in projection areas (Demotes-Mainard et al., 1986; Neumann and Landgraf, 1989;Landgraf et al., 1990, 1991) but also at the site of their synthesis (Neumann et al., 1993a; Landgraf, 1995; Landgraf et al., 1995b; Ludwig, 1995).
So far, however, little information is available about the intrahypothalamic release of these neuropeptides under physiological rather than pharmacological conditions. Although some authors have monitored changes in the release of OXT within the SON and PVN during parturition and in response to suckling in the lactating rat (Moos et al., 1989; Neumann et al., 1993a), there are no reports about changes in AVP release within the hypothalamus in a similar, naturally occurring and challenging situation. The present study was designed to demonstrate changes in the intrahypothalamic release of AVP in response to ethologically relevant stimuli. Because (1) central AVP seems to be critically involved in the general stress response of the organism, especially with respect to the regulation of the HPA system (van Dijk et al., 1981; Kalsbeek et al., 1992); (2) the PVN represents the hypothalamic origin of the HPA system, integrating autonomic, endocrine, and behavioral responses to stress (Kiss, 1988; Swanson, 1991); and (3) AVP is synthesized and released within this nucleus in detectable amounts (Landgraf, 1995; Ludwig, 1995), in a first series of experiments we studied AVP release within the PVN of male Wistar rats during exposure to emotional stressors (social defeat, novel cage). To evaluate the extent to which both the HPA system and the HNS were activated by emotional stress, we measured plasma ACTH, corticosterone, OXT, and AVP. In addition, we monitored the release patterns of AVP within the SON and of OXT within the PVN, which are thought to reflect the state of activity of the magnocellular nonapeptidergic system. In two additional series of experiments, we investigated possible sources of AVP detected in dialysates from the PVN and the physiological significance of intra-PVN-released AVP.
Parts of the present study have been published previously in abstract form (Wotjak et al., 1995).
MATERIALS AND METHODS
Experiments were carried out on adult male Wistar rats [320–370 gm body weight (b.w.)] that were housed in groups of six in the breeding unit of the Max Planck Institute under standard laboratory conditions (12:12 light/dark cycle with lights on at 7:00 A.M., 22 ± 1°C, 60% humidity, and food and water ad libitum) for at least 1 week after delivery from the supplier (Charles River, Sulzfeld, Germany).
Surgery
Surgery was performed under halothane (experiments 1 and 3) or urethane anesthesia (experiment 2: ethyl carbamate, 1.2 gm/kg b.w. i.p., 25% w/v solution).
Microdialysis. Before its implantation, a U-shaped microdialysis probe (dialysis membrane: molecular cutoff of 18 kDa; Hemophan, Gambro Dialysatoren, Hechingen, Germany) (for a detailed description, see Neumann et al., 1993b) was flushed and filled with sterile Ringer’s solution (147.1 mm Na+, 2.25 mm Ca2+, 4 mm K+, 155.6 mm Cl−, pH 7.4; Fresenius, Bad Homburg, Germany). The probe was then stereotaxically implanted according to the atlas of Paxinos and Watson (1986) with its tip aimed at either the right PVN (experiment 1: 1.5 mm caudal to bregma, 1.6 mm lateral to midline, 8.9 mm beneath the surface of the skull, angle of 10° to avoid sagittal sinus damage; nose: −3.5 mm) or the right SON (experiment 1: 0.6 mm caudal to bregma, 1.7 mm lateral to midline, 9.4 mm beneath the surface of the skull) or between the two PVN (experiment 3: 1.5 mm caudal to bregma, 1.3 mm lateral to midline, 8.9 mm beneath the surface of the skull, angle of 10°). In experiment 2, two microdialysis probes were lowered into the brain so that their tips reached the right SCN (1.4 mm anterior to bregma, 1.1 mm lateral to midline, 9.8 mm beneath the surface of the skull, angle of 6°, nose +5 mm) or the right PVN. The probes were secured with dental cement to two stainless steel screws inserted into the skull. Afterward, two pieces of PE-20 polyethylene tubings (adapters, 5 cm long each) were filled with Ringer’s solution, connected to the probes, and fixed with dental cement. At the end of surgery, animals received a subcutaneous injection of an antibiotic (Tardomycel, Bayer, Leverkusen, Germany) and were housed singly in polycarbon cages (23 × 39 × 36 cm3) until testing.
Jugular venous catheter. Rats were implanted with jugular venous catheters under aseptic conditions (experiments 1 and 3). The jugular vein was exposed by blunt dissection, and a small incision was made using iridectomy scissors. The catheter, consisting of a silicone tubing (3.5 cm long; Dow Corning) and a PE-50 polyethylene tubing (15 cm long), was inserted into the vein ∼3 cm in the cardial direction, ligated to the vessel, tunneled subcutaneously, and exteriorized at the neck of the animal. The cannula was filled with sterile saline containing gentamicin (30,000 IU/rat; Centravet, Bad Bentheim, Germany).
Stressors
Social defeat. This procedure, adapted with modifications from Miczek (1979), consisted of placing the experimental animal in the home cage of a dominant male resident (Wistar rats from our own breeding colony, 500–600 gm b.w., housed together with a female) that had been trained to be aggressive toward intruders. The intruder was attacked and subdued by the residents within the first 5 min of the exposure encounter. Immediately after the first attack, intruder and residents were separated by wire mesh, allowing visual and olfactory contact, but preventing further physical contact (Fig.1). Social defeat was considered successful if the intruder showed submissive body postures according to Koolhaas et al. (1980) or freezing behavior for at least 15 min during the 30 min stress exposure.
Fig. 1.
Social defeat. The experimental rat was exposed to a dominant male rat (resident, housed together with a female) for 30 min. The resident attacked the intruder rat, which subsequently showed submissive body postures. Wire mesh prevented physical contact between the intruder and the other animals and put emphasis on the emotional component of the stressor. The intruder was dialyzed during the stress exposure.
Novel cage. In this paradigm, animals were placed in a novel cage. Similar to social defeat conditions, wire mesh was inserted that restricted the experimental animal in one part of the cage.
Experiments
After surgery, rats were handled for 3 min twice a day to familiarize them with the microdialysis and blood collection procedures and to minimize nonspecific stress responses during the experiments with conscious animals. Experiments were performed between 8:00 A.M. and 2:00 P.M.
Experiment 1: effects of emotional stressors on central and peripheral release of AVP and OXT and on HPA system activity. Two days after surgery, one adapter of the microdialysis probe was connected to a microinfusion pump with a PE-20 tubing. The other adapter was equipped with a tube holder that allowed sample collection in a 1.5 ml Eppendorf tube (containing 10 μl 0.1N HCl) (Fig. 1). Microdialysis probes were initially perfused with 3.3 μl/min of sterile Ringer’s solution for 2.5 hr. During this time period, sample collection was simulated by changing the Eppendorf tubes every 30 min and discarding the dialysates. After this adaptation period, five consecutive 30 min dialysates were collected and immediately stored on dry ice. Animals were exposed to one of the two different emotional stressors (social defeat or novel cage) during the third dialysis interval.
To monitor stress-mediated alterations in endocrine parameters, plasma ACTH, corticosterone, AVP, OXT, and lactate concentrations were measured in two other groups of animals. On the fourth day after surgery, the end of the jugular venous catheter was opened and attached to extension tubing that was connected to a plastic syringe. The catheter was then flushed with sterile heparinized saline (20 IU/ml; Ratiopharm, Ulm, Germany). Thereafter, the rats remained undisturbed for 2.5–3 hr. Blood samples (0.6 ml) were taken 30 min before and then at 15, 45, and 105 min after onset of a 30 min exposure to one of the two stressors and replaced by sterile saline. Blood samples were collected in prechilled tubes containing EDTA and a protease inhibitor (10 μl Trasylol, Bayer) and centrifuged (5 min, 4000 rpm, 4°C). Plasma samples were stored at −80°C until measurement of ACTH (50 μl plasma), corticosterone (10 μl), lactate (50 μl), and AVP and OXT (160 μl).
Experiment 2: effects of stimulation of the SCN on AVP release within the PVN. After implantation of the microdialysis probes, the urethane anesthetized animals were placed on heating pads to ensure a stable body temperature of 37°C. Microdialysis probes were connected to a microinfusion pump (see experiment 1) and perfused with 3.3 μl/min of Ringer’s solution for 2 hr. Afterward, seven consecutive 30 min dialysates were collected and stored on dry ice. The SCN was locally stimulated by dialyzing with a high K+solution (56 mm in Ringer’s solution) (The concentration of Na+ was reduced to keep the osmolality of the dialysis medium in a physiological range.) during the third dialysis interval and with 1 m NaCl–hypertonic Ringer’s during the fifth dialysis interval. The PVN was simultaneously dialyzed with Ringer’s solution. The dialysis medium of the PVN was switched to hypertonic solution during the seventh dialysis interval to confirm the precise localization and proper functioning of the microdialysis probeante mortem (Neumann et al., 1993a).
Experiment 3: physiological significance of AVP released within the PVN. Four days after surgery, animals equipped with both a jugular venous catheter and a microdialysis probe placed between the two PVN were connected to a blood sampling syringe and a microinfusion pump (see experiment 1). Two hours later, the microdialysis probes were perfused with Ringer’s solution for 30 min. Thereafter, the microdialysis medium was switched to Ringer’s solution (controls) or Ringer’s solution containing a mixture of the V1 AVP receptor antagonist d(CH2)5Tyr(Me)AVP (10 μg/ml, Dr. M. Manning, Medical College of Ohio, Toledo, OH) and a CRH receptor antagonist (α-helical CRH9–41; 10 μg/ml, Sigma, Deisenhofen, Germany) by changing the tubings at the adapter, and animals were dialyzed for 30 min (Presupposing a comparable passage of the V1 or CRH receptor antagonists and AVP through the dialysis membrane, a total amount of ∼5 ng of the antagonists was delivered into the PVN area during a 30 min perfusion period.) (Engelmann et al., 1992). Rats were then exposed to social defeat for another 30 min under ongoing microdialysis with the respective dialysis medium. Immediately after the stress exposure, animals were disconnected from the microinfusion pump/blood sampling syringes and tested on the elevated plus-maze according to the procedure described by Liebsch et al. (1995). The following behavioral parameters were measured for 5 min by means of a video setup: (1) entries into open arms (ratio of open-arm entries to total number of entries into all arms); (2) time spent on the open arms (ratio of time spent on open arms to total time spent on all arms); and (3) overall activity (total number of entries into enclosed arms). At the end of the plus-maze test, rats were transferred to their home cages.
A total of four blood samples (0.2 ml) replaced by sterile saline was collected at 15, 45, 75, and 105 min after initiation of the microdialysis procedure (t = 0 min). Plasma samples were treated as in experiment 1.
Histology
Animals were killed with an overdose of halothane at the end of the experiments. Brains were removed, frozen in prechilledn-methylbutane on dry ice, and stored at −80°C. For histological verification of the probes’ placement, brains were sectioned in a cryostat, and 25 μm coronal sections were stained with cresyl violet.
Radioimmunoassays and measurement of plasma lactate
AVP and OXT content was measured in lyophilized dialysates and plasma samples after extraction by highly sensitive and selective radioimmunoassays (detection limit: 0.1 pg/sample; cross-reactivity of the antisera with other related peptides, including AVP or OXT, was <0.7%) (for a detailed description, see Landgraf et al., 1995b). Plasma ACTH and corticosterone were measured using commercially available kits (ICN Biomedicals) according to the respective protocol. Plasma lactate concentrations were measured enzymatically (MPR1 Lactat, Boehringer Mannheim, Mannheim, Germany).
Statistics
Experimental subjects were included in the statistical analysis only if (1) the microdialysis probes had been localized in the respective target brain area (Fig. 2), and/or (2) social defeat was successful (for criteria, see Stressors). The microdialysis data of experiments 1 and 2 are expressed as a percentage of averaged baseline values. All data are presented as mean ± SEM. Statistical analysis was performed with a statistical software package (GB-Stat version 5.4, Dynamic Microsystems, Silver Spring, MD). Statistical significance was determined between the groups using two-way ANOVA for repeated measures (experiment 1: Stressor × Time; experiment 3: Pharmacological Treatment × Time) and within the groups (experiments 1–3) by one-way ANOVA for repeated measures, followed by Tukey’s t test or Fisher’s lowest significant difference test, if appropriate. Plus-maze behavior was analyzed using a completely randomized one-way ANOVA (experiment 3), and the plasma lactate concentrations were compared with the paired t test (experiment 1). p < 0.05 was considered to be statistically significant.
Fig. 2.

Representative coronal sections of the rat brain showing the localization of the tip of the microdialysis probes (arrowhead) in the PVN (a), SON (b), and SCN (c), as well as between the two PVN (d).
RESULTS
Experiment 1: effects of emotional stressors on central and peripheral release of AVP and OXT and on HPA system activity
The AVP content of dialysates collected in the PVN under basal conditions was comparable in the two groups that were then exposed to either social defeat or a novel cage (0.64 ± 0.12 and 0.61 ± 0.08 pg/dialysate, respectively). With respect to the influence of the emotional stressors on AVP release within the PVN, the two-way ANOVA revealed a significant effect of both factors (Stressor:F(1,32) = 7.57, p = 0.009; Time:F(4,128) = 6.52, p < 0.001) and a significant interaction (F(4,128) = 2.51,p = 0.044). The post hoc test showed a significant difference in the AVP content of the dialysates collected during exposure to the stressors (p < 0.05). Social defeat caused an increase in AVP release within the PVN (F(4,68) = 5.73, p < 0.001, to 211 ± 32%) that was still elevated 30 min after offset of the stressor (fourth collection interval, to 185 ± 30%,p < 0.05) and returned to prestimulation values during the fifth collection interval (to 106 ± 14%, not significant). In contrast, exposure to a novel cage had no significant impact on AVP release within the PVN (F(4,60) = 0.95,p = 0.440) either during (to 123 ± 11%, not significant) or 30 min after the stress exposure (to 114 ± 16%, not significant) (Fig. 3).
Fig. 3.
Effect of emotional stress on AVP content of 30 min dialysates collected consecutively in the PVN of freely moving rats. Data are expressed as a percentage of baseline (100%,dotted line). Animals were exposed to a dominant male rat (social defeat) or to a novel cage during the third dialysis interval. +, p < 0.05 versus novel cage; *p < 0.05; **p < 0.01 versus dialysates 1 and 2.
Social defeat failed to affect either the AVP content in dialysates collected in the SON (averaged baseline value: 2.70 ± 0.86 pg/dialysate; F(4,16) = 0.46, p= 0.762) (Fig. 4a) or the OXT content of dialysates collected in the PVN (averaged baseline value: 1.36 ± 0.32 pg/dialysate; F(4,24) = 0.65,p = 0.626) (Fig. 4b).
Fig. 4.
Effect of social defeat on AVP content of dialysates collected in the SON (a;n = 5) and on OXT content of dialysates collected in the PVN (b; n = 7). Data are expressed as a percentage of baseline (100%, dotted line). Animals were exposed to social defeat during the third dialysis interval.
With respect to endocrine parameters, no significant differences were detectable between the effects of social defeat and exposure to a novel cage on plasma ACTH, corticosterone, AVP, and OXT (statistics not shown). As shown in Table 1, both exposure to social defeat and exposure to a novel cage caused an increase in plasma ACTH (F(3,15) = 3.92, p = 0.029;F(3,15) = 6.05, p = 0.036) and corticosterone (F(3,12) = 5.85,p = 0.027; F(3,12) = 3.91,p = 0.036), whereas plasma AVP (F(3,15) = 1.63, p = 0.233;F(3,15) = 1.05, p = 0.396) and OXT (F(3,15) = 0.63, p = 0.600;F(3,15) = 2.12, p = 0.130) remained almost unchanged. Plasma lactate concentrations before and during social defeat did not differ significantly (p = 0.558; Table 1).
Table 1.
Plasma concentrations of ACTH, corticosterone, AVP, OXT, and lactate in conscious male rats under basal conditions and in response to an emotional stressor
| Hormone | Stressor | Basal concentrations | Concentrations at different times after onset of the stress exposure | ||
|---|---|---|---|---|---|
| 15 min | 45 min | 105 min | |||
| ACTH (pg/ml) | Social defeat (n = 6) | 25.7 ± 2.4 | 415 ± 159* | 351 ± 258* | 106 ± 110 |
| Novel cage (n = 6) | 25.2 ± 2.4 | 164 ± 41.0† | 106 ± 49.6* | 32.9 ± 3.4 | |
| Corticosterone (ng/ml) | Social defeat (n = 5) | 9.4 ± 8.1 | 386 ± 84.0* | 346 ± 134* | 238 ± 102 |
| Novel cage (n = 5) | 4.6 ± 3.7 | 269 ± 56.0† | 197 ± 71.0* | 119 ± 88.2 | |
| AVP (pg/ml) | Social defeat (n = 6) | 5.0 ± 1.6 | 2.6 ± 0.7 | 2.1 ± 0.7 | 2.0 ± 1.0 |
| Novel cage (n = 6) | 3.2 ± 0.6 | 2.6 ± 1.1 | 2.0 ± 0.8 | 2.4 ± 0.6 | |
| OXT (pg/ml) | Social defeat (n = 6) | 11.8 ± 4.3 | 13.6 ± 3.2 | 11.9 ± 2.5 | 14.3 ± 6.3 |
| Novel cage (n = 6) | 7.9 ± 0.9 | 12.3 ± 2.8 | 9.7 ± 2.3 | 8.9 ± 2.3 | |
| Lactate (μg/ml) | Social defeat (n = 6) | 56.9 ± 2.2 | 61.7 ± 8.9 | ||
p < 0.05,
p < 0.01 versus basal.
Experiment 2: effects of stimulation of the SCN on AVP release within the PVN
AVP release within the SCN was increased during dialysis of the nucleus with either high K+ solution (to 277 ± 74%;F(3,15) = 3.608, p = 0.038; averaged baseline value: 1.23 ± 0.35 pg/sample) or 1m NaCl–hypertonic solution (to 527 ± 112%;F(6,30) = 7.61, p < 0.01; Fig.5). Similarly, dialysis of the PVN with hypertonic medium caused a significant increase in AVP release within the PVN (to 480 ± 190%; F(6,30) = 3.20,p = 0.015; averaged baseline value: 0.59 ± 0.26 pg/sample). However, stimulation of the SCN with high K+ or hypertonic medium was not accompanied by significant changes in the AVP content of dialysates collected simultaneously in the ipsilateral PVN, and PVN stimulation, in turn, had no effect on AVP release within the SCN (Fig. 5).
Fig. 5.
AVP content of consecutive 30 min dialysates collected simultaneously in the SCN and PVN of urethane-anesthetized rats (n = 6). Data are expressed as a percentage of baseline (100%, dotted line). The SCN was dialyzed with high K+ solution (56 mm) during the third dialysis interval and with 1 m NaCl–hypertonic medium during the fifth dialysis interval, whereas the PVN was dialyzed with 1m NaCl–hypertonic medium during the seventh dialysis interval. a, p < 0.05 versus dialysates 1 and 2; b, p < 0.01 versus dialysates 1, 2, 4, and 7; c,p < 0.01 versus dialysates 1, 2, 3, 4, and 7;d, p < 0.01 versus all other dialysates collected in the same region.
Experiment 3: physiological significance of AVP released within the PVN
As shown in Figure 6, basal plasma ACTH levels during dialysis with Ringer’s solution were not significantly different in the two groups of rats perfused subsequently with either the antagonists or Ringer’s solution. Changing the tubing at the adapter and continuing dialysis of the PVN with Ringer’s solution had no significant influence on the plasma ACTH concentration in the control group (increase to ∼140%; F(1,5) = 1.92, p = 0.224). In contrast, administration of the mixed V1/CRH receptor antagonists into the PVN was followed by an increase in plasma ACTH content (to ∼280%,F(1,7) = 31.33, p < 0.001), which was approximately twice as high as in the control group (Pharmacological Treatment: F(1,12) = 24.32,p < 0.001; Time: F(1,12) = 24.43, p < 0.001; Interaction:F(1,12) = 10.60, p = 0.006). The effect of the pharmacological treatment on plasma ACTH remained statistically significant throughout the experiment, i.e., during social defeat and after exposure to the elevated plus-maze (Pharmacological Treatment: F(1,12) = 5.33,p = 0.039; Time: F(2,24) = 30.40, p < 0.001; Interaction:F(2,24) = 0.88, p = 0.425).
Fig. 6.
Effect of mixed AVP (V1) and CRH receptor antagonists on basal and stress-induced plasma ACTH levels. After the rats had been dialyzed with Ringer’s solution for 30 min (white horizontal bar), the antagonists were administered directly into the PVN via inverse microdialysis (hatched horizontal bar). Animals were exposed to social defeat att = 60 min for 30 min during ongoing microdialysis with either Ringer’s solution (control group) or Ringer’s solution containing the antagonists, and then tested on the elevated plus-maze (pm) for 5 min. A total of four blood samples was taken at 15, 45, 75, and 105 min after initiation of the microdialysis procedure. The pharmacological treatment caused a significant increase in basal plasma ACTH concentration (*p < 0.01 vs blood sample 1 and vs the respective blood sample of the control group). This effect remained statistically significant during social defeat and after exposure to the elevated plus-maze.
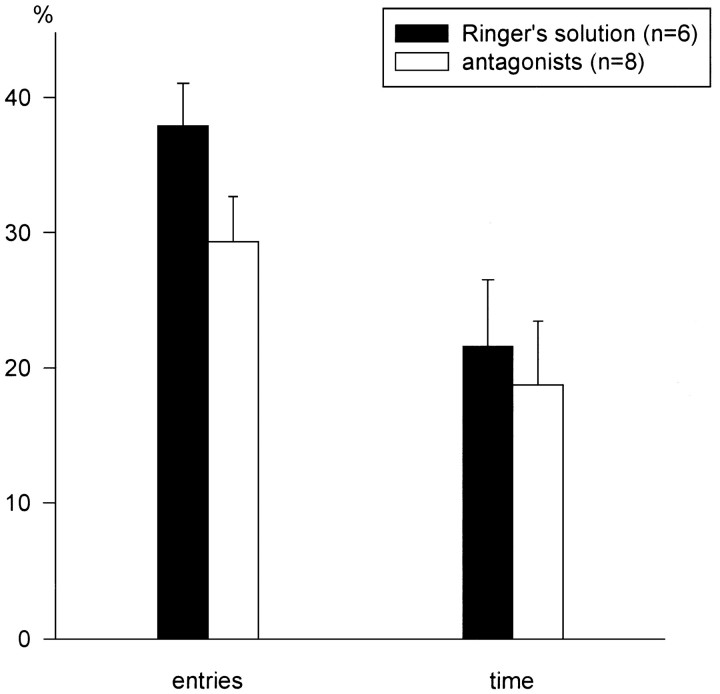
Antagonist- and vehicle-treated animals did not differ significantly in their behavior on the elevated plus-maze, as evidenced by a similar number of entries into (F(1,12) = 3.39,p = 0.090) and a similar amount of time spent on the open arms (F(1,12) = 0.17, p = 0.683) (Fig. 7). The overall activity did not differ between the groups (11.3 ± 1.10 and 9.7 ± 1.20 entries into closed arms, respectively; F(1,12) = 1.07,p = 0.319).
Fig. 7.
Lack of effects of the mixed AVP and CRH receptor antagonists administered into the PVN on rats’ anxiety-related behavior. The elevated plus-maze test was performed immediately after social defeat, as shown in Figure 6. Parameters measured: percentage of total arm entries that were entries into the open arms and percentage of total time that was spent on the open arms.
DISCUSSION
Central release of AVP and OXT
The present study demonstrates for the first time that AVP is released within the rat PVN not only after pharmacological stimulation but also in response to an ethologically relevant emotional stressor. Confrontation of the experimental animals with dominant residents and the resulting social defeat caused a significant increase in AVP release within the PVN (Fig. 3). During the exposure, the intruder and the resident rats were separated by a wire mesh screen after the first attack. Despite this barrier, ongoing threats from the residents produced a marked emotional stress in the intruder rat with a negligible physical component, which was evident in the activation of the HPA system (increased levels of plasma ACTH and corticosterone; Table 1), the freezing behavior/submissive body postures of the intruder rat (Fig. 1), and unchanged plasma lactate concentrations (Table 1). In contrast to social defeat, exposure to a novel cage failed to change the release of AVP within the PVN (Fig. 3), apparently because a novel cage is less stressful for the animals than an aggressive conspecific (Table 1). Hence, alterations in extracellular AVP concentrations might have been too small to be clearly detected in the dialysates even with a highly sensitive radioimmunoassay.
The increase in AVP release within the PVN in response to social defeat was not accompanied by similar changes either in OXT release within the same nucleus or in AVP release within the SON (Fig. 4a,b). These findings seem to correspond with unchanged plasma AVP and OXT concentrations during and after social defeat (Table 1), pointing to an unaffected HNS. In this context, it is noteworthy that AVP release into the blood was suppressed in response to emotional stressors under selected experimental conditions (Yagi, 1992). Interestingly, in the present study there was a similar tendency to reduced plasma AVP concentrations after social defeat (Table 1).
Until now, the only studies investigating the stress-induced intracerebral release of AVP and OXT reported changes in nonapeptide concentration in the CSF during strong physical/pharmacological stress (increase in AVP and OXT concentration) and in response to emotional stress (increase in OXT but not AVP concentration) (Láczi et al., 1984; Iványi et al., 1991). At first glance, the latter result seems to contradict the findings of the present study. However, a major problem in interpreting data obtained from measurements of selected neuropeptides in the CSF arises from the fact that a variety of locally occurring release patterns are “integrated” in the CSF. Moreover, because the neuropeptides diffuse from their sites of release through the extracellular space to the ventricles, where they are further diluted in the CSF, it is likely that their concentration in the CSF decreases with increases in the distance between the site of release and the site of CSF collection (Simon-Oppermann et al., 1987). Hence, stress-induced release of OXT in the brainstem, which has been assumed by Callahan et al. (1989), would be more likely to be reflected in CSF samples taken from the cisterna magna than in dialysates collected in the PVN.
Origin of AVP released within the PVN
Two findings support the hypothesis that parvocellular neurons of the SCN project to and release AVP within the PVN area. (1) Immunohistochemical studies demonstrated a vasopressinergic innervation of ventral parts of the PVN and of the dorsomedial nucleus of the hypothalamus (de Vries et al., 1985; Buijs et al., 1993), which is localized near the PVN. (2) Pharmacological and lesion studies suggested that endogenous AVP originating from the SCN might be involved in the regulation of the HPA system at the level of the PVN/dorsomedial nucleus of the hypothalamus (Kalsbeek et al., 1992). To investigate the extent to which this intrahypothalamic projection was responsible for the increase in extracellular AVP within the PVN in response to social defeat and whether AVP collected by microdialysis in this nucleus derived at least partially from AVP release within the dorsomedial nucleus of the hypothalamus, we stimulated neurons of the SCN and concomitantly monitored the AVP release within both the SCN and PVN. Although dialysis of the SCN with depolarizing agents triggered a significant increase in local AVP release, supporting the findings ofKubota et al. (1996), no similar changes could be observed in the ipsilateral PVN dialyzed simultaneously with Ringer’s solution (Fig.5, dialysates 3 and 5). Because the efferents of the SCN represent the only known vasopressinergic innervation of the PVN, the results of this experiment indicate that AVP released within the PVN, e.g., in response to social defeat, most likely originated from intranuclear sources. At present, it is impossible to determine whether AVP was released from parvocellular or magnocellular neurons. Both cell types are generally capable of releasing the nonapeptide locally as shown in the SCN (Kalsbeek et al., 1995; Kubota et al., 1996) and SON (Pow and Morris, 1989; Ludwig, 1995).
Physiological significance of AVP released within the PVN
The relatively high concentration of AVP in the extracellular fluid of the PVN, together with the expression of V1 receptors within or adjacent to this brain area (Ostrowski et al., 1994), suggests a local autocrine/paracrine action of the neuropeptide. Therefore, in a first attempt to elucidate the physiological significance of basal and stress-induced AVP release on HPA system activity, we administered a mixture of V1 and CRH receptor antagonists into the PVN and measured the effects on plasma ACTH levels and the emotionality of the animals. Because (1) CRH may be released within the hypothalamus in response to selected stimuli (Merlo Pich et al., 1993a); (2) CRH and AVP seem to interfere mutually with their intrahypothalamic release (Bernardini et al., 1994); and (3) intracerebroventricular (ICV) injections of both peptides have clear synergistic effects on the investigative behavior of rats (Elkabir et al., 1990), we included a CRH receptor antagonist, together with the V1 antagonist in the dialysis medium, to prevent possible interfering or even counter-regulating effects of CRH. The continuous administration of both antagonists increased ACTH secretion into the blood in resting animals and during stress (Fig. 6), pointing to an inhibitory effect of intra-PVN-released AVP and/or CRH. Taking into account that (1) central CRH has rather stimulatory effects on HPA system activity (Ono et al., 1985; Arnold et al., 1992), and (2) in anesthetized rats, ICV injections of an AVP antagonist (Plotsky et al., 1984), but not of a CRH antagonist (Plotsky et al., 1985), stimulated the release of CRH into portal blood, i.e., of the predominant secretagogue of ACTH under basal conditions (Whitnall, 1993) and during social defeat (Merlo Pich et al., 1993b), the effects observed in the present study are most likely attributable to the action of the V1 antagonist. Therefore, we hypothesize that AVP released within the PVN both under basal conditions and in response to an emotional stressor provides a negative tonus on the HPA system probably by inhibiting release of ACTH secretagogues from the median eminence into portal blood (Plotsky et al., 1984) and hence of ACTH from the corticotropes. This hypothesis is supported by findings of Kalsbeek et al. (1992, 1996), who demonstrated that the same V1 antagonist as used in the present study exerted a stimulatory effect on plasma concentrations of corticosterone after its administration into the area of the PVN/dorsomedial nucleus of the hypothalamus. However, the exact mechanisms by which AVP released within the PVN influences the HPA system and the site of action remain to be elucidated.
The effects of central AVP on the regulation of the HPA system are a subject of controversy. Whereas ICV injections of synthetic AVP in picogram amounts were followed by a decrease in plasma ACTH and corticosterone concentrations (van Dijk et al., 1981), opposite effects were observed after ICV injections at higher dosages (nanogram–microgram range) (van Dijk et al., 1981; Bugajski et al., 1995). A possible explanation of this phenomenon is that low amounts of synthetic AVP reach periventricular brain structures near the injection site (including the PVN) in concentrations high enough to partially inhibit ACTH release. In contrast, AVP injected in higher dosages might reach brain structures relatively far from the injection site, where it could superimpose such inhibitory effects by stimulating release of ACTH directly at the corticotropes (Antoni, 1993) or indirectly via its influence on blood pressure and heart rate (Andretta-van Leyden et al., 1990; Berecek and Swords, 1990). Compared with these pharmacological studies, the continuous administration of the antagonists directly into the PVN via inverse microdialysis might provide a more suitable approach to investigating the physiological significance of AVP released within a distinct brain region. In this context, it is of importance that the plasma ACTH levels were almost unaffected by the microdialysis procedure per se (Fig. 6, samples 1 and 2 of the control group; Table 1).
To elucidate behavioral consequences of AVP or CRH release within the PVN, we tested the rats on the elevated plus-maze after administration of the antagonists and social defeat. The mixed antagonists had no significant effects on anxiety-related behavior of the experimental animals (Fig. 7), which argues against a critical influence of intra-PVN-released AVP or CRH on the emotionality of rats. Although stimulation of the PVN might trigger release of AVP also within the septum (Neumann et al., 1988), where this neuropeptide probably exerts anxiogenic effects (Landgraf et al., 1995a), AVP released within the PVN is unlikely to be critically involved in this extrahypothalamic projection. Additionally, the results of the present study support earlier observations of a dissociation between the activity of the HPA system and the animals’ behavior on the elevated plus-maze (Merlo Pich et al., 1993b; File et al., 1994).
Conclusion
In conclusion, emotional stress produced by a confrontation with a dominant conspecific may be relayed to PVN neurons, which then respond with an increased release of AVP into the extracellular fluid of this nucleus. Once locally released, the neuropeptide could exert a negative tonus on the HPA system via inhibition of ACTH secretion from the adenohypophysis by as yet unknown mechanisms. Taking into consideration that AVP released into the portal blood might become the primary secretagogue of ACTH during chronic emotional stress (de Goeij et al., 1992), we herewith suggest that the same neuropeptide influences the activity of the HPA system in two completely different ways at the level of the PVN and the median eminence/adenohypophysis. Although the fine-tuned coordination of this regulatory pattern remains to be elucidated, it underscores the need to monitor the dynamics of AVP release into different compartments. The proposed novel mechanism of regulating ACTH secretion opens up a wide range of studies, which could include experiments investigating the effects of physical stress on AVP release within the PVN and SON, as well as the extent to which this regulatory principle is involved in coping strategies and alterations of the HPA system observed in psychiatric disorders (Holsboer, 1995;Abelson and Curtis, 1996).
Footnotes
This work was supported by Volkswagen Stiftung. M.K. is a recipient of an Overseas Research Scholar Grant of the Japanese Ministry of Education. We thank Julia Ganster and Gabriele Kohl for superb technical assistance, Dr. Maurice Manning for kindly providing the V1 antagonist, and Dr. Mario Engelmann for helpful comments on this manuscript and expert advice on statistical analysis of the data.
Correspondence should be addressed to Carsten T. Wotjak, Max Planck Institute of Psychiatry, Kraepelinstrasse 2, 80804 Munich, Germany.
REFERENCES
- 1.Abelson JL, Curtis GC. Hypothalamic-pituitary-adrenal axis activity in panic disorder: prediction of long-term outcome by pretreatment cortisol levels. Am J Psychiatry. 1996;153:69–73. doi: 10.1176/ajp.153.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G, Assenmacher I. Radioautographic studies on the neurohypophysial projections of the supraoptic and paraventricular nuclei in the rat. Cell Tissue Res. 1981;219:525–534. doi: 10.1007/BF00209991. [DOI] [PubMed] [Google Scholar]
- 3.Andretta-van Leyden S, Averill DB, Ferrario CM. Cardiovascular actions of vasopressin at the ventrolateral medulla. Hypertension [Suppl] 1990;15:I102–I106. doi: 10.1161/01.hyp.15.2_suppl.i102. [DOI] [PubMed] [Google Scholar]
- 4.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 5.Arnold FJL, de Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- 6.Berecek KH, Swords BH. Central role for vasopressin in cardiovascular regulation and the pathogenesis of hypertension. Hypertension. 1990;16:213–224. doi: 10.1161/01.hyp.16.3.213. [DOI] [PubMed] [Google Scholar]
- 7.Bernardini R, Chiarenza A, Kamilaris TC, Renaud N, Lempereur L, Demitrack L, Gold PW, Chrousos GP. In vivo and in vitro effects of arginine-vasopressin receptor antagonists on the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;60:503–508. doi: 10.1159/000126787. [DOI] [PubMed] [Google Scholar]
- 8.Bugajski J, Borycz J, Glod R, Bugajski AJ. Crowding stress impairs the pituitary-adrenocortical responsiveness to the vasopressin but not corticotropin-releasing hormone stimulation. Brain Res. 1995;681:223–228. doi: 10.1016/0006-8993(95)00297-4. [DOI] [PubMed] [Google Scholar]
- 9.Buijs RM, Markman M, Nunes-Cardoso B, Hou Y-X, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- 10.Callahan MF, Kirby RF, Cunningham JT, Eskridge-Sloop SL, Johnson AK, McCarty R, Gruber KA. Central oxytocin systems may mediate a cardiovascular response to acute stress in rats. Am J Physiol. 1989;256:H1369–H1377. doi: 10.1152/ajpheart.1989.256.5.H1369. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham ET, Jr, Sawchenko PE. Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci. 1991;14:406–411. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- 12.de Goeij DCE, Dijkstra H, Tilders FJH. Chronic psychological stress enhances vasopressin, but not corticotropin-releasing factor, in the external zone of the median eminence of male rats: relationship to subordinate status. Endocrinology. 1992;131:847–853. doi: 10.1210/endo.131.2.1322285. [DOI] [PubMed] [Google Scholar]
- 13.Demotes-Mainard J, Chauveau J, Rodriguez F, Vincent JD, Poulin DA. Septal release of vasopressin in response to osmotic and electrical stimulation in rats. Brain Res. 1986;381:314–321. doi: 10.1016/0006-8993(86)90082-x. [DOI] [PubMed] [Google Scholar]
- 14.de Vries GJ, van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 15.de Wied D, Diamant M, Fodor M. Central effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- 16.Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effect of separate or combined infusions of corticotropin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept. 1990;28:199–214. doi: 10.1016/0167-0115(90)90018-r. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann M, Ludwig M, Landgraf R. Microdialysis administration of vasopressin antagonists into the septum during pole-jumping behavior in rats. Behav Neural Biol. 1992;58:51–57. doi: 10.1016/0163-1047(92)90907-l. [DOI] [PubMed] [Google Scholar]
- 18.Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 19.File SE, Zangrossi H, Sanders FL, Mabbutt PS. Raised corticosterone in the rat after exposure to the elevated plus-maze. Psychopharmacology. 1994;113:543–546. doi: 10.1007/BF02245237. [DOI] [PubMed] [Google Scholar]
- 20.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 21.Holsboer F. Neuroendocrinology of mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 957–969. [Google Scholar]
- 22.Iványi T, Wiegant VM, de Wied D. Differential effects of emotional and physical stress on the central and peripheral secretion of neurohypophysial hormones in male rats. Life Sci. 1991;48:1309–1316. doi: 10.1016/0024-3205(91)90527-i. [DOI] [PubMed] [Google Scholar]
- 23.Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580:62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 24.Kalsbeek A, Buijs RM, Engelmann M, Wotjak CT, Landgraf R. In vivo measurement of a diurnal variation in vasopressin release in the rat suprachiasmatic nucleus. Brain Res. 1995;682:75–82. doi: 10.1016/0006-8993(95)00324-j. [DOI] [PubMed] [Google Scholar]
- 25.Kalsbeek A, van der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 26.Kiss JZ. Dynamism of chemoarchitecture in the hypothalamic paraventricular nucleus. Brain Res Bull. 1988;20:699–708. doi: 10.1016/0361-9230(88)90080-9. [DOI] [PubMed] [Google Scholar]
- 27.Koolhaas JM, Schuurman T, Wiepkema PR. The organization of intraspecific agonistic behavior in the rat. Prog Neurobiol. 1980;15:247–268. doi: 10.1016/0301-0082(80)90024-6. [DOI] [PubMed] [Google Scholar]
- 28.Kubota M, Landgraf R, Wotjak CT (1996) Release of vasopressin within the rat suprachiasmatic nucleus: no effect of a V1/V2 antagonist. NeuroReport, in press. [DOI] [PubMed]
- 29.Láczi F, Gaffori O, Fekete M, de Kloet ER, de Wied D. Levels of arginine-vasopressin in cerebrospinal fluid during passive avoidance behavior in rats. Life Sci. 1984;34:2385–2391. doi: 10.1016/0024-3205(84)90426-0. [DOI] [PubMed] [Google Scholar]
- 30.Landgraf R. Intracerebrally released vasopressin and oxytocin: measurement, mechanisms and behavioural consequences. J Neuroendocrinol. 1995;7:243–253. doi: 10.1111/j.1365-2826.1995.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 31.Landgraf R, Malkinson TJ, Veale WL, Lederis K, Pittman QJ. Vasopressin and oxytocin in rat brain in response to prostaglandin fever. Am J Physiol. 1990;259:R1056–R1062. doi: 10.1152/ajpregu.1990.259.5.R1056. [DOI] [PubMed] [Google Scholar]
- 32.Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- 33.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligonucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995a;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landgraf R, Kubota M, Holsboer F, Wotjak CT. Release of vasopressin and oxytocin within the brain and into blood: microdialysis and antisense targeting. In: Saito T, Kurokawa K, Yoshida S, editors. Neurohypophysis: recent progress of vasopressin and oxytocin research. Elsevier; Amsterdam: 1995b. pp. 243–256. [Google Scholar]
- 35.Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig M. Functional role of intrahypothalamic release of oxytocin and vasopressin: consequences and controversies. Am J Physiol. 1995;268:E537–E545. doi: 10.1152/ajpendo.1995.268.4.E537. [DOI] [PubMed] [Google Scholar]
- 37.Merlo Pich E, Koob GF, Heilig M, Menzaghi F, Vale W, Weiss F. Corticotropin-releasing factor release from the mediobasal hypothalamus of the rat as measured by microdialysis. Neuroscience. 1993a;55:695–707. doi: 10.1016/0306-4522(93)90435-i. [DOI] [PubMed] [Google Scholar]
- 38.Merlo Pich E, Heinrichs SC, Rivier C, Miczek KA, Fisher DA, Koob GF. Blockade of pituitary-adrenal axis activation induced by peripheral immunoneutralization of corticotropin-releasing factor does not affect the behavioral response to social defeat stress in rats. Psychoneuroendocrinology. 1993b;18:495–507. doi: 10.1016/0306-4530(93)90043-k. [DOI] [PubMed] [Google Scholar]
- 39.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 40.Moos F, Poulain DA, Rodriguez F, Guerné Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- 41.Neumann I, Landgraf R. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious rat during suckling. J Neuroendocrinol. 1989;1:305–308. doi: 10.1111/j.1365-2826.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 42.Neumann I, Schwarzberg H, Landgraf R. Measurement of septal release of vasopressin and oxytocin by the push-pull technique following electrical stimulation of the paraventricular nucleus of rats. Brain Res. 1988;462:181–184. doi: 10.1016/0006-8993(88)90603-8. [DOI] [PubMed] [Google Scholar]
- 43.Neumann I, Russell J, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993a;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 44.Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993b;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- 45.Ono N, Bedran de Castro JC, McCann SM. Ultrashort-loop positive feedback of corticotropin (ACTH)-releasing factor to enhance ACTH release in stress. Proc Natl Acad Sci USA. 1985;82:3528–3531. doi: 10.1073/pnas.82.10.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostrowski NL, Lolait SJ, Young WS. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 47. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1986. Academic; Sydney: Press. [DOI] [PubMed] [Google Scholar]
- 48.Plotsky P. Pathways to the secretion of adrenocorticotropin: a view from the portal. J Neuroendocrinol. 1991;3:1–9. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 49.Plotsky PM, Bruhn TO, Vale W. Central modulation of immunoreactive corticotropin-releasing factor secretion by arginine vasopressin. Endocrinology. 1984;115:1639–1641. doi: 10.1210/endo-115-4-1639. [DOI] [PubMed] [Google Scholar]
- 50.Plotsky PM, Bruhn TO, Otto S. Central modulation of immunoreactive arginine vasopressin and oxytocin secretion into the hypophysial-portal circulation by corticotropin-releasing factor. Endocrinology. 1985;116:1669–1671. doi: 10.1210/endo-116-4-1669. [DOI] [PubMed] [Google Scholar]
- 51.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 52.Simon-Oppermann C, Eriksson S, Simon E, Gray DA. Gradient of arginine vasopressin concentration but not angiotensin II concentration between cerebrospinal fluid of anterior 3rd ventricle and cisterna magna in dogs. Brain Res. 1987;424:163–168. doi: 10.1016/0006-8993(87)91206-6. [DOI] [PubMed] [Google Scholar]
- 53.Sofroniew MV. Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends Neurosci. 1983;6:467–472. [Google Scholar]
- 54.Swanson LW. Biochemical switching in hypothalamic circuits mediating responses to stress. Prog Brain Res. 1991;87:181–200. doi: 10.1016/s0079-6123(08)63052-6. [DOI] [PubMed] [Google Scholar]
- 55.van Dijk AMA, Lodewijks HMJM, van Ree JM, van Wimersma Greidanus TB. Inhibitory and stimulatory action of vasopressin on the secretion of corticotrophin in rats: structure-activity study. Life Sci. 1981;29:1107–1116. doi: 10.1016/0024-3205(81)90198-3. [DOI] [PubMed] [Google Scholar]
- 56.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 57.Wotjak CT, Kubota M, Engelmann M, Neumann I, Landgraf R. Physical and emotional stressors stimulate the release of vasopressin within the hypothalamic paraventricular nucleus of rats: a microdialysis study. Soc Neurosci Abstr. 1995;21:806.7. [Google Scholar]
- 58.Yagi K. Suppressive vasopressin response to emotional stress. Jpn J Physiol. 1992;42:681–703. doi: 10.2170/jjphysiol.42.681. [DOI] [PubMed] [Google Scholar]