Abstract
Nerve growth factor (NGF)-induced differentiation in PC12 cells is accompanied by changes in the expression of voltage-dependent Ca2+ channels. Ca2+ channels are multimeric complexes composed of at least three subunits (α1, β, and α2δ) and are involved in neuronal migration, gene expression, and neurotransmitter release. Although attempts have been undertaken to elucidate NGF regulation of Ca2+ channel expression, the changes in subunit composition of these channels during differentiation still remain uncertain. In the present study, patch-clamp recordings show that in addition to the previously documented L-type and N-type Ca2+ currents, undifferentiated PC12 cells also express an ω-agatoxin-IVA-sensitive (P/Q-type) component. In addition, the corresponding mRNA encoding the pore-forming α1 subunits for these channels (C, B, and A, respectively) was detected. Likewise, mRNA for three distinct auxiliary β subunits (1, 2, 3) were also found, β3 protein being dominantly expressed. Immunoprecipitation experiments show that the N-type Ca2+ channel is associated with either a β2 or β3 subunit and that NGF increases the channel expression without affecting its β subunit association. These results (1) indicate that the diversity of Ca2+ currents in PC12 cells arise from the expression of three distinct α1and three different β subunit genes; (2) support a model for heterogenous β subunit association of the N-type Ca2+channel in a single cell type; and (3) suggest that the regulation of the N-type Ca2+ channel during NGF-mediated differentiation involves an increase in the number of functional channels with no apparent changes in subunit composition.
Keywords: calcium channels, α1B subunit, β subunit, PC12, nerve growth factor, P/Q-type, N-type, ω-agatoxin-IVA, ω-conotoxin GVIA
Voltage-dependent Ca2+ channels play an important role in regulating cellular Ca2+ concentration in excitable cells (Tsien et al., 1995). These Ca2+channels not only are central in controlling neurotransmitter release, excitation–contraction coupling, and excitation–secretion coupling, they are also involved in gene expression and neuronal migration (Beam et al., 1992; Komuro and Rakic, 1992; Ghosh et al., 1994; Dunlap et al., 1995). Several voltage-dependent Ca2+ channels have been identified and carry out diverse functions: T-, L-, N-, P-, Q-, and R-type (Llinas et al., 1992; Zhang et al., 1993; Basarsky et al., 1994). The structure of the Ca2+ channels was elucidated with the purification of skeletal muscle L-type and brain N-type channel, which are composed of at least three protein subunits (α1, β, and α2δ) (Leung et al., 1987;Takahashi et al., 1987; McEnery et al., 1991; Witcher et al., 1993).
The α1 subunit forms the Ca2+ channel pore and binds Ca2+ channel blockers (Tanabe et al., 1987;Hockerman et al., 1995). Six different α1 genes have been identified thus far (S, A, B, C, D, and E), and with the exception of S, all are expressed in the nervous system (Birnbaumer et al., 1994). The class B α1 gene encodes for the N-type Ca2+ channel (Dubel et al., 1992; Williams et al., 1992;Witcher et al., 1993), whereas the product of class A α1gene is a component of the P/Q-type Ca2+ channel (Mori et al., 1991; Liu et al., 1996).
The β subunit directly associates with the α1 subunit (De Waard et al., 1994; Pragnell et al., 1994) and is essential for normal function and localization of the α1 subunit (Castellano et al., 1993a,b; Olcese et al., 1994; Chien et al., 1995). Similar to the multitude of the α1 subunits, at least four different β genes (1, 2, 3, and 4) have been identified (Birnbaumer et al., 1994).
Rat PC12 pheochromocytoma cell line has been a model system to study neuronal differentiation (Greene and Tischler, 1976; Shafer and Atchison, 1991; Chao, 1992). PC12 cells are chromaffin-like cells that begin to resemble sympathetic neurons when exposed to nerve growth factor (NGF). Previously, functional N- and L-type Ca2+channels were detected in PC12 cells (Usowicz et al., 1990; Avidor et al., 1994). In addition, it has been shown that NGF increases mRNA expression of several Ca2+ channel α1subunits (Lievano et al., 1994). Although in the highly heterogeneous brain, it has been reported recently that β3, β4, and β1b subunits all are capable of associating with the α1B subunit to form distinct brain N-type channels (Scott et al., 1996), it is not clear whether different β subunits can associate with the N-type channels in a single cell type. Furthermore, it is uncertain whether there is any change of the subunit composition of the Ca2+ channels during NGF-induced differentiation. To address these questions, we have investigated, at the molecular level, the expression of Ca2+ channel α1 and β subunits in PC12 cells. In addition, we have characterized the β subunit composition of the PC12 N-type Ca2+ channel during NGF-induced differentiation.
MATERIALS AND METHODS
Cell culture. PC12 cells (American Type Culture Collection, Rockville, MD) were grown on rat collagen type I (Collaborative Biomedical Products, Bedford, MA) coated dishes in RPMI 1640 medium supplemented with 5% horse serum and 10% fetal calf serum in a 5% CO2 air-humidified atmosphere. The medium was changed every 3 d, and the cells were subcultured approximately every 10 d in a ratio of 1:6. Cells were plated 1 d before the NGF (Promega, Madison, WI) addition. NGF (50 ng/ml) was added to the culture medium for a 3 d period to induce PC12 differentiation.
Patch-clamp experiments. For electrophysiological recording, undifferentiated PC12 cells were plated on poly-l-lysine-coated glass coverslips (10 × 25 mm) and grown in RPMI 1640 medium supplemented with 5% fetal bovine serum, 1% l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. After 3–5 d in culture, the cells were subjected to the standard whole-cell patch-clamp technique (Hamill et al., 1981) using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Current signals were filtered at 1 kHz (internal four-pole Bessel filter), digitized at 50 kHz, and analyzed with pClamp software (Axon Instruments). Patch pipettes were pulled from borosilicate glass capillaries (KIMAX-51; Kimble Division, Owens-Illinois, Toledo, OH) on a horizontal puller (Sutter Instrument, Novato, CA), and pipette tips were fire-polished with a microforge (Narishige, Tokyo, Japan). Typical electrode resistances were 2–5 MΩ. The bath solution contained (in mm): 20 BaCl2, 125 tetraethylammonium chloride (TEA-Cl), 10 HEPES, and 10 glucose, pH 7.3. The internal (patch-pipette) solution consisted of (in mm): 130 CsCl, 2 MgCl2, 11 EGTA, 20 HEPES, 2 Na2ATP, 0.1 GTP, and 10 glucose, pH 7.3. After establishing the whole-cell mode, capacitative transients were canceled with the amplifier. Currents were obtained by applying 150 msec test pulses every 30 sec from a holding potential of −90 mV. Leakage currents were usually less than −20 pA at holding potential. Data were leak-subtracted on line by a standard P/4 procedure. ω-agatoxin-IVA (ω-Aga-IVA, Peptides International, Louisville, KY) was prepared as a 10 μm stock in distilled water, and aliquots were stored at −20°C. A fresh aliquot was diluted in the bath solution for each experiment to give a final concentration of 200 nm. All experiments were performed at 20–22°C.
Membrane preparations and immunoblot analysis. Crude rat brain membranes and cardiac microsome membranes were prepared as detailed elsewhere (Sakamoto and Campbell, 1991). PC12 cell membranes were prepared as follows. Preconfluent PC12 cells were washed with PBS and harvested with a cell scraper. Cells were collected by centrifugation at 500 × g and homogenized in buffer A (5 mm HEPES, pH 7.4, 0.3 m sucrose, 0.1 mm phenylmethylsulfonyl fluoride, 0.75 mmbenzamidine, 0.6 μg/ml pepstatin A, 0.5 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.01 mg/ml lysozyme). Cell membranes were collected by centrifugation at 135,000 × g for 30 min, resuspended in buffer A, and stored at −80°C. PC12 and rat tissue membranes (100–250 μg of protein/lane) were resolved on 3–12% gradient SDS–polyacrylamide gels and transferred to nitrocellulose. ECL detection system (Amersham, Arlington Heights, IL) was used according to the manufacturer’s instructions.
Total RNA isolation, RT-PCR, and sequencing. Total RNA was isolated by homogenization in RNAzol according to the protocol of Cinna/Biotecx (Friendswood, TX) followed by chloroform extraction. The RNA was stored at −80°C as precipitates in 70% ethanol. To eliminate any possible contamination by genomic DNA, the total RNA was treated with DNase RQ 1 (Promega, Madison, WI) for 30 min at 37°C before reverse transcription PCR (RT-PCR).
cDNA was synthesized from 2 μg total RNA by 400 U Moloney murine leukemia virus reverse transcriptase in a total volume of 30 μl. Typically, 4 μl of cDNA was used in 50–100 μl PCR. The PCR reactions were usually carried out for 32–36 cycles of 94°C 1 min, 56°C 1 min, and 72°C 1 min. The primers used are as follows: rat β1b forward 5′-TCCAGGGACCCTACCTTGTTTCC-3′ (1391–1413, rat β1b GenBank no. X61394); rat β1breverse 5′-CCTCCAGCTCATTCTTATTGCGC-3′ (1806–1828); rat β2 forward 5′-TCGGATCCGAAGAAGAA- CCTTGTCTGG-3′ (1759–1777, GenBank no. 80545); rat β2 reverse 5′-TCGAATTCAGTAGCGATCCTTAGATTTATGC-3′ (2087–2110); rat β3 forward 5′-GTGGTGTTGGATGCTGAC-3′ (892–909, GenBank no. M88751); rat β3 reverse 5′-ATTGTGGTCATGCTCCGA-3′ (1483–1500); rat β4 forward 5′-TTGGATCCACAGCTCTCTCACCGTA- TCC-3′ (1457–1479, GenBank no. L02315), rat β4 reverse 5′-TAGGAT- CCAGGGTAGTGATCTCGGCTG-3′ (1639–1664); rat α1A forward 5′-CCAGTCTGTGGAGATGAGAGAAATGGG-3′ (6042–6068, GenBank no.M64373); rat α1A reverse 5′-TTTGGAGGGCAGGTCACCCG- ATTG-3′ (6412–6435); rat α1B forward 5′-GCCGTCTCAGCCGCG- GCCTTTCT-3′ (6668–6690, GenBank no. M92905); α1B reverse 5′-CAAAGGTGAGTGTATCCTCAGGC-3′ (6810–6832); α1Cforward 5′-GGAAGGATCCGAGCGAAAGAAGCTGGC-3′ (3064–3090 in rat α1C rbc-I, GenBank no. M67516); α1C reverse 5′-AGGTGAATTCGG- CCCACAGGCATCTCG-3′ (3307–3333 rat α1Crbc-I); α1D-1 forward 5′-GGAGAGGAGGGCAAACGAAACACTAGC-3′ (1892–1918, GenBank no. M57682); α1D-1 reverse 5′-CGTACACACCGGAACACAGAGA- CGC-3′ (2364–2388); α1D-2forward 5′-GTGCCCTGCACACAGTAGGC- GC-3′ (485–506); α1D-2 reverse 5′-GGCACGGGCAGGTCGGCT- GTTAG-3′ (816–838); α1E forward 5′-TCGGATCCAAATGTGAAG- AGGAGCGTATC-3′ (2569–2597, GenBank no. L15453), α1E reverse 5′-AGTCAAGCTTCACGTCAGGGATGGCGACTG-3′ (3262–3291).
The β1 and β3 RT-PCR products from PC12 total RNA were directly sequenced by the dideoxy chain termination method using total PCR products as sequencing templates (Barnard et al., 1994). The β1 PCR product was also subcloned into pBluescript, and its sequence was confirmed by cycle sequencing from both directions. All others were subcloned into pGEX 2T or pBluescript and sequenced by the dideoxy chain termination method according to United States Biochemicals (Cleveland, Ohio).
Enrichment of β2 subunit using β2antibody affinity column. Affinity-purified rabbit 143 (β2) antibody was coupled to Hydrazide Avidgel AX according to the manufacturer’s instructions (Unisyn Technologies, San Diego, CA). PC12 membranes (25 mg) were solubilized for 1 hr at 4°C in solubilization buffer (1% digitonin, 1 m NaCl, 10 mm HEPES, pH 7.4, 0.75 mm benzamidine, 0.6 μg/ml pepstatin A, 0.5 μg/ml aprotinin, 0.5 μg/ml leupeptin). The solubilized materials were sedimented by centrifugation in a Beckman TL 100 ultracentrifuge for 10 min at 400,000 × g. Then the supernatant was diluted eightfold with 10 mm HEPES containing the above-mentioned protease inhibitors. The diluted material (50 ml) was applied to the antibody affinity column. After washing the column extensively with 50 ml of buffer B (10 mm HEPES, pH 7.4, 0.1% digitonin, 100 mmNaCl), the column was eluted with glycine buffer (50 mmglycine, pH 2.5, 100 mm NaCl, 0.1% digitonin) containing the protease inhibitors. A 125 μl volume of 2 m Tris, pH 8.0, was added to every milliliter of eluate immediately after the elution. The eluted material was concentrated with Centricon-100 (Amicon, Beverly, WA) to 1–2 ml, and 0.1 ml per lane was loaded onto SDS-polyacrylamide gel for analysis.
Immunoprecipitation of N-type Ca2+ channels.PC12 cell membranes were solubilized and diluted as described above. The diluted extract was labeled with 50 pm[125I]ω-conotoxin GVIA (ω-CgTX) (Amersham, Arlington Heights, IL) for 1 hr at room temperature. Subsequently, aliquots (1 ml) of the labeled extract were incubated overnight at 4°C with 50 μl antibody–protein G Sepharose beads (Pharmacia, Piscataway, NJ). The beads were extensively washed, and total binding was quantified by γ counting. Nonspecific binding was determined by the addition of 1000-fold excess unlabeled ω-CgTX (Bachem, Torrance, CA) before the addition of the labeled toxin. The nonspecific counts were subtracted from total counts to give specific counts. Each sample point represents the average of triplicates.
RESULTS
Detection of P/Q-type Ca2+ channels
Ca2+ currents in undifferentiated PC12 cells consist of dihydropyridine (L-type) and ω-CgTX (N-type)-sensitive components (Usowicz et al., 1990), as well as a component that is resistant to both pharmacological agents (Rane and Pollock, 1994). Likewise, it is known that undifferentiated PC12 cells resemble normal chromaffin cells (Greene and Tischler, 1976), which in addition to N- and L-type Ca2+ currents, possess a component sensitive to the P/Q-type Ca2+ channel blocker ω-Aga-IVA (Artalejo et al., 1994; Gandia et al., 1995). To investigate the presence of P/Q-type functional Ca2+ channels, ω-Aga-IVA was applied to PC12 cells, and whole-cell Ba2+ inward currents were recorded. As described previously (Randall and Tsien, 1995), although some of the ω-Aga-IVA sensitive current is inhibited quickly, blockage increases progressively until a stable plateau is reached ∼5 min after toxin application. Therefore, to ensure a saturating degree of inhibition and to minimize the impact of the rundown on Ca2+ current, cells were preincubated for 30 min with 200 nm ω-Aga-IVA before initiating whole-cell recording. This concentration of the toxin produces maximal block of P/Q-type Ca2+ currents (Pearson et al., 1995).
The average amplitude of Ba2+ currents (IBa) in the control condition and after the preincubation with ω-Aga-IVA is plotted as a function of membrane potential (Vm) in Figure1A. In both cases, the peak current increases steeply over the range between −20 and 10 mV, reaching a maximum value at +20 mV. The shape of these current–voltage (I–V) relationships suggests that PC12 cells exhibit a large component of high-voltage-activated Ca2+ channels, whereas low-voltage-activated Ca2+ channels are virtually absent. As illustrated in the I–V curve indicated by filled circles, the preincubation with ω-Aga-IVA induced a decrease in peak current amplitude at all potentials examined.
Fig. 1.
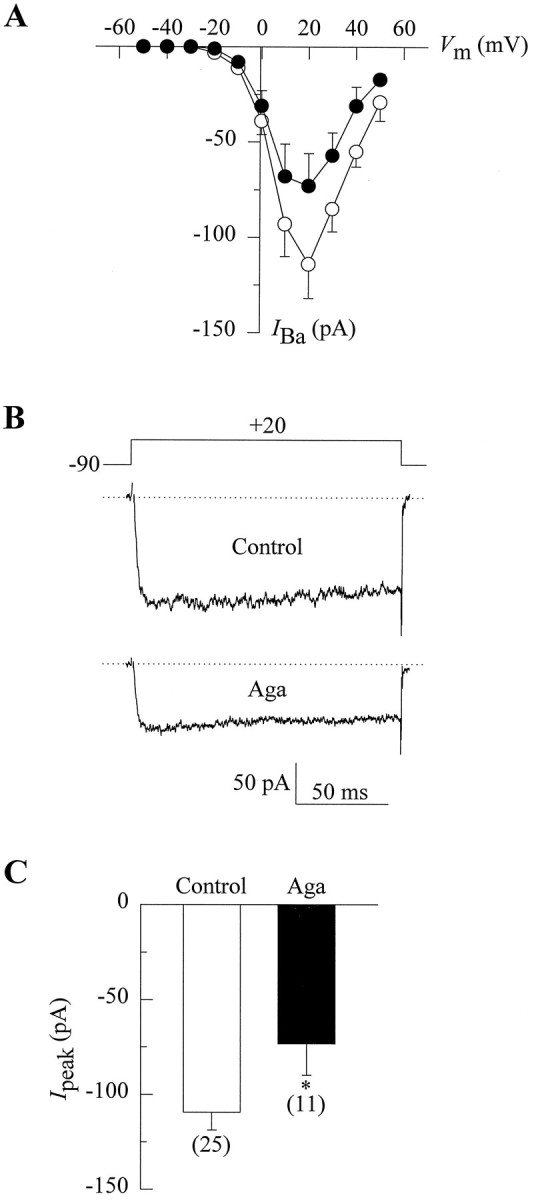
Voltage dependence of Ba2+ current in PC12 cells and its inhibition by ω-Aga-IVA. A, Peak current–voltage relationships obtained from two groups of cells after 30 min incubation in the absence (open circles) or presence of 200 nm ω-Aga-IVA (filled circles). The currents were recorded in response to 150 msec depolarization from a holding potential of −90 mV with 10 mV increase in the pulse amplitude per step. Symbols represent mean peak current values of sham preincubated (n = 9) and toxin-treated cells (n = 11). B, Illustrative traces of peak inward current from a control (upper trace) and an ω-Aga-IVA-treated (lower trace) cell. The voltage protocol is shown above the traces, and the dotted line represents baseline current.C, Comparison of peak current amplitudes at +20 mV in both control and ω-Aga-IVA-treated cells. Data are given as mean ± SE, and the number of recorded cells is indicated inparentheses. Asterisks denote significant differences (p < 0.05).
Typical Ba2+ currents from two distinct PC12 cells are shown in Figure 1B, one cell subjected to a sham preincubation (upper trace) and the other preincubated with ω-Aga-IVA (lower trace), as described above. Under the conditions of these experiments, test pulses elicited a rapidly activating inward sustained current, the average amplitude of which was significantly reduced in the toxin-preincubated cells. On average,IBa was inhibited ∼33% from a mean control value of −109 ± 9 pA to −73 ± 17 pA on treatment with ω-Aga-IVA (Fig. 1C).
To gain insight into the ω-Aga-IVA sensitive component of the whole-cell Ca2+ channel current in PC12 cells, we recorded Ba2+ currents immediately before and after 1–6 min of exposure to 200 nm ω-Aga-IVA in individual cells. The results of these experiments indicated that PC12 cells differ markedly in their response to the toxin. We found that ω-Aga-IVA was capable of blocking a fraction of Ca2+ channel currents in about one-third of the investigated cells (n = 16) over a 6 min period. The curve indicated by filled circles in Figure2A shows the inhibition of ω-Aga-IVA-sensitive current, as evidenced by a decrease in normalized total current from 1.0 to 0.62 ± 0.07 after 6 min toxin exposure. Note that under our recording conditions, IBaamplitude in control cells (open circles) decreased from 1.0 to 0.91 ± 0.02 with no detectable changes in waveform. A straight line provided a close fit to these rundown data, which were given to demonstrate the specific inhibitory effect of ω-Aga-IVA.
Fig. 2.
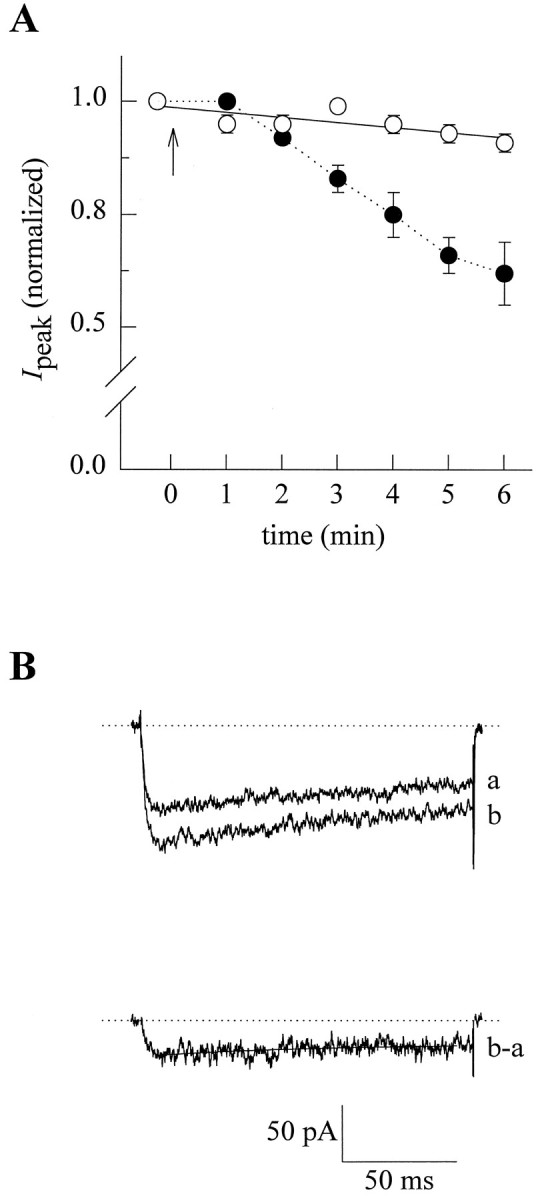
Properties of the ω-Aga-IVA-sensitive Ca2+ channel current in PC12 cells. A, Plot of peak IBa versus time in which recordings were sequentially taken before and after ω-Aga-IVA application.Filled circles represent average current blocked by the toxin (n = 5). Currents were obtained by applying 150 msec steps from −90 to +20 mV, and peak currents were normalized by the values observed before toxin application. Arrowindicates ω-Aga-IVA addition. Open circles denote time-dependent changes in the amplitude of the current (rundown) in control cells, and the straight line represents the best fit to the data. B, Representative superimposed traces of Ba2+ currents taken from one of the cells indicated inA, before (b) and 6 min after exposure to 200 nm ω-Aga-IVA (a). Lower trace exemplifies the currents (b-a) obtained by subtracting the current in the absence and in the presence of the toxin and represents the ω-Aga-IVA-sensitive component. The inactivation phase of these currents was fitted with a single exponential (solid line).
Upper traces in Figure 2B show representative currents from one ω-Aga-IVA responsive cell immediately before (b) and 6 min after (a) the application of the toxin. Subtraction of the current remained after exposure to the toxin from the current before treatment enabled us to dissect out the blocked component, as illustrated in lower trace (b-a). According to this analysis, ω-Aga-IVA-sensitive current averaged 51 ± 10 pA in amplitude and accounted for 32 ± 2% of the totalIBa. In addition, the inactivation phase of these subtracted currents were fitted with a single exponential equation of the form: Aexp(−t/τ) +C, where A is the initial amplitude (pA),t is time (msec), τ is the time constant for inactivation, and C is a constant. The application of the toxin resulted in the inhibition of a current component that exhibited a degree of decay of 17.5 ± 3.1% over the 150 msec voltage step and an average time constant (τ) of 88.7 ± 9.4 msec.
mRNA expression of multiple α1 and β subunits in PC12 cells
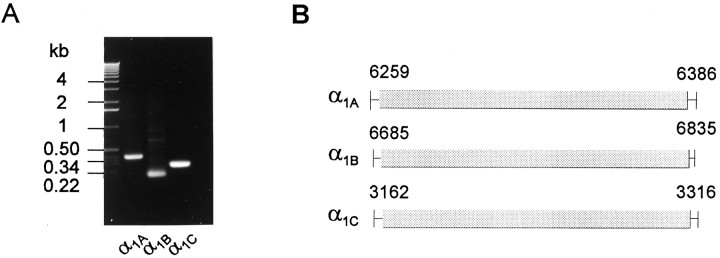
Because several different types of Ca2+ channels are present in PC12 cells, these cells should express mRNA of various α1 subunits. RT-PCR was performed on PC12 total RNA to investigate the α1 and β subunit genes expressed in these cells. PCR of total RNA, without reverse transcription, was performed as a negative control in parallel with RT-PCR (data not shown). RT-PCR with three pairs of α1 subunit primers amplified PC12 cDNA of the predicted sizes (Fig.3A). Sequencing of these PCR products confirmed the expression of three α1 subunit genes (A, B, C) in PC12 cells (Fig.3B). The sequences of the RT-PCR products are identical to nucleotide 6259–6386 of rat α1A (GenBank accession no.M64373), nucleotide 6685–6835 of rat α1B (GenBank accession no. M92905), and nucleotide 3162–3316 of a splice variant rbc-I of rat α1C (GenBank accession no. M67516) (Snutch et al., 1991), respectively. These data are in agreement with the expression of L-type (α1C), N-type (α1B), and P/Q-type (α1A) Ca2+ channels. α1D and α1E subunits were not detected using RT-PCR either because of their absence or very low level of expression.
Fig. 3.
mRNA expression of three Ca2+ channel α1 subunits in PC12 cells. A, RT-PCR from PC12 cell total RNA with α1A, α1B, and α1C primers. The PCR products were separated on a 1% agarose gel. The molecular weight standards are on theleft. B, Schematic representation of the regions of published rat α1A, α1B, and α1C rbc-I sequences, which are identical to the sequences of PC12 α1A, α1B, and α1CRT-PCR products, respectively. The numbers stand for the nucleotide positions in the published rat sequences.
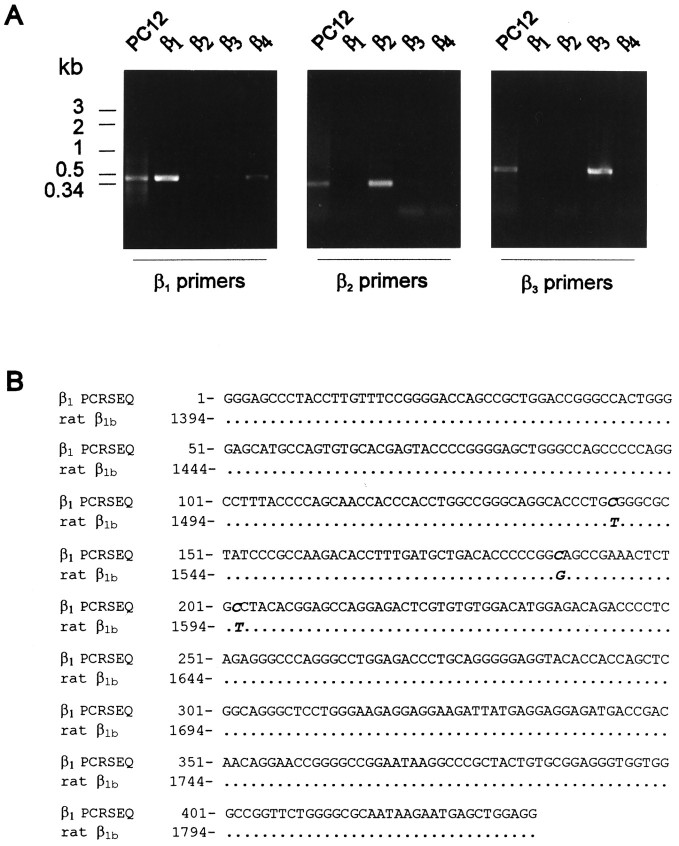
Because Ca2+ channel β subunits have two highly homologous domains, two pairs of primers that specifically amplified a portion of β2 or β3 control cDNA in PCR reactions were used to amplify PC12 cDNA (Fig.4A). PCR of PC12 cDNA with these primers amplified DNA fragments of identical molecular weights as the products of control β cDNA. The sequence of β2 RT-PCR product from PC12 cells is 100% identical to nucleotide 1759–1990 of rat β2 (GenBank accession no. M80545) in a 232-nucleotide stretch. The β3 RT-PCR product is 100% identical to nucleotide 984–1122 of rat β3 (GenBank accession no.M88751) in the 143 bases sequenced. The β1 primers efficiently amplify control β1 cDNA, although they also weakly amplify control β4 cDNA. However, no β4 sequence was identified in sequencing 14 subcloned β1 PCR products. The sequence of the β1RT-PCR product is 99% identical to rat β1b (GenBank accession no. X61394) (Pragnell et al., 1991) with only three nucleotide differences between the PCR product and the rat β1b sequence in a stretch of 435 nucleotides (Fig.4B). Two of the three single nucleotide changes (T1537→C and T1595→C) result in W492R and V511A amino acid changes. The last two different nucleotides (C188, C202) in PC12 β1 are the same as the corresponding nucleotides in human β1 sequence (Collin et al., 1993). Therefore, PC12 cells express a β1 isoform that is very similar to the β1b isoform expressed in brain. β4 was not detected using either the β1 primers or a pair of β4 primers, suggesting an absence or extremely low level of expression. These results demonstrate that PC12 cells express mRNA that encode for β1, β2, and β3 subunits.
Fig. 4.
Expression of mRNA of three Ca2+channel β subunits in PC12 cells. A, RT-PCR from PC12 cell total RNA with β1, β2, and β3 primers. Left panel, PCR products obtained from PC12 cDNA, control β1, β2, β3, and β4 cDNA with β1primers. Middle panel, PCR products amplified with β2 primers from the same DNA samples as left panel.Right panel, PCR products amplified with β3 primers from the same DNA samples as left panel.B, Alignment of β1 sequence of PC12 RT-PCR product with rat β1b sequence. The different nucleotides are in italics, and dots represent identical nucleotides.
Association of different β subunits with the α1B subunit
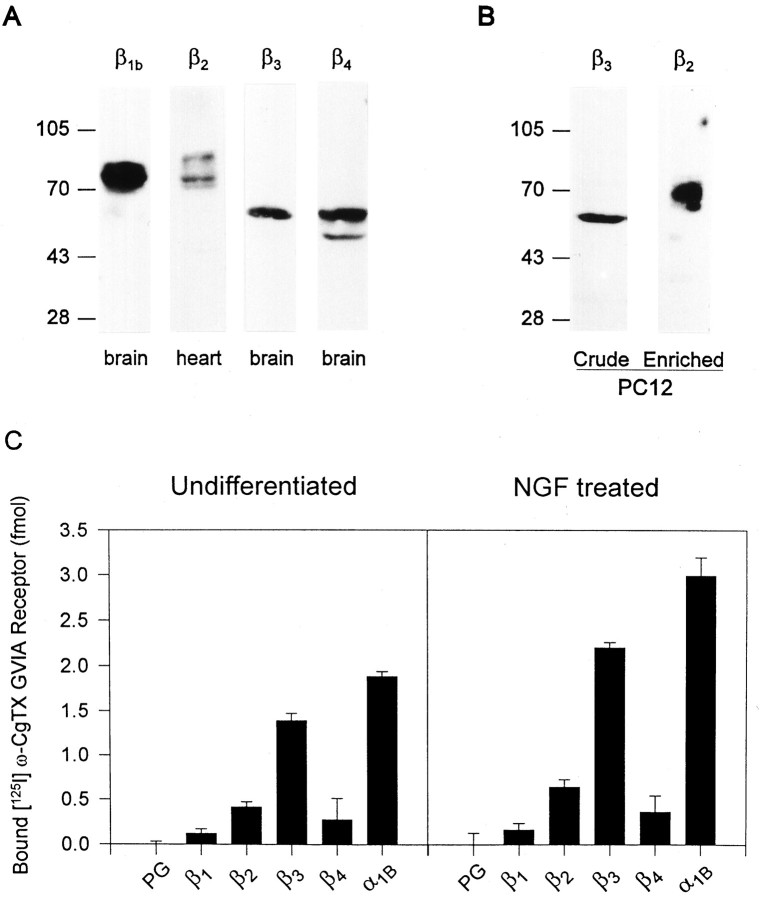
The N-type Ca2+ channel represents the most abundant Ca2+ channel in PC12 cells (Usowicz et al., 1990) and has been shown to be heterogeneous in its β subunit composition in brain (Scott et al., 1996). Because several β subunits are expressed in PC12 cells, it is important to identify the β subunit(s) associated with the N-type channel. We first examined the protein expression of these β subunits in PC12 cells. Four β subtype-specific antibodies were produced against β1b, β2, β3, and β4 subunits, respectively (Liu et al., 1996; Scott et al., 1996). These polyclonal β subunit-specific antibodies recognize a β1 subunit of 78 and 80 kDa, a β3 subunit of 58 kDa, and a β4 subunit of 59 and 55 kDa in crude rat brain membranes, and β2subunits of 87, 74, and 70 kDa in rat cardiac microsome membranes (Fig.5A). The β2 antibody only detected the 74 kDa β2 in rat brain (data not shown). These results demonstrate that the four β subunit-specific antibodies recognize native rat β subunit proteins. There is also evidence that at least four β subunits are present in rat whole brain (β1, β2, β3, and β4) and that β2 subunit is present in rat cardiac tissue. The multiple bands seen in immunoblot of β1, β2, and β4 subunit may be attributable to post-translational modification or multiple β subunit isoforms (Collin et al., 1993; Chien et al., 1995). These same four polyclonal β antibodies were used to detect the β subunits in PC12 cells. β3 subunit was easily detected from crude membranes of both undifferentiated and differentiated PC12 cells, whereas no other β subunits were ever detected (Fig. 5B). This suggests a dominant expression of β3 subunit in PC12 cells. An ∼10-fold enrichment by a β2 antibody affinity column was required to visualize the β2 subunit by immunoblot analysis. As shown in Figure 5B, a 70 kDa β2 subunit appeared in the eluate of the β2antibody affinity column. The size of this β2 subunit is comparable to the post-translational modified form of this subunit expressed in human embryonic kidney cells (Chien et al., 1995).
Fig. 5.
β3 subunit is dominantly expressed and is the major form of β subunit of the N-type Ca2+channels in PC12 cells. A, Four β subunit-specific antibodies recognize native β subunits from rat tissues. Shown are ECL immunoblots of crude rat tissue membranes stained by four affinity-purified β subunit-specific antibodies. Crude rat brain membranes were probed with polyclonal antibodies to β1, β3, and β4 subunits, and rat cardiac microsome membranes were probed with polyclonal antibodies to β2 subunit. B, Expression of β3 and β2 subunit protein in PC12 cells. Shown are an ECL immunoblot of crude PC12 membranes stained with affinity-purified β3 subunit antibody and an ECL immunoblot of the eluate from a β2 antibody affinity column stained with β2 antibody. C, Association of different β subunits with the α1Bsubunit of the PC12 N-type Ca2+ channel. Shown is the immunoprecipitation of [125I]ω-CgTX GVIA receptors by protein G (PG), four β subunit antibodies, and polyclonal antibodies against α1B subunit from undifferentiated and differentiated PC12 cells. The error bar represents the SE of three replicates.
Because expression of the α1B subunit of the N-type Ca2+ channel is upregulated by NGF (Lievano et al., 1994), we investigated whether the β subunit composition of this channel is modified by NGF treatment. To examine the interaction between α1B and β subunits in PC12 cells, the above-mentioned four β subunit-specific antibodies and polyclonal antibodies against α1B GST fusion protein (Y006) were used for immunoprecipitation of [125I]ω-CgTX-labeled N-type Ca2+ channels (Fig. 5C). Polyclonal antibodies against β3 immunoprecipitated 74 ± 4% (n = 3) of [125I]ω-CgTX labeled receptors from undifferentiated PC12 membrane extracts, followed by β2 antibody 22 ± 3% (n = 3). No immunoprecipitation was detected with protein G beads alone used as a negative control. In addition, polyclonal antibodies against β1b and β4 subunits were unable to immunoprecipitate significant amounts of labeled receptors from undifferentiated PC12 membrane extracts (<10%), which may result from either the low level of expression or the sensitivity of the methods. Therefore, the α1B subunits are associated with either β3 or β2 subunits in the N-type Ca2+ channels of undifferentiated PC12 cells.
NGF treatment induced striking morphological changes in PC12 cells. After 3 d of NGF treatment, >70% of PC12 cells in culture had grown neurites longer than the cell diameter (data not shown). Lievano et al. (1994) has reported that the upregulation of the α1B mRNA expression reached the maximum plateau as early as day 1 of NGF application. Therefore, the association of the different β subunits with the α1B subunit was examined in the 3 d NGF-treated PC12 cells (Fig. 5C). The total expression of α1B subunits per milligram of membrane protein was increased ∼59%. Notably, the polyclonal antibody against β3 immunoprecipitated 74 ± 2% (n = 3) of [125I]ω-CgTX-labeled receptors, followed by β2 antibody 21 ± 3% (n = 3). Again, polyclonal antibodies against β1b and β4 subunits immunoprecipitated negligible amounts of [125I]ω-CgTX-labeled receptors. Therefore, the same proportion of α1B subunits in both undifferentiated and differentiated PC12 cells is associated with either the β3 or β2 subunits, although the expression of N-type Ca2+ channels increased significantly after NGF treatment.
DISCUSSION
In this paper, we provide evidence for the presence of ω-Aga-IVA-sensitive Ca2+ channels in undifferentiated PC12 cells. This component of the whole-cell Ca2+ current has not been reported previously in this cell line. Our results confirm previous reports (Usowicz et al., 1990; Rane and Pollock, 1994) that describe global Ca2+ channel current in undifferentiated PC12 cells as consisting of a large component of high-voltage-activated Ca2+ current and no low-voltage-activated Ca2+current. In addition, preincubation experiments indicated that exposure to nanomolar concentrations of ω-Aga-IVA resulted in a significant decrease in average peak IBa amplitude (Fig. 1). This inhibitory effect was confirmed when IBawas sequentially recorded from patch-clamped cells before and after toxin application, particularly in cells with large Ca2+channel activity.
Sequential recording experiments also reveal that PC12 cell cultures were heterogeneous in their response to the spider toxin. This functional heterogeneity was characterized by the presence of a subset of cells apparently insensitive to ω-Aga-IVA during 6 min exposure. It is known that ω-Aga-IVA blockage of Ca2+ channel current in neurons is greatly time-dependent (Pearson et al., 1995;Randall and Tsien, 1995), thus differences in sensitivity to the toxin among PC12 cells could account for the lack of response observed in some cases: less susceptible cells could not be inhibited after short periods of toxin exposure. If this were the case, long periods of time would be necessary to recruit the entire fraction of ω-Aga-IVA-sensitive cells; however, as illustrated in Figure2A, blockage in PC12 cells is gradual, and Ca2+ channel activity runs down during continuous whole-cell recording, hampering the analysis of slow-developing effects of the toxin in individual cells. A second possibility could be that PC12 cultures were indeed composed of ω-Aga-IVA-sensitive and -insensitive cell subpopulations. The presence of subpopulations with differential expression of distinct Ca2+ channel types has been documented previously in neurons (Christenson et al., 1993) and endocrine cells (Felix et al., 1993) in culture. Likewise, a combination of both possibilities cannot be ruled out.
Taken together, these data indicate that ω-Aga-IVA partially inhibits a high-threshold Ca2+ current in undifferentiated PC12 cells and strongly suggest that a significant proportion of the current in these cells can be attributed to the activity of P/Q-type Ca2+ channels in their plasma membrane. Interestingly, when analyzed by subtraction (lower trace in Fig.2B), the waveform of the current blocked by ω-Aga-IVA showed attributes described previously for the current induced by α1A Ca2+ channel subunit expression in Xenopus oocytes (Sather et al., 1993; Stea et al., 1994; De Waard and Campbell, 1995), and it is comparable in relative magnitude and the degree of decay to the Q-type Ca2+ channel current in cerebellar neurons (Randall and Tsien, 1995). Although we have no information so far regarding the possible functional significance of the ω-Aga-IVA-sensitive current component in PC12 cells, it may be associated with neurotransmitter release as has been documented in chromaffin cells (Artalejo et al., 1994).
The presence of functional ω-Aga-IVA-sensitive Ca2+current is in agreement with the expression of the α1Asubunit mRNA in undifferentiated PC12 cells. In addition, mRNA expression of α1B and α1C (isoform rbc-I) is consistent with the presence of N-type and L-type Ca2+currents in PC12 cells, and the detection of specific receptors for125I-ω-CgTX (N-type) and [3H]PN200-110 (L-type) in the PC12 membranes (data not shown). Therefore, the expression of these different α1 subunits provides the molecular basis for the heterogeneity of Ca2+ currents in PC12 cells. Furthermore, our data demonstrate that not only several α1 subunits (α1A, α1B, and α1C) but also multiple β subunits are expressed in the undifferentiated PC12 cells. Using a sensitive RT-PCR assay, mRNA expression of three β subunits (β1, β2, and β3) was detected from PC12 cell. In contrast, control experiments from RNA without reverse transcription failed to amplify any signal, indicating that RT-PCR products were amplified from PC12 cDNA. The inability to detect β1 in immunoblot analysis is probably attributable to technical difficulty because of a low expression level of Ca2+ channel subunits. The novel finding that three α1 and three β subunits are expressed in PC12 cells suggests that multiple α1 and β subunits are likely to be expressed in other neuronal cell types, because several different types of Ca2+ currents can be recorded from many neurons such as sympathetic neurons and cerebellum granule cells (Mintz and Bean, 1993; Pearson et al., 1995; Randall and Tsien, 1995). Considering the relative uniformity of PC12 cells, it is possible that a single PC12 cell may express multiple α1and β subunits, which raises the question how these α1and β subunits are assembled in a single cell.
Protein level of the Ca2+ channel subunits in PC12 cells is very low for immunoblot analysis. However, β3 and an α2δ subunit (data not shown) can be detected on ECL immunoblot. There did not appear to be a significant change of expression level per milligram of total protein for β3 or the α2δ subunit after NGF treatment. This may be attributable to the experimental error associated with ECL immunoblot analysis, which makes it difficult to detect less than a twofold change in protein level. On the other hand, immunoprecipitation experiments showed ∼59% increase of the α1B subunit after NGF treatment. Similarly, the level of the β subunits associated with the N-type channel increased in approximately the same proportion after NGF induction.
In our study, NGF treatment did not significantly alter the β subunit composition of the N-type Ca2+ channels despite an increase in total number of N-type channels. β3 subunit remains the major β subunit of the N-type Ca2+ channel after NGF treatment. This indicates that NGF regulates the expression of different β subunits in a similar manner. Furthermore, this suggests that NGF upregulates the expression of functional N-type channels without changing the properties of the channels.
Our results indicate that either β3 or β2subunits are associated with the α1B subunits of the N-type Ca2+ channel in PC12 cells, which is in agreement with the β subunit heterogeneity of N-type Ca2+ channels observed in rabbit brain (Scott et al., 1996). Although we detected the same order of association (β3>β4>β1b) for different β subunits with the α1B subunit in rat brain tissue as in rabbit brain (data not shown), the order of association for β subunits in PC12 cells (β3>β2) is clearly different from that of mammalian brain. Whereas numerous neuronal cell types are present in brain, PC12 cells are rather homogeneous, although individual cells may differ to some extent. It is therefore important to demonstrate that in a single cell type, the α1Bsubunit is associated with either of the two β subunits. In addition, this is the first evidence that β2 subunit can associate with the N-type channel. Noticeably, the association of β3 subunit with the α1B subunit correlates with the dominant expression of this subunit in PC12 cells suggesting a role for the expression level of β subunits in determining the β subunit association with the calcium channels.
The diversity of Ca2+ channel currents in PC12 cells arise from the expression of at least three α1 subunits and three β subunits. Whereas α1 subunits determine the major properties of the current type (N, L, P/Q), different β subunits may further modify the properties of the Ca2+channels. In support of a previous finding that three of brain β subunits are associated with the N-type channel (Scott et al., 1996), at least two populations of N-type channels (α1Bβ3 and α1B β2) are present in a single PC12 cell line that may differ in their function and/or localization. However, NGF does not appear to modify the fraction of each population over the 3 d period we examined. Therefore, the properties of the N-type channels resulting from the β subunit association remain unchanged before and after NGF induced differentiation. In the future, PC12 cells can serve as a neuronal cell model for additional investigation of the regulation and function of N-, L-, and P/Q-type Ca2+ channels during development.
Footnotes
K.P.C. is an Investigator of The Howard Hughes Medical Institute. H.L. is supported by a predoctoral fellowship from the American Heart Association, Iowa Affiliate. R.F. is supported by a postdoctoral fellowship from the Human Frontier Science Program. We thank the University of Iowa DNA Core Facility for DNA sequencing.
Correspondence should be addressed to Dr. Kevin P. Campbell, The Howard Hughes Medical Institute, University of Iowa College of Medicine, 400 EMRB, Iowa City, IA 52242.
Dr. De Waard’s present address: INSERM U374, Institute Jean Roche, Marseille Cedex 20, France 13916.
Dr. De Witcher’s present address: Protein Products, Protein Biochemistry, Pioneer Hi-Bred International Inc., 7300 NW 62nd Avenue, Johnston, IA 50131-1004.
REFERENCES
- 1.Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channels trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 2.Avidor B, Avidor T, Schwartz L, De Jongh KS, Atlas D. Cardiac L-type Ca2+ channel triggers transmitter release in PC12 cells. FEBS Lett. 1994;342:209–213. doi: 10.1016/0014-5793(94)80502-4. [DOI] [PubMed] [Google Scholar]
- 3.Barnard GF, Puder M, Begum NA, Chen LB. PCR product sequencing with [α-33P] and [α-32P] dATP. Biotechniques. 1994;16:572–573. [PubMed] [Google Scholar]
- 4.Basarsky TA, Parpura V, Haydon PG. Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci. 1994;14:6402–6411. doi: 10.1523/JNEUROSCI.14-11-06402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beam KG, Adams BA, Niidome T, Numa S, Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992;360:169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated Ca2+ channels. Neuron. 1994;13:503–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 7.Castellano A, Wei XY, Birnbaumer L, Perez-Reyes E. Cloning and expression of a third calcium channel β subunit. J Biol Chem. 1993a;268:3450–3455. [PubMed] [Google Scholar]
- 8.Castellano A, Wei XY, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993b;268:12359–12366. [PubMed] [Google Scholar]
- 9.Chao MV. Growth factor signaling: where is the specificity? Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 10.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 11.Christenson J, Hill RH, Bongianni F, Grillner S. Presence of low voltage activated calcium channels distinguishes touch from pressure sensory neurons in the lamprey spinal cord. Brain Res. 1993;608:58–66. doi: 10.1016/0006-8993(93)90774-h. [DOI] [PubMed] [Google Scholar]
- 12.Collin T, Wang JJ, Nargeot J, Schwartz A. Molecular cloning of three isoforms of the L-type voltage-dependent calcium channel β subunits from normal human heart. Circ Res. 1993;72:1337–1344. doi: 10.1161/01.res.72.6.1337. [DOI] [PubMed] [Google Scholar]
- 13.De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol (Lond) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Waard M, Pragnell M, Campbell KP. Calcium channel regulation by a conserved β subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 15.Dubel SJ, Starr TVB, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the α1 subunit of an ω-conotoxin sensitive calcium channel. Proc Natl Acad Sci USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–96. [PubMed] [Google Scholar]
- 17.Felix R, Horta J, Cota G. Comparison of lactotrope subtypes of neonatal and adult male rats: plaque assays and patch clamp studies. Am J Physiol. 1993;265:E121–E127. doi: 10.1152/ajpendo.1993.265.1.E121. [DOI] [PubMed] [Google Scholar]
- 18.Gandia L, Borges R, Albillos A, Garcia AG. Multiple calcium channels subtypes in isolated rat chromaffin cells. Pflügers Arch. 1995;430:55–63. doi: 10.1007/BF00373839. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- 20.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Hockerman GH, Johnson BD, Scheuer TS, Catterall WA. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels. J Biol Chem. 1995;270:22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- 23.Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- 24.Leung AT, Imagawa T, Campbell KP. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent Ca2+ channel from rabbit skeletal muscle. J Biol Chem. 1987;262:7943–7946. [PubMed] [Google Scholar]
- 25.Liévano A, Bolden A, Horn R. Calcium channels in excitable cells: divergent genotypic and phenotypic expression of α1-subunits. Am J Physiol. 1994;267:C411–C424. doi: 10.1152/ajpcell.1994.267.2.C411. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, De Waard M, Scott VES, Gurnett CA, Lennon VA, Campbell KP. Identification of three subunits of the high affinity ω-conotoxin MVIIC sensitive Ca2+ channels. J Biol Chem. 1996;271:13804–13810. [PubMed] [Google Scholar]
- 27.Llinas R, Sugimori M, Hillman DE, Cherksey B. Distribution and functional significance of the P-type voltage-dependent Ca2+ channels in the mammalian nervous system. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 28.McEnery MW, Snowman AM, Sharp AH, Adams ME, Snyder SH. Purified ω-conotoxin GVIA receptor of rat brain resembles a dihydropyridine-sensitive L-type calcium channel. Proc Natl Acad Sci USA. 1991;88:11095–11099. doi: 10.1073/pnas.88.24.11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintz IM, Bean B. Block of calcium channels in rat neurons by synthetic ω-Aga-IVA. Neuropharmacology. 1993;32:1161–1169. doi: 10.1016/0028-3908(93)90010-z. [DOI] [PubMed] [Google Scholar]
- 30.Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 31.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel β subunit sets rates of channel inactivation independently of the subunit’s effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 32.Pearson HA, Sutton KG, Scott RH, Dolphin AC. Characterization of Ca2+ channel currents in cultured rat cerebellar granule neurones. J Physiol (Lond) 1995;482:493–509. doi: 10.1113/jphysiol.1995.sp020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pragnell M, Sakamoto J, Jay SD, Campbell KP. Cloning and tissue specific expression of the brain calcium channel β subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 34.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1 subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 35.Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rane SG, Pollock JD. Fibroblast growth factor-induced increases in calcium currents in the PC12 pheochromocytoma cell line are tyrosine phosphorylation dependent. J Neurosci Res. 1994;38:590–598. doi: 10.1002/jnr.490380511. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto J, Campbell KP. A monoclonal antibody to the β subunit of the skeletal muscle dihydropyridine receptor immunoprecipitates the brain ω-conotoxin GVIA receptor. J Biol Chem. 1991;266:18914–18919. [PubMed] [Google Scholar]
- 38.Sather WA, Tanabe T, Zhang J-F, Mori Y, Adams ME, Tsien RW. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel α1 subunits. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 39.Scott VES, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, Campbell KP. β subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- 40.Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–492. [PubMed] [Google Scholar]
- 41.Snutch TP, Tomlinson WJ, Leonard JP, Gilbert MM. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 42.Stea A, Tomlinson J, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain α1 calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi M, Seagar MJ, Jones JF, Reber BFX, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanabe TH, Takeshima A, Mikami V, Flockerzi V, Takahashi K, Kangawa M, Kojima H, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 45.Tsien RW, Lipscombe D, Madison D, Bley K, Fox A. Reflections on Ca2+-channel diversity, 1988–1994. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 46.Usowicz MM, Porzig H, Becker C, Reuter H. Differential expression by nerve growth factor of two types of Ca2+ channels in rat phaeochromocytoma cell lines. J Physiol (Lond) 1990;426:95–116. doi: 10.1113/jphysiol.1990.sp018128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an ω-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 48.Witcher DR, DeWaard M, Sakamoto J, Franzini-Armstrong C, Pragnell M, Kahl SD, Campbell KP. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J-F, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]