Abstract
The role of the mesolimbic dopaminergic system in the reinforcement of learning suggests that dopamine should be able to modulate activity-dependent synaptic plasticity. We have examined the effect of D1/D5 agonists on early long-term potentiation (LTP) (40 min) in the CA1 region of hippocampal slices. D1/D5 agonists (+)bromo-APB, 6-chloro-PB, and dihydrexidine increased the magnitude of LTP in a synapse-specific manner (by ∼10, 15, and 20%, respectively). This D1/D5 effect was mimicked by a low dose (10 μm) of the adenylyl cyclase activator forskolin. The D1/D5 antagonist (+)SCH 23390 reduced early LTP. In catecholamine-depleted slices, LTP was smaller by ∼20-25% and could not be decreased further by D1/D5 antagonist. Under these conditions, D1/D5 agonist 6-chloro-PB and forskolin produced a larger enhancement of LTP (20–25%), restoring it to the control level. At the same dose, dideoxyforskolin did not affect early LTP. The D1/D5 agonist effect was completely blocked by the D1/D5 antagonist (+)SCH 23390. These results indicate that dopamine produces a synapse-specific enhancement of early LTP through D1/D5 receptors and cAMP.
Keywords: CA1, cAMP, catecholamine depletion, D1/D5 dopamine receptors, early LTP, field EPSP, forskolin, hippocampus
Substantial progress is being made in understanding the mechanisms of activity-dependent synaptic plasticity and its role in learning and memory (for review, see Nicoll and Malenka, 1995). An important aspect of this problem is the effect of the ascending neuromodulatory systems on plasticity. These modulatory inputs have been severed from the slice preparation, but their role can be examined by direct application of the modulator to the slice. Recent works point to the importance of neuromodulators in long-term potentiation (LTP) in different subregions of the hippocampus (Dunwiddie et al., 1992; Burgard et al., 1993; Villani and Johnston, 1993; Auerbach and Segal, 1994; Dahl and Li, 1994; Maeda et al., 1994). Most dramatically, cholinergic modulation powerfully gates synaptic plasticity and changes the rules of synaptic modification in the CA1 region (Huerta and Lisman, 1995, 1996).
Several lines of evidence suggest that dopamine is likely to modulate synaptic plasticity. The mesolimbic dopaminergic system lies at the core of the brain reward mechanisms involved in electrical self-stimulation, place conditioning, intracranial drug self-application, and natural reinforcement (Cooper, 1991; Wise, 1996). This system seems to provide the feedback that allows the generation of activity patterns that lead to satisfaction of appetitive needs, and it plays an important role in addictive behaviors. The evidence for this has been strengthened by recent studies of the brainstem dopaminergic neurons. In unconditioned animals, these cells fire when food or juice is given. In animals conditioned with a tone that precedes food, the cells fire when tone is given rather than when the food is given (Schultz et al., 1993). Theoretical models show how the underlying changes in information flow could be produced by an effect of dopamine on activity-dependent synaptic potentiation (Sutton and Barto, 1981;Friston et al., 1994; Montague et al., 1996).
The available physiological evidence provides some support for a role of dopamine in synaptic modification, particularly in long-term depression in the striatum (Calabresi et al., 1992) and hippocampus (Chen et al., 1995). The only report for a role in synaptic potentiation comes from work on the CA1 region of hippocampus. Frey and coworkers have demonstrated that blockade of either D2 or D1 receptor decreases the magnitude of late phases of LTP, 2 hr and more after the induction (Frey et al., 1990, 1991). This late phase seems to involve the effects of cAMP on protein synthesis and glycoprotein fucosylation (Angenstein et al., 1992; Frey et al., 1993). Perfusion of the slice with high concentrations (50–100 μm) of D1/D5 agonists without any tetanus can itself imitate the late phases of LTP, an effect that is blocked by inhibitors of protein synthesis (Huang and Kandel, 1995). Importantly, this late synaptic enhancement occurs without strong synaptic stimulation, suggesting that this form of potentiation is not activity-dependent or synapse- specific.
We have been prompted to examine the role of dopamine in the early stages of LTP, because theory indicates that what is needed to produce behavioral modifications are iterative synaptic modifications in which the changes in one set of synapses influence subsequent changes in other synapses. Both theory (Montague et al., 1996) and experiment (Shultz et al., 1993) show that conditioned behavior changes with each trial. Because trials were spaced only seconds apart, the underlying synaptic modifications must develop on this time scale. Furthermore, theory suggests that the changes must be activity-dependent and synapse-specific. It was therefore of interest to study the effect of dopamine on early LTP, a rapidly developing, activity-dependent, synapse-specific modification. We have concentrated our initial efforts on D1/D5 dopamine receptors for several reasons. First, D1/D5 receptors seem to be implicated more strongly in the mechanisms of reinforcement (Cooper, 1991). Second, they increase cAMP (Kebabian and Calne, 1979;Kimura et al., 1995), which is important for early LTP (Blitzer et al., 1995). Third, D1/D5 receptors are positioned on neuronal spines, a location strategically advantageous for affecting synaptic plasticity (Huang et al., 1992; Smiley et al., 1994).
MATERIALS AND METHODS
Transverse hippocampal slices (400 μm thick) were prepared from 17- to 25-d-old Long–Evans rats. During the experiment, slices were placed on a nylon net and perfused on both sides with artificial cerebrospinal fluid (ACSF) using a pump with a flow rate of 1.5–2.25 ml/min. ACSF contained (in mm): 120 NaCl, 26 NaHCO4, 1 NaH2PO4, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, and 10 d-glucose. Before entry into the recording chamber, ACSF was saturated with the gas mixture of 95% oxygen/5% carbon dioxide and heated to 29.2–30.2°C.
A glass recording electrode filled with ACSF (r = 0.2–0.3 MΩ) was placed in stratum radiatum of the CA1 region. To stimulate independent inputs to the same cell population, two monopolar stimulating electrodes (glass pipettes filled with ACSF;r = 0.25–0.35 MΩ) were positioned on both sides of the recording electrode ∼200-250 μm apart from each other. Experiments were controlled by PC through LM-900 interface (Dagan Corporation, Minneapolis, MN) using a custom-made AXOBASIC program. The strength of stimuli was 50–60% of the population spike threshold for both test and tetanic stimulation. The tetanus consisted of 10 bursts of four stimuli (100 Hz), with 30 msec intervals between bursts, and lasted 0.6 sec altogether. Test stimulation alternated between two stimulating electrodes throughout the experiment at constant frequency (0.1 Hz). After a stable baseline (15–30 min) was established, LTP was induced by single tetanus and observed for 40 min after the tetanus.
R(+)- and S(−)-6-bromo-APB hydrobromide (bromo-APB) and R(+)SCH 23390 and S(−)SCH 23388 were a gift from the National Institute of Mental Health Chemical Synthesis Program at RBI (Natick, MA). Dihydrexidine was provided for this study by Interneuron Pharmaceuticals (Lexington, MA), and A-77636 was a donation from Abbott Laboratories (Abbott Park, IL). All other dopaminergic drugs, water soluble analog 7β-deacetyl-7β-[γ-(morpholino) butyryl]-forskolin, hydrochloride, and 1,9-dideoxy-forskolin were purchased from RBI. 7β-Deacetyl-7β-[γ-(morpholino) butyryl]-forskolin was chosen for this study because water-soluble analogs display fewer side effects than forskolin on glucose transport and nicotinic receptors (Laurenza et al., 1989).
The depletion of catecholamines was achieved by a recently described two-step procedure (O’Donnell and Grace, 1993). Rats were injected with reserpine (5 mg/kg, s.c.) 24 hr before the experiment to cause the depletion of catecholamine stores. Then, at least 1 hr before the experiment, 100 μm of tyrosine hydroxylase inhibitordl-α-methyl-π-tyrosine methyl ester hydrochloride was added to ACSF to block new synthesis of dopamine and noradrenaline. The inhibitor was present starting with incubation time and throughout the experiment. When control and depleted animals were compared, experiments were performed within 2 weeks under the same conditions on animals of the same age and, where possible, from the same litters.
In most cases, drugs were dissolved in the ACSF for a stock solution. Ascorbic acid (0.02%) was added to a stock solution of dihydrexidine. Water-insoluble bromo-APB and 1,9-dideoxy-forskolin were initially dissolved in dimethylsulfoxide (DMSO) so that the final concentration of DMSO during perfusion did not exceed 0.02%. In this case, in the control experiment tetanus was applied after 5 min perfusion with 0.02% DMSO in ACSF. In our experience, most D1/D5 agonists in stock solutions appeared to loose activity slowly, even when stored at −20°C, so we preferred not to use drugs that were stored in the freeze for >2-3 weeks.
All drugs solutions in ACSF were prepared immediately before each experiment in small volumes (25 ml) from frozen stocks, oxygenated in the separate reservoir, and delivered in the perfusion media for a short time (5–10 min). Only one drug application was performed per slice. Model experiments with methylene blue (50–200 μm) showed that dye was reaching the recording chamber within 12–15 sec after the start of the perfusion and was washed out within 1.5–2.5 min after it was switched off.
For statistical analysis, responses were first collected and averaged in 5 min blocks: 15 min of baseline and 40 min after the tetanus. Field EPSP (fEPSP) slope (mV/msec) and fiber volley amplitude (mV) were calculated, and data for each experiment were normalized relative to baseline. Having “control” and “drug-affected” LTP in each experiment (see Fig. 1) allowed us to analyze normalized results of individual experiments for each drug using two-way ANOVA for repeated measurements (df = 1 for drug and df = 7 for time after the tetanus factors) and a paired t test for means as apost hoc criterion in the EXCEL program package. For some graphic presentations (Figs. 2, 4, 5, and 7), data were collected and averaged in 1 min blocks. All figures show means ± SEM.
Fig. 1.
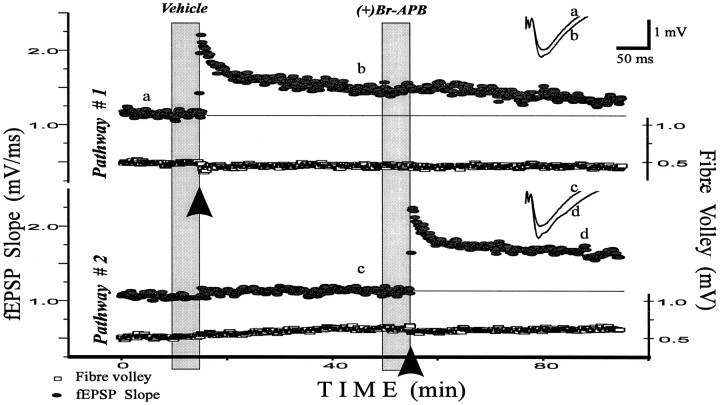
D1/D5 agonist (+)bromo-APB facilitates early LTP of fEPSP in CA1 hippocampal region. This is an example of the general experimental design used in this paper. Both pathways are alternately stimulated at 0.1 Hz. First, LTP was evoked by a tetanus onPathway #1 in control ACSF [0.022% DMSO solution in ACSF was applied before this tetanus, because it served as a vehicle for a subsequent (+)bromo-APB application ((+)Br-APB)]. Thirty-five minutes later, the D1/D5 agonist (+)bromo-APB (5 μm) was perfused for 5 min, and a tetanus was then given on Pathway #2. The potentiation was larger than in control. Inserts compare fEPSPs before and 30 min after the tetanus in control (a, b) and after drug application (c, d). Application time is shown by filled vertical columns; tetani are indicated by thearrows. We randomly varied whether control or test substances were given first. The amplitude of the fiber volley (Fibre volley, lower traces in pairs), an indicator of the number of axons stimulated, was not affected by vehicle or drugs.
Fig. 2.
D1/D5 dopamine agonist (+)bromo-APB increases early LTP, whereas inactive enantiomer (−)bromo-APB is ineffective. For graphic presentation, fEPSPs were collected in 1 min blocks; maximal slopes were calculated and then normalized relative to the baseline average 15 min before the tetanus. The time of drug application is marked by the horizontal bar, and the moment of tetanus is indicated by the small arrow. Only averaged data (mean ± SEM) are presented. A, Summary of five experiments comparing the magnitude of LTP on the same slices with vehicle (one pathway) and after the perfusion of active enantiomer of D1/D5 dopamine agonist (+)Bromo-APB (5 μm). B, Inactive enantiomer of D1/D5 dopamine agonist (−)Bromo-APB does not affect early LTP induction. Averaged data (n = 4).
Fig. 4.
D1/D5 dopamine antagonist (+)SCH 23390 decreases early LTP in control slices, whereas inactive enantiomer (−)SCH 23388 is ineffective. Calculation procedures and markings as in Figure 2.A, Summary of seven similar experiments comparing the magnitude of LTP on the same slices in control conditions and after the perfusion of an active enantiomer of the antagonist (+)SCH 23390 (5 μm). B, Inactive enantiomer of D1/D5 dopamine antagonist (−)SCH 23388does not affect early LTP (n = 4).
Fig. 5.
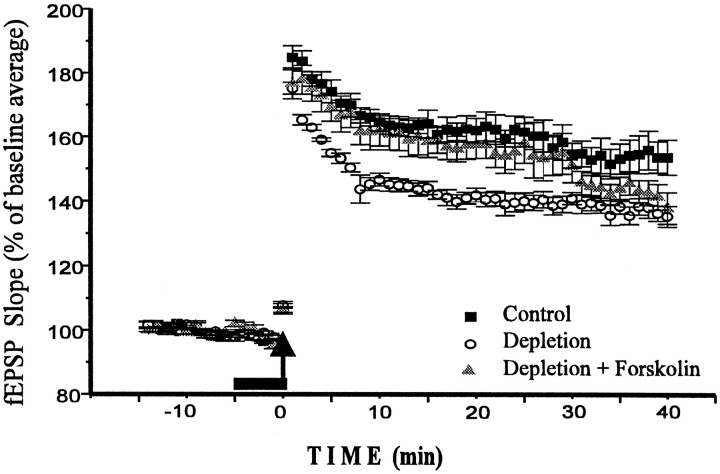
Catecholamine depletion decreases LTP. Forskolin application restores LTP to control level. Calculations and markings are as in Figure 2. Note that Depletion andDepletion + Forskolin experiments were performed on the same slice (n = 5), whereas Controlwas taken from nondepleted rats of the same age and carried out under the same conditions (n = 5). All experiments were performed within a 1.5 week period without interruption by other experiments. We did not note any differences in slice condition, fEPSP threshold or amplitude, or population spike threshold between control and depleted slices.
Fig. 7.
In depleted slices, D1/D5 agonist 6-chloro-PB substantially increases early LTP. The D1/D5 antagonist (+)SCH 23390 blocks the agonist effect. Averaged data (mean ± SEM). Calculations and markings are as in Figure 2. A, The D1/D5 agonist 6-chloro-PB (6-Cl-PB), 10 μm, facilitates early LTP on depleted slices (n = 4). B, The D1/D5 antagonist (+)SCH 23390 (+SCH 23390), 5 μm(gray bar), blocks 6-chloro-PB-dependent increase in early LTP (n = 4).
RESULTS
Effect of D1/D5 agonists on early LTP
Recordings of the fEPSPs were made in the stratum radiatum of the CA1 region in response to the stimulation of two independent pathways. Figure 1 shows a representative experiment with D1/D5 agonist application and illustrates the protocol used throughout this study. A tetanus was given to one pathway after a control solution was present for 5 min. The tetanus induced LTP in this pathway; 35 min later, (+)bromo-APB was applied. No effect on the baseline responses was observed for this or any other drug used in this study. Five minutes after (+)bromo-APB application, a tetanus was given to the second pathway. This caused LTP in this pathway and no change in the other pathway. None of the drugs affected the amplitude of the fiber volley, an indicator of axon excitability. The order of drug and control presentation was alternated in replicates of the same experiment. The mean magnitude of control LTP did not depend on whether it was induced by the first or the second tetanus.
In Figure 2A, the responses of the two pathways have been normalized to the baseline average (15 min before the tetanus) and summarized for five similar experiments. It can be seen that LTP was larger in the presence of (+)bromo-APB than in control. LTP was increased by 5–13% in different time intervals after LTP induction (F = 21.735; p < 0.001; Figs. 2B, 3). This effect was stereospecific, because the same dose of inactive enantiomer, (−)bromo-APB, did not affect the LTP (n = 4;F = 0.008; p > 0.977; Fig.2B). Two other D1/D5 agonists, 6-chloro-PB, 10 μm (n = 5; F = 12.337;p < 0.001), and dihydrexidine, 10 μm(n = 5; F = 13.114; p< 0.001), had larger effects, ranging between 12 and 22% (Fig. 3). The effect of A-77636, a new D1/D5 agonist, was not statistically significant at 5 μm (n = 4;F = 0.560; p > 0.45); a higher dose was not tested.
Fig. 3.
Other D1/D5 agonists and the adenylyl cyclase stimulator forskolin also enhance early LTP. All drugs were applied for 5 min before the tetanus and washed out immediately after.Columns represent the results of post hocpaired t test after two-way ANOVA for repeated measurements (% of LTP after drug application minus % of LTP in control input on the same slice) for the baseline and four 5 min periods after the tetanus. The time of drug application is marked by the horizontal bar, and the moment of tetanus is indicated by the small arrow. F,p, and n values for each drug are listed in Results. Levels of significance:○p < 0.1; *p < 0.05; **p < 0.01.
Effect of D1/D5 antagonists
Previous research (Frey et al., 1990) demonstrated that endogenous dopamine in the slice is released during the tetanization. If this dopamine can enhance the early stages of LTP through D1/D5 receptors, then dopamine antagonists should decrease the magnitude of early LTP. Figure 4 shows this to be the case. The D1/D5 antagonist (+)SCH 23390 at the dose 5 μm decreased early LTP by 5–12%. Figure 4A shows the average of all experiments (n = 7; F = 8.822;p < 0.004). Inactive enantiomer of the antagonist (−)SCH 23388 at the same dose did not produce any significant effect (n = 4; F = 0.1563; Fig.4B).
Forskolin also increases early LTP
The main intracellular action of the D1 receptor family is to increase cAMP production (Kebabian and Calne, 1979; Kimura et al., 1995). It was therefore of interest to determine whether early LTP could be increased by forskolin, a direct activator of adenylyl cyclase. As with dopaminergic drugs, forskolin was applied 5 min before a tetanus, and washout started immediately after the tetanus. For our experiments we deliberately chose a concentration of forskolin (10 μm) that does not affect the baseline synaptic response or membrane excitability (Dunwiddie et al., 1992; Chaves-Noriega and Stevens, 1992). We found that forskolin affected neither the fEPSP nor the fiber volley evoked by 0.1 Hz test stimuli. As Figure 3 shows, however, forskolin was as effective as D1/D5 agonists in increasing early LTP (n = 4; F = 22.822; p < 0.001).
Effects of catecholamine depletion
The reduction of LTP by D1/D5 antagonist suggests that dopamine contained within the slice may be released during a tetanus and may enhance early LTP. If this is the case, procedures that deplete endogenous dopamine levels should depress LTP. To reduce endogenous dopamine, we used the catecholamine depletion method developed byO’Donnell and Grace (1993). Comparison of LTP in control and depleted slices from animals of the same age (Fig. 5) shows a substantial (∼20-25%) decrease of the magnitude of early LTP after catecholamine deprivation (F = 163.993;p < 0.001). A post hoc t test confirms significant differences for all time intervals after the tetanus. If the method we used for depletion were successful, we would expect that application of D1/D5 antagonist would no longer have any effect on early LTP. Figure 6A shows that in slices from depleted animals, the D1/D5 antagonist (+)SCH 23390 (5 μm) no longer affected potentiation (n= 4; F = 0.893; p > 0.34).
Fig. 6.
D1/D5 antagonist is ineffective in depleted slices, whereas the LTP-facilitating effect of D1/D5 agonist is enhanced. Calculation procedures and markings as in Figure 3. All data points are shown. A, The active form of D1/D5 antagonist(+)SCH 23390 (5 μm; n= 7) significantly decreases early LTP in control slices but not in depleted slices (n = 4). B, D1/D5 agonist facilitates the induction of LTP. The effect is stronger in depleted slices (n = 4; df = 1;F = 56.815) than in control conditions (n = 4; df = 1; F = 12.337).
The results above suggest that in control slices LTP is enhanced by endogenous dopamine released by tetanus. This could diminish the effect of exogenous D1/D5 agonists. One would therefore expect that the action of exogenous dopamine agonists would be greater in depleted slices. Figures 6B and 7A show that the D1/D5 agonist 6-chloro-PB (10 μm) became more effective in depleted slices (n = 4; F= 49.994; p < 0.001). It increased early LTP by 20–25%, compared with the average of 15% in control slices (Fig. 3).
To confirm D1/D5 agonist action, it was important to check whether we could block it with a specific antagonist. In control slices, D1/D5 agonists and antagonist had opposite effects on the early LTP. This means that the results of agonist plus antagonist coapplication would be difficult to interpret. After depletion, however, antagonist by itself was not active. This gave us the opportunity to see whether antagonist could block the agonist action. In the same experimental paradigm, 10 min before one of tetani we introduced a D1/D5 antagonist, (+)SCH 23390 (5 μm), and then 5 min later added 10 μm 6-chloro-PB to the perfusion media. A tetanus was then given, and both drugs were removed immediately. Figure 7Bshows that D1/D5 antagonist entirely blocks the effect of 6-chloro-PB. Although the “drugs–control” difference was still positive (F = 5.849; p < 0.05), in no separate time interval did it reach statistical significance according to a paired t test.
Like the D1/D5 agonist 6-chloro-PB, forskolin (10 μm) also had a larger effect in depleted slices than in control slices (n = 5; F = 52.453;p < 0.001). As a result, the magnitude of LTP in depleted animals after forskolin application did not differ from control LTP (Fig. 5). In all intervals after the tetanus, the difference between “depletion” and “depletion + forskolin” conditions was significant, with p < 0.04–0.005. This difference declined at the interval of 30–35 min (p < 0.07); however, we doubt that this decline is meaningful, because we did not observe it in the different set of experiments under the same conditions. To check whether this effect of forskolin depends on adenylyl cyclase activation, we performed a separate experiment with dideoxyforskolin (10 μm), the analog that displays most of the effects of forskolin except for adenylyl cyclase activation (Laurenza et al., 1989). Dideoxyforskolin did not affect early LTP (n = 4; F = 0.619; p > 0.43).
The effect of D1/D5 agonist on LTP might conceivably arise from a large enhancement of a small component of LTP that is NMDA channel-independent (Grover and Teyler, 1990). If this were the case, the agonist would produce significant LTP even after the block of NMDA channels. We found, however, that in depleted slices in the presence of 100 μm ±APV, a tetanus produced no significant LTP with or without 6-chloro-PB (10 μm).
In some models of dopamine action in reinforcement, the dopamine arrives after synaptic activity and yet leads to the selective strengthening of active synapses. To study whether the enhancement of LTP by dopamine that we have studied can operate in this way, we applied D1/D5 agonist after the tetanus. These experiments were carried out in depleted slices. D1/D5 agonist 6-chloro-PB, 10 μm, was perfused for 5 min starting immediately after the tetanus on one pathway. The other pathway served as a control. As we mentioned in Materials and Methods, the agonist should arrive at the slice in ∼15-25 sec after the tetanus. Under these conditions, D1/D5 agonist did not affect early LTP (n = 5;F = 0.969; p > 0.33).
With rare exceptions, we observed a decrease in the magnitude of LTP during a 40 min period after a single tetanus (Figs. 1, 2, 4, 5, and 7) in both control and depleted slices. Two-way ANOVA confirms this observation, showing a significant effect of the time factor for most experiments. On the other hand, in no case did we observe a significant time–drug interaction. That means that drug effects appear immediately after the tetanus and persist throughout the observation period after the tetanus.
DISCUSSION
D1/D5 activation enhances the early phase of LTP
Our findings provide strong evidence that the early phase of LTP is enhanced by dopamine D1/D5 agonists. The enhancement was produced in control slices by three different types of D1/D5 agonists at low concentrations (5–10 μm). In some individual experiments it was as high as 30%. On average, in control slices with a 5 μm concentration of (+)bromo-APB the increase was ∼10%; with two other agonists and a 10 μm dose it was 15–20%. In depleted slices, where the agonist effect is no longer occluded by endogenous dopamine, LTP was increased by up to 40% (25% on average). The observed enhancement of LTP requires tetanic stimulation and is specific to the stimulated pathways. It is thus the type of plasticity required to be of computational importance in reinforcement of learning. Previous work indicates that D1/D5 antagonists strongly reduce the late phase of LTP (Frey et al., 1990,1991, 1993). Thus dopamine may be important for both early and late phases of LTP in the hippocampus.
Endogenous dopamine affects early LTP
Previous work has shown by direct measurement that endogenous dopamine is released in normal slices during tetanic stimulation (Frey et al., 1990). Consistent with this finding, our results show that D1/D5 antagonist can decrease early LTP in control slices. Similar antagonist effects on early LTP can be noted in the experiments on late LTP (Frey et al., 1990, 1991; Huang and Kandel, 1995), although they were not commented on by the authors. Furthermore, depletion of catecholamines (dopamine and noradrenaline) decreases early LTP and abolishes the effect of D1/D5 antagonist. Taken together, these results suggest that the release of endogenous dopamine in normal slices acts to enhance the magnitude of early LTP. It is important to note, however, that the decrease in LTP produced by depletion of catecholamines is larger than that produced by dopamine antagonists, suggesting that both endogenous catecholamines may act to enhance LTP in normal slices.
Stanton and Sarvey (1985) found that catecholamine depletion affects LTP in the dentate gyrus but not in the CA1 region. The difference with our results may stem from the different depletion methods. They aimed mostly at the noradrenergic system, injecting 6-hydroxydopamine bilaterally into dorsal noradrenergic bundle. Slices were prepared after the 14–21 d delay necessary to allow degeneration of catecholamine axons. Dopamine levels were not measured in this experiment, and noradrenaline depletion was only ∼83%. The procedure we used (see Materials and Methods) was reported to achieve a 92–95% depletion of dopamine in dopamine-enriched nucleus accumbens (O’Donnell and Grace, 1993).
Dopamine in the hippocampus
Our work indicates the importance of dopamine in hippocampal synaptic plasticity and together with other recent work should help to dispel the idea that dopamine is unimportant in hippocampal function. The hippocampus receives dopaminergic input from both the substantia nigra and the ventral tegmental area to the hilus area and CA1-subiculum region (Gasbarri et al., 1994; Goldsmith and Joyce, 1994). Both D2 (Brouwer et al., 1992; Mengod et al., 1992; Yokoyama et al., 1994) and D1 (Gingrich et al., 1992; Huang et al., 1992) receptor families are found in the hippocampus. It now seems that the D1 type of receptor found in the hippocampus is partially the D5 subtype (Meador-Woodruff et al., 1992; Laurier et al., 1994; Sokoloff and Schwartz, 1995). Much of the enzymatic machinery that is associated with dopaminergic target cells is present in the hippocampus. This includes dopamine uptake sites (Mennicken et al., 1992), DARPP-32 (Sakagami et al., 1994), Ca2+-inhibitable adenylyl cyclase (Mons and Cooper, 1994), and calmodulin-dependent phosphodiesterase PDE1B1 (Polli and Kincaid, 1994). In vivo microdialysis studies have shown dopaminergic effects on the extracellular acetylcholine release in the hippocampus (Nilsson et al., 1992;Imperato et al., 1993). Finally, activation of NMDA receptors affects extracellular dopamine concentration and metabolism in the hippocampus (Whitton et al., 1994).
Mechanism of the dopamine effect
We have found that the enhancement of early LTP by D1/D5 agonists can be mimicked by forskolin. This is true in both the normal and the depleted slice, where the effect of D1/D5 agonist and forskolin is even greater (∼ 20–25%). As Figure 5 shows, forskolin restored LTP in depleted slices to the control level. Dideoxyforskolin on the other hand was inactive. Similar effects of forskolin on θ-burst-induced LTP of fEPSPs was described previously by Arai and Lynch (1992). The ability of forskolin to mimic the effect of D1/D5 agonist is consistent with the established view that D1/D5 receptors are coupled to adenylyl cyclase (Kebabian and Calne, 1979; Kimura et al., 1995) and produce their ultimate effect through a cAMP-dependent process.
Our results do not themselves indicate whether the D1/D5 or forskolin actions are presynaptic or postsynaptic. The recent finding, however, that early LTP can be inhibited by interfering with the cAMP pathway in the postsynaptic cell (Blitzer et al., 1995), supports the view that the D1/D5 action is postsynaptic. Indeed, the available evidence indicates that most D1/D5 receptors are postsynaptic and located on spines, precisely the location where the biochemistry of synaptic plasticity occurs (Huang et al., 1992; Smiley et al., 1994; Bergson et al., 1995).
Given the importance of postsynaptic depolarization in LTP induction (Wingstrom et al., 1986; Gustafsson et all., 1987), one way that dopamine might enhance LTP is by enhancing this depolarization during the tetanic stimulation (Yang and Seamans, 1996). The literature on this subject is not clear. Depolarization and the blockade of afterhyperpolarization (AHP) was described for high doses of dopamine (>1-5 μm) and long exposures (Gribkoff and Ashe, 1984;Malenka and Nicoll, 1986; Pedarzani and Storm, 1995). With lower doses (1 μm or less), however, the AHP is increased, and spontaneous and depolarization-evoked spike activity is decreased (Bernardo and Prince, 1982; Stanzione et al., 1984; Pockett, 1985). Only intracellular experiments with specific D1 and D2 agonists can directly answer this question.
A second way dopamine might increase LTP is by enhancing the NMDA channels that are crucial for LTP induction. This possibility seems quite likely in view of the recent finding that hippocampal NMDA channels are upregulated by the cAMP-dependent protein kinase PKA (Raman et al., 1996). Work in striatal cells also shows that the NMDA channel function is enhanced by dopamine acting on D1 receptors (Levine et al., 1996).
Third, dopamine acting through cAMP might also affect the biochemistry of LTP more directly. Phosphatase I inhibitors (I1 and DARPP-32) are the substrates for PKA. Phosphatase 1 is known to be involved in synaptic plasticity (Mulkey et al., 1994), possibly through its action on Ca2+-calmodulin-kinase II (Lisman, 1989,1994). More generally, substantial biochemical evidence suggests that cAMP metabolism is powerfully controlled by neuronal activity and neuromodulators. At least three different types of adenylyl cyclases are found in the CA1 pyramidal cells, allowing the regulation of cAMP levels by intracellular Ca2+, Gs, Gi, and βγ subunits of G-proteins, by protein kinase C (Cooper et al., 1995), and by the degree of membrane depolarization (Reddy et al., 1995). More than 70% of neuronal cAMP-dependent protein kinase A, mostly IIb isoform, is concentrated in the postsynaptic density and dendritic cytoskeletal elements (Francis and Corbin, 1994) where it is coanchored with protein phosphatase 2B and protein kinase C by the common anchoring protein (Coghlan et al., 1995; Klauck et al., 1996). The disruption of PKA anchoring affects the regulation of glutamate receptor channels (Rosenmund et al., 1994). During normal LTP induction, cAMP elevation is known to occur (Chetkovich and Sweatt, 1993; Frey et al., 1993), and intracellularly applied PKA inhibitors can block early LTP under some conditions (Blitzer et al., 1995).
Functional significance
Is the effect of dopaminergic modulation on LTP large enough to be of functional significance? In depleted slices the effect can be as great as 20–35%. This is certainly significant if one takes as a yardstick the work on LTD, which often produces smaller effects on synaptic strength. It is possible that dopaminergic modulation on particular synaptic inputs may be larger than our measurements indicate. Electron microscopic study in the frontal cortex and the hippocampus in monkeys shows that only 20% of pyramidal spines have D1 receptors and <5% have D5 labeling (Bergson et al., 1995). If the same is true for the rat, the D1/D5 effect on early LTP may be much larger at these spines, but may be diluted out in fEPSP recordings by synapses that have no D1/D5 dopaminergic modulation.
Another important issue about dopamine effects on synaptic modification relates to timing. A critical aspect of some models of reinforcement is the ability of dopamine to strengthen active synapses, even if it arrives after the activity. We have tested this possibility and failed to find any effect of dopamine; however, the rapidity with which dopamine can be applied to the slice and the delay for dopamine to diffuse into the slice leave open the possibility that the sensitivity to dopamine might occur during a very short period after activity. We should point out, however, that the existing biochemical data indicate that the actions of dopamine are inherently slow: 2–5 min are required to activated adenylyl cyclase and to accumulate sufficient cAMP for PKA stimulation (Seamon and Daly, 1986; Chetkovich and Sweatt, 1993; Frey et al., 1993). On this basis one could argue that the period of dopamine action must be minutes long, and if so, we should have detected it. An altogether different view more consistent with our data is that information is processed in two stages (Buzsaki, 1989). During the actual conditioning, information is stored. It is then replayed after the reward. If a memory is replayed during dopamine application, the synapses involved would be strengthened by the dopaminergic action we have described in this paper.
Footnotes
This work was supported by National Institutes of Health Grant 5 R01 NS27337-7. We gratefully acknowledge the support of the W. M. Keck Foundation and generous donations of dopaminergic drugs from the National Institute of Mental Health Synthesis Program at RBI (Natick, MA), Abbott Laboratories (Abbott Park, IL), and Interneuron Pharmaceuticals (Lexington, MA). We thank Drs. John W. Kebabian, Perry B. Molinoff, and Kenneth W. Locke for helpful discussions on preliminary results and advice on dopaminergic drugs. We are also grateful to Dr. P. Read Montague for remarks on this manuscript.
Correspondence should be addressed to John E. Lisman, Biology Department, Center for Complex Systems, Brandeis University, 415 South Street, Waltham, MA 02254.
REFERENCES
- 1.Angenstein F, Matthies H, Staek S, Reymann KG, Staak S. The maintenance of hippocampal long-term potentiation is paralleled by a dopamine-dependent increase in glycoprotein fucosylation. Neurochem Int. 1992;21:403–408. doi: 10.1016/0197-0186(92)90191-s. [DOI] [PubMed] [Google Scholar]
- 2.Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–2040. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- 4.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardo LS, Prince DA. Dopamine action on hippocampal pyramidal cells. J Neurosci. 1982;2:415–423. doi: 10.1523/JNEUROSCI.02-04-00415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitzer RD, Wong T, Nouranifar R, Iengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer N, Dijken HV, Ruiters HJ, VanVilligen J-D, Horst GJT. Localization of dopamine D2 receptor mRNA with non-radioactive in situ hybridization histochemistry. Neurosci Lett. 1992;142:223–227. doi: 10.1016/0304-3940(92)90378-k. [DOI] [PubMed] [Google Scholar]
- 8.Burgard EC, Cote TE, Sarvey JM. Muscarinic depression of synaptic transmission and blockade of norepinephrine-induced long-lasting potentiation in the dentate gyrus. Neuroscience. 1993;54:377–389. doi: 10.1016/0306-4522(93)90259-i. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi P, Maj R, Mercuri NB, Bernardi G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett. 1992;142:95–99. doi: 10.1016/0304-3940(92)90628-k. [DOI] [PubMed] [Google Scholar]
- 11.Chavez-Noriega LE, Stevens CF. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 1992;574:85–92. doi: 10.1016/0006-8993(92)90803-h. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Fujii S, Ito K-I, Kato H, Kaneko K, Miyakawa H. Activation of dopamine D1 receptors enhances long-term depression of synaptic transmission induced by low frequency stimulation in rat hippocampal CA1 neurons. Neurosci Lett. 1995;188:195–198. doi: 10.1016/0304-3940(95)11430-5. [DOI] [PubMed] [Google Scholar]
- 13.Chetkovich DM, Sweatt JD. NMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 14.Coghlan VM, Perrino BA, Howard M, Landeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DMF, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 16.Cooper SJ. Interactions between endogenous opioids and dopamine: implications for reward and aversion. In: Wilner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: from motivation to action. Wiley; New York: 1991. pp. 331–366. [Google Scholar]
- 17.Dahl D, Li J. Induction of long-lasting potentiation by sequenced application of isoproterenol. NeuroReport. 1994;5:657–660. doi: 10.1097/00001756-199401000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. Long-term increases in excitability in the CA1 region of rat hippocampus induced by β-adrenergic stimulation: possible mediation by cAMP. J Neurosci. 1992;12:506–517. doi: 10.1523/JNEUROSCI.12-02-00506.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis SH, Corbin JD. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 1994;56:237–272. doi: 10.1146/annurev.ph.56.030194.001321. [DOI] [PubMed] [Google Scholar]
- 20.Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- 21.Frey U, Matties H, Reimann KL, Matties H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the CA1 region in vitro. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- 22.Frey U, Huang Y-Y, Kandel ER. Effects of cAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Tononi G, Reeke GN, Sporns O, Edelman GM. Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience. 1994;59:229–243. doi: 10.1016/0306-4522(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 24.Gasbarri A, Packard MG, Campana E, Pasitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 25.Gingrich JA, Dearry A, Falardeau P, Bates MD, Fremeau RT, Caron MG. Localization and molecular cloning of D1 dopamine receptor. Neurochem Int [Suppl] 1992;20:9–15. doi: 10.1016/0197-0186(92)90204-5. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith SK, Joyce JN. Dopamine D2 receptor expression in hippocampus and parahippocampal cortex of rat, cat, and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus. 1994;4:354–373. doi: 10.1002/hipo.450040318. [DOI] [PubMed] [Google Scholar]
- 27.Gribkoff VK, Ashe JH. Modulation by dopamine of population responses and cell membrane properties of hippocampal CA1 neurons in vitro. Brain Res. 1984;292:327–338. doi: 10.1016/0006-8993(84)90768-6. [DOI] [PubMed] [Google Scholar]
- 28.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different pattern of afferent stimulation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 29.Gustafsson B, Wingstrom H, Abraham WC, Huang Y. Long-term potentiation in the hippocampus using depolarizing current pulses as a conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci USA. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y-Y, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillations in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 33.Huerta PT, Lisman JE. Low frequency stimulation at the troughs of θ-oscillation induces long-term depression of previously potentiated CA1 synapses. J Neurophysiol. 1996;75:877–884. doi: 10.1152/jn.1996.75.2.877. [DOI] [PubMed] [Google Scholar]
- 34.Imperato A, Obinu MC, Gessa GL. Stimulation of both D1 and D2 receptors facilitates in vivo acetylcholine release in the hippocampus. Brain Res. 1993;618:341–345. doi: 10.1016/0006-8993(93)91288-4. [DOI] [PubMed] [Google Scholar]
- 35.Kebabian J, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 36.Kimura K, Sela S, Bouvier C, Grandy D, Sidhu A. Differential coupling of D1 and D5 dopamine receptors to guanine nucleotide binding proteins in transfected GH4C1 rat somatomammotrophic cells. J Neurochem. 1995;64:2118–2124. doi: 10.1046/j.1471-4159.1995.64052118.x. [DOI] [PubMed] [Google Scholar]
- 37.Klauck TM, Faux MG, Labudda K, Langenberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 38.Laurenza A, Sutkowski EM-H, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action. Trends Pharmacol Sci. 1989;10:442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- 39.Laurier LG, O’Dowd BF, George SR. Heterogeneous tissue-specific transcription of dopamine receptor subtype messenger RNA in rat brain. Mol Brain Res. 1994;25:344–350. doi: 10.1016/0169-328x(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 40.Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Westphal H. Modulatory action of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisman J. A mechanism for the Hebb and anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994;17:406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 43.Maeda T, Kaneko S, Satoh M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slice. Neurosci Res. 1994;18:277–282. doi: 10.1016/0168-0102(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 44.Malenka RC, Nicoll RA. Dopamine decreases the calcium-activated afterhyperpolarization in hippocampal CA1 pyramidal cells. Brain Res. 1986;379:210–215. doi: 10.1016/0006-8993(86)90773-0. [DOI] [PubMed] [Google Scholar]
- 45.Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ. Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett. 1992;145:209–212. doi: 10.1016/0304-3940(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 46.Mengod G, Villaro MT, Landwehrmeyer GB, Martinez-Mir MI, Niznik HB, Sunahara RK, Seenam P, O’Dowd BF, Probst A, Palacios JM. Visualization of dopamine D1, D2, and D3 receptor mRNA in human and rat brain. Neurochem Int [Suppl] 1992;20:33–43. doi: 10.1016/0197-0186(92)90208-9. [DOI] [PubMed] [Google Scholar]
- 47.Mennicken F, Savasta M, Peretti-Renucci R, Feuerstein C. Autoradiographic localization of dopamine uptake sites in the rat brain with 3H-GBR 12935. J Neural Transm. 1992;87:1–14. doi: 10.1007/BF01253106. [DOI] [PubMed] [Google Scholar]
- 48.Mons N, Cooper DMF. Selective expression of one Ca2+-inhibitable adenylyl cyclase in dopaminergically innervated rat brain regions. Mol Brain Res. 1994;22:236–244. doi: 10.1016/0169-328x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 49.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 51.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson OG, Leanza G, Bjorklund A. Acetylcholine release in the hippocampus: regulation by monoaminergic afferents as assessed by in vivo microdialysis. Brain Res. 1992;584:132–140. doi: 10.1016/0006-8993(92)90886-e. [DOI] [PubMed] [Google Scholar]
- 53.O’Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedarzani P, Storm JF. Dopamine modulates the slow Ca2+-activated current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol. 1995;74:2749–2753. doi: 10.1152/jn.1995.74.6.2749. [DOI] [PubMed] [Google Scholar]
- 55.Pockett S. Dopamine changes the shape of action potentials in hippocampal pyramidal cells. Brain Res. 1985;342:386–390. doi: 10.1016/0006-8993(85)91143-6. [DOI] [PubMed] [Google Scholar]
- 56.Polli JW, Kincaid RL. Expression of calmodulin-dependent phosphodiesterase isoform (PDE1B1) correlates with brain regions having extensive dopaminergic innervation. J Neurosci. 1994;14:1251–1261. doi: 10.1523/JNEUROSCI.14-03-01251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raman IM, Tong G, Jahr GE. β-Adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 58.Reddy R, Smith D, Wayman G, Wu Z, Villacres EC, Storm DR. Voltage-sensitive adenylyl cyclase activity in cultured neurons: a calcium independent phenomenon. J Biol Chem. 1995;270:14340–14346. doi: 10.1074/jbc.270.24.14340. [DOI] [PubMed] [Google Scholar]
- 59.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 60.Sakagami H, Ebina K, Kondo H. Re-examination of the ontogeny in the gene expression of DARPP-32 in the rat brain. Mol Brain Res. 1994;25:67–72. doi: 10.1016/0169-328x(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 61.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seamon KB, Daly JW. Forskolin: its biological and chemical properties. In: Greengard P, Robinson GA, editors. Advances in cyclic nucleotide and protein phosphorylation research, Vol 20. Raven; New York: 1986. pp. 1–150. [PubMed] [Google Scholar]
- 63.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokoloff P, Schwartz J-C. Novel dopamine receptors half a decade latter. Trends Pharmacol Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 65.Stanton PK, Sarvey JM. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985;5:2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanzione P, Calabresi P, Mercuri N, Bernardi G. Dopamine modulates CA1 hippocampal neurons by elevating the threshold for spike generation: an in vitro study. Neuroscience. 1984;13:1105–1116. doi: 10.1016/0306-4522(84)90291-4. [DOI] [PubMed] [Google Scholar]
- 67.Sutton RS, Barto AG. Towards a modern theory of adaptive networks: expectation and prediction. Physiol Rev. 1981;88:135–170. [PubMed] [Google Scholar]
- 68.Villani F, Johnston D. Serotonin inhibits induction of long-term potentiation at commissural synapses in hippocampus. Brain Res. 1993;606:304–308. doi: 10.1016/0006-8993(93)90998-3. [DOI] [PubMed] [Google Scholar]
- 69.Whitton PS, Malione S, Biggs CS, Fowler LJ. N-methyl-d-aspartate receptor modulate extracellular dopamine concentration and metabolism in rat hippocampus and striatum in vivo. Brain Res. 1994;635:312–316. doi: 10.1016/0006-8993(94)91453-2. [DOI] [PubMed] [Google Scholar]
- 70.Wingstrom H, Gustafsson B, Huang YY, Abraham WC. Hippocampal LTP is induced by pairing single afferent volleys with intracellulary injected depolarizing current pulses. Acta Physiol Scand. 1986;126:317–319. doi: 10.1111/j.1748-1716.1986.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 71.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 72.Yang CR, Seamans JK. Dopamine D1 receptor actions in the layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendrito-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokoyama C, Okamura H, Nakajima T, Taguchi J-I, Ibata Y. Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J Comp Neurol. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]