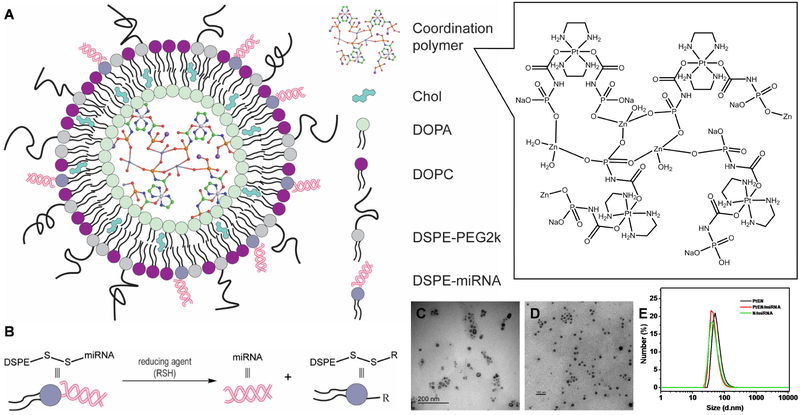
Abstract
Though early detection and treatment of primary tumors has significantly improved in recent years, metastatic disease remains among the most significant challenges in cancer therapy. Cancer cells can disseminate before the primary tumor is detected to form micro or gross metastases, requiring toxic systemic therapies. To prevent and suppress metastases, we have developed a nontoxic, long-circulating nanoscale coordination polymer (NCP) protecting microRNA (miRNA) in circulation and releasing it in tumors. PtIV(en)2 [en=ethylenediamine] containing NCPs (PtEN) can release a nontoxic, kinetically inert PtII(en)2 compound and carbon dioxide which aids the endosomal escape of its miRNA cargo, miR-655-3p. Without the presence of the PtEN core, the miRNA showed cellular uptake but no effect. When transfected into human colorectal HCT116 cells by NCPs, this oligometastatic miRNA limited proliferation and epithelial-to-mesenchymal transition (EMT) by preventing β-catenin nuclear translocation and tumor cell invasion. Systemic administrations of PtEN/miR-655-3p sustained effective transfection to reduce liver colonization and tumor burden in a xenogenic hepatic metastatic model of HCT116 without any observable toxicity.
Keywords: nontoxic, nanovehicle, nanoscale coordination polymers, miRNA, metastasis, miR-655-3p
Graphical Abstract

Introduction
Metastases are responsible for ~90% of cancer deaths, accounting for nearly 1,500 deaths each day in the United States.1-3 The metastatic process is proposed as the last step of cancer progression, but it has recently been found that cancer cells will disseminate much earlier in both animal models and human patients.4-6 Due to the potential risks of metastasis, patients are often treated with radiotherapy, systemic adjuvant chemotherapy, or targeted therapies after surgery to eliminate residual tumors and prevent or treat metastatic disease.7-10 However, these treatment modalities are accompanied by significant toxicities and can lead to overtreatment of patients, particularly in young breast, colon cancer, and prostate cancer patients.11-15 As cancer cells can form undetectable micrometastases, systemic therapies that can specifically treat tumors or suppress growth without damaging healthy tissues are a high priority in advancing cancer therapy to treat metastatic disease. Nanoparticles are an ideal platform for these purposes due to improvements in circulation half-lives and preferential accumulation in cancerous tissues via the enhanced permeability and retention effect.
One promising strategy has been microRNAs (miRNAs), short non-coding regions of RNA commonly found in cancer-associated genomic regions.16-20 The miRNA profile in cancerous tumors is often found to be deregulated compared to normal tissues, opening a window for both diagnosis and therapy.20-24 Specifically, the chromosomal locus 14q32, which is frequently translocated in myeloma patients, encodes miR-655-3p, a miRNA regulating key metastatic pathways including the epithelial-to-mesenchymal transition (EMT).25, 26 It was found to be significantly down-regulated in human hepatocellular carcinomas, among other cancers, compared to healthy livers.27 Low expression of miR-655-3p was clinically associated with poor prognosis and a metastatic phenotype.25, 27 However, patients with only limited metastases, or oligometastasis, and associated with more favorable outcomes were found to overexpress miR-655-3p, suggesting there may be a therapeutic benefit to its ectopic expression.25, 28
Though the tumor suppressing capabilities of miR-655-3p have been demonstrated in a number of cell lines, miRNAs are unstable in systemic circulation and require often toxic vehicles for delivery and transfection.26, 29-31 Delivery of small RNA therapeutics has also been plagued by poor pharmacokinetics and unexpected immunotoxicity,21, 31-35 but the recent successes of two oligonucleotides in clinical trials for transtheyretin (TTR)-mediated amyloidosis demonstrate the potential of gene therapy if these limitations can be overcome.33, 36-41 One is a lipid nanoparticle delivering RNAi, which has inspired a number of platforms for the delivery of small molecule therapeutics to tumors in vivo.42-50 The pharmacokinetics of drugs encapsulated in nanoparticles can be altered by reducing binding to serum proteins or cells.
We have recently used cytotoxic nanoscale coordination polymers (NCPs) for the delivery of double-stranded RNAs for cancer treatment.51, 52 Specifically, NCP-encapsulated small interfering RNAs (siRNAs) could be effectively shielded from degradation in sera, while maintaining gene silencing in vivo. Drug resistance gene-targeting siRNAs potentiated cisplatin-carrying NCPs resulting in substantially improved anticancer efficacy in animal models. 51
In this work, we take advantage of platinum coordination chemistry to design a nontoxic vehicle for effective miRNA delivery in vitro and in vivo. NCPs with prolonged blood circulation and triggered release allow for the direct investigation of the molecular mechanisms of miR-655-3p on human colorectal cancer HCT116 cells. Only NCPs containing the Pt(IV) coordination polymer showed effective miRNA delivery. The miRNA-carrying NCPs limited cell proliferation, nuclear localization of β-catenin, and tumor cell invasion in vitro. NCP/miRNAs showed favorable biodistribution and minimal toxicity, allowing for in vivo transfection to effectively limit epithelial-to-mesenchymal transition (EMT) and suppress the formation and growth of tumor colonies in a xenogenic hepatic metastasis model of colorectal cancer.
Results
[PtII(en)2]Cl2 synthesis and incorporation into NCPs.
Dichlorobis(ethylenediamine)platinum(II), [PtII(en)2]Cl2, was synthesized according to literature and characterized by NMR and mass spectrometry (Fig. S1-2).53 [PtII(en)2]Cl2 was converted into [PtIV(en)2(OH)2]Cl2 by treatment with 10% H2O2 in water at 70 °C. The bis(phosphonic acid) compound, [PtIV(en)2bp]Cl2, was synthesized by treatment of [PtIV(en)2(OH)2]Cl2 with diethoxyphosphinyl isocyanate followed by hydrolysis with Me3SiBr and methanol in accordance with analogous PtIV complexes (Fig. S3-5).54, 55 All of these new PtIV complexes were characterized by NMR and mass spectrometry.
To prepare PtIV(en)2-containing NCPs (PtEN), [PtIV(en)2bp]Cl2 was deprotonated and polymerized with ZnII ions in the presence of the phospholipid 1,2-dioleoyl-sn-glycero-3-phosphate sodium salt (DOPA) via reverse microemulsion. A self-assembled lipid bilayer in the presence of cholesterol and the phospholipids 1,2-dioleyl-sn-gylcero-3-phosphocholine (DOPC) and 1,2-diastearoyl-sn-gylcero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (DSPE-PEG2k) in a 2:2:1 molar ratio yielded nontoxic PtEN. Cholesterol ordered and stabilized the DOPC and DSPE-portion of DSPE-PEG2k, while the PEG portion endowed “stealth” properties to evade clearance by mononuclear phagocyte system (MPS). The Pt loading was determined to be 9.2 wt% by inductively coupled plasma-mass spectrometry (ICP-MS). The Z-average diameter, PDI, and ζ-potential of PtEN were determined by dynamic light scattering (DLS) to be 96.9±0.7, 0.14±0.01, and −15.2±2.0 mV in water. In PBS, the ζ-potential of PtEN was determined to be slightly negative at −1.85+0.261 mV.
PtEN is nontoxic to cells because [PtII(en)2]Cl2 neither coordinates nor intercalates with DNA.
The most prominent members of the Pt family of anticancer drugs form inter- and intra-strand Pt-DNA adducts to interfere with DNA replication. [PtIV(en)2bp]Cl2 was designed as a nontoxic, kinetically inert core for PtEN due to the low spin d*****6 electronic configuration of octahedral Pt complexes. While other PtIV prodrugs release active PtII species to bind to DNA,56, 57 the resultant [PtII(en)2]2+ does not have a substitution-labile coordination site and cannot bind to DNA. To test our hypothesis, we first investigated the kinetics of [PtIV(en)2bp]2+ reduction and Pt-en dissociation.
In the presence of 5mM ascorbate at 37°C, [PtIV(en)2bp]Cl2 can be reduced to [PtII(en)2]2+ in 5h by electron transfer from ascorbate to the PtIV center via the axial ligands (Fig. 1a, Fig. S6). This allows for generation of CO2 as a product, akin to the previously reported PtIV prodrug of cisplatin, Pt(NH3)2Cl2(bp) (Fig. S8). [PtII(en)2]2+ is highly stable as Pt-en coordination will not dissociate in an aqueous solution at 37°C for up to two weeks. (Fig. S7). The chemically inert [PtII(en)2]Cl2 is thus unable to bind to DNA. Charged PtII complexes with two N-N’ bidentate ligands have also been investigated as metallointercalators.58, 59 The planar, aromatic rings must be on the size of a base pair, ~3.4Å, to insert into the DNA bases.59 The planar [PtII(en)2]2+ could not displace ethidium bromide (EtBr) intercalated with DNA, with consistent fluorescence from EtBr-bound DNA at Pt:DNA ratios ranging from 0.01:1 to 10:1 (Fig. S9).
Figure 1. PtEN are stable and nontoxic in vitro and in vivo.
(A) Reduction of [PtIV(en)2bp]Cl2 to [PtII(en)2]2+ in the presence of ascorbate. (B) Negligible cytotoxicity in HCT116 cells treated with [PtII(en)2]Cl2, [PtIV(en)2bp]2+, or PtEN at up to 100μM equivalents of Pt for up to 72 h (n=6). Biodistribution, tumor uptake (C) and blood concentration (D) of Pt over time after i.v. injection of PtEN in CT26 tumor-bearing BALB/c mice at a dose of 3.0 mg/kg Pt. Data are expressed as means±SD (n=3).
As a result, no cytotoxicity was observed on HCT116 cells by MTS assay. Negligible changes in cell viability were observed after treatment with [PtII(en)2]Cl2, [PtIV(en)2bp]Cl2, or PtEN at up to 100μM after up to 72 h incubation (Fig. 1b). [PtII(en)2]Cl2 and [PtIV(en)2bp]Cl2 were further directly compared against cisplatin in platinum-sensitive H460 cells by MTS assay. The IC50 of CDDP was determined to be 5.58±0.26 μM compared to > 100 μM, as determined by ~100% cell viability at this dose (Fig. S10). These data indicate that any observed anticancer effects from PtEN delivered payloads are intrinsic to the payload itself.
PtEN shows long circulation and high tumor accumulation without observable toxicity.
The pharmacokinetic properties of intravenously injected PtEN were investigated on subcutaneous murine colorectal cancer CT26 tumor-bearing BALB/c mice. As shown in Fig. 1d, PtEN did not significantly accumulate in the lung, liver, or kidney (< 10% ID/g). In contrast, there was high tumor uptake of PtEN (13.8±1.4 %ID/g) 24 h post injection and a long blood circulation half-life of 14.3±2.8 h (Fig. 1d-e). Even after ten daily doses of PtEN (10 × 3 mg/kg Pt), no significant toxicity was observed by monitoring mouse body weights and activities (Fig. S11). Thirty days after the last dose, the mice were sacrificed to investigate the PtEN clearance and toxicity in organs with significant accumulation. There was significantly less PtEN in major mononuclear phagocyte system organs, with < 1% ID/g in the liver and kidneys (Fig. S12). The histology of five major organs with relatively high PtEN accumulation showed no obvious signs of damage or significant differences compared to those of 5% dextrose-treated mice (Fig. S13). These data indicate that PtEN is a nontoxic nanovehicle platform, which is ideal for delivering biological materials such as miRNAs.
PtEN/miRNA synthesis.
Cationic lipids are typically required to encapsulate negatively charged mi- and siRNAs, leading to rapid clearance and short circulation half-lifes. However, NCP/miRNA retains a slightly negative surface charge by incorporating an Alexa647-labeled miRNA mimic (Alexa), nontargeted miRNA (NT), miR-655-3p thiolated on the 5’ end of the sense strand and conjugated to DSPE as previously described. 51, 52 PtEN/miRNAs were prepared in the presence of cholesterol, DOPC, DSPE-PEG2k, and DSPE-conjugated miRNAs (DSPE-miRNA, Fig. 2a). The miRNA was loaded at 8.6 wt % into the lipid bilayer shell with 89% efficiency, as determined by Quant-iT RiboGreen RNA kit. DSPE-PEG2k was used to protect the DSPE-miRNAs from degradation by nucleases in physiological environments, but allowed for intracellular cleavage of DSPE-miRNA by reducing agents (Fig. 2b). The Z-average diameter, PDI, and ζ-potential of PtEN/miR-655-3p were determined to be 99.2±0.4, 0.15±0.01, and −18.8±1.9 mV in water, with similar size and shape to PtEN (Fig. 2c-e). A control lipid nanoparticle formulation containing DSPE-NT (N/NT) or DSPE-miR-655-3p (N/655) was synthesized by the same procedure in the absence of PtEN.
Figure 2. Preparation and characterization of PtEN/miRNA.
(A) Schematic representation of PtEN/miRNA carrying a nontoxic coordination polymer in the core and miRNA in the lipid shell, protected by DSPE-PEG2k. (B) Schematic illustration of miRNA release from DSPE-miRNA. (C-D) TEM image of PtEN (C) and PtEN/miRNA (D) showing the approximate size and monodispersity of the spherical nanostructure. Bar = 100 nm. (E) Number-average size distribution of PtEN, PtEN/miRNA, and N/miRNA in H2O.
PtEN delivers miR-655-3p for cytotoxicity, but not regulated cell death.
The cell uptake of PtEN was not affected by loading miRNA onto the particle surface (Fig. S14). This was visualized by confocal laser scanning microscopy (CLSM) of PtEN/Alexa rapidly internalized by HCT116 cells (Fig 3a). Cells were incubated with PtEN/Alexa for up to 2h, fixed, stained with Lysotracker Green and DAPI to visualize the endosome/lysosome and nuclei. PtEN/Alexa taken up by the HCT116 cells could release their payload into the cytoplasm, which was observed as a decrease in the colocalization between PtEN/Alexa and the endosome/lysosome by confocal laser scanning microscopy (Fig. 3b). Over 24 h, this led to significant uptake of Alexa when delivered as PtEN/Alexa or N/Alexa with no observed uptake of the Alexa647-miRNA mimic alone (Fig. 3c-d).
Figure 3. Endosomal escape of PtEN/miR-655-3p inhibits tumor cell proliferation.
(A) Time-dependent endosomal escape of PtEN/Alexa (red fluorescence) in HCT116 cells by CLSM. Alexa overlap with endo/lysosomes stained by Lysotracker Green (green fluorescence) can be observed as yellow fluorescence. Bar = 10μm. (B) Percentage co-localization of Alexa with endo/lysosomes over time in (A) as quantified by Image J (n=3). (C, D) Cellular uptake of Alexa by HCT116 cells dosed with the Alexa647-miRNA mimic or PtEN/Alexa by flow cytometry. (E) In vitro cytotoxicity of PtEN/miR-655-3p on HCT116 cells after 72h by MTS assay (n=6). (F) Reduced cell proliferation after treatment of HCT116 with PtEN/miR-655-3p for 24 h by cell counting (n=3). Data are expressed as means±SD. * p < 0.05, ** p < 0.01, *** p < 0.001.
PtEN/miR-655-3p at a dose of 50 nM miRNA only showed slight cytotoxicity (89.0±3.5% cell viability) in HCT116 cells after incubation for 24 h, but significantly decreased cell viability after 72 h (32.4±7.3% cell viability). In contrast, cells treated with PtEN/NT or [PtII(en)2]Cl2 plus miR-655-3p showed no impact or increase in cell viability, respectively (Fig. 3e, Fig. S15). This confirms that neither Pt(en)2Cl2 nor PtEN are inherently toxic and that miR-655-3p requires a transfection agent for activity.
To elucidate the mechanisms behind the observed cytotoxicity, we probed treated HCT116 cells for Annexin V and propidium iodide (PI) to assess apoptosis and necrosis, respectively. Though there have been reports of cytotoxicity by necrosis by liposomes producing large amounts of CO2,60-62 the CO2 released as a byproduct of the reduction of [PtIV(en)2bp]Cl2 was not cytotoxic. We did not observe any apoptosis/necrosis in HCT116 cells dosed with PtEN, PtEN/NT or PtEN/mi655-3p for up to 72 h (Fig. S16, Table S1).
PtEN/miR-655-3p inhibits HCT116 cell adherence and proliferation.
Given that PtEN/miR-655-3p does not directly induce cytotoxicity by apoptosis or necrosis, we hypothesized that miR-655-3p may suppress cell proliferation by impacting cell cycle regulation and DNA synthesis. HCT116 cells treated with PBS, [PtII(en)2]Cl2 plus miR-655-3p, or PtEN/NT for 24 h had proliferated to 1.7-1.9 times of the seed amount, compared to 0.96±0.17 times of adherent cells in the group treated with PtEN/miR-655-3p (Fig. 3f). Interestingly, miRNA-carrying nanoparticles without the NCP core had no effect on cell proliferation despite strong uptake due to the inability to escape from the endosome (Fig. 3c-d, f). After 72h, PtEN/miR-655-3p treated cells proliferated 5.5±1.2 times compared to 11.4-12.0 times for controls (Fig. S17).
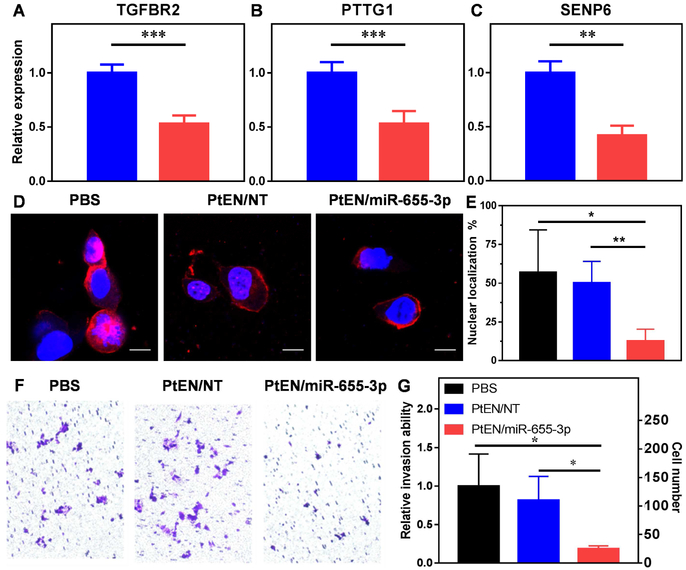
To probe the mechanisms behind reduced proliferation, mRNA levels of several target genes were investigated by real-time qPCR. HCT116 cells transfected with PtEN/miR-655-3p for 48 h showed significant downregulation of TGFBR2 (53% of PtEN/NT, p=0.0001, Fig. 4a), whereas transfection with N/655 did not show any effect on TGFBR2 expression (Fig. S18). TGF-β signaling by TGF-β ligand binding to TGFBR2 is a known regulator of the G1/S checkpoint, which arrests normal cell cycle progression but promotes metastatic progression in cancer cells.63 The G1 checkpoint regulates whether a cell will enter the S phase committing to cell division or enter G0 to become quiescent/senescent. One observed molecular effect associated with cell cycle exit is the downregulation of PTTG1,64 which is decreased in HCT116 cells treated with PtEN/miR-655-3p (53% of PtEN/NT, p=0.0009, Fig. 4b). Interestingly, SENP6 was also downregulated (42% of PtEN/NT, p=0.0028), which leads to reduced DNA synthesis and increased DNA breaks (Fig. 4c).65
Figure 4. In vitro transfection leads to suppression of nuclear β-catenin and cancer cell invasion.
(A-C) mRNA expression levels of miR-655-3p target genes (A) TGFBR2, (B) PTTG1, and (C) SENP6 treated with PtEN/NT (blue) or PtEN/miR-655-3p (red) after 48 h (n=4). (D) Representative immunofluorescence images and (E) quantification of β-catenin localization in HCT116 cells (n=4-5). Scale bar = 10μm. (F) Representative images of crystal violet-stained invading HCT116 cells after treatment with PBS, PtEN/NT or PtEN/miR-655-3p by transwell invasion assay. (G) Quantification of invading cells in (F) represented as a ratio over PBS control and as discrete cell counts (n=3). Data are expressed as means±SD. * p < 0.05; ** p < 0.01, *** p < 0.001.
PtEN/miR-655-3p inhibits nuclear accumulation of β-catenin and cancer cell invasion.
Wnt/β-catenin signaling has been correlated with EMT, cancer cell proliferation, and metastasis. In a study of spontaneous breast cancer in mice, normal and precancerous mammary tissue showed β-catenin localization in the cell membrane and cytoplasm, respectively, compared to cancerous tissue displaying β-catenin in the cytoplasm and nuclei of cells.66 After treatment with PtEN/miR-655-3p for 48h, HCT116 cells showed significantly decreased nuclear β-catenin (Fig. 4d-e). The fluorescence signal is primarily sequestered to the cell membrane after treatment with PtEN/miR-655-3p, whereas β-catenin is non-specifically found across the cell in PBS or PtEN/NT treated cells (Fig. 4d). Nuclear β-catenin is typically associated with cancer progression and metastasis due to its effects on transcription, whereas β-catenin sequestration at the cell membrane suggests decreased EMT activity.67 PtEN/miR-655-3p represses invasion of HCT116 cells in a transwell assay, reducing the number of invading cells by 81.4%, from 134.67±56.0 to 25.0±5.0 for PBS and PtEN/miR-655-3p treated groups, respectively (Fig. 4f-g, Fig. S19). Expectedly, PtEN/NT did not significantly affect the number of invading cells (134.67±56.0 vs. 109.42 67±42.0 for PBS and PtEN/NT, respectively).
In vivo toxicity and anti-metastatic efficacy of PtEN/miR-655-3p.
Inspired by our in vitro results, we next evaluated the possibility of PtEN/miR-655-3p for in vivo anti-metastatic activity. The serum levels of pro-inflammatory cytokines in immunocompetent BALB/c mice did not significantly increase after dosing with PtEN or PtEN/miR-655-3p, suggesting the miRNA therapy does not cause immunotoxicity by cytokine release (Fig. S20). After three doses of PtEN or PtEN/miR-655-3p with 1.875 mg/kg miRNA and 4.5 mg/kg Pt given once every three days, no liver damage was observed by histology in mice (Fig. S21). Liver damage will also lead to increased levels of ALT and AST in the blood, Furthermore, mice treated with PBS, PtEN, or PtEN/miR-655-3p all showed normal levels of serum aspartate transaminase (AST) and alanine transaminase (ALT) (Fig. S22). The tumor accumulation of miRNA payloads was further investigated in a liver metastasis model of HCT116 doubly labeled with luciferase and tdTomato (HCT116-L2T) with multiple nodules developing inside the liver. Athymic nude mice were given splenic injections of HCT116-L2T, after which the spleens were removed and the HCT116-L2T cells primarily metastasized to the liver. Intraperitoneally injected PtEN/Alexa was found to primarily co-localize with the liver tumors, with minimal Alexa signal observed in the lung, heart, or kidney (Fig. S23-24). Even when there were few or small liver tumors, PtEN/Alexa showed low accumulation in normal liver parenchyma (Fig. 5a). ICP-MS analysis of the lung, heart, or kidney confirmed minimal deposition in these organs after 3 or 24 h, with < 3 %ID Pt/g (Fig. S25), consistent with our subcutaneous biodistribution where PtEN showed higher accumulation in the tumor than other healthy tissues.
Figure 5. PtEN/miR-655-3p inhibits liver metastasis colony formation and proliferation.
(A) Ex vivo fluorescence images of the distribution of PtEN/Alexa (excitation/emission 640/680, green fluorescence) in the tumors (excitation/emission 535/580, red fluorescence) of livers harvested 3 or 24 h after injection of PtEN/Alexa. Co-localization can be observed as yellow fluorescence. (B, C) Representative bioluminescence images of mice intraperitoneally injected with 5% dextrose (w/v) control, PtEN/NT, or PtEN/miR-655-3p once every 3 days starting one day after tumor inoculation by intrasplenic injection of HCT116-L2T cells followed by splenectomy. The mice were dosed a total of nine times. Tumor burden of mice (B) 7 days or (C) 28 days post tumor inoculation. (D) Quantification of tumor burden by bioluminescence signal for mice imaged weekly beginning 7 days post tumor inoculation. (E) Quantification of the ex vivo fluorescence of the livers harvested 28 days post tumor inoculation. Data are expressed as means±SD (n=8). * p < 0.05; ** p < 0.01.
After establishing that the miRNA-loaded particles were safe, the anti-metastatic activity of PtEN/miR-655-3p was evaluated on the metastatic model of HCT116-L2T. Beginning one day after intrasplenic injection, the mice were treated with PtEN/NT or PtEN/miR-655-3p in a 5% dextrose (w/v) solution at equivalent doses of 625 μg/kg miRNA and 1.5 mg/kg Pt once every three days. Mice treated with 5% dextrose (w/v) alone served as control. The low toxicity of both the Pt core and the miRNA allowed for repeated doses throughout the entirety of the study for a total of nine doses. Beginning 7 days after tumor inoculation by intrasplenic injection, mice were injected weekly with luciferin, anesthetized with 2% isoflurane (v/v) to visualize tumors by bioluminescence imaging (Fig. 5b-c, Fig. S25-28). The tumor burden of PtEN/miR-655-3p-treated mice was significantly lower than either control group at the end of the study, as quantified by bioluminescence (Fig. 5d). 28 days post intrasplenic injection, the mice were euthanized for ex vivo quantification of tumor burden. These results further confirmed our in vivo imaging results, with a significant decrease in both liver weight and ex vivo fluorescence compared to both PtEN/NT and 5% dextrose treated mice (Fig. 5e, Fig. S29-30).
The in vivo transfection of PtEN/miR-655-3p were further validated in the HCT116-L2T system. 28 days after establishing the liver metastasis model, mice received a single dose of PtEN/NT or PtEN/miR-655-3p at a miRNA dose of 625 μg/kg, equivalent to the efficacy dose. 2 days after treatment, the liver was excised and the tumor was isolated from half the liver metastases for RNA extraction with Trizol. The mRNA levels of miR-655-3p regulated genes were found to be down-regulated, similar to in vitro transfected cells (Fig. 6a-c). The other half of the liver and associated tumor was sectioned and stained for histology and immunofluorescence of β-catenin (Fig. 6d). The localization of β-catenin in mice treated with PtEN/miR-655-3p primarily showed localized in the cell membrane in both the tumor and liver tissue. There was similar sequestration of β-catenin to the membrane in the liver parenchyma of PtEN/NT-treated mice, whereas there was nonspecific distribution of β-catenin in the labeled tumor cells.
Figure 6. In vivo transfection of PtEN/miR-655-3p.
(A-C) mRNA expression levels of miR-655-3p target genes (A) TGFBR2, (B) PTTG1, and (C) SENP6 (n=6 for PtEN/NT; n=5 for PtEN/miR-655-3p). (D) Representative histology and immunofluorescence images of β-catenin (green fluorescence) in the liver parenchyma and the tumor nodules (tdTomato red fluorescence) in livers treated once with PtEN/NT or PtEN/miR-655-3p. Scale bar = 200μm for H&E staining; scale bar = 20μm for immunofluorescence. * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Discussion
Though there has been significant progress in early detection and personalized medicines for cancer treatment, cancer metastases have remained a preeminent challenge. Metastases can occur long before detectable both in animal models and human patients, emphasizing the need for effective treatment strategies.69, 70 Currently, patients are treated systemically with radiotherapy, chemotherapies, or targeted therapies to stop or slow the growth of tumors, but the associated toxicities limit the frequency and total dose which can be given.7-10 In some cases, the aggressive regimen represents overtreatment with minimal gains.11-15 As endogenous molecules often downregulated by cancers, miRNAs represent a promising class of gene therapy to prevent and control metastasis without introducing significant toxicities. A nanoplatform for miRNA delivery with a long circulation half-life can both lower the total and frequency of drug dose by increasing effective drug exposure, allowing for thorough preparation against and facile management of metastatic disease.
The transient nature of miRNA delivery endows unique advantages and disadvantages to this therapy. By not directly integrating into the genome, there are arguably less off-target gene mutations or chromosomal aberrations. However, long-term management of a disease such as cancer requires repeated treatments to prevent and/or control tumor metastasis. Although conventional transfection agents are accompanied by cytotoxicity and initiation of the innate immune response,31 we have demonstrated that mice can tolerate multiple successive treatments of both the PtEN carrier and PtEN/miRNA treatment without inducing any obvious signs of toxicity.
Though miRNAs can act as either tumor suppressors or promoters, miR-655-3p is known to regulate genes involved in suppressing cellular adhesion, invasion, and motility.26, 29 The multitude of targets is less likely to lead to compensatory mechanisms of resistance which impede current chemo- and targeted therapies.31 As miR-655-3p is specifically down-regulated by cancer cells compared to healthy tissues, some accumulation in normal cells should not drastically change the cellular levels of miRNA to alter activity whereas cancer cell proliferation will be significantly inhibited.20
Ectopic expression of miR-655-3p has inhibited tumor migration and invasion of multiple cancer cell lines, but miRNAs have thus far had limited application in living systems due to low stability in serum. An effective vehicle for miRNA delivery can protect it from degradation, but release the cargo into the cytoplasm of tumor cells of which it preferentially accumulates. Typically, cationic lipids are required for efficient escape from the endosome, but negatively affect blood circulation of nanoparticles leading to two opposing requirements. Ionis’s antisense oligonucleotide products, such as Inotersen, are given subcutaneously to circumvent the issues with stability in blood circulation, but has broad applicability as its target, TTR, accumulates in all major organs. Even Alnylam’s Patisiran, a lipid nanoparticle formulation, is primarily uptaken by hepatocytes, limiting its use to liver disease.
To this end, we have developed a nontoxic nanocarrier that maintains its structural integrity over its long circulation time, but will fall apart upon endocytosis, exposing the miRNA and the PtEN core. The exposed Pt(IV) compound was designed to enable endosomal escape upon degradation, releasing carbon dioxide and an inert, nontoxic, charged Pt(II) compound without a coordination site for Pt binding or intercalation. A neutral charge lipid nanoparticle formulation of miRNA led to significant uptake, but lack of efficacy even in vitro, underscoring the advantages of the PtEN core. Both Pt and the Alexa-miRNA mimic showed higher accumulation in tumor cells than in the liver, leading to suppression of liver metastases of colorectal cancer in mice treated with PtEN/miR-655-3p. We anticipate PtEN can thus be applied to a variety of therapeutic miRNAs outside the liver in addition to other therapeutics currently limited by poor pharmacokinetics or stability in circulation.
In summary, we have demonstrated that a nontoxic platinum-containing self-assembled core-shell nanoparticle can deliver miRNA to effectively suppress cancer metastasis in a xenogenic hepatic metastasis model of colorectal cancer. PtEN is distinctly superior to existing nanocarriers as it is inherently nontoxic and non-immunogenic, but can carry high loadings of miRNA with a long 14h circulation half-life and favorable biodistribution and tumor accumulation after systemic injection. PtEN/miRNA can be transfected into cultured cells or tumors without cationic lipids by loading miRNA onto the lipid shell to protect from degradation in sera, while releasing the cargo into the cytoplasm of the tumor cells. PtEN/miR-655-3p suppressed cancer cell proliferation and EMT by limiting β-catenin nuclear translocation and resultant cell invasion in vitro. Systemic administration of PtEN/miR-655-3p transfected circulating and/or invading tumor cells in vivo, limiting liver colonization by HCT116 cells.
The successful application of PtEN/miR-655-3p to suppress tumor metastasis provides proof of concept for an effective nontoxic delivery vehicle. The versatility of this platform provides a significant step towards overcoming the complex barriers inhibiting successful application of promising biologic therapies, such as nucleic acids and peptides.
Methods
Synthesis of [PtII(en)2bp]Cl2.
K2PtCl4 (0.5g) was stirred in the dark with excess ethylenediamine (en; 4mL) in 10mL H2O at room temperature for 2 h. The yellow solid was collected by vacuum filtration and then refluxed in the dark with excess en (0.8mL) in 10mL H2O for 2 h. The resultant white solid was collected by vacuum filtration and washed with cold H2O and EtOH. Yield for [PtII(en)2]Cl2: 77%. 1H NMR in D2O: δ 2.60 (triplet, 8H). The M/Z of [M-H]+ for [PtII(en)2]2+ was determined to be 314.1 (expected 314.1).
[PtII(en)2]Cl2 (0.35g) was reacted with 2.5mL 30% H2O2 in 5mL H2O at 70°C for 5 h in the dark. EtOH was added to the solution and cooled to −20°C overnight to yield a white solid, [PtIV(en)2(OH)2]Cl2. The solid was collected and washed twice with cold EtOH. Yield: 71%. 1H NMR in D2O: δ 2.94 (triplet, 8H). The M/Z of [M-H]+ for [PtIV(en)2(OH)2]2+ was determined to be 348.1 (expected 348.1).
[PtIV(en)2(OH)2]Cl2 (0.28 g) was dissolved in minimal DMF, to which 4 equivalents of diethoxyphosphinyl isocyanate (0.45 mL) was added at 4°C. The mixture was allowed to warm to room temperature and then reacted overnight in the dark. The solution was filtered, and the resulting white product [PtIV(en)2bis(phosphoester)]Cl2 was precipitated by addition of Et2O. The product was washed twice with cold Et2O. Yield: 65%. 1H NMR in D2O: δ 4.18 (m, 8H); δ 2.98 (m, 8H); δ 1.33 (t, 12H). The M/Z of [M-H]+ for [PtIV(en)2bis(phosphoester)]2+ was determined to be 706.3 (expected 706.2).
Trimethylsilyl bromide (TMSBr, 0.58mL) was added slowly to a solution of [PtIV(en)2bis(phosphoester)]Cl2 (0.30 g) in DMF (3mL) at 4 °C and stirred at r.t. in the dark under N2 protection for 18 h. A yellow solid was collected after addition of dichloromethane and then washed twice with additional dichloromethane. The solid was dissolved in MeOH and stirred overnight to hydrolyze the TMS-ester intermediate. The final product, [PtIV(en)2bp]Cl2 (bp = bisphosphonic acid [OCONHP(O)(OH)2]2), was collected by precipitation with dichloromethane and then washed twice with dichloromethane. Yield: 80%. 1H NMR in D2O: δ 3.08 (t, 8H). The M/Z of [M-H]+ for [PtIV(en)2bp]2+ was determined to be 594.1 (expected 594.3).
Preparation and characterization of PtEN/miRNAs.
A microemulsion of 0.27mL aqueous Zn(NO3)2·6H2O (100 mg/mL) was added to 10mL surfactant (0.3 M TritonX-100, 1.5 M hexanol in cyclohexane). This was added dropwise to a vigorously stirring microemulsion of 0.27mL aqueous [PtIV(en)2bp]Cl2 (25 mg/mL, pH=4) and 40μL of DOPA (200mg/mL in CHCl3) in 10mL surfactant. The solution was stirred in the dark for thirty minutes at room temperature, followed by precipitation with 20mL EtOH. DOPA-coated PtEN was collected by centrifugation at 12,000 rpm (16,743 ×g) for 20 minutes at 4C. The supernatant was discarded and the pellet was washed once with 50% cyclohexane/EtOH, once with 50% THF/EtOH, and then finally dispersed into THF and filtered through a 0.2μm PTFE filter.
PtEN was prepared by adding a THF solution (80 μL) of cholesterol, DSPC (cholesterol/DOPC=1:2 in molar ratio), 20 mol% DSPE-PEG2k, and DOPA-coated NCP to 500 μL of 30% (v/v) EtOH/H2O at 50 °C. The mixture was stirred at 1,700 rpm for 1 min. THF and EtOH were completely evaporated, and the PtEN solution was allowed to cool down to room temperature. PtEN/miRNA was similarly prepared with 10 μL DSPE-miRNA (2 mg/mL) added to EtOH/DEPC-H2O at a 10:1 weight ratio of DOPA-PtEN:DSPE-miRNA. The suspensions were prepared in water or 5% dextrose for in vitro and in vivo studies, respectively.
Toxicity analysis and Pt retention.
BALB/c mice were intraperitoneally injected with PtEN at a Pt dose of 3 mg/kg daily for a total of 10 doses. 30 days after the final dose, the mice were sacrificed and their blood, livers, spleens, lungs, kidneys, and bladders were harvested. A portion of the organs were embedded into optimal cutting temperature (OCT) medium, sectioned into 5 μM slices with a cryostat (NX50, Thermo, USA), and stained with haemotoxylin and eoisin (H&E) for histological examination by light microscopy. The rest of the organs were digested with concentrated nitric acid to determine Pt concentration by ICP-MS.
Cell uptake and cellular distribution of PtEN/miRNA.
5 × 105 HCT116 cells/well were seeded onto 6-well plates and cultured overnight. The Pt uptake was detected in cells treated with PtEN or PtEN/NT at equivalent Pt concentrations of 25 μM for 24 h. After incubation, the cells were collected, washed three times with PBS, counted with a hemocytometer and digested with concentrated nitric acid to determine Pt concentration by ICP-MS. The miRNA uptake was detected in cells treated with PBS, Alexa, or PtEN/Alexa at equivalent Alexa concentrations of 18 nM for 24 h. After incubation, the cells were collected, washed three times with PBS, and then analyzed for AlexaFluor647 fluorescence with the APC filter by flow cytometry (LSR II 3-8, BD, USA).
The intracellular distribution of PtEN/Alexa after internalization was evaluated by seeding 1 × 105 HCT116 cells/well onto 6-well plates with cover slips and cultured overnight. The culture medium was replaced with PtEN/Alexa at a concentration of 18 nM miRNA in serum-free culture medium and incubated for 15 min, 30 min, 1 h, or 2 h. The cells were washed three times with PBS, stained with 100 nM Lysotracker DND-26 for 2 h at 37°C, fixed with 4% paraformaldehyde, and stained with 1 μg/mL DAPI for 15 min at room temperature. The DAPI (blue), Lysotracker (green), and Alexa (red) fluorescence were observed by confocal microscopy (CLSM; FV1000, Olympus, Japan) and analyzed for colocalization by ImageJ.
Establishment of a xenogenic hepatic metastasis tumor model.
Liver metastases of colorectal cancer were established as previously described.68 Briefly, 2 × 106 HCT116-L2T cells stably transfected with luciferin and tdTomato were slowly injected into the spleens of athymic nude mice, respectively, anesthetized with 2% isoflurane in oxygen (v/v). The spleens were removed 5 min later to reduce tumor growth in the spleen and the abdominal cavity.
In vivo anti-metastatic efficacy.
Beginning 24 h after intrasplenic injection of HCT116-L2T, athymic nude mice were dosed with 5% dextrose (w/v), PtEN/NT, or PtEN/miR-655-3p at equivalent doses of 625 μg/kg miRNA once every three days until day 28. Once weekly, the mice were anesthetized with 2% isoflurane in oxygen (v/v) and subjected to bioluminescence imaging by an IVIS Spectrum 200 (Xenogen, USA). On day 28, the mice were euthanized and the livers were harvested for ex vivo fluorescence and liver weight.
Data availability.
The authors declare that all the data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author upon request.
Statistical Analysis.
Group sizes (N≥6) were chosen to ensure proper statistical ANOVA analysis for efficacy studies. Student’s t-tests were used to determine if the variance between groups is similar. Group sizes of N=3-4 were chosen for characterization that was not subject to statistical analysis. Statistical analysis was performed using OriginPro (OriginLab Corp.). Statistical significant was calculated using two-tailed Student’s t-tests and defined as * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. Animal experiments were not performed in a blinded fashion and are represented as mean ± SD.
Supplementary Material
Acknowledgements
We thank Dr. Demin Liu, Dr. Chunbai He, and Mr. Kui Yang for experimental help and helpful discussion. We acknowledge the NIH CBI Training Grant (NIH 5T32GM008720-15), the National Cancer Institute (1R01CA216436-01A1), the University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599), and the Ludwig Institute for Metastasis Research for funding support.
Footnotes
Supplementary Information accompanies this paper at http://www.nature.com/naturecommunications
Competing financial interests: W. L. is the founder of Coordination Pharmaceuticals, Inc., which licenses the NCP technology from the University of Chicago. All other authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seyfried TN & Huysentruyt LC On the origin of cancer metastasis. Crit Rev Oncog 18, 43–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehlen P & Puisieux A Metastasis: a question of life or death. Nat Rev Cancer 6, 449–458 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS & Theodorescu D Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol 5, 206–219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanger N et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer 129, 2522–2526 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Rocken M Early tumor dissemination, but late metastasis: insights into tumor dormancy. J Clin Invest 120, 1800–1803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narod SA, Iqbal J, Giannakeas V, Sopik V & Sun P Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol 1, 888–896 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Nelson VM & Benson AB 3rd Status of targeted therapies in the adjuvant treatment of colon cancer. J Gastrointest Oncol 4, 245–252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midgley R & Kerr DJ Adjuvant chemotherapy for stage II colorectal cancer: the time is right! Nat Clin Pract Oncol 2, 364–369 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Steeg PS Targeting metastasis. Nat Rev Cancer 16, 201–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandi G et al. Adjuvant chemotherapy for resected colorectal cancer metastases: Literature review and meta-analysis. World J Gastroenterol 22, 519–533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chibaudel B, Bonnetain F, Tournigand C & de Gramont A Maintenance treatment in metastatic colorectal cancer. Lancet Oncol 16, e583–584 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS et al. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol 9, 471–478 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Esserman LJ et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 15, e234–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen KJ, Gotzsche PC, Kalager M & Zahl PH Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis. Ann Intern Med 166, 313–323 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Kneuertz PJ et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 150, 402–409 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Price C & Chen J MicroRNAs in Cancer Biology and Therapy: Current Status and Perspectives. Genes Dis 1, 53–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy KB MicroRNA (miRNA) in cancer. Cancer Cell Int 15, 38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schetter AJ, Okayama H & Harris CC The role of microRNAs in colorectal cancer. Cancer J 18, 244–252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Bader AG, Brown D & Winkler M The promise of microRNA replacement therapy. Cancer Res 70, 7027–7030 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H et al. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev 81, 142–160 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Esau CC & Monia BP Therapeutic potential for microRNAs. Adv Drug Deliv Rev 59, 101–114 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Bader AG, Brown D, Stoudemire J & Lammers P Developing therapeutic microRNAs for cancer. Gene Ther 18, 1121–1126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng CJ et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 518, 107–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uppal A et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget 6, 3540–3552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harazono Y et al. miR-655 Is an EMT-suppressive microRNA targeting ZEB1 and TGFBR2. PLoS One 8, e62757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao XQ, Liang B, Jiang K & Zhang HY Down-regulation of miR-655-3p predicts worse clinical outcome in patients suffering from hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 21, 748–752 (2017). [PubMed] [Google Scholar]
- 28.Niibe Y & Hayakawa K Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 40, 107–111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G et al. MicroRNA-655-3p functions as a tumor suppressor by regulating ADAM10 and beta-catenin pathway in Hepatocellular Carcinoma. J Exp Clin Cancer Res 35, 89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raemdonck K, Vandenbroucke RE, Demeester J, Sanders NN & De Smedt SC Maintaining the silence: reflections on long-term RNAi. Drug Discov Today 13, 917–931 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Gao DY & Huang L In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81, 128–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garzon R, Marcucci G & Croce CM Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 9, 775–789 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zatsepin TS, Kotelevtsev YV & Koteliansky V Lipid nanoparticles for targeted siRNA delivery - going from bench to bedside. Int J Nanomedicine 11, 3077–3086 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaczmarek JC, Kowalski PS & Anderson DG Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med 9, 60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett JC & Rossi JJ RNA-based therapeutics: current progress and future prospects. Chem Biol 19, 60–71 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayaraman M et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 51, 8529–8533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coelho T et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369, 819–829 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Suhr OB et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis 10, 109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams D et al. Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol 17, 181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizk M & Tuzmen S Update on the clinical utility of an RNA interference-based treatment: focus on Patisiran. Pharmgenomics Pers Med 10, 267–278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benson MD, Ackermann EJ & Monia BP Treatment of transthyretin cardiomyopathy with a TTR-specific antisense oligonucleotide (IONIS-TTRRx). Amyloid 24, 134–135 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Chen W & Yang L Targeted Delivery with Imaging Assessment of siRNA Expressing Nanocassettes into Cancer. Methods Mol Biol 1372, 49–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho YS et al. Targeted delivery of siRNA-generating DNA nanocassettes using multifunctional nanoparticles. Small 9, 1964–1973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnaby SN et al. Design Considerations for RNA Spherical Nucleic Acids (SNAs). Bioconjug Chem 27, 2124–2131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosi NL et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Garbuzenko OB et al. Combinatorial treatment of idiopathic pulmonary fibrosis using nanoparticles with prostaglandin E and siRNA(s). Nanomedicine 13, 1983–1992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen SA et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med 5, *****209ra152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anchordoquy TJ et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano 11, 12–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Yang Z, Patanavanich S, Xu B & Chau Y Controlling self-assembly within nanospace for peptide nanoparticle fabrication. Soft Matter 4, 1617–1620 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Goodwin TJ & Huang L Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 35, 2550–2557 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He C, Poon C, Chan C, Yamada SD & Lin W Nanoscale Coordination Polymers Codeliver Chemotherapeutics and siRNAs to Eradicate Tumors of Cisplatin-Resistant Ovarian Cancer. J Am Chem Soc 138, 6010–6019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oshima G et al. In Vivo Delivery and Therapeutic Effects of a MicroRNA on Colorectal Liver Metastases. Mol Ther 25, 1588–1595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drew HDK *****332. The behaviour of chelate groupings attached to platinum and to palladium. Journal of the Chemical Society (Resumed), 2328–2331 (1932). [Google Scholar]
- 54.Liu D, Poon C, Lu K, He C & Lin W Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat Commun 5, 4182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poon C, Duan X, Chan C, Han W & Lin W Nanoscale Coordination Polymers Codeliver Carboplatin and Gemcitabine for Highly Effective Treatment of Platinum-Resistant Ovarian Cancer. Mol Pharm 13, 3665–3675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chin CF, Wong DY, Jothibasu R & Ang WH Anticancer platinum (IV) prodrugs with novel modes of activity. Curr Top Med Chem 11, 2602–2612 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Johnstone TC, Suntharalingam K & Lippard SJ The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem Rev 116, 3436–3486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu HK & Sadler PJ Metal complexes as DNA intercalators. Acc Chem Res 44, 349–359 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Zeglis BM, Pierre VC & Barton JK Metallo-intercalators and metallo-insertors. Chem Commun (Camb), 4565–4579 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung MF et al. A liposomal system capable of generating CO2 bubbles to induce transient cavitation, lysosomal rupturing, and cell necrosis. Angew Chem Int Ed Engl 51, 10089–10093 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Han HD et al. Therapeutic efficacy of doxorubicin delivery by a CO2 generating liposomal platform in breast carcinoma. Acta Biomater 24, 279–285 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Chen KJ et al. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano 7, 438–446 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Blobe GC, Schiemann WP & Lodish HF Role of transforming growth factor beta in human disease. N Engl J Med 342, 1350–1358 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Ishitsuka Y et al. Pituitary tumor-transforming gene 1 enhances proliferation and suppresses early differentiation of keratinocytes. J Invest Dermatol 132, 1775–1784 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Dou H, Huang C, Singh M, Carpenter PB & Yeh ET Regulation of DNA repair through deSUMOylation and SUMOylation of replication protein A complex. Mol Cell 39, 333–345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D et al. The role of beta-catenin in the initiation and metastasis of TA2 mice spontaneous breast cancer. J Cancer 8, 2114–2123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamieson C, Sharma M & Henderson BR Targeting the beta-catenin nuclear transport pathway in cancer. Semin Cancer Biol 27, 20–29 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Oshima G et al. Imaging of tumor clones with differential liver colonization. Sci Rep 5, 10946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Husemann Y et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Kang Y & Pantel K Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 23, 573–581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author upon request.