Abstract
The neostriatum and its connections control the sequential organization of action (“action syntax”) as well as simpler aspects of movement. This study focused on sequential organization of rodent grooming. Grooming syntax provides an opportunity to study how neural systems coordinate natural patterns of serial order. The most stereotyped of these grooming patterns, a “syntactic chain,” has a particularly stereotyped order that recurs thousands of times more often than could occur by chance. The purpose of the present study was to identify the crucial site within the striatopallidal system where lesions disrupt the syntax or serial order of syntactic grooming chains without disrupting constituent movements. Small excitotoxin lesions were made using quinolinic acid at bilateral sites within the dorsolateral, dorsomedial, ventrolateral, or ventromedial neostriatum, or in the ventral pallidum or globus pallidus of rats. An objective technique for mapping functional lesions was used to quantify cell death and to map precisely those lesions that disrupted grooming syntax. Our results identified a single site within the anterior dorsolateral neostriatum, slightly more than a cubic millimeter in size (1.3 × 1.0 × 1.0 mm), as crucial to grooming syntax. Damage to this site did not disrupt the ability to emit grooming actions. By contrast, damage to sites in the ventral pallidum and globus pallidus impaired grooming actions but left the sequential organization of grooming syntax intact. Neural circuits within this crucial “action syntax site” seem to implement sequential patterns of behavior as a specific function.
Keywords: neostriatum, globus pallidus, basal ganglia, pallidum, movement, sensorimotor, sequence, serial order, syntax, lesion, quinolinic acid, excitotoxin, stereology, grooming, neuroethology
Traditionally, the neostriatum was thought to be involved primarily in simple motor functions such as movement initiation and execution (Wilson, 1914; Denny-Brown, 1962); however, the neostriatum also participates in more complex motor functions such as behavioral sequencing (Cools, 1980; Marsden, 1982; Evarts et al., 1984; Berridge and Fentress, 1987; Kermadi and Joseph, 1995), sensorimotor modulation (Schneider and Lidsky, 1981; Lidsky et al., 1985; Schallert and Hall, 1988), and motivational-sensorimotor integration (Hall and Schallert, 1988; Berridge and Cromwell, 1990;Mogenson and Yang, 1991; Bakshi and Kelley, 1993; Schultz et al., 1995). The present study was undertaken specifically to examine neuroanatomical constraints on the control of behavioralsequencing by the neostriatum.
Studies of clinical populations support the hypothesis that the neostriatum plays a role in the sequencing of action (Agostino et al., 1992; Bradshaw et al., 1992). Some Parkinson’s and Huntington’s disease patients have difficulty in sequencing action into ordered combinations (Marsden, 1984; Oepen et al., 1985; Folstein, 1989;Harrington and Haaland, 1991; Montgomery and Buchholz, 1991; Brown et al., 1993). For example, when Parkinson’s disease patients were asked to perform a series of heterogeneous hand postures, they committed significantly more errors in sequential ordering than control subjects did (Harrington and Haaland, 1991).
Animal studies have provided more direct evidence for the hypothesis that the neostriatum is involved in the sequencing of action (Cools, 1980; Van den Brecken and Cools, 1982; Cools, 1985; Sabol et al., 1985;Whishaw et al., 1986; Berridge and Fentress, 1987; Mansbach et al., 1988; Pisa, 1988; Jaspers et al., 1990; Nakamura et al., 1990; Berridge and Whishaw, 1992; Gardiner and Kitai, 1992; Kimura et al., 1992;Aldridge et al., 1993; Kermadi et al., 1993; Pellis et al., 1993; Wiener, 1993; Mittler et al., 1994; Baunez et al., 1995; Kermadi and Joseph, 1995). Most animal studies of the role of the neostriatum in sequential coordination have examined the relation of neostriatal activity to the performance of learned sequences of behavior, such as reaching for food. Such studies effectively demonstrate the importance of the neostriatum to patterned sequences of behavior; however, they leave open the question of whether the neostriatum facilitates behavioral sequences directly by coordinating the organization of serial patterns, a function Lashley (1951) termed “action syntax,” or less directly by mediating motor learning and memory.
Neuroethological studies of species-specific action sequences depend less on explicit training than learned sequences do, and so they can help dissociate memory deficits from the actual serial coordination processes that generate action syntax. Natural species-specific sequences, such as those used in rodent self-grooming, exhibit serial patterns that are rule-governed, predictable, and to a large degree coordinated endogenously by the brain (Fentress, 1972; Sachs, 1988;Berridge, 1990; Fentress, 1992). In rats, the most highly stereotyped sequences of grooming, called “syntactic chains,” combine up to 25 actions into a predictable order that occurs 13,000 times greater than chance (Berridge and Fentress, 1986; Berridge et al., 1987).
The serial order of grooming syntax depends crucially on the neostriatum (Berridge and Fentress, 1987; Berridge, 1989b; Berridge and Whishaw, 1992; Aldridge et al., 1993). In early postnatal development, syntactic chains emerge coincidentally with the maturation of projections to neostriatum (Colonnese et al., in press). In adults, the sequential organization of syntactic chains is disrupted by large excitotoxin lesions of the neostriatum (Berridge and Fentress, 1987), by 6-hydroxydopamine lesions of the nigrostriatal tract (Berridge, 1989b), and by neostriatal ablation (Berridge and Whishaw, 1992), even though the capacity to emit grooming actions is not impaired by these lesions. By comparison, lesions to other motor structures such as motor cortex (M1 and M2), prefrontal cortex, or the cerebellum do not result in sequential disruption of syntactic grooming chains, even though such lesions produce other motor consequences (Berridge and Whishaw, 1992). Such evidence demonstrates that the neostriatum makes a special intrinsic contribution to grooming syntax, above and beyond that of motor cortex and related structures.
It is clear from both neuroanatomical and functional studies that the striatopallidal system is segregated into heterogeneous compartments (Graybiel and Ragsdale, 1978; Haber et al., 1985; Alexander et al., 1986; Nauta, 1989; Delong, 1990; Hazrati and Parent, 1992; Haber et al., 1993; Hoover and Strick, 1993). The goal of the present study was to determine whether any particular neuroanatomical regionof the neostriatum or pallidum is especially crucial for sequential coordination. This was approached by creating small targeted excitotoxin lesions in prespecified regions and identifying the crucial striatopallidal region in which neuron death produced sequential impairment.
MATERIALS AND METHODS
Rats were housed individually on a 14 hr light/10 hr dark cycle throughout the experiment. Small bilateral lesions were made in one of seven selected targets within the striatopallidal system. A functional lesion mapping procedure for identifying neuroanatomical sites responsible for behavioral deficits, the “modified fractionator” procedure (Cromwell and Berridge, 1993, 1994), was used to delineate the crucial site in which neuron death disrupts grooming syntax.
Surgery
Sprague–Dawley male rats (n = 108) were anesthetized with halothane (by placing the rat in a small enclosed chamber above a halothane-soaked paper towel) followed by methoxyfluorane delivered by a gas anesthesia system for small animals (given when the rat was placed in the stereotaxic and continued throughout the surgery; Lasiter and Garcia, 1984). Atropine sulfate (3 mg/kg, i.p.) and bicillin (30,000 U, i.m.) were given before surgery.
The four quadrants of the neostriatum (dorsomedial, dorsolateral, ventromedial, ventrolateral), the globus pallidus, and the ventral pallidum were each targeted for bilateral lesions in separate groups of rats. Because preliminary results from a pilot study (Cromwell and Berridge, 1990) had indicated that the dorsolateral quadrant might be especially crucial to syntactic sequences of action, the dorsolateral quadrant was subdivided into separate anterior and posterior targets. Coordinates for lesions intended to damage the anterior dorsolateral neostriatum were anterior-posterior (AP) +1.0 mm anterior to bregma, lateral (L) ±1.7 mm lateral to bregma, ventral (V) −4.5 mm below the skull (n = 8 rats); for the posterior dorsolateral neostriatum: AP −1.0, L ±2.9, V −4.7 (n = 8 rats); for the dorsomedial neostriatum: AP +1.0, L ±1.6, V −4.2 (n = 8 rats); for the ventrolateral neostriatum: AP +1.0, L ±3.6, V −6.6 (n = 8 rats); for the ventromedial neostriatum: AP +1.0, L ±1.6, V −4.2 (n = 8 rats); for the globus pallidus: AP −1.0, L ±2.6, V −7.5 (n = 8); and for the ventral pallidum: AP −1.0, L ±2.5, V −8.0 (n = 8 rats).
With bregma and λ in the same horizontal plane, bilateral skull holes were drilled, and a 30 gauge injection needle was lowered to the appropriate level. Lesions were made using the excitotoxin quinolinic acid (10 μgm freshly dissolved in 1 μl of PBS, pH 7.4. The injection was made during a 3 min period, and the needle was left in place for an additional 5 min. For each site, a group of eight vehicle control rats received bilateral infusions of the PBS alone, without excitotoxin.
Postsurgical maintenance
Diazepam (8 mg/kg) was given within 30 min of the first injection to minimize damage outside the injection site attributable to excitoconvulsive activity. A second injection of diazepam (8 mg/kg) was given 1 hr later. All rats were provided each day with 250 ml of cereal mash (commercial baby cereal freshly mixed with water), 5 chow pellets, and 40 ml of water. Intake was monitored by counting the number of pellets, the approximate amount of mash eaten (e.g., 50%), and the amount of water drunk within a 24 hr period, and by weighing each rat daily. Rats were considered aphagic if neither chow pellets nor mash were eaten. If a rat lost weight after surgery, an intubation procedure was initiated to maintain good health. For each 5 gm of weight lost, a rat was intubated with 12 ml of vitamin-supplemented sweetened milk solution, up to three intubations per day. This prevented dehydration or malnutrition and helped maintain good physical condition during the experiment.
Behavioral testing
Grooming sequences were videotaped during 10 min test sessions on alternate days (between 2 and 6 P.M.) starting 2 d after surgery. Grooming was elicited by lightly spraying the dorsal side of the torso of the rat with a water mist. The rat was placed on a transparent plastic floor under which a mirror was positioned to reflect a close-up view of the head and upper body of the rat into the lens of a video camera. The rat was allowed to habituate for 5 min to the situation before its fur was sprayed. Trials were repeated during subsequent days until a total of at least 10–12 min of grooming behavior, cumulative across days, had been videotaped for each rat (mean = 15 d). All videotaped grooming sequences were scored as described below.
Grooming syntax
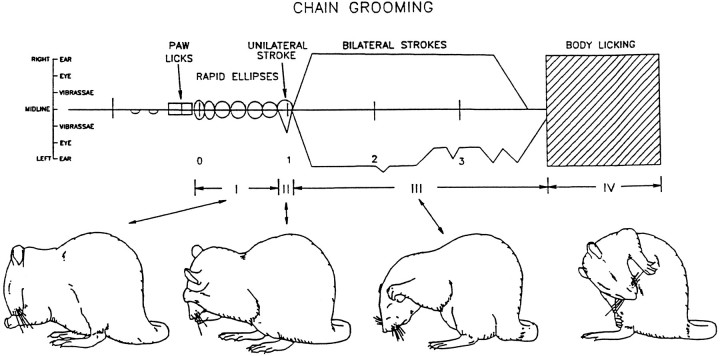
The serial organization of syntactic grooming chains arranges at least 15–25 forepaw strokes and licking actions into four consecutive sequential phases (Fig. 1) as follows. Phase I: A concatenation of five to nine small, rapid bilateral forepaw strokes (“ellipses”) around the nose and mouth at a rate of 6–7 Hz. Ellipse stroke movements at this speed are extremely rare outside of syntactic chains. The concatenation of multiple ellipse strokes, faster than 6 Hz, virtually never occurs during nonchain grooming (Berridge, 1990). A fast series of Phase I ellipse strokes thus serves as an excellent marker for the initiation of syntactic chains.Phase II: A short bout of one to four small or medium paw strokes along the mystacial vibrissae, usually performed by one unilateral paw or by both paws tracing asymmetric amplitudes.Phase III: A repetitive series of 3–10 large bilaterally symmetrical strokes, which may extend behind the ears and most of the head. Phase IV: A bout of body licking over the lateral and ventral torso (Fig. 1). Once the initial components of Phase I appear, the entire sequence follows to Phase IV with a completion rate of 85–95% for normal rats (Berridge et al., 1987).
Fig. 1.
Choreographed “syntactic chain” sequence of grooming actions. A choreographic transcription of a prototypical syntactic grooming chain shows the moment-by-moment trajectories of forelimb strokes over the face and the occurrence of other grooming actions. Drawings (bottom) display the actions that typify each phase of syntactic chains. To read the choreographic transcription, time proceeds from left to right. Thehorizontal axis represents the position of the rat’s nose, and stroke trajectories over the face are depicted relative to the nose. Deviations of the lines above (right paw) and below (left paw) the horizontal axis represent the elevation (level of the eye, the ear, etc.) reached by each forepaw during a stroke. Small rectangles denote paw licks. Large rectangle denotes body licking (adapted from Aldridge et al., 1990).
Behavioral video analysis
Videotapes were analyzed in slow motion (frame by frame to one tenth actual speed) by independent, trained observers blind to the experimental condition of each rat, and they were scored using a computer-aided event-recording procedure and a choreographic grooming notation system (Berridge et al., 1987; Berridge, 1990) (sample notation of syntactic chain shown in Fig. 1). Grooming behavior was analyzed for the following features.
(1) Occurrence of syntactic chain sequences. The beginning of each syntactic chain was counted to assess the propensity to engage in patterned sequences of action. Chain initiation was defined as the occurrence of a full Phase I: a bout of five to nine consecutive bilateral “ellipses” (small rapid strokes in which the paws trace a tight elliptical trajectory around the mouth) emitted at a rate of at least 6 Hz. To qualify as a syntactic chain initiation, Phase I had to be followed immediately by either a Phase II stroke, namely a unilateral stroke or an asymmetrical bilateral stroke over the vibrissae, or a Phase III stroke, namely large amplitude overhand strokes over the ears or eyes, performed simultaneously with both paws. Phase III typically comprises a set of 3–10 large-trajectory bilateral strokes.
(2) Efficacy of syntactic completion. Once initiated, grooming chains were analyzed for syntactic completion rates for each lesion group to assess the ability to implement full sequential patterns. A “syntactically perfect” complete chain was defined as one that progressed through Phases I, II, III, and IV (body licking, in which the rat lowered its head after the last Phase III stroke and turned sideways in order to bring its tongue in contact with its flank or back), without interruption and within 5 sec of Phase I.
In intact rats, certain kinds of “imperfect” syntactic completion are seen on rare occasions, and the incidence of imperfect completion is increased by some neocortical or cerebellar brain lesions (Berridge and Whishaw, 1992). For example, after such lesions an extraneous action may intrude between one of the phases (e.g., paw licking between Phases III and IV), or a phase may be skipped (e.g., Phase I connects directly with Phase III and Phase II is not seen), or Phase IV body licking may be replaced by paw or forelimb licking. Imperfect chains were tallied separately, and the perfect and imperfect completion rates were determined for each lesion group and their respective controls. Finally, “incomplete” chains were considered to be those in which the rat reverted to sequentially flexible grooming within the chain or in which the rat simply stopped grooming before Phase IV.
A factor that has been found previously to be important in influencing syntactic completion is the order of chain emission within a grooming bout (i.e., whether a chain is the first, second, etc., to be emitted within that bout). Initial chains of a test trial are less likely to be completed than are subsequent chains: a “warm-up” effect (Berridge, 1990; Berridge and Whishaw, 1992). For each lesion group, the first chain and subsequent chains were analyzed separately to determine whether an effect of specific striatopallidal damage interacted with this factor.
(3) Simple motor impairments: movement frequency. To detect motor impairments in the ability to perform grooming actions, detailed counts were made of the actual number of forelimb strokes, paw licks, and body licks emitted during each videotaped grooming bout. The amplitude of forelimb strokes was scored as either large (passing above the eye), medium (highest point between vibrissae and eye), or small (not extending above vibrissae). The laterality of each stroke was scored as either bilateral (both paws following symmetrical trajectories) or unilateral (a single paw or both paws following asymmetrical trajectories). This was tabulated in a separate slow-motion video analysis using a computer-assisted keyboard scoring procedure (Berridge et al., 1987). The videotape was played at one tenth speed, and a key was pressed that corresponded to each grooming action at onset and offset. Deficits in the ability to control paw or tongue movements would be expected to be reflected in the distortion of the relative frequency and timing of one type of action relative to the others, or in a global reduction of all grooming actions.
(4) Ellipse timing and syntactic chain completion. Variation in the speed of ellipses emitted in Phase I can predict whether the entire chain will be completed syntactically to Phase IV. Completed syntactic chains have a faster rate of Phase I ellipses than chains that fail to be completed syntactically (Berridge, 1990). To determine whether this relationship between ellipse rate and chain completion remains intact after lesions to discrete striatopallidal subregions, the ellipse cycle length was compared for complete and incomplete chains for each lesion group. To discover whether ellipse rate varied with chain order (first chain of a video session vs later chains of the same session), the effect of chain order on ellipse duration and chain completion was also examined.
(5) Microstructure of syntactic chains. Finally, the stroke-by-stroke microstructure of each syntactic grooming chain was transcribed using a detailed choreographic notation system that depicts a moment-by-moment flow of paw trajectories and other grooming actions (Berridge and Fentress, 1986; Berridge et al., 1987; Berridge, 1990). The microstructure of syntactic chains of rats from each lesion group was analyzed statistically in terms of the number of forelimb strokes contained within Phases I, II, and III of the chain, the symmetry of the stroke trajectories made by the two forepaws, and the latency after Phase I to complete the chain by the onset Phase IV body licking.
Histological procedures
At the conclusion of testing, each rat was anesthetized deeply and perfused intracardially with 0.9% saline followed by 10% formalin in PBS, pH 7.4. Brains were removed and stored in a 30% sucrose solution (10% formalin). Rats that died before perfusion were decapitated, and their brains were removed and soaked in formalin for at least 7 d. The brains were blocked, frozen, and sliced in 30 μm slices with a sliding microtome. Alternate slices were saved for cresyl violet staining, to label neuron cell bodies, or for glial fibrillary acidic protein immunoreactivity (GFAP-IR) staining, to label astrocytes. Cresyl violet: Slices were mounted directly onto gelatin-coated slides for Nissl staining. The slides were dipped in xylene and ethanol baths (70%, 95%, and 100%) for cleaning and defatting. After being dipped in cresyl violet, the slides were taken through the final set of alcohols and xylenes before coverslipping using permount. GFAP: Slices were rinsed in three consecutive washes (PBS with 1% bovine serum albumin and 0.03% Triton X-100), transferred to 1.5 ml centrifuge tubes containing rabbit anti-GFAP (primary, diluted 1:500; Dako, Carpinteria, CA), and placed on a rototorque to turn slowly for 20–24 hr at 5°C. The slices were rinsed again, rotated for 1 hr in peroxidase conjugated to goat anti-rabbit IgG (secondary, diluted 1:100; Dako), re-rinsed, and bathed in freshly prepared 3,3-diaminobenzidine tetrahydrochloride before mounting.
Stereological lesion analysis
The lesions of rats that displayed grooming syntax deficits were analyzed in detail using the modified fractionator technique for mapping functional lesions, which was described earlier (Cromwell and Berridge, 1993, 1994). Briefly, this modified fractionator procedure for assessing functional lesions was carried out in three stages.
Stage 1. Normal reference maps. First, to obtain accurate normal baseline neuron averages, the striatopallidal region was divided into 251 fractions. The normal neuronal density of each fraction in control rats was calculated using a modification of the fractionator technique of Gundersen et al. (1988) (n = 8 control rats) (Fig. 2 and Table 1). For each fraction, an exhaustive neuronal count was made of a 250 × 250 × 30 μm “core sample,” a 250 μm2 area of a 30-μm-thick tissue section whose position was randomly chosen within the fraction. The microscope was set at 400× magnification, and all neurons within the core sample were counted using a computerized image-analysis system (JAVA, Jandel). Neuronal density varied in normal animals from 12 neurons per core sample in the globus pallidus to 154 neurons per core sample in certain fractions from the ventrolateral neostriatum; however, neuronal density never differed by >25% between different rats for the same fraction (Table 1). This consistency in fraction neuronal number across animals allowed us to set a criterion for the detection of lesions. It meant that decrements in neuronal density significantly beyond 25% of the normal value for that fraction (e.g., by 50% or more) denoted pathological neuron loss.
Fig. 2.
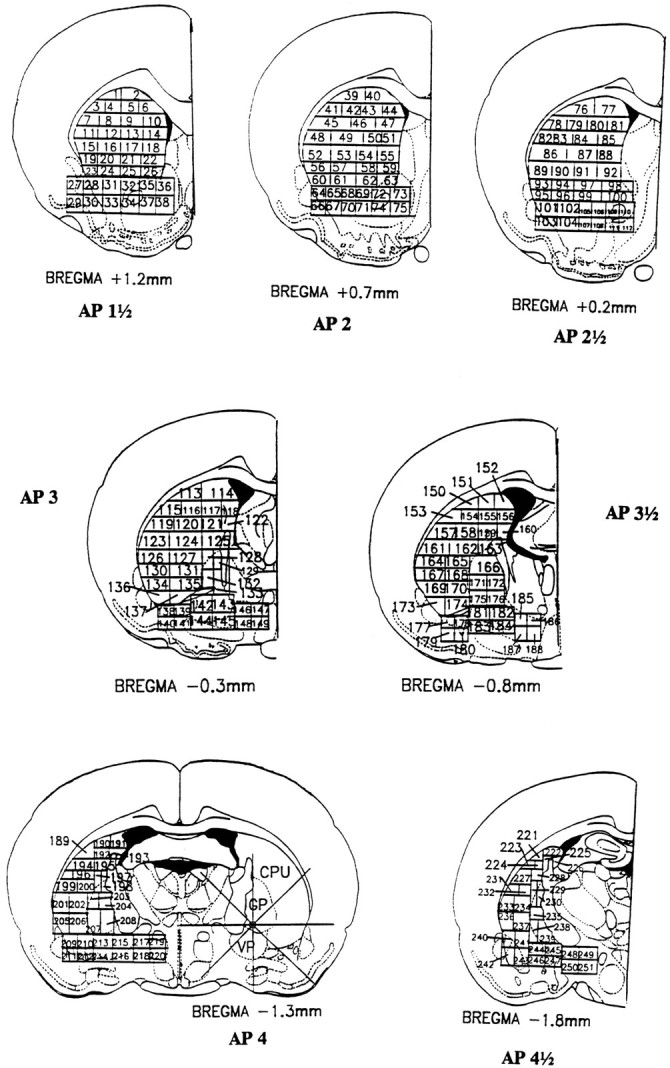
Map of fraction assignment within the striatopallidal system used for this study. Neuronal densities were calculated separately from core samples for each fraction. Baseline neuronal densities ranged from 12 neurons for fractions in the globus pallidus to >150 neurons in the ventrolateral neostriatum per 250 × 250 × 30 μm core sample (Table 1). For any given numbered fraction, however, neuronal density varied across different control rats by <25%.
Table 1.
Normal neuronal densities for striatopallidal fractions
| Bregma +1.2 mm | Bregma +0.7 mm | Bregma +0.2 mm | Bregma −0.3 mm | Bregma −0.8 mm | Bregma −1.3 mm | Bregma −1.8 mm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction no. | Density | Fraction no. | Density | Fraction no. | Density | Fraction no. | Density | Fraction no. | Density | Fraction no. | Density | Fraction no. | Density |
| 1 | 60 ± 15 | 39 | 75 ± 19 | 76 | 76 ± 18 | 113 | 70 ± 12 | 150 | 60 ± 10 | 189 | 65 ± 13 | 221 | 52 ± 13 |
| 2 | 65 ± 17 | 40 | 81 ± 18 | 77 | 45 ± 12 | 114 | 101 ± 25 | 151 | 79 ± 12 | 190 | 64 ± 10 | 222 | 63 ± 15 |
| 3 | 71 ± 17 | 41 | 53 ± 16 | 78 | 36 ± 8 | 115 | 55 ± 14 | 152 | 79 ± 12 | 191 | 64 ± 10 | 223 | 52 ± 10 |
| 4 | 66 ± 16 | 42 | 69 ± 11 | 79 | 63 ± 11 | 116 | 56 ± 12 | 153 | 51 ± 10 | 192 | 54 ± 9 | 224 | 32 ± 6 |
| 5 | 70 ± 18 | 43 | 59 ± 11 | 80 | 49 ± 9 | 117 | 54 ± 12 | 154 | 55 ± 12 | 193 | 68 ± 12 | 225 | 59 ± 9 |
| 6 | 75 ± 19 | 44 | 52 ± 12 | 81 | 55 ± 10 | 118 | 51 ± 13 | 155 | 59 ± 13 | 194 | 40 ± 10 | 226 | 61 ± 10 |
| 7 | 60 ± 7 | 45 | 63 ± 11 | 82 | 48 ± 10 | 119 | 64 ± 14 | 156 | 81 ± 19 | 195 | 57 ± 13 | 227 | 49 ± 10 |
| 8 | 60 ± 7 | 46 | 58 ± 8 | 83 | 48 ± 10 | 120 | 64 ± 14 | 157 | 59 ± 13 | 196 | 58 ± 4 | 228 | 44 ± 5 |
| 9 | 65 ± 11 | 47 | 46 ± 7 | 84 | 50 ± 9 | 121 | 40 ± 13 | 158 | 59 ± 13 | 197 | 44 ± 13 | 229 | 51 ± 12 |
| 10 | 64 ± 13 | 48 | 46 ± 7 | 85 | 46 ± 8 | 122 | 60 ± 12 | 159 | 45 ± 12 | 198 | 33 ± 7 | 230 | 59 ± 17 |
| 11 | 59 ± 11 | 49 | 57 ± 11 | 86 | 42 ± 8 | 123 | 75 ± 10 | 160 | 66 ± 16 | 199 | 75 ± 14 | 231 | 39 ± 7 |
| 12 | 61 ± 11 | 50 | 51 ± 13 | 87 | 45 ± 9 | 124 | 57 ± 12 | 161 | 55 ± 12 | 200 | 41 ± 3 | 232 | 12 ± 3 |
| 13 | 58 ± 9 | 51 | 51 ± 13 | 88 | 18 ± 2 | 125 | 76 ± 16 | 162 | 61 ± 17 | 201 | 77 ± 13 | 233 | 77 ± 14 |
| 14 | 58 ± 9 | 52 | 68 ± 11 | 89 | 52 ± 9 | 126 | 70 ± 13 | 163 | 48 ± 8 | 202 | 69 ± 10 | 234 | 56 ± 14 |
| 15 | 75 ± 12 | 53 | 59 ± 12 | 90 | 52 ± 9 | 127 | 80 ± 12 | 164 | 54 ± 7 | 203 | 15 ± 4 | 235 | 12 ± 2 |
| 16 | 70 ± 12 | 54 | 72 ± 12 | 91 | 66 ± 11 | 128 | 26 ± 4 | 165 | 37 ± 8 | 204 | 15 ± 4 | 236 | 94 ± 15 |
| 17 | 67 ± 11 | 55 | 72 ± 12 | 92 | 66 ± 11 | 129 | 24 ± 3 | 166 | 12 ± 3 | 205 | 84 ± 21 | 237 | 63 ± 17 |
| 18 | 67 ± 11 | 56 | 64 ± 10 | 93 | 58 ± 11 | 130 | 103 ± 19 | 167 | 54 ± 7 | 206 | 72 ± 18 | 238 | 21 ± 10 |
| 19 | 71 ± 14 | 57 | 45 ± 10 | 94 | 78 ± 14 | 131 | 79 ± 15 | 168 | 37 ± 8 | 207 | 19 ± 4 | 239 | 13 ± 4 |
| 20 | 55 ± 10 | 58 | 54 ± 12 | 95 | 84 ± 17 | 132 | 25 ± 8 | 169 | 76 ± 12 | 208 | 21 ± 7 | 240 | 58 ± 14 |
| 21 | 68 ± 11 | 59 | 51 ± 13 | 96 | 71 ± 15 | 133 | 24 ± 3 | 170 | 62 ± 6 | 209 | 63 ± 10 | 241 | 75 ± 12 |
| 22 | 69 ± 12 | 60 | 64 ± 10 | 97 | 58 ± 11 | 134 | 154 ± 33 | 171 | 15 ± 3 | 210 | 36 ± 12 | 242 | 65 ± 15 |
| 23 | 71 ± 9 | 61 | 45 ± 10 | 98 | 78 ± 14 | 135 | 79 ± 15 | 172 | 17 ± 4 | 211 | 101 ± 21 | 243 | 58 ± 14 |
| 24 | 55 ± 10 | 62 | 54 ± 12 | 99 | 84 ± 17 | 136 | 103 ± 19 | 173 | 76 ± 12 | 212 | 34 ± 8 | 244 | 23 ± 7 |
| 25 | 68 ± 11 | 63 | 51 ± 13 | 100 | 71 ± 15 | 137 | 79 ± 15 | 174 | 62 ± 6 | 213 | 28 ± 6 | 245 | 18 ± 7 |
| 26 | 69 ± 12 | 64 | 81 ± 16 | 101 | 103 ± 20 | 138 | 154 ± 31 | 175 | 15 ± 3 | 214 | 18 ± 5 | 246 | 26 ± 6 |
| 27 | 55 ± 9 | 65 | 84 ± 12 | 102 | 104 ± 13 | 139 | 96 ± 22 | 176 | 17 ± 4 | 215 | 35 ± 10 | 247 | 36 ± 7 |
| 28 | 79 ± 16 | 66 | 73 ± 15 | 103 | 96 ± 17 | 140 | 24 ± 3 | 177 | 82 ± 18 | 216 | 34 ± 7 | 248 | 41 ± 9 |
| 29 | 81 ± 20 | 67 | 82 ± 14 | 104 | 93 ± 15 | 141 | 25 ± 3 | 178 | 53 ± 16 | 217 | 48 ± 12 | 249 | 87 ± 13 |
| 30 | 85 ± 21 | 68 | 103 ± 16 | 105 | 88 ± 13 | 142 | 29 ± 5 | 179 | 37 ± 6 | 218 | 86 ± 13 | 250 | 42 ± 6 |
| 31 | 76 ± 15 | 69 | 90 ± 14 | 106 | 67 ± 16 | 143 | 32 ± 6 | 180 | 36 ± 4 | 219 | 44 ± 10 | 251 | 59 ± 5 |
| 32 | 91 ± 25 | 70 | 45 ± 17 | 107 | 23 ± 4 | 144 | 29 ± 5 | 181 | 24 ± 6 | 220 | 77 ± 12 | ||
| 33 | 75 ± 17 | 71 | 76 ± 20 | 108 | 48 ± 12 | 145 | 56 ± 14 | 182 | 35 ± 12 | ||||
| 34 | 62 ± 12 | 72 | 66 ± 14 | 109 | 85 ± 12 | 146 | 76 ± 15 | 183 | 26 ± 4 | ||||
| 35 | 101 ± 25 | 73 | 112 ± 21 | 110 | 71 ± 13 | 147 | 81 ± 24 | 184 | 30 ± 5 | ||||
| 36 | 95 ± 20 | 74 | 105 ± 21 | 111 | 73 ± 14 | 148 | 36 ± 10 | 185 | 40 ± 3 | ||||
| 37 | 88 ± 15 | 75 | 108 ± 23 | 112 | 51 ± 8 | 149 | 65 ± 13 | 186 | 42 ± 10 | ||||
| 38 | 89 ± 16 | 187 | 54 ± 12 | ||||||||||
| 188 | 75 ± 14 | ||||||||||||
Stage 2a. Lesion center identification. “Moderate neuron loss” was judged to exist if a fraction lost at least 50% of its neurons. “Severe neuron loss” was judged to exist if the fraction had lost at least 80% of its neurons. Fractions that had the most severe neuron loss were labeled as the center of the lesion.
Stage 2b. Lesion border mapping. Once the center of the lesion had been located, eight radial arms emanating from the center along the major compass points (0°, 45°, 90°, 135°, etc.) were drawn using the video image analysis system. Core sample counts were taken along each line at 250 μm steps (see above) until the neuron density rose above 50% of the normal level for that fraction, which was labeled as the border of the lesion.
Stage 3. Subtraction of noncrucial sites of damage. To identify the crucial site responsible for the sequential grooming deficit, a composite map of total shared damage was made first. This was accomplished by adding the mapped lesions of each rat together, producing a large composite “group lesion” that wassufficient to produce the syntactic deficit. Then areas in which only some symptomatic rats, but not others, had damage were subtracted. These unshared areas, by definition, were undamaged in some symptomatic rats and thus were not strictly necessary for the behavioral deficit. The remaining composite lesion identified the “crucial site” for producing syntactic grooming deficits.
Measurement of GFAP-IR
By using a computerized densitometry video analysis based on pixel darkness, the reactive gliosis was quantified, and the darkest 10% and 20% of the pixels were used, respectively, to identify the lesion center and shell. Maps of lesions constructed by gliosis analysis were compared with maps constructed by neuronal density analysis.
Statistics
Behavioral data were examined using a two-tailed ANOVA and specific post hoc tests (Newman–Keuls or Mann–Whitney U). For a comparison of neuron count numbers, a two-factor (treatment by region) ANOVA was used to examine whether there were significant effects of cell loss or region of damage. This was followed by a posteriori Newman–Keuls tests to examine the differences between particular groups for each region.
RESULTS
Behavioral results
Overall grooming
Sham-lesion control groups did not vary significantly from each other by any of the behavioral measures discussed below, regardless of their anatomical site of vehicle injection, and so they were combined to form a single control group (n = 40) for statistical comparison to excitotoxin lesion groups. Among lesion groups there were significant differences in the overall time spent grooming (F(6,81) = 0.629; p < 0.05). Pair-wise comparisons showed that rats with either globus pallidus lesions or ventral pallidum lesions spent less time grooming than controls and all other lesion groups (vehicle control rats = 21% of observation trial spent grooming; GP lesions = 12 ± 2%, VP lesions = 6 ± 1%; Newman–Keuls, p < 0.05 each). This phenomenon of decreased grooming activity after pallidal lesions has been noted previously (Norton, 1976) and may reflect a postural deficit (Campbell and Dill, 1974; DeLong and Coyle, 1979; Labuszewski et al., 1981; Schneider and Olazabal, 1984; Feve et al., 1993). By contrast, rats with neostriatal lesions did not differ from control rats in time spent grooming (dorsolateral lesion group = 21.1 ± 2.1%, dorsomedial lesion group = 17.8 ± 2.4%, ventromedial lesion group = 20.1 ± 2.0%, and ventrolateral lesion group = 20.4 ± 2.1%).
Individual grooming bout durations were examined for all groups to determine whether the temporal pattern of discrete bouts had been changed by the lesions. A grooming bout was defined as any continuous display of grooming (including both chain and nonchain) that contained no pauses >5 sec. The duration of individual grooming bouts did not vary significantly among groups (F(6,81) = 1.84; p = 0.10; ±SEM = 26 ± 4 sec), although rats that had pallidal lesions seemed to have a nonsignificant trend toward a reduced bout duration (globus pallidus = 18 ± 6.0 sec; ventral pallidum = 19 ± 7.0 sec).
Syntactic grooming chains: chain initiation
Chain initiation was defined as the occurrence of Phase I (four or more tight elliptical trajectories around the nose performed with both paws simultaneously, at a rate of at least 5.5 Hz) followed by Phase II (one or more unilateral medium-size strokes), and/or Phase III (one or more bilateral, large amplitude strokes). When considered in terms of rate of syntactic chain initiation per time spent grooming, no lesion group differed from controls (F(6,87) = 1.649; p > 0.1; mean = 1.3 ± 0.5 chains/min of grooming). In terms of absolute number of chains emitted per minute of observation, by comparison, rats with pallidal damage had lower absolute rates of chain emission (globus pallidus lesion group = 5.0 ± 0.9 chains, ventral pallidum lesion group = 7.0 ± 1.3 chains, vs control group = 10.0 ± 0.9 chains or neostriatal lesion group = 10.0 ± 0.7 chains; Newman–Keuls,p < 0.05); however, given that this deficit disappeared when time spent grooming was taken into consideration, the reduction in absolute chain initiation for globus pallidus and ventral pallidum groups seemed to be merely a secondary consequence of the overall reduction of grooming duration produced by pallidal lesions.
Syntactic efficacy: chain completion rates
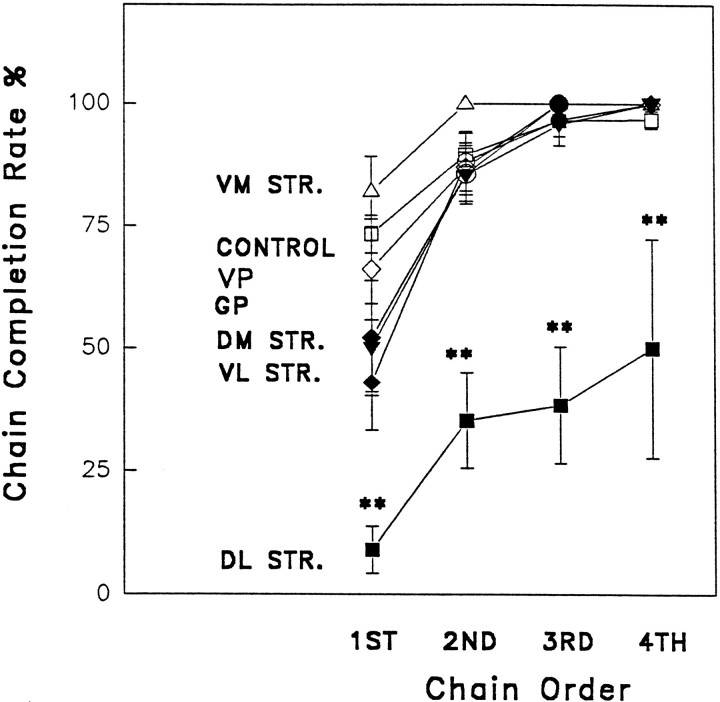
The percentage of syntactic chains completed perfectly to Phase IV differed significantly depending on lesion site (Fig. 3) (ANOVA, F(6,87) = 9.876; p < .01). Only rats with anterior dorsolateral neostriatum lesions had a significant impairment in grooming syntax (pair-wise comparison to controls and to all other lesion groups; Newman–Keuls,p < .05). Vehicle control rats completed 81 ± 3% of their chains perfectly to Phase IV. By contrast, the syntactic completion rate of the anterior dorsolateral group was reduced dramatically to a mere 24 ± 5%, less than one third of the control value. Lesions to other sites did not reduce the rate of syntactic completion (posterior dorsolateral neostriatum lesion group = 71.0 ± 4.3%, dorsomedial neostriatum = 77.0 ± 5.5%, ventromedial neostriatum = 92.0 ± 5.4%, ventrolateral neostriatum = 70.0 ± 7.5%, globus pallidus lesion group = 76.0 ± 5.5%, ventral pallidum lesion group = 72.0 ± 12.2%). The initial chain emitted within a test session was less likely than later chains to be completed syntactically, regardless of group (F(6, 81) = 15.78; p < 0.001) (Fig. 3), as has been reported before (Berridge, 1990; Berridge and Whishaw, 1992). Rats with damage to the ventrolateral neostriatum or medial neostriatum had marginal decreases only in initial chain completion (p < 0.05), whereas later chains were normal. This “first chain only” deficit is similar to the effect of cortical or cerebellar lesions (Berridge and Whishaw, 1992), and it reflects merely an exaggerated “warm-up” deficit. Only the anterior dorsolateral neostriatal group had syntactic completion deficits that extended to later chains within a grooming bout (p < 0.05), indicating that this was the only lesion to produce a reliable action syntax deficit.
Fig. 3.
Sequential organization after excitotoxin lesions: rates of syntactic chain completion. The percentage of first, second, third, and fourth grooming chains begun within a session that were completed syntactically (i.e., Phases I, II, III, and IV without interruption). Only rats with lesions of the anterior dorsolateral neostriatum had a reliable disruption of grooming syntax throughout a session. Abbreviations: VM STR., ventromedial neostriatum;VP, ventral pallidum; GP, globus pallidus;DM STR., dorsomedial neostriatum; VL STR., ventrolateral neostriatum; DL STR., anterior dorsolateral neostriatum.
Even if the criterion for chain completion was expanded to include “imperfectly completed” chains, in which the rat ended the chain with a Phase IV mutation by licking its paws or forelimbs instead of its torso, rats with bilateral dorsolateral neostriatum damage still had a significant impairment in chain completion (imperfect + perfect completion = 29 ± 5%, compared with sham-injected controls = 88 ± 4%; ANOVA F(1,14) = 73.67; p < .01). Thus the syntactic deficit of this group does not reflect mere response substitution but rather reflects a true failure of sequential pattern completion.
Frequency of grooming actions: assessment of motor impairment
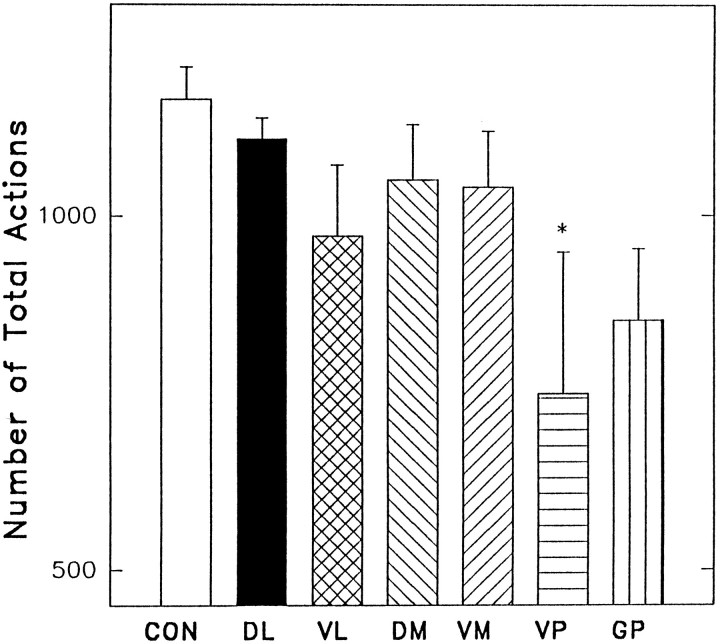
Is the grooming syntax disruption after dorsolateral neostriatum lesions attributable to a simple inability to perform “late chain” Phase III or Phase IV component actions (i.e., large amplitude forepaw strokes and body licking)? To answer this question, the total number of grooming actions (forelimb strokes, body licks, paw licks) per minute of grooming observation were counted by computer-assisted slow-motion analysis and compared between groups. The number of total grooming actions (all categories combined) varied among the lesion groups (F(6,81) = 3.73; p < 0.01) (Fig. 4); however, it was the ventral pallidum lesion group that had reduced overall grooming actions (control group = 1163 ± 148, ventral pallidal group = 749 ± 92; Newman–Keuls, p < 0.01) (Fig. 4), not the dorsolateral neostriatum group that had shown syntax failure. In some instances, rats with ventral pallidal lesions would seem to go through a period of “grooming arrest” similar to that described originally by Levitt and Teitelbaum (1975)for rats with large electrolytic lesions of the lateral hypothalamus (that likely extended into the ventral pallidum). In this condition, a rat would slump over and seem to fall asleep during behavior. In many cases, the halt would come in the middle of a grooming bout (and sometimes in mid-stroke), hence the label “grooming arrest.” These rats, however, essentially had normal grooming syntax, as described above. By contrast, the anterior dorsolateral neostriatum group had impaired action syntax but emitted grooming actions at a rate (1107 ± 113) that did not differ from controls.
Fig. 4.
Number of grooming actions emitted by each group. Actions emitted both within and outside of syntactic chains are included in this analysis. Rats with lesions of the ventral pallidal region had a significant decrease in the number of grooming actions. Abbreviations: Con, control; others as in Figure 3.
Forelimb strokes form the constituent action for Phases II and III of syntactic chains. Regarding the overall emission of small, medium, or large face-wash strokes during grooming, the dorsolateral neostriatum lesion group did not differ from controls (small forelimb strokes = 34 ± 5%, medium forelimb strokes = 22 ± 3%, and large forelimb strokes = 44 ± 4). By contrast, for the pallidal lesion groups, the total number of face-wash strokes was reduced in every category compared with controls (Newman–Keuls, p < 0.05). Therefore, it seems that syntactic deficits produced by dorsolateral neostriatum lesions are not attributable to an inability to produce Phase II or Phase III actions; anterior dorsolateral neostriatum lesions produce grooming syntax deficits but not stroke deficits, whereas ventral pallidum lesions produce deficits in the motor generation of forelimb strokes but not in the sequential organization of strokes into syntactic chains.
Body licks, directed to the back and flank of the torso of the rat, constitute the Phase IV action that normally terminates syntactic chains. To discover whether grooming syntax deficits are attributable to an inability to perform the action required for Phase IV completion, the frequency and duration of body lick bouts (both inside and outside the chain) were compared across groups. A body lick bout was defined as an occurrence of continuous body licking that lasted at least 2 sec and had no pauses >2 sec. Rats with anterior dorsolateral neostriatal lesions did not differ from sham-injected control groups in either number or duration of body lick bouts (number: controls = 0.6 ± 0.1 bouts/min of grooming vs dorsolateral neostriatum = 0.5 ± 0.1 bouts,F(1,14) = 3.4, p = 0.08; duration: controls = 4.4 ± 0.5 sec versus dorsolateral neostriatum = 5.3 ± 0.3 sec, F(1,14) = 2.2, p = 0.1). These results indicate that the dramatic impairment of chain completion is not a result of an inability to perform the Phase IV constituent action, body licking, but instead reflects a specific failure of action syntax.
Microstructure of syntactic chains
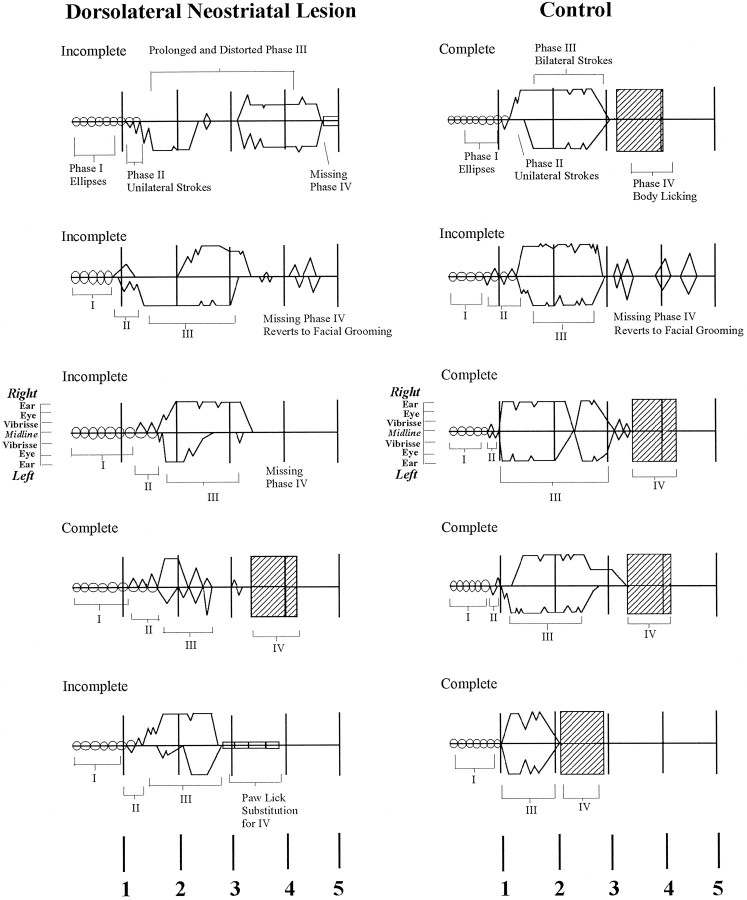
The duration of syntactic chains, from the first Phase I ellipse stroke to the onset of Phase IV body licking that terminates the chain, varied across lesion and control groups (F(6,81) = 2.92; p < 0.05). Rats with anterior dorsolateral neostriatum lesions emitted chains of prolonged duration (when they were completed syntactically; control = 3.2 ± 0.6 sec vs dorsolateral neostriatum = 3.8 ± 0.1; Newman–Keuls,p < 0.05). An increase in chain duration was seen also in rats with ventrolateral neostriatum lesions and rats with ventral pallidal lesions (ventrolateral neostriatum = 3.9 ± 0.1 sec, pallidum = 3.9 ± 0.3; Newman–Keuls, p < 0.05 for both). The slight increase in the duration of syntactic chains for these groups was not caused by an increase in the number of strokes per grooming chain. Stroke number was assessed by counting the number of zero crossings from forepaw trajectory ascent to descent (represented as peaks in choreographic notation of grooming chains) (Fig. 5) for every completed chain. There was no difference among groups in the number of strokes in Phases I, II, or III for any lesion groups compared with controls (F(6,81) = 2.21;p = 0.05). Rats with dorsolateral neostriatum lesions emitted an average of 23 ± 0.9 strokes per chain, which is comparable to the control average of 23 ± 0.6 strokes per chain. Thus the slight increase in chain duration after these specific small lesions may perhaps be attributable to small expansions of component durations or intervals rather than to an increased number of components.
Fig. 5.
Examples of notated chains in control animals (right) and rats with lesions of the dorsolateral neostriatum (left). The position of the paws in relation to the face during each stroke is indicated by stroke-amplitude marker near the third chain for each group. Time (in seconds) is shown at thebottom. Choreographic notation symbols as in Figure 1.
The most temporally stereotyped chain components are elliptical paw strokes performed during Phase I, which trace rapid elliptical trajectories around the nose with both paws simultaneously. An analysis of ellipse stroke duration showed that only the ventral pallidum lesion group had a slower rate of emission for Phase I ellipses than the control group did (VP lesion group: mean = 5.1 ellipses/sec vs control group mean of 6.8; F(6,81) = 4.69; Newman–Keuls, p < 0.05). Rats with dorsolateral neostriatum lesions, by contrast, did not differ from controls (rats with lesions, 6.18 ± 0.2, compared with dorsolateral neostriatum sham-injected controls, 6.5 ± 0.07; ANOVAF(1,14) = 3.39; p > 0.05). The increase in ventral pallidal ellipse duration is consistent with the results above, indicating that ventral pallidum lesions produce general motor deficits in component movements, whereas dorsolateral neostriatum lesions do not. It may also partly explain the increased chain duration, at least for rats with ventral pallidal lesions.
Inspection of “syntax failures,” in which rats with anterior dorsolateral lesions began a syntactic grooming chain but failed to complete it syntactically, showed that failures were of several types. In ∼14% of cases, the rat emitted Phases I and II and initiated Phase III, but rather than perform the ordinary Phase IV component of body licking, the rat “replaced” it with licking directed instead to the forepaw, as described earlier. In an additional 30% of failures, dorsolateral rats failed to show any form of Phase IV, but instead reverted back to sequentially flexible patterns of facial grooming without interruption. In these cases, the series of Phase III large-amplitude strokes over the face was prolonged beyond its normal duration and merged imperceptibly into a rich series of facial strokes that were unpredictable in magnitude, laterality, or sequence. Finally, in 56% of syntactic failures, rats with dorsolateral neostriatal lesions simply halted action during the chain, typically while they were within Phase III. In these cases, the rat paused for up to several seconds, often after emitting a body shake. After slightly more than half of such pause interruptions, the rat began a new bout of facial grooming. In the remaining cases, it began instead to engage in another activity, or it sat quietly.
Stereological lesion mapping
Each lesion group had extensive bilateral neuron depletion in the appropriate striatopallidal target region, ranging from 62% in the dorsomedial neostriatum group (i.e., 38% of neurons survived) to >81% in the ventromedial neostriatum and ventral pallidal groups. Rats in the anterior dorsolateral neostriatum lesion group had an average bilateral neuron loss of 79 ± 5%.
For the rats in the anterior dorsolateral neostriatum lesion group that showed deficits in grooming syntax (n = 8), individual lesion maps were made, using boundary criteria of 80% and 50% depletion cutoffs to identify lesion center and shell. The individual lesion maps were then superimposed on one another to create a composite lesion for the group. Then, to eliminate regions that were not strictly necessary to the syntax failure, any region that was left undamaged in one or more rats was subtracted away from the composite lesion. The remaining shared lesion showed the crucial site of damage within the dorsolateral neostriatal region that disrupted grooming syntax (Fig.6). This site was in the anterior dorsolateral corner of the neostriatum, immediately subjacent to the corpus callosum (Fractions 39, 42, 76, and 79). The stereotaxic center was +0.7 mm anterior to bregma, ±3.5 mm lateral to the midline, and −4.0 mm below dura (on the basis of the atlas of Paxinos and Watson, 1982). The maximal lateral diameter of the crucial site was ∼1.3 mm, the dorsoventral diameter was 1.0 mm, and the AP diameter was 1 mm.
Fig. 6.
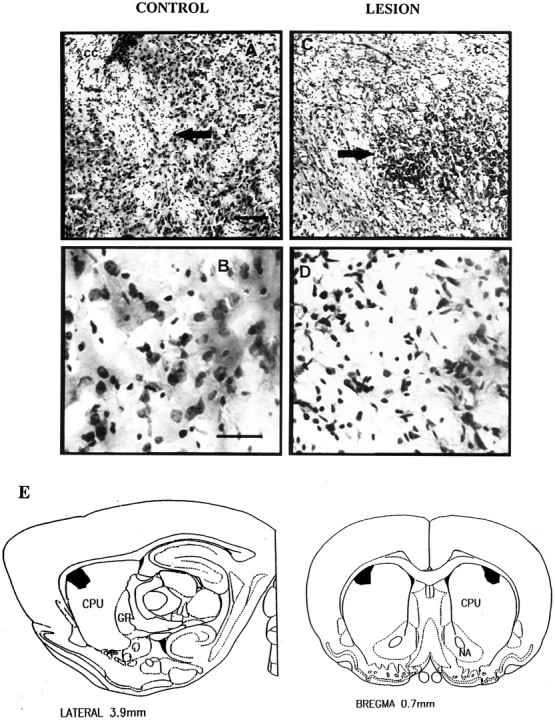
Crucial syntax site and photomicrographs of lesions within the dorsolateral neostriatum. Photomicrographs show (A) low magnification (10×) of the anterior dorsolateral neostriatum of a vehicle-injected control rat (arrow points to location of vehicle microinjection; cc denotes corpus callosum). B, High magnification (40×) of the same anterior dorsolateral neostriatal region in vehicle-injected control rat. Note lack of neuronal death. C, Low magnification of anterior dorsolateral neostriatum in rat that received an excitotoxin lesion and had impaired grooming syntax. (Arrow points to center of excitotoxin lesion; cc denotes corpus callosum).D, High magnification of dorsolateral neostriatum after an excitotoxin lesion that impaired grooming syntax. Note paucity of neurons compared with those in B. Scale bars: Aand C, 140 μm; B and D, 40 μm.E, Map of the crucial “grooming syntax site.” Atlas view shows boundaries of the site, identified by the modified fractionator procedure, in which loss of >50% of neurons is associated with specific deficits in the sequential organization of syntactic grooming chains.
Every rat that showed a deficit in grooming syntax had at least 57% bilateral neuronal depletion throughout this crucial site (range, 57–95%). Increased severity of neuronal depletion much above 57% did not seem to result in an increased degree of behavioral deficit. Instead, the impairment of grooming syntax was related to cell loss from the crucial anterior dorsolateral site in a “step function” fashion. For example, rats with neuron loss between 60% and 70% depletion had behavioral syntactic chain completion rates of 7–12%, which was as great a behavioral deficit as that seen in rats that had neuron loss above 80% depletion.
The dorsolateral neostriatum was the only region with damage in all rats that had deficits in grooming syntax. Four of the eight rats had damage extending to the neighboring dorsomedial region of the neostriatum. Only one of eight rats had bilateral damage in the ventrolateral neostriatum. Damage to the overlying neocortex was seen in both lesion and control groups, primarily attributable to passage of the injection needle. Both control and dorsolateral neostriatal groups had damage to the overlying frontal cortex in ∼75% of rats. Twenty-five percent of rats with dorsolateral neostriatal lesions had additional cortical damage extending posteriorly <1 mm into somatosensory cortex. The average cross-sectional area of cortical damage in control rats was 0.7 ± 0.5 mm2, and for rats with dorsolateral neostriatal lesions it was 2.0 ± 0.5 mm2. It is unlikely that damage to the neocortex contributed to the behavioral deficits of grooming syntax, however, because neocortical damage such as aspiration of primary and secondary motor cortex, prefrontal cortex, or even complete decortication doesnot impair grooming syntax (Berridge and Whishaw, 1992). It therefore seems reasonable to ascribe the deficits observed in the present study to neostriatal damage rather than to neocortical damage.
No shrinkage of the neostriatum could be detected after the excitotoxin lesions used in this study by area measures of either the neostriatum or of lateral ventricle expansion. Comparison of control area size to groups that had behavioral deficits showed no difference between the groups. Ventricle area was 0.3 ± 0.04 mm2 for controls, 0.5 ± 0.1 mm2 for the dorsolateral lesion group, and 0.4 ± 0.05 mm2 for the ventral pallidal lesion group [ANOVA F(2,18) = 0.395, not significant (NS)]. Total neostriatal area was 12.9 ± 0.3 mm2 for the controls, 12.5 ± 0.6 mm2 for the dorsolateral lesion group, and 13.3 ± 0.5 mm2 for the ventral pallidal lesion group (F(2, 16) = 0.445, NS). It may be that the relatively small volume of tissue lost by neuron death was replaced by gliosis after these relatively small excitotoxin lesions.
A separate mapping of lesions based on GFAP-IR staining confirmed the pattern of results that was indicated by the neuron analysis. Dense GFAP-IR was measured throughout the crucial site within the dorsolateral neostriatum of every rat that had impaired grooming syntax. Other lesion groups had dense GFAP-IR staining in the appropriate target regions. Much lighter GFAP-positive staining was observed in the neocortex immediately dorsal to the striatopallidal target site in each group. These results support the conclusion that damage to the shared crucial site within the dorsolateral neostriatum is necessary and sufficient to produce an impairment of the serial organization of syntactic grooming chains in the rat.
DISCUSSION
The results of this study indicate that a single site within the anterior dorsolateral neostriatum is uniquely crucial to striatopallidal coordination of the serial order of grooming behavior. Lesions that produced a deficit in the sequential coordination of syntactic chains destroyed 57% or more of neurons bilaterally in this 1.3 × 1.0 × 1.0 mm anterior dorsolateral site. The impairment of grooming syntax was not attributable to a simple motor inability to make the constituent movements of the behavioral sequence. That can be deduced from the double dissociation between syntax deficits and movement deficits produced by lesions of the anterior dorsolateral neostriatum and the pallidum, respectively. Damage to the crucial site in dorsolateral neostriatum impaired grooming syntax but not movement, whereas damage to globus pallidus or ventral pallidum impaired grooming movements but not syntax. In other words, the serial coordination of lawful sequences of grooming action is localized as a function within the neostriatum. This conclusion does not imply that behavioral functions other than grooming syntax are not mediated by circuits within the crucial syntax site or that other neostriatal neurons outside of the crucial site might also code aspects of action syntax (Aldridge et al., 1993), but it does seem that only neurons within this site are absolutely required for the neostriatum to implement the grooming syntax pattern into actual behavior.
The contribution of the neostriatum to action syntax seems to originate intrinsically within the dorsolateral neostriatum, rather than being conducted passively from the neocortex. Destruction of motor cortex, prefrontal cortex, or the entire neocortex fails to produce reliable disruption of grooming syntax (Berridge and Whishaw, 1992). Only neostriatal damage results in syntactic disruption (Berridge and Whishaw, 1992). It can now be added that syntactic disruption occurs only when neostriatal damage extends to the crucial dorsolateral site identified here.
The deficit in grooming syntax produced in this study by small lesions of the dorsolateral neostriatum was comparable in magnitude to deficits seen in earlier studies after much greater damage to the neostriatum and related systems, such as that produced by large kainic acid lesions of the neostriatum and globus pallidus, aspiration of the entire neostriatum plus neocortex, or decerebrate transection above the midbrain (Berridge and Fentress, 1987; Berridge, 1989a,b; Berridge and Whishaw, 1992). It is striking that damage to the single neostriatal site identified here produced a deficit in grooming syntax (50% reduction in rates of syntactic completion) that was as great as the deficit reported after loss of the entire forebrain via decerebration (Berridge, 1989a). This equivalence of impact highlights the importance to grooming syntax of the anterior dorsolateral neostriatum.
Our results indicate that it is lesion location and not lesion severity (at least not above a threshold of ∼60% cell loss) or lesion size (at least not beyond the boundaries of the crucial dorsolateral site) that determines whether a grooming syntax deficit is produced by neostriatal damage. There was no correlation of the degree of behavioral sequencing deficit with lesion diameter or with severity of suprathreshold neuronal depletion; however, bilateral damage of the site on both sides of the brain seemed to be necessary to produce the deficit.
Anatomy and function of the dorsolateral neostriatum
The neostriatopallidal system is composed anatomically of several parallel corticostriatal “loops,” and this anatomical differentiation within the neostriatum has been proposed to produce functional specialization (Divac, 1972; Alexander et al., 1986; Alheid and Heimer, 1988; Nauta, 1989; Flaherty and Graybiel, 1993; Hoover and Strick, 1993; Rajakumar et al., 1993; Pierce and Rebec, 1995). The dorsolateral, dorsomedial, ventromedial, and ventrolateral regions of the neostriatum receive distinct projections from different areas of neocortex (Selemon and Goldman-Rakic, 1985; McGeorge and Faull, 1989). Cortical input to the dorsolateral neostriatum arises from primary motor cortex, primary sensory cortex, secondary sensory cortex, and medial agranular cortex (McGeorge and Faull, 1989). The anterior sector of the dorsolateral neostriatum (1.2–0.7 mm anterior to bregma) receives densest input from motor cortex (McGeorge and Faull, 1989); however, as mentioned above, the deficits in grooming syntax reported here are not produced by aspiration lesions of motor cortex (M1 and M2;Berridge and Whishaw, 1992), indicating that the neostriatal role in grooming syntax is not merely a passive consequence of neocortical inputs. Other afferents into the dorsal neostriatum include the subthalamic nucleus (Beckstead, 1983), the substantia nigra (Gerfen et al., 1982), the midbrain raphe nucleus (Parent et al., 1981), the thalamus (Sadikot et al., 1992), the globus pallidus (Staines et al., 1981), the locus coeruleus (Parent, 1986), and the basolateral nucleus of the amygdala (Kelley et al., 1982). The special importance of the neostriatum to grooming syntax possibly might derive from its unique capacity to “bind” these diverse sources of afferentation (Graybiel and Kimura, 1995).
Role of the neostriatum in grooming syntax
The dorsolateral neostriatum is not strictly required for the brain to generate grooming actions. Even decerebrate animals can emit all the component actions involved in grooming (Bard and Macht, 1958;Grill and Norgren, 1978; Berntson et al., 1988; Berridge, 1989a). Similarly, the dorsolateral neostriatum does not seem to be required absolutely to generate the serial order pattern of syntactic grooming chains. Decerebrate rats produce syntactically completed chains at rates that exceed chance, although most decerebrate grooming chains fail to be completed syntactically (Berridge, 1989a). Such observations indicate that the basic central pattern generators for the serial order of syntactic grooming chains are contained to a large degree within the brainstem. It is not so much for the generation of the serial order pattern that the neostriatum is needed as for theimplementation of that pattern in the normal flow of behavior.
The dorsolateral neostriatum has been implicated in motor, sensory, and integrative aspects of function (Kelley et al., 1982; Lidsky et al., 1985; Schallert and Hall, 1988; West et al., 1990; Carelli and West, 1991; Flaherty and Graybiel, 1991; Brown et al., 1993; Mittler et al., 1994; Brown et al., 1995). Recent electrophysiological evidence indicates that neurons within the dorsolateral neostriatum may code the serial pattern of motor sequences such as eye movements, reaching, locomotion, or grooming (West et al., 1990; Aldridge et al., 1993;Kermadi and Joseph, 1995). For example, Kermadi and Joseph (1995)reported that sequential order was specifically coded by a significant percentage of neurons within the monkey caudate nucleus responding during the orientation, movement, or postsaccade components of a serial eye movement and reaching task. Regarding grooming syntax in particular, Aldridge et al. (1993) found that ∼85% of sampled neurons within the dorsolateral neostriatum had firing patterns correlated specifically with the serial pattern of syntactic grooming chains. These neurons were not activated in the same way when the same grooming actions were emitted in different serial order. Neurons that coded grooming syntax specifically seemed to be clustered predominantly within the anterior dorsolateral neostriatum, consistent with our finding that a site in this region is crucial to the control of action syntax (Aldridge et al., 1993).
Many researchers have suggested that the neostriatum may implement action via the modulation of competing central generators and sensorimotor control systems (Iversen, 1979; Cools et al., 1980; Cools, 1985; Lidsky et al., 1985; Berridge and Fentress, 1987; Schallert and Hall, 1988; Albin et al., 1989; Graybiel and Kimura, 1995; Jackson and Houghton, 1995). For syntactic grooming chains in particular, there is evidence that the balance of motor control temporarily switches away from sensory guided systems and toward central pattern-generating circuits during the performance of the serial pattern (Berridge and Fentress, 1986). Circuits within the anterior dorsolateral neostriatum site might promote grooming syntax in a hierarchical fashion by phasically modulating the balance between central pattern generators and sensorimotor systems. This could be performed, for instance, by channeling motor control to syntactic pattern-generating circuits at the beginning of a syntactic chain, while suppressing other competing signals, but then allowing control to revert to a more sensory-guided mode at the end of the pattern (Berridge and Fentress, 1987; Berridge and Whishaw, 1992).
Clinical implications
Although akinesia, dyskinesia, and chorea are the most well known consequences of diseases related to the striatum, deficits may also extend to functions involving memory and cognition in patients with Parkinson’s disease (Heindel et al., 1989; Sprengelmeyer et al., 1995), Huntington’s disease (Butters et al., 1985; Willingham and Koroshetz, 1993; Gabrieli, 1995), or Gilles de la Tourette’s syndrome (Georgiou et al., 1995). The serial coordination of action syntax studied here is intermediate in complexity between simple movement and abstract cognition. Disorders of action syntax accompany some human striatopallidal diseases. Patients with Parkinson’s disease, for instance, have particular difficulty performing some forms of sequential movement tasks (Benecke et al., 1987; Stelmach et al., 1987;Harrington and Haaland, 1991; Agostino et al., 1992), and even simple movement execution deficits may depend on the sequential pattern in which the movements are embedded (Georgiou et al., 1993, 1994). On the basis of such studies, for example, Georgiou et al. (1994) concluded that Parkinson’s disease patients have special difficulty in using internal programs to guide the sequence of action, a deficit remarkably similar to the action syntax deficit produced by neostriatal lesions in our study. Similarly, Huntington’s disease patients show deficits on a complex mirror-tracing task, interpreted by the authors to reflect a deficit in “perceptual-motor sequencing, the rapid selection of perceptual, cognitive, and motor operations that allows for good performance and skill learning” (Gabrieli, 1995; italics added).
It has even been suggested that a role for the striatopallidal system in human language and thought may have evolved from its original role in coordinating the serial order of action (Marsden, 1982; Rapoport and Wise, 1988; Berridge and Whishaw, 1992; Aldridge et al., 1993; Graybiel et al., 1994). Striatum-related disruptions appear in actual human language syntax as well as in action sequence. For example, Lieberman et al. (1992) reported that some late-stage Parkinson’s disease patients had difficulty comprehending spoken language when syntax was relatively complex (“the dog was chased by the cat”) but not when syntax was simple (“the cat chased the dog”), suggesting a striatopallidal involvement in real language syntax. In a review of the role of the neostriatum in coordinating action serial order, Marsden (1992) has suggested, based primarily on a consideration of Parkinson’s disease symptoms, that the “normal operation of the basal ganglia … may be the orderly and rational sequencing of the individual components of motor and cognitive plans.” Diseases such as Gilles de la Tourette’s syndrome and obsessive–compulsive disorder are associated with dramatically unusual sequences of movement, utterances, and thought. The neural origins of these disorders are not clear but are thought by many to involve the striatopallidal system (Cummings and Frankel, 1985; Frankel et al., 1986; Luxenberg et al., 1988; Rapoport and Wise, 1988; Trimble, 1989;Rapoport, 1991; Resnick, 1992). The explanation of why only some forms of human striatopallidal disease influence the sequential organization of behavior may have to do with the functional heterogeneity of the striatopallidal system and with the localization of action syntax control within the neostriatum demonstrated here. Sequential symptoms might depend at least in part on the location of neuropathology, which in turn may depend on the selective pattern of neuronal dysfunction of each disease at a particular stage (Albin et al., 1990; Owen and Leigh, 1992; Bhatia and Marsden, 1994; Mufson and Branabur, 1994; Hedreen and Folstein, 1995). An understanding of how anatomical and neuronal patterns of damage produce specific patterns of sequential and other symptoms will allow for a better understanding of the etiology of these human diseases. Our results lead to the prediction that sequential symptoms may be linked to anatomically specific patterns of damage in the human striatopallidal system.
Conclusions
A specific disruption in the ability to coordinate the serial order of grooming sequences, without a corresponding deficit in simple motor control, was produced by neuron death within a 1 mm3 site in the anterior dorsolateral neostriatum. Damage to this site produced a deficit in grooming syntax but spared the ability to perform the component grooming actions. By contrast, lesions in either the globus pallidus or ventral pallidum produced a general motor deficit, which disrupted emission of grooming movements but did not disrupt the serial pattern of action syntax. This double dissociation of action syntax from simpler aspects of motor control supports the functional heterogeneity of the striatopallidal system. Localization of sequential control within the striatopallidal system may have implications for clinical disorders of behavioral sequencing produced by human basal ganglia disease.
Footnotes
This work was supported by National Institutes of Mental Health predoctoral fellowship MH-09838, National Science Foundation Grant IBN 9319933, and National Institutes of Health Grants NS-23959 and NS-31650. We thank Rick Roberts, Joel Zimmer, Amy Andersen, Chul Lee, Jackie Ramirez, Adrianna Kampfner, and Natasha Gorbundhun for their help in behavioral testing, video analysis, and histological analysis. We are grateful to Professor J. Wayne Aldridge for helpful comments on an earlier version of this manuscript.
Correspondence should be addressed to Kent Berridge, University of Michigan, Department of Psychology, Ann Arbor, MI 48109-1109.
Dr. Cromwell’s present address: Mental Retardation Research Center, University of California at Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90025.
REFERENCES
- 1.Agostino R, Beradelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- 2.Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington’s disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–365. doi: 10.1002/ana.410270403. [DOI] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge JW, Berridge KC, Herman M, Zimmer L. Neuronal coding of serial order: syntax of grooming in the neostriatum. Psychol Sci. 1993;4:391–395. [Google Scholar]
- 5.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 6.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 7.Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology. 1993;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- 8.Baunez C, Nieoullon A, Amalric M. Dopamine and complex sensorimotor integration: further studies in a conditioned motor task in the rat. Neuroscience. 1995;65:375–384. doi: 10.1016/0306-4522(94)00498-t. [DOI] [PubMed] [Google Scholar]
- 9.Beckstead RM. A reciprocal axonal connection between the subthalamic nucleus and the neostriatum in the cat. Brain Res. 1983;275:137–142. doi: 10.1016/0006-8993(83)90425-0. [DOI] [PubMed] [Google Scholar]
- 10.Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 11.Berntson GG, Jang JF, Ronica AE. Brainstem system and grooming behaviors. Ann NY Acad Sci. 1988;525:350–362. doi: 10.1111/j.1749-6632.1988.tb38619.x. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav Brain Res. 1989a;33:241–253. doi: 10.1016/s0166-4328(89)80119-6. [DOI] [PubMed] [Google Scholar]
- 13.Berridge KC. Substantia nigra 6-OHDA lesions mimic striatopallidal disruption of syntactic grooming chains: a neural systems analysis of sequence control. Psychobiology. 1989b;17:377–385. [Google Scholar]
- 14.Berridge KC. Comparative fine structure of action: rules of form and sequence in the grooming patterns of six rodent species. Behavior. 1990;113:1–22. [Google Scholar]
- 15.Berridge KC, Cromwell HC. Motivational-sensorimotor interaction controls aphagia and exaggerated treading after striatopallidal lesions. Behav Neurosci. 1990;104:778–795. doi: 10.1037//0735-7044.104.5.778. [DOI] [PubMed] [Google Scholar]
- 16.Berridge KC, Fentress JC. Contextual control of trigeminal sensorimotor function. J Neurosci. 1986;9:325–330. doi: 10.1523/JNEUROSCI.06-02-00325.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge KC, Fentress JC. Disruption of natural grooming chains after striatopallidal lesions. Psychobiology. 1987;15:336–342. [Google Scholar]
- 18.Berridge KC, Whishaw IQ. Cortex, striatum and cerebellum: control of serial order in a grooming sequence. Exp Brain Res. 1992;90:275–290. doi: 10.1007/BF00227239. [DOI] [PubMed] [Google Scholar]
- 19.Berridge KC, Fentress JC, Parr H. Natural syntax rules control action sequence of rats. Behav Brain Res. 1987;23:59–68. doi: 10.1016/0166-4328(87)90242-7. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia KP, Marsden CD. The behavioral and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 21.Bradshaw JL, Phillips JG, Dennis C, Mattingley JB, Andrewes D, Chiu E, Pierson JM, Bradshaw Initiation and execution of movement sequences in those suffering from and at-risk of developing Huntington’s disease. J Clin Exp Neuropsychol. 1992;14:179–192. doi: 10.1080/01688639208402822. [DOI] [PubMed] [Google Scholar]
- 22.Brown LL. Somatotopic organization in rat striatum: evidence for a combinatorial map. Proc Natl Acad Sci USA. 1992;15:7403–7407. doi: 10.1073/pnas.89.16.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LL, Feldman SM, Divac I, Hand PJ, Lidsky TI. A distributed network of context dependent functional units in the rat neostriatum. In: Percheron G, McKenzie JS, Feger J, editors. The basal ganglia IV. Plenum; New York: 1995. pp. 215–228. [Google Scholar]
- 24.Brown RG, Jahanshahi M, Marsden CD. The execution of bimanual movements in patient with Parkinson’s, Huntington’s and cerebellar disease. J Neurol Neurosurg Psychiatry. 1993;56:295–297. doi: 10.1136/jnnp.56.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butters N, Wolfe J, Martone M, Granholm E, Cermack LS. Memory disorders associated with Huntington’s disease: verbal recall, verbal recognition, procedural memory. Neuropsychology. 1985;23:729–743. doi: 10.1016/0028-3932(85)90080-6. [DOI] [PubMed] [Google Scholar]
- 26.Campbell KM, Dill RE. Trunk rigidity and limb hypotonia produced in squirrel monkeys by direct cholinergic stimulation of the globus pallidus. Exp Neurol. 1974;42:555–565. doi: 10.1016/0014-4886(74)90078-8. [DOI] [PubMed] [Google Scholar]
- 27.Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309:231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- 28.Colonnese MT, Stallman EL, Berridge KC (1996) Ontogeny of action syntax in altricial and precocial rodents: grooming sequences by rat and guinea pig pups. Behaviour, in press.
- 29.Cools AR. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: facilitation of processes involved in selecting the best strategy in a stressful situation. Behav Brain Res. 1980;1:361–378. doi: 10.1016/0166-4328(80)90035-2. [DOI] [PubMed] [Google Scholar]
- 30.Cools AR. Brain and behavior: hierarchy of feedback systems and control of input. In: Bateson PPG, Klopfer PH, editors. Perspectives in ethology. Plenum; New York: 1985. pp. 109–168. [Google Scholar]
- 31.Cummings JL, Frankel M. Gilles de la Tourette syndrome and the neurological basis of obsessions and compulsions. Biol Psychiatry. 1985;20:1117–1126. doi: 10.1016/0006-3223(85)90011-3. [DOI] [PubMed] [Google Scholar]
- 32.Cromwell HC, Berridge KC (1990) Anterior lesions of the corpus striatum produce a disruption of stereotypic grooming sequences in the rat. Soc Neurosci Abstr. 16:233.
- 33.Cromwell HC, Berridge KC. Where does damage produce enhanced aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 34.Cromwell HC, Berridge KC. Mapping of globus pallidus and ventral pallidum lesions that produce hyperkinetic treading. Brain Res. 1994;668:16–29. doi: 10.1016/0006-8993(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 35.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 36.DeLong MR, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT. Role of basal ganglia in limb movements. Hum Neurobiol. 1984;2:235–244. [PubMed] [Google Scholar]
- 37.Delong MR, Coyle JT. Globus pallidus lesions in the monkey produced by kainic acid: histologic and behavioral effects. Appl Neurophysiol. 1979;42:95–97. doi: 10.1159/000102350. [DOI] [PubMed] [Google Scholar]
- 38.Denny-Brown D. Oxford UP; London: 1962. The basal ganglia and their relation to disorders of movement. . [Google Scholar]
- 39.Divac I. Neostriatum and functions of prefrontal cortex. Acta Neurobiol Exp. 1972;32:461–477. [PubMed] [Google Scholar]
- 40.Divac I, Diemer NH. Prefrontal system in the rat visualized by means of labeled deoxyglucose: further evidence for functional heterogeneity of the neostriatum. J Comp Neurol. 1980;190:1–13. doi: 10.1002/cne.901900102. [DOI] [PubMed] [Google Scholar]
- 41.Evarts EV, Kimura M, Wurtz RH, Hikosaka O. Behavioral correlates of activity in basal ganglia neurons. Trends Neurosci. 1984;56:447–453. [Google Scholar]
- 42.Fentress JC. Development and patterning of movement sequences in inbred mice. In: Kiger J, editor. The biology of behavior. Oregon State University; Corvallis: 1972. pp. 83–132. [Google Scholar]
- 43.Fentress JC. Emergence of pattern in the development of mammalian movement sequences. J Neurobiol. 1992;23:1529–1556. doi: 10.1002/neu.480231011. [DOI] [PubMed] [Google Scholar]
- 44.Feve A, Fenelon G, Wallays C, Remy P, Guillard A. Axial motor disturbances after hypoxic lesions of the globus pallidus. Mov Disord. 1993;8:321–326. doi: 10.1002/mds.870080311. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system: projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- 46.Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13:1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folstein S (1989) Huntington’s disease: a disorder of families. Baltimore: The John Hopkins University.
- 48.Frankel M, Cummings JL, Robertson MM, Trimble MR, Hill MA, Benson DR. Obsessions and compulsions in Gilles de la Tourette’s syndrome. Neurology. 1986;36:378–382. doi: 10.1212/wnl.36.3.378. [DOI] [PubMed] [Google Scholar]
- 49.Gabrieli J. Contribution of the basal ganglia to skill learning and working memory in humans. In: Houl JA, Davis JL, Beiser DB, editors. Models of information processing in the basal ganglia. MIT; Cambridge: 1995. pp. 277–294. [Google Scholar]
- 50.Gardiner TW, Kitai ST. Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Exp Brain Res. 1992;88:517–530. doi: 10.1007/BF00228181. [DOI] [PubMed] [Google Scholar]
- 51.Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, Bradshaw JA. Reduction in external cues and movement sequencing in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57:368–370. doi: 10.1136/jnnp.57.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA, Chiu E. Advance information and movement sequencing in Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1995;58:184–191. doi: 10.1136/jnnp.58.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of Parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- 54.Gerfen CR, Staines WA, Arbuthnott GW, Fibiger HC. Crossed connections of the substantia nigra in the rat. J Comp Neurol. 1982;207:283–303. doi: 10.1002/cne.902070308. [DOI] [PubMed] [Google Scholar]
- 55.Graybiel AM, Kimura M. Adaptive neural networks in the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. MIT; Cambridge: 1995. pp. 103–116. [Google Scholar]
- 56.Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 58.Grill HJ, Norgren R. Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:299–312. doi: 10.1016/0006-8993(78)90570-x. [DOI] [PubMed] [Google Scholar]
- 59.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 60.Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- 61.Haber SN, Kowall NW, Vonsattel JP, Bird ED, Richardson EP., Jr Gilles de la Tourette’s syndrome. A postmortem neuropathological and immunohistochemical study. J Neurol Sci. 1986;75:225–241. doi: 10.1016/0022-510x(86)90097-3. [DOI] [PubMed] [Google Scholar]
- 62.Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol. 1993;329:111–128. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- 63.Hall S, Schallert T. Striatal dopamine and the interface between orienting and ingestive functions. Physiol Behav. 1988;44:469–471. doi: 10.1016/0031-9384(88)90307-1. [DOI] [PubMed] [Google Scholar]
- 64.Harrington DL, Haaland KA. Sequencing in Parkinson’s disease. Brain. 1991;114:99–115. [PubMed] [Google Scholar]
- 65.Hazrati LN, Parent A. The striatopallidal projection displays a high degree of anatomical specificity in the primate. Brain Res. 1992;592:213–227. doi: 10.1016/0006-8993(92)91679-9. [DOI] [PubMed] [Google Scholar]
- 66.Hedreen JC, Folstein SE. Early loss of neostriatal striosome neurons in Huntington’s disease. J Neuropathol Exp Neurol. 1995;54:105–120. doi: 10.1097/00005072-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. J Neurosci. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 69.Iversen LL. The chemistry of the brain. Sci Am. 1979;241:134–149. doi: 10.1038/scientificamerican0979-134. [DOI] [PubMed] [Google Scholar]
- 70.Jackson S, Houghton G. Sensorimotor selection and the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. MIT; Cambridge: 1995. pp. 337–368. [Google Scholar]
- 71.Jaspers RMA, de Vries TJ, Cools AR. Enhancement in switching motor patterns following local application of the glutamate agonist AMPA into the cat caudate nucleus. Behav Brain Res. 1990;37:237–246. doi: 10.1016/0166-4328(90)90135-2. [DOI] [PubMed] [Google Scholar]
- 72.Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat: an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- 73.Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. J Neurophysiol. 1995;74:911–933. doi: 10.1152/jn.1995.74.3.911. [DOI] [PubMed] [Google Scholar]
- 74.Kermadi I, Jurquet Y, Arzi M, Joseph JP. Neural activity in the caudate nucleus of monkeys during spatial sequencing. Exp Brain Res. 1993;94:352–356. doi: 10.1007/BF00230305. [DOI] [PubMed] [Google Scholar]
- 75.Kimura M, Aosaki T, Hu Y, Ishida A, Watanabe K. Activity of primate putamen neurons is selective to the mode of voluntary movement: visually guided, self-initiated, or memory guided. Exp Brain Res. 1992;89:473–477. doi: 10.1007/BF00229870. [DOI] [PubMed] [Google Scholar]
- 76.Labuszewski T, Lockwood R, McManus R, Edelstein FE, Lidsky TI. Role of postural deficits in oro-ingestive problems caused by globus pallidus lesions. Exp Neurol. 1981;74:93–110. doi: 10.1016/0014-4886(81)90151-5. [DOI] [PubMed] [Google Scholar]
- 77.Lashley KS. The problem of serial order in behavior. In: Jefferies LA, editor. Cerebral mechanisms of behavior. Wiley; New York: 1951. pp. 112–136. [Google Scholar]
- 78.Lasiter PS, Garcia J. A methoxyflurane delivery system for stereotaxic surgery. Brain Res Bull. 1984;13:457–460. doi: 10.1016/0361-9230(84)90097-2. [DOI] [PubMed] [Google Scholar]
- 79.Levitt DA, Teitelbaum P. Somnolence, akinesia and sensory activation of motivated behavior in the lateral hypothalamic syndrome. Proc Natl Acad Sci. 1975;72:2819–2823. doi: 10.1073/pnas.72.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lidsky TI, Manetto C, Schneider JS. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res. 1985;356:133–146. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- 81.Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, Jiminez EB. Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain Lang. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- 82.Luxenberg JS, Swedo SE, Flament MF, Friedland RP, Rapoport J, Rapoport SI. Neuroanatomical abnormalities in obsessive-compulsive disorder detected with quantitative x-ray computed tomography. Biol Psychiatry. 1988;145:1089–1093. doi: 10.1176/ajp.145.9.1089. [DOI] [PubMed] [Google Scholar]
- 83.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 84.Marsden CD. The mysterious motor function of the basal ganglia. Neurology. 1982;32:524–532. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- 85.Marsden CD. Which motor disorder in Parkinson’s disease indicates the true motor function of the basal ganglia? In: Evered D, O’Conner M, editors. Functions of the basal ganglia. Pitman; London: 1984. pp. 225–237. [DOI] [PubMed] [Google Scholar]
- 86.Marsden CD. Dopamine and basal ganglia disorders in humans. Semin Neurosci. 1992;4:171–178. [Google Scholar]
- 87.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 88.Mittler T, Cho J, Peoples LL, West MO. Representation of the body in the lateral striatum of the freely moving rat: single neurons related to licking. Exp Brain Res. 1994;98:163–167. doi: 10.1007/BF00229122. [DOI] [PubMed] [Google Scholar]
- 89.Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- 90.Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 91.Montgomery EB, Buchholz SR. The striatum and motor cortex in motor initiation and execution. Brain Res. 1991;549:222–229. doi: 10.1016/0006-8993(91)90461-4. [DOI] [PubMed] [Google Scholar]
- 92.Mufson EJ, Brandabur MM. Sparing of NADPH-diaphorase striatal neurons in Parkinson’s and Alzheimer’s diseases. NeuroReport. 1994;5:705–708. doi: 10.1097/00001756-199402000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura S, Muramatsu S, Yoshida M. Role of the basal ganglia in manifestation of rhythmical jaw movement in rats. Brain Res. 1990;535:335–338. doi: 10.1016/0006-8993(90)91620-v. [DOI] [PubMed] [Google Scholar]
- 94.Nauta WJH. Reciprocal links of the corpus striatum with the cerebral cortex and the limbic system: a common substrate for movement and thought? In: Mueller, editor. Neurology and psychiatry: a meeting of the minds. Karger; Basel: 1989. pp. 43–63. [Google Scholar]
- 95.Norton S. Hyperactive behavior after lesions of the globus pallidus. Brain Res Bull. 1976;1:193–202. doi: 10.1016/0361-9230(76)90069-1. [DOI] [PubMed] [Google Scholar]
- 96.Oepen G, Mohr U, Willmes K, Thoden U. Huntington’s disease: visuomotor disturbances in patients and offspring. J Neurol Neurosurg Psychiatry. 1985;48:426–433. doi: 10.1136/jnnp.48.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Owen AM, Leigh JM. Frontostriatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 98.Parent A. Wiley; New York: 1986. Comparative neurobiology of the basal ganglia. Wiley series in neurobiology (Northcutt RG, ed). . [Google Scholar]
- 99.Parent A, Decarries L, Beaudet A. Organization of ascending serotonin systems in the rat brain. A radiographic study after intraventricular administration of 3H-5-hydroxy-tryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 100.Paxinos G, Watson C. Academic; New York: 1982. The rat brain in stereotaxic coordinates. . [DOI] [PubMed] [Google Scholar]
- 101.Pellis SM, Castaneda E, Mckenna MM, Tran-Nguyen LTL, Whishaw IQ. The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neurosci Lett. 1993;158:13–15. doi: 10.1016/0304-3940(93)90600-p. [DOI] [PubMed] [Google Scholar]
- 102.Pierce RC, Rebec GV. Iontophoresis in the neostriatum of awake, unrestrained rats: differential effects of dopamine, glutamate, and ascorbate on motor-and nonmotor-related neurons. Neuroscience. 1995;67:313–324. doi: 10.1016/0306-4522(95)00012-8. [DOI] [PubMed] [Google Scholar]
- 103.Pisa M. Motor functions of the striatum in the rat: critical role of the lateral region in tongue and forelimb reaching. Neuroscience. 1988;24:453–463. doi: 10.1016/0306-4522(88)90341-7. [DOI] [PubMed] [Google Scholar]
- 104.Rajakumar N, Elisevich K, Flumerfelt BA. Compartmental origin of the striato-entopeduncular projection in the rat. J Comp Neurol. 1993;331:286–296. doi: 10.1002/cne.903310210. [DOI] [PubMed] [Google Scholar]
- 105.Rapoport J. Recent advances in obsessive-compulsive disorder. Neuropsychopharmacology. 1991;5:1–20. [PubMed] [Google Scholar]
- 106.Rapoport J, Wise SP. Obsessive-compulsive disorder: evidence for basal ganglia dysfunction. Psychopharmacol Bull. 1988;20:380–384. [PubMed] [Google Scholar]
- 107.Resnick SM. Positron emission tomography in psychiatric illness. Curr Dir Psychol Sci. 1992;1:92–98. [Google Scholar]
- 108.Sabol KE, Neill DB, Wages SA, Church WH, Justice JB. Dopamine depletion in a striatal subregion disrupts performance of a skilled motor task in the rat. Brain Res. 1985;335:33–43. doi: 10.1016/0006-8993(85)90273-2. [DOI] [PubMed] [Google Scholar]
- 109.Sachs BD. The development of grooming and its expression in adult animals. Ann NY Acad Sci. 1988;525:1–17. doi: 10.1111/j.1749-6632.1988.tb38591.x. [DOI] [PubMed] [Google Scholar]
- 110.Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafiscular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320:228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- 111.Schallert T, Hall S. “Disengage” sensorimotor deficit following apparent recovery from unilateral dopamine depletion. Behav Brain Res. 1988;30:15–24. doi: 10.1016/0166-4328(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 112.Schneider JS, Lidsky TI. Processing of somatosensory information in striatum of behaving cats. J Neurophysiol. 1981;45:841–851. doi: 10.1152/jn.1981.45.5.841. [DOI] [PubMed] [Google Scholar]
- 113.Schneider JS, Olazabal UE. Behaviorally specific limb use deficits following globus pallidus lesions in rats. Brain Res. 1984;308:341–346. doi: 10.1016/0006-8993(84)91075-8. [DOI] [PubMed] [Google Scholar]
- 114.Schultz W, Apicella P, Ljungberg T, Romo R. Activity of monkey striatal and dopamine neurons during the performance of delayed response tasks. In: Percheron G, Mckenzie JS, Feger J, editors. The basal ganglia IV. Plenum; New York: 1995. pp. 305–316. [Google Scholar]
- 115.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sprengelmeyer R, Canavan AGM, Lange HW, Homberg V. Associative learning in degenerative neostriatal disorders: contrasts in explicit and implicit remembering between Parkinson’s and Huntington’s diseases. Mov Disord. 1995;10:51–65. doi: 10.1002/mds.870100110. [DOI] [PubMed] [Google Scholar]
- 117.Staines WA, Atmadja S, Fibiger HC. Demonstration of pallidostriatal pathway by retrograde transport of HRP-labeled lectin. Brain Res. 1981;206:446–450. doi: 10.1016/0006-8993(81)90545-x. [DOI] [PubMed] [Google Scholar]
- 118.Stelmach GE, Worringham CJ, Strand EA. The programming and execution of movement sequences in Parkinson’s disease. Int J Neurosci. 1987;36:55–65. doi: 10.3109/00207458709002139. [DOI] [PubMed] [Google Scholar]
- 119.Trimble M (1989) Psychopathology and movement disorders: a new perspective on the Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry 52[Suppl]:90–95. [DOI] [PMC free article] [PubMed]
- 120.Van den Brecken JH, Cools AR. Evidence for a role of the caudate nucleus in the sequential organization of behavior. Behav Brain Res. 1982;4:319–327. doi: 10.1016/0166-4328(82)90058-4. [DOI] [PubMed] [Google Scholar]
- 121.Weiner SI. Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci. 1993;13:3802–3817. doi: 10.1523/JNEUROSCI.13-09-03802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.West MO, Carelli RE, Pomerantz M, Cohen SM, Gardiner JP, Chapin JK, Woodward DJ. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990;64:1233–1246. doi: 10.1152/jn.1990.64.4.1233. [DOI] [PubMed] [Google Scholar]
- 123.Whishaw IQ, O’Conner WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate putamen to skilled forelimb use in the rat. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 124.Willingham DB, Koroshetz W. Evidence for dissociable motor skills in Huntington’s disease patients. Psychobiology. 1993;21:173–182. [Google Scholar]
- 125.Wilson SAK. An experimental research into the anatomy and physiology of the corpus striatum. Brain. 1914;36:427–492. [Google Scholar]