Abstract
Placental and marsupial mammals exist in three states of consciousness: waking, non-REM sleep, and REM sleep. We now report that the echidna Tachyglossus aculeatus, a representative of the earliest branch of mammalian evolution (the monotremes), does not have the pattern of neuronal activity of either of the sleep states seen in nonmonotreme mammals. Echidna sleep was characterized by increased brainstem unit discharge variability, as in REM sleep. However, the discharge rate decreased and the EEG was synchronized, as in nonREM sleep. Our results suggest that REM and non-REM sleep evolved as a differentiation of a single, phylogenetically older sleep state. We hypothesize that the physiological changes that occur during postnatal sleep development parallel certain aspects of the changes that have occurred during the evolution of sleep–waking states in mammals.
Keywords: sleep, monotreme, evolution, phylogeny, development, REM
Living monotremes such as the echidna and platypus represent the earliest offshoot of the mammalian evolutionary line. For this reason, these egg-laying mammals have provided many insights and constraints that have contributed to our understanding of mammalian evolution. Their structural stasis deduced from the fossil record, their plesiomorphic physiological mechanisms, and the lack of speciation in the monotreme line all indicate that monotreme species have modes of physiological function that are more similar to those of the common mammalian ancestor than any other existing group of mammals (Griffiths, 1968, 1978; Kemp, 1982; Archer et al., 1992; Pasqual et al., 1992; Westerman and Edwards, 1992).
There are three monotreme species, the duck-billed platypus,Ornithorhynchus anatinus, the long-beaked echidna,Zaglossus bruijni, and the short-beaked echidna (spiny anteater), Tachyglossus aculeatus. The platypus is aquatic and presents many technical difficulties for chronic recording. The long-beaked echidna of New Guinea is not readily available for study. The short-beaked echidna is the only monotreme in which sleep has been studied.
Allison et al. (1972) found high-voltage cortical electroencephalograms (EEG) throughout sleep in the echidna, resembling the EEG of the non-REM sleep state seen in placental and marsupial mammals. Arousal threshold was elevated during periods of high-voltage EEG, as in sleep in other mammals, and was lowest during periods of low-voltage EEG, as in waking. Periods of low-voltage EEG occurring immediately after high-voltage sleep, called PS?, were studied carefully to determine whether they might represent REM (paradoxical) sleep. Pyriform cortex rhythmic activity and hippocampal theta are maximal in active waking and REM sleep and are reduced or absent in quiet waking (QW) in all mammals in which these parameters have been studied. Whereas the active waking pattern of other mammals was seen in echidna active waking, the pyriform rhythmic activity and hippocampal theta seen in REM sleep was not seen in PS?. Brain temperature did not increase in PS? as it does in most mammals in REM sleep. Deprivation of PS? did not result in a rebound as it does for REM sleep. Somatic evoked responses in PS? resembled the waveform seen in QW rather than that of REM sleep. Finally, the arousal threshold in PS? was not elevated as it is in most mammalian species in REM sleep, but was similar to that of waking. These findings led Allison et al. to conclude that PS? was QW. Based on their observations, they concluded that the echidna does not have REM sleep. They hypothesized that REM sleep evolved after non-REM sleep in the mammalian line. Their conclusions have been an important consideration in subsequent theories of REM sleep function (Crick and Mitchison, 1983; Vertes, 1986; Siegel and Rogawski, 1988; Winson, 1990).
Slow wave variables suggest that sleep in the echidna resembles non-REM sleep. However, cortical EEG is just one indicator of sleep state. The two sleep states seen in nonmonotreme mammals, REM and non-REM sleep, are the product of different patterns of neuronal activity. The difference in discharge pattern in REM and non-REM sleep can be seen most easily in the brainstem reticular formation.
In nonmonotreme mammals, the great majority of brainstem reticular neurons have high and irregular discharge rates during active waking. Rate is reduced and more regular during QW. Discharge rate is minimal and most regular during non-REM sleep. This discharge pattern underlies the regularity of physiological control in this state (Siegel, 1994). REM sleep is characterized by fast and irregular discharge in most brainstem neurons. This discharge pattern produces the rapid eye movements, twitches, and other aspects of REM sleep (Siegel, 1994;Zepelin, 1994). The postural eye movement and other “epiphenomena” of sleep may differ in their expression among mammalian species. However, the underlying pattern of unit activity change during REM and nonREM sleep is similar in all placental and marsupial mammalian species, in all brainstem reticular and cortical areas (Huttenlocher, 1961; Evarts, 1964; Findlay and Hayward, 1969; Desiraju, 1972; Hobson et al., 1974; Siegel et al., 1977, 1979, 1983; Siegel and Tomaszewski, 1983; Chase and Morales, 1990; Steriade et al., 1993; Siegel, 1994;Zepelin, 1994).
The finding of a single sleep state in the echidna characterized by cortical slow waves raises the question of the nature of neuronal activity during sleep in this monotreme mammal. Does brainstem neuronal discharge show the non-REM sleep pattern of other mammals? Or might the cortical slow waves accompany a REM sleep-like state in the brainstem? The answer to this question would shed light on the issue of how the neuronal activity patterns of REM and non-REM sleep evolved.
In the present studies, we examined brainstem neuronal activity in the unrestrained echidna across the sleep cycle to determine the nature of the echidna sleep state. Some of this work has been presented in preliminary form (Siegel et al., 1994).
MATERIALS AND METHODS
Three short-beaked echidnas, one male and two female, were captured near Brisbane, Australia. The echidnas were all adults weighing between 2.3 and 3.2 kg. Echidnas are found in all regions of Australia and are not considered threatened or endangered. They are maintained easily in captivity. However, the use of echidnas for exhibition in zoos and for scientific purposes in Australia is tightly regulated by the State Minister of Environment and Heritage.
The echidnas were adapted to an enclosure at the University of Queensland that measured 1 m2. The enclosure was in a room with an opening in the roof that allowed natural light cycles and temperature conditions. Infrared LEDs provided illumination for video observation using a charge-coupled video camera with the infrared filter removed (Manger and Pettigrew, 1995). The enclosure was covered with dirt or shredded paper to a depth of 15 cm. Echidnas were fed a mixture of Chum dog food, uncooked egg, and milk. Studies were performed in December, January, and February between 7 A.M. and 11 P.M., with temperatures in the enclosure ranging from 20 to 28°C. Animals were active during the night, typically eating all their food between 11 P.M. and 7 A.M.
After an adaptation period of at least 2 months, the animals were anesthetized with Ketamine–Xylazine. Echidnas cannot be held in a standard stereotaxic frame. Their ear canals are at an acute angle to the stereotaxic planes and are of such small size that they will not permit the introduction of ear bars. The small size and lateral placement of the eyes also prevent use of the orbits as a restraint point. To use stereotaxic technique, we cemented a stainless steel bar to the frontal portion of the skull to immobilize the head during surgery. The bar, attached to a lockable universal joint, was clamped with Nicad magnets to the stereotaxic frame. The skull then was oriented with the ventral surface of the upper beak in the horizontal plane. The intersection of the sagittal sinus and cerebellum then was exposed to provide a stereotaxic reference point. A stereotaxic atlas was developed using the skulls and brain of two additional echidnas. Microwire electrodes, diameter 32 μ, mounted on movable microdrives, were constructed using techniques described previously (Siegel et al., 1977).
Unit recording electrodes were placed in the pontine tegmentum, an area that has been shown to be critically involved in REM-sleep generation. Electrodes also were placed in the midbrain reticular formation, the brain region in which sleep-cycle discharge has been most thoroughly studied in placental mammals (Huttenlocher, 1961; Hobson et al., 1974;Siegel et al., 1979, 1983; Siegel and Tomaszewski, 1983; Steriade et al., 1993; Siegel, 1994). The recording arrays sampled from a 6 mm rostro-caudal and 4 mm medio-lateral expanse of the reticular formation, including midbrain, pontine, medial and medio-lateral regions of the nucleus reticularis pontis oralis, and midbrain reticular formation, as defined by the location of the cranial nerve nuclei and other brainstem anatomical landmarks. Pairs of 1 mm diameter screw electrodes with 3 mm separation were placed on the dura mater over parietal and sensorimotor cortex for EEG recording. The eyes of the echidna face laterally. Wire hook electrodes were placed just lateral and medial to the orbit of the left eye with a 6 mm separation for electro-oculogram (EOG) recording. Four stranded stainless steel wires were placed in the dorsal neck musculature for electromyogram (EMG) recording. A thermocouple was placed in the cerebellum to continuously monitor brainstem temperature. A counterbalance system supported the recording cables and allowed the echidna to move freely throughout the enclosure during spontaneous waking and sleep episodes. Physiological variables were recorded and digitized for subsequent computer analysis. Animals were healthy and maintaining or gaining weight over the course of the study.
RESULTS
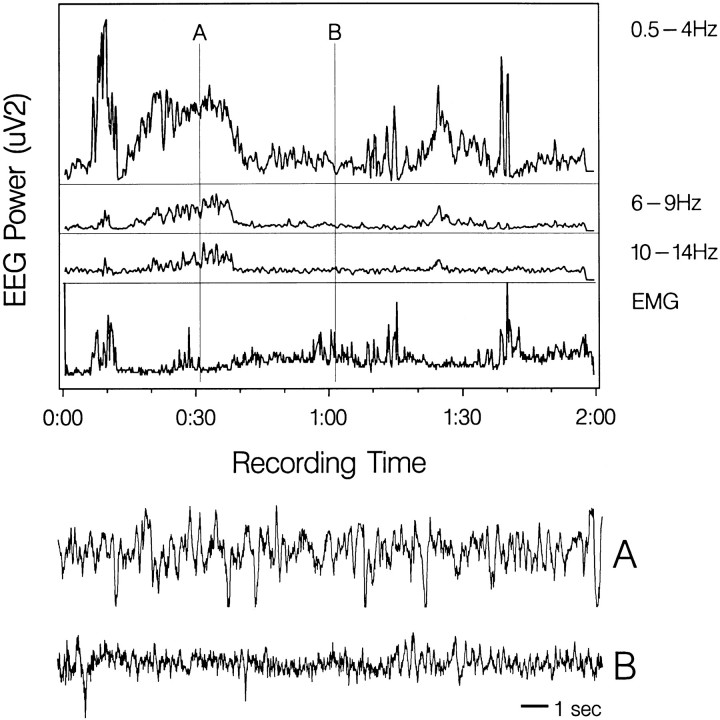
As reported by Allison et al. (1972), we saw long periods of high- voltage EEG during behavioral quiescence. The time course of EEG power changes during sleep was similar to that of non-REM sleep in nonmonotreme mammals (Fig. 1). Allison et al. previously had emphasized a lack of EEG spindles in the echidna. With our sensorimotor cortex recording derivations, we did see activity in the 6–9 and 10–14 Hz range. However, the distinct waxing–waning envelope of the feline spindle wave was not seen in the raw EEG signal.
Fig. 1.
Power distribution of sensorimotor EEG recorded continuously for 2 hr during sleep in the echidna. Sample A, Indicated on the power distribution plots and expanded below is from sleep; sample B is from waking.
Neck muscle tone was absent whenever the echidna was not actively moving. Thus, there was little or no additional reduction in tone with the high-voltage EEG of sleep.
We saw no periods of EOG activity during sleep with high-voltage EEG waves. In an attempt to elicit waking eye movement, we held an implanted echidna and rotated it rapidly in the sagittal and horizontal planes in a manner that elicits vigorous vestibulo-ocular reflexes in the cat (Siegel et al., 1983). We saw no eye movement by direct visual observation of the eyes or with our EOG recordings. We saw no periods of EOG activity with low-voltage EEG when the animal was not actively locomoting. These observations are in accord with the conclusion ofAllison et al. (1972) that the echidna does not have large-amplitude eye movements. Periods during which the echidnas had little movement or EMG activity but showed a low-voltage EEG tended to follow active periods during which the echidnas ate, locomoted, and explored the environment. Gross movements of the body produced significant activity on the EOG leads. This appeared to be a movement artifact. The sharp quills of the echidna, which cover its entire body and head, are movable. They are another source of EMG artifact recordable on the EOG channel. The tongue of the echidna, used to capture ants, can be projected 18 cm from its mouth. The tongue movement path runs close to the eyes and also is a potential source of activity in electrodes placed to record eye movements. We acquired usable brain temperature data on one of the animals and found decreased brain temperature during sleep, as reported by Allison et al. (1972).
A total of 43 units were recorded, 26 from the midbrain reticular formation and 17 from the subcoeruleus/reticularis pontis oralis region of the pons. Units were recorded for a minimum of 3 hr and a maximum of 30 hr.
Active waking was characterized by high and irregular discharge rates. During QW, the EEG remained low voltage, and reticular formation unit activity slowed and became more regular. Thus, during waking, echidna unit discharge was like that of placental mammals.
Mean unit activity rates were 4.7 Hz in QW and 3.3 Hz in sleep. Sleep discharge rates were significantly lower than the rates in QW (F = 3.8, p <.05). Therefore, the rate decrease during sleep in the echidna resembled that seen in brainstem reticular units in placental mammals during non-REM sleep (Hobson et al., 1974;Siegel and McGinty, 1976; Siegel, 1979, 1994; Siegel et al., 1979,1983; Siegel and Tomaszewski, 1983; McGinty and Siegel, 1992; Steriade et al., 1993).
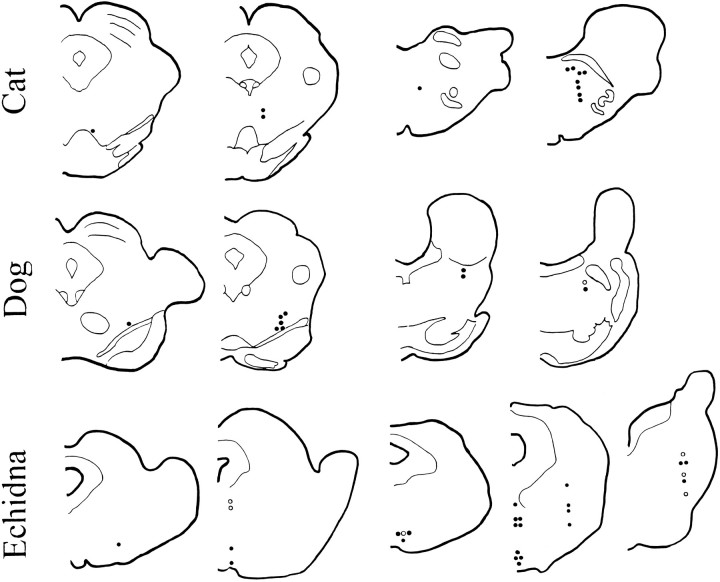
We saw no major difference in the rate or pattern of sleep-cycle discharge as a function of the location of the units within the medial reticular formation of the echidna (Figs. 2, 3). Figure2 includes evaluations of changes in variability in digitized echidna data (n = 22, with 17 increasing variability in sleep), as well as in an additional six cells in which a polygraph record of multiple sleep cycles was made and the change in variability with sleep assessed by inspection of the paper record (five of six increased variability in sleep). In nonmonotreme mammals, reticular cells decrease variability in the transition from waking to non-REM sleep and increase variability in REM sleep (Huttenlocher, 1961; Hobson et al., 1974; Siegel and McGinty, 1977; Siegel, 1979). In the echidna, 79% of units increased discharge variability in the transition from waking to sleep with high-voltage EEG. Cells with this pattern were distributed throughout the brainstem reticular formation of the echidna. Therefore, the differences we report cannot be attributable to our sampling of a different neuronal population.
Fig. 2.
Location of recorded neurons in the echidna, cat, and dog. Filled circles indicate units that increased variability in sleep in the echidna or in REM sleep in the cat and dog, relative to QW.
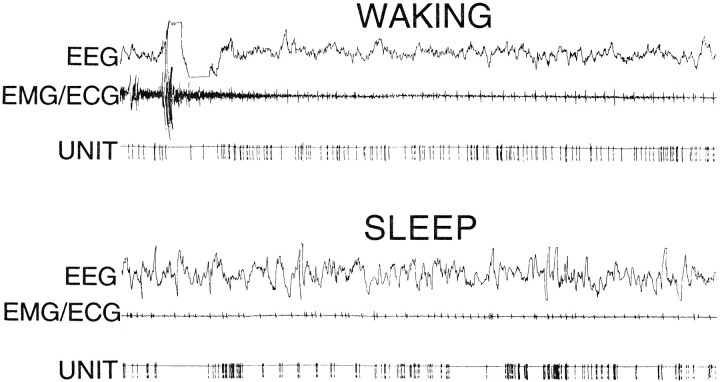
Fig. 3.
Unit discharge of a representative neuron recorded in the nucleus reticularis pontis oralis of the echidna during waking and sleep. Note irregularity of neuronal discharge during sleep. EEG, EMG–ECG (electromyogram–electrocardiogram) unit, pulse output of window discriminator triggered by neuron. Duration of recordings is 30 sec.
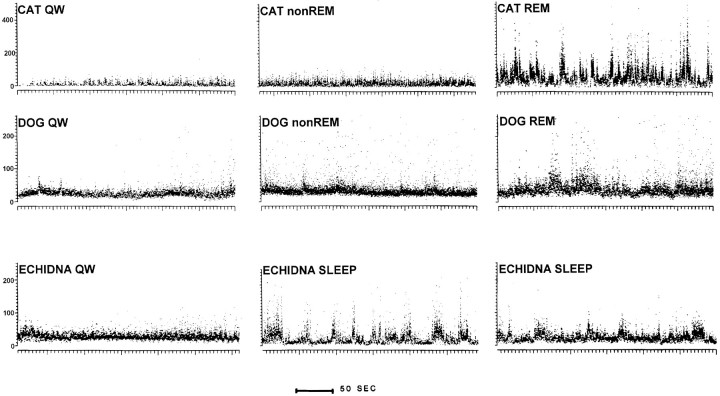
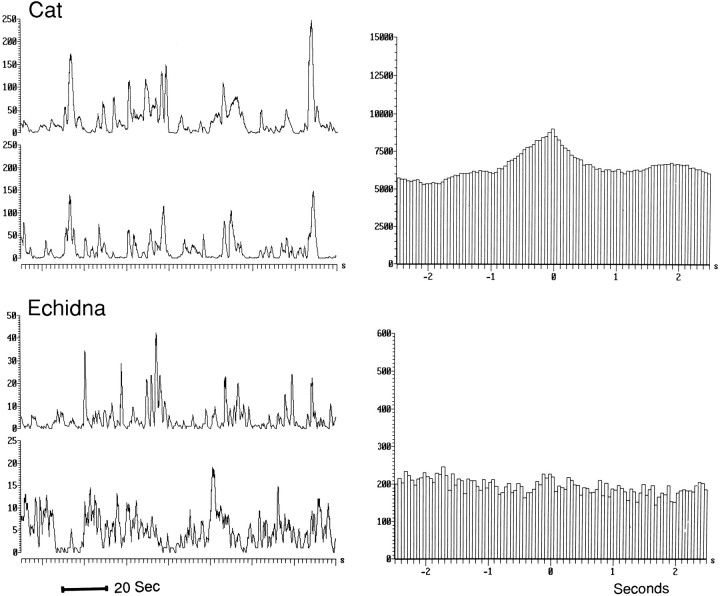
Figures 3 and 4 show the discharge of echidna units during waking and sleep. Figure 4 also shows representative units recorded in the same brainstem regions in the cat and dog. In placental mammals, virtually all midbrain and pontine units show unchanged or decreased discharge variability in non-REM sleep relative to QW and increased variability only in REM sleep.
Fig. 4.
Instantaneous compressed rate plots of representative units recorded in nucleus reticularis pontis oralis of the cat, dog, and echidna. Each point represents the discharge rate for the previous interspike interval. In cat QW and non-REM sleep, the discharge rate is low and relatively regular. The rate increases and becomes highly variable during REM sleep. A similar pattern can be seen in a unit recorded in the dog. In the echidna, sleep is characterized by variable unit discharge rates.
The increased variability of echidna reticular formation unit discharge in sleep relative to QW was quantified by calculating the variability in discharge frequency during consecutive 10 sec epochs. The echidna data were compared with unit data collected for other studies in our laboratory. Cat units were recorded in mongrel cats (Siegel and Tomaszewski, 1983; Siegel et al., 1983). Dog units were noncataplexy-related units recorded in narcoleptic Dobermans (Siegel et al., 1991). We selected cat and dog units recorded from anatomical locations approximating the anatomical locations of the echidna units (Fig. 2). Both cat and dog units were recorded in unrestrained, unstimulated, and undrugged animals during spontaneous sleep cycles using the same 32 μ microwire recording techniques used in the echidna (Siegel and McGinty, 1976; McGinty and Siegel, 1992). Ten reticular cells in the dog, 13 in the cat, and the 22 echidna cells for which we were able to digitize records across the sleep cycle were compared.
The levels of variability of reticular unit discharge in REM sleep did not differ significantly in the dog and cat; neither did levels of discharge variability in non-REM sleep (p >0.1, Mann–Whitney U test). These data were pooled for the subsequent comparisons. Variability of discharge in QW also did not differ significantly in the dog and cat. Variability of discharge in QW in the echidna did not differ from the pooled cat and dog QW values. The increased variability of reticular unit discharge in sleep in the echidna differed significantly from the decreased variability seen in non-REM sleep in the cat and dog (p <0.001, Mann–WhitneyU test comparing the direction of change from waking to sleep). The level of reticular unit discharge variability in the echidna during sleep was significantly greater than that during non-REM sleep in the cat and dog (p <0.027, Mann–WhitneyU test). Variability in echidna sleep was significantly less than the variability in dog and cat REM sleep (p <0.001). In summary, whereas the increase of variability in the echidna was in the direction of the change seen in REM sleep and the variability was greater than that in dog and cat non-REM sleep, the magnitude of the variability increase seen in sleep in the echidna was lower than that in REM sleep.
Figure 5 plots sleep variance against QW variance in the cat, dog, and echidna. The points for the echidna can be seen to fall between the points from REM and non-REM sleep in the cat and dog. This illustrates graphically that the increased discharge variability during sleep in the echidna is intermediate between the pattern seen in non-REM sleep and REM sleep in cats and dogs.
Fig. 5.
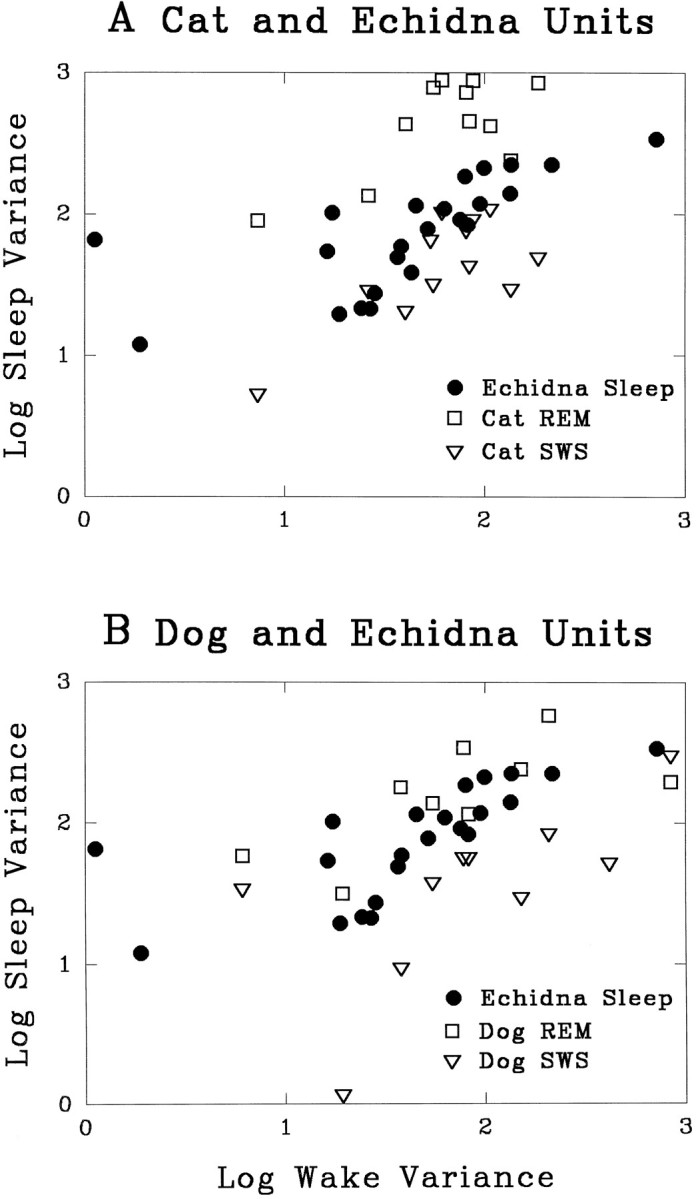
QW variance of the number of neuronal action potentials in consecutive 10 sec epochs plotted against sleep variance. Echidna units are plotted with REM sleep and non-REM sleep values in the cat (A) and dog (B). Note that the majority of the points representing echidna sleep fall between those of cat or dog units recorded in REM sleep and non-REM sleep.
We did not see any periods of phasic motor activity in the EMG or in our infrared video observations during these periods of unit discharge irregularity. The complete atonia during sleep may have blocked the motor expression of phasic brainstem unit activity. Another explanation for the lack of motor activity is that whereas the bursting activity of brainstem neurons during REM sleep is synchronized across the entire neuronal population in placental mammals (Siegel et al., 1981), bursting was not synchronized in the echidna. Cross-correlation analyses at 1, 25, and 200 msec binwidth of 20 units recorded in 10 pairs revealed that these echidna units, even when recorded from the same microwire, fired their bursts asynchronously in sleep. Figure6 compares a cross-correlation of a representative cell pair recorded from the pontine reticular formation of the echidna with a cross-correlation of a typical pair of cat pontine reticular units recorded in our laboratory using identical techniques (Siegel et al., 1981). A peak, indicating correlated firing of units, is present during REM sleep in the cat, but was not present in any of the echidna pairs we recorded.
Fig. 6.
Rate histogram and cross-correlogram of discharge in a pair of cat reticularis pontis oralis units recorded during REM sleep (top), compared with a pair of echidna reticularis pontis oralis units recorded during sleep (bottom). Counts per second on y-axis on left. Cross-correlograms of each pair computed at 50 msec binwidth are shown atright. Unit pairs in both the cat and echidna were recorded from adjacent microwires on a single bundle of 32 μm microwires. Whereas most cat and dog units fire synchronously and are cross-correlated during REM sleep (Siegel et al., 1981), none of the echidna unit pairs was cross-correlated in sleep.
DISCUSSION
We find that the pattern of brainstem neuronal activity during sleep in the echidna does not resemble that seen in the sleep of placental mammals. While the discharge rate of the neuronal population decreases, discharge variability increases. This period of increased variability is accompanied by a high-voltage cortical EEG. The neuronal activity pattern of REM sleep, which consists of low-voltage EEG, increased discharge rate and variability, and synchrony of firing in reticular units does not occur. However, neither does the neuronal activity pattern seen in nonmonotremes during non-REM sleep, which consists of decreased discharge rate and decreased variability during a period of high-voltage cortical EEG. Instead, sleep in the echidna has aspects of both non-REM and REM sleep states.
We hypothesize that since the divergence of monotreme and nonmonotreme mammals 130 million years ago, there has been a differentiation of this primordial sleep state into two states; whereas the primordial state had reduced discharge rate and increased variability simultaneously, the subsequently evolved states alternate temporally. Non-REM has a low discharge rate and low rate variability, whereas REM sleep has a high discharge rate and very high rate variability.
Our study was aimed at an analysis of neuronal activity during the echidna sleep state described by Allison et al. (1972), not at a reexamination of their conclusion that the echidna lacked a REM sleep state. A recent abstract reports evidence for REM sleep with low-voltage EEG and rapid eye movements in the echidna (Berger et al., 1995). However, in contrast to the studies by Allison et al., arousal thresholds were not tested in this putative sleep state. Therefore, this criterion for distinguishing sleep from waking was unavailable. The Berger et al. study used only needle electrodes placed outside the cranium and, therefore, did not include the hippocampal, pyriform cortex, and brain temperature measures that allowed Allison et al. to conclude that the echidna did not have REM sleep. Berger et al. also did not repeat Allison et al.’s PS? deprivation test or somatosensory-evoked responses that contributed to the latter’s conclusion that echidnas do not have REM sleep. Our observation of lack of signal on EOG electrodes placed close to the eye during vestibular stimulation suggests that the potentials recorded by the more widely spaced bilateral needle electrode derivations used in Berger’s study were movement artifact rather than eye movement, indicating a waking state. Our unit recordings indicate reticular bursting during active waking (as the animal locomoted) and in periods of high-voltage EEG with the animal quiescent, but uniformly low levels of variability during periods of low-voltage cortical EEG without movement, as in QW. All of these considerations are most consistent with the conclusion that the low-voltage EEG periods are waking rather than REM sleep.
REM and non-REM sleep usually are viewed as completely distinct states. Thus, an often-quoted statement is that REM sleep is as different from non-REM sleep as non-REM sleep is from waking (Dement, 1972). Indeed, this appears to be true at the descriptive level. However, there also are important links between REM and non-REM sleep. Across mammalian species, REM sleep time is correlated positively with non-REM sleep time (Zepelin and Rechtschaffen, 1974). Studies of REM–non-REM cyclicity show that even within the cycle of an individual animal, REM sleep duration is highly correlated with, and can be predicted by, previous non-REM sleep duration (Uchida et al., 1992; Benington and Heller, 1994). The current study is consistent with the idea that REM sleep and non-REM sleep are linked evolutionarily and, perhaps, functionally.
Adult nonmonotreme mammals have a high ratio of REM sleep to non-REM sleep discharge rates in the brainstem reticular formation. Corner and Bour (1984) have shown that this pattern develops postnatally, in the first few weeks of life. In the current study, we show that the rate and variability of discharge in medial reticular cells during sleep in the echidna are lower than those in REM sleep and higher than those of non-REM sleep in the cat and dog. This pattern resembles more closely the neonatal pattern. Therefore, a state of reduced reticular variability is shared by monotremes and young nonmonotreme mammals. We hypothesize that the developmental differentiation of this state into REM and non-REM sleep parallels the phylogenetic differentiation of these states from the primordial sleep state.
Footnotes
This work was supported by the Medical Research Service of the Veterans Administration, US Public Health Service Grants NS32819 and NS14610, and the Australian Research Council Special Research Centres Budget. We thank Joel Benington for help with spectral analysis of the echidna EEG. This research was carried out according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes under Queensland National Parks and Wildlife permits T00803 and K01782.
Correspondence should be addressed to Jerome Siegel, Department of Psychiatry, UCLA, Neurobiology Research 151A3, VAMC, 16111 Plummer Street, North Hills, CA 91343.
REFERENCES
- 1.Allison T, Van Twyver H, Goff WR. Electrophysiological studies of the echidna, Tachyglossus aculeatus . I. Waking and sleep. Arch Ital Biol. 1972;110:145–184. [PubMed] [Google Scholar]
- 2.Archer M, Jenkins F, Hand S, Murray P, Godthelp H. Description of the skull and non-vestigial dentition of a miocene platypus [Obdurodon dicksoni (N. Sp.)] from Riversleigh, Australia, and the problem of monotreme origins. In: Augee M, editor. Platypus and echidnas. Royal Zoological Society of NSW; Mosman, Australia: 1992. pp. 15–27. [Google Scholar]
- 3.Benington J, Heller HC. Does the function of REM sleep concern non-REM sleep of waking? Prog Neurobiol. 1994;44:433–449. doi: 10.1016/0301-0082(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 4.Berger RJ, Nicol SC, Andersen NA, Phillips NH. Paradoxical sleep in the echidna. Sleep Res. 1995;24A:199. [Google Scholar]
- 5.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–84. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 6.Corner MA, Bour HL. Postnatal development of spontaneous neuronal discharge in the pontine reticular formation of free-moving rats during sleep and wakefulness. Exp Brain Res. 1984;54:66–72. doi: 10.1007/BF00235819. [DOI] [PubMed] [Google Scholar]
- 7.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 8.Dement WC (1972) Some must watch while some must sleep. Stanford: Alumni Association.
- 9.Desiraju T. Discharge properties of neurons of the parietal association cortex during states of sleep and wakefulness in the monkey. Brain Res. 1972;47:69–75. doi: 10.1016/0006-8993(72)90252-1. [DOI] [PubMed] [Google Scholar]
- 10.Evarts EV. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- 11.Findlay ALR, Hayward JN. Spontaneous activity of single neurons in the hypothalamus of rabbits during sleep and waking. J Physiol (Lond) 1969;201:237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths M. Pergamon; New York: 1968. Echidnas. . [Google Scholar]
- 13.Griffiths M. Academic; New York: 1978. The biology of the monotremes. . [Google Scholar]
- 14.Hobson JA, McCarley RW, Pivik T, Freedman R. Selective firing by cat pontine brain stem neurons in desynchronized sleep. J Neurophysiol. 1974;37:497–511. doi: 10.1152/jn.1974.37.3.497. [DOI] [PubMed] [Google Scholar]
- 15.Huttenlocher PR. Evoked and spontaneous activity in single units of medial brain stem during natural sleep and waking. J Neurophysiol. 1961;24:451–468. [Google Scholar]
- 16.Kemp T. Academic; London: 1982. Mammal-like reptile and the origin of mammals. [Google Scholar]
- 17.Manger PR, Pettigrew JD. Electroreception and the feeding behaviour of the platypus [Ornithorhynchus anatinus (Monotremata mammalia)]. Philos Trans R Soc Lond [Biol] 1995;347:359–381. [Google Scholar]
- 18.McGinty DJ, Siegel JM. Brain neuronal unit discharge in freely-moving animals: methods and application in the study of sleep mechanisms. Prog Psychobiol Physiol Psychol. 1992;15:85–140. [Google Scholar]
- 19.Pascual R, Archer M, Jaureguizar EO, Prado J, Godthelp H, Hand S. The first non-Australian monotreme: an early paleocene South American platypus (Monotremata ornithorhynchidae ). In: Augee M, editor. Platypus and echidnas. Royal Zoological Society of NSW; Mosman, Australia: 1992. pp. 1–14. [Google Scholar]
- 20.Siegel JM. Behavioral functions of the reticular formation. Brain Res Rev. 1979;1:69–105. doi: 10.1016/0165-0173(79)90017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Saunders; Philadelphia: 1994. pp. 125–144. [Google Scholar]
- 22.Siegel JM, McGinty DJ. Brainstem neurons without spontaneous unit discharge. Science. 1976;193:240–242. doi: 10.1126/science.180599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel JM, McGinty DJ. Pontine reticular formation neurons: relationship of discharge to motor activity. Science. 1977;196:678–680. doi: 10.1126/science.193185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel JM, Rogawski MA. A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res Rev. 1988;13:213–233. doi: 10.1016/0165-0173(88)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel JM, Tomaszewski KS. Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol. 1983;50:696–716. doi: 10.1152/jn.1983.50.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel JM, McGinty DJ, Breedlove SM. Sleep and waking activity of pontine gigantocellular field neurons. Exp Neurol. 1977;56:553–573. doi: 10.1016/0014-4886(77)90321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 1979;179:49–60. doi: 10.1016/0006-8993(79)90488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel JM, Nienhuis R, Wheeler RL, McGinty DJ, Harper RM. Discharge pattern of reticular formation unit pairs in waking and REM sleep. Exp Neurol. 1981;74:875–891. doi: 10.1016/0014-4886(81)90260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel JM, Tomaszewski KS, Wheeler RL. Behavioral organization of reticular formation: studies in the unrestrained cat. II. Cells related to facial movements. J Neurophysiol. 1983;50:717–723. doi: 10.1152/jn.1983.50.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel JM, Nienhuis R, Fahringer H, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science. 1991;262:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel JM, Manger P, Nienhuis R, Fahringer HM, Pettigrew J. Novel sleep state organization in the echidna: implications for the evolution of REM and non-REM sleep. Soc Neurosci Abstr. 1994;20:1218. doi: 10.1523/JNEUROSCI.16-10-03500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 33.Uchida S, Maloney T, Feinberg I. Beta (20–28 Hz) and delta (0.3–3 Hz) EEGs oscillate reciprocally across NREM and REM sleep. Sleep. 1992;4:352–358. doi: 10.1093/sleep/15.4.352. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP. A life-sustaining function for REM sleep: a theory. Neurosci Biobehav Rev. 1986;10:371–6. doi: 10.1016/0149-7634(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 35.Westerman M, Edwards D. DNA hybridization and the phylogeny of monotremes. In: Augee M, editor. Platypus and echidnas. Royal Zoological Society of NSW; Mosman, Australia: 1992. pp. 28–34. [Google Scholar]
- 36.Winson J. The meaning of dreams. Sci Am. 1990;263:86–96. doi: 10.1038/scientificamerican1190-86. [DOI] [PubMed] [Google Scholar]
- 37.Zepelin H. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Philadelphia: 1994. pp. 69–80. [Google Scholar]
- 38.Zepelin H, Rechtschaffen A. Mammalian sleep, longevity and energy metabolism. Brain Behav Evol. 1974;10:425–470. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]