Abstract
Genetic approaches in Drosophila have advanced our understanding of the molecular mechanisms of different forms of learning, including habituation, but relevant neural components have not been explored. We show that a well defined neural circuit that underlies an escape response can be habituated, providing for the first time excellent opportunities for studying physiological parameters of learning in a functional circuit in the fly. Compared with other forms of conditioning, relatively little is known of the physiological mechanisms of habituation. The giant fiber pathway mediates a jump-and-flight escape response to visual stimuli. The jump may also be triggered electrically at multiple sites in the tethered fly. This response shows parameters of habituation, including frequency-dependent decline in responsiveness, spontaneous recovery, and dishabituation by a novel stimulus, attributable to plasticity in the brain.
Mutations of rutabaga that diminish cAMP synthesis reduced the rate of habituation, whereas dunce mutations that increase cAMP levels led to a detectable but moderate increase in habituation rates. Surprisingly, habituation was extremely rapid indunce rutabaga double mutants. This corresponds to the extreme defects seen in double mutants in other learning tasks, and demonstrates that defects of the rutabaga anddunce products interact synergistically in ways that could not have been predicted on the basis of simple counterbalancing biochemical effects. Although habituation is localized to afferents to the giant fiber, cAMP mutations also affected performance of thoracic portions of the pathway on a millisecond time scale that did not account for behavioral plasticity. More significantly, spontaneous recovery and dishabituation were not as clearly affected as habituation in mutants, indicating that these processes may not overlap entirely in terms of cAMP-regulating mechanisms.
The analysis of habituation of the giant fiber response in available learning and memory mutants could be a crucial step toward realizing the promise of memory mutations to elucidate mechanisms in neural circuits that underlie behavioral plasticity.
Keywords: Drosophila, giant fiber, habituation, learning and memory mutants, cAMP, rutabaga, dunce
In seeking to understand the neural substrates of learning, the study of habituation holds special promise because of its simplicity. Habituation is a reduction in the response to a stimulus over time that is not attributable to sensory adaptation or motor fatigue (Thompson and Spencer, 1966). There is increasing recognition of the potential of genetic approaches for defining neural mechanisms of learning, because consistent physiological and behavioral defects can be induced as a consequence of defined biochemical perturbations. In Drosophila melanogaster, more than half a dozen genes have been identified, the mutations of which primarily affect learning and memory (Dudai et al., 1976; Aceves-Pina et al., 1983; Tully and Quinn, 1985; Boynton and Tully, 1992; Dura et al., 1993). Genetic technology provides a means for examining how biochemical components of membrane excitability, synaptic transmission, or regulation of neuronal growth are involved in learning. However, although there has been great progress in defining the effects of memory mutations at both biochemical and behavioral levels, their physiological effects have been studied only in reduced preparations and in cell culture (Delgado et al., 1991; Zhong and Wu, 1991a; Wang et al., 1994; Zhao and Wu, 1994). There has not been an accessible system established inDrosophila to study the neural substrates that directly mediate behavioral plasticity.
The giant fiber-mediated escape response in Drosophila has been extensively described, and the development and physiology of the underlying circuit are understood in some detail (Levine and Tracey, 1973; Levine, 1974; Tanouye and Wyman, 1980; Wyman et al., 1984;Trimarchi and Schneiderman, 1993, 1995a,b). Appropriate visual or mechanical stimulation evokes a stereotyped jump-and-flight response, associated with activity in descending giant fiber neurons and a consistent spike pattern in leg and flight muscles. Electrodes, placed in the eyes to provide a defined path of stimulus current across the head, can bypass sensory receptors to trigger the circuit at the giant fiber neurons (short-latency response) (Tanouye and Wyman, 1980;Gorczyca and Hall, 1984) or, with lower-intensity stimulation, at giant fiber afferents (long-latency response) (Elkins and Ganetzky, 1990;Trimarchi and Schneiderman, 1993). Habituation has been demonstrated in escape responses of arthropods, including crayfish (Zucker, 1972;Krasne and Teshiba, 1995), crickets (May and Hoy, 1991), and odorant-induced jump in flies (Tully and Koss, 1992). The jump-and-flight response to visual stimulation in Drosophilais also plastic, showing characteristics of habituation in tethered flies (see Results). The work described here shows that the electrically induced long-latency response also attenuates in a manner that satisfies criteria for habituation and that this occurs within pathways afferent to the giant fiber.
The two best-described Drosophila “memory genes” arerutabaga (rut) and dunce(dnc), which encode an adenylyl cyclase (Aceves-Pina et al., 1983; Levin et al., 1992) and a cAMP-specific phosphodiesterase (Dudai et al., 1976; Chen et al., 1986), respectively. They affect modulation of cAMP metabolism (Byers et al., 1981; Livingstone et al., 1984), which has been implicated in synaptic plasticity in several systems (Aplysia: Klein and Kandel, 1980; Schacher et al., 1993; crayfish: Dixon and Atwood, 1989; Drosophila: Zhong and Wu, 1991a; Zhong et al., 1992; mouse: Huang et al., 1994). However, the roles of cAMP in habituation have not been established. Mutations ofrut and dnc lead to defects in both associative and nonassociative conditioning paradigms (Dudai et al., 1976; Booker and Quinn, 1981; Duerr and Quinn, 1982; Tempel et al., 1983; Tully and Quinn, 1985; Corfas and Dudai, 1989; Rees and Spatz, 1989; Tully and Koss, 1992). In the present study, we show that mutations of both loci also affect habituation of the electrically stimulated giant fiber response. Unlike other behaviors that show habituation in the fly, the giant-fiber response is carried by an identified, accessible circuit. This provides the first direct physiological demonstration of the involvement of rut and dnc in plasticity of a central circuit in which cellular mechanisms contributing to behavioral plasticity can be readily analyzed.
Some of these results have appeared in abstract form (Engel and Wu, 1994a).
MATERIALS AND METHODS
Mutants. Alleles of rut and dnccontained in rut1, y rut2, y dnc2 ec f, y dncM11 cv v f, y dncM14 cv v f, and y dncM14 cv v rut1 stocks have been described previously (Dudai et al., 1976; Mohler, 1977;Aceves-Pina et al., 1983; Livingstone et al., 1984; Zhong et al., 1992;Zhong and Wu, 1993a). Control strains used were Canton-S or, in a few trials, cn bw, w, In(1)FM7,y sc w B, or C(1)RM,y f in a Canton-S background (Lindsley and Zimm, 1992), or Oregon-R. Results for mutant alleles within each locus (rut or dnc) have been pooled in figures and tables because they did not differ significantly.
Preparation and recording. Preparation of flies, stimulation, recording, and analysis of muscle responses were performed as described previously (Engel and Wu, 1992, 1994b) with some modifications. Legs were waxed together (except for experiments in Fig.3) to inhibit flight and prevent sweeping of a leg across the wings, which could dishabituate the long-latency response. The experimental Faraday cage was covered with black plastic to reduce ambient light because strong illumination was found to inhibit the long-latency response. Stimulation (0.1 msec pulse, Grass S8, Quincy MA) was passed between uninsulated tungsten electrodes inserted in the eyes (anode normally in left eye). Signals were recorded from the right tergotrochanteral (TTM) jump muscle and left dorsal longitudinal a (DLMa) flight muscle (Miller, 1950), which are innervated by the same side of the giant fiber pathway (Levine, 1974; Wyman et al., 1984) (Fig. 1A). An arrangement of pulse generators feeding into a pen recorder provided a convenient means to distinguish spike latency classes in trial records. A negative pulse, triggered by the stimulator synch output, and a positive pulse, triggered by the DLM muscle spike, went to the same channel of the pen recorder (Gould-Brush 220, Cleveland, OH). The delay of the stimulator-triggered pulse could be adjusted so that it would be canceled on the pen record by DLM responses of the desired latency class. Precise latency values were measured as described previously (Engel and Wu, 1992, 1994b).
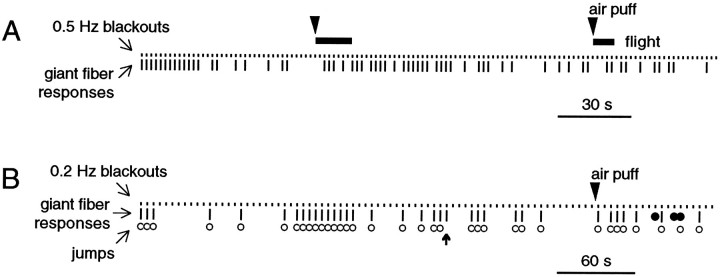
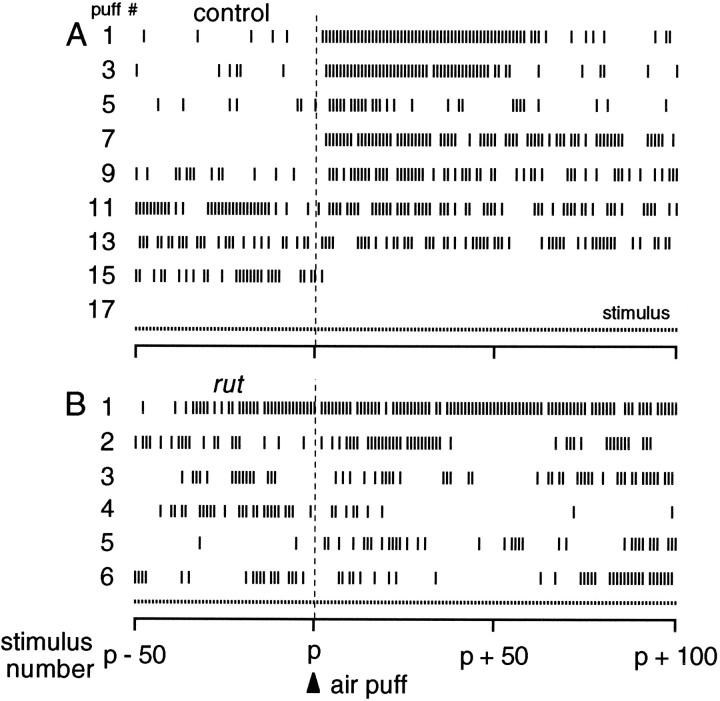
Fig. 3.
Habituation-like attenuation of the visually induced response. A, Giant-fiber-triggered leg and flight muscle responses in a tethered fly. Short ticks show timing of 20 msec darkenings of LED illumination (“blackouts”), andlong ticks indicate giant fiber responses detected in muscles. Response probability diminished but was dishabituated by an air puff (arrowhead); a second air puff had less effect.Bars indicate brief episodes of tethered flight in response to air puffs. B, Jumps corresponded with muscle responses in a second fly with legs unrestrained. Jumps (open circles) were normally readily detected by movement of a paper square glued to mesothoracic legs; one muscle response was not associated with a clear jump (bold arrow). Three filled circles indicate stimuli for which muscle responses could not be determined, because of movement after the air puff; all other muscle responses or failures were unambiguous.
Fig. 1.
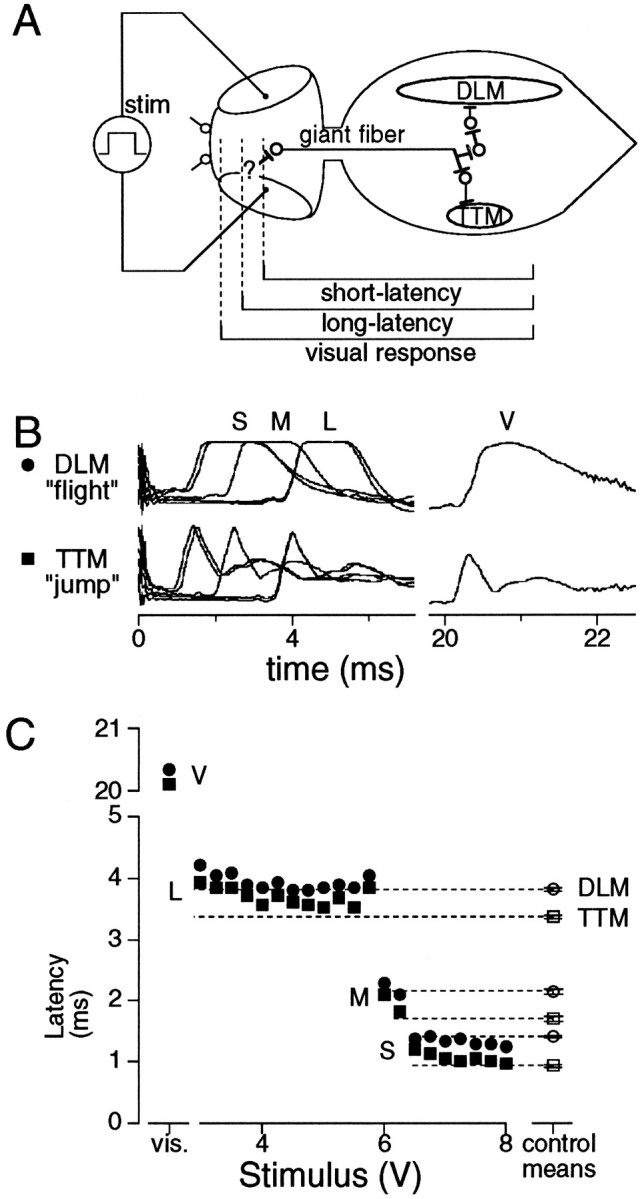
Illustration of the giant fiber response stimulated at different sites. A, Schematic representation of the giant fiber pathway, showing one side of the bilaterally symmetrical circuit. Stimulation of the cervical giant fiber triggers responses in tergotrochanteral (TTM) “jump” and dorsal longitudinal (DLM) “flight” muscles. Short- and long-latency responses result from electrical stimulation of the pathway at different points, as indicated by brackets.B, Muscle spikes recorded in visual response (V) evoked by lights-off, and long-(L), intermediate-(M), and short-latency (S) responses in the same fly. Note similar TTM/DLM interlatency for each response. Spike shapes differ in visual response because of some deterioration of the impaled muscles. C, The three distinct classes of response latency are triggered by different stimulus voltages. Long- and short-latency responses were seen in nearly all flies; intermediate-latency responses were seen less consistently (see text). Data in filled symbols from the same fly as B.Open symbols are mean ± SEM of the shortest latency of each class measured in all control flies (see Table 1).
Testing protocols. To minimize habituation from handling and threshold tests before a trial, flies were rested after mounting for at least 1 hr in a humid chamber before setting up for recording. After assessing response thresholds using interstimulus intervals (ISI) of 30 sec, flies were rested for 5 min before habituation testing. Three classes of response were identified, with progressively greater thresholds: long-latency, intermediate-latency, and short-latency. Although absolute latency values varied with temperature (Fig. 2) (Nelson and Wyman, 1990), these response classes were easily distinguished in individual flies (Fig.1B,C). For long-latency response trials, stimulus intensity was set near the top of the long-latency stimulus range. The DLM muscle spike was used to indicate success or failure of the long-latency response. To avoid the possibility of using damaged flies, those few flies in which at least two consecutive responses were not obtained were excluded from the analysis.
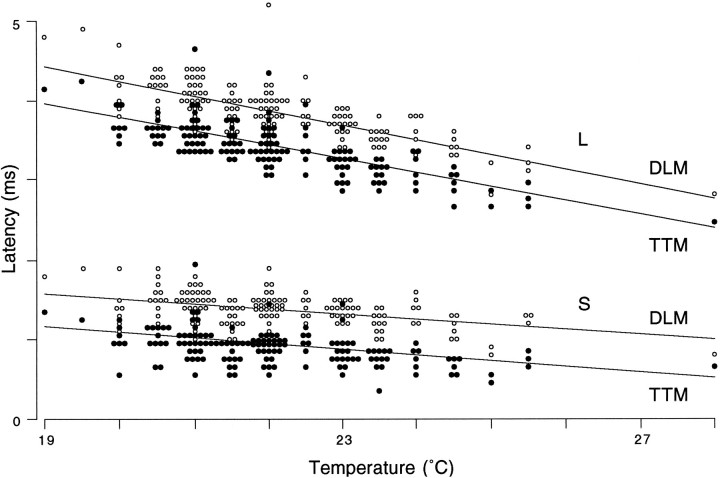
Fig. 2.
Response latency varied with temperature. Points indicate shortest muscle-response latencies of each class measured in 152 control flies; slopes show least-squares regression on data between 20 and 25.5°C. Temperature effects were significant (p < 0.001 for each muscle/latency classification;t test comparing slopes with zero). For both DLM (open symbols) and TTM (filled symbols), the effect of temperature was more pronounced in long-latency (L) than short-latency (S) responses, consistent with the presence of additional afferent neurons in the long-latency path [p < 0.001; test for homogeneity of the four slopes (Sokal and Rohlf, 1981)].
Two types of dishabituating stimulation were used. Air puffs were provided by gently squeezing a rubber bulb connected by tubing to a pipette nozzle mounted 2 cm to the anterior left of the fly. Light flashes of 200 msec were produced by a green (565 nm) light-emitting diode (LED) (HLMP 3950, Chicago Miniature Lamp, Buffalo Grove, IL) positioned 2 cm from the left eye driven by 40 mA of current (150 mcd/20 mA) from a regulated power supply gated by a relay switch.
To trigger visually evoked responses in white-eyed (Wyman et al., 1984) (w or cn bw) flies, constant illumination provided by the same LED was interrupted by 20 msec openings of the relay switch. To allow monitoring of jumps in visual trials, tethered flies held a small square of tape or had a square of paper glued to mesothoracic legs.
To measure long-latency response refractory periods, twin-pulse stimuli were given every 15 sec or longer, with ISI adjusted from 100 msec to find the shortest ISI that gave a twin response in at least one of three attempts. Short-latency response refractory periods and following frequency with 50% failures (FF50) were determined as described previously (Gorczyca and Hall, 1984; Engel and Wu, 1992). Further details of testing protocols have been described elsewhere (Engel, 1995).
RESULTS
Electrical response initiated at different sites
Stimulation by electrodes placed in the eyes (Fig. 1A) gave rise to three classes of response latency (Table 1) by triggering the giant fiber pathway at distinct sites associated with different thresholds. All three classes showed the typical muscle spike pattern of the giant fiber response, which is also evoked by visual stimulation (Fig. 1B,C). The short-latency response arises from direct activation of the giant fibers by a strong stimulus, whereas late responses are attributed to recruitment of afferent pathways (Levine, 1974; Tanouye and Wyman, 1980; Elkins and Ganetzky, 1990; Trimarchi and Schneiderman, 1993). Consistent with this, we found that the long-latency response has greater temperature dependence than the short-latency response for both flight and jump muscles (DLM and TTM in Fig. 2). Previous researchers, using a variety of electrode placements, have tended to refer to any late response as “long-latency,” without distinguishing between classes. The long-latency response as used here (DLM latency ≥ 3.0 msec) is commonly obtained when stimulating electrodes are placed in the eyes, as in these experiments (Levine, 1974; Trimarchi and Schneiderman, 1993). A distinct intermediate-latency response (Fig. 1B,C) was seen in 28% (68 of 247) of control flies that showed long- and short-latency responses and, in those flies, the intermediate-latency response was less reliable than long- or short-latency responses (Engel, 1995). All three classes of response were also seen inrut, dnc, and dnc rut mutants, and latencies did not differ significantly from controls in any mutant (Table 1). The intermediate-latency response was not well suited as a model of habituation because it was not seen in the majority of flies (Table 1). Of the two reliably induced response classes, triggered at different points in the giant fiber pathway, the long-latency response, but not the short-latency response, attenuated with characteristics of habituation (shown below).
Table 1.
Mean latencies of giant fiber response classes in control and cAMP pathway mutants
| Genotype | Long-latency | Intermediate-latency | Short-latency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLM | TTM | DLM | TTM | DLM | TTM | |||||||
| Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | |
| Controls | 3.82 | 247 | 3.37 | 217 | 2.16 | 68 /247 | 1.70 | 64 /217 | 1.40 | 247 | 0.94 | 217 |
| (±0.024) | (±0.024) | (±0.038) | (±0.038) | (±0.013) | (±0.014) | |||||||
| rut | 3.77 | 83 | 3.36 | 76 | 2.11 | 39 /83 | 1.66 | 36 /76 | 1.40 | 83 | 0.97 | 76 |
| (±0.033) | (±0.030) | (±0.042) | (±0.031) | (±0.020) | (±0.016) | |||||||
| dnc | 3.87 | 71 | 3.43 | 65 | 2.23 | 24 /71 | 1.68 | 22 /65 | 1.39 | 71 | 0.90 | 65 |
| (±0.043) | (±0.039) | (±0.066) | (±0.060) | (±0.027) | (±0.025) | |||||||
| dnc rut | 3.84 | 48 | 3.41 | 44 | 2.21 | 15 /48 | 1.72 | 15 /44 | 1.33 | 48 | 0.89 | 44 |
| (±0.053) | (±0.047) | (±0.083) | (±0.071) | (±0.030)* | (±0.027) | |||||||
Means ± SEM, in msec, of shortest latency of each class measured for each fly. Mutants did not differ from controls in any category (two-tailed t tests). Only trials in which both short- and long-latency responses were measured are included; intermediate-latency responses were seen in a minority of trials, as indicated by the ratios of occurrence over total observations (see text). In some cases, only DLM responses were measured. Mutant alleles includerut1, rut2; dnc2, dncM11, dncM14; dncM14rut1.
Habituation of the long-latency response
The visually induced giant fiber response appears to habituate when stimulated repeatedly in tethered flies and shows dishabituation by a novel stimulus, such as an air puff (Fig.3A). This plasticity of the visually induced response in jump and flight muscles correlates to observable leg movements (Fig. 3B). These factors led us to examine the electrically induced giant fiber response for characteristics of habituation.
The probability of the long-latency response diminishes earlier and more abruptly at higher stimulus frequencies. The time course of attenuation is apparent in the response–probability plots in Figure4A. To provide a consistent level of habituation for recovery tests, trials were ended after five consecutive failures. Because this led to a range of trial lengths, to combine results for response–probability plots in Figure4A, each preparation was taken to have failed for every stimulus after five consecutive failures. Plotting the mean number of stimuli to attain criteria of one to five consecutive failures (Fig.4B) shows that attenuation is more abrupt at higher frequencies (note log scale). Median numbers of stimuli to attain five consecutive failures (Table 2) allow comparisons with relatively little influence from outlying values and also correspond closely to points at which response probabilities fell to 50% (Fig.4A).
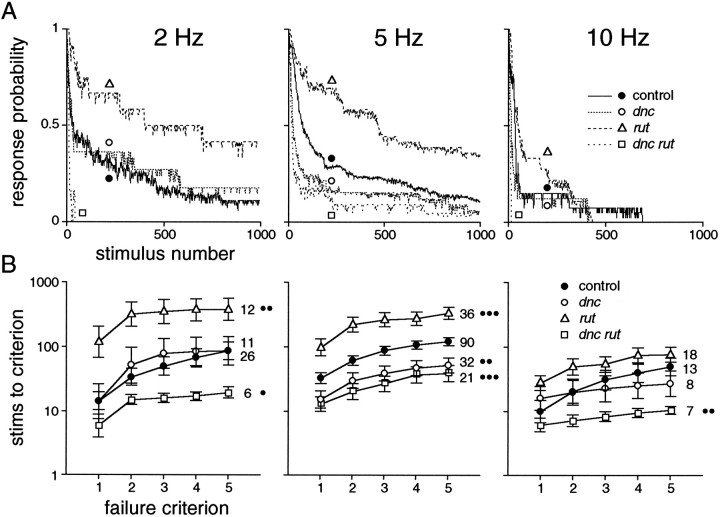
Fig. 4.
Kinetics of habituation of the long-latency response. A, Frequency-dependent decrement of response probability. Three-point running averages of pooled responses (numbers of flies are indicated in B). Trials of individual flies were terminated after attaining criterion (five consecutive failures) and were considered to fail thereafter in this set of plots; this accounts for abrupt changes of slope. Symbols are for identification of curves. B, Numbers of stimuli to attain criteria of one to five consecutive failures. Mean ± SEM of log-transformed values for the numbers of flies indicated; a value of 1000 was used if a fly had not reached a criterion by that point. Two-tailed t test comparisons of five-failure values versus controls: *p < 0.05; **p < 0.01; ***p < 0.001. Comparing the difference between one and five consecutive failures shows that failure was not only earlier but also more abrupt at 10 Hz than at lower frequencies (note vertical log scale). From these plots, it is clear that habituation is more rapid at higher stimulus frequencies and that rut mutants are resistant to habituation, whereasdnc and dnc rut mutants are abnormally susceptible.
Table 2.
Quantification of habituation, recovery, and dishabituation in controls and cAMP pathway mutants
| Genotype | Habituation | Recovery | Dishabituation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median number of stimuli to five consecutive failures (N) | Median number of stimuli to five failures at 5 Hz | By light flash | By air puff | ||||||
| 2 Hz | 5 Hz | 10 Hz | 1st/120 sec (N) | 1st/30 sec/5 sec (N) | Rising | Increase (mean ± SD) | Rising | Increase (mean ± SD) | |
| Controls | 42 (26) | 85 (90) | 39 (13) | 39 /34 (15) | 85 /47/29 (15) | 3 of 4 | 7.00 ± 5.00 | 7 of 16 | 8.29 ± 7.48 |
| rut | 404 (12) | 473 (36) | 64 (18) | 473/66 (8) | 464 /77/29 (15) | 6 of 9 | 12.67 ± 7.17 | 7 of 17 | 12.43 ± 6.90 |
| dnc | 37 (11) | 30 (32) | 19 (8) | 43 /47 (10) | 19 /19/11 (16) | 4 of 5 | 8.75 ± 5.19 | 3 of 14 | 3.00 ± 1.00 |
| dnc rut | 19 (6) | 23 (21) | 9 (7) | 21/28 (5) | 28 /26/14 (15) | 5 of 6 | 4.00 ± 2.45 | 3 of 14 | 5.00 ± 4.58 |
Habituation: medians of numbers of stimuli to attain habituation criterion of five consecutive failures for all trials shown in Figure4. Recovery: median values for recovery trials shown in Figure 5; “1st” means initial conditioning bout, and “120 sec,” “30 sec,” and “5 sec” refer to recovery intervals. Dishabituation: “rising” gives number of flies from Figure 7 in which thefirst flash or puff led to increased responsiveness; “increase” is the mean increase in number of responses (25 stimuli after vs before the flash or puff) in the first test in “rising” trials.
The habituation process is a gradual decrease in response probability, not an abrupt loss of response. Electrical response thresholds tested after habituation trials were generally close to pretrial values (data not shown). Changing the stimulus voltage up or down could lead to increased response probabilities in some habituated flies, but this was not seen consistently.
Properties of habituation in cAMP pathway mutants were similar to controls, but kinetics of habituation and asymptotic response probabilities differed (Fig. 4). In rut mutant flies, habituation occurred more slowly. Habituation was more rapid indnc, and still more rapid in dnc rut double mutants. These differences were most evident at 5 Hz stimulation, perhaps in part because of the larger sample size at 5 Hz (Fig. 4). After the first instance of two consecutive failures, the length of strings of failures tended to increase more abruptly in all mutants than in controls (note flatter stimuli-to-criterion plots between two- and five-failure criteria in Fig. 4B). These results indicate that cAMP pathways mediated by the products of rutand dnc are important in habituation of this response. The nonadditivity of defects in single- and double-mutant flies implies that developmental and regulatory factors must be considered in the specific cellular functions of these enzymes in the long-latency pathway (see Discussion).
Spontaneous recovery and dishabituation
Spontaneous recovery is an indication that attenuation is not attributable to deterioration of the preparation, and its time course may provide information about the process of habituation. Figure5A shows response–probability plots of trials in which animals were stimulated at 5 Hz, habituated to five consecutive failures, and allowed to recover for 120 sec before retesting. The 120 sec recovery period was sufficient to restore initial response levels in all genotypes. Shorter recovery periods revealed incomplete recovery and faster rehabituation in certain genotypes. Flies were conditioned once, recovered for 30 sec, conditioned again, recovered for 5 sec, and conditioned a third time (Fig. 5B). After 30 sec, response probabilities recovered fully, but subsequent habituation was more rapid; after 5 sec, initial response probabilities were diminished in dnc and dnc rut mutants. Median numbers of stimuli to attain five consecutive failures are given in Table 2.
Fig. 5.
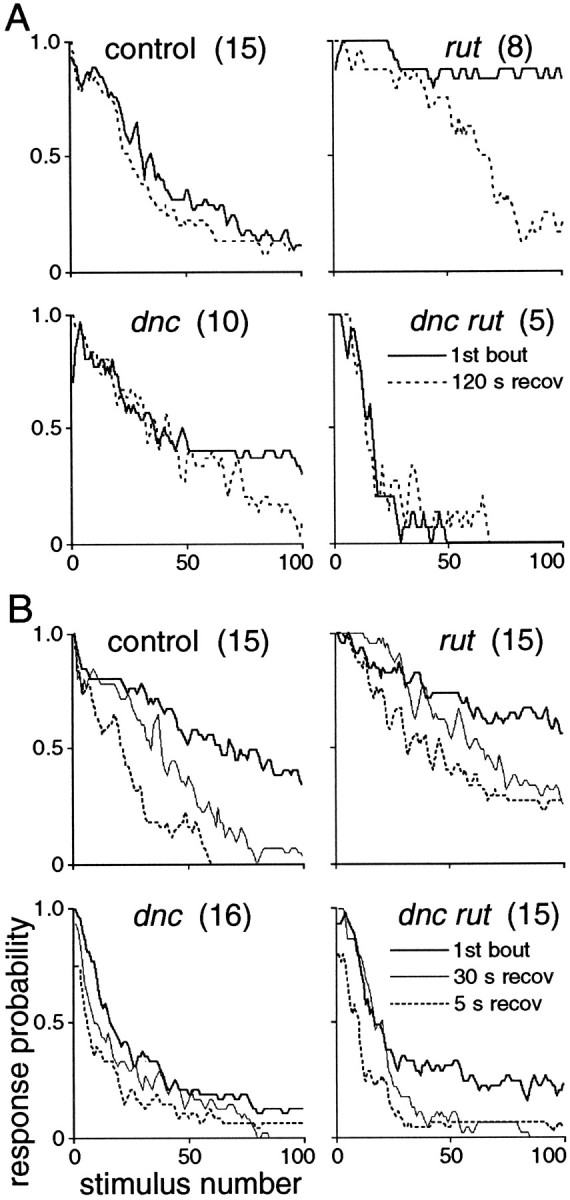
Spontaneous recovery from habituation of the long-latency response. A, Overlay comparison of 120 sec recovery after 5 Hz stimulation. Flies were habituated to five-failure criterion (solid line), then recovered for 120 sec before being stimulated again (dashed lines). Compared with the conditioning first bout, rut mutants habituated more rapidly after 120 sec recovery. B, Flies were habituated (heavy line), allowed to recover for 30 sec and habituated again (light line), then allowed to recover for 5 sec and habituated a third time. dnc and dnc rut did not recover to initial response levels in 5 sec. Sample sizes inparentheses. Three-point running average as in Figure4.
It is worth noting that rut appeared to rehabituate more quickly even after 120 sec of recovery (Fig. 5A, Table 2) and that dnc and dnc rut appeared to require >5 sec to recover fully (Fig. 5B). The prolonged stimulation required to bring rut flies to habituation criterion (Table2) may lead to more stringent conditioning than experienced by other genotypes. On the other hand, dnc and dnc rutneeded more time than controls to recover, although they habituated more rapidly. Nevertheless, substantial recovery in 5 sec did occur indnc and dnc rut mutants, indicating that their rapid habituation is probably not attributable to generalized weakness.
One characteristic of habituation identified by Thompson and Spencer (1966) is “habituation beyond zero,” in which recovery is reduced when stimulation extends beyond the point at which responses are lost. An example is shown in Figure 6. In some extended trials, initial habituation was interrupted by a brief period of increased response probability within the trend of long-term decline (Fig. 6) (Engel, 1995). This could indicate coincident processes of habituation and sensitization (Groves and Thompson, 1970), which must be studied further. However, it can be noted here that this pattern of transitory spontaneous recovery during extended trials was seen in mutants as well as in controls.
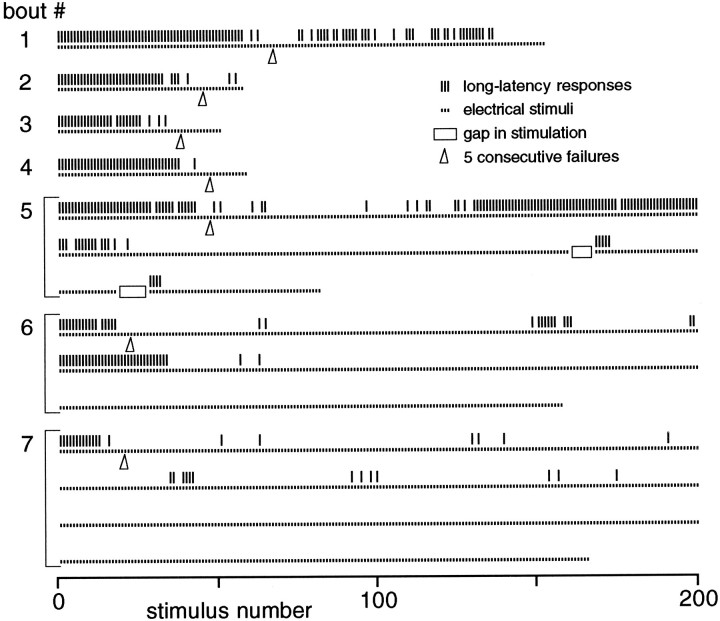
Fig. 6.
Habituation beyond zero. Repeated bouts of stimulation (10 Hz) were given with 60 sec recovery intervals.Dots indicate stimuli, ticks indicate long-latency responses, arrowheads show fifth consecutive failure, and a bracket indicates a continuous bout carried to multiple lines in the plot. When habituation was prolonged well beyond the cessation of responses (bouts 5–7), subsequent recovery was reduced. Note also the transient increase in responsiveness after habituation in bouts 1, 5, and 6 (see text), and recovery produced by brief stimulus pauses near the end of bout 5.
In addition to spontaneous recovery, another important parameter of habituation is dishabituation, or recovery induced by a novel stimulus. Dishabituation helps to distinguish habituation from sensory adaptation or motor fatigue. We identified several types of stimuli that could induce recovery of the long-latency response in a habituated preparation. These included stroking of the wing with an eyelash probe or with the fly’s own leg in grooming in trial experiments (normally the legs were waxed together; see Materials and Methods); an air puff, especially when this triggered a burst of wing buzzing; and various sorts of visual stimulation.
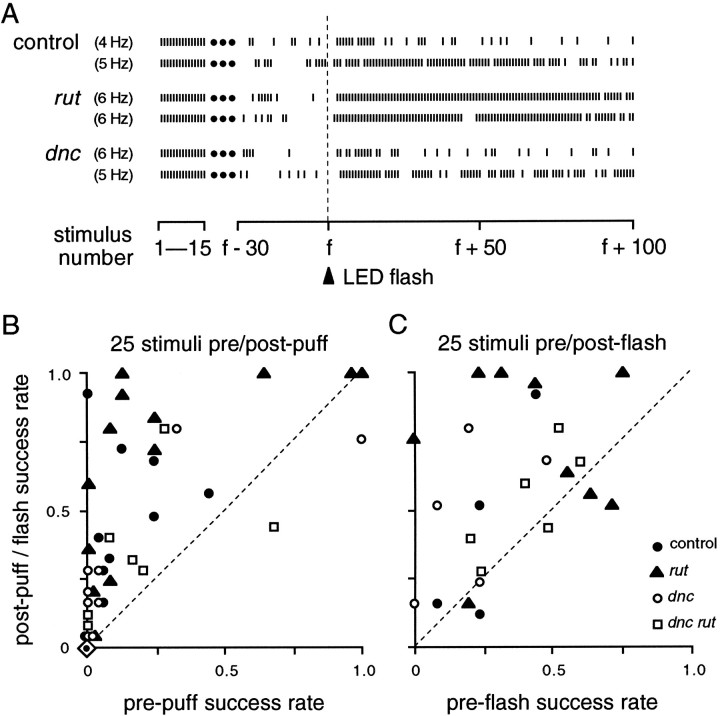
Two types of stimulus, air puffs and light flashes, were standardized and used to examine dishabituation of the long-latency response to electrical stimulation. As with the visually induced response (Fig. 3), the electrically induced response can be dishabituated in both mutants and controls (Fig. 7A). The response pattern during habituation normally shows considerable variability from trial to trial, as is apparent in other figures (Figs. 4B, 6, 8); each dishabituating stimulus led to recovery in many cases but reduced responsiveness in others. Nevertheless, dishabituation could be demonstrated in two operationally distinct ways (Fig.7B,C).
Fig. 7.
Dishabituation of the long-latency response.A, Examples of dishabituation by a 200 msec flash from LED.Ticks indicate responses to the initial 15 stimuli and to the 30 before and 100 after the flash. B, Comparison of response probability for 25 stimuli before and after an air puff.Points above the dashed line reflect increased response probabilities after the puff. When a fly was tested more than once, the greatest increase obtained is shown. Because the response to a puff (or light flash, as seen in A) was often slightly delayed, post-puff counts began with the sixth stimulus after the puff. Dishabituation was less common in dnc and dnc rutmutants. Puffs were delivered a few seconds after attainment of five consecutive failures at 5 Hz; data points with high “pre-puff” values resulted when response probabilities increased spontaneously after five failures (compare Fig. 6, bouts 1, 5, 6). Overlapping data points indicate the same values. Some flies, indicated here by a single symbol (dot within diamond), showed no responses to 25 stimuli before or after puffs: controls, 6 of 16; rut, 1 of 14; dnc, 7 of 16; dnc rut, 8 of 15. C, Comparison of response probability for 25 stimuli before and after a flash. As in B, postflash counts began with the sixth stimulus after the flash. Dishabituation was seen in controls and all mutant genotypes. Stimulus frequencies ranged from 1 to 10 Hz, selected to give a steady preflash response probability between 0.2 and 0.8 for each fly, although in some cases, response probability then dropped below 0.2 before the flash was given.
Fig. 8.
Habituation of dishabituation. Successive air puffs (indicated by the vertical dashed line) were delivered during a 5 Hz stimulus bout. Dishabituation of the long-latency electrical response diminished with repeated puffs. Dotsindicate electrical stimuli; ticks indicate responses. Plots show multiple dishabituation episodes from a single extended stimulus bout. A, Control fly, showing every second puff.B, rut mutant.
Because of better experimental control of the dishabituating stimulus, light flash trials were made over a range of pretest response probabilities at different frequencies of electrical stimulation. Dishabituation was demonstrated throughout the range of responsiveness (Fig. 7C). In contrast, air puffs were given during 5 Hz trials, and initial puffs were given within a few seconds after attaining the five-failure habituation criterion. As in the case of using light flashes, the degree of dishabituation after air puffs also varied. Although the trend among all genotypes was increased responsiveness after puffs (Fig. 7B), responsiveness sometimes decreased, and some flies responded neither before nor after the puff (Fig. 7B, Table 2). However, the paradigm using air puffs allowed more distinction between genotypes, in general being most effective for rut and least effective for dnc anddnc rut (Fig. 7B, Table 2). In conclusion, stimuli of two distinct sensory modalities led to dishabituation, dishabituation could be observed in all mutant genotypes, and differences between genotypes could be seen in the air-puff paradigm.
Habituation of the dishabituation response is another common parameter of habituating systems (Thompson and Spencer, 1966). As illustrated in Figure 8, because of the intrinsic fluctuation of responses as shown above, a large number of sequential air puffs is required to demonstrate the trend of dishabituation becoming less effective over time. The effect was seen in mutant and control flies (Fig. 8) and in light flash trials as well.
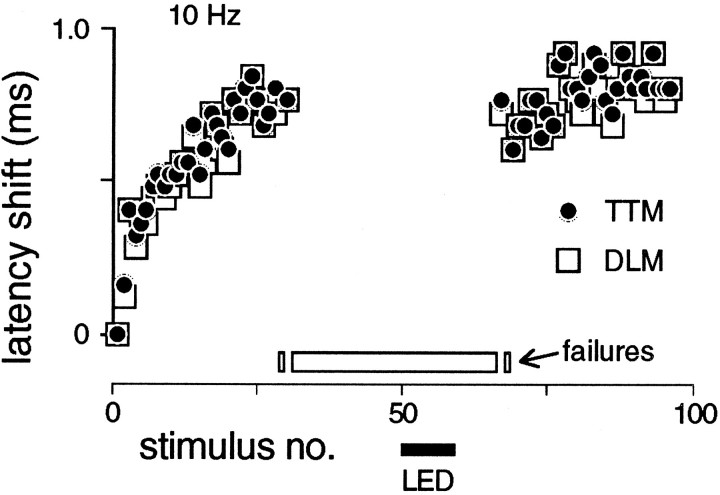
A universal consequence of repetitive stimulation was a rapid increase in latency of up to 2 msec (Fig. 9). This latency shift occurred early in the giant fiber pathway because different thoracic branches of the pathway shifted together (Fig. 9) (Engel, 1995), and it could represent a systematic artifact (e.g., stimulus electrode polarization). The shift was much more rapid than habituation (Fig. 9) and did not differ between genotypes (Table 3), suggesting that the latency shift and habituation are not directly related. Therefore, the similarity of latencies before and after light-induced dishabituation (Fig. 9) indicates that dishabituation involves recovery of the original long-latency pathway rather than recruitment of a parallel route.
Fig. 9.
Coincident failures and latency shift of jump and flight muscles in the long-latency response. The plot shows an increase in latency of the long-latency response in contralateral DLM and TTM muscles, with failures indicated by open bars at thebottom of the plot. Stimulus frequency was 10 Hz; symbols for muscle fibers are given in figure. Note concomitant failures of DLM and TTM. Furthermore, the latency shift is preserved after dishabituation by a 1 sec LED flash, indicating that the same pathway carries the recovered response. Latency shifts (Table 3) and coupled muscle failures were seen in all genotypes; this fly wasdncM11.
Table 3.
Refractory periods and latency shift of the giant fiber response in controls and cAMP pathway mutants
| Genotype | LL latency shift (msec) | LL refractory period (msec) | SL refractory period (msec) | |||||
|---|---|---|---|---|---|---|---|---|
| DLM | (DLM and TTM)† | DLM‡ | TTM | |||||
| Mean | N | g-Mean | N | g-Mean | N | g-Mean | N | |
| Controls | 0.83 | 9 | 86 | 37 | 5.2§ | 41 | 3.3§ | 48 |
| (±0.033) | (72–103) | (5.0–5.5) | (3.2–3.3) | |||||
| rut | 0.77 | 13 | 27 | 30 | 4.9 | 15 | 3.6 | 12 |
| (±0.055) | (25–28)*** | (4.4–5.3) | (3.5–3.8) | |||||
| dnc | 0.77 | 5 | 42 | 22 | 5.3 | 15 | 3.7 | 15 |
| (±0.083) | (36–49)** | (5.0–5.6) | (3.5–3.9)* | |||||
| dnc rut | 0.88 | 5 | 43 | 16 | 4.3 | 9 | 3.3 | 8 |
| (±0.074) | (38–47)* | (3.7–5.1) | (3.1–3.6) | |||||
LL, Long-latency; SL, short-latency. Latency shift: arithmetic means (±SEM) of shifts averaged between the 21st and 64th DLM responses (after shift had reached maximum, compare Fig. 9) in 5 Hz trials. LL refractory period, SL refractory period: geometric means (log-transformed to improve normality) (SEM range in parentheses). ND, Not determined. *p < 0.05, **p < 0.01, ***p < 0.001; two-tailed t test of mutant versus controls.
†LL refractory periods are identical for DLM and TTM.
‡Short-latency DLM (not TTM) refractory periods may be underestimates because of interference by double-spiked muscle action potentials (Engel and Wu, 1992).
§Because previously published Canton-S short-latency response results (Engel and Wu, 1992) did not differ significantly from seven new control trials (two-tailed t tests, 95% criterion), these data are combined here.
Attenuation of the long-latency response satisfies several of Thompson and Spencer’s (1966) criteria for habituation. Attenuation is more rapid at higher stimulus frequencies (Fig. 4), and there is spontaneous recovery (Fig. 5). Habituation is more rapid after recovery (Fig.5B), and recovery is diminished after extended habituation (“beyond zero,” Fig. 6). Novel stimuli can lead to dishabituation (Fig. 7), and this effect also habituates (Fig. 8).
Delimiting the site of habituation
Several lines of inference place the site of habituation in the brain rather than in the thoracic portions of the giant fiber pathway. For most trials, recordings were made from DLM and TTM contralateral muscles, which are innervated by the same giant fiber (Fig.1A), and DLM failures were always accompanied by TTM failures (Fig. 9) (Engel, 1995). This pattern of synchronous DLM and TTM failures, which indicates that habituation of the giant fiber pathway occurs before its bifurcation in the thorax (Fig.1A), was seen in mutants as well as in controls. Moreover, contralateral DLM flight muscles failed in synchrony (tested in 6 flies) (Engel, 1995), confirming that habituation occurs ahead of the point in the thorax at which the contralateral giant fiber pathways are coupled (King and Wyman, 1980; Benshalom and Dagan, 1985).
The most direct evidence that habituation occurs in the brain comes from the short-latency response, which results from direct activation of the giant fibers in the head. After the long-latency response had been habituated, an increase in stimulus voltage invariably gave short-latency responses, and these could be driven for long periods at high frequencies before failures occurred (Fig. 10). These patterns of short-latency versus long-latency response attenuation kinetics and synchronous muscle failures were seen in mutant as well as in control flies, showing that gross connectivity in the circuit is not likely altered in these mutants.
Fig. 10.
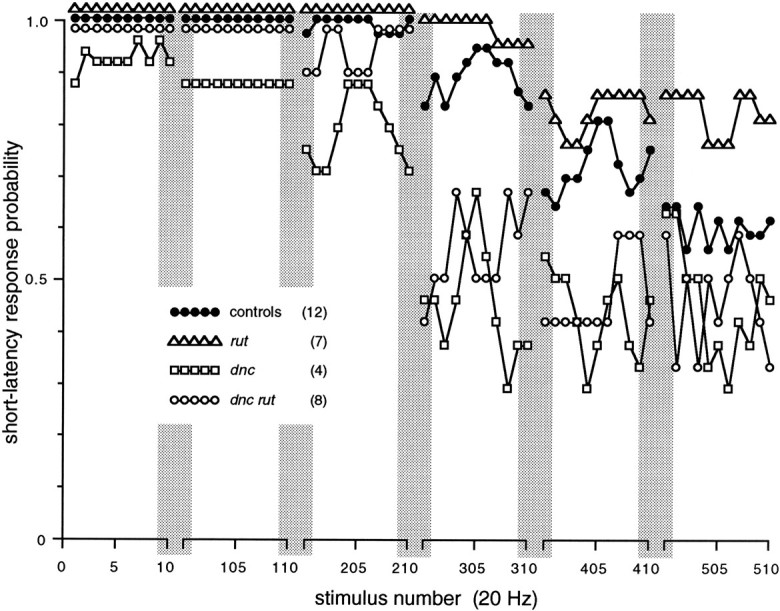
Short-latency response failures at high frequency. The short-latency response is less labile than the long-latency response and can be driven for hundreds of stimuli at high frequencies. Plot shows 10-stimulus excerpts from three-point running averages of pooled DLM responses to 20 Hz stimulation (sample sizes inparentheses). Control and dnc lines are slightly offset in first three excerpts. Note that although these failures occur at a later stage in the giant fiber pathway, the ranking of resistance to failures resembles that for the long-latency response (Fig.4).
Habituation of this circuit may be important in modulating the sensitivity of the escape response, and it occurs in the head, where sensory inputs converge (Strausfeld and Bacon, 1983; Milde and Strausfeld, 1990), rather than in the less labile thoracic portion of the pathway. Visual interneurons and antennal primary mechanoreceptors are electrically coupled to giant fibers in dipterans (Strausfeld and Bassemir, 1983; Bacon and Strausfeld, 1986). Although placing stimulating electrodes in eyes produces reliable long-latency responses, electrical stimulation probably bypasses retinular cells and is directed by the high-impedance retinal basal membrane along a path to giant fiber afferent pathways. Because the long-latency response is ∼2.5 msec later than the short-latency response (Table 1), at least one chemical synapse must be interposed. Therefore, habituation of the electrically induced long-latency response likely occurs at interneurons that may participate in habituation of visually induced escape as well (Fig. 3). However, the visually induced response takes at least 15 msec longer than the long-latency response (Fig. 1), indicating that additional connections are interposed. Furthermore, the long-latency response resists failure at stimulus frequencies an order of magnitude greater (compare Figs. 3 and 4), and spontaneous recovery of the visually induced response is slower (data not shown). Thus, other components of the sensory pathway probably also contribute to habituation of the visually induced response.
Following frequencies and refractory periods of short- and long-latency responses
Although rut and dnc affect habituation of the long-latency response in the brain, we wondered whether such cAMP mutations would also alter plasticity in the thoracic stage of the pathway, which can be activated directly using greater stimulus voltages to produce the short-latency response. In fact, even though attenuation of the short-latency response occurs after more stimuli and at higher frequencies, it is affected by these mutations in directions that parallel defects in the long-latency response, occurring more slowly in rut and more rapidly in dnc anddnc rut mutants (Fig. 10). This implies that these mutations can have a consistent effect on sustained responsiveness in different synapses with very different response properties.
In contrast to habituation paradigms using sustained stimulus trains, twin-pulse refractory period protocols measure the limit of the ability to respond to repeated stimulation at short intervals. Refractory periods of the long-latency response in rut mutants were much shorter than in controls, but refractory periods were also shortened in dnc and dnc rut mutants (Table 3), in contrast to their effects on habituation rates (Fig. 4, Table 2). Apparently, the twin-pulse refractory period is limited by different processes than govern attenuation of the response during sustained repetitive stimulation.
Refractory periods of the short-latency response did not differ between mutants and controls in the same way as the long-latency response, nor in parallel to attenuation of the short-latency response with repetitive stimulation. A significant difference was seen only in the short-latency TTM response of dnc mutants, in which the refractory period was prolonged (Table 3). Thus, cAMP cascade mutations alter certain long-latency and short-latency response properties that are not immediately related to habituation.
DISCUSSION
Plasticity of the electrically induced long-latency giant fiber response fulfills criteria for habituation (Thompson and Spencer, 1966) in a circuit with a well established link to an escape response (Levine, 1974; Trimarchi and Schneiderman, 1995a,b,c). Although the habituation occurs in the brain, other segments of the circuit show different forms of activity-dependent plasticity. This system has great potential to provide information about the physiological bases of behavioral plasticity in a genetic context, beginning with the role of cAMP-dependent pathways studied here.
cAMP cascade mutations and habituation
Although cAMP has been implicated in synaptic plasticity and learning (Dixon and Atwood, 1989; Zhong and Wu, 1991a; Klein, 1993;Huang et al., 1994; Yin et al., 1994), its role in habituation has been largely unexplored. Drosophila memory mutants have provided good evidence for cAMP involvement in habituation, but there is no consistent pattern of mutant effects on habituation in different behavioral paradigms. Habituation of odor-induced jumps is slowed inrut mutants and not clearly altered in dncmutants (Tully and Koss, 1992), paralleling effects on the giant fiber jump response (Fig. 4), even though these are thought to be mediated by different circuits (Trimarchi and Schneiderman, 1995a,c). Habituation of a proboscis extension reflex may be reduced in both rutand dnc mutants (Duerr and Quinn, 1982), although those results could also indicate defective retention of conditioning in the mutants. However, landing (Rees and Spatz, 1989) habituates more rapidly in both rut and dnc mutants. A thoracic grooming reflex in decapitated flies habituates and dishabituates normally but recovers more rapidly in a rut mutant (Corfas and Dudai, 1989). This lack of consistency is not surprising because habituation may depend on specific arrangements of connectivity in different circuits. This underscores the importance of studying physiological effects of memory mutations in circuits directly related to behavior.
Our results prompt an expanded notion of the scope of rutand dnc influence. Both genes’ products are expressed throughout neuropil but concentrated in mushroom bodies (Nighorn et al., 1991; Han et al., 1992), brain structures implicated in olfactory associative conditioning and defective in dnc andrut mutants (Heisenberg et al., 1985; Balling et al., 1987;de Belle and Heisenberg, 1994). The mushroom bodies are not part of the giant fiber pathway, although their projections may intersect the circuit (Strausfeld and Bacon, 1983). It is known that rutand dnc mutations have physiological effects in peripheral neurons and muscles (Corfas and Dudai, 1990a; Zhong and Wu, 1991a,1993a; Zhong et al., 1992), even though their expression in the mushroom bodies is more readily demonstrated immunohistochemically (Nighorn et al., 1991; Han et al., 1992). Our results from the giant fiber pathway suggest that these genes may play roles in synaptic plasticity throughout the CNS.
Effects of these mutations on long-latency habituation could not have been simply extrapolated from their known biochemistry. The products ofrut and dnc are antagonistic, and their mutations have opposing effects on overall cAMP levels (Byers, 1979; Byers et al., 1981; Livingstone et al., 1984). Yet, although habituation rates were markedly slowed in rut single mutants and only moderately increased in dnc, in the dnc rutdouble mutant habituation was very rapid. Normal response latencies and strong refractory periods and recovery from habituation in the double mutant indicates that this extreme defect is not attributable to general disruptions of the giant fiber pathway.
Effects of rut and dnc are also nonadditive in paradigms such as olfactory associative conditioning (Tully and Quinn, 1985), where defects in dnc rut are worse than either single-mutant. Explanations for the complexity of rut anddnc mutant phenotypes might include enzyme compartmentalization and colocalization with downstream targets, kinetics of cAMP regulation, compensatory interactions with other second messengers, or details of neural connectivity, which will require further investigation.
Finally, it is worth noting that the mutations studied here did not affect recovery or dishabituation (Fig. 5, 7) as clearly as they did habituation (Fig. 4). This implies that these processes are not entirely overlapping with respect to cAMP pathways.
cAMP and the physiology of synaptic plasticity
Short-term habituation has been related to homosynaptic depression in spinal interneurons in frogs (Thompson and Glanzman, 1976) and primary mechanosensory neurons of crayfish (Zucker, 1972) andAplysia (Castellucci et al., 1970; Castellucci and Kandel, 1974; Klein et al., 1980; Bailey and Chen, 1988). InAplysia, cAMP contributes to synaptic facilitation (Brunelli et al., 1976; Klein and Kandel, 1980). In contrast, results inrut mutants imply that a cAMP signal promotes habituation of the giant fiber response in Drosophila (Fig. 4). However, it is not known whether this habituation is homosynaptic. Polysynaptic pathways and inhibitory input contribute to plasticity, for instance, in Aplysia (Fischer and Carew, 1993; Schacher et al., 1993;Trudeau and Castellucci, 1993) and crayfish (Krasne and Teshiba, 1995).
Effects of cAMP in synaptic plasticity in Drosophila have also been explored in neuromuscular junctions (Zhong and Wu, 1991a;Zhong et al., 1992), where their influence is frequency-dependent (Zhong and Wu, 1991a). It is interesting that stimulus frequencies that facilitate these peripheral synapses apparently depress central synapses in the giant fiber pathway. Twin-pulse facilitation and post-tetanic potentiation of neuromuscular excitatory junctional currents (EJCs) are reduced in both rut and dncmutants (Zhong and Wu, 1991a), but for different reasons:rut EJCs require abnormally high stimulus levels for partial conditioning, whereas unconditioned dnc EJCs resemble conditioned wild-type EJCs but are not strengthened further by stimulation.
Physiological bases of the mutant effects are unclear, but evidence points to direct, indirect, and chronic or developmental mechanisms.rut and dnc mutations alter K+ currents in larval muscles (Zhong and Wu, 1991b) and cultured neurons, leading to increased excitability (Zhao and Wu, 1994). In Drosophila, cAMP may directly gate K+ channels (Delgado et al., 1991), modulate K+ and Ca2+ channels (Alshuaib and Byerly, 1992; Wright and Zhong, 1995) possibly via PKA or other kinases (Dévay and Friedrich, 1987; Dévay et al., 1989; Drain et al., 1991; Asztalos et al., 1993), or alter gene expression via CREB/CREM pathways (Yin et al., 1994). It should be noted that a number of synaptic vesicle proteins that regulate the release process are also known targets of PKA (for review, seeSüdhof, 1995), although their biochemical alterations have not been demonstrated in these mutants. Furthermore, heightened cAMP levels in dnc mutants activate PKA indirectly by increasing proteolysis of regulatory subunits (Müller and Spatz, 1989), and expression of a G-protein subunit that may regulate cation currents is enhanced in rut and dnc mutants (Guillén et al., 1990), showing that these mutations have far-ranging biochemical effects.
Although some physiological effects of cAMP mutations can be mimicked by acute pharmacological treatments (Corfas and Dudai, 1990b; Zhong and Wu, 1991a, 1993a), others appear chronic (Zhong and Wu, 1993b), and developmental morphological effects have been documented in mechanosensory and motor axon terminals (Corfas and Dudai, 1991; Zhong et al., 1992) and mushroom body tracts (Balling et al., 1987). Chronic and developmental effects could contribute to mutant defects in habituation by changing properties of the giant fiber pathway.
These results suggest several directions for further exploration. By analyzing additional mutations and their interactions withrut and dnc, it should be possible to identify regulators and targets of cAMP pathways. For example, rutadenylyl cyclase was recently shown to be potentiated by Ca/CaM and receptor-coupled G-protein pathways (Livingstone et al., 1984; Levin et al., 1992; Feany and Quinn, 1995; Zhong, 1995). Other possibilities include ion channels, which are altered by many available mutations (Wu and Ganetzky, 1992), as well as messengers that could act in parallel pathways of habituation, such as CaM Kinase-II (Griffith et al., 1993). Enhancer-trap methods (O’Kane and Gehring, 1987) using tissue-specific promoters to localize gene expression could provide more detailed information about where different molecules act in the circuit. Those methods can also be used to mark identified neurons in culture, where cellular and synaptic properties could be studied and correlated with the response in vivo (Zhao and Wu, 1994; Wright and Zhong, 1995). Finally, because sensory pathways of several modalities appear to converge on the giant fiber pathway in flies (Strausfeld and Bacon, 1983; Bacon and Strausfeld, 1986; Milde and Strausfeld, 1990), this system should allow us to examine mechanisms of sensory integration in plasticity. Similar molecular mechanisms may mediate associative and nonassociative conditioning in Aplysia (Fitzgerald et al., 1990), and both types of conditioning are altered by memory mutants inDrosophila (Dudai et al., 1976; Duerr and Quinn, 1982;Aceves-Pina et al., 1983; Tempel et al., 1983; Tully and Quinn, 1985;Corfas and Dudai, 1989; Rees and Spatz, 1989; Tully and Koss, 1992). Future experiments exploring the possibility of associative conditioning of the giant fiber response could extend the advantages of this system to studying the relationships between these different conditioning paradigms.
Footnotes
This work was supported by National Institutes of Health Grants NS26528 and HD18577 to C.F.W. We thank Ms. Jisue Lee for assistance with data collection and Dr. Marla B. Sokolowski for critical comments.
Correspondence should be addressed to Chun-Fang Wu, Department of Biological Sciences, University of Iowa, 136 Biology Building, Iowa City, IA 52242-1324.
REFERENCES
- 1.Aceves-Pina EO, Booker R, Duerr JS, Livingstone MS, Quinn WG, Smith RF, Sziber PP, Tempel BL, Tully TP. Learning and memory in Drosophila , studied with mutants. Cold Spring Harb Symp Quant Biol. 1983;48:831–840. doi: 10.1101/sqb.1983.048.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Alshuaib WB, Byerly L. Sensitivity of calcium and potassium currents in Drosophila embryonic neurons to cyclic AMP. Soc Neurosci Abstr. 1992;18:1502. [Google Scholar]
- 3.Asztalos Z, von Wegerer J, Wustmann G, Dombrádi V, Gausz J, Spatz H-C, Friedrich P. Protein phosphatase 1-deficient mutant Drosophila is affected in habituation and associative learning. J Neurosci. 1993;13:924–930. doi: 10.1523/JNEUROSCI.13-03-00924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon JP, Strausfeld NJ. The Dipteran “giant fibre” pathway: neurons and signals. J Comp Physiol [A] 1986;158:529–548. [Google Scholar]
- 5.Bailey CH, Chen M. Morphological basis of short-term habituation in Aplysia . J Neurosci. 1988;8:2452–2459. doi: 10.1523/JNEUROSCI.08-07-02452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987;4:65–73. [PubMed] [Google Scholar]
- 7.Benshalom G, Dagan D. Drosophila neural pathways: genetic and electrophysiological analysis. J Comp Physiol [A] 1985;156:13–23. [Google Scholar]
- 8.Booker R, Quinn WG. Conditioning of leg position in normal and mutant Drosophila . Proc Natl Acad Sci USA. 1981;78:3940–3944. doi: 10.1073/pnas.78.6.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boynton S, Tully T. latheo , a new gene involved in associative learning and memory in Drosophila melanogaster , identified from P element mutagenesis. Genetics. 1992;131:655–672. doi: 10.1093/genetics/131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelli M, Castellucci VF, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia : possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 11.Byers D (1979) Studies on learning and cyclic AMP phosphodiesterase of the dunce mutant of Drosophila melanogaster. PhD thesis, California Institute of Technology.
- 12.Byers D, Davis RL, Kiger JAJ. Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster . Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 13.Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia . Proc Natl Acad Sci USA. 1974;71:5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellucci VF, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia . Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 15.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci USA. 1986;83:9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corfas G, Dudai Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila . J Neurosci. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corfas G, Dudai Y. Adaptation and fatigue of a mechanosensory neuron in wild-type Drosophila and in memory mutants. J Neurosci. 1990a;10:491–499. doi: 10.1523/JNEUROSCI.10-02-00491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corfas G, Dudai Y. Pharmacological evidence for the involvement of the cAMP cascade in sensory fatigue in Drosophila . J Comp Physiol [A] 1990b;167:437–440. doi: 10.1007/BF00192579. [DOI] [PubMed] [Google Scholar]
- 19.Corfas G, Dudai Y. Morphology of a sensory neuron in Drosophila is abnormal in memory mutants and changes during aging. Proc Natl Acad Sci USA. 1991;88:7252–7256. doi: 10.1073/pnas.88.16.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 21.Delgado R, Hidalgo P, Diaz F, Latorre R, Labarca P. A cyclic AMP-activated K+ channel in Drosophila larval muscle is persistently activated in dunce . Proc Natl Acad Sci USA. 1991;88:557–560. doi: 10.1073/pnas.88.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dévay P, Friedrich P. Cyclic AMP-induced phosphorylation of 27.5 kDa protein(s) in larval brains of normal and memory-mutant Drosophila melanogaster . J Neurogenet. 1987;4:275–284. [PubMed] [Google Scholar]
- 23.Dévay P, Pintér M, Kiss I, Faragó A, Friedrich P. Protein kinase C in larval brain of wild-type and dunce memory-mutant Drosophila . J Neurogenet. 1989;5:119–126. doi: 10.3109/01677068909066202. [DOI] [PubMed] [Google Scholar]
- 24.Dixon D, Atwood HL. Adenylate cyclase system is essential for long-term facilitation at the crayfish neuromuscular junction. J Neurosci. 1989;9:4246–4252. doi: 10.1523/JNEUROSCI.09-12-04246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drain P, Folkers E, Quinn WG. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron. 1991;6:71–82. doi: 10.1016/0896-6273(91)90123-h. [DOI] [PubMed] [Google Scholar]
- 26.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duerr JS, Quinn WG. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Natl Acad Sci USA. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dura J-M, Preat T, Tully T. Identification of linotte , a new gene affecting learning and memory in Drosophila melanogaster . J Neurogenet. 1993;9:1–14. doi: 10.3109/01677069309167272. [DOI] [PubMed] [Google Scholar]
- 29.Elkins T, Ganetzky B. Conduction in the giant nerve fiber pathway in temperature-sensitive paralytic mutants of Drosophila . J Neurogenet. 1990;6:207–219. doi: 10.3109/01677069009107111. [DOI] [PubMed] [Google Scholar]
- 30.Engel JE (1995) Effects of second messenger and excitability mutations upon identified neural circuits underlying activity-dependent plasticity of behavior in Drosophila. PhD thesis, University of Iowa.
- 31.Engel JE, Wu C-F. Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila . J Comp Physiol [A] 1992;171:93–104. doi: 10.1007/BF00195964. [DOI] [PubMed] [Google Scholar]
- 32.Engel JE, Wu C-F. Repetitive conditioning of the Drosophila giant fiber response is altered in a mutant with learning deficiencies. Soc Neurosci Abstr. 1994a;20:803. [Google Scholar]
- 33.Engel JE, Wu C-F. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. J Comp Physiol [A] 1994b;175:267–278. doi: 10.1007/BF00192986. [DOI] [PubMed] [Google Scholar]
- 34.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac . Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 35.Fischer TM, Carew TJ. Activity-dependent potentiation of recurrent inhibition: a mechanism for dynamic gain control in the siphon withdrawal reflex of Aplysia . J Neurosci. 1993;13:1302–1314. doi: 10.1523/JNEUROSCI.13-03-01302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzgerald K, Wright WG, Marcus EA, Carew TJ. Multiple forms of non-associative plasticity in Aplysia : a behavioural, cellular and pharmacological analysis. Philos Trans R Soc Lond Biol. 1990;329:171–178. doi: 10.1098/rstb.1990.0162. [DOI] [PubMed] [Google Scholar]
- 37.Gorczyca M, Hall JC. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. J Neurogenet. 1984;1:289–313. doi: 10.3109/01677068409107093. [DOI] [PubMed] [Google Scholar]
- 38.Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioural plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 39.Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 40.Guillén A, Jallon J-M, Fegrentz J-A, Pantaloni C, Bockaert J, Homburger V. A Go-like protein in Drosophila melanogaster and its expression in memory mutants. EMBO J. 1990;9:1449–1455. doi: 10.1002/j.1460-2075.1990.tb08261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han P-L, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 42.Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y-Y, Li X-C, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 44.King DG, Wyman RJ. Anatomy of the giant fibre pathway in Drosophila . I. Three thoracic components of the pathway. J Neurocytol. 1980;9:753–770. doi: 10.1007/BF01205017. [DOI] [PubMed] [Google Scholar]
- 45.Klein M. Differential cyclic AMP dependence of facilitation at Aplysia sensorimotor synapses as a function of prior stimulation: augmentation versus restoration of transmitter release. J Neurosci. 1993;13:3793–3801. doi: 10.1523/JNEUROSCI.13-09-03793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein M, Kandel ER. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia californica . Proc Natl Acad Sci USA. 1980;77:6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein M, Shapiro E, Kandel ER. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- 48.Krasne FB, Teshiba TM. Habituation of an invertebrate escape reflex due to modulation by higher centers rather than local events. Proc Natl Acad Sci USA. 1995;92:3362–3366. doi: 10.1073/pnas.92.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levin LR, Han P-L, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 50.Levine J. Giant neuron input in mutant and wild type Drosophila . J Comp Physiol. 1974;93:265–285. [Google Scholar]
- 51.Levine J, Tracey D. Structure and function of the giant motoneuron of Drosophila melanogaster . J Comp Physiol. 1973;87:213–235. [Google Scholar]
- 52.Lindsley DL, Zimm GG. Academic; San Diego: 1992. The genome of Drosophila melanogaster . . [Google Scholar]
- 53.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga , a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 54.May ML, Hoy RR. Habituation of the ultrasound-induced acoustic startle response in flying crickets. J Exp Biol. 1991;159:489–499. doi: 10.1242/jeb.159.1.489. [DOI] [PubMed] [Google Scholar]
- 55.Milde JJ, Strausfeld NJ. Cluster organization and response characteristics of the giant fiber pathway of the blowfly Calliphora erythrocephala . J Comp Neurol. 1990;294:59–75. doi: 10.1002/cne.902940106. [DOI] [PubMed] [Google Scholar]
- 56.Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster . In: Demerec M, editor. Biology of Drosophila. Hafner; New York: 1950. pp. 420–534. [Google Scholar]
- 57.Mohler JD. Developmental genetics of the Drosophila egg. Identification of 59 sex-linked cistrons with maternal effects of embryonic development. Genetics. 1977;85:259–272. doi: 10.1093/genetics/85.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller U, Spatz H-C. Ca2+-dependent proteolytic modification of the cAMP-dependent protein kinase in Drosophila wild-type and dunce memory mutants. J Neurogenet. 1989;6:95–114. doi: 10.3109/01677068909107104. [DOI] [PubMed] [Google Scholar]
- 59.Nelson JC, Wyman RJ. Examination of paralysis in Drosophila temperature-sensitive paralytic mutations affecting sodium channels; a proposed mechanism of paralysis. J Neurobiol. 1990;21:453–469. doi: 10.1002/neu.480210307. [DOI] [PubMed] [Google Scholar]
- 60.Nighorn A, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- 61.O’Kane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila . Proc Natl Acad Sci USA. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rees CT, Spatz H-C. Habituation of the landing response of Drosophila wild-type and mutants defective in olfactory learning. J Neurogenet. 1989;5:105–118. doi: 10.3109/01677068909066201. [DOI] [PubMed] [Google Scholar]
- 63.Schacher S, Kandel ER, Montarolo P. cAMP and arachidonic acid simulate long-term structural and functional changes produced by neurotransmitters in Aplysia sensory neurons. Neuron. 1993;10:1079–1088. doi: 10.1016/0896-6273(93)90056-w. [DOI] [PubMed] [Google Scholar]
- 64.Sokal R, Rohlf F. Freeman; New York: 1981. Biometry, 2nd Ed. . [Google Scholar]
- 65.Strausfeld NJ, Bacon JP. Multimodal convergence in the central nervous system of Dipterous insects. In: Horn H, editor. Multimodal convergences in sensory systems. Gustav Fischer; StuttgartNew York: 1983. pp. 47–76. [Google Scholar]
- 66.Strausfeld NJ, Bassemir UK. Cobalt-coupled neurons of a giant fibre system in Diptera. J Neurocytol. 1983;12:971–991. doi: 10.1007/BF01153345. [DOI] [PubMed] [Google Scholar]
- 67.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 68.Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila . J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- 69.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila . Proc Natl Acad Sci USA. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson RF, Glanzman DL. Neural and behavioral mechanisms of habituation and sensitization. In: Tighe TJ, Leaton RN, editors. Habituation: perspectives from child development, animal behavior, and neurophysiology. Wiley; New York: 1976. pp. 49–93. [Google Scholar]
- 71.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 72.Trimarchi JR, Schneiderman AM. Giant fiber activation of an intrinsic muscle in the mesothoracic leg of Drosophila melanogaster . J Exp Biol. 1993;177:149–167. doi: 10.1242/jeb.177.1.149. [DOI] [PubMed] [Google Scholar]
- 73.Trimarchi JR, Schneiderman AM. Flight initiations in Drosophila melanogaster are mediated by several distinct motor patterns. J Comp Physiol [A] 1995a;176:355–364. doi: 10.1007/BF00219061. [DOI] [PubMed] [Google Scholar]
- 74.Trimarchi JR, Schneiderman AM. Initiation of flight in the unrestrained fly, Drosophila melanogaster . J Zool (Lond) 1995b;235:211–222. [Google Scholar]
- 75.Trimarchi JR, Schneiderman AM. Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol. 1995c;198:1099–1104. doi: 10.1242/jeb.198.5.1099. [DOI] [PubMed] [Google Scholar]
- 76.Trudeau L-E, Castellucci VF. Functional uncoupling of inhibitory interneurons plays an important role in short-term sensitization of Aplysia gill and siphon withdrawal reflex. J Neurosci. 1993;13:2126–2135. doi: 10.1523/JNEUROSCI.13-05-02126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tully T, Koss S. Habituation of the jump reflex to olfactory cues in normal and mutant Drosophila . Soc Neurosci Abstr. 1992;8:942. [Google Scholar]
- 78.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster . J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu C-F. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 80.Wright NJD, Zhong Y. Characterization of K+-currents and the cAMP-dependent modulation in cultured Drosophila mushroom body neurons identified by lacZ expression. J Neurosci. 1995;15:1025–1034. doi: 10.1523/JNEUROSCI.15-02-01025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu C-F, Ganetzky B. Neurogenetic studies of ion channels in Drosophila . In: Narahashi T, editor. Ion channels, Vol. 3. Plenum; New York: 1992. pp. 261–314. [DOI] [PubMed] [Google Scholar]
- 82.Wyman RJ, Thomas JB, Salkoff L, King DG. The Drosophila giant fiber system. In: Eaton RC, editor. Neural mechanisms of startle behavior. Plenum; New York: 1984. pp. 133–161. [Google Scholar]
- 83.Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila . Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 84.Zhao M-L, Wu C-F. Altered spike activity in cultured “giant” neurons derived from dunce and rutabaga mutants of Drosophila . Soc Neurosci Abstr. 1994;20:803. [Google Scholar]
- 85.Zhong Y. Mediation of PACAP-like neuropeptide transmission by coactivation of RAS/RAF and cAMP signal transduction pathways in Drosophila . Nature. 1995;375:588–592. doi: 10.1038/375588a0. [DOI] [PubMed] [Google Scholar]
- 86.Zhong Y, Wu C-F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991a;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 87.Zhong Y, Wu C-F. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag . Science. 1991b;252:1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]
- 88.Zhong Y, Wu C-F. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila . J Neurogenet. 1993a;9:15–27. doi: 10.3109/01677069309167273. [DOI] [PubMed] [Google Scholar]
- 89.Zhong Y, Wu C-F. Modulation of different K+ currents in Drosophila : a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci. 1993b;13:4669–4679. doi: 10.1523/JNEUROSCI.13-11-04669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong Y, Budnik V, Wu C-F. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zucker RS. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972;35:621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]