Abstract
Parkinson’s disease (PD) is characterized by degeneration of dopamine (DA)-containing nigro-striatal neurons. Loss of the antioxidant glutathione (GSH) has been implicated in the pathogenesis of PD. Previously, we showed that the oxidant hydrogen peroxide inhibits vesicular uptake of DA in nigro-striatal neurons. Hydrogen peroxide is scavenged by GSH and, therefore, we investigated a possible link between the process of vesicular storage of DA and GSH metabolism. For this purpose, we used rat pheochromocytoma-derived PC12 cells, a model system applied extensively for studying monoamine storage mechanisms. We show that depletion of endogenous DA stores with reserpine was accompanied in PC12 cells by a long-lasting, significant increase in GSH content the extent of which appeared to be inversely related to the rate of GSH synthesis. A similar increase in GSH content was observed after depletion of DA stores with the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine. In the presence of α-methyl-p-tyrosine, refilling of the DA stores by exogenous DA reduced GSH content back to control level. Lowering of PC12 GSH content, via blockade of its synthesis with buthionine sulfoximine, however, led to a significantly decreased accumulation of exogenous [3H]DA without affecting uptake of the acetylcholine precursor [14C]choline. These data suggest that GSH is involved in the granular storage of DA in PC12 cells and that, considering the molecular characteristics of the granular transport system, it is likely that GSH is used to protect susceptible parts of this system against (possibly DA-induced) oxidative damage.
Keywords: Parkinson’s disease, oxidative stress, glutathione, dopamine, PC12 cells, neurotransmitter uptake, vesicular storage
Parkinson’s disease (PD) is characterized primarily by a loss of dopamine (DA) in the striatum caused by degeneration of DAergic neurons in the zona compacta of the substantia nigra (SN) (Gibb and Lees, 1991). Although the cause of PD is still unknown, oxidative stress has been implicated as a pathogenetic factor. According to this so called “free radical hypothesis,” the degeneration of the nigro-striatal system in PD is related to the relatively high exposure of these neurons to reactive oxygen species (ROS), in particular hydrogen peroxide (H2O2), produced during both the enzymatic (monoamine oxidase-catalyzed) and nonenzymatic (auto-oxidative) breakdown of DA (Adams and Odunze, 1991;Olanow, 1992). Thus, DAergic cell death in PD may be caused by an overproduction of ROS and/or a diminished protection against them. Evidence to support the “free radical hypothesis” has come from postmortem investigations of PD brains, which consistently show an increase in the indices of oxidative stress in the SN at the time of death (Hirsch et al., 1991; Jenner, 1993). One of these indices, possibly even preceding the loss of DA (Dexter et al., 1994), is a decrease in the level of glutathione (GSH) in the Parkinsonian SN (Riederer et al., 1989; Sofic et al., 1992; Jenner, 1993). GSH, the most abundant free thiol in mammalian cells (Meister and Andersson, 1983), is considered to be a major antioxidant in the brain. Thus, GSH has been shown to detoxify (organic and inorganic) hydroperoxides including the H2O2 formed during the oxidative metabolism of DA (Spina and Cohen, 1988, 1989).
To prevent breakdown of DA before its release from presynaptic endings, the transmitter is taken up and stored in presynaptic granules. This process is effected through the action of a transporter protein (Johnson, 1988). Interference with granular storage, for instance via blockade of the ligand-binding site on the transporter, leads to an enhanced turnover of DA, inducing considerable oxidative stress as a consequence of H2O2 production (Spina and Cohen, 1989). Recently, we have shown that, apart from generating oxidative stress, H2O2 is able to inhibit storage of DA in the nigro-striatal system through an as yet undefined interaction with the granular uptake mechanism (Langeveld et al., 1995). Interestingly, the DA storage system appeared to be substantially more sensitive to H2O2 compared with that of other transmitters, such as noradrenaline (NA) and acetylcholine (ACh) (Langeveld et al., 1995). Based on these data, we proposed that the degeneration of the nigro-striatal system in PD may be the outcome of a self-sustaining cycle that is initiated by lowering of the capacity to scavenge the H2O2 released during normal DA metabolism, leading to a decrease in the granular storage and enhanced breakdown of DA, inducing more oxidative stress, disturbance of neuronal function and, eventually, cell death. Considering our observation that H2O2preferentially interferes with presynaptic storage of DA and the fact that, in the mammalian brain, H2O2 is detoxified mainly by GSH (Maker et al., 1981; DiMonte et al., 1992), whose levels are decreased in PD, we initiated a series of in vitro experiments to investigate a possible linkage between granular uptake of DA and GSH metabolism. For this purpose, we used a rat pheochromocytoma-derived PC12 cell line (Greene and Tischler, 1976) which, in contrast to tissue slices or primary neuronal cultures, consists of a homogeneous population of DA containing cells that have been demonstrated to synthesize, store, and release DA in a similar manner as neurons (Greene and Rein, 1977). Moreover, these cells are known to contain both GSH and the enzyme systems involved in its metabolism (Pan and Perez-Polo, 1993; Sampath et al., 1994).
MATERIALS AND METHODS
Materials. Radiolabeled DA and choline were obtained from Amersham International (Little Chalfont, UK).dl-α-methyl-p-tyrosine (αMPT) was from Research Biochemicals (Natick, MA), and reserpine was from De Onderlinge Pharmaceutische Groothandel (Utrecht, The Netherlands). Tissue culture media and supplements were obtained from Gibco Netherlands BV (Breda, The Netherlands), whereas all other chemicals and drugs, unless noted otherwise, were obtained from Sigma (St. Louis, MO).
Cell culture. PC12 cells, originally described by Greene and Tischler (1976), were grown in 80 cm2 tissue culture flasks containing DMEM/Ham’s F-10 (1:1) supplemented with 15% fetal calf serum (FCS), 2 mml-glutamine, penicillin (100 U/ml), and streptomycin (50 μg/ml) at 37°C under an atmosphere of 5% CO2/95% air. After 1 week, in which the culture medium had been changed once or twice, the medium was removed and the cells were trypsinized according to Van Muiswinkel et al. (1995). After trypsinization, viable cells in the resulting cell suspension were counted by trypan blue exclusion in a hemocytometer. Subsequently, cells were plated in 12-well culture dishes, containing 1 ml of culture medium in each well, at a density of 2.5 × 105cells/well for all experiments. Before culturing, both culture flasks and dishes had been precoated with poly-l-lysine (100 μg/ml). Investigations were started when the cells had been in the culture dishes for at least 24 hr and the cultures were maintained, without a change of medium, for a maximum of 4 d depending on the type of experiment (see Results).
GSH assay: sample preparation. Samples were prepared for the measurement of cellular total GSH content, that is, reduced GSH + oxidized glutathione (GSSG), according to the method described byRedegeld et al. (1988), with the exception that 5-sulfosalicylic acid (SSA) was used instead of perchloric acid to extract GSH. Preparation of the samples was performed at 4°C. Cells were rinsed free of culture medium or phosphate buffer (see below) before 250 μl of 2.5% (w/v) SSA was added. Subsequently, culture dishes were quickly frozen (−70°C) and thawed to ensure cell lysis. The lysate was centrifuged to remove denaturated protein. From the supernatant a volume of 100 μl was taken to which 10 μl of H2O and, after 5 min, 20 μl of K3PO4 (3 m, pH 13.5) were added for incubation at room temperature. After a 10 min period, the samples were then neutralized with 50 μl of a 10% (w/v) SSA solution. In a subset of samples, instead of H2O, 10 μl of 11 mmN-ethyl-maleimide (NEM) was added to determine GSSG content. In these samples, alkaline hydrolysis by K3PO4 was used for inactivation of excess NEM.
GSH assay: enzyme recycling procedure. GSH was determined spectrophotometrically according to Tietze (1969) with adaptations byBaker et al. (1990). The procedure was performed at room temperature. In short, 50 μl of GSH or GSSG standards, samples, or blanks was pipetted into the wells of a microtiter plate. A buffer of 100 mm sodium phosphate and 1 mm EDTA (Fluka Chemie, Buchs, Switzerland; pH 7.5) was used to prepare a reaction mixture consisting of 0.225 mm5,5′-dithio-bis(2-nitro-benzoic acid) (DTNB), 0.3 mm NADPH (tetrasodium salt, Boehringer Mannheim, Mannheim, Germany), and 2.8 U/ml GSSG reductase. Subsequently, 100 μl of freshly prepared reaction mixture was added to each of the wells. The absorbance at 405 nm (TitertekPlus Microplate Reader, ICN Biomedicals, Amsterdam, The Netherlands) was measured immediately after the addition of the reaction mixture and at intervals of 1 min thereafter during 6 min. Total GSH and GSSG content was calculated from the rate of increase in absorbance.
Cellular catecholamine content. Cells were rinsed free from culture medium or phosphate buffer (see below) and subsequently lysed by adding 400 μl of ice-cold perchloric acid (0.2 N). After this procedure, the culture dishes were frozen and thawed and the lysate then centrifuged as described above. Aliquots of the supernatants were stored at −20°C before the analysis of catecholamine content by HPLC with electrochemical detection. HPLC analysis was performed essentially as described previously (Westerink and Mulder, 1981; Van Muiswinkel et al., 1995), using a Guardcolumn (Phase Sep, Deeside, UK) in combination with a reverse-phase column filled with Spherisorb (3 μm, SRODS2; Phase Sep) and an amperometric electrochemical detector (Antec, Leiden, The Netherlands). The mobile phase consisted of 0.05 mcitric acid, 0.05 m phosphoric acid, 1.5 mmoctanesulfonic acid (sodium salt monohydrate, Janssen Chimica, Beerse, Belgium), 0.2 mm EDTA, and 5% (v/v) methanol, pH 2.5. The detector potential was set at +750 mV versus a K/KCl reference electrode. Catecholamine content was calculated against standards that had been subjected to the same treatment as the samples.
Cell survival assay. To determine cell survival, we used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (Langeveld et al., 1992) with some adaptations. An MTT stock solution was prepared and stored at −20°C. On the day of the assay, 100 μl of MTT solution (5 mg/ml) was added to each well of the culture dishes containing 1 ml of culture medium. Subsequently, incubation of the cultures took place for 2 hr at 37°C under an atmosphere of 5% CO2/95% air. Thereafter, the medium was removed and replaced by 2 ml of dimethylsulfoxide (DMSO) to which 0.5% (v/v) FCS had been added. After formazan solubilization by vibration on a plate shaker, a 150 μl sample was drawn from this solution and pipetted into a microtiter plate. The absorbance of each well was measured at 540 nm using the TitertekPlus microplate reader described above. Absorbance data were calculated by subtracting the mean of “background” readings obtained from identical incubations in the absence of cells.
Radioligand uptake. Cells were rinsed free of culture medium and preincubated for 30 min at 37°C in 500 μl of a phosphate buffer, pH 7.3, consisting of (in mm): NaCl 137, KCl 2.7, Na2HPO4 8, KH2PO4 1.5, MgCl2 0.5, CaCl2 1.2, andd-(+)-glucose 5. To this buffer 0.5% (w/v) bovine serum albumin (BSA) had been added. Subsequently, the cultures were washed and incubated for 20 min with BSA containing buffer to which [3H]DA (specific activity 45 Ci/mmol; final concentration 65 nm) and/or [14C]choline (specific activity 55 mCi/mmol; final concentration 8.5 μm) had been added. Thereafter, cultures were washed seven times with buffer before the accumulated radioactivity was extracted by a 30 min incubation with acidified ethanol (95% ethanol/5% 0.1 N HCl) and quantified by liquid scintillation counting (Packard TriCarb 1900 AC liquid scintillation analyzer, Groningen, The Netherlands). If uptake inhibitors were used, they were added to the buffer at the start of the preincubation period and remained present throughout the experiment.
Protein content. Protein content was determined, according to the method of Bradford (1976) and using BSA as a standard, in parallel cultures to those used for the above described measurements.
Statistical analysis. Statistical analysis was performed using a two-tailed Student’s t test.
RESULTS
Effect of reserpine on GSH content
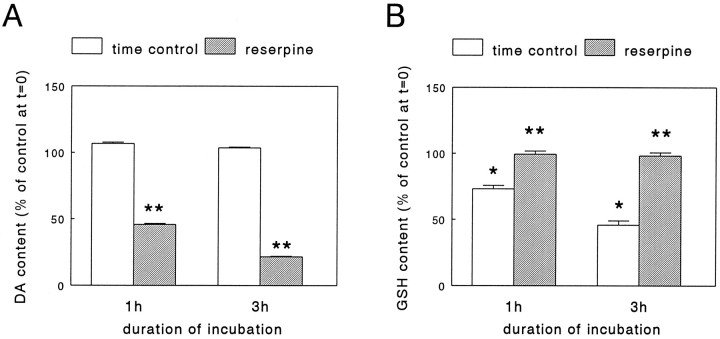
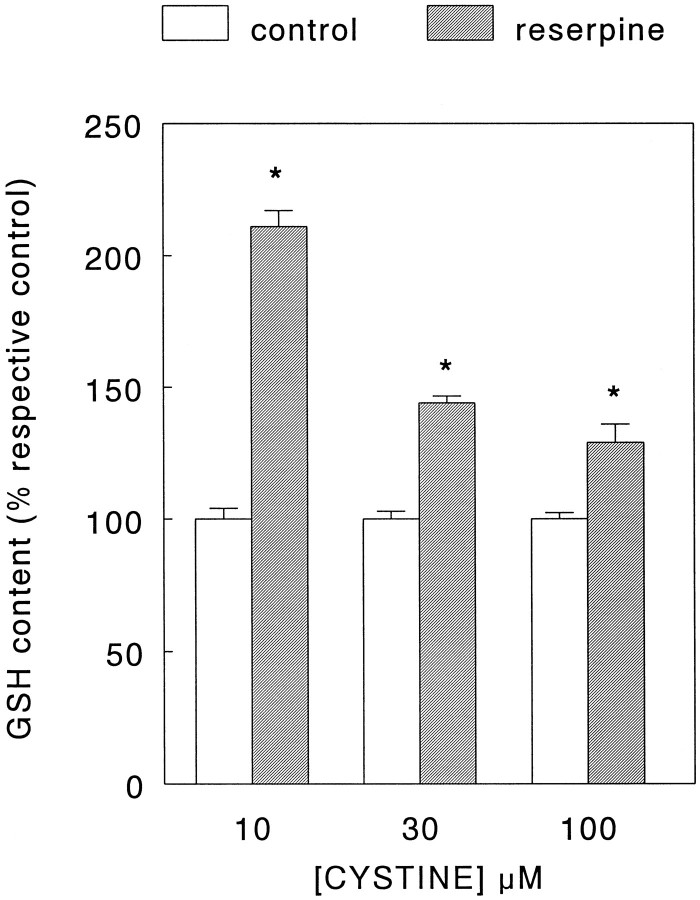
Under our culture conditions, ∼95% of the total catecholamine content of the PC12 cells consisted of DA (data not shown). As expected, incubation of the cells for either 1 or 3 hr in a phosphate buffer (see Materials and Methods) to which the granular uptake inhibitor reserpine (50 nm; Carlsson, 1965) had been added led to a time-dependent decrease of DA content (Fig.1A). However, whereas incubation of the PC12 cells in the buffer time-dependently lowered total GSH levels, this phenomenon was not observed when reserpine was present (Fig.1B). The decrease in GSH content under control condition during incubation with buffer was prevented by addition of cystine (10–100 μm) which, after uptake, is reduced inside the cell into the GSH precursor cysteine. In fact, a 3 hr incubation with cystine induced a concentration-dependent increase in GSH levels which, between experiments, averaged from 0.7–2.0 nmol/mg protein in the absence of cystine to 8.7–18.3 nmol/mg protein in the presence of 100 μm cystine (see legend to Fig.2). In the presence of cystine, a statistically significant elevation of GSH content was noted after addition of reserpine (50 nm, Fig. 2), without alteration of its DA content decreasing action (data not shown). Interestingly, this effect of reserpine appeared to be inversely related to the concentration of cystine in the buffer and thus, possibly, to cellular GSH levels. In contrast to cystine, incubation of cells for 3 hr with buffer containing 100 μml-buthionine-S,R-sulfoximine (BSO), an inhibitor of GSH synthesis (Meister, 1991), led to a decrease of GSH content below control values (see legend to Table 1). However, the presence of BSO did not prevent the reserpine (50 nm)-mediated effect on GSH levels from occurring (Table 1). In all of the above described experiments, the GSSG levels remained below the limit of detection (0.05 nmol/well). Thus, we concluded that most of the GSH present in the cells was in the reduced form and decided to refrain from any further attempts at measurement of GSSG in the follow-up experiments. Furthermore, in this set of experiments no extracellular GSH was detected in the incubation buffer under either control or treatment conditions.
Fig. 1.
Effect of reserpine on dopamine (DA) content (A) and glutathione (GSH) content (B) of PC12 cells. PC12 cells, cultured for 2 d, were rinsed free of culture medium and incubated for either 1 or 3 hr at 37°C in a phosphate buffer (see Materials and Methods) in the absence or presence of 50 nmreserpine, respectively. Subsequently, the cells were lysed and the DA, GSH, and protein content in parallel cultures was determined as detailed in Materials and Methods. Data represent the mean ± SEM (n = 5–6) from two independent experiments. Between experiments, at the start of incubation (i.e.,t = 0), DA content averaged from 1.8 to 3.3 nmol/mg protein, whereas GSH content averaged from 1.6 to 3.1 nmol/mg protein. Therefore, the data are expressed as percentage of DA or GSH content att = 0. *p < 0.001 versus control at t = 0; **p < 0.001 versus respective time control.
Fig. 2.
Effect of reserpine on glutathione (GSH) content of PC12 cells in the presence of cystine. PC12 cells, cultured for 2 d, were rinsed free of culture medium and incubated for 3 hr in a phosphate buffer (see Materials and Methods) at 37°C with 10, 30, or 100 μm cystine in the absence (control) or presence of 50 nm reserpine, respectively. Subsequently, the cells were lysed and the GSH and protein content in parallel cultures was determined as detailed in Materials and Methods. Data represent the mean ± SEM (n = 9) from three independent experiments. Between experiments, the GSH content after a 3 hr incubation in the absence of reserpine averaged from 0.7 to 2.0 nmol/mg protein (no cystine), 1.5 to 4.4 nmol/mg protein (10 μm cystine), 4.1 to 11.1 nmol/mg protein (30 μm cystine), and 8.7 to 18.3 nmol/mg protein (100 μm cystine). Therefore, the data are expressed as percentage of respective GSH content at each cystine concentration in the absence of reserpine. *p < 0.005 versus respective control.
Table 1.
Effect of BSO on the reserpine-induced increase in glutathione content in PC12 cells
| Treatment | Glutathione content (% respective control) |
|---|---|
| No drug | 100 ± 3.4 |
| Reserpine (50 nm) | 222 ± 14.5* |
| BSO (100 μm) | 100 ± 7.6 |
| BSO (100 μm) + reserpine (50 nm) | 276 ± 9.2* |
After 2 d of culture, PC12 cells were rinsed free of medium and incubated for 3 hr at 37°C in a phosphate buffer (see Materials and Methods) in the absence of drugs or in the presence of reserpine alone, BSO alone, or the combination of BSO and reserpine, respectively. Data represent the mean ± SEM (n = 5–6) from two independent experiments and are expressed, in case of reserpine alone, as percentage of glutathione content after 3 hr in the absence of drugs and, in case of the combination of BSO and reserpine, as percentage of glutathione content after 3 hr in the presence of BSO alone. In the absence of drugs, the glutatione content after a 3 hr incubation amounted to 45.8 ± 3.2% from control at t = 0 hr, whereas in the presence of BSO glutathione content after a 3 hr incubation amounted to 26.7 ± 2.4% from control (mean ± SEM; p < 0.001 vs incubation in absence of drugs).
*p < 0.001 vs respective control.
Treatment of PC12 cells with reserpine (50 nm) added to the culture medium for up to 3 d induced a decrease of cellular DA content down to ∼1.5% of (time)control values (Table2). The maximum level of DA depletion was already attained after a 24 hr incubation period and remained stable throughout the experiment. At the same time, in the presence of reserpine, the levels of the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) also decreased, albeit to a much smaller extent than those of DA, thus demonstrating ongoing DA breakdown in the absence of granular storage (Table 2). Concomitant with these reserpine-induced effects on DA metabolism, a lasting and statistically significant increase in cellular total GSH content was observed after incubation with the drug for 1, 2, or 3 d (Table 2). Under these circumstances, however, no effect of reserpine treatment on cell survival was detected, as measured with the MTT assay [0.65 ± 0.009 vs 0.68 ± 0.009, 0.80 ± 0.003 vs 0.77 ± 0.02, 0.93 ± 0.01 vs 0.97 ± 0.02; mean ± SEM (n = 3) of absorbance in control versus reserpine treatment group after 1, 2, or 3 d of incubation with reserpine, respectively].
Table 2.
Effect of treatment of PC12 cells for up to 3 d with reserpine on the GSH, DA, and DOPAC content
| Treatment | Content (% respective control) | ||
|---|---|---|---|
| GSH | DA | DOPAC | |
| 24 hr no drug | 100 ± 12.3 | 100 ± 0.3 | 100 ± 0.8 |
| 24 hr reserpine (50 nm) | 424 ± 2.82_a | 1.3 ± 0.052_a | 92.8 ± 1.12_b |
| 48 hr no drug | 100 ± 13.8 | 100 ± 1.5 | 100 ± 5.3 |
| 48 hr reserpine (50 nm) | 296 ± 5.52_a | 1.4 ± 0.052_a | 85.4 ± 1.0 |
| 72 hr no drug | 100 ± 8.3 | 100 ± 0.9 | 100 ± 1.5 |
| 72 hr reserpine (50 nm) | 414 ± 1.72_a | 1.4 ± 0.022_a | 88.0 ± 1.22_c |
After an initial 24 hr drug-free culture period, without a change of medium (see Materials and Methods), PC12 cells were kept in culture for an additional 24, 48, or 72 hr period in the absence (no drug) or presence of 50 nm reserpine, respectively. At the end of each incubation period, samples were prepared for determination of GSH, DA, and DOPAC content in parallel cultures, respectively, as detailed in Materials and Methods. Subsequent to preparation, all samples were stored at −20°C to ensure that determination of GSH, DA, and DOPAC content would take place on the same day. Data represent the mean ± SEM of a typical experiment performed in triplicate and are expressed as percentage of respective control (i.e., the value obtained for the respective incubation period in the absence of drug).
p < 0.001 vs respective control.
p < 0.01 vs respective control.
p < 0.005 vs respective control.
Effect of αMPT on GSH content
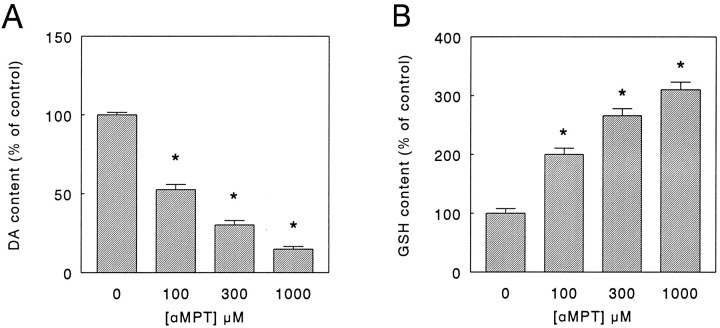
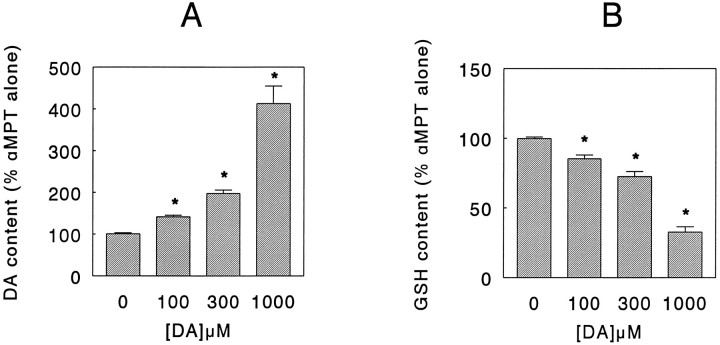
To rule out the possibility that the reserpine-induced elevation of GSH levels was (partly) attributable to enhanced DA turnover and not to inhibition of granular uptake and/or storage, we incubated the PC12 cells for 24 hr with the tyrosine hydroxlase (TH) inhibitor α-methyl-p-tyrosine (αMPT) (Spector et al., 1965) added to the culture medium. Under these conditions, αMPT (0.1–1 mm) induced a concentration-dependent lowering of the DA level down to ∼15% of control value (Fig.3A), accompanied, however, by a decrease in DOPAC content below the detection limit at all drug concentrations used (data not shown). Concurrently, similar to the results obtained with reserpine, compared with control values total GSH content increased in a concentration-dependent manner in the presence of αMPT (Fig.3B). After depletion of intracellular DA by treatment for 24 hr with 1 mm αMPT, incubation of the cells for 20 min in a phosphate buffer (see Materials and Methods) containing DA (0.1–1 mm) again increased the intracellular DA level while simultaneously decreasing GSH level in a concentration-dependent manner (Fig. 4). In contrast, PC12 cells that were not pretreated with αMPT but were incubated for 20 min with DA under similar experimental conditions showed only a marginal increase in intracellular DA content and no change in GSH level (data not shown).
Fig. 3.
Effect of α-methyl-p-tyrosine (αMPT) on dopamine (DA) content (A) and glutathione (GSH) content (B) of PC12 cells. After an initial 24 hr drug-free culture period, without a change of medium (see Materials and Methods), PC12 cells were kept in culture for an additional 24 hr period in the absence (0 μm) or presence of 100, 300, or 1000 μm αMPT, respectively. Subsequently, the cells were lysed and the DA, GSH, and protein content in parallel cultures was determined as detailed in Materials and Methods. Data represent the mean ± SEM (n = 5–6) from two independent experiments and are expressed as percentage of control (i.e., content after 48 hr of culture in the absence of αMPT). Under control conditions, between experiments the DA content averaged from 3.7 to 4.0 nmol/mg protein, whereas the GSH content averaged from 1.5 to 1.6 nmol/mg protein, respectively. *p < 0.001 versus control.
Fig. 4.
Effect of exogenous dopamine (DA) on the DA content (A) and glutathione (GSH) content (B) of PC12 cells incubated in the presence of α-methyl-p-tyrosine (αMPT). After an initial 24 hr drug-free culture period, without a change of medium (see Materials and Methods), PC12 cells were kept in culture for an additional 24 hr period in the absence or presence of 1 mm αMPT. Subsequently, the cells were rinsed free of culture medium and incubated for 20 min at 37°C in a phosphate buffer (see Materials and Methods) in the absence (0 μm) or presence of 100, 300, or 1000 μm DA, respectively. Thereafter, the cells were lysed and the DA, GSH, and protein content in parallel cultures was determined as detailed in Materials and Methods. Data represent the mean ± SEM (n = 6) from two independent experiments and are expressed as percentage of DA or GSH content in cells treated only with αMPT. In control cultures (i.e., cells not treated with any drug), the DA content amounted to 5.3 ± 0.5 nmol/mg protein, whereas in cultures treated only with αMPT, the DA content amounted to 0.8 ± 0.1 nmol/mg protein (mean ± SEM). In control cultures, the GSH content amounted to 2.1 ± 0.3 nmol/mg protein, whereas in cultures treated only with αMPT the GSH content amounted to 4.6 ± 0.2 nmol/mg protein (mean ± SEM). *p < 0.001 versus αMPT alone (i.e., 0 μm DA).
To control for possible “nonspecific,” non-DA-related drug effects on cellular GSH metabolism, human D384 glioma cells (Langeveld et al., 1992), not containing any detectable DA, were cultured in DMEM/F-10 containing 10% FCS and exposed to either reserpine or αMPT in the concentrations used for the experiments with PC12 cells. Incubation of the cells for up to 24 hr with either of the drugs did not induce any significant changes in cellular GSH content (data not shown). Furthermore, it is important to note that, under none of the above described experimental conditions in PC12 and D384 cells, neither reserpine nor αMPT affected cell survival as estimated by measurement of protein content (data not shown).
Effect of BSO on DA uptake
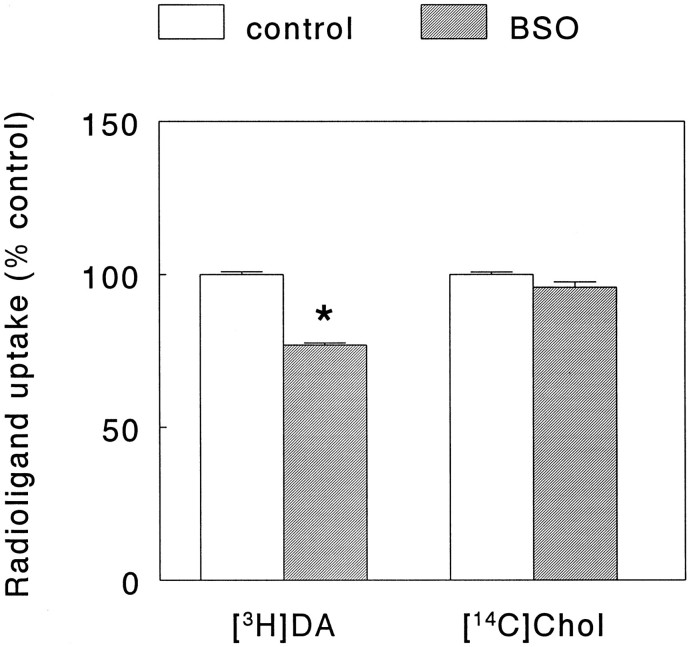
To check whether radiolabeled DA and choline uptake in PC12 cells, cultured under our experimental conditions, was mediated via a high-affinity membrane transport system, the Na+concentration in the incubation buffer was lowered by isomolar replacement of NaCl by LiCl. Under these conditions, [3H]DA uptake was lowered to 18.2 ± 0.7% of the control value [mean ± SEM (n = 3); Student’st test, p < 0.001], and [14C]choline uptake was lowered to 25 ± 0.3% of control [mean ± SEM (n = 3); Student’st test, p < 0.001]. Moreover, incubation of the cells with either the DA uptake inhibitor GBR 12909 (10 μm) (Andersen, 1989) or the NA uptake inhibitor desmethylimipramine (10 μm) (Iversen, 1973) reduced radiolabeled DA uptake down to 0.8 ± 0.04 and 1.0 ± 0.03% of control, respectively [mean ± SEM (n = 3); Student’s t test, p < 0.001], whereas incubation with the choline uptake inhibitor hemicholinium-3 (1 mm) (Birks and MacIntosh, 1957) reduced choline uptake to 26.6 ± 0.8% of control [mean ± SEM (n = 3); Student’s t test, p < 0.001]. Exposure of the PC12 cells for 24 hr to 10 μm BSO added to the culture medium, depleted the cellular GSH content down to 15.3 ± 1.2% of control [mean ± SEM (n = 6); Student’s t test, p < 0.001]. Consistent with previous data (Pan and Perez-Polo, 1993), in the presence of this relatively low concentration of BSO no effect was observed on cell survival (measured as protein content in treated and untreated groups), which contrasted with the results obtained after a 24 hr incubation with higher concentrations of the drug, where clear toxicity was induced without an additional effect on GSH levels in the remaining cells (data not shown). After the incubation with BSO (10 μm), the high-affinity uptake of [3H]DA was decreased by ∼25%, whereas no significant effect on [14C]choline uptake occurred (Fig. 5). At the same time, in BSO-treated cultures a slight yet statistically significant lowering of the DA content accompanied by an enhancement of DA turnover was noted [DA content: 94.5 ± 1.0% vs control; DOPAC/DA ratio: 108 ± 1.5% vs control (mean ± SEM, n = 6; Student’s t test,p < 0.01)].
Fig. 5.
Effect of buthionine sulfoximine (BSO) on the uptake of [3H]dopamine (DA) and [14C]choline (Chol) in PC12 cells. After an initial drug-free culture period of 48 hr, without a change of medium (see Materials and Methods), PC12 cells were kept in culture for an additional period of 24 hr in the absence (control) or presence of 10 μm BSO, respectively. Subsequently, the culture medium was removed and radioligand uptake was determined using a phosphate buffer to which both [3H]dopamine and [14C]choline had been added, as detailed in Materials and Methods. Data represent the mean ± SEM (n = 6) from two independent experiments and are expressed as percentage of control (i.e., uptake of either radioligand after 72 hr of culture in the absence of BSO). Under control conditions, the uptake of [3H]dopamine and [14C]choline amounted to 497,000 ± 10,900 and 11,600 ± 531 dpm (mean ± SEM), respectively. *p < 0.001 versus uptake of [3H]dopamine under control condition.
DISCUSSION
In general, DA, after its synthesis, is taken up and stored in specialized subcellular organelles, the storage granules, to ensure its regulated release via exocytosis. Most information about the granular transport mechanism(s) and storage mechanism(s) of DA (and monoamines in general) has been obtained by using chromaffin granules isolated from adrenal medullary cells or PC12 cells as experimental substrate (Roda et al., 1980). Thus, it is presently known that the DAergic granular transport system consists of at least two components: (1) a so-called vesicular monoamine transporter (VMAT), structurally distinct from the plasma membrane DA transporter, and (2) a vacuolar-type ATP-driven H+ pump, which provides the electrochemical gradient on which the transporter depends for its function (Johnson, 1988; Schuldiner, 1994). Drugs such as reserpine and tetrabenazine deplete intracellular DA stores by selectively interfering with transmitter uptake via the VMAT. In the present set of experiments, we used reserpine because it reportedly induces a long-lasting decrease in the DA level of PC12 cells at lower concentrations than tetrabenazine and, moreover, in contrast to tetrabenazine, specifically blocks the transmitter (DA) recognition site on the VMAT (Erickson and Eiden, 1993; Schuldiner et al., 1993).
Our data show that, as reported previously (Greene and Rein, 1977), inhibition of DA transport across the storage granule membrane in PC12 cells by a maximally effective concentration of reserpine (our unpublished observations) induces a fast, almost total, and long-lasting depletion of intracellular DA without affecting cell survival. This demonstrated that, in PC12 cells cultured under our conditions the major part of intracellularly synthesized, endogenous DA is sequestered in the storage granules and does not exert any toxic effect on the cells, as is observed in case of exogenous DA (Michel and Hefti, 1990). Interestingly, the reserpine-mediated emptying of the granular DA stores was accompanied by a substantial and sustained rise in the intracellular total GSH (i.e., reduced GSH + GSSG) content. Using the GSH precursor cystine to stimulate GSH formation, the extent of this reserpine-induced effect appeared to depend on the amount of GSH being synthesized in the cell and suggested the existence of a (thus far unknown) distinct pool of cytoplasmic GSH intimately linked to the DA storage compartment of PC12 cells. This interpretation of our data is supported moreover by the fact that, irrespective of the amount of GSH present after incubation with increasing doses of cystine, at each cystine concentration the reserpine-mediated rise in GSH content, when calculated as absolute amounts increase, appeared to be very similar. Because it had been reported previously that reserpine affects the cellular GSH status by enhancing DA turnover (Spina and Cohen, 1989), we investigated, in addition, the effect of depletion of DA stores through inhibition of its synthesis. Although blockade of TH activity effectively lowered intracellular DA levels and, in contrast to reserpine, also reduced DA breakdown, again a significant increase in GSH content was detected that was of comparable magnitude to that observed with reserpine. Because inhibition of GSH formation did not prevent the observed effect, we concluded that the increased GSH level in the presence of reserpine was not attributable to enhanced synthesis but may be caused by a reduction in the use of GSH for cell function as a result of the emptying of the granular DA stores. This conclusion is supported moreover by our data showing that, after an increase of GSH content caused by depletion of stored DA with αMPT, direct refilling of the DA stores by incubation with exogenous DA immediately reduced the GSH level of the cells back to control values (i.e., GSH content of cells not treated with αMPT).
Although the described experiments demonstrated a relationship between the granular storage of DA and cellular GSH homeostasis, additional data were needed to establish whether GSH itself plays a role in the granular transport of DA. In central and peripheral neurons, released DA is recovered from the synapse via translocation into the cytoplasm by a Na+-dependent membrane transporter (Rudnick and Clark, 1993). Subsequently, the DA taken up is protected from metabolic breakdown by storage in so-called presynaptic vesicles by the above described mechanism. Adrenal medulla-derived cells such as the PC12 cell line are known to preferentially accumulate (radiolabeled) DA from the external medium in the chromaffin granules in a way similar to that of DAergic neurons (Greene and Rein, 1977; Rudnick and Clark, 1993). Our results show that depletion of GSH, by inhibition of its synthesis with the γ-glutamylcysteine synthetase inhibitor BSO (Meister, 1991), significantly reduces the Na+-dependent cellular uptake of radiolabeled DA in PC12 cells. In addition to DA (and NA), PC12 cells are known to transport (radiolabeled) choline, the precursor of ACh, across the plasma membrane using a transporter molecule (Melega and Howard, 1981). In our experiments, depletion of GSH had no effect on the uptake of radiolabeled choline. Thus, although our experimental setup does not allow us to differentiate between the transport of DA across the plasma membrane or the chromaffin granule membrane, in our opinion, by inference, it is more likely that the diminished uptake of DA reflects a lesion in the chromaffin granule membrane transport system rather than in its plasma membrane counterpart. Contrary to expectation, however, the supposed defect in DA storage led only to a small, although statistically significant, rise in the indices of DA metabolism, i.e., a decrease in the steady-state level of endogenous DA in combination with an increase in DA turnover. Although further investigation of this issue is needed, the apparent contradiction may be explained by the fact that, under our experimental conditions, the depletion of GSH over a 24 hr time period caused only a relatively small decrease in DA accumulation of which the effect on endogenous DA metabolism is still unknown.
Interestingly, the above described data are very similar to those obtained previously by Elroy-Stein and Groner (1988), who found a 50–80% reduction in the uptake of monoamines, including DA, but no change in the uptake of choline in transformed PC12 cells overexpressing the human Cu/Zn-superoxide dismutase gene. Using elegant techniques, they demonstrated that the impaired monoamine accumulation was caused by a diminished electrochemical gradient across the chromaffin granule membrane in the transformed clones. As mentioned already, the electrochemical gradient is the driving force for the transport of monoamines, such as DA, into the storage granules and is generated by a vacuolar-type, ATP-dependent H+ pump. This subclass of proton pumps, including the one present in the chromaffin granule membrane, is known to be extremely sensitive to sulfhydryl modification by oxidants such as H2O2 (Rudnick, 1986), against which it is protected by reductants like GSH (Dschida and Bowman, 1995). Thus, malfunction of the pump may be attributable either to increased production of ROS, such as occurs in cells with a high superoxide dismutase activity (Elroy-Stein and Groner, 1988;Schickler et al., 1989), or to decreased protection, for instance, after reduction of cellular GSH levels. Moreover, it is conceivable that oxidative damage to the proton pump also forms the basis of our previous observations demonstrating a preferential inhibition of the vesicular storage of DA in the nigro-striatal system of the rat brain by H2O2 (Langeveld et al., 1995). At present, experiments along the lines described by Elroy-Stein and Groner (1988)are being implemented in our laboratory to identify, possibly, a proton pump deficit as the cause of the diminished uptake of DA in GSH depleted PC12 cells.
In conclusion, our data, taken together, strongly suggest that GSH is involved in the uptake of DA in the chromaffin granules of PC12 cells. Although further research is clearly warranted and alternative explanations are available, considering the fact that DA is easily oxidized (Bindoli et al., 1992), it is tempting to propose a mechanism in which GSH is used to protect susceptible parts of the transport system against the potentially toxic interaction with DA oxidation products during DA uptake. Apparently, during this process GSH is transformed into a compound, for instance, a glutathionyl conjugate (Nappi and Vass, 1994), which escapes detection by our enzyme recycling method, which is rather specific for GSH and GSSG (Redegeld et al., 1988). Thus, a reduction in granular storage of DA, in our experiments induced by reserpine or αMPT, may lead to an increase in measurable GSH content. Conversely, a lowering of GSH content will leave the granular DA transport system relatively unprotected against oxidative damage, resulting in a diminished storage of the transmitter.
Notwithstanding the fact that differences in molecular structure and function of VMATs have been noted (Peter et al., 1994) and that, in general, data obtained in adrenal medulla-derived PC12 cells are not directly extrapolatable to DAergic neurons, concentration of DA in synaptic vesicles of neurons is thought to involve similar mechanisms as those active in chromaffin granules (Schuldiner, 1994). GSH is present in neurons (Pileblad et al., 1991), and in PD loss of GSH has been implicated as an early event in the pathogenesis of the disorder (Dexter et al., 1994). Looking at our results, it seems plausible to suggest that the consequent shift in the neuronal redox balance will initiate the self-sustaining cycle already described in the introductory remarks, in which a dysfunction in vesicular storage of DA, probably at the level of the proton pump, eventually leads to nigro-striatal cell death through massive oxidative stress. In this context, it is of interest to note that oxidative degeneration of DAergic neurons indeed has been observed after interference with the vesicular electrochemical gradient (Cubells et al., 1994).
Footnotes
We thank Dr. Robbert J. Slingerland (University of Amsterdam) for providing us with the PC12 cell line.
Correspondence should be addressed to Dr. Benjamin Drukarch, Research Institute Neurosciences Vrije Universiteit, Department of Neurology, Boechorststraat 7, 1081 BT Amsterdam, The Netherlands.
REFERENCES
- 1.Adams JD, Odunze IN. Oxygen free radicals and Parkinson’s disease. Free Radic Biol Med. 1991;10:161–169. doi: 10.1016/0891-5849(91)90009-r. [DOI] [PubMed] [Google Scholar]
- 2.Andersen PH. The dopamine uptake inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 3.Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- 4.Birks RI, MacIntosh FC. Acetylcholine metabolism at nerve-endings. Br Med Bull. 1957;13:157–161. doi: 10.1093/oxfordjournals.bmb.a069605. [DOI] [PubMed] [Google Scholar]
- 5.Bindoli A, Rigobello MP, Deeble DJ. Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic Biol Med. 1992;13:391–405. doi: 10.1016/0891-5849(92)90182-g. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson A. Drugs which block the storage of 5-hydroxytryptamine and related amines. Handb Exp Pharmacol. 1965;19:529–592. [Google Scholar]
- 8.Cubells JF, Rayport S, Rajendran G, Sulzer D. Metamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Daniel SE, Lees AJ, Schapira AHV, Jenner P, Marsden CD. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- 10.DiMonte DA, Chan P, Sandy MS. Glutathione in Parkinson’s disease: a link between oxidative stress and mitochondrial damage? Ann Neurol. 1992;32:S111–S115. doi: 10.1002/ana.410320719. [DOI] [PubMed] [Google Scholar]
- 11.Dschida WJA, Bowman BJ. The vacuolar ATPase: sulfite stabilization and the mechanism of nitrate inactivation. J Biol Chem. 1995;270:1557–1563. doi: 10.1074/jbc.270.4.1557. [DOI] [PubMed] [Google Scholar]
- 12.Elroy-Stein O, Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase: implication for gene dosage effects in Down’s syndrome. Cell. 1988;52:259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- 13.Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibb WRG, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psych. 1991;54:388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene LA, Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheochromocytoma cells. Brain Res. 1977;129:247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch EC, Brandel JP, Galle P, Javoy-Agid F. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: an X-ray microanalysis. J Neurochem. 1991;56:446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- 18.Iversen LL. Catecholamine uptake processes. Br Med Bull. 1973;29:130–135. doi: 10.1093/oxfordjournals.bmb.a070982. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P (1993) Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson’s disease. Acta Neurol Scand 87[Suppl 146]:6–13. [PubMed]
- 20.Johnson RG., Jr Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiol Rev. 1988;68:233–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- 21.Langeveld CH, Van Waas MP, Stoof JC, Sutanto W, De Kloet ER, Wolbers JG, Heimans JJ. Implication of glucocorticoid receptors in the stimulation of human glioma cell proliferation by dexamethasone. J Neurosci Res. 1992;31:524–531. doi: 10.1002/jnr.490310316. [DOI] [PubMed] [Google Scholar]
- 22.Langeveld CH, Schepens E, Stoof JC, Bast A, Drukarch B. Differential sensitivity to hydrogen peroxide of dopaminergic and noradrenergic neurotransmission in rat brain slices. Free Radic Biol Med. 1995;19:209–217. doi: 10.1016/0891-5849(95)00014-o. [DOI] [PubMed] [Google Scholar]
- 23.Maker HS, Weiss C, Silides DJ, Cohen G. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem. 1981;36:589–593. doi: 10.1111/j.1471-4159.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 24.Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal: applications in research and therapy. Pharmacol Ther. 1991;51:155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- 25.Meister A, Andersson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 26.Melega WP, Howard BD. Choline and acetylcholine metabolism in PC12 secretory cells. Biochemistry. 1981;20:4477–4483. doi: 10.1021/bi00518a036. [DOI] [PubMed] [Google Scholar]
- 27.Michel PP, Hefti F. Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res. 1990;26:428–435. doi: 10.1002/jnr.490260405. [DOI] [PubMed] [Google Scholar]
- 28.Nappi AJ, Vass E. The effects of glutathione and ascorbic acid on the oxidations of 6-hydroxydopa and 6-hydroxydopamine. Biochim Biophys Acta. 1994;1201:498–504. doi: 10.1016/0304-4165(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 29.Olanow CW. An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol. 1992;32:S2–S9. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- 30.Pan Z, Perez-Polo JR. Role of nerve growth factor in oxidant homeostasis: glutathione metabolism. J Neurochem. 1993;61:1713–1721. doi: 10.1111/j.1471-4159.1993.tb09808.x. [DOI] [PubMed] [Google Scholar]
- 31.Peter D, Jimenez J, Liu Y, Kim J, Edwards RH. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J Biol Chem. 1994;269:7231–7237. [PubMed] [Google Scholar]
- 32.Pileblad E, Eriksson PS, Hansson E. The presence of glutathione in primary neuronal and astroglial cultures from rat cerebral cortex and brain stem. J Neural Transm. 1991;86:43–49. doi: 10.1007/BF01250374. [DOI] [PubMed] [Google Scholar]
- 33.Redegeld FA, Van Opstal MA, Houdkamp E, Van Bennekom WP. Determination of glutathione in biological material by flow-injection analysis using an enzymatic recycling reaction. Anal Biochem. 1988;174:489–495. doi: 10.1016/0003-2697(88)90048-6. [DOI] [PubMed] [Google Scholar]
- 34.Riederer P, Sofic E, Rausch W-D, Schmidt B, Reynolds GP, Jellinger K, Youdim MBH. Transition metals, ferritin, glutathione, and ascorbic acid in Parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 35.Roda LG, Nolan JA, Seung UK, Hogue-Angeletti RA. Isolation and characterization of chromaffin granules from a pheochromocytoma (PC 12) cell line. Exp Cell Res. 1980;128:103–109. doi: 10.1016/0014-4827(80)90392-4. [DOI] [PubMed] [Google Scholar]
- 36.Rudnick G. ATP-driven H+ pumping into intracellular organelles. Annu Rev Physiol. 1986;48:403–413. doi: 10.1146/annurev.ph.48.030186.002155. [DOI] [PubMed] [Google Scholar]
- 37.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 38.Sampath D, Jackson GR, Werrbach-Perez K, Perez-Polo JR. Effects of nerve growth factor on glutathione peroxidase and catalase in PC12 cells. J Neurochem. 1994;62:2476–2479. doi: 10.1046/j.1471-4159.1994.62062476.x. [DOI] [PubMed] [Google Scholar]
- 39.Schickler M, Knobler H, Avraham KB, Elroy-Stein O, Groner Y. Diminished serotonin uptake in platelets of transgenic mice with increased Cu/Zn-superoxide dismutase activity. EMBO J. 1989;8:1385–1392. doi: 10.1002/j.1460-2075.1989.tb03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuldiner S. A molecular glimpse of vesicular monoamine transporters. J Neurochem. 1994;62:2067–2078. doi: 10.1046/j.1471-4159.1994.62062067.x. [DOI] [PubMed] [Google Scholar]
- 41.Schuldiner S, Liu Y, Edwards RH. Reserpine binding to a vesicular amine transporter expressed in Chinese hamster ovary fibroblasts. J Biol Chem. 1993;268:29–34. [PubMed] [Google Scholar]
- 42.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 43.Spector S, Sjoerdsma A, Udenfriend S. Blockade of endogenous norepinephrine synthesis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther. 1965;147:86–95. [PubMed] [Google Scholar]
- 44.Spina MB, Cohen G. Exposure of school synaptosomes tol-dopa increases levels of oxidized glutathione. J Pharmacol Exp Ther. 1988;247:502–507. [PubMed] [Google Scholar]
- 45.Spina MB, Cohen G. Dopamine turnover and glutathione oxidation: implications for Parkinson’s disease. Proc Natl Acad Sci USA. 1989;86:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tietze F. Enzymatic method for quantification of total and oxidized glutathione: applications to mammalian blood and other tissue. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 47.Van Muiswinkel FL, Jongenelen CAM, Schepens HTWJ, Stoof JC, Drukarch B. Effects of chronic activation of dopamine D-2 receptors in cultures of rat fetal dopaminergic neurons: indications for alterations in functional activity. Dev Brain Res. 1995;85:128–136. doi: 10.1016/0165-3806(94)00207-g. [DOI] [PubMed] [Google Scholar]
- 48.Westerink BHC, Mulder TBA. Determination of picomole amounts of dopamine, noradrenaline, 3,4-dihydroxyphenylalanine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5- hydroxyindoleacetic acid in nervous tissue after one-step purification on Sephadex G-10, using high-performance liquid chromatography with a novel type of electrochemical detection. J Neurochem. 1981;36:1449–1462. doi: 10.1111/j.1471-4159.1981.tb00586.x. [DOI] [PubMed] [Google Scholar]