Abstract
The mesolimbic dopaminergic system plays a primary role in mediating the euphoric and rewarding effects of most abused drugs. Chronic cocaine use is associated with an increase in dopamine neurotransmission resulting from the blockade of dopamine uptake and is mediated by the activation of dopamine receptors. Recent studies have suggested that the D3 receptor subtype plays a pivotal role in the reinforcing effects of cocaine. The D3receptor-preferring agonist 7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT) is a reinforcer in rhesus monkeys trained to self-administer cocaine, but not in cocaine-naive monkeys. In vitro autoradiographic localization of [3H]-(+)-7-OH-DPAT binding in the human brain demonstrated that D3 receptors were prevalent and highly localized over the ventromedial sectors of the striatum. Pharmacological characterization of [3H]-(+)-7-OH-DPAT binding to the human nucleus accumbens demonstrated a rank order of potency similar to that observed for binding to the cloned D3 receptor expressed in transfected cell lines. Region-of-interest analysis of [3H]-(+)-7-OH-DPAT binding to the D3 receptor demonstrated a one- to threefold elevation in the number of binding sites over particular sectors of the striatum and substantia nigra in cocaine overdose victims as compared with age-matched and drug-free control subjects. The elevated number of [3H]-(+)-7-OH-DPAT binding sites demonstrates that adaptive changes in the D3 receptor in the reward circuitry of the brain are associated with chronic cocaine abuse. These results suggest that the D3 receptor may be a useful target for drug development of anti-cocaine medications.
Keywords: cocaine, human, brain, D3 receptor, (+)-7-OH-DPAT, density
The reinforcing effects of cocaine are mediated by the potentiation of dopamine (DA) neurotransmission. Cocaine binds to the presynaptic DA transporter and inhibits the reuptake of released DA (Ritz et al., 1987; Reith et al., 1989; Kuhar et al., 1991). Increased intrasynaptic DA interacts with pre- and postsynaptic DA receptors to initiate a sequence of events that mediate the reinforcing effects of cocaine (Koob and Bloom, 1988; Kuhar et al., 1991; Pulvirenti and Koob, 1994). DAergic signaling is mediated by five receptor subtypes distinguished by their unique molecular and pharmacological properties and distinct anatomical locations. Interest has focused on the D3 receptor because of the association of this DA receptor subtype with the mesolimbic reward circuits in brain.
The DAergic system in the nucleus accumbens is a neuroanatomical substrate for cocaine reinforcement (Koob and Bloom, 1988; Robledo et al., 1992). In the human brain, D3 receptor mRNA and binding sites are prevalent throughout the ventral and medial sectors of the striatum (Landwehrmeyer et al., 1993b; Murray et al., 1994). The human D3 receptor cDNA encodes a protein with 400 amino acids, which shares 46% homology overall and 78% homology within the transmembrane (TM) domains of the human D2 receptor (Giros et al., 1990; Sokoloff et al., 1990, 1992a,c). The D3receptor gene is structurally complex, with the coding sequence interrupted by introns that may be spliced alternatively to generate receptor isoforms (Giros et al., 1990, 1991; Sokoloff et al., 1992a). Three variants of the human D3 receptor have been identified to date, including the D3(TM3-del) (Snyder et al., 1991), D3(TM4-del) (Nagai et al., 1993), and D3nf (Liu et al., 1994). Although the functional significance of less abundant and shorter mRNA species is still unclear, one possibility is that atypical regulatory processing of the mRNA in response to chronic agonist or antagonist stimulation may lead to the expression of truncated D3 receptor proteins (Liu et al., 1994).
Although the precise contribution of each of the DA receptor subtypes to the behavioral effects of cocaine is not understood fully, recent studies suggest that there are a number of promising DAergic subtype-selective agents that deserve further evaluation as potential therapies for cocaine abuse (Robledo et al., 1992; Pulvirenti and Koob, 1994; Roberts and Ranaldi, 1995). Studies by Caine and Koob (1993,1995) have suggested that the D3 receptor may be a primary mediator of the reinforcing effects of cocaine. D3receptor-preferring agonists, although not self-administered by drug-naive monkeys, are reinforcing in monkeys that have been trained to self-administer cocaine (Nader and Mach, 1996). D1 (SKF 81297) and putative D3 (7-OH DPAT) agonists exert qualitatively different aspects of the reinforcing stimulus produced by cocaine (Self et al., 1996), and the D3 antagonist (DS 121) attenuates the motivation to self-administer cocaine in rats (Roberts and Ranaldi, 1995). Chronic exposure to cocaine leads to regulatory adaptations in the regional complement of specific DA receptor subtypes, which in turn may affect the expression of the reinforcing effects of cocaine. In the present study, we used [3H]-(+)-7-OH-DPAT in vitro for ligand binding and autoradiographic mapping to investigate the regulatory effects of cocaine on the D3 receptor in human brain.
MATERIALS AND METHODS
Materials. [3H]-(+)-7-OH DPAT was purchased from Amersham (Arlington Heights, IL). All unlabeled drugs were obtained from Research Biochemicals (Natick, MA), with the exception of the pentazocine isomers that were supplied by the National Institute on Drug Abuse Drug Supply (Rockville, MD), and (+) AJ76, which was generously supplied by Upjohn (Kalamazoo, MI). Tritium standards and Hyperfilm for autoradiographic studies were purchased from Amersham.
Neuropathological tissue specimens. Postmortem neuropathological specimens were obtained at autopsy from age-matched and drug-free control subjects (n = 9; mean age = 30.0 ± 2.8 years; mean autolysis = 15.0 ± 1.6 hr), cocaine overdose (CO) victims (n = 6; mean age = 32.2 ± 2.2 years; mean autolysis = 18.5 ± 2.4 hr), and excited delirium (ED) victims (n = 6; mean age = 32.3 ± 2.3 years; mean autolysis = 11.2 ± 1.1 hr). Medicolegal investigations of the deaths were conducted by forensic pathologists who evaluated the scene environment and circumstances of death, performed autopsies on the victims, and determined the cause and manner of death (Mittelman and Wetli, 1984). The circumstances of death and toxicology data were reviewed carefully before a death was classified as a CO (Escobedo et al., 1991; Wetli et al., 1996). In a similar manner, controls were selected from those whose deaths were not caused by cocaine, with no cocaine or metabolites detected in toxicology screens of blood or brain tissues. All cases were evaluated for common drugs of abuse and alcohol, and positive urine screens were confirmed by quantitative analysis of blood. Alcohol was detected in two of the control subjects (0.01–0.05%) and in one of the CO victims (0.05%). The cocaine toxicity cases selected for the present study had evidence of a number of different variables of chronic cocaine use, determined on the basis of review of the previous arrest records and treatment admissions as well as on pathological signs (i.e., perforation of the nasal septum). Blood cocaine was quantified using gas–liquid chromatography with a nitrogen detector. Frozen brain regions were sampled for quantitation of cocaine and benzoylecgonine using gas chromatography/mass spectroscopy techniques (Hernandez et al., 1994). Neuropathological analysis was carried out to verify the absence of any gross or histopathological abnormalities.
Ligand binding assays. Putative D3 receptors were labeled using the procedure described by Burris et al. (1994), with some minor modifications. Briefly, tissue punches from the nucleus accumbens were weighed, homogenized in (1:20, w/v) ice-cold 10 mm Tris-HCl buffer, pH 7.4, 5 mm EDTA, and centrifuged for 20 min at 32,000 × g. Membranes were washed once in Tris-HCl buffer, pH 7.7, 1.0 mm EDTA, and resuspended in assay buffer that contained 50 mm Tris, pH 7.7, 2 mm MgCl2, and 50 mm NaCl. The guanine nucleotide GTP (300 μm) was included in the assay tubes to enhance the selectivity of [3H]-(+)-7-OH-DPAT binding to D3 receptors over D2 receptors. For saturation binding, increasing concentrations of [3H]-(+)-7-OH-DPAT were incubated with nucleus accumbens membranes (5 mg tissue original wet weight) in the presence and absence of 10 μm (+)-butaclamol for 2 hr at 25°C. Competition binding assays were conducted with various concentrations of competitor incubated in the presence of 1 nm [3H]-(+)-7-OH-DPAT for 2 hr at 25°C. The binding reaction was terminated by dilution with 4 ml of ice-cold 50 mm Tris-HCl, pH 7.7, and bound radioligand was separated from free radioligand by vacuum filtration through 934AH filters presoaked in 0.1% polyethyleneimine. Filters were washed three times with 4 ml of ice-cold buffer and counted by a Beckman Scintillation counter at 50% efficiency.
In vitro autoradiography. Half-hemisphere coronal sections of the human brain were cut on a Hacker/Bright sledge microtome cryostat at 50 μm, thaw-mounted on gelatin-coated slides, and dried under reduced pressure at 4°C. Adjacent sections were stained with Nissl substance and acetylcholinesterase for cytoarchitecture. For D3 receptor autoradiography, slide-mounted tissue sections were incubated with 1 nm[3H]-(+)-7-OH-DPAT in the presence of 300 μm GTP for 2 hr at 25°C. Nonspecific binding was determined in the presence of 10 μm (+)-butaclamol. At the end of the incubation, tissue sections were washed in two changes of ice-cold assay buffer followed by a quick rinse in ice-cold distilled water to dissociate nonspecifically bound ligand. Tissue sections were dried under a stream of cool air and apposed with tritium standards to Hyperfilm for 7–8 weeks at 4°C.
Data analysis. For analysis of binding data, equilibrium binding constants were determined from the saturation binding data using the iterative, nonlinear curve-fitting program EBDA/LIGAND, (Biosoft, Elsevier). The best fit to a one- or two-site model was based on the partial F-test. The rank order of potency for [3H]-(+)-7-OH-DPAT binding was determined by competition binding analysis; IC50 and Ki values were determined using DRUG (EBDA) and LIGAND, respectively. Differences in D3 receptor densities between control subject and experimental groups were analyzed for statistical significance by the Student’s t test.
For analysis of D3 receptor autoradiography, films were scanned using a Howtek Scanmaster 3 at 400 dots per inch using a transparency illuminator. The resulting tagged image file format for RGB color files were converted to pseudocolor format in specific activity units using the IMAGE (version 1.44; National Institutes of Health Shareware) and BRAIN (version 1.6; Drexel University) programs. After background subtraction, two-dimensional pseudocolor maps were created to allow radioactivity levels (in fmol/mg) to be superimposed on the sections (Kuhar et al., 1986).
RESULTS
Characteristics of fatal CO victims
CO deaths are defined as deaths that were investigated and on the basis of medical judgment were attributed to the toxic effects of cocaine alone or in combination with alcohol. Cocaine fatalities were identified and classified as part of an ongoing case-control study of the toxicology reports, scene descriptions, supplemental background information, and autopsy findings (Escobedo et al., 1991). On the basis of this analysis, CO cases demonstrating evidence of significant underlying cardiac pathology, cerebrovascular disorders, or polydrug abuse were eliminated from the study. CO deaths presenting with preterminal ED have been included in this study as a comparison group. This syndrome is composed of four components that appear in sequence: hyperthermia, delirium with agitation, respiratory arrest, and death (Wetli and Fishbain, 1985; Wetli et al., 1996).
The concentration of cocaine and its principal metabolite benzoylecgonine (BE) were measured in blood and brain samples obtained at autopsy. All cocaine fatalities had quantifiable levels of cocaine in blood and brain. The average (mean ± SEM) blood levels of cocaine and BE were 9.2 ± 3.8 and 8.1 ± 2.2 mg/l in the CO victims. The ED victims exhibited 10-fold lower levels of cocaine (0.6 ± 0.2 mg/l) and 4-fold lower levels of BE (1.9 ± 0.5 mg/l) in blood. The levels of cocaine and BE were measured also in brain tissue specimens from occipital cortex (Brodman’s areas 17 and 18). The mean cocaine and BE levels in the CO group were 12.8 ± 4.0 and 3.8 ± 1.3 mg/kg tissue, respectively. Similar to the observations in blood samples, the ED victims exhibited ∼10-fold lower levels of cocaine (1.2 ± 0.3 mg/kg) and twofold lower levels of BE (1.9 ± 0.5 mg/kg) in brain. Elevated body temperatures were recorded for five of the fatal ED victims (range, 101.7–110.0°C; mean ± SEM, 104.4 ± 1.5°C).
Visualization of D3 receptor distribution in human brain
Pharmacological studies with [3H]-(+)-7-OH-DPAT demonstrate that it has a 100-fold higher affinity for binding to the cloned D3 receptor as compared with the cloned D2 receptor expressed in transfected cell lines. Binding studies conducted in brain in regions enriched in the native D2 and D3 receptors have indicated that [3H]-(+)-7-OH-DPAT demonstrates selectivity for the D3 subtype when the receptors are dissociated from their respective G-proteins (Large and Stubbs, 1994). This selectivity profile (D3 > D2) is not seen in the absence of guanine nucleotides, because the high-affinity G-protein-coupled state of the D2 receptor is left-shifted and overlaps in binding affinity with that of the D3 receptor. Because guanine nucleotides have a minimal effect on agonist binding to the D3 receptor (twofold right-shift), but markedly decrease agonist binding to the D2 receptor (100-fold right-shift), it is possible to achieve selective labeling of the D3receptor in the presence of GTP (Burris et al., 1994). Under these assay conditions, [3H]-(+)-7-OH-DPAT labels a single population of binding sites in the human nucleus accumbens with an affinity value (KD) of 1.3 ± 0.3 nm (mean ± SEM) and a density (Bmax) of 2.8 ± 0.3 pmol/gm tissue original wet weight. The pharmacological profile for binding of [3H]-(+)-7-OH-DPAT to the D3 receptor in human nucleus accumbens is shown in Table 1. The putative D3 agonists (+)-7-OH-DPAT and PD128907 demonstrated the highest potencies for inhibition of [3H]-(+)-7-OH-DPAT binding. Quinpirole and DA exhibited twofold lower potency as compared with (+)-7-OH-DPAT and PD128907. The D2 receptor antagonists (−)-eticlopride and spiperone were the most potent inhibitors of [3H]-(+)-7-OH-DPAT binding. Pimozide, raclopride, (+)-butaclamol, and domperidone had lower potencies, whereas (+)-AJ 76 and clozapine were the least potent of the dopamine antagonists. The isomers of the ς-active drug pentazocine inhibited binding of [3H]-(+)-7-OH-DPAT with micromolar potencies. The overall rank order of inhibition of [3H]-(+)-7-OH-DPAT binding observed in human nucleus accumbens correlated significantly with the Kivalues reported previously for the cloned D3 receptor (r = 0.98; p < 0.001; Table 1).
Table 1.
Pharmacological profile of [3H]-(+)-7-OH-DPAT binding to the D3 receptor
| Competitor | Human nucleus accumbens | Cloned D3receptor | |
|---|---|---|---|
| nH | KI, (nm) | KI, (nm) | |
| Dopamine agonists | |||
| (+)-7-OH DPAT | 0.67 | 3.1 ± 1.1 | 0.8 ± 0.11_a |
| PD 128907 | 0.70 | 6.5 ± 0.2 | |
| (−) Quinpirole | 0.68 | 12.8 ± 0.9 | 3.7 ± 0.21_a |
| 5.1 ± 0.31_b | |||
| 29.4 ± 4.01_c | |||
| Dopamine | 0.84 | 15.6 ± 2.3 | 43.9 ± 4.41_a |
| 25.0 ± 3.01_b | |||
| Dopamine antagonists | |||
| (−) Eticlopride | 0.95 | 0.1 ± 0.03 | 0.2 ± 0.021_d |
| Spiperone | 0.72 | 0.3 ± 0.07 | 0.3 ± 0.041_d |
| 0.6 ± 0.051_b | |||
| Pimozide | 0.84 | 2.5 ± 1.5 | 3.7 ± 0.51_b |
| Raclopride | 0.78 | 2.9 ± 0.8 | 3.5 ± 0.31_b |
| (+) Butaclamol | 0.81 | 3.7 ± 0.6 | 11.2 ± 0.81_d |
| 4.1 ± 1.21_c | |||
| Domperidone | 0.77 | 8.5 ± 0.4 | 9.5 ± 0.51_b |
| (+) AJ76 | 0.77 | 94.4 ± 0.5 | |
| Clozapine | 0.85 | 304.6 ± 8.5 | 389.0 ± 31.01_a |
| 480.0 ± 47.41_c | |||
| 180.0 ± 17.01_b | |||
| (+) Pentazocine | 1.00 | 1144.7 ± 341.3 | |
| (−) Pentazocine | 0.82 | 3646.4 ± 443.5 | |
The potency values for inhibition of [3H]-(+)-7-OH DPAT (1 nm) binding to nucleus accumbens membranes are shown.
D3-HEK293 cells (Burris et al., 1994).
D3-CHO cells (Sokoloff et al., 1990, 1992d).
D3MN9D cells (MacKenzie et al., 1994).
D3-CCL1.3 (MacKenzie et al., 1994).
The regional distribution of the D3 receptor was mapped in half-hemisphere coronal sections of the human brain. In vitro autoradiographic localization of [3H]-(+)-7-OH-DPAT binding demonstrated high densities of D3 receptors in the nucleus accumbens and the ventromedial sectors of the striatum (Fig. 1). Moderate densities were observed in the dorsal sectors of the anterior caudate and putamen. Low levels of labeling were apparent also in the hypothalamus, reticular thalamus, and substantia nigra. Low levels of labeling were observed in the entorhinal and cingulate gyri and over the frontal and parietal lobes.
Fig. 1.
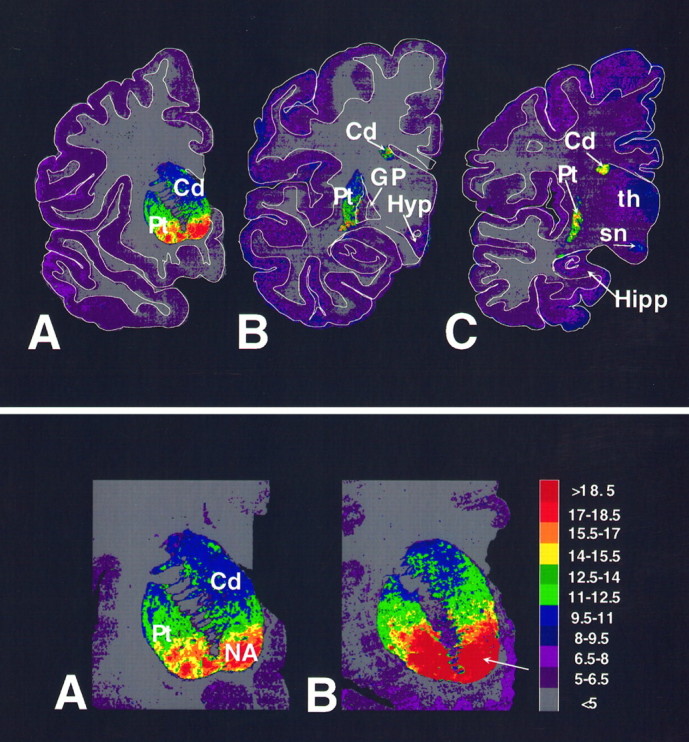
Top. Autoradiographic localization of [3H]-(+)-7-OH-DPAT binding in representative half-hemisphere coronal sections of human brain. Computer-generated color coding of the autoradiograms from a series of half-hemisphere coronal sections of the human brain at three different anterior to posterior levels through the striatum is shown. The pseudocolor codes represent a rainbow scale (red = high densities;yellow to green = intermediate densities; blue to purple= low densities). High densities of the D3 receptor were observed in the ventral sectors of the striatum, with the most prevalent labeling in the nucleus accumbens. Moderate labeling was seen in the substantia nigra. Cd, Caudate; GP, globus pallidus; Hipp, hippocampus; Hyp, hypothalamus; Pt, putamen; sn, substantia nigra; th, thalamus.
Regulation of the D3 receptor by cocaine
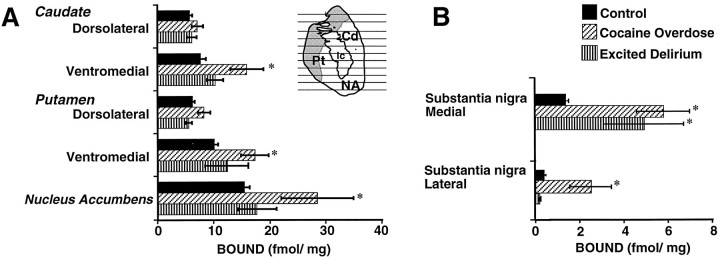
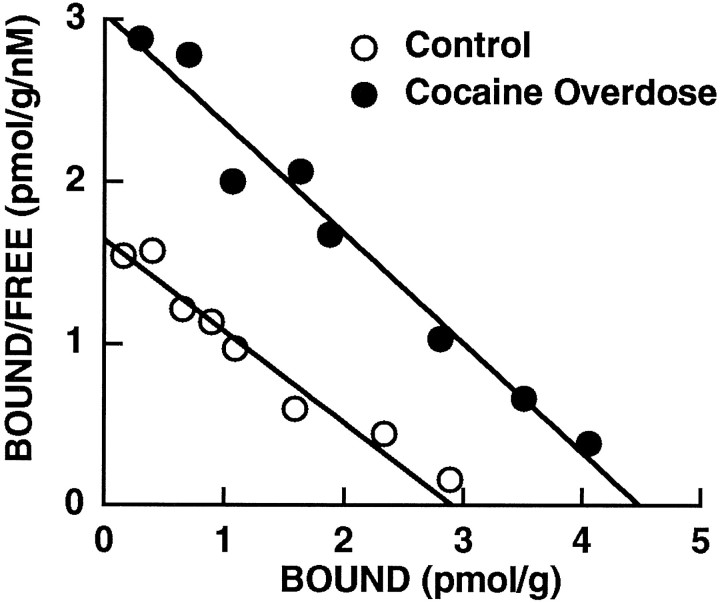
Quantitative in vitro autoradiography was used to map and quantify D3 receptor densities in human CO victims. Binding of [3H]-(+)-7-OH-DPAT was elevated approximately twofold in the ventromedial sectors of the anterior caudate and putamen and in the nucleus accumbens of the CO victims as compared with drug-free and age-matched control subjects (Fig.2). The intensity of [3H]-(+)-7-OH-DPAT labeling was increased also in the lateral and medial divisions of the substantia nigra in the CO victims. Binding of [3H]-(+)-7-OH-DPAT was not elevated significantly in the anterior ventral striatum of the ED subgroup; however, preliminary studies indicate a two- to threefold elevation in receptor densities over the cell body fields in the medial division of the substantia nigra. Quantitative densitometric measurements of [3H]-(+)-7-OH-DPAT binding revealed significant elevations in the densities of D3 receptors throughout the mesolimbic sectors of the striatum in the CO victims as compared with the drug-free and age-matched control subjects (Fig. 3; Student’s t test; p < 0.05). These findings were confirmed further by saturation analysis of [3H]-(+)-7-OH-DPAT binding in membrane homogenates from the nucleus accumbens. The affinity for [3H]-(+)-7-OH-DPAT binding was not different in the CO victims (KD = 1.5 ± 0.2 nm;n = 6) or the ED victims (1.7 ± 0.4 nm; n = 6) as compared with drug-free control subjects (KD = 1.3 ± 0.3 nm; n = 5). Figure 4illustrates the lack of a change in the affinity for [3H]-(+)-7-OH-DPAT binding to the D3 receptor in the nucleus accumbens of a representative CO victim as compared with a representative drug-free and age-matched control subject. The saturation binding density for the CO victims (4.6 ± 0.4 pmol/gm) when compared with the drug-free control subjects (2.8 ± 0.3 pmol/gm) was significantly elevated (Student’s t test;p < 0.001). The density for [3H]-(+)-7-OH-DPAT binding to the nucleus accumbens in the ED victims was not significantly different (4.0 ± 1.0 pmol/gm) from control values.
Fig. 2.
Bottom. Pseudocolor density maps of [3H]-(+)-7-OH-DPAT binding to the D3 receptor in the anterior striatum of (A) a representative drug-free control subject and (B) a representative CO victim. Note the significant increase in the density of the D3 receptors throughout the mesolimbic sectors of the striatum. The color bar at the rightdepicts the density of radioligand binding sites in fmol/mg tissue equivalence units.
Fig. 3.
Summary of the region-of-interest densitometric measurements of [3H]-(+)-7-OH-DPAT binding in the dopaminergic (A) terminal regions and (B) cell body fields from Control subjects (n = 9), Cocaine Overdose deaths (n = 6), and Excited Deliriumvictims (n = 6). The density of the D3receptor was determined in the substantia nigra and throughout the striatum using [3H]-(+)-7-OH-DPAT. The quantitative densitometric measurements demonstrate elevated D3 receptor densities in the ventral sectors of the striatum, including the nucleus accumbens, and in the lateral and medial sectors of the substantia nigra of the CO deaths. Black bars represent values for drug-free and age-matched controls; stripped bars, CO deaths; stippled bars, ED subgroup. Significant differences from control values, *p < 0.05. Cd, Caudate; ic, internal capsule;Pt, putamen; NA, nucleus accumbens.
Fig. 4.
Rosenthal plots of [3H]-(+)-7-OH-DPAT binding to nucleus accumbens in a representative control subject and a CO victim. This figure illustrates that there was no change in the affinity for [3H]-(+)-7-OH-DPAT binding to the D3receptor, but an increase in the density of sites in the CO victim as compared with a representative age-matched and drug-free control subject.
DISCUSSION
We have investigated the effect of cocaine exposure on the affinity and number of D3 receptors in human brain. The regulatory profile shown here provides additional support for a role of the D3 receptor in the modulation of addictive behaviors, including cocaine dependence. In human CO victims, D3receptor number was increased as compared with drug-free and age-matched control subjects in the nucleus accumbens, mesolimbic sectors of the caudate and putamen, and substantia nigra. These findings suggest that cocaine use may lead to an adaptive elevation in D3 receptor density in response to elevated synaptic DA levels.
Pharmacological signature and anatomical locations of the D3 receptor in human brain
The specificity of [3H]-(+)-7-OH-DPAT labeling in human brain was confirmed by saturation analysis and competition binding assays. Saturation analysis revealed a single high-affinity binding site with a KD value comparable to that observed for the cloned D3 receptor (Levesque et al., 1992;Chio et al., 1994; MacKenzie et al., 1994; Pilon et al., 1994). Competition binding assays demonstrated a rank order of potency [(−)-eticlopride ≥ spiperone > pimozide = raclopride = (+)-7-OH-DPAT = (+)-butaclamol ≥ PD 128907 ≥ domperidone ≥ (−)-quinpirole = dopamine > (+)-AJ 76 > clozapine] similar to the cloned D3receptor (Sokoloff et al., 1990, 1992c,d; Burris et al., 1994; Mackenzie et al., 1994). Previous studies have suggested that 7-OH-DPAT may bind also to ς receptors (Wallace and Booze, 1995); however, the ς isomers (+)- and (−)-pentazocine demonstrated low micromolar potency for inhibition of [3H]-(+)-7-OH-DPAT binding. Taken together, these studies confirm that [3H]-(+)-7-OH-DPAT binding in human brain demonstrates a pharmacological signature characteristic of the D3receptor.
DAergic competitors exhibited Hill coefficients (nH) < 1, suggesting negative cooperativity, the recognition of multiple affinity states, or distinct receptor subtypes. Both agonists and antagonists had lownH values, and GTP was present in all assays, indicating that the binding was not to high- and low-affinity “states” of a single receptor subtype; however, 7-OH DPAT and its tetralin derivative 7-OH-PIPAT also bind to D2 and 5-HT1A receptors (Burris et al., 1994). The lownH values may represent a minor labeling component attributable to the recognition by the radioligand of the uncoupled states of the D2 and 5-HT1Areceptors. Neither of these explanations are likely, because the relative occupancies by the radioligand would be negligible for these G-protein-coupled receptors stabilized in their low-affinity conformations by sodium ion and guanine nucleotides. Alternatively, the low nH values may represent binding to different D3 receptor isoforms. The binding affinity of DAergic ligands may vary among the D3 receptor isoforms. D3(TM3-del) and D3(TM4-del), which encode truncated D3 receptors, do not retain sufficient tertiary structure to bind DAergic ligands (Snyder et al., 1991; Nagai et al., 1993). The D3nf receptor, however, which has a frameshift deletion in the coding region of the i3 loop, may bind DAergic ligands. A purported D3 receptor isoform, equivalent to the rat and mouse D3L receptor, may exist also in human brain (Park et al., 1995). Heterogeneity in the regulatory processing of the D3 core protein may lead to alterations in the recognition domain for the radioligand, providing an alternative explanation for the low nH values.
Autoradiographic localization of [3H]-(+)-7-OH-DPAT binding demonstrated that D3 receptors were prevalent over the limbic sectors of the human striatum. The present findings confirm and extend previous studies that have demonstrated enriched densities of D3 receptors in human and rat ventral striatum using the D2/D3 sensitive radioligands [125I]iodosulpiride, [3H]CV 205 502, and [125I]epidepride (Landwehrmeyer et al., 1993a,b; Parsons et al., 1993; Hillefors-Berglund and Von Euler, 1994; Murray et al., 1994; Booze and Wallace, 1995). Overall, D3 mRNA expression closely correlates with the localization of D3 binding sites in human brain (Landwehrmeyer et al., 1993b). The unique anatomical localization of the D3 receptor shown here in human brain is in agreement with a previous study (Murray et al., 1994) and provides additional support for a role for the human D3receptor in substance abuse.
Adaptive increase in D3 receptor density by cocaine
Quantitative in vitro autoradiography demonstrated a marked elevation in D3 receptor number in CO victims as compared with drug-free and age-matched control subjects. Although a marked elevation in D3 receptor density was observed in all CO victims, the density was not increased reliably in every subject included in the ED subgroup. The reason for heterogeneity within this subgroup of cocaine fatalities is not fully understood, although it may be related to previous history and pattern of cocaine use. Recent and repeated use of cocaine may be necessary to elevate D3receptor density. Alternatively, differences in the molecular processing of D3 receptors attributable to defects in alternatively spliced transcripts might explain the lack of an increase in the D3 binding sites in the certain ED victims. It is interesting to note that a different mRNA species has been found in the cortices of chronic schizophrenic patients (Schmauss et al., 1993), suggesting the possibility that similar alterations in D3receptor expression may be involved in the psychopathology of the cocaine delirium syndrome.
Because cocaine does not interact with the D3 receptor, the changes in D3 receptor number must be a secondary response to the interaction of cocaine with the DA transporter. Chronic high DA levels that result from the binge use of cocaine may lead to an adaptive increase in D3 receptor number. Chronic treatment of C6 glioma cells transfected with the D3 receptor cDNA with DA results in an elevation in D3 receptor number (Cox et al., 1995). These findings suggest that the adaptive increase in D3 receptor density observed over the mesolimbic sectors of the striatum in cocaine fatalities may be regulated by synaptic levels of DA. Recent studies by Meador-Woodruff and colleagues (1995) demonstrated no change in D3 mRNA in human cocaine abusers. These findings suggest that D3 mRNA and binding sites may be differentially regulated by cocaine exposure. Chronic treatment of C6 glioma cells transfected with D3 cDNA with DA agonists demonstrated no change in D3 mRNA abundance, although the receptor number was increased (Cox et al., 1995). The elevation in D3 receptor density was blocked by treatment with cycloheximide in this study. These observations suggest that the adaptive increase in the D3 receptor density observed in the present study reflects an increase in receptor protein synthesis. Alternatively, elevated [3H]-(+)-7-OH DPAT binding may reflect a selective increase in one of the D3 receptor isoforms. The abundance of different mRNA splice variants may not be discerned by in situ hybridization. D3-specific probes may hybridize to all of the alternative splice variants, including the truncated D3 receptors (Fishburn et al., 1993). Because DAergic ligands may or may not bind to the proteins generated from the truncated splice variants, a dissociation between mRNA levels and binding sites may be observed. In keeping with this suggestion, the abundance of D2s receptor isoform is altered after interruption of DA transmission (Martres et al., 1992). An elevation in D2 receptor density, but not D2mRNA levels, was observed after chronic treatment with haloperidol. Quantitation of the D2 receptor splice variants by PCR methods revealed an increase in D2s mRNA. Additional studies are needed to determine whether this regulatory pattern for the D3 receptor occurs in the human brain.
The role of the D2 and D3 receptors in cocaine dependence
The advent of subtype-selective ligands for the members of the D1 and D2 receptor families has made it possible to begin to discern the specific role of the DA receptor subtypes in cocaine dependence (Roberts and Ranaldi, 1995; Self et al., 1996). The elevation in D3 receptor densities observed in the present study contrasts with our previous observations in human brain postmortem, which showed no change in D2 receptor densities measured with [3H]raclopride in CO victims (Staley et al., 1995). The effects of cocaine exposure on the D3 receptor has not been studied in animals. Regulatory alterations in D2-like binding sites after chronic cocaine treatment have been shown using radioligands that did not discriminate between the D2 and D3 receptor subtypes. Administration of cocaine in a binge-like regimen showed a transient increase in the binding of [3H]raclopride in the olfactory tubercle, nucleus accumbens, and caudate–putamen (Unterwald et al., 1994). Elevations in radiolabeled spiperone binding was observed in the nucleus accumbens, olfactory tubercle, and substantia nigra after chronic administration of cocaine (Goeders and Kuhar, 1987;Kleven et al., 1990; Peris et al., 1990; Ziegler et al., 1991). The lack of selectivity of the radioligands and the observed elevations in regions rich in D3 receptors, suggests that it is the D3 receptor and not the D2 receptor that is upregulated after chronic cocaine exposure.
Implications for cocaine dependence
The neuroadaptations of the D3 receptor that result from repeated activation of DA transmission attributable to chronic binge use of cocaine may contribute to the development of cocaine dependence. Putative D3 receptor agonists decrease cocaine self-administration in rats (Caine and Koob, 1993, 1995) and monkeys (Nader and Mach, 1996). D3 receptor agonists substitute for the discriminative stimulus effects of cocaine and produce place preference, indicating that the D3 receptor may mediate some of the subjective effects of cocaine (Mallet and Beninger, 1994;Acri et al., 1995). Furthermore, 7-OH-DPAT functions as a reinforcer in monkeys trained to self-administer cocaine, but not in cocaine-naive monkeys (Nader and Mach, 1996). The behavioral studies are confounded by the lack of purported selectivity of 7–OH-DPAT for D3(or D2-like) receptors in vivo (Large and Stubbs, 1994; Self et al., 1996). Because selective labeling of the D3 receptor can be achieved in vitro, it may be suggested that our demonstration of an adaptive increase in human D3 receptor densities by cocaine exposure may link this DA receptor subtype to the reinforcing effects of cocaine and the development of cocaine dependence.
The search for pharmacotherapies for cocaine addiction has focused primarily on drugs that target DAergic synapses. Both DA agonists and antagonists have failed to demonstrate therapeutic efficacy in cocaine dependence (Roberts and Ranaldi, 1995). Although DA agonists reduce craving, they may be reinforcing, and although DA antagonists attenuate reinforcement, compliance is hindered by dysphoria and extrapyramidal side effects. The close association of the D3 receptor with mesolimbic DAergic circuits suggests that partial blockade of the D3 receptor may selectively decrease the rewarding effects of cocaine without contributing to the dysphoria associated with cocaine withdrawal.
Footnotes
This study was supported by the National Institute on Drug Abuse (DA06227). We acknowledge the expert technical assistance of Margaret Basile and Qinjie Ouyang.
Correspondence should be addressed to Deborah C. Mash, Ph.D., Department of Neurology (D4-5), University of Miami School of Medicine, P.O. Box 016960, Miami, FL 33101.
REFERENCES
- 1.Acri JB, Carter SR, Alling K, Geter-Douglas B, Dijkstra D, Wikstrom H, Katz JL, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol. 1995;281:R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- 2.Booze RM, Wallace DR. Dopamine D2 and D3 receptors in rat striatum and nucleus accumbens: use of 7-OH-DPAT and [125I]-iodosulpiride. Synapse. 1995;19:1–13. doi: 10.1002/syn.890190102. [DOI] [PubMed] [Google Scholar]
- 3.Burris KD, Filtz TM, Chumpradit S, Kung M-P, Foulon C, Hensler JG, Kung HF, Molinoff PB. Characterization of [125I](R)-trans -7-hydroxy-2-[ N -propyl- N -(3′-iodo-2′-propenyl)amino]tetralin binding to dopamine D3 receptors in rat olfactory tubercle. J Pharmacol Exp Ther. 1994;268:935–942. [PubMed] [Google Scholar]
- 4.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D3 dopamine receptors. Science. 1993;260:1814–1815. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 5.Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose–effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- 6.Chio CL, Lajiness ME, Huff RM. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- 7.Cox BA, Rosser MP, Kozlowski MR, Duwe KM, Neve RL, Neve KA. Regulation and functional characterization of a rat recombinant dopamine D3 receptor. Synapse. 1995;21:1–9. doi: 10.1002/syn.890210102. [DOI] [PubMed] [Google Scholar]
- 8.Escobedo LG, Ruttenber AJ, Agocs MM, Anda RF, Wetli CV. Emerging patterns of cocaine use and the epidemic of cocaine overdose deaths in Dade County, Florida. Arch Pathol Lab Med. 1991;115:900–905. [PubMed] [Google Scholar]
- 9.Fishburn CS, Belleli D, David C, Carmon S, Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J Biol Chem. 1993;268:5872–5878. [PubMed] [Google Scholar]
- 10.Giros B, Martres M-P, Sokoloff P, Schwartz J-C. cDNA cloning of the human dopaminergic D3 receptor and chromosome identification. C R Acad Sci. 1990;311:501–508. [PubMed] [Google Scholar]
- 11.Giros B, Martres M-P, Pilon C, Sokoloff P, Schwartz J-C. Shorter variants of the D3 dopamine receptor produced through various patterns of alternative splicing. Biochem Biophys Res Commun. 1991;176:1584–1592. doi: 10.1016/0006-291x(91)90469-n. [DOI] [PubMed] [Google Scholar]
- 12.Goeders NE, Kuhar MJ. Chronic cocaine administration induced opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res. 1987;7:207–216. [PubMed] [Google Scholar]
- 13.Hernandez A, Andollo W, Hearn WL. Analysis of cocaine and metabolites in brain using solid phase extraction and full-scanning and GC/ion trap mass spectrometry. Forensic Sci Int. 1994;65:149–156. doi: 10.1016/0379-0738(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 14.Hillefors-Berglund M, Von Euler G. Pharmacology of dopamine D3 receptors in the islands of Calleja of the rat using quantitative receptor autoradiography. Eur J Pharmacol. 1994;261:179–183. doi: 10.1016/0014-2999(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 15.Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2dopamine receptors in rat brain. Brain Res. 1990;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF, Bloom FE. Molecular and cellular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 17.Kuhar MJ, DeSouza EB, Unnerstall JR. Neurotransmitter receptor mapping by autoradiography and other methods. Annu Rev Neurosci. 1986;9:27–59. doi: 10.1146/annurev.ne.09.030186.000331. [DOI] [PubMed] [Google Scholar]
- 18.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 19.Landwehrmeyer B, Mengod G, Palacios JM. Differential visualization of dopamine D2 and D3 receptor sites in rat brain: a comparative study using in situ hybridization histochemistry and ligand binding autoradiography. Eur J Neurosci. 1993a;5:145–153. doi: 10.1111/j.1460-9568.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 20.Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding site in human brain. Mol Brain Res. 1993b;18:187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- 21.Large CH, Stubbs CM. The dopamine D3receptor: Chinese hamsters or Chinese whispers. Trends Pharmacol Sci. 1994;15:46–47. doi: 10.1016/0165-6147(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 22.Levesque D, Diaz J, Pilon C, Martres M-P, Giros B, Souil E, Schott D, Morgat J-L, Schwartz J-C, Sokoloff P. Identification, characterization and localization of the dopamine D3receptor in rat brain using 7-[3H]hydroxy-N,N -di- n -propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, Bergson C, Levenson R, Schmauss C. On the origin of mRNA encoding the truncated dopamine D3-type receptor D3nf and detection of D3nf-like immunoreactivity in human brain. J Biol Chem. 1994;269:29220–29226. [PubMed] [Google Scholar]
- 24.MacKenzie RG, VanLeeuwen D, Pugsley TA, Shih Y-H, Demattos S, Tang L, Todd RD, O’Malley KL. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. Eur J Pharmacol. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 25.Mallet PE, Beninger RJ. 7-OH-DPAT produced place conditioning in rats. Eur J Pharmacol. 1994;261:R5–R6. doi: 10.1016/0014-2999(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 26.Martres MP, Sokoloff P, Giros B, Schwartz JC. Effects of dopaminergic neurotransmission interruption on the D2receptor isoform in various cerebral tissue. J Neurochem. 1992;58:673–679. doi: 10.1111/j.1471-4159.1992.tb09770.x. [DOI] [PubMed] [Google Scholar]
- 27.Meador-Woodruff JH, Little KY, Damask SP, Watson SJ. Effects of cocaine on D3 and D4 receptor expression in the human striatum. Biol Psychiatry. 1995;38:263–266. doi: 10.1016/0006-3223(95)00099-3. [DOI] [PubMed] [Google Scholar]
- 28.Mittleman RE, Wetli CV. Death caused by recreational cocaine use: an update. JAMA. 1984;252:1889–1893. [PubMed] [Google Scholar]
- 29.Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- 31.Nagai Y, Ueno S, Saeki Y, Soga F, Yanagihara T. Expression of the D3 dopamine receptor gene and a novel variant transcript generated by alternative splicing in human peripheral blood lymphocytes. Biochem Biophys Res Commun. 1993;194:368–374. doi: 10.1006/bbrc.1993.1829. [DOI] [PubMed] [Google Scholar]
- 32.Park BH, Fishburn S, Carmon S, Accili D, Fuchs S. Structural organization of the murine D3dopamine receptor gene. J Neurochem. 1995;64:482–486. doi: 10.1046/j.1471-4159.1995.64020482.x. [DOI] [PubMed] [Google Scholar]
- 33.Parsons B, Stanley M, Javitch J. Differential visualization of dopamine D2 and D3 receptors in rat brain. Eur J Pharmacol. 1993;234:269–272. doi: 10.1016/0014-2999(93)90963-i. [DOI] [PubMed] [Google Scholar]
- 34.Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahniser NR. Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J Pharmacol Exp Ther. 1990;253:35–43. [PubMed] [Google Scholar]
- 35.Pilon C, Levesque D, Dimitriadou V, Griffon N, Martre M-P, Schwartz J-C, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108–15 neuroblastoma-glioma hybrid cell line. Eur J Pharmacol. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 36.Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci. 1994;15:374–379. doi: 10.1016/0165-6147(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 37.Reith MEA, Kramer HK, Sershen H, Lajtha A. Cocaine competitively inhibits catecholamine uptake into brain synaptic vesicles. Res Commun Subst Abuse. 1989;10:205–208. [Google Scholar]
- 38.Ritz MC, Lamb SR, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DCS, Ranaldi R. Effect of dopaminergic drugs on cocaine reinforcement. Clin Neuropharmacol. 1995;18:S84–S95. [Google Scholar]
- 40.Robledo P, Maldonado-Lopez R, Koob GF. Role of the dopamine receptors in the nucleus accumbens in the rewarding properties of cocaine. Ann NY Acad Sci. 1992;654:509–512. doi: 10.1111/j.1749-6632.1992.tb26015.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Natl Acad Sci USA. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1-like and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 43.Snyder LA, Roberts JL, Sealfon SC. Alternative transcripts of the rat and human dopamine D3 receptors. Biochem Biophys Res Commun. 1991;180:1031–1035. doi: 10.1016/s0006-291x(05)81169-6. [DOI] [PubMed] [Google Scholar]
- 44.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 45.Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, Schwartz JC (1992a) Localization and function of the D3 dopamine receptor. Arzneimittel-Forschung/Drug Res 42:224–230. [PubMed]
- 46.Sokoloff P, Martre MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992b;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- 47.Sokoloff P, Levesque D, Martre M-P, Lannfelt L, Diaz G, Pilon C, Schwartz J-C (1992c) The dopamine D3 receptor as a key target for antipsychotics. Clin Neuropharmacol 15:456A–457A. [DOI] [PubMed]
- 48.Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres M-P, Giros B, Schwartz J-C. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992d;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 49.Staley JK, Wetli CV, Ruttenber AJ, Hearn WL, Mash DC. Altered dopaminergic synaptic markers in cocaine psychosis and sudden death. NIDA Res Monogr. 1995;153:491. [Google Scholar]
- 50.Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1397. [PubMed] [Google Scholar]
- 51.Wallace DR, Booze RM. Identification of D3and sigma receptors in the rat striatum and nucleus accumbens using [3H]-7-OH DPAT and carbetapentane. J Neurochem. 1995;64:700–710. doi: 10.1046/j.1471-4159.1995.64020700.x. [DOI] [PubMed] [Google Scholar]
- 52.Wetli CV, Fisbain DA. Cocaine-induced psychosis and sudden death in recreational cocaine users. J Forensic Sci. 1985;30:873–880. [PubMed] [Google Scholar]
- 53.Wetli CV, Mash DC, Karch SB. Cocaine-associated agitated delirium and the neuroleptic malignant syndrome. Am J Emerg Med. 1996;14:425–428. doi: 10.1016/S0735-6757(96)90066-2. [DOI] [PubMed] [Google Scholar]
- 54.Zeigler S, Lipton J, Toga A, Ellison G. Continuous cocaine administration produces persisting changes in brain neurochemistry and behavior. Brain Res. 1991;552:27–35. doi: 10.1016/0006-8993(91)90655-f. [DOI] [PubMed] [Google Scholar]