Abstract
The suprachiasmatic nucleus (SCN) is a circadian oscillator and a critical component of the mammalian circadian system. It receives afferents from the retina and the mesencephalic raphe. Retinal afferents mediate photic entrainment of the SCN, whereas the serotonergic afferents originating from the midbrain modulate photic responses in the SCN; however, the serotonin (5HT) receptor subtypes in the SCN responsible for these modulatory effects are not well characterized. In this study, we tested the hypothesis that 5HT1B receptors are located presynaptically on retinal axon terminals in the SCN and that activation of these receptors inhibits retinal input.
The 5HT1B receptor agonists TFMPP and CGS 12066A, administered systemically, inhibited light-induced phase shifts of the circadian activity rhythm in a dose-dependent manner at phase delay and phase advance time points. This inhibition was not affected by previous systemic application of either the selective 5HT1A receptor antagonist (+)WAY 100135 or by the 5HT2 receptor antagonist mesulergine, whereas pretreatment with the nonselective 5HT1 antagonist methiothepin significantly attenuated the effect of TFMPP. TFMPP also produced a dose-dependent reduction in light-stimulated Fos expression in the SCN, although a small subset of cells in the dorsolateral aspect of the caudal SCN were TFMPP-insensitive. TFMPP (1 mm) infused into the SCN produced complete inhibition of light-induced phase advances. Finally, bilateral orbital enucleation reduced the density of SCN 5HT1B receptors as determined using [125I]-iodocyanopindolol to define 5HT1Bbinding sites. These results are consistent with the interpretation that 5HT1B receptors are localized presynaptically on retinal terminals in the SCN and that activation of these receptors by 5HT1B agonists inhibits retinohypothalamic input.
Keywords: suprachiasmatic nucleus, circadian rhythm, presynaptic, 5HT1B, TFMPP, CGS 12066A, c-fos, photic entrainment, retinal afferents, [125I]-iodocyanopindolol
The hypothalamic suprachiasmatic nucleus (SCN) is a critical component of the mammalian circadian system. Converging lines of investigation have provided support for the role of the SCN as a circadian oscillator (Turek, 1985; Meijer and Rietveld, 1989; Klein et al., 1991; van den Pol and Dudek, 1993). Most recently, transplanted fetal or neonatal anterior hypothalamic tissue containing the SCN has been shown to be capable of restoring a circadian rhythm of activity to rodents rendered arrhythmic by SCN destruction (Sawaki et al., 1984;Lehman et al., 1987; DeCoursey and Buggy, 1988; Boer and Griffioen, 1990; Ralph et al., 1990; Saitoh et al., 1991; Sollars and Pickard, 1994; 1995; Sollars et al., 1995).
The functional utility of the SCN oscillatory system is derived from its ability to be synchronized, or “entrained,” to the 24 hr environmental day/night cycle. Entrainment provides for stable and appropriate phasing of the SCN circadian oscillator with the environment, thereby, in effect, enabling recognition of local time. Thus, the SCN circadian oscillator is said to function as a biological clock (Pittendrigh and Daan, 1976). In the absence of rhythmic photic cues (i.e., constant dark conditions), the clock free-runs with a period slightly greater or less than 24 hr, drifting in and out of synchrony with periodic events in the external environment.
Entrainment of the SCN to the 24 hr day/night cycle is accomplished by a daily resetting mechanism. Light exposure early in the subjective night phase delays the oscillator, whereas light exposure late in the subjective night results in phase advances. During the subjective day, the SCN circadian oscillator is insensitive to light (Daan and Pittendrigh, 1976). This phase resetting process is mediated by a projection from the retina to the SCN, the retinohypothalamic tract (RHT) (Hendrickson et al., 1972; Moore and Lenn, 1972; Pickard, 1982).
The SCN is also innervated by serotonergic fibers arising from the mesencephalic raphe (Azmitia and Segal, 1978; Moore et al., 1978;Steinbusch, 1981; Meyer-Bernstein and Morin, 1996); however, neither the role of this very concentrated 5HT input to the SCN nor the functional organization of the 5HT receptor subtypes in the SCN is well understood. Serotonergic innervation of the SCN is not required for the expression of circadian rhythms (Block and Zucker, 1976), although depletion of 5HT in the hamster SCN alters the phase angle of entrainment (Smale et al., 1990). Systemic administration of the nonselective serotonin agonist quipazine lowers the activity of photically responsive SCN neurons (Miller and Fuller, 1990) and attenuates light-induced SCN Fos expression (Selim et al., 1993). Microiontophoretic application of 5HT or 5HT1A/7 agonists to the SCN in anesthetized hamsters inhibits photic responses in SCN cells (Ying and Rusak, 1994). Moreover, Rea and colleagues (1994) have shown that the 5HT1A/7 receptor agonist 8-OH-DPAT can attenuate several aspects of the photic response of the SCN. 5HT1A/7 receptors mediating 8-OH-DPAT effects are most likely located on the soma and dendritic processes of SCN neurons (Kiss et al., 1984; Bosler and Beaudet, 1985; Bosler, 1989; Chalmers and Watson, 1991; Lovenberg et al., 1993; Kawahara et al., 1994).
5HT1B binding sites have also been reported in the SCN in relatively high density (Manrique et al., 1993, 1994; Prosser et al., 1993), although SCN neurons express little 5HT1B mRNA (Roca et al., 1993), suggesting that a large percentage of these 5HT1B receptors are not synthesized in the SCN. These findings are consistent with data indicating that 5HT1Breceptors are located predominately on axon terminals in the brain, including retinal axon terminals (Boschert et al., 1994), where they appear to inhibit glutamatergic neurotransmission. We therefore hypothesized that 5HT1B receptors located on RHT axon terminals might serve to regulate RHT neurotransmission in the SCN. To test this hypothesis, we evaluated (1) the level of SCN 5HT1B receptors after enucleation; (2) the ability of 5HT1B agonists and antagonists to modulate light-induced behavioral phase shifts; and (3) the effect of 5HT1Bagonists on light-induced Fos expression in the SCN , a cellular correlate of light-induced behavioral responses .
MATERIALS AND METHODS
Animals. Syrian hamsters (Mesocricetus auratus, male; Charles River, Wilmington, MA) were housed in groups of six and maintained under a light/dark (LD) cycle of 14 hr/10 hr (LD 14:10; lights out at 2 A.M.) for at least 2 weeks before experiments. Illuminance at cage level was ∼200 lux, and food and water were freely available.
Activity rhythms. After at least two weeks in LD 14:10, hamsters were transferred to individual cages equipped with activity wheels and maintained in constant dark (DD) conditions until the experiment was terminated. Wheel-running activity was monitored continuously as described previously (Pickard et al., 1982; Rea et al., 1993b) using a Zenith 248 computer running DATAQUEST III data acquisition software (Minimitter, Sunriver, OR). Activity records were generated in the standard manner: each day’s activity was presented beneath the previous day’s activity and analyzed using CIRCADIA software (Behavioral Cybernetics, Cambridge, MA) running on a Macintosh IIci computer.
The onset of wheel-running activity is designated as circadian time (CT) 12 and was used as a phase reference point for the timing of photic stimulation, as described previously (Rea et al., 1994). The onset of wheel-running activity on the day of light stimulation was predicted by extrapolation of the least squares line through the activity onsets for at least 5 d preceding the day of stimulation.
Light-induced phase shifts. After at least 10 d in DD (typically 10–12 d), groups of hamsters received injections followed by light stimulation at either CT 14 (2 circadian hours after predicted activity onset; 1 circadian hour = τ/24) or CT 19 (7 circadian hours after predicted activity onset). Groups of hamsters received (1 ml/kg, i.p.) injections of either vehicle (0.9% saline for TFMPP or 65% EtOH for CGS 12066A) or 5HT1B agonists (TFMPP or CGS 12066A) (0.1–0.2 ml) 30 min before light exposure. In some experiments, 5HT antagonists ((+)WAY 100135, mesulergine, and methiothepin) or vehicle were delivered intraperitoneally 30 min before agonists. In addition, some animals received intracerebral injections of vehicle (0.9% saline) or TFMPP (0.3 μl) 10 min before light stimulation. All injections were performed under dim red illumination (<1 lux). Each animal received 10 min of white light at an average illuminance of 20 lux at CT 14 or CT 19 using a light stimulation apparatus, as described previously (Rea et al., 1994). After light stimulation, animals were returned to their wheel-running cages in DD. Animals that received drug injections without light treatment were handled as described above and returned to their wheel-running cages in DD immediately after injection.
Intracerebral injections. Surgical procedures for cannula placement were described previously (Rea et al., 1993a). Briefly, under deep anesthesia, animals were placed in a Kopf stereotaxic apparatus, and 26 ga cannula guides containing 33 ga stylets were implanted to a depth of 2.9 mm below the dura, secured with dental cement, and closed with sutures. Animals recovered from the surgery under LD 14:10 conditions for 1 week and were then transferred to running-wheel cages and placed in DD. After at least 10 d in DD, at the appropriate phase of the circadian cycle, a 33 ga infusion cannula attached to a 1 μl Hamilton syringe was lowered to a position just dorsal to the SCN (extending 4.4 mm beyond the tip of the indwelling guide cannula), and 300 nl of TFMPP (1 mm) or vehicle was delivered. Cannula placement was verified histologically at the termination of behavioral data collection as described previously (Weber et al., 1995).
Quantitation of phase shifts. Animals remained in DD for 10–14 d after photic stimulation. Phase shifts were calculated as the difference between the projected times of activity onset (CT 12) on the day after stimulation as determined by (1) extrapolation of the least squares line calculated from activity onset data collected during the 5 d before and including the day of stimulation and (2) back-extrapolation of the least squares line through five activity onsets beginning as soon as a steady-state free-run was resumed (usually days 2–6 were used and never later than days 4–8 after stimulation) (Pittendrigh and Daan, 1976).
Light-induced Fos expression. Hamsters were maintained in DD in wheel-running cages as described above. After 10–11 d in DD, animals received intraperitoneal injections of either TFMPP or vehicle 30 min before light exposure (20 lux for 10 min as described above) at CT 19. After light stimulation, animals were returned to their cages in DD. Ninety minutes after the onset of light stimulation, animals were anesthetized in the dark and prepared for immunocytochemical demonstration of Fos as described previously (Rea et al., 1994). All Fos-immunoreactive (Fos-ir) cell nuclei that were stained above background in both SCN throughout the rostrocaudal extent of the nucleus were counted by two investigators, and the counts were averaged and expressed as Fos-ir cells/SCN.
Quantitative 5HT1B receptor autoradiography. Hamsters were either mono- or binocularly enucleated under deep anesthesia. After removal of the eyeball, the orbit was packed with gelfoam, and the eyelids were sutured. It has been reported that the density of 5HT1B binding sites in the rat SCN varies with time of day (Prosser et al., 1993). Therefore, enucleated animals and intact controls were placed in DD conditions in running-wheel cages after surgery. After 7 d in DD, all animals were killed by decapitation under dim red light at CT 18–18.5. Brains were removed rapidly, dipped briefly in ice-cold saline, frozen on dry ice, and stored at −80°C until use. 5HT1B receptor autoradiography in the SCN and superior colliculus (SC), as defined by125I-iodocyanopindolol (125I-ICYP) binding (Offord et al., 1988), was conducted on 16 μm sections cut in the coronal plane on a Jung Frigocut 2800N cryostat, as described byManaker and Verderame (1990). Briefly, frozen sections were brought to 4°C in a refrigerator, preincubated in ice-cold buffer (50 mm Tris-HCL/2.5 mm MgCl2) for 10 min, and then incubated in the same buffer containing ∼100 pm125I-ICYP (specific activity 2200 Ci/mmol) and 30 μm isoproterenol (to block β-adrenergic receptors) at room temperature for 60 min. Under these conditions,125I-ICYP has been shown to label a single binding site that displays the pharmacological profile of 5HT1Breceptors (Offord et al., 1988). Incubation in an excess of unlabeled 5HT (20 μm) was used to define nonspecific binding. After incubation, sections were washed twice in ice-cold buffer, dipped in cold distilled water to remove buffer salts, and dried rapidly on a slide warmer. Dried slides were apposed to Amersham Hyperfilm (Amersham, Arlington Heights, IL) for 18–20 hr, and the exposed film was developed in Kodak D-19 for 2 min. Analysis of autoradiograms was performed using a computerized microdensitometry system using National Institutes of Health Image software. A calibration curve was generated using commercially available 125I standards, and results were expressed as tissue equivalent activities using rat brain gray matter values provided by the supplier. 125I-ICYP binding was determined throughout the rostrocaudal extent of the SCN. Analyses were conducted on the bilateral SCN as a single structure, because the retinal input is bilateral, overlapping, and approximately equal from both eyes (Pickard, 1982). A separate analysis was conducted on the ventromedial aspect of the bilateral SCN. Data are expressed as specific binding in femtomoles per milligram protein.
Statistical analysis. Statistical significance was determined using Student’s t test and by ANOVA, and differences between means were tested post hoc for significance (p < 0.05) using the Neuman–Keuls test.
Drugs and reagents. TFMPP {1-[3-(trifluoromethyl)phenyl]-piperazine}, CGS 12066A {7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1.2-a]quinoxaline}, DOI, methiothepin, and mesulergine were obtained from Research Biochemicals International (Natick, MA). (+)WAY 100135 was generously supplied by Wyeth-Ayerst. Serotonin and isoproterenol were obtained from Sigma (St. Louis, MO). [125I]-ICYP was purchased from Amersham.
RESULTS
5HT1B receptors in the hamster SCN and SC: distribution and effects of enucleation
High-affinity binding of 125I-ICYP, in the presence of isoproterenol, was observed throughout the rostrocaudal extent of the SCN and the SC. In the SCN, however, the binding density is much higher in the ventromedial aspects of the nucleus compared with the dorsolateral region (Fig. 1). The density of125I-ICYP binding sites in the SCN also appears greater in the more caudal aspects of the nucleus. In the SC,125I-ICYP binding is heavily distributed in the stratum griseum superficiale (SGS), with a much lower density in the stratum opticum and in the deep laminae of the SC (Fig. 2). Bilateral enucleation resulted in a 35% reduction in the125I-ICYP binding in the ventromedial SCN and a >50% reduction in 125I-ICYP binding in the SGS of the SC (Table1). Monocular enucleation produced a marked reduction in the SGS of the contralateral SC, with a slight reduction in the SGS ipsilateral to the enucleation. Monocular enucleation produced no detectable change in 5HT1B receptor density in the SCN (Table 1).
Fig. 1.
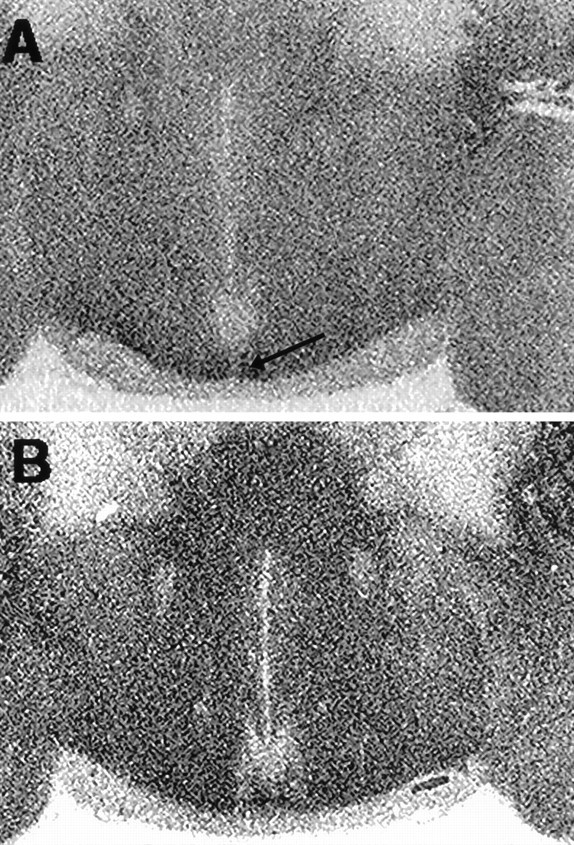
Distribution of [125I]-ICYP binding sites in the hamster SCN. A, Autoradiogram of a coronal section through the caudal SCN of a normal hamster, killed after 7 d in DD at CT 18, illustrates the dense 5HT1B receptor binding in the ventral and ventromedial aspects of the SCN (arrow) and the relatively sparse binding in the dorsolateral aspect of the nucleus. B, Autoradiogram of a similar section through the caudal SCN of an enucleated hamster killed at CT 18, 7 d after removal of RHT afferents, illustrates the decrease in [125I]-ICYP binding in the ventral and ventromedial SCN relative to the SCN in A where RHT afferents are intact (see Table 1).
Fig. 2.
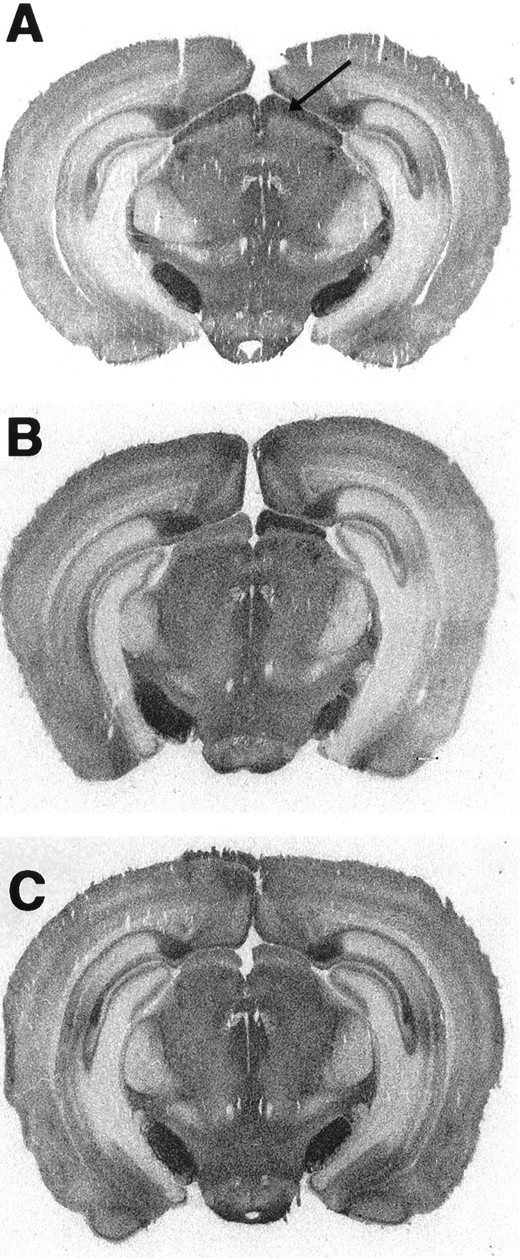
Distribution of [125I]-ICYP binding sites in the hamster SC. A, Autoradiograph of a coronal section through the SC of a normal hamster illustrates the dense 5HT1B receptor binding in the retinorecipient region of the SC, the SGS (arrow). Dense [125]I-ICYP binding is also apparent in the substantia nigra and subiculum.B, Autoradiograph illustrating the effect of monocular enucleation on [125I]-ICYP binding in the SCS. Note the reduction in 5HT1B receptor binding density in the SGS (left side) contralateral to the removed eye.C, Autoradiograph illustrating the effect of binocular enucleation on [125I]-ICYP binding in the SCS. Note the reduction in 5HT1B receptor binding density bilaterally in the SGS compared to that in the intact hamster (A).
Table 1.
Effects of enucleation on 5HT1B receptors in the suprachiasmatic nucleus (SCN) and superior colliculus (SC)
| Intact | Unilateral | Bilateral | |
|---|---|---|---|
| SC | L 6.49 ± 0.24 | C 2.39 ± 0.19 | L 3.04 ± 0.16 |
| R 6.36 ± 0.31 | I 5.04 ± 0.39* | R 2.98 ± 0.11 | |
| SCN | 5.57 ± 0.71 | 5.66 ± 0.15 | 3.60 ± 0.80† |
All values expressed as femtomoles per milligram protein (mean ± SEM), n = 4/group. Unilateral, monocular enuceation; Bilateral, bilateral enucleation; L, left; R, right; C, contralateral to the enucleated eye; I, ipsilateral to the enucleated eye.
p < 0.001 C versus I;
p < 0.05 bilateral versus intact.
Effects of systemic 5HT1B agonists on light-induced phase shifts
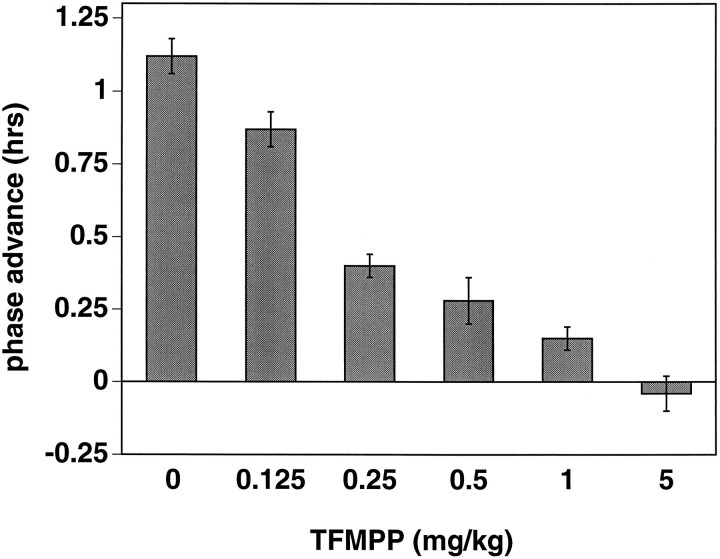
Hamsters that received intraperitoneal injections of vehicle 30 min before light stimulation at CT 19 exhibited large, stable phase advances of the free-running activity rhythm as expected (Fig.3). Injection of TFMPP (5 mg/kg body weight) 30 min before light stimulation completely blocked light-induced phase advances [−0.04 ± 0.06 hr (mean ± SEM) (TFMPP + light;n = 6) vs +1.16 ± 0.09 hr (vehicle + light;n = 11); p < 0.001] (Fig. 3). The effect of TFMPP on light-induced phase advances was dose-dependent over a dose range of 0.125–5.0 mg/kg; injection of 0.125 mg/kg, the lowest dose injected, produced a 25% reduction in phase shifts (Fig.4). Injection of 5 mg/kg TFMPP alone at CT 18.5 did not significantly alter the phase of the activity rhythm (+0.01 ± 0.05 hr; n = 3) (Fig. 3).
Fig. 3.
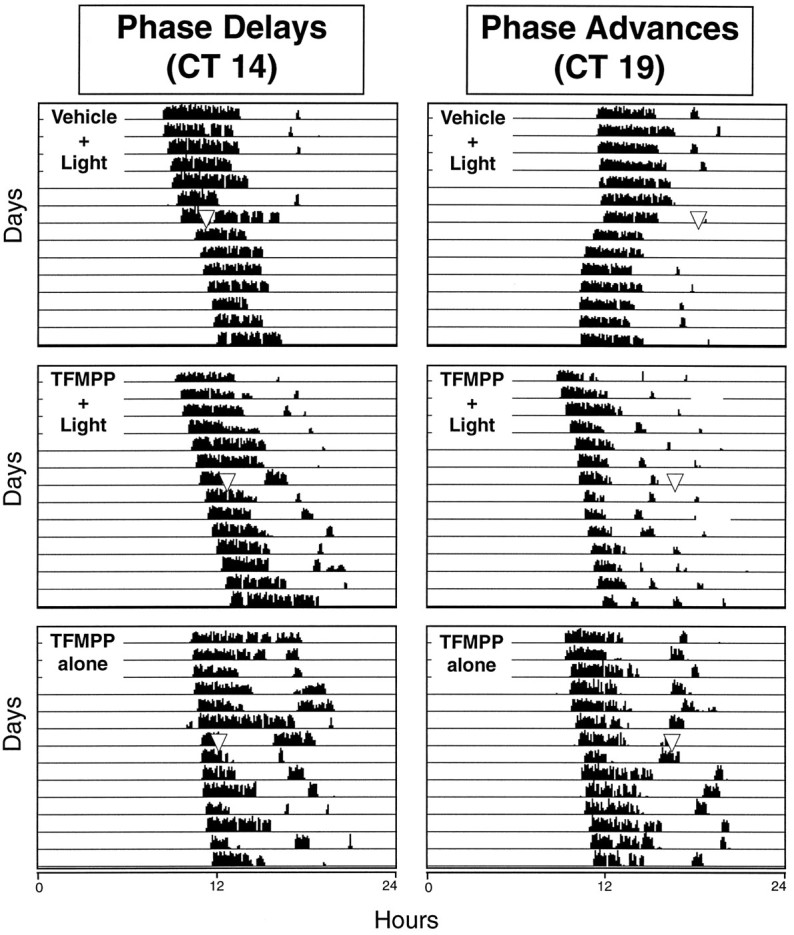
The effect of systemic administration of TFMPP on light-induced phase shifts of the circadian rhythm of wheel-running activity is illustrated in representative actograms. Hamsters were maintained in DD throughout the experiment and received injections of vehicle or TFMPP (5 mg/kg, i.p.) at either CT 13.5 or CT 18.5, followed by brief light exposure (10 min at 20 lux) at CT 14 to elicit phase delays (left) or at CT 19 to elicit phase advances (right). Approximate time of light stimulation is indicated by the inverted triangles(top and middle rows). Inverted triangles in the bottom row indicate approximate time of TFMPP injection.
Fig. 4.
Dose-dependent effect of systemic administration of TFMPP on light-induced phase advances of the free-running activity rhythm. Data represent the mean ± SEM of four to five animals/TFMPP group. Light-induced phase shifts in all TFMPP-treated groups are significantly smaller compared with the vehicle (0 mg/kg) + light group (n = 11) (p < 0.05).
Animals that received intraperitoneal injections of vehicle 30 min before light stimulation at CT 14 exhibited the expected phase delays of the free-running activity rhythm (Fig. 3). TFMPP (5 mg/kg, i.p.) injected 30 min before light stimulation at CT 14 completely blocked the phase-delaying effects of light on the circadian activity rhythm [−0.05 ± 0.07 hr (TFMPP + light; n = 6) vs −0.67 ± 0.10 hr (vehicle + light); n = 6;p < 0.001]. This dose of TFMPP alone at CT 13.5 did not significantly alter the phase of the circadian activity rhythm (0.02 and 0.22 hr; n = 2) (Fig. 3).
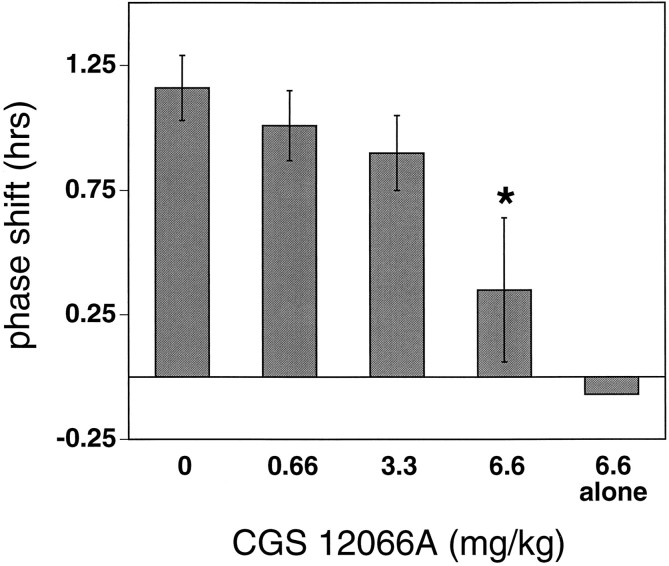
In another set of experiments, CGS 12066A was administered intraperitoneally 30 min before light stimulation at CT 19 to examine the effects of this more selective 5HT1B agonist on light-induced phase advances. CGS 12066A attenuated the phase-shifting effect of light at CT 19 in a dose-dependent manner, with the phase shifts at the highest dose tested (6.6 mg/kg) significantly reduced compared with vehicle-injected controls [+0.35 ± 0.29 hr (CGS 12066A + light); n = 4 vs +1.16 ± 0.13 hr (vehicle + light); n = 5; p < 0.02; Fig. 5]. CGS 12066A administered at CT 18.5 in the absence of light had no significant effect on the phase of the circadian activity rhythm (+0.09 and −0.23 hr; n = 2) (Fig. 5).
Fig. 5.
The effect of systemic administration of CGS 12066A on light-induced phase advances of the circadian rhythm of wheel-running activity. Hamsters were maintained in DD throughout the experiment and received intraperitoneal injections of vehicle or CGS 12066A at CT 18.5, followed by brief light exposure (10 min at 20 lux) at CT 19. Data represent mean ± SEM of four to six animals/group (drug alone group, n = 2). Light-induced phase advances are significantly reduced in the 6.6 mg/kg + light group compared to the vehicle + light group (*p < 0.02).
Effects of 5HT1A and 5HT2 antagonists and a 5HT2 agonist on TFMPP inhibition of light-induced phase advances at CT 19
TFMPP is a well characterized 5HT1B receptor agonist (Lucki et al., 1989; Chopin et al., 1994); however, in addition to its affinity for 5HT1B receptors, TFMPP also has a relatively high affinity for 5HT1A and 5HT2Creceptors (Chopin et al., 1994; Hoyer et al., 1994), both of which have been described in the rat SCN (Prosser et al., 1993; Roca et al., 1993). Unfortunately, a selective 5HT1B receptor antagonist is not yet available (Hoyer et al., 1994). Therefore, a series of experiments was conducted to determine the effects of selective 5HT1 and 5HT2 receptor antagonists on the ability of TFMPP to inhibit light-induced phase advances at CT 19.
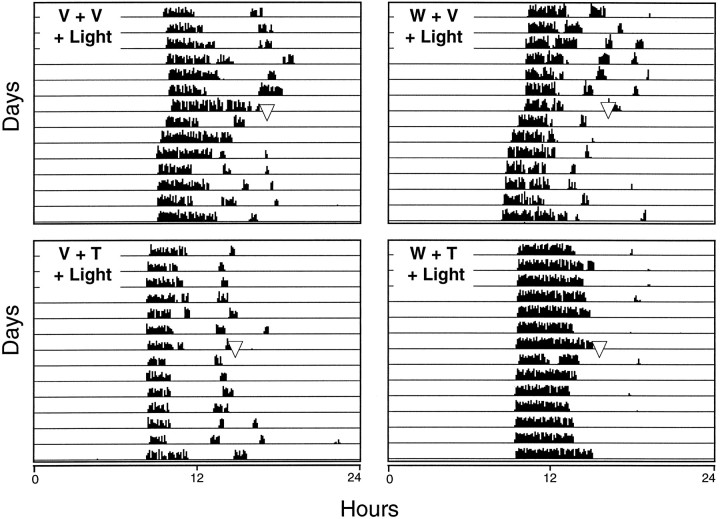
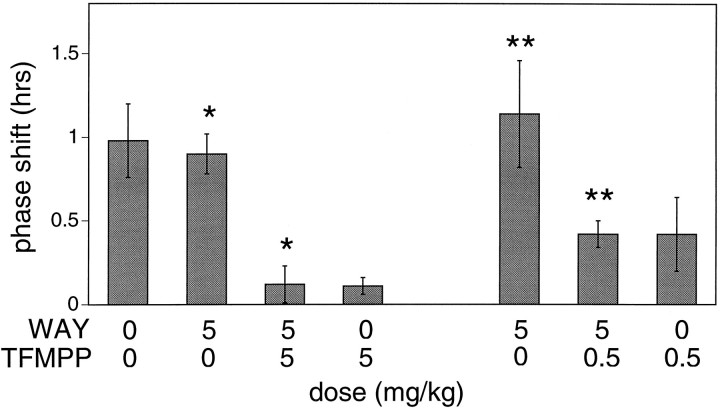
(+)WAY 100135, a selective 5HT1A receptor antagonist, administered (5 mg/kg, i.p.) 30 min before the systemic injection of TFMPP (5 mg/kg or 0.5 mg/kg) had no effect on TFMPP inhibition of light-induced phase shifts at CT 19 (Fig. 6). Phase shifts generated after (+)WAY 100135 + TFMPP + light were similar to phase shifts generated after vehicle + TFMPP + light [+0.12 ± 0.11 hr (n = 6) vs +0.11 ± 0.06 hr (n = 6) and +0.42 ± 0.08 hr (n = 5) vs 0.42 ± 0.22 hr (n = 4) for TFMPP doses of 5 and 0.5 mg/kg, respectively] (Fig. 7). (+)WAY 100135 administered alone had no significant effect on the phase of the free-running activity rhythm (−0.08 ± 0.12; n = 3). The inability of the selective 5HT1A antagonist (+)WAY 100135 to reduce TFMPP inhibition of light-induced phase shifts while blocking the effects of the 5HT1A/7 agonist 8-OH-DPAT is consistent with the interpretation that TFMPP is acting via 5HT1B receptors.
Fig. 6.
The effect of pretreatment with the 5HT1A antagonist (+)WAY 100135 on TFMPP inhibition of light-induced phase shifts of the circadian rhythm of wheel-running activity is illustrated in representative actograms. Hamsters were maintained in DD throughout the experiment and received injections of vehicle (V) or (+)WAY 100135 (W) (5 mg/kg, i.p.) at CT 18, followed by vehicle or TFMPP (T) (5 mg/kg, i.p.) at CT 18.5, followed by light stimulation (10 min at 20 lux) at CT 19. Pretreatment with (+)WAY 100135 had no effect on TFMPP inhibition of light-induced phase advances of the circadian activity rhythm (compare bottom left panelwith bottom right panel). (+)WAY 100135 administration had no significant effect on light-induced phase shifts compared with vehicle-treated animals (compare top left panel with top right panel).
Fig. 7.
Effect of (+)WAY 100135 on TFMPP inhibition of light-induced phase advances at CT 19. Data represent the mean ± SEM of four to six animals/group. Systemic pretreatment with the 5HT1A antagonist (+)WAY 100135 (WAY; 5 mg/kg) had no significant effect on the ability of TFMPP to inhibit light-induced phase shifts at CT 19 at TFMPP doses of either 5 mg/kg (left side; *p< 0.001) or 0.5 mg/kg (right side; **p < 0.05). (+)WAY 100135 by itself did not significantly affect light-induced phase shifts (not shown).
To ascertain whether the effects of TFMPP on light-induced phase shifts might be mediated via its affinity for the 5HT2C receptor, the 5HT2 antagonist mesulergine was injected (5 mg/kg, i.p.) before TFMPP and light at CT 19 in a manner similar to that described above for (+)WAY 100135. Mesulergine injected before TFMPP and light had no significant effect on TFMPP inhibition of light-induced phase advances at CT 19 at TFMPP doses of 5 and 0.5 mg/kg (Table 2). In a separate experiment, the selective 5HT2 agonist DOI (5 mg/kg), injected 30 min before light stimulation at CT 19, had no effect on phase advances of the circadian activity rhythm [+1.42 ± 0.27 hr (n = 6); vehicle + light vs +1.10 ± 0.31 hr (n = 6); DOI + light; p > 0.4]. DOI alone produced small and variable phase shifts (+0.22 ± 0.13 hr; n = 4). The mesulergine and DOI results taken together support the interpretation that TFMPP inhibition of light-induced phase shifts is not mediated through the 5HT2C receptor.
Table 2.
The effect of a 5HT2 receptor antagonist, mesulergine, on TFMPP inhibition of light-induced phase shifts at CT 19
| Second injection | First injection Vehicle | First injection Mesulergine |
|---|---|---|
| Vehicle | 1.44 ± 0.24 (6) | 1.24 ± 0.21 (8) |
| TFMPP (0.5 mg/kg) | 0.39 ± 0.13 (9) | 0.59 ± 0.14 (8) |
| TFMPP (5.0 mg/kg) | 0.16 ± 0.02 (5) | 0.33 ± 0.10 (6) |
Animals were maintained in DD throughout and were pretreated with systemic vehicle or mesulergine (5 mg/kg) (first injection) at CT 18 followed by vehicle or two doses of TFMPP (second injection) at CT 18.5 followed by light-stimulation (10 min at 20 lux) at CT 19. Mesulergine had no significant effect on attenuating the inhibition of TFMPP on light-induced phase shifts at either dose of TFMPP (p> 0.13 and p > 0.28). n = animals/group.
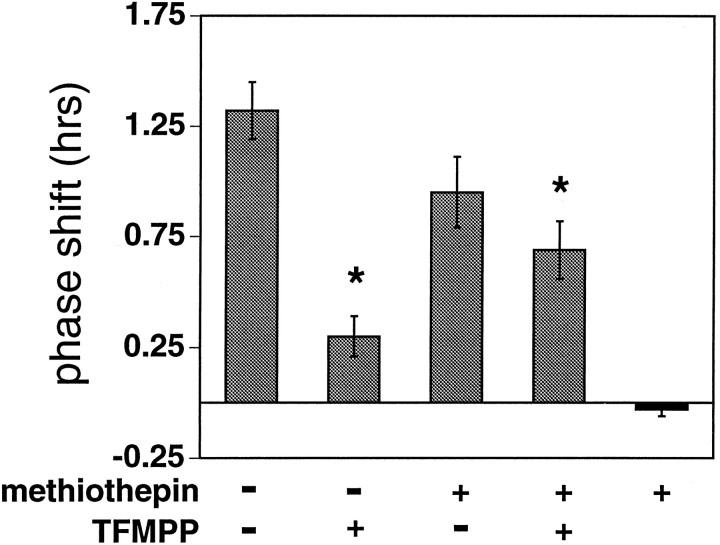
Effects of the nonselective 5HT1 antagonist methiothepin on TFMPP inhibition of light-induced phase advances at CT 19
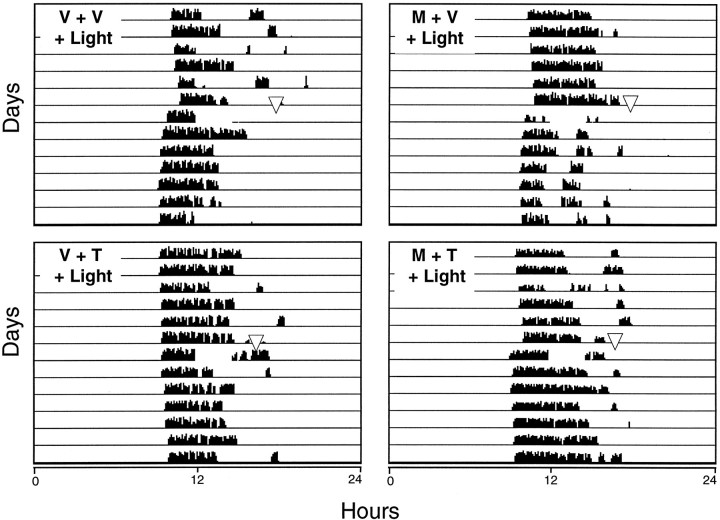
To further examine whether the effect of TFMPP on light-induced behavioral phase shifts is mediated by its affinity to 5HT1B receptors, the 5HT1 antagonist methiothepin, which has affinity for both the 5HT1A and 5HT1B receptors, was injected before TFMPP administration and light, as described above. Methiothepin (5 mg/kg, i.p.) significantly attenuated the inhibition produced by TFMPP at 1.0 mg/kg [+0.28 ± 0.10 hr (n = 9); vehicle + TFMPP + light vs +0.69 ± 0.13 hr (n = 10); methiothepin + TFMPP + light; p < 0.02]. In addition, methiothepin itself had no significant effect on light-induced phase shifts, and methiothepin alone had no effect on the phase of the circadian activity rhythm (Figs. 8, 9). These results again are consistent with the interpretation that the effect of TFMPP on light-induced phase shifts is mediated through 5HT1Breceptors.
Fig. 8.
The effect of pretreatment with the nonselective 5HT1 antagonist methiothepin (M) on TFMPP (T) inhibition of light-induced phase shifts of the circadian rhythm of wheel-running activity is illustrated in representative actograms. Hamsters were maintained in DD throughout the experiment and received injections of vehicle (V) or methiothepin (5 mg/kg, i.p.) at CT 18, followed by vehicle or TFMPP (5 mg/kg, i.p.) at CT 18.5, and light stimulation (10 min at 20 lux) at CT 19. Pretreatment with methiothepin significantly attenuated the ability of TFMPP to inhibit light-induced phase advances of the circadian activity rhythm (compare bottom left panel with top right panel). Methiopthepin had no significant effect on light-induced phase shifts (compare top right panel withtop left panel). Lost data on the day after light stimulation resulted from temporary equipment malfunction.
Fig. 9.
Effect of the nonselective 5HT1antagonist methiothepin on TFMPP inhibition of light-induced phase advances at CT 19. Data represent the mean ± SEM of 9–10 animals/group. Systemic pretreatment with methiothepin (5 mg/kg) (animals that received methiothepin are indicated by +) significantly attenuated the ability of TFMPP (1 mg/kg) (animals that received TFMPP are indicated by +) to inhibit light-induced phase shifts. Phase shifts of the methiothepin (+) plus TFMPP (+) group were significantly larger than the vehicle [methiothepin (−)] plus TFMPP (+) group (*p < 0.02). Methiothepin by itself did not significantly affect light-induced phase shifts (p > 0.1). Methiothepin administration in the absence of light (darkened bar; n = 5) had no effect on the phase of the free-running activity rhythm.
Effects of TFMPP delivered directly into the SCN region on light-induced phase shifts at CT 19
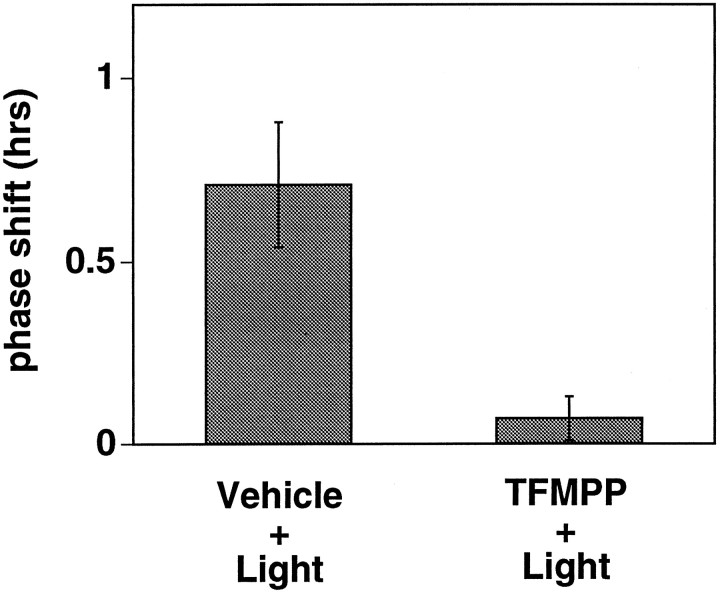
To begin to address the question of the site of action of systemically administered TFMPP, animals were implanted with chronic indwelling cannula, and TFMPP (1 mm in 0.3 μl) was injected directly into the SCN region. TFMPP injected 10 min before light stimulation at CT 19 significantly inhibited light-induced phase shifts compared with animals injected with vehicle 10 min before light stimulation [+0.07 ± 0.06 hr (n = 6); TFMPP + light vs +0.71 ± 0.17 hr (n = 5); vehicle + light; p < 0.001] (Fig. 10).
Fig. 10.
Effect of local infusion of 1 mmTFMPP into the SCN region on light-induced phase advances at CT 19. TFMPP was infused into the SCN 10 min before light stimulation (10 min at 20 lux) at CT 19. Data represent the mean ± SEM of five to six animals/group. Local TFMPP infusion into the SCN significantly inhibited light-induced phase advances (p < 0.001).
Effects of systemic TFMPP on light-induced Fos expression in the SCN at CT 19
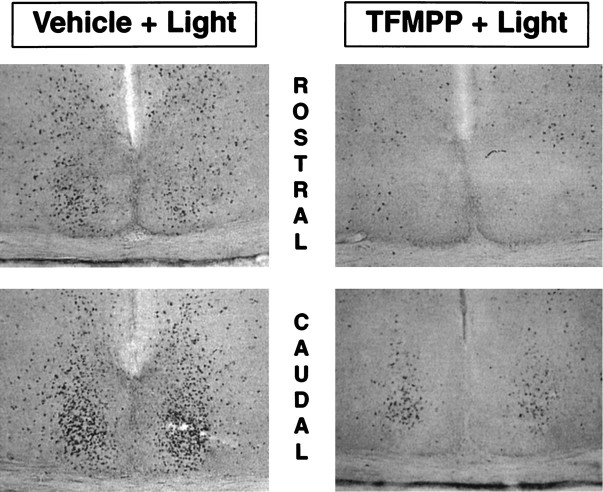
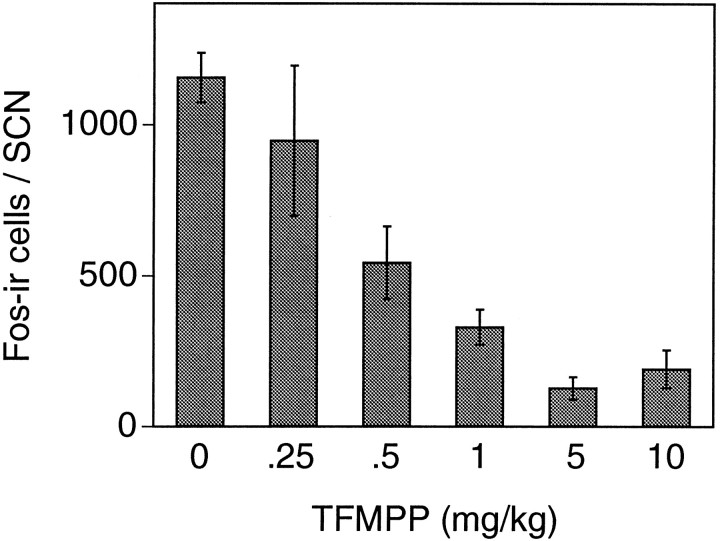
Light stimulation at CT 19 of vehicle-injected animals produced the characteristic pattern of Fos expression within the SCN region (Fig. 11). Fos-ir cell nuclei were distributed throughout the rostrocaudal extent of the SCN, with a higher concentration in the caudal third of the nucleus. As described previously (Rea, 1989; Abe et al., 1991), many Fos-ir cells were also noted surrounding the cytoarchitectonic boundaries of the SCN extending into the periventricular region. Injection of TFMPP 30 min before light stimulation at CT 19 reduced the number of Fos-ir cells in the SCN in a dose-dependent manner, whereas it did not affect Fos expression in the regions surrounding the SCN (Figs. 11, 12). After the injection of increasing concentrations of TFMPP before light stimulation, fewer Fos-ir cells were noted in the ventral and medial aspects of the nucleus. At the two highest doses administered (5 and 10 mg/kg; note that 5 mg/kg completely blocks light-induced behavioral phase shifts), virtually no Fos-ir cells were noted in the rostral division of the nucleus, with the remaining Fos-ir cells restricted to the dorsolateral portion of the caudal SCN (Fig. 11). The number of Fos-ir cells/SCN in this restricted dorsolateral patch of the caudal SCN in animals receiving 5 and 10 mg/kg TFMPP were 127 ± 37 (n = 5) and 191 ± 63 (n = 3), respectively, which represents ∼10-15% of the total number of SCN cells expressing Fos after vehicle injection and light stimulation (1155 ± 81; n = 9). Injection of TFMPP alone at the highest dose administered before light stimulation (10 mg/kg) did not induce Fos expression in the SCN (data not shown). The relationship between the magnitude of behavioral phase shifts and the number of SCN cells expressing detectable levels of Fos at varying doses of TFMPP administered before light stimulation was highly correlated (Figs. 4,12).
Fig. 11.
Representative photomicrographs illustrating the effect of systemic TFMPP administration on light-induced Fos expression in the SCN. Fos-ir cells are distributed throughout the rostrocaudal SCN in the vehicle-injected animal after light stimulation at CT 19 (left). Pretreatment with 5 mg/kg TFMPP completely eliminates light-induced Fos expression in the rostral SCN (right top) and much of the caudal SCN (right bottom). In the caudal third of the SCN, however, a small population of SCN cells in the dorsolateral aspect of the nucleus continue to express Fos after light stimulation at CT 19, despite pretreatment with TFMPP (right bottom).
Fig. 12.
Dose-dependent effect of systemic administration of TFMPP on light-induced Fos expression in the SCN. Data represent the mean ± SEM of three to five animals/TFMPP group. The number of light-stimulated Fos-ir cells in the SCN is significantly reduced at TFMPP doses of 0.5 mg/kg and higher (p < 0.05) relative to the vehicle (0 mg/kg) group (n = 9). TFMPP alone did not induce Fos expression in the SCN (not shown).
DISCUSSION
The present study demonstrates that 5HT agonists with an affinity for the 5HT1B receptor subtype, administered systemically or directly into the SCN, inhibit light-induced phase shifts of the circadian activity rhythm. It was also shown that systemic injection of TFMPP before light stimulation inhibits expression of thec-fos gene product in the SCN. These results, taken together with the additional observation that bilateral enucleation reduces 5HT1B receptor binding in the SCN, are consistent with the interpretation that 5HT1B receptors are localized presynaptically on RHT axon terminals in the SCN and that activation of these receptors elicits an inhibition of retinohypothalamic neurotransmission.
The interpretation that the effects of TFMPP on light-induced phase shifts involve activation of 5HT1B receptors is supported by the inability of pretreatment with either the selective 5HT1A antagonist (+)WAY 100135 (Cliffe et al., 1993) or the selective 5HT2A/2C antagonist mesulergine (Hoyer et al., 1994) to diminish the inhibitory effects of TFMPP. It is important to note that (+)WAY 100135 has been shown to block the inhibitory effects of the 5HT1A/7 receptor agonist 8-OH-DPAT on light-induced phase shifts (Weber et al., 1996). Additional support for the interpretation that these effects of TFMPP are mediated via its affinity for the 5HT1B receptor subtype is provided by the finding that pretreatment with the nonselective 5HT1A/1Bantagonist methiothepin significantly reduced TFMPP inhibitory effects on light-induced phase shifts. Moreover, systemic application of the pyrroloquinoxaline CGS 12066A, a more selective 5HT1Breceptor agonist with relatively little 5HT1A activity and negligible 5HT2 affinity (Neale et al., 1987), also inhibited light-induced phase shifts.
5HT1B receptors are localized primarily on axon terminals in the CNS. In several well characterized neuronal structures (e.g., hippocampus, cerebellum, caudate-putamen, and retina), 5HT1B receptor mRNA, determined by in situhybridization, is localized in neuronal cell bodies (Voight et al., 1991; Jin et al., 1992; Maroteaux et al., 1992; Boschert et al., 1994), whereas 5HT1B binding sites, determined by autoradiography, are found in regions receiving efferent projections from these cell bodies (Hoyer et al., 1985; Boulenguez et al., 1991; Segu et al., 1991;Palacios et al., 1992; Boschert et al., 1994). Thus, for example, 5HT1B mRNA is found in the cell bodies of retinal ganglion cells, although no 5HT1B binding sites have been detected in the retina (Boschert et al., 1994). Conversely, target sites of ganglion cell retinofugal projections [e.g., SCN, SC, lateral geniculate nucleus (LGN)] exhibit high to moderate levels of 5HT1B binding sites (Manrique et al., 1993, 1994; Prosser et al., 1993; Boschert et al., 1994; Mooney et al., 1994), whereas these same retinorecipient regions express very little or no 5HT1B receptor mRNA (Roca et al., 1993; Boschert et al., 1994). Our finding of a decrease in 5HT1B binding sites in the SC after enucleation is in agreement with previous work suggesting that 5HT1B receptors are located on retinal axon terminals (Segu et al., 1986; Waeber and Placios, 1990; Mooney et al., 1994).
The finding presented herein that 5HT1B binding sites decrease in the SCN after bilateral enucleation suggests further that presynaptic 5HT1B receptors might be a general property of the majority of retinal axon terminals in the brain, including optic fibers of the RHT. Because the RHT seems to originate from a subset of retinal ganglion cells distinct from those that give rise to the major visual projections to the SC and LGN (Pickard, 1982; Pickard et al., 1982; Card et al., 1991; Moore et al., 1995), the demonstration that 5HT1B presynaptic receptors are common to the majority of optic fibers would indicate that the morphological type of retinal ganglion cell does not define the population of ganglion cells that synthesize 5HT1B presynaptic receptors. The data also suggest that 5HT1B receptors are not located on all retinal ganglion cells. The inability of TFMPP to eliminate all light-induced Fos expression in the SCN at a dose higher than that necessary to completely inhibit light-induced behavioral phase shifts (10 mg/kg) suggests that a subset of RHT axons projecting to a restricted region of the SCN continue to release neurotransmitter in response to photic stimulation of the retina. Our inability to detect a change in 5HT1B binding in the SCN after monocular enucleation may indicate compensatory changes in 5HT1B binding sites (pre- or postsynaptic) in the SCN after removal of one eye, or it may simply reflect a level of reduction in 5HT1B binding sites that borders the limits of the resolution of our assay.
Activation of 5HT1B receptors causes the inhibition of neurotransmitter release. In the hippocampus, 5HT1Breceptors located on cholinergic terminals inhibit acetylcholine release (Maura and Raiteri, 1986); in the midbrain, activation of 5HT1B receptors inhibits GABA release onto dopamine-containing neurons (Johnson et al., 1992), and in the cingulate cortex, 5HT1B receptors presynaptically inhibit the release of excitatory amino acids at synapses onto prefrontal pyramidal neurons (Tanaka and North, 1993). Rhoades and co-workers have also presented evidence from single unit recordings from the hamster SC demonstrating 5HT1B presynaptic inhibition of retinotectal excitatory amino acid neurotransmission (Huang et al., 1993; Mooney et al., 1994). There is now general agreement that the 5HT1B receptor is localized predominantly on axon terminals in the brain (Hen, 1992; Boschert et al., 1994; Saudou and Hen, 1994;Doucet et al., 1995).
The synthesis of neurotransmitter receptors in retinal ganglion cells followed by their retinofugal transport and subsequent insertion into the presynaptic axon terminal is not a phenomenon unique to the 5HT1B receptor subtype. There is substantial evidence that nicotinic acetylcholine receptors are synthesized in the retina and transported to the optic tectum in the goldfish, frog, and chick (Henley et al., 1986; Sargent et al., 1989; Brito et al., 1992). Immunocytochemical localization of neuronal nicotinic receptors has also been described in the entire visual system of the rat, including the retina, optic nerve and tract, and all of the major terminal fields of the optic nerve except the SCN (Swanson et al., 1987). There is also electrophysiological data suggesting that GABAB receptors are located presynaptically on RHT terminals in the SCN (Jiang et al., 1995). Interestingly, the GABAB receptor agonist baclofen, injected systemically, also blocks light-induced phase shifts of the hamster circadian activity rhythm (Ralph and Menaker, 1989).
The ability of TFMPP applied directly to the SCN region to block light-induced phase shifts, and the reduction in 5HT1Bbinding sites in the SCN after bilateral enucleation, suggests that TFMPP acts at the level of the SCN to inhibit photic phase shifts and is consistent with the activation of 5HT1B receptors localized on RHT axon terminals in the SCN. There is, however, very little morphological evidence demonstrating 5HT axo–axonic synapses in the SCN. The number of 5HT varicosities making conventional synapses on somas and dendrites in the SCN is relatively high (45%) compared with other regions of the visual system. Although analogous to other retinorecipient regions, virtually no 5HT axon terminals have been reported to make axo–axonic synapses in the SCN (Kiss et al., 1984;Bosler and Beaudet, 1985; Bosler, 1989), but Ugrumov and colleagues (1994) recently described 5HT-immunopositive axons establishing axo–axonic synapses in the SCN of the young rat. Despite the paucity of data illustrating conventional 5HT axo–axonic synapses in the SCN, the synaptic organization of 5HT axon varicosities in retinorecipient structures such as the SCN and SC seems to be in accord with the well documented observation in the cerebral cortex that 5HT varicosities are rarely engaged in morphologically differentiated synaptic junctions (Smiley and Goldman-Rakic, 1996). In the SCN as well as in the cerebral cortex, however, 5HT varicosities are frequently observed to be in apposition to non-5HT axonal terminals, and thus these appositional contacts may provide the structural basis for the presynaptic control of other transmitters by 5HT (Beaudet and Descarries, 1978; Seguela et al., 1989). The recent production of an antibody directed against the mouse 5HT1B receptor will provide a useful tool for identifying these receptors on axon terminals in the SCN (Grimaldi et al., 1995).
Fos expression in the SCN after light exposure at night represents a cellular correlate of the behavioral response of the SCN circadian oscillator to light (Rea, 1989; Aronin et al., 1990; Colwell et al., 1990; Kornhauser et al., 1990; Rusak et al., 1990; Rea et al., 1993a,1993b) and seems to be required for photic phase shifting (Wollnik et al., 1995). The ability of TFMPP to inhibit light-induced Fos expression in the SCN in a dose-dependent manner indicates that the site of action of this compound is “upstream” of the signal transduction processes that lead to c-fos expression and is consistent with a presynaptic site of action on RHT terminals. Several lines of investigation suggest that excitatory amino acids mediate fast excitatory neurotransmission at RHT synapses in the SCN (Kim and Dudek, 1991; Castel et al., 1993; Rea et al., 1993a,b); several glutamatergic receptor antagonists applied systemically or locally in the SCN region block the phase-shifting effects of light on locomotor activity and reduce Fos expression in the SCN (Colwell et al., 1990; Abe et al., 1991; Vindlacheruvu et al., 1992; Rea et al., 1993a; Mikkelsen et al., 1995). These antagonists, however, fail to completely eliminate Fos expression in the SCN, with the remaining Fos-expressing cells localized to a discrete region in the dorsolateral aspect of the caudal SCN (Abe et al., 1991; Vindlacheruvu et al., 1992), very similar to the dorsolateral region of the SCN noted in the present study where light-induced Fos expression remains despite pretreatment with TFMPP. Taken together, these results suggest that a small subset of retinal ganglion cells innervating the dorsolateral portion of the caudal SCN may be neurochemically distinct from the remainder of the retinal ganglion cells comprising the RHT. Indeed, Treep and co-workers (1995) suggested recently that the dorsolateral SCN may receive selective input from retinal ganglion cells that send bifurcating axonal projections to both the SCN and IGL (Pickard, 1985). Moreover, the SCN localization of the calcium binding protein calbindin corresponds to this dorsolateral region of the caudal SCN in the hamster (Silver et al., 1996). Thus, it seems that the dorsolateral aspect of the caudal SCN of hamsters is neurochemically and neuroanatomically distinct from the remainder of the nucleus and is innervated by a specific subset of RHT axons.
In summary, the present findings suggest that activation of 5HT1B receptors located on retinal axon terminals in the SCN inhibit the effect of light on circadian phase and on Fos expression in the SCN. We therefore propose that 5HT1Breceptors play an important role in the modulation of retinal input to the SCN by serotonin.
Footnotes
This work was supported by Air Force Office of Scientific Research (AFOSR) Grant 92-AL-004 (M.A.R.). G.E.P. is an AFOSR University Resident Research Program Fellow. E.T.W. is a National Research Council Associate. A.F.R. was an Air Force Summer Research Fellow. We acknowledge the excellent technical assistance of Matt Cato and Anna Marie Michel.
Correspondence should be addressed to Michael A. Rea, Biological Rhythms and Integrative Neuroscience Institute, 2504 Gillingham Road, Suite 25, Armstrong Laboratory (CFTO), Brooks AFB, TX 78235-5104.
Gary E. Pickard’s current address: Department of Anatomy and Neurobiology, Colorado State University, Fort Collins, CO 80523-1670.
REFERENCES
- 1.Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett. 1991;127:9–12. doi: 10.1016/0304-3940(91)90881-s. [DOI] [PubMed] [Google Scholar]
- 2.Aronin N, Sagar SM, Sharp FR, Schwartz WJ. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc Natl Acad Sci USA. 1990;87:5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azmitia EC, Segal M. An autoradiographic analysis of differential ascending projections of the dorsal and median raphe nuclei of the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 4.Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- 5.Block M, Zucker I. Circadian rhythms of rat locomotor activity after lesions of the midbrain raphe nuclei. J Comp Physiol. 1976;109:235–247. [Google Scholar]
- 6.Boer GJ, Griffioen HA. Developmental and functional aspects of grafting of the suprachiasmatic nucleus in the Brattleboro rat. Eur J Morphol. 1990;28:330–345. [PubMed] [Google Scholar]
- 7.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominately on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 8.Bosler O. Ultrastructural relationships of serotonin and GABA terminals in the rat suprachiasmatic nucleus: evidence for a close interconnection between two afferent systems. J Neurocytol. 1989;18:105–113. doi: 10.1007/BF01188429. [DOI] [PubMed] [Google Scholar]
- 9.Bosler O, Beaudet A. VIP neurons as prime synaptic targets for serotonin afferents in rat suprachiasmatic nucleus: a combined radioautographic and immunocytochemical study. J Neurocytol. 1985;14:749–763. doi: 10.1007/BF01170826. [DOI] [PubMed] [Google Scholar]
- 10.Boulenguez P, Chauveau J, Segu L, Morel A, Delaage M, Lanoir J. Pharmacological characterization of serotonin-O-carboxymethyl-glycyl-tyrosinamide, a new selective indolic ligand for 5-hydroxytryptamine 5-HT1B and 5-HT1D binding sites. J Pharmacol Exp Ther. 1991;259:1360–1365. [PubMed] [Google Scholar]
- 11.Brito LRG, Keyser KT, Lindstrom JM, Karten HJ. Immunohistochemical localization of nicotinic acetylcholine receptor subunits in the mesencephalon and diencephalon of the chick (Gallus gallus). J Comp Neurol. 1992;317:325–340. doi: 10.1002/cne.903170402. [DOI] [PubMed] [Google Scholar]
- 12.Card JP, Whealy ME, Robbins AK, Moore RY, Enquist LW. Two α-herpes virus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 13.Castel M, Belenky MA, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain: a combined in situ hybridization/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 15.Chopin P, Chantal M, Briley M. Neuropharmacology of 5-hydroxytryptamine1B/D receptor ligands. Pharmacol Ther. 1994;62:385–405. doi: 10.1016/0163-7258(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 16.Cliffe IA, Brightwell CI, Fletcher A, Forster EA, Mansell HL, Reilly Y, Routledge C, White AC. (S)-N-tert-Butyl-3-(4-(2-methoxyphenyl)-piperazine-1-yl)-2-phenylpropanamide [(S)-WAY-100135]: a selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. J Med Chem. 1993;36:1509–1510. doi: 10.1021/jm00062a028. [DOI] [PubMed] [Google Scholar]
- 17.Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- 18.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- 19.DeCoursey PJ, Buggy J. Restoration of circadian locomotor activity in arrhythmic hamsters by fetal SCN transplants. Comp Endocrinol. 1988;7:49–54. [Google Scholar]
- 20.Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotoninergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- 21.Grimaldi B, Cloez-Tayarani I, Fillion MP, Mazie JC, Hen R, Fillion G. Production and characterization of an antibody directed against the mouse 5HT1B receptor. Neurosci Res. 1995;24:97–101. doi: 10.1016/0168-0102(95)00974-4. [DOI] [PubMed] [Google Scholar]
- 22.Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992;13:160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscope study of retinohypothalamic connections. Z Zellforsch. 1972;135:1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- 24.Henley JM, Lindstrom JM, Oswald RE. Acetylcholine receptor synthesis in retina and transport to optic tectum in goldfish. Science. 1986;232:1627–1629. doi: 10.1126/science.3715468. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer D, Engel G, Kalkman HO. Characterization of the 5-HT1B recognition site in rat brain: binding studies with [125I]iodocyanopindolol. Eur J Pharmacol. 1985;118:1–12. doi: 10.1016/0014-2999(85)90657-0. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 27.Huang XG, Mooney RD, Rhoades RW. Effects of serotonin on retinotectal-, corticotectal-, and glutamate-induced activity in the superior colliculus of the hamster. J Neurophysiol. 1993;70:723–732. doi: 10.1152/jn.1993.70.2.723. [DOI] [PubMed] [Google Scholar]
- 28.Jiang ZG, Allen CN, North RA. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience. 1995;64:813–819. doi: 10.1016/0306-4522(94)00429-9. [DOI] [PubMed] [Google Scholar]
- 29.Jin H, Oksenberg D, Ashkenazi A, Peroutka SJ, Duncan AM, Rozmahel R, Yang Y, Mengold G, Palacios JM, O’Dowd BF. Characterization of the human 5-hydroxytryptamine1B receptor. J Biol Chem. 1992;267:5735–5738. [PubMed] [Google Scholar]
- 30.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawahara F, Saito H, Katsuki H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J Physiol (Lond) 1994;478:67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol (Lond) 1991;44:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiss J, Leranth C, Halaz B. Serotonergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus: a combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci Lett. 1984;44:119–124. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- 34.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. Oxford UP; New York: 1991. [Google Scholar]
- 35.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 36.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant: immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovenberg TW, Baron BM, deLecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 38.Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoromethylphenyl)piperazine on locomotor activity. J Pharmacol Exp Ther. 1989;249:155–164. [PubMed] [Google Scholar]
- 39.Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- 40.Manrique C, Segu L, Hery F, Hery M, Faudon M, Francois-Bellan AM. Increase of central 5HT1B binding sites following 5,7-dihydroxytryptamine axotomy in the adult rat. Brain Res. 1993;623:345–348. doi: 10.1016/0006-8993(93)91452-x. [DOI] [PubMed] [Google Scholar]
- 41.Manrique C, Francois-Bellan AM, Segu L, Becquet D, Hery M, Faudon M, Hery F. Impairment of serotonergic transmission is followed by adaptive changes in 5HT1B binding sites in the rat suprachiasmatic nucleus. Brain Res. 1994;663:93–100. doi: 10.1016/0006-8993(94)90466-9. [DOI] [PubMed] [Google Scholar]
- 42.Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression and localization in motor control centers. Proc Natl Acad Sci USA. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maura G, Raiteri M. Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol. 1986;129:333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- 44.Meijer JH, Rietveld WJ. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989;69:671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- 45.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikkelsen JD, Larsen PJ, Mick G, Vrang N, Ebling FJP, Maywood ES, Hastings MH, Moller M. Gating of retinal inputs through the suprachiasmatic nucleus: role of excitatory neurotransmission. Neurochem Int. 1995;27:263–272. doi: 10.1016/0197-0186(95)00039-b. [DOI] [PubMed] [Google Scholar]
- 47.Miller JD, Fuller CA. The response of suprachiasmatic neurons of the rat hypothalamus to photic and serotonergic stimulation. Brain Res. 1990;515:155–162. doi: 10.1016/0006-8993(90)90590-8. [DOI] [PubMed] [Google Scholar]
- 48.Mooney RD, Shi MY, Rhoades RW. Modulation of retinotectal transmission by presynaptic 5-HT1B receptors in the superior colliculus of the adult hamster. J Neurophysiol. 1994;72:3–13. doi: 10.1152/jn.1994.72.1.3. [DOI] [PubMed] [Google Scholar]
- 49.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 50.Moore RY, Halaris AE, Jones BA. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 51.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 52.Morin LP, Blanchard J. Depletion of brain serotonin by 5,7-DHT modifies hamster circadian rhythm response to light. Brain Res. 1991;566:173–185. doi: 10.1016/0006-8993(91)91696-x. [DOI] [PubMed] [Google Scholar]
- 53.Neale RF, Fallon SL, Boyar WC, Wasley JWF, Martin LL, Stone GA, Glaeser BS, Sinton CM, Williams M. Biochemical and pharmacological characterization of CGS 12066B, a selective serotonin-1B agonist. Eur J Pharmacol. 1987;136:1–9. doi: 10.1016/0014-2999(87)90772-2. [DOI] [PubMed] [Google Scholar]
- 54.Offord SJ, Ordway GA, Frazer A. Application of [125I]Iodocyanopindolol to measure 5-hydroxytryptamine-1B receptors in the brain of the rat. J Pharmacol Exp Ther. 1988;244:144–153. [PubMed] [Google Scholar]
- 55.Palacios JM, Waeber C, Bruinvels AT, Hoyer D. Direct visualization of serotonin1D receptors in the human brain using a new iodinated radioligand. Mol Brain Res. 1992;13:175–178. doi: 10.1016/0169-328x(92)90060-o. [DOI] [PubMed] [Google Scholar]
- 56.Pickard GE. The afferent connection of the suprachiasmatic nucleus of the golden hamster with emphasis on retinohypothalamic projection. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- 57.Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- 58.Pickard GE, Turek FW, Lamperti AA, Silverman AJ. The effect of neonatally administered monosodium glutamate (MSG) on the development of retinofugal projections and the entrainment of circadian locomotor activity. Behav Neural Biol. 1982;34:433–444. doi: 10.1016/s0163-1047(82)91873-8. [DOI] [PubMed] [Google Scholar]
- 59.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 60.Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system; I. In vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- 61.Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 63.Rea MA. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989;23:577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- 64.Rea MA, Buckley B, Lutton LM. Local administration of EEA antagonists blocks light-induced phase shifts and c-fos expression in hamster SCN. Am J Physiol. 1993a;34:R1191–R1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- 65.Rea MA, Michel AM, Lutton LM. Is Fos expression necessary and sufficient to mediate light-induced phase advances of the suprachiasmatic circadian oscillator? J Biol Rhythms. 1993b;8:S59–S64. [PubMed] [Google Scholar]
- 66.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roca AL, Weaver DR, Reppert SM. Serotonin receptor gene expression in the rat suprachiasmatic nuclei. Brain Res. 1993;608:159–165. doi: 10.1016/0006-8993(93)90789-p. [DOI] [PubMed] [Google Scholar]
- 68.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 69.Saitoh Y, Matsui Y, Nihonmatsu I, Kawamura H. Cross-species transplantation of the suprachiasmatic nuclei from rats to Siberian chipmunks (Eutamias sibiricus) with suprachiasmatic lesions. Neurosci Lett. 1991;123:77–81. doi: 10.1016/0304-3940(91)90162-m. [DOI] [PubMed] [Google Scholar]
- 70.Sargent PB, Pike SH, Nadel DB, Lindstrom JM. Nicotinic acetylcholine receptor-like molecules in the retina, retinotectal pathway, and optic tectum of the frog. J Neurosci. 1989;9:565–573. doi: 10.1523/JNEUROSCI.09-02-00565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saudou F, Hen R. 5-hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int. 1994;25:503–532. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 72.Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res. 1984;1:67–72. doi: 10.1016/0168-0102(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 73.Segu L, Abdelkefi J, Dusticier G, Lanoir J. High-affinity serotonin binding sites: autoradiographic evidence for their location on retinal afferents in the rat superior colliculus. Brain Res. 1986;384:205–217. doi: 10.1016/0006-8993(86)91156-x. [DOI] [PubMed] [Google Scholar]
- 74.Segu L, Chauveau J, Boulenguez P, Morel A, Lanoir J, Delaage M. Synthesis and pharmacological study of radioiodinated serotonin derivative specific of 5HT1B and 5HT1D binding sites of the central nervous system. C R Acad Sci [III] 1991;312:655–661. [PubMed] [Google Scholar]
- 75.Seguela P, Watkins KC, Descarries L. Ultrastructural relationships of serotonin axon terminals in the cerebral cortex of the adult rat. J Comp Neurol. 1989;289:129–142. doi: 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- 76.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibition of light-induced fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Res. 1993;621:181–188. doi: 10.1016/0006-8993(93)90105-v. [DOI] [PubMed] [Google Scholar]
- 77.Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. NeuroReport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- 78.Smale L, Michels KM, Moore RY, Morin LP. Destruction of the hamster serotonergic system by 5,7-DHT: effects on circadian rhythm phase, entrainment, and response to triazolam. Brain Res. 1990;515:9–19. doi: 10.1016/0006-8993(90)90570-2. [DOI] [PubMed] [Google Scholar]
- 79.Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 80.Sollars PJ, Pickard GE. Neural heterografts as a model for the study of mammalian circadian behavior. In: Marwah J, Teitelbaum H, Prasad KN, editors. Neural transplantation, CNS neuronal injury and regeneration: recent advances. CRC; Boca Raton, FL: 1994. pp. 161–181. [Google Scholar]
- 81.Sollars PJ, Pickard GE. Vasoactive intestinal peptide efferent projections of the suprachiasmatic nucleus in anterior hypothalamic transplants: correlation with functional recovery of circadian behavior. Exp Neurol. 1995;136:1–11. doi: 10.1006/exnr.1995.1078. [DOI] [PubMed] [Google Scholar]
- 82.Sollars PJ, Kimble DP, Pickard GE. Restoration of circadian behavior by anterior hypothalamic heterografts. J Neurosci. 1995;15:2109–2122. doi: 10.1523/JNEUROSCI.15-03-02109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 84.Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunocytochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka E, North RA. Cocaine enhancement of the action of 5-hydroxytryptamine in rat cingulate cortex in vitro. Neurosci Lett. 1993;163:50–52. doi: 10.1016/0304-3940(93)90226-b. [DOI] [PubMed] [Google Scholar]
- 86.Treep JA, Abe H, Rusak B, Goguen DM. Two distinct retinal projections to the hamster suprachiasmatic nucleus. J Biol Rhythms. 1995;10:299–307. doi: 10.1177/074873049501000403. [DOI] [PubMed] [Google Scholar]
- 87.Turek FW. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- 88.Ugrumov MV, Popov AP, Vladimirov SV, Kasmambetova S, Novodjilova AP, Tramu G. Development of the suprachiasmatic nucleus in rats during ontogenesis: serotonin-immunopositive fibers. Neuroscience. 1994;58:161–165. doi: 10.1016/0306-4522(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 89.van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- 90.Vindlacheruvu RR, Ebling FJP, Maywood ES, Hastings MH. Blockade of glutamatergic neurotransmission in the suprachiasmatic nucleus prevents cellular and behavioral responses of the circadian system to light. Eur J Neurosci. 1992;4:673–679. doi: 10.1111/j.1460-9568.1992.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 91.Voight MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991;10:4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waeber CW, Palacios JM. 5-HT1 receptor binding sites in the guinea pig superior colliculus are predominantly of the 5-HT1D class and are presynaptically located on primary retinal afferents. Brain Res. 1990;528:207–211. doi: 10.1016/0006-8993(90)91659-5. [DOI] [PubMed] [Google Scholar]
- 93.Weber ET, Gannon RL, Michel AM, Gillette MU, Rea MA. Nitric oxide synthase inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 1995;692:137–142. doi: 10.1016/0006-8993(95)00685-j. [DOI] [PubMed] [Google Scholar]
- 94.Weber ET, Michel AM, Gannon RL, Rea MA. Local serotonin agonists dose-dependently attenuate light-induced phase advances of circadian activity rhythms in hamsters. Soc Neurosci Abstr. 1996;22:1141. [Google Scholar]
- 95.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen KH, Herdegen T. Block of c-Fos and JunB expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. J Neurosci. 1995;7:388–93. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 96.Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]