Abstract
The ferret retinogeniculate projection segregates into eye-specific layers during the first postnatal week and into ON/OFF sublaminae, which receive inputs from either on-center or off-center retinal ganglion cells, during the third and fourth postnatal weeks. The restriction of retinogeniculate axon arbors into eye-specific layers appears to depend on action potential activity (Shatz and Stryker, 1988) but does not require activation of NMDA receptors (Smetters et al., 1994). The formation of ON/OFF sublaminae is also activity-dependent and is disrupted by in vivo blockade of NMDA receptors (Hahm et al., 1991). To investigate a possible mechanism whereby blockade of postsynaptic NMDA receptors in the lateral geniculate nucleus (LGN) results in changes in the size and position of presynaptic axon arbors, we tested the role of the diffusible messenger nitric oxide (NO) in the development of the retinogeniculate pathway. We found previously that NO synthase (NOS) is transiently expressed in LGN cells during the refinement of retinogeniculate projections (Cramer et al., 1995). In this study, treatment with NG-nitro-l-arginine (l-NoArg), an arginine analog that inhibits NOS, during the third and fourth postnatal weeks resulted in an overall pattern of sublamination that was significantly reduced compared with normal and control animals. Single retinogeniculate axon arbors were located in the middle of eye-specific layers rather than toward the inner or outer half as in normal or control animals. The effect of NOS inhibition was not a consequence of the hypertensive effect of l-NoArg. In contrast to the effect of l-NoArg on the formation of ON/OFF sublaminae, treatment with l-NoArg during the first postnatal week did not disrupt the formation of eye-specific layers. Biochemical assays indicated significant inhibition of NOS during both treatment periods. These data suggest that NO acts together with NMDA receptors in activity-dependent refinement of connections during a specific phase of retinogeniculate development.
Keywords: diffusible messenger, visual system, lateral geniculate nucleus (LGN), pattern formation, eye-specific layers, ON/OFF sublaminae, neuronal activity
The precise pattern of connections underlying adult visual processing in mammals arises from the refinement of less specific connectivity present early in development. The mechanisms by which diffuse connections are refined rely at least in part on neuronal activity. In the ferret, retinogeniculate connections are refined in two distinct phases. During the first postnatal week, retinal axons within the lateral geniculate nucleus (LGN) segregate into eye-specific layers (Linden et al., 1981). During the third and fourth postnatal weeks, afferents to the A and A1 layers, which receive input from the contralateral and ipsilateral eye, respectively, further segregate into sublaminae. The inner sublamina receives inputs from on-center retinal ganglion cells, whereas the outer sublamina receives inputs from off-center retinal ganglion cells (Stryker and Zahs, 1983; Hahm and Sur, 1988). Blockade of afferent activity with tetrodotoxin disrupts the formation of eye-specific layers in the cat retinogeniculate projection (Shatz and Stryker, 1988) and the formation of ON/OFF sublaminae in the ferret (Cramer and Sur, 1996). The formation of ON/OFF sublaminae requires NMDA receptor activation (Hahm et al., 1991) during the third postnatal week, whereas the formation of eye-specific layers during the first postnatal week does not (Smetters et al., 1994). The sequential segregation of retinogeniculate afferents into eye-specific layers in an NMDA receptor-independent manner and into ON/OFF sublayers in an NMDA receptor-dependent manner provides an opportunity to investigate how neuronal activity effects changes in synaptic connections in the LGN and how activity may be transduced via different biochemical pathways during different phases of development.
In retinofugal development, there is strong evidence that presynaptic modifications result from changes in postsynaptic activity. Postsynaptic NMDA receptors contribute significantly to retinogeniculate synaptic transmission (Sillito et al., 1990; Kwon et al., 1991; Esguerra et al., 1992; Ramoa and McCormick, 1994). NMDA receptor antagonists infused into the ferret LGN disrupt the pattern of sublamination of presynaptic retinogeniculate axons; retinal ganglion cell axon arbors are either abnormally large or are restricted in size but terminate in inappropriate locations within the LGN (Hahm et al., 1991). In rats, blockade of NMDA receptors in the superior colliculus leads to abnormal branching of retinocollicular axons (Simon et al., 1992). In the frog retinotectal system, application of NMDA to the tectum reduces the branching of retinal axon arbors and the number of retinotectal synapses (Cline and Constantine-Paton, 1990; Yen et al., 1995). Thus, postsynaptic activity in target structures significantly influences the structure and contacts of presynaptic retinal axons, suggesting a role for a retrograde messenger. Nitric oxide (NO) has been proposed as a diffusible retrograde messenger in the maintenance of hippocampal long-term potentiation (LTP) (Bohme et al., 1991;O’Dell et al., 1991; Schuman and Madison, 1991; Haley et al., 1992; cf. Gribkoff and Lum-Ragan, 1992) and, by analogy, in activity-dependent refinement of connections in the visual pathway during development (Montague et al., 1991; Wu et al., 1994; Cramer and Sur, 1995).
Because NO synthase (NOS), the synthetic enzyme for NO, requires Ca2+ (for review, see Garthwaite, 1991; Bredt and Snyder, 1992; Vincent and Hope, 1992), NO may be produced as a consequence of Ca2+ influx through NMDA receptors. NOS activity, which can be revealed using NADPH–diaphorase histochemistry (Dawson et al., 1991; Hope et al., 1991), is developmentally regulated in the ferret LGN (Cramer et al., 1995). NO is thus at its highest levels in postsynaptic cells during sublaminar refinement. In the present study, we have assessed the role of NO in the formation of both eye-specific layers and ON/OFF sublaminae in the ferret retinogeniculate pathway. We find that NO has a role in the latter process of ON/OFF sublamination, which also involves NMDA receptors, but not in the earlier formation of eye-specific layers, which does not involve NMDA receptors.
MATERIALS AND METHODS
ON/OFF sublamination. Two separate methods were used to study the role of NO in the formation of the overall pattern of ON/OFF sublaminae. First, ferret kits received daily intraperitoneal injections of l-NoArg in three doses: low (0.04 mg/kg/d), intermediate (0.4 mg/kg/d), and high (4–40 mg/kg/d). Control animals received NG-nitro-d-arginine methyl ester (d-NAME) (40 mg/kg/d) or l-NoArg +l-Arginine (40 mg/kg/d of each) daily starting at postnatal day 14 (P14) and continuing through P26. Normal controls received no intraperitoneal injections. On P24, animals were given atropine (3 mg/kg, s.c.) and anesthetized with ketamine (30–40 mg/kg, i.m.). The left eyelid was opened, and an intravitreal injection of 5–10 μl of 4–5% WGA–HRP in distilled water was made into the left eye. At P26, animals were given an overdose of sodium pentobarbital (>100 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde for 5–10 min. Brains were removed, equilibrated in 30% sucrose in 0.1 m phosphate buffer, pH 7.4, containing up to 0.5% paraformaldehyde, and sectioned at 50 μm in the horizontal plane using a freezing microtome. Sections were processed using tetramethylbenzidine (TMB) to reveal HRP histochemically (Mesulam, 1978).
In a second set of experiments, l-NoArg was administered focally over the LGN via osmotic minipumps. P14 ferrets were anesthetized with ketamine (40 mg/kg) and diazepam (0.3 to 0.5 mg/kg, i.p.) or midazolam (0.3 to 0.5 mg/kg, i.m.) and also given atropine (3 mg/kg). An incision was made in the scalp, and an osmotic minipump (Alzet model 2002) containing saline or l-NoArg (0.5 mm) was inserted underneath the skin on the back of the neck. The minipump was connected to a 22 gauge cannula. A small hole was made in the cranium with a 26 gauge needle, and the cannula was inserted through the hole from the dorsal surface through the cortex and above the thalamus, slightly anterior to the LGN, with the tip of the cannula in close proximity to the LGN. Areas connected to the LGN were posterior to the point of entry of the cannula; it is thus unlikely that connected areas were affected by drug treatment, and the issue of mechanical or surgical damage was addressed directly in control experiments using saline minipumps. The cannula was glued in place and covered with dental acrylic, and the wound was sutured. Animals were treated with antibiotics prophylactically during the survival period (until P26). Intraocular injections, histology, and analysis were performed as in animals receiving intraperitoneal injections. The cannulae were tested to ensure that there were no blockages. Alternate sections were processed for Nissl substance to assess the placement of the cannulae.
To assess sublamination, each section through the LGN was given a score on a scale from 0 to 3, based on the proportion of the labeled A layer that contained a pale staining region between inner and outer sublaminae. This assessment was done objectively by a “blind” observer, who did not know which treatment group the sections belonged to. A single score for each animal was obtained from the mean of all scored sections.
Assessment of blood pressure effects. We measured blood pressure in normal animals during the fourth postnatal week and in age-matched animals treated daily from P14 with l-NoArg (40 mg/kg, i.p.). Animals were anesthetized with 0.5 mg/kg midazolam intramuscularly and 50 mg/kg ketamine intramuscularly. The left carotid artery was exposed and cannulated with a 24 gauge catheter attached by saline-filled tubing to a Gould P23 pressure transducer. Blood pressure measurements were displayed digitally on a Gould SP1405 pressure monitor. Measurements were noted every 30 sec for up to 20 min. Animals were then killed with sodium pentobarbital (>100 mg/kg, i.p.).
To determine whether changes in blood pressure account for the effect of l-NoArg on sublamination, we administered 40 mg/kg/d, i.p., l-NoArg together with verapamil (5 mg/kg/d, i.p.), an antihypertensive calcium channel blocker, from P14 to P26. Preliminary acute experiments suggested that this dose of verapamil reversed the pressor effects of l-NoArg. An injection of WGA–HRP was made in the left eye at P24, and blood pressure measurements were made immediately before perfusion on P26. Tissue was processed and analyzed as described above.
Effects of NOS blockade on individual axon arbors.Sublamination of retinogeniculate afferents was assessed at the level of individual retinal ganglion cell axon arbors in animals treated systemically with 40 mg/kg/d l-NoArg or 40 mg/kg/dd-NAME from P14 to P21. Axon labeling and analysis of axon arbors were done at P21 according to the methods outlined in Hahm et al. (1991), so that the effects of NOS blockade could be compared with the effects of NMDA receptor blockade. Ferrets were given an overdose (>100 mg/kg, i.p.) of sodium pentobarbital and were perfused transcardially with chilled, oxygenated (95% O2/5% CO2) artificial ACSF containing (in mm): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 10 dextrose, 20 NaHCO3, 1.2 MgSO4, 2.5 CaCl2, pH 7.4. The brain was removed quickly and placed in chilled ACSF. The cortices were removed, and the diencephalon was hemisected. The pia mater overlying the optic tract was removed, and two or three small deposits of HRP were made in the optic tract overlying the ventral portion of the LGN using pulled glass pipettes coated with concentrated HRP. Diencephalon halves were placed in an aquarium containing ACSF and continuously perfused with 95% O2/5% CO2 at room temperature for 3–5 hr to allow transport of HRP. The tissue was then immersion-fixed in 2% paraformaldehyde/1% glutaraldehyde overnight, allowed to equilibrate in 30% sucrose in 0.1 m phosphate buffer, and sectioned in the horizontal plane at 100 μm. Sections were processed using diaminobenzidine (DAB) histochemistry with cobalt chloride enhancement (Adams, 1981). Briefly, sections were incubated in 0.1 m Tris buffer, pH 7.4, for 5 min; transferred to 1% CoCl2 in Tris buffer for 20 min; rinsed in Tris buffer; transferred to 0.1 m phosphate buffer, pH 7.4; and incubated in DAB (0.03% in phosphate buffer) alone for 20 min and then with 0.03% H2O2 for 20 min. Sections were rinsed in several changes of phosphate buffer, dehydrated through a graded series of alcohols, cleared in Histoclear, and coverslipped.
Axon arbors that were well filled and clearly distinguishable from other axons were reconstructed using a camera lucida with a 63× oil immersion objective. The area of arbors was determined for the polygon formed by the outer branches of the arbor using a drawing tablet with a Neuron Tracing System (Eutectic Electronics). For each section, a line was drawn bisecting the A layer. The sublamination index (Hahm et al., 1991) was determined for each axon as the proportion of the axon arbor area in the half of the A layer containing the majority of that arbor; the sublamination index thus varied from 0.5 (no sublamination) to 1 (complete sublamination).
Eye-specific layers. To study the effect of NOS blockade on the formation of eye-specific layers, animals received intraperitoneal injections of l-NoArg, 20 mg/kg/d, from P1 to P7. This dose was at least as effective at increasing brain [arginine]/[citrulline] as the dose that significantly disrupted ON/OFF sublamination at a later age (see Results). Control animals received d-NAME (20 mg/kg/d); normal, untreated animals were also included in the study. At P7, animals were anesthetized with hypothermia, and both eyelids were opened. The left eye was injected with 5 μl of 5% WGA–HRP (Sigma, St. Louis, MO) in saline or distilled water; the right eye was injected with 5 μl of 1% cholera toxin B (CTB) subunit (List Biological Labs, Campbell, CA) in distilled water. At P8, animals received an overdose of barbiturate anesthesia and were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. After equilibration in 30% sucrose in 0.1m phosphate buffer, brains were sectioned in the horizontal plane at 40 or 50 μm.
One series of sections was used to reveal HRP using TMB, and the series of adjacent sections was used to reveal CTB, using the method described by Angelucci et al. (1996). Briefly, sections were washed in PBS, pH 7.4, rinsed in methanol and 0.3% hydrogen peroxide to quench endogenous and injected peroxidase activity, treated with 0.1m glycine, preincubated in 2–8% normal rabbit serum (NRS, Vector Laboratories, Burlingame, CA) and 2.5% bovine serum albumin (BSA), then incubated in goat anti-CTB primary antibody (List) at a concentration of 1:4000 for 1–2 d at room temperature in a solution containing 2% NRS, 2.5% BSA, and 2% Triton X-100. The primary antibody was rinsed, and the sections were incubated in a solution containing biotinylated rabbit anti-goat antibodies (Vector Laboratories), 2% NRS, 2.5% BSA, and 1% Triton X-100. The Vector ABC kit was used to label the antigen–antibody complex with HRP, together with DAB or the Vector VIP substrate to visualize HRP. Sections were dehydrated in a graded series of alcohol, cleared in Histoclear, and coverslipped.
Camera lucida drawings were made of TMB-stained and CTB-stained material, and drawings of adjacent sections were superimposed to assess the degree of segregation according to the eye of origin. Because tissue stained with the two methods had slightly different extents of shrinkage, the magnification was adjusted to bring adjacent sections in register, using the lateral edges of the nucleus.
HPLC measurements of [arginine] and [citrulline]. To assess the effectiveness of systemic administration ofl-NoArg in the inhibition of brain NOS activity, we injected two animals daily from P14 to P28 with l-NoArg (40 mg/kg, i.p.) and two animals daily from P1 to P8. These animals and three control littermates in each age group were perfused with sterile saline, and the brains were homogenized in 1N perchloric acid buffer. Because NOS produces citrulline from arginine during NO synthesis, we used the ratio [arginine]/[citrulline] as an indicator of NOS activity. Citrulline and arginine concentrations were detected by HPLC with electrochemical detection after precolumn derivatization witho-phtalaldehyde, essentially as described previously for glutamate (Bogdanov and Wurtman, 1994). Amino acid derivates were separated on a 3 μm C18 ODS 80 × 4.6 mm column and detected using an ESA 5200A coulometric detector with an ESA 5014 dual-electrode analytical cell. The first electrode was set at +200 mV and the second at +400mV. The mobile phase delivered at 1.2 ml/min was 0.1m sodium dibasic phosphate buffer, 25% methanol, and 5% acetonitrile, pH 6.4. Standards of arginine and citrulline at known concentrations were also run. The concentration of these amino acids was determined using the area under the peak corresponding to the elution time of the standards.
RESULTS
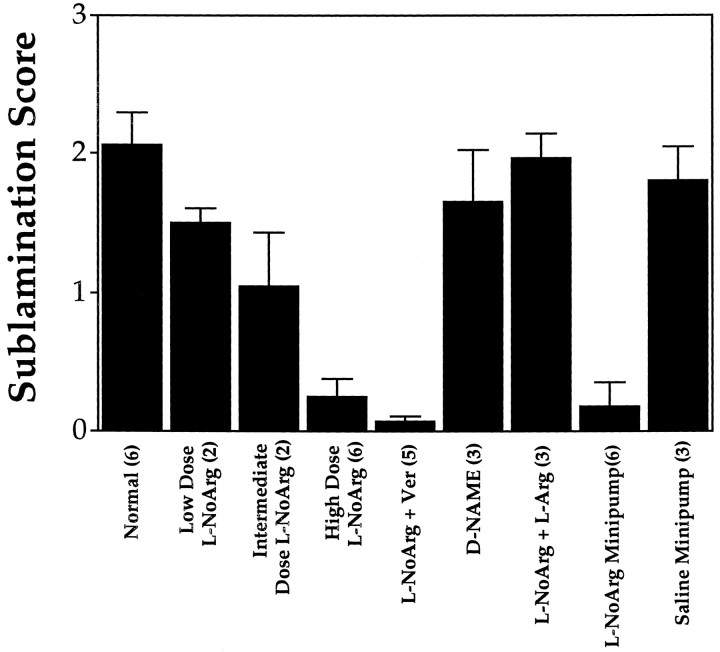
In the first part of this study, we examined the role of NO in the segregation of ON/OFF sublaminae. One group of animals received daily, systemic injections of l-NoArg, a NOS inhibitor, from P14 to P26. Treatment with a high dose of l-NoArg (4–40 mg/kg/d) disrupted formation of sublaminae. Sublamination in normal animals is clearly visible in horizontal sections through the LGN, whereas in treated animals, clearly delineated sublaminae are not evident (Fig. 1A,B). A sublamination score was assigned to each section of the LGN containing visible A and A1 layers. The score ranged from 0 to 3, based on the fraction of the A layer clearly divided by a pale staining intersublaminar zone that approximately bisected the A layer longitudinally; e.g., a score of 1 indicated that one-third of the A layer contained a pale staining intersublaminar zone. The score for each animal was obtained by averaging the scores from each section. On average, 10 sections were scored through each LGN. The scored sections spanned the depth of the LGN containing both A and A1 layers. We found no systematic differences in sublamination scores at different depths within the LGN. Sublamination scores from animals in all conditions are summarized in Figure 2. The mean sublamination score (± SEM) for animals treated with the high dose of l-NoArg was 0.24 ± 0.13 (n = 6). This value was significantly less than that seen in normal animals, 2.1 ± 0.24 (n = 6; p < 0.001, Student’st test). In addition, treated animals had significantly lower sublamination scores than two control groups. One control group (n = 3) received 40 mg/kg/d l-NoArg together with 40 mg/kg/d l-arginine, the normal substrate for the NOS binding site. In these animals (Fig. 1C), the mean sublamination score was 1.96 ± 0.18, which was significantly higher than scores from l-NoArg-treated animals (p < 0.0001). Because addition of excessl-arginine can overcome the effects of NOS blockade, this control supports the assumption that l-NoArg is acting on NOS (Garthwaite, 1991). Another control group (n = 3) received 40 mg/kg/d of d-NAME, an inactive enantiomer. The sublamination score of this group was 1.65 ± 0.37, which was also significantly higher than that of the high dose l-NoArg group (p < 0.01).
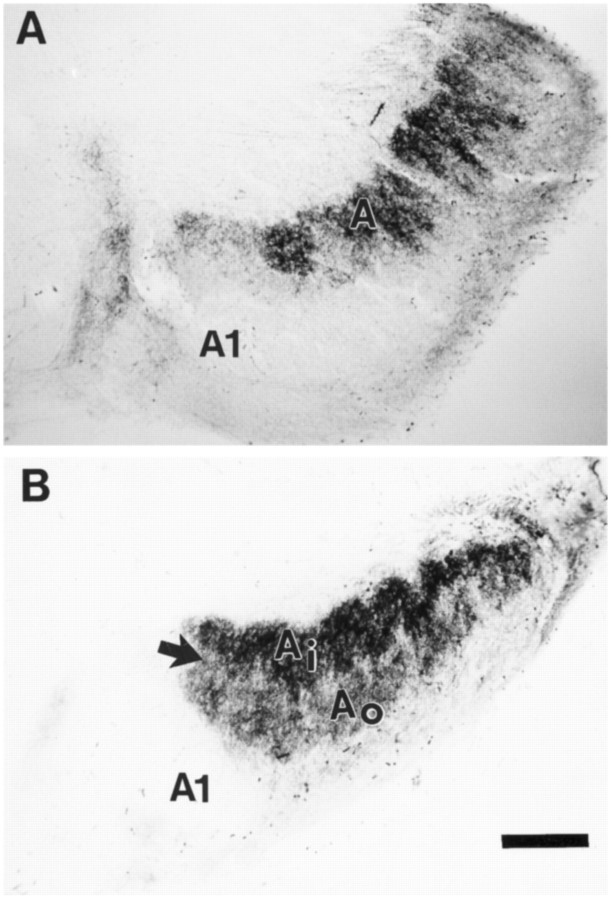
Fig. 1.
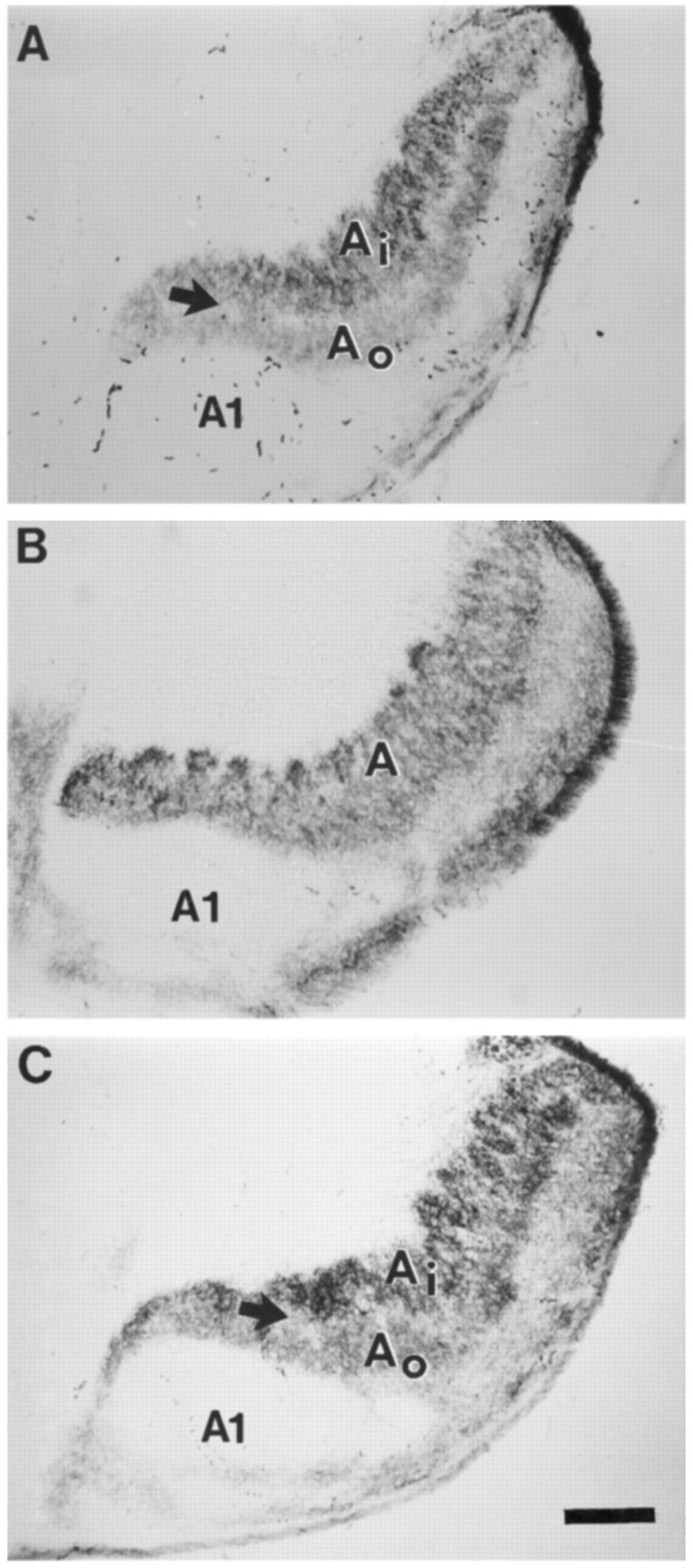
Horizontal sections through the LGN of P26 ferrets after intraocular injections of WGA–HRP in the contralateral eye, viewed with bright-field microscopy. A, Normal ferret LGN. The dark arrow in the contralateral projection to layer A shows a pale staining region between on and off sublaminae. For simplicity, C layers are not labeled in the micrographs.B, LGN from an animal treated with the high dose ofl-NoArg between P14 and P26. A pale staining region between sublaminae is not evident. C, LGN from a control animal treated with l-NoArg together with l-Arg. A pale staining intersublaminar zone is visible. InA–C, the A andA1 layers are indicated. The inner (Ai) and outer (Ao) sublaminae are labeled inA and C where they are visible. Scale bars (shown in C): 200 μm.
Fig. 2.
Summary of sublamination scores (with SEM) for all experiments in this study. Numbers in parenthesesindicate the number of animals in each treatment group. Higher scores signify greater sublamination. Sublamination scores decreased with increasing doses of l-NoArg. The highest dose of systemically applied l-NoArg produced a significant reduction in sublamination compared with normal. Systemic treatment with l-NoArg together with verapamil (Ver) to reverse the pressor effect ofl-NoArg resulted in disrupted sublamination. Control animals treated with d-NAME or l-NoArg together with l-arginine (l-Arg) showed no effect. Focal application of l-NoArg using surgically implanted osmotic minipumps also reduced sublamination, whereas minipumps containing saline had no effect.
To examine the dose-dependence of the effect of l-NoArg on sublamination, an additional group of animals received intermediate (0.4 mg/kg/d; n = 2) or low (0.04 mg/kg/d;n = 2) doses of l-NoArg (Fig. 2). The intermediate-dose group had a sublamination score (± SEM) of 1.04 ± 0.4, and the low-dose group had a sublamination score of 1.5 ± 0.05. When data from all animals receiving systemic injections ofl-NoArg were examined using a linear regression of the sublamination score versus the logarithm of the dose, a significant correlation was found (r = 0.87, p < 0.01). Thus, there is a dose-dependent inhibitory effect of systemically applied l-NoArg on retinogeniculate sublamination.
Animals receiving intraperitoneal injections were monitored, and their overall growth was compared with that of normal animals. Animals receiving high doses of l-NoArg appeared healthy and gained as much weight (as a fraction of their weight before treatment) as animals receiving low doses and animals receiving d-NAME. Animals approximately doubled their weights between P14 and P26. Overall brain morphology appeared normal in all animals.
Although intraperitoneal application of l-NoArg demonstrably reduces NOS activity in the brain (see below), it remains possible that the reduction of sublamination in the LGN results secondarily from systemic or indirect effects of the drug rather than from a direct effect on NOS in LGN cells. Because NO is a vasodilator, one possible systemic effect of l-NoArg is hypertension. We addressed this possibility by measuring mean arterial blood pressure directly during the fourth postnatal week in four animals treated from P14 with daily injections of 40 mg/kg l-NoArg and in four normal, age-matched controls. The normal mean arterial blood pressure (± SEM) was 57.6 ± 4.6 mmHg, and the mean arterial blood pressure in the group treated with l-NoArg was 83.1 ± 4.8 mmHg. The mean arterial blood pressure increased significantly after the treatment period (p < 0.01, Student’s t test). We then treated five animals from P14 to P26 with 40 mg/kg/d l-NoArg together with 5 mg/kg/d verapamil to counteract the hypertensive effect of l-NoArg. The mean arterial blood pressure in this group was 60.0 ± 1.63 mmHg, which did not differ from that of normal animals (p > 0.6). We assessed sublamination in animals treated with l-NoArg together with verapamil. The mean sublamination score in these animals was 0.07 ± 0.03, which was significantly less than scores from normal animals (p < 0.001) and did not differ from scores from animals treated with l-NoArg alone (p = 0.28). The effects of NOS blockade on sublamination are thus unlikely to result from changes in mean arterial blood pressure.
To address the possibility that other indirect effects of systemically applied l-NoArg underlie the observed disruption of sublamination, we applied l-NoArg (0.5 mm) directly over the LGN between P14 and P26 in six animals using surgically implanted osmotic minipumps that released 0.5 μl of solution per hour. In these animals (Fig.3A), sublamination was disrupted to an extent similar to that seen with systemic application (sublamination score = 0.17 ± 0.07; p < 0.001 comparing treated and normal animals). Animals treated with control minipumps containing only saline (Fig. 3B) had a mean sublamination score of 1.80 ± 0.25 (n = 3); this score was significantly greater than that obtained from animals treated focally with l-NoArg (p < 0.001) and did not differ from scores obtained from normal animals (p = 0.5). Nissl staining confirmed that the cannulae were correctly positioned above the LGN. Because the minipumps delivered small regulated amounts of l-NoArg to the LGN (we estimate that the entire LGN has a uniform steady-state concentration of the drug), these results support the view that the effect ofl-NoArg on sublamination results from a reduction of NO in the LGN. In animals treated with l-NoArg through osmotic minipumps, the disruptive effect on sublamination was restricted to the infused side, whereas sublamination in the opposite LGN appeared normal (Fig. 4), confirming the local nature of the effect of NOS blockade.
Fig. 3.
Horizontal sections through the LGN of P26 ferrets after labeling with WGA–HRP in the contralateral eye.A, LGN treated with focal application of 0.5 mml-NoArg from P14 to P26 via an implanted cannula attached to an osmotic minipump. The staining is uniform, and no pale staining in the intersublaminar zone is evident.B, Control LGN after implantation of a cannula attached to an osmotic minipump containing 0.9% saline. As in Figure 1, thearrow indicates the intersublaminar zone, and the A and A1 layers and sublaminae Ai and Ao are labeled. Scale bars (shown inB): 200 μm.
Fig. 4.
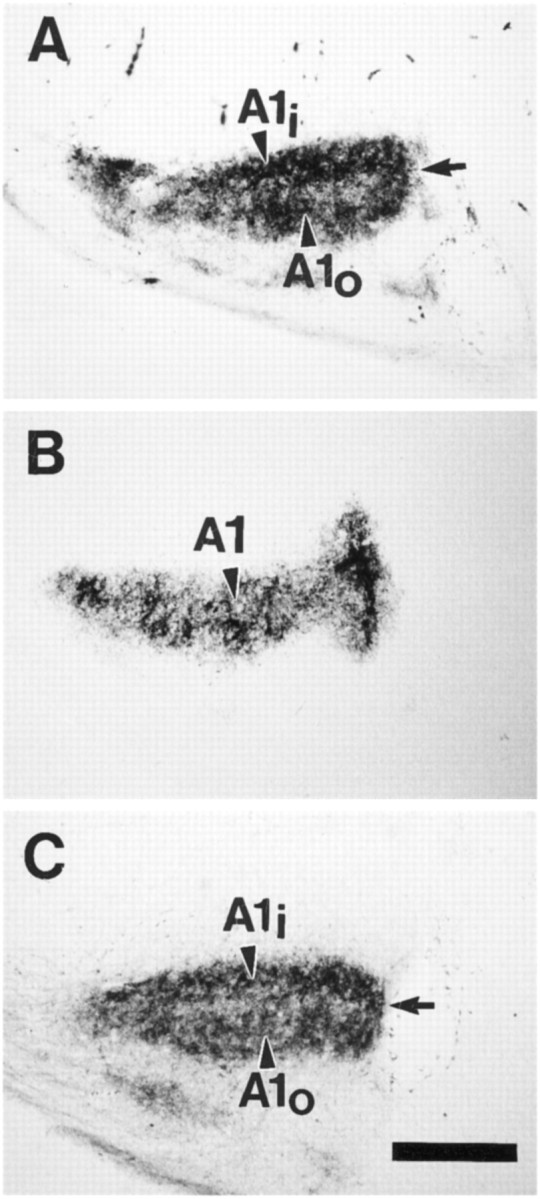
Horizontal sections through the LGNs of P26 ferrets ipsilateral to an intraocular WGA–HRP injection.A, A normal, untreated animal; sublamination is evident in the ipsilateral A1 layer, and sublaminae (A1i andA1o) are evident. The arrow indicates the intersublaminar zone. B, An animal treated withl-NoArg systemically. Sublamination is disrupted in the ipsilateral projection. C, An animal in which the contralateral LGN was treated with focal application ofl-NoArg. Sublamination is evident (A1i andA1o), and the intersublaminar zone is indicated by thearrow. The effects of l-NoArg are thus restricted to the infused side after minipump implantation.
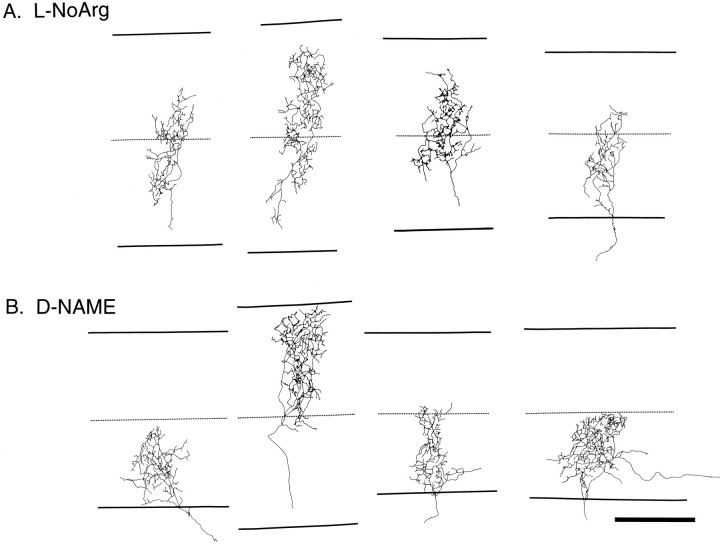
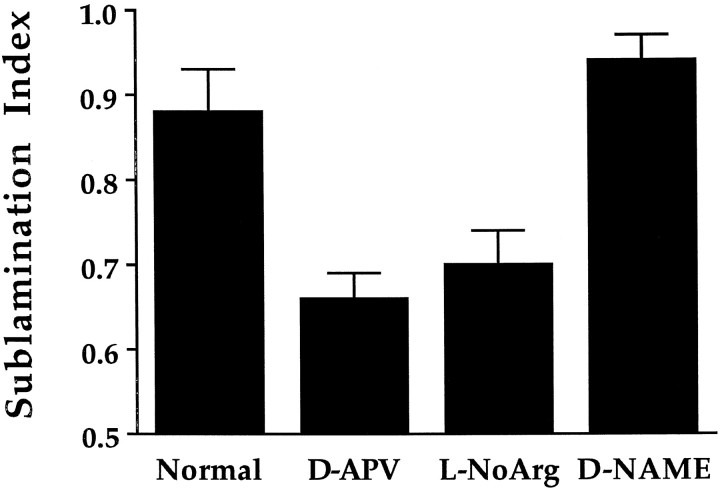
The pattern of sublamination seen after WGA–HRP eye injection results from the labeling of most or all retinal ganglion axons from the eye, and the pale staining intersublaminar region appears to result in normal animals from the relatively sparse termination of axon arbors within that zone (Hahm et al., 1991). We sought to determine how blockade of NOS affects individual axon arbors terminating within the LGN. Axon arbors were reconstructed in four animals treated with 40 mg/kg/d l-NoArg from P14 to P21 and three control animals treated with 40 mg/kg/d d-NAME. Three to four axons were drawn from each brain; reconstructions were made without knowledge of the treatment group from which the tissue was taken. Fourteen axons were drawn from the l-NoArg-treated group, and 11 axons were drawn from the d-NAME-treated group (Fig.5). The mean sublamination index for thel-NoArg group was 0.7 ± 0.04 (SEM), and the mean sublamination index for the d-NAME group was 0.94 ± 0.03. This difference was statistically significant (p < 0.0001, Student’s t test) and remained significant when sublamination indices from axons of the same brain were pooled together so that each animal was considered a single datum (p < 0.01); l-NoArg-treated animals in this case had a mean sublamination index of 0.7 ± 0.03; d-NAME-treated animals had a sublamination index of 0.94 ± 0.02. Sublamination indices forl-NoArg-treated animals in the present study were similar to those found for d-APV-treated animals in the study byHahm et al. (1991); similarly, the sublamination indices ford-NAME-treated animals were similar to those found for normal animals (Fig. 6). As with d-APV treatment (Hahm et al., 1991), axons treated with l-NoArg were abnormally large and/or positioned incorrectly in the intersublaminar zone (Fig. 5), consistent with the interpretation that NOS blockade and NMDA receptor blockade inhibit sublamination in a similar way.
Fig. 5.
Camera lucida tracings of individual retinogeniculate axon arbors reconstructed after labeling with HRP.A, Four representative arbors from animals treated with 40 mg/kg/d l-NoArg from P14 to P21. The borders of the A layer are shown as bold lines above and below each axon arbor; the dashed line bisects these borders and represents an approximation of the sublaminar boundary. The axon arbors in this treatment group extend into both inner and outer sublaminae.B, Four representative arbors from animals treated with 40 mg/kg/d of the inactive isomer d-NAME. These axon arbors tend to be restricted to one-half of the A layer, corresponding to one sublamina. Scale bars (shown in B): 100 μm.
Fig. 6.
Histogram summarizing the sublamination indices at P21 in normal animals and animals treated with drugs from P14 to P21. The sublamination index denotes the fraction of the total arbor area in the half of the layer containing the majority of that arbor. The two bars on the left (normal animals and animals treated with the NMDA receptor blocker d-APV) are data taken fromHahm et al. (1991); error bars indicate SEM. NOS blockade withl-NoArg and NMDA receptor blockade with d-APV result in a similar reduction of the sublamination index, whereas treatment with the control drug d-NAME does not reduce the sublamination index.
We then examined the effect of NOS blockade on the formation of eye-specific layers during the first postnatal week. In this part of the study, it was necessary to use only systemic application of NOS inhibitors, because neonatal animals are too small to accommodate an implanted cannula and osmotic minipump. Animals were treated from P1 to P7 with 20 mg/kg/d l-NoArg (n = 7) or 20 mg/kg/d of the the control drug d-NAME (n = 1), or were left untreated as normal controls (n = 4). The dose of l-NoArg used effectively disrupted sublamination (see above) and had an inhibitory effect on brain NOS (see below). We examined the LGN of these animals at P8, after anterograde labeling of retinogeniculate projections at P7 with intraocular injection of WGA–HRP in one eye and CTB subunit in the other eye. Alternate sections were processed using TMB histochemistry to visualize WGA–HRP (Mesulam, 1978) or immunohistochemistry to visualize CTB (Angelucci et al., 1996). Segregation of inputs from the two eyes was evident in every case. The staining patterns of left and right eye projections to the two LGNs within a single brain were complementary in sections stained using either TMB (Fig.7) or CTB immunohistochemistry. Moreover, within each LGN, the staining pattern of TMB was complementary to that of CTB and together, these labeled the full extent of the LGN (Fig.8). Treated animals did not differ from control animals in weight; animals in both groups increased their weight by 240% during the first postnatal week. Overall brain morphology appeared similar in normal and treated animals.
Fig. 7.
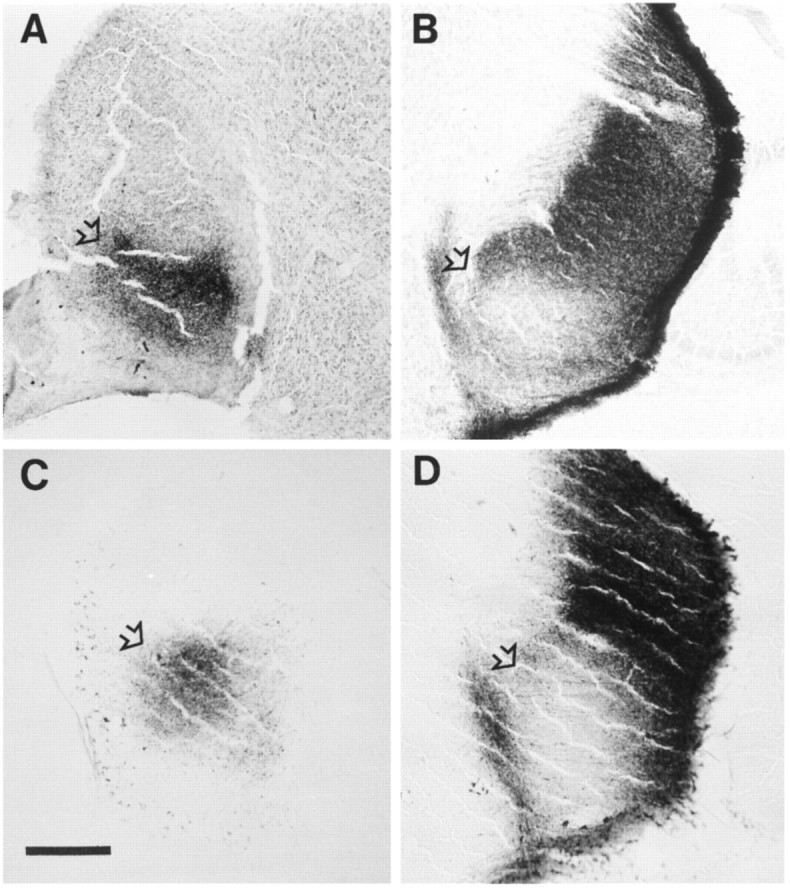
Horizontal sections through the LGNs of P8 animals after anterograde labeling of retinogeniculate projections using intraocular injections of WGA–HRP in the left eye. A,B, Sections from the left (A) and right (B) LGNs of a normal P8 ferret. C,D, Sections through the left (C) and right (D) LGNs of a ferret treated with 20 mg/kg/dl-NoArg from P1 to P8. InA–D, the open arrowsindicate the ipsilateral projection zone, or layer A1. The left and right LGNs show a complementary pattern of labeling in both normal and drug-treated animals. Scale bars (shown in C): 200 μm.
Fig. 8.
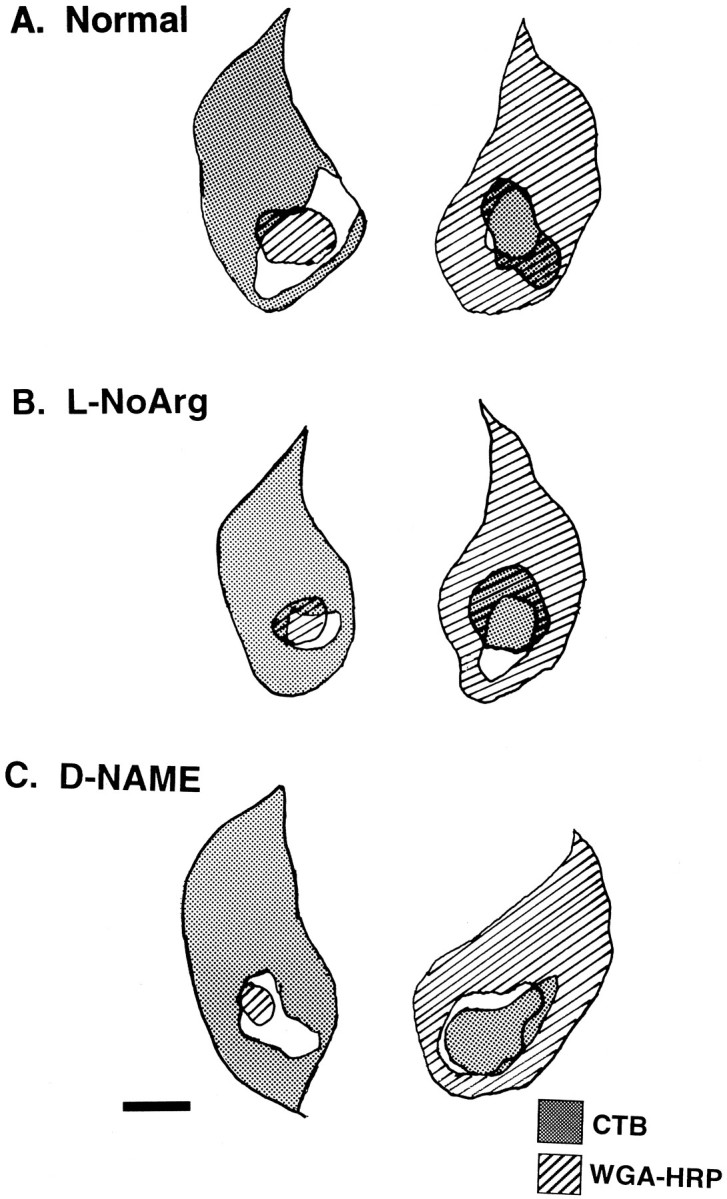
Camera lucida tracings of representative P8 left and right LGN sections in a normal animal (A), an animal treated with the NOS inhibitor l-NoArg (B), and an animal treated with the inactive isomer d-NAME (C). Drawings superimpose adjacent 40 or 50 μm sections processed to reveal WGA–HRP after anterograde transport from the left eye or CTB subunit after transport from the right eye. In all cases, the labeling pattern is similarly complementary, consistent with normal eye-specific layer segregation. Scale bar, 300 μm.
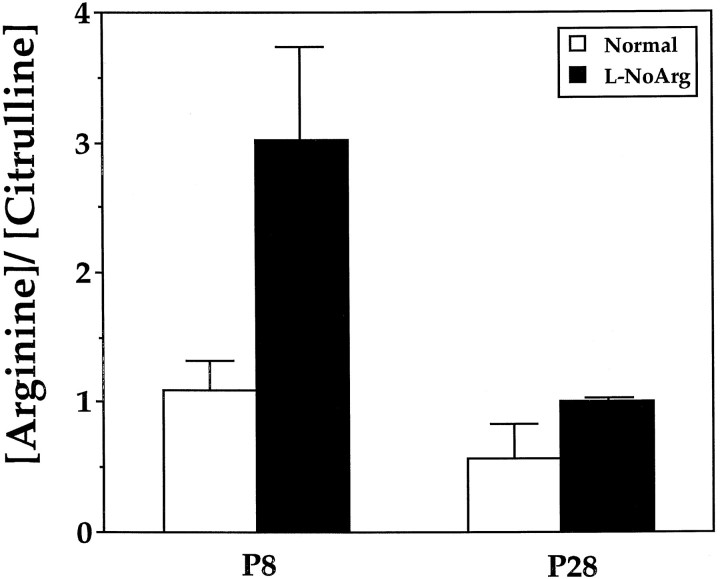
We assessed the effectiveness of systemically appliedl-NoArg on NOS activity in the brain during the period of eye-specific layer formation as well as during the period of ON/OFF sublamination. Intraperitoneal application of l-NoArg inhibits NOS activity in the brains of adult rats (Dwyer et al., 1991); our experiments extend this result to young ferrets. To assess NOS activity, we used HPLC to measure the relative concentrations of arginine and citrulline in brain homogenates of treated and normal animals. Citrulline is produced from arginine by NOS stoichiometrically along with NO; although there are other sources and sinks for both arginine and citrulline, the only pathway by which arginine is converted to citrulline in the brain is via NOS (Ohta et al., 1994). The [arginine]/[citrulline] ratio is thus a meaningful measure of NOS activity. In our material, the [arginine]/[citrulline] ratio for animals treated with 40 mg/kg/d l-NoArg from P14 to P28 (n = 2) was 1.0 ± 0.02, and the ratio for normal, age-matched animals (n = 3) was 0.57 ± 0.26. Treatment with l-NoArg thus increased the [arginine]/[citrulline] ratio by a factor of 1.75. When animals received 20 mg/kg/d l-NoArg from P1 to P8 (n = 2), the [arginine]/[citrulline] ratio was 3.0 ± 0.73, whereas normal, age-matched controls (n = 3) had a ratio of 1.1 ± 0.23 (Fig.9). At this age, treatment with l-NoArg increased the [arginine]/[citrulline] ratio by a factor of 2.8. Across ages, l-NoArg treatment resulted in a significantly increased [arginine]/[citrulline] ratio compared with normal brains (p < 0.02, two-way ANOVA with drug treatment and age as two factors). The ratio was higher at P8 compared with P28 (p < 0.02), possibly reflecting the relatively lower NOS activity at early ages (Cramer et al., 1995). The effectiveness of the drug at P8 versus P28 (the interactive effect of drug treatment and age) appeared to be similar (p = 0.08). Changes in [citrulline] are also a useful measure of NOS activity. In our P28 material,l-NoArg treatment reduced [citrulline] to 86% of normal levels, and in our P8 material, l-NoArg treatment reduced [citrulline] to 60% of normal levels. These decreases in [citrulline] are similar to those found by Ohta et al. (1994) in extracellular dialysates of adult rat brain after treatment withl-NoArg, and are consistent with the findings we obtained using [arginine]/[citrulline]. These measurements indicate that systemic application of l-NoArg significantly reduces NOS activity in the brain during both developmental periods.
Fig. 9.
Histogram summarizing the effect of NOS blockade on relative concentrations of arginine and citrulline in P8 and P28 ferret brain homogenates. The [arginine]/[citrulline] ratio significantly increased after application of l-NoArg (p < 0.02, two-way ANOVA). Error bars indicate SEM.
DISCUSSION
Taken together, our results support a role for NO in retinogeniculate development specifically during the period of ON/OFF sublamination when NMDA receptor activation is also required and when NADPH–diaphorase levels are high in LGN cells. Although NADPH–diaphorase staining is developmentally regulated in LGN cells, staining is present in the neuropil in the LGN at all ages (Cramer et al., 1995). Neuropil staining arises from cholinergic brainstem projections (Bickford et al., 1993). Because blockade of NOS at early ages does not interfere with the formation of eye-specific layers, it is unlikely that NO produced in the neuropil is involved in early retinogeniculate pattern formation. A developmental role for NO has also been found in the chick retinotectal pathway, where NOS expression is highest during the removal of a transient ipsilateral projection (Williams et al., 1994) and blockade of NOS results in the retention of this projection (Wu et al., 1994). NO is thus required for the refinement of eye-specific projections from the retina to the optic tectum in the chick. Interestingly, we show that in the retinogeniculate pathway of the ferret, NO is not required for eye-specific layer refinement, but for a later, NMDA receptor-dependent part of the pathway that segregates according to on- or off-center receptive field properties. Thus, although NO has a role in the development of both the chick retinotectal pathway and the ferret retinogeniculate pathway, the nature of its role may differ, and these differences suggest that several different mechanisms are involved in activity-dependent refinement of the visual system.
Activity-dependent remodeling of retinogeniculate connections during development may resemble activity-dependent synaptic plasticity in certain regions of the adult brain (Cramer and Sur, 1995). In particular, LTP in the CA1 region of the hippocampus may share mechanisms in common with developmental remodeling (Goodman and Shatz, 1993). Postsynaptic activation of NMDA receptors is required to initiate LTP (Bliss and Collingridge, 1993), whereas maintenance of LTP may require presynaptic upregulation of neurotransmitter release (Bekkers and Stevens, 1990; Malinow and Tsien, 1990; Malgaroli et al., 1995). The development of sublaminae in the LGN represents an example of activity-dependent remodeling of connections that may show mechanistic similarities to LTP in the hippocampal CA1 region. Sublamination requires postsynaptic activation of NMDA receptors, which are present and involved in synaptic potentiation in the developing LGN (Mooney et al., 1993). Whereas presynaptic NMDA receptors may be present in visual cortex (Aoki et al., 1994), there is no evidence for their presence in the LGN. Physiological evidence suggests that NMDA receptors are postsynaptic, because holding postsynaptic LGN cells at hyperpolarizing membrane potentials reduces the NMDA component of the postsynaptic current (Esguerra et al., 1992; Ramoa and McCormick, 1994). Additionally, the NMDA antagonist d-APV selectively inhibits the NMDA receptor-mediated component of retinogeniculate EPSPs (Esguerra et al., 1992). Presynaptic NMDA receptors would be expected to increase transmitter release after Ca2+ influx; thus blocking presynaptic receptors would influence all components of the EPSP. By analogy with studies of LTP in the CA1 region of the hippocampus, NO may act downstream of the NMDA receptor, diffusing to the presynaptic terminal to transmit information about postsynaptic activation. In LTP, this signal might result in increased neurotransmitter release (Bekkers and Stevens, 1990; Malinow and Tsien, 1990; Malgaroli et al., 1995), whereas in retinogeniculate development, this signal may result in regulation of presynaptic arbor size and position, because both NMDA receptor blockade (Hahm et al., 1991) and NOS blockade (in the present study) result in inappropriately placed retinogeniculate axon arbors. Determination of the extent of the similarity in the biochemical mechanisms underlying activity-dependent refinement of connections and LTP will require additional investigation.
Possible substrates for NO in the presynaptic terminal during LTP include guanylyl cyclase (East and Garthwaite, 1991), adenosine diphosphate ribosyl transferase (ADPRT) (Schuman et al., 1994), or a combination of both (Abe et al., 1994). It is not known whether either of these potential substrates has a role in development or how they could induce changes in the size and position of afferent axon arbors. NO is known to activate a cGMP-gated cation conductance in retinal ganglion cells via a NO-sensitive guanylyl cyclase (Ahmad et al., 1994), resulting in an increase in intracellular [Ca2+], which could in turn regulate axonal structure (Mattson and Kater, 1987). A possible mode of action with respect to ADPRT is that it acts on growth-associated protein 43 (GAP-43; Coggins et al., 1993; cf. Luo and Vallano, 1995); this protein is expressed in growing axons during development and regeneration (Skene and Willard, 1981; Meiri et al., 1988; Moya et al., 1988) and may have a role in remodeling synaptic connections (Erzurumlu et al., 1990; for review, see Benowitz and Routtenberg, 1987). Another possibility suggested by a study of cultured dorsal root ganglia (Hess et al., 1993) is that NO inhibits growth cone elongation by inhibiting palmitoylation of proteins such as GAP-43 and SNAP-25; this effect is independent of guanylyl cyclase. Although the effect of NO on protein palmitoylation in the developing ferret LGN is not known, NO may influence GAPs via several different pathways.
An alternative mechanism for the action of NO on developing retinogeniculate axons is related to the role of NO in regulating local blood flow. Although our results suggest that increases in blood pressure after application of l-NoArg do not account for the disruption of development in the LGN, NO is involved in the neuronal activity-dependent regulation of local blood flow in the brain (for review, see Iadecola, 1993), and an inhibitor of neuronal NOS decreases regional blood flow in the brain (Kelly et al., 1995). NO produced by NOS in response to NMDA receptor activation might thus regulate the access of presynaptic terminals to circulating factors that are necessary for the control of presynaptic structure.
Although NOS is present in a subset of LGN neurons, it is likely that the retrograde action of NO is both local and diffuse. NO might be released from LGN neurons and influence synaptic stability in the retinal ganglion cell axons innervating them. In addition, NO might diffuse to nearby cells; a mechanism for cooperative strengthening of nearby inputs has been demonstrated in hippocampal LTP by Schuman and Madison (1994). Moreover, as described above, local increases in blood flow as a consequence of NO release would influence a region that includes numerous synapses. Thus, NO might have a local effect on a subset of inputs to the LGN and a more diffuse effect on a larger number of inputs.
The restriction of retinogeniculate arbors into eye-specific layers and subsequently into ON/OFF sublaminae involves the progressive elaboration of connections in appropriate regions of the LGN and the retraction and loss of connections from inappropriate regions. Blocking NMDA receptors causes not only an increase in the proportion of retinogeniculate arbors in the inappropriate sublamina but also a concomitant increase in the number and density of postsynaptic dendritic spines (Rocha and Sur, 1995). Although NO is likely to be produced in the postsynaptic cell after activation of NMDA receptors, it is available simultaneously to both presynaptic axons and postsynaptic cells. It seems reasonable to propose that the coordinated regulation of presynaptic and postsynaptic sites in the LGN during sublamina formation is mediated by NO. On this hypothesis, NMDA receptors and NO provide a signal specifying correlated inputs and coactive presynaptic and postsynaptic terminals. When NMDA receptors are active and NO is present, contacts would stabilize; when NMDA receptors or NOS is blocked, contacts would continue to turn over and reorganize. The present experiments demonstrate that blocking NOS indeed prevents the restriction of retinogeniculate afferent arbors during an NMDA receptor-dependent phase of development; it would be of interest to examine whether blocking NOS leads to an upregulation of spines on postsynaptic dendrites as well.
Our results in the ferret LGN contrast with studies of cat visual cortex, in which ocular dominance plasticity requires NMDA receptor activation (Bear et al., 1990) but does not seem to require NO (Gillespie et al., 1993; Daw et al., 1994). Consistent with this difference, NADPH–diaphorase staining is developmentally regulated in LGN neurons but not in visual cortical neurons. In the cortex, staining is present in a small subset of cells at birth and at every age examined through 6 postnatal weeks in the ferret (Cramer et al., 1995). Moreover, at 4 postnatal weeks, the density of NADPH–diaphorase-labeled cells in the LGN is much higher than that in the visual cortex.
Finally, our results, together with those of Smetters et al. (1994), suggest that the segregation of retinal afferents into eye-specific layers proceeds independently of NO or NMDA receptor activation and indicate that other activity-dependent mechanisms act during this phase. Interestingly, this demonstrates that rather different mechanisms can be used to regulate sequential aspects of development in the same pathway. NMDA receptors and NO are well suited to mediate the later sharpening of connections into ON/OFF sublaminae, which is accompanied by the maturation of photoreceptors and bipolar cells in the ferret retina and their functional connections to retinal ganglion cells (Greiner and Weidman, 1981) (cf. Maslim and Stone, 1986). Additionally, the distinct burst frequencies of on and off retinal ganglion cells become evident during this period of segregation (Wong and Oakley, 1996) and may thus establish patterns of correlated activity on which NMDA receptors and NO act to shape the adult projection pattern.
Footnotes
This work was supported by a National Institutes of Health (NIH) National Research Service Award to K.S.C. and by NIH Grants EY07023 and EY11512 to M.S. We thank S. Kuffler for technical assistance, Dr. R. P. Marini for assistance with surgical procedures, C. I. Moore and C. D. Hohnke for helpful comments on this manuscript, and Dr. R. J. Wurtman for allowing us to carry out biochemical assays in his laboratory.
Correspondence should be addressed to Dr. Karina S. Cramer, Department of Brain and Cognitive Sciences, E25-235, Massachusetts Institute of Technology, Cambridge, MA 02139.
REFERENCES
- 1. Abe K, Mizutani A, Saito H. Possible role of nitric oxide in long-term potentiation in the dentate gyrus. Takagi H, Toda N, Hawkins RD. Nitric oxide: roles in neuronal communication and neurotoxicity 1994. 149 159 Japan Scientific; Tokyo: Societies. [Google Scholar]
- 2.Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 4.Angelucci A, Clasca F, Sur M. Anterograde axonal tracing with the subunit B of cholera toxin: a highly sensitive immunohistochemical protocol for revealing fine axonal morphology in adult and neonatal brains. J Neurosci Methods. 1996;65:101–112. doi: 10.1016/0165-0270(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 5.Aoki C, Venkatesan C, Go C-G, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bear MF, Kleinschmidt A, Gu Q, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz LI, Routtenberg A. A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity. Trends Neurosci. 1987;10:527–532. [Google Scholar]
- 9.Bickford ME, Gunluk AE, Guido W, Sherman SM. Evidence that cholinergic axons from the parabrachial region of the brainstem are the exclusive source of nitric oxide in the lateral geniculate nucleus of the cat. J Comp Neurol. 1993;334:410–430. doi: 10.1002/cne.903340307. [DOI] [PubMed] [Google Scholar]
- 10.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 11.Bogdanov MB, Wurtman RJ. Effects of systemic or oral ad libitum monosodium glutamate administration on striatal glutamate release, as measured using microdialysis in freely moving rats. Brain Res. 1994;660:337–340. doi: 10.1016/0006-8993(94)91309-9. [DOI] [PubMed] [Google Scholar]
- 12.Bohme GA, Bon C, Stutzmann J-M, Doble A, Blanchard J-C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991;199:379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- 13.Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 14.Cline HT, Constantine-Paton M. NMDA receptor agonists and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coggins PJ, McLean K, Nagy A, Zwiers H. ADP-ribosylation of the neuronal phosphoprotein B-50/GAP-43. J Neurochem. 1993;60:368–371. doi: 10.1111/j.1471-4159.1993.tb05862.x. [DOI] [PubMed] [Google Scholar]
- 16.Cramer KS, Sur M. Activity-dependent remodeling of connections in the mammalian visual pathway. Curr Opin Neurobiol. 1995;5:106–111. doi: 10.1016/0959-4388(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 17.Cramer KS, Moore CI, Sur M. Transient expression of NADPH-diaphorase in the lateral geniculate nucleus of the ferret during early postnatal development. J Comp Neurol. 1995;353:306–316. doi: 10.1002/cne.903530211. [DOI] [PubMed] [Google Scholar]
- 18.Cramer KS, Sur M (1996) Blockade of afferent impulse activity disrupts on/off sublamination in the ferret lateral geniculate nucleus. Dev Brain Res, in press. [DOI] [PubMed]
- 19.Daw NW, Reid SNM, Czepita D, Flavin H. A nitric oxide synthase (NOS) inhibitor reduces the ocular dominance shift after monocular deprivation. Soc Neurosci Abstr. 1994;20:1428. [Google Scholar]
- 20.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwyer MA, Bredt DS, Snyder SH. Nitric oxide synthase: irreversible inhibition by l-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991;176:1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- 22.East SJ, Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the l-arginine-nitric oxide pathway. Neurosci Lett. 1991;123:17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- 23.Erzurumlu RS, Jhaveri S, Benowitz LI. Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex. J Comp Neurol. 1990;292:443–456. doi: 10.1002/cne.902920310. [DOI] [PubMed] [Google Scholar]
- 24.Esguerra M, Kwon YH, Sur M. Retinogeniculate EPSPs recorded intracellularly in the ferret lateral geniculate nucleus in vitro: role of NMDA receptors. Vis Neurosci. 1992;8:545–555. doi: 10.1017/s0952523800005642. [DOI] [PubMed] [Google Scholar]
- 25.Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie DC, Ruthazer ES, Dawson TM, Snyder SH, Stryker MP. Nitric oxide synthase inhibition does not prevent ocular dominance plasticity. Soc Neurosci Abstr. 1993;19:893. doi: 10.1113/jphysiol.1996.sp021510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell [Suppl] 1993;10:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 28.Greiner JV, Weidman TA. Histogenesis of the ferret retina. Exp Eye Res. 1981;33:315–332. doi: 10.1016/s0014-4835(81)80055-3. [DOI] [PubMed] [Google Scholar]
- 29.Gribkoff VK, Lum-Ragan JT. Evidence for nitric oxide synthase inhibitor-sensitive and insensitive hippocampal synaptic potentiation. J Neurophysiol. 1992;68:639–642. doi: 10.1152/jn.1992.68.2.639. [DOI] [PubMed] [Google Scholar]
- 30.Hahm J-O, Sur M. The development of individual retinogeniculate axons during laminar and sublaminar segregation in the ferret LGN. Soc Neurosci Abstr. 1988;14:460. [Google Scholar]
- 31.Hahm J-O, Langdon RB, Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991;351:568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- 32.Haley JE, Wilcox GL, Chapman PF. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992;8:211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- 33.Hess DT, Patterson SI, Smith DS, Skene JHP. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 1993;366:562–565. doi: 10.1038/366562a0. [DOI] [PubMed] [Google Scholar]
- 34.Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- 36.Kelly PA, Ritchie IM, Arbuthnott GW. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole: effects upon local cerebral blood flow and glucose use in the rat. J Cereb Blood Flow Metab. 1995;15:766–773. doi: 10.1038/jcbfm.1995.96. [DOI] [PubMed] [Google Scholar]
- 37.Kwon YH, Esguerra M, Sur M. NMDA and non-NMDA receptors mediate visual responses of neurons in the cat’s lateral geniculate nucleus. J Neurophysiol. 1991;66:414–428. doi: 10.1152/jn.1991.66.2.414. [DOI] [PubMed] [Google Scholar]
- 38.Linden DC, Guillery RW, Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Vallano ML. Arachidonic acid, but not sodium nitroprusside, stimulates presynaptic protein kinase C and phosphorylation of GAP-43 in rat hippocampal slices and synaptosomes. J Neurochem. 1995;64:1808–1818. doi: 10.1046/j.1471-4159.1995.64041808.x. [DOI] [PubMed] [Google Scholar]
- 40.Malgaroli A, Ting AE, Wendland B, Bergamaschi A, Villa A, Tsien RW, Scheller RH. Presynaptic component of long-term potentiation visualized at individual hippocampal synapses. Science. 1995;268:1624–1628. doi: 10.1126/science.7777862. [DOI] [PubMed] [Google Scholar]
- 41.Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 42.Maslim J, Stone J. Synaptogenesis in the retina of the cat. Brain Res. 1986;373:35–48. doi: 10.1016/0006-8993(86)90313-6. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. J Neurosci. 1987;7:4034–4043. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meiri KF, Willard M, Johnson MI. Distribution and phosphorylation of the growth-associated protein GAP-43 in regenerating sympathetic neurons in culture. J Neurosci. 1988;8:2571–2581. doi: 10.1523/JNEUROSCI.08-07-02571.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesulam M-M. Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a noncarcinogenic blue reaction product with superior sensitivity for visualizing neuronal afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 46.Montague PR, Gally JA, Edelman GM. Spatial signaling in the development and function of neural connections. Cereb Cortex. 1991;1:199–220. doi: 10.1093/cercor/1.3.199. [DOI] [PubMed] [Google Scholar]
- 47.Mooney R, Madison DV, Shatz CJ. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993;10:815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- 48.Moya KL, Benowitz LI, Jhaveri S, Schneider GE. Changes in rapidly transported proteins in developing hamster retinofugal axons. J Neurosci. 1988;8:4445–4454. doi: 10.1523/JNEUROSCI.08-12-04445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohta K, Shimazu K, Komatsumoto S, Araki N, Shibata M, Fukuuchi Y. Modification of striatal arginine and citrulline metabolism by nitric oxide synthase inhibitors. NeuroReport. 1994;5:766–768. doi: 10.1097/00001756-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocha M, Sur M. Rapid acquisition of dendritic spines by visual thalamic neurons after blockade of NMDA receptors. Proc Natl Acad Sci USA. 1995;92:8026–8030. doi: 10.1073/pnas.92.17.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 54.Schuman EM, Madison DV. Locally distributed synaptic potentiation in the hippocampus. Science. 1994;263:532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- 55.Schuman EM, Meffert MK, Schulman H, Madison DV (1994) An ADP-ribosyltransferase as a potential target for nitric oxide action in hippocampal long-term potentiation. Proc Natl Acad Sci USA 11958–11962. [DOI] [PMC free article] [PubMed]
- 56.Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- 57.Sillito AM, Murphy PC, Salt TE, Moody CI. Dependence of retinogeniculate transmission in cat on NMDA receptors. J Neurophysiol. 1990;63:347–355. doi: 10.1152/jn.1990.63.2.347. [DOI] [PubMed] [Google Scholar]
- 58.Simon DK, Prusky GT, O’Leary DDM, Constantine-Paton M. N-methyl-d-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci USA. 1992;89:10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skene JHP, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smetters DK, Hahm J-O, Sur M. An N-methyl-d-aspartate receptor antagonist does not prevent eye-specific segregation in the ferret retinogeniculate pathway. Brain Res. 1994;658:168–178. doi: 10.1016/s0006-8993(09)90023-3. [DOI] [PubMed] [Google Scholar]
- 61.Stryker MP, Zahs K. ON and OFF sublaminae in the lateral geniculate nucleus of the ferret. J Neurosci. 1983;3:1943–1951. doi: 10.1523/JNEUROSCI.03-10-01943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent SR, Hope BT. Neurons that say NO. Trends Neurosci. 1992;15:108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- 63.Williams CV, Nordquist D, McLoon SC. Correlation of nitric oxide synthase expression with changing patterns of axonal projections in the developing visual system. J Neurosci. 1994;14:1746–1755. doi: 10.1523/JNEUROSCI.14-03-01746.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong ROL, Oakley DM. Changing patterns of spontaneous bursting activity of On and Off retinal ganglion cells during development. Neuron. 1996;16:1087–1095. doi: 10.1016/s0896-6273(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 65.Wu HH, Williams CV, McLoon SC. Involvement of nitric oxide in the elimination of a transient retinotectal projection in development. Science. 1994;265:1593–1596. doi: 10.1126/science.7521541. [DOI] [PubMed] [Google Scholar]
- 66.Yen L, Sibley JT, Constantine-Paton M. Analysis of synaptic distribution within single retinal axonal arbors after chronic NMDA treatment. J Neurosci. 1995;15:4712–4725. doi: 10.1523/JNEUROSCI.15-06-04712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]