Abstract
The neuron moves protein and membrane from the cell body to the synapse and back via fast and slow axonal transport. Little is known about the mechanism of microtubule movement in slow axonal transport, although cytoplasmic dynein, the motor for retrograde fast axonal transport of membranous organelles, has been proposed to also slide microtubules down the axon. We previously showed that most of the cytoplasmic dynein moving in the anterograde direction in the axon is associated with the microfilaments and other proteins of the slow component b (SCb) transport complex. The dynactin complex binds dynein, and it has been suggested that dynactin also associates with microfilaments. We therefore examined the role of dynein and dynactin in slow axonal transport. We find that most of the dynactin is also transported in SCb, including dynactin, which contains the neuron-specific splice variant p135Glued, which binds dynein but not microtubules. Furthermore, SCb dynein binds dynactin in vitro. SCb dynein, like dynein from brain, binds microtubules in an ATP-sensitive manner, whereas brain dynactin binds microtubules in a salt-dependent manner. Dynactin from SCb does not bind microtubules, indicating that the binding of dynactin to microtubules is regulated and suggesting that the role of SCb dynactin is to bind dynein, not microtubules. These data support a model in which dynactin links the cytoplasmic dynein to the SCb transport complex. Dynein then may interact transiently with microtubules to slide them down the axon at the slower rate of SCa.
Keywords: axonal transport, slow component b, dynein, dynactin, microtubule, microfilament, motor protein
Axonal transport, the movement of proteins and subcellular structures from the cell body through the axon to the synapse, has been divided into separate fast and slow components (Grafstein and Forman, 1980; Brady, 1991). Membranous organelles and their associated proteins travel in fast anterograde and retrograde transport. Slow anterograde transport is composed of two subcomponents. Slow component a (SCa), which travels at 0.1–1.0 mm/d, consists mainly of tubulin and the neurofilament subunit polypeptides (Hoffman and Lasek, 1975). Slow component b (SCb), which travels at 2–8 mm/day, includes actin, regulatory proteins, and metabolic enzymes (Black and Lasek, 1979). The coherent transport of the proteins in these two components suggests that the SCa polypeptides are transported as microtubules (MTs) and neurofilaments, whereas SCb represents the movement of a microfilament-based transport complex (Black and Lasek, 1980; Tytell et al., 1981; Brady, 1991).
In contrast to the movement of membranous organelle in fast axonal transport, very little is known about the mechanisms of slow axonal transport. It has been proposed that motor proteins slide the different cytoskeletal polymers toward the synapse (Lasek, 1986; Brady, 1991;Vallee and Bloom, 1991). Dynein generates sliding between the MTs of flagellar axonemes, and the polarity of dynein force generation and the orientation of the MTs, minus end toward the cell body, are consistent with the hypothesis that SCa MT movement toward the synapse is generated by dynein (Heidemann et al., 1981; Gibbons, 1988; Brady, 1991). We recently showed that most of the dynein transported in the anterograde direction is associated with SCb, leading us to propose a model in which dynein is attached to the microfilaments or another protein of the SCb transport complex and interacts transiently with MTs, sliding the plus ends of axonal MTs toward the synapse at the slower rate of SCa (Lasek, 1986; McQuarrie et al., 1986; Dillman et al., 1996).
The p150Glued subunit of dynactin binds cytoplasmic dynein in vitro (Karki and Holzbaur, 1995;Vaughan and Vallee, 1995), and genetic disruption of subunits of dynein and dynactin suggests that they interact in vivo (Muhua et al., 1994; Plamann et al., 1994; Schroer, 1994; McGrail et al., 1995). A major component of dynactin is a short filament composed of actin-related protein 1 (Arp1; Schafer et al., 1994), and it has been suggested that either conventional or novel actin-binding proteins might mediate an interaction between the Arp1 filament and microfilaments (Schroer, 1994; Mullins et al., 1996). To examine whether dynactin is involved in axonal transport, perhaps as a link between dynein and the SCb transport complex, we analyzed the axonal transport of dynactin. We found that the bulk of the axonally transported dynactin is associated with SCb. Biochemically distinct forms of dynein and dynactin are associated with fast component and SCb. Furthermore, SCb dynein is capable of binding to dynactin, and SCb dynein binds MTs in an ATP-sensitive manner. Brain dynactin binding to MTs is ATP-insensitive, whereas SCb dynactin does not bind MTs. Our results indicate the presence of biochemically and functionally distinct pools of dynein and dynactin and support a model in which dynactin cross-links dynein to the microfilaments of the SCb transport complex. These results are discussed in terms of models for the role of dynein and dynactin in slow axonal transport.
MATERIALS AND METHODS
Radiolabeling and isolation of axonally transported proteins. Axonally transported proteins were radiolabeled as described previously (Dillman et al., 1996); 1 mCi of Tran35S-label (ICN Biomedicals, Costa Mesa, CA) was injected into the vitreous of the left eye of adult male Sprague Dawley rats (Hilltop, Scottdale, PA). Four rats were used for each time point (4 mCi of total label/time point). To analyze proteins associated with membranous organelles in fast anterograde axonal transport, we isolated the optic nerves 4 hr after injection. To examine the proteins of SCb or the leading edge of the wave of MTs and neurofilaments in SCa, we isolated the optic nerves 4 and 21 d after injection, respectively. For the segmental analysis of SCb transport, rat optic nerves and tracts were isolated 2, 4, and 6 d after injection. The optic nerves and tracts were divided into three ∼5 mm segments (Fig.2A). Segment 1 corresponds to the proximal half of the optic nerve. Segment 2 corresponds to the distal half of the optic nerve. Segment 3 corresponds to the optic chiasm and the proximal portion of the optic tract.
Fig. 2.
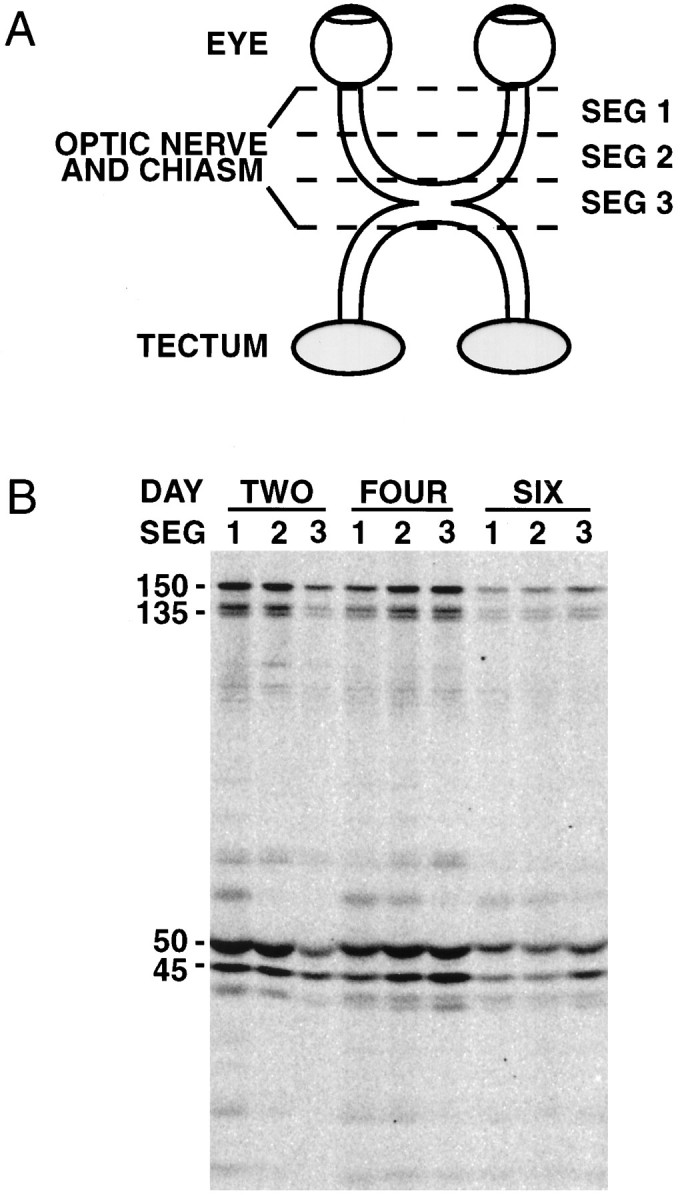
Association of dynactin with axonal transport slow component b. Segmental analysis was performed to confirm the association of dynactin with SCb. Axonally transported proteins were radiolabeled with Tran35S-label via intravitreal injection.A, The radiolabeled optic nerves and tracts were removed at specified times after injection and divided into three ∼5 mm segments, as diagramed. Segment 1 corresponds to the proximal half of the optic nerve. Segment 2 corresponds to the distal half of the optic nerve. Segment 3 corresponds to the optic chiasm and the proximal portion of the optic tract. The segments were homogenized in lysis buffer. Dynactin was then immunoprecipitated and analyzed by SDS-PAGE and storage phosphor autoradiography. B, An autoradiograph showing the radiolabeled dynactin polypeptides immunoprecipitated from segments of the optic nerve and tract at the indicated times after injection. Above each set of three lanes is the number of days (DAY) after injection that the optic nerve and tract were isolated (TWO,FOUR, and SIX). Numerals (1, 2, 3) indicate the isolated segment (SEG) of the optic nerve or tract analyzed in that lane. The major dynactin subunits and isoforms are indicated to the left of the gel: p150Glued (150), p135Glued (135), p50 (50), and Arp1 (45). Increasing amounts of each of the dynactin subunits are seen in the more distal segments with increasing time after injection.
Immunoprecipitation and electrophoretic procedures. Isolated optic nerves or segments were pooled and homogenized in Triton X-100 lysis buffer (Dillman and Pfister, 1994; Dillman et al., 1996). The entire cytoplasmic dynein complex was immunoprecipitated by using monoclonal antibody 74.1 as described previously (Dillman and Pfister, 1994; Dillman et al., 1996). The entire dynactin complex was immunoprecipitated by using monoclonal antibody p50 (Paschal et al., 1993; Echeverri et al., 1996). Kinesin was immunoprecipitated by using monoclonal antibody H2 (Pfister et al., 1989). The immunoprecipitation procedure was found to be 95–99% efficient, on the basis of quantitation of sequential immunoprecipitations. SDS-PAGE (8% acrylamide, or 4% acrylamide and 8 m urea), two-dimensional gel electrophoresis, and visualization of radiolabeled proteins with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) were performed as described previously (Dillman and Pfister, 1994; Pfister et al., 1996a,b). For Western blotting, proteins were transferred to Polyscreen PVDF [poly(vinylidene difluoride); DuPont NEN Research Products, Boston, MA] as described previously (Pfister et al., 1996a,b). The p150Glued subunit of dynactin and its isoforms were identified by using the affinity-purified rabbit polyclonal antibody UP235 (Karki and Holzbaur, 1995) and visualized with the SuperSignal chemiluminescent detection system (Pierce, Rockford, IL).
Phosphatase treatment. Cytoplasmic dynein immunoprecipitated from radiolabeled rat optic nerve bound to protein A beads was treated with 200 U of Lambda phosphatase (New England Biolabs, Beverly, MA) for 40 min at 30°C and analyzed by two-dimensional gel electrophoresis as described previously (Pfister et al., 1996a,b).
MT binding assays. Rat tissues, either whole brain or radiolabeled optic nerves isolated 4 d after intravitreal injection of five rats with 1 mCi/eye (both eyes) of Tran35S-label (10 mCi of total label), were pooled and homogenized in PHEM buffer (50 mm HEPES, 50 mmPIPES, 2 mm MgCl2, 1 mm EDTA), apyrase, and inhibitors of proteases, kinases, and phosphatases (Dillman and Pfister, 1994). The MT binding assay was similar to that described by others (Lye et al., 1987; Paschal et al., 1987). A high-speed supernatant (HSS; 100,000 rpm in a TLA100.3 rotor) (Beckman Instruments, Palo Alto, CA) for 10 min) was prepared. Taxol (Calbiochem, San Diego, CA) was added to brain HSS to polymerize MTs; exogenous Taxol-stabilized MTs (Williams and Lee, 1982) were added to optic nerve HSS. After incubation, the MTs were centrifuged through a sucrose cushion (55,000 rpm in a TLA100.3 rotor for 20 min), yielding an MT-depleted supernatant (MDS) and an MT pellet (MTP). The MTP was resuspended in PHEM buffer and the solution centrifuged (as above), producing a PHEM supernatant and a PHEM-washed MT pellet. The MT pellet was resuspended in 5 mm MgATP and centrifuged as above, producing an ATP supernatant and ATP-extracted MT pellet. The pellet was resuspended in 10 mm MgATP and centrifuged, resulting in a second ATP supernatant and ATP-extracted MT pellet. This pellet was resuspended in 1.0 m NaCl and centrifuged, yielding a NaCl supernatant and NaCl-extracted MT pellet, or in one experiment, the second ATP-extracted MT pellet was first resuspended in 0.6 m NaCl and centrifuged before the 1.0 m NaCl extraction of the MT pellet. In several later experiments (Fig. 8) the ATP washes were eliminated, and the MT pellet was resuspended directly in 1.0 m NaCl. Dynein heavy chain and the p150Glued isoforms in each fraction were analyzed with SDS-PAGE and storage phosphor autoradiography or Western blotting. Alternatively, the dynein and dynactin were immunoprecipitated from each of the supernatant fractions and then analyzed via SDS-PAGE by using storage phosphor autoradiography for radiolabeled samples and silver staining for unlabeled samples (Wray et al., 1981).
Fig. 8.
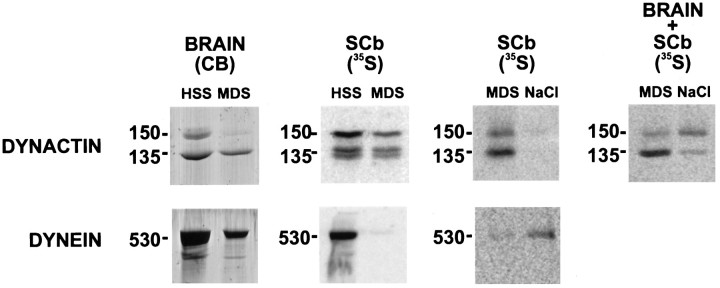
Regulation of SCb dynactin binding to MTs. Rat brain (BRAIN), radiolabeled optic nerves (SCb), or a mixture of brain and radiolabeled optic nerves (BRAIN + SCb) were homogenized in PHEM buffer supplemented with phosphatase inhibitors, kinase inhibitors, and apyrase; high-speed supernatants (HSS) were prepared from the homogenized tissue as in Figure 7. Taxol was added to the brain HSS to assemble MTs, and Taxol-stabilized MTs were added to the radiolabeled optic nerve HSS. After incubation, the MTs were pelleted, creating an MT-depleted supernatant (MDS) and MT pellet. Proteins bound to the MT pellets were eluted with 1.0 mNaCl, and the supernatant (NaCl) was separated from the microtubules by centrifugation. Dynein and dynactin were immunoprecipitated from these supernatants; the immunoprecipitated protein was eluted from the beads with sample buffer, and the polypeptides were resolved by SDS-PAGE. Top row, Analysis of DYNACTIN; bottom row, analysis of DYNEIN. Brain polypeptides were visualized with Coomassie blue (CB). Radiolabeled SCb optic nerve polypeptides were visualized by storage phosphor autoradiography (35S). The 530 kDa dynein heavy chain (530) and the dynactin p150Glued(150) and p135Glued(135) isoforms are indicated to the leftof each panel of two gel lanes. As was seen in Figure 7, most of the brain dynein and p150Glued dynactin are found in the starting HSS, but not the MDS, demonstrating that they bind MTs. SCb dynein is found in the HSS, but not the MDS, confirming that it binds MTs. The SCb p150Glued dynactin from optic nerves is found in MDS, but not the NaCl supernatant, demonstrating that it does not bind MTs. In contrast, when SCb optic nerve is cohomogenized with rat brain, there is a significant amount of the p150Glued dynactin band present in the NaCl supernatant and less of the p150Glued dynactin band present in the MDS, demonstrating that a factor in brain alters p150Glued dynactin binding to MTs.
p150Glued ligand beads binding assay.p150Glued beads and BSA beads were prepared as described previously (Karki and Holzbaur, 1995). Rat optic nerves radiolabeled for 4 d were pooled and homogenized in PHEM buffer, and a HSS was prepared as described above. Packed bovine serum albumin (BSA; 50 μl) ligand beads (2 mg ligand/ml beads) were washed two times with PHEM buffer, and then equal volumes of high-speed supernatant were added to the BSA-beads and the samples were rocked at 4°C for 3 hr. The BSA-beads were pelleted and the supernatant added to either p150Glued ligand beads or BSA-beads and rocked at 4°C for 3 hr. The p150Gluedbeads were pelleted and washed five times with 100 mm NaCl in PHEM buffer. The beads were eluted with sample buffer (Laemmli, 1970) and analyzed via SDS-PAGE and fluorography.
Quantitative analyses. Quantitation of radioactivity associated with individual polypeptides was performed by autoradiography with storage phosphor screens and ImageQuant image analysis software (Molecular Dynamics), as described previously (Dillman and Pfister, 1994; Dillman et al., 1996). The amount of radiolabeled protein associated with each time point of the SCb study was expressed as a percentage of the total radiolabeled protein from all time points of the SCb study (Elluru et al., 1995; Dillman et al., 1996). Quantitation of bulk radiolabeled protein was performed by TCA-precipitation of radiolabeled rat optic nerve homogenates and scintillation counting (Mans and Novelli, 1961).
RESULTS
Characterization of the axonal transport of dynactin
Dynactin is a large multisubunit protein complex consisting of at least nine distinct components (Gill et al., 1991; Paschal et al., 1993; Schafer et al., 1994), including p45 (Arp1), p50 (dynamitin), and p150 (the Drosophila Glued polypeptide) and its alternative splice variant p135. Arp1 forms a short actin-like filament to which the other dynactin subunits are bound (Schafer et al., 1994), and p150Glued binds both MTs and cytoplasmic dynein (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995; Waterman-Storer et al., 1995).
To examine the role of dynactin in mediating the binding of dynein to the SCb transport complex, we determined the axonal transport profile of dynactin by using the rat optic nerve axonal transport system (Brady and Lasek, 1982; Dillman and Pfister, 1994; Elluru et al., 1995;Dillman et al., 1996). Tran35S-label was injected into the vitreous of rat eyes, incorporated into proteins in the cell bodies of the retinal ganglion cells, and transported anterogradely along the axons that comprise the optic nerve. The dynactin complex was immunoprecipitated from the radiolabeled optic nerves at various times after injection with an antibody to the p50 subunit of dynactin (see Fig. 2B; Paschal et al., 1993) and analyzed by SDS-PAGE and storage phosphor autoradiography. Figure1A shows an autoradiograph of a portion of a gel analyzing the relative amounts of the p50 subunit of the dynactin complex associated with the different rate components of axonal transport. To examine the dynactin associated with the membranous organelles of fast axonal transport, we removed the optic nerves 4 hr after injection. To examine the dynactin associated with SCb and SCa, we removed the optic nerves 4 or 21 d after injection, respectively. Quantitation of the amount of p50 associated with the different rate components of axonal transport (Fig.1B) revealed that ∼90% of the axonally transported dynactin is associated with SCb of slow axonal transport. Only a small amount of the dynactin (<10%) is associated with the membranous organelles of fast axonal transport. The remainder of the dynactin (<5%) is probably contained in the trailing edge of the wave of SCb transport, because no dynactin was detected when the SCa wave of proteins was analyzed after using longer time intervals (20–60 d after injection; J. F. Dillman and K. K. Pfister, unpublished observations).
Fig. 1.
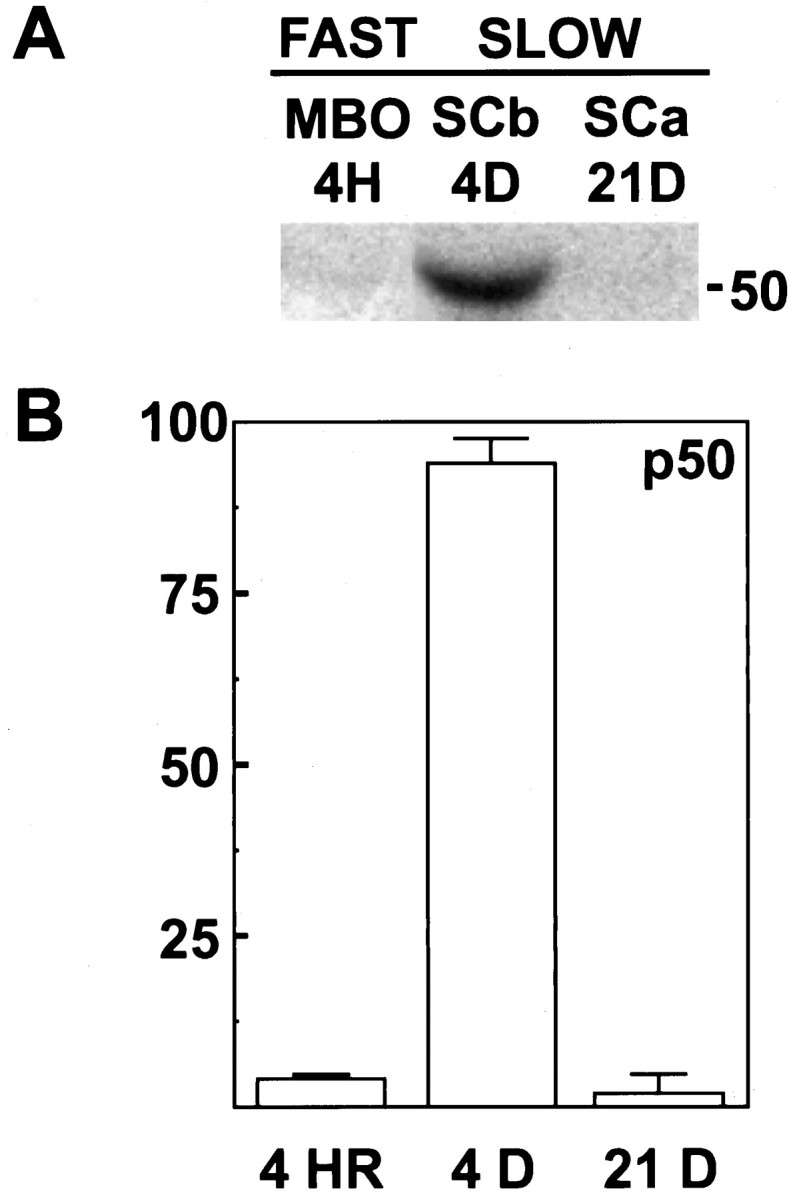
Rate of axonal transport of dynactin. Proteins transported along the axon were radiolabeled by intravitreal injection of Tran35S-label. The optic nerve was isolated at the specified times after injection and homogenized in lysis buffer; the dynactin complex was immunoprecipitated and analyzed via SDS-PAGE and storage phosphor autoradiography. A, Portion of an autoradiograph of a gel showing the radiolabeled p50 subunit of dynactin immunoprecipitated from rat optic nerve at various times after injection. Lane 1, To analyze the presence of the p50 subunit of dynactin (50) associated with the fast axonal transport (FAST) of membranous organelles (MBO), a 4 hr (4H) time interval was used. Lanes 2, 3, Two time intervals were used to analyze the association of dynactin with the slow components (SLOW). For association with the microfilaments of the slow component b (SCb) transport complex, a 4 d (4D) time interval was used. For association with the leading edge of the MTs and neurofilaments of slow component a (SCa), a 21 d (21D) interval was used. B, Quantitation of the p50 subunit for the dynactin complex present in each rate component of anterograde axonal transport, as described in Materials and Methods (n = 2). The bulk of the dynactin p50 (∼90%) is associated with the 4 d time point.
To confirm the association of dynactin with SCb, we performed a segmental analysis. This analysis tracks the time course of the movement of dynactin through three continuous ∼5 mm segments of the optic nerve and tract over several days (Fig.2A) (Elluru et al., 1995; Dillman et al., 1996). The results shown in Figure 2B are those expected for a protein moving through the optic nerve and tract at SCb rates. Two days after injection of Trans35S-label, most of the dynactin is seen in segments 1 and 2, the segments most proximal to the retina. At 4 d, most of the dynactin is seen in segments 2 and 3, the segments most distal to the retina. By 6 d, most of the dynactin has left the optic nerve segments, with the largest remaining amount found in segment 3.
Comparison of the axonal transport of dynactin and cytoplasmic dynein
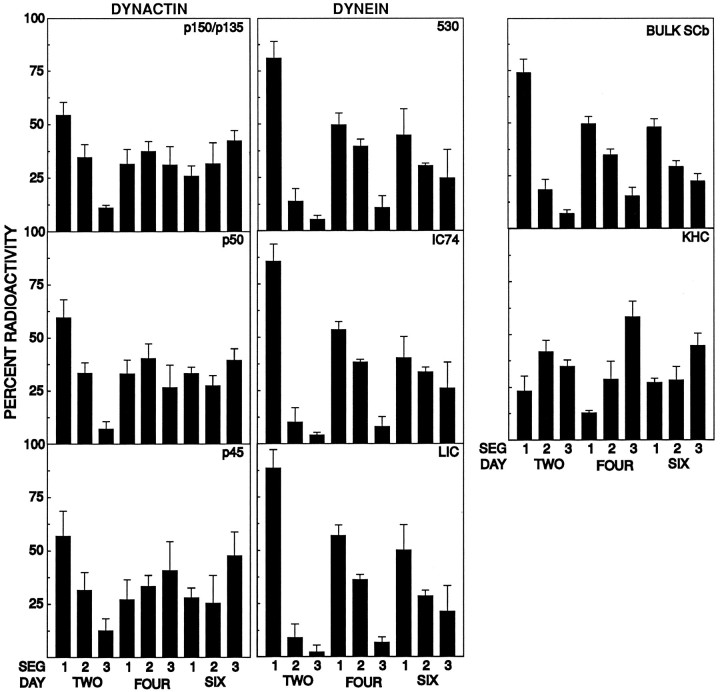
To analyze further the transport of dynactin in SCb, we quantified the relative amount of the major dynactin subunits in each optic nerve segment (Fig. 3, left). The individual subunits exhibit similar transport kinetics, suggesting that they are moving coherently, that is, moving as a complex, not individual polypeptides. Analysis of the transport of the major subunits of cytoplasmic dynein, the 530 kDa heavy chain, the 74 kDa intermediate chain (IC74), and the light intermediate chains (LIC; Fig. 3,center) demonstrates that they are also moving coherently.
Fig. 3.
Quantitative comparison of the SCb transport segmental analysis of dynactin, cytoplasmic dynein, kinesin, and bulk protein. Dynactin, cytoplasmic dynein, and kinesin were immunoprecipitated sequentially from homogenates of individual segments of optic nerves removed at 2, 4, and 6 d after injection, as described above. Bulk SCb protein was determined from TCA-precipitable counts of radiolabeled tissue homogenates from the segments. Quantitation of the indicated polypeptides from the storage phosphor autoradiographs was performed with ImageQuant analysis software, as described in Materials and Methods. The average of three trials (n = 3) is shown. Left(DYNACTIN), Quantitation of the major radiolabeled dynactin subunit polypeptides: p150Glued and p135Gluedisoforms (p150/p135), p50, and Arp1 (p45). Center(DYNEIN), Quantitation of the cytoplasmic dynein subunit polypeptides: dynein heavy chain (530), the 74 kDa intermediate chain (IC74), and the 53–59 kDa set of light intermediate chains (LIC).Right, Top, Quantitation of bulk radioactive TCA-precipitable protein present in the rat optic nerve and tract (BULK SCb) at the indicated times after injection.Right, Center, Quantitation of the kinesin heavy chain (KHC). Dynactin and cytoplasmic dynein have transport kinetics consistent with SCb transport, as opposed to kinesin, representative of the transport kinetics of proteins transported with fast component.
The transport kinetics of each of the cytoplasmic dynein subunits are nearly identical to the transport kinetics of bulk SCb protein. Dynactin, however, seems to be moving slightly faster than dynein. That is, it is moving closer to the leading edge of the wave of SCb protein than to the peak of bulk SCb protein. The presence of variation in the distribution of individual proteins within the SCb wave was analyzed in detail by Garner and Lasek (1982). Their interpretation is that different protein components of SCb have different affinities for the SCb transport complex machinery. Thus, those components that have high affinity for the SCb transport complex are tightly bound to it and are seen at the leading edge of the SCb wave. Those components that have lower affinities for the SCb transport complex dissociate from it more frequently and spread out into the middle and trailing edge of the SCb wave. Their analysis, in combination with our data, predicts that dynactin is tightly associated with the SCb transport complex, whereas the slightly slower transport rate of dynein suggests that it spends some time dissociated from the SCb transport complex and dynactin.
Neither dynactin nor cytoplasmic dynein has transport kinetics similar to the kinesin heavy chain, the motor for fast anterograde transport of membranous organelles. A segmental analysis, with time points appropriate to examine SCa (20–60 d), was used to compare the transport kinetics of cytoplasmic dynein and tubulin (a major component of SCa), and this analysis demonstrated that cytoplasmic dynein is not a component of SCa (Dillman and Pfister, unpublished observations).
Comparison of isoform differences of dynactin and dynein subunits from the SCb and fast anterograde axonal compartments
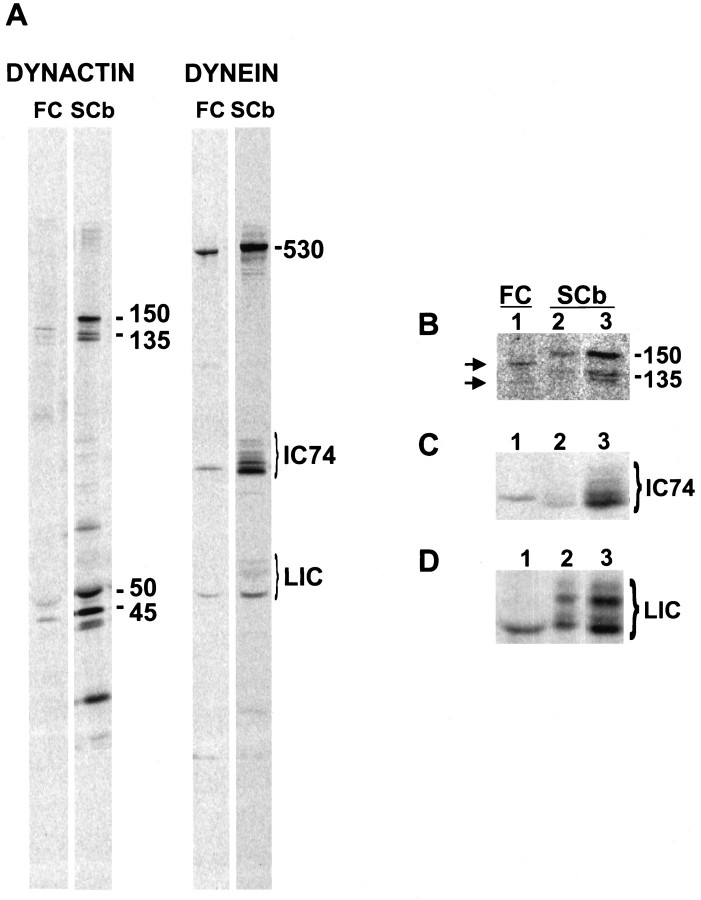
Although the majority of the axonally transported dynactin and cytoplasmic dynein is associated with SCb of slow axonal transport, our results indicate that there is a small pool of both dynactin and dynein associated with the membranous organelles of anterograde fast axonal transport (for dynactin, Fig. 1; for dynein, Dillman et al., 1996). Because the membranous organelles of fast component and SCb represent functionally distinct axonal compartments (Brady, 1991; Vallee and Bloom, 1991), we compared these two pools of dynactin and dynein to determine whether they are biochemically distinct, as well. When all the polypeptides from an experiment such as that shown in Figure 1 were compared, we found differences in the isoform composition of various subunits of the dynein and dynactin complexes (Fig.4A). Specifically, there seemed to be different isoforms of the dynactin p150Gluedsubunit and the dynein intermediate chain (IC74) and light intermediate chain (LIC) subunits in the fast component and SCb pools.
Fig. 4.
Different isoforms of dynactin p150Glued and the dynein IC74 and LICs are associated with SCb and the membranous organelles of anterograde fast component. A, The left pair of gel lanes (DYNACTIN) shows a comparison of the dynactin subunits associated with fast component (FC) and slow component b (SCb). The polypeptides are indicated to theright of the lanes: the p150Glued and p135Glued isoforms (p150/p135), p50 (p50), and Arp1 (p45). The right pair of gel lanes (DYNEIN) shows a comparison of the dynein subunits associated with fast component (FC) and slow component b (SCb). The dynein heavy chain (530), IC74 intermediate chain isoforms (IC74), and light intermediate chains (LIC) are indicated to the right of the lane. B, Isoforms of dynactin p150Glued. C, Isoforms of dynein intermediate chains. D, Isoforms of dynein light intermediate chains. Lane 1, Analysis of the isoforms associated with the fast component (FC). Lanes 2, 3, Analysis of the isoforms associated with slow component b (SCb). Lane 2 was loaded with equal CPM of protein with respect to lane 1.Lane 3 shows the same sample as lane 2, except that the loading was increased to better visualize the more numerous isoforms associated with SCb.
To further analyze the isoform differences, we analyzed equal amounts of radiolabeled dynactin or dynein from the fast component and SCb pools by SDS-PAGE. The dynactin p150Gluedisoforms moving in SCb, p150Glued, and p135Glued are the major isoforms found in brain (Figs. 4B, 7C). When dynactin associated with the membranous organelles of fast component was analyzed, a band of slightly greater electrophoretic mobility than p150Glued and a band of slightly greater electrophoretic mobility than p135Glued was seen. These dynactin subunits from anterograde fast axonal transport are probably either alternative splice variants of p150Glued or the result of post-translational modification, because they are immunoprecipitated with affinity-purified UP235, a broad-specificity polyclonal antibody to p150Glued (our unpublished observations). With SDS-PAGE analysis, there seems to be a single dynein IC74 isoform associated with the membranous organelles of anterograde fast component, whereas SCb dynein contains many IC74 isoforms (Fig.4C). The fastest migrating LIC (∼53 kDa) of dynein is the major LIC associated with the membranous organelles of anterograde fast component, whereas SCb dynein contains the entire repertoire of LIC isoforms (Fig. 4D).
Fig. 7.
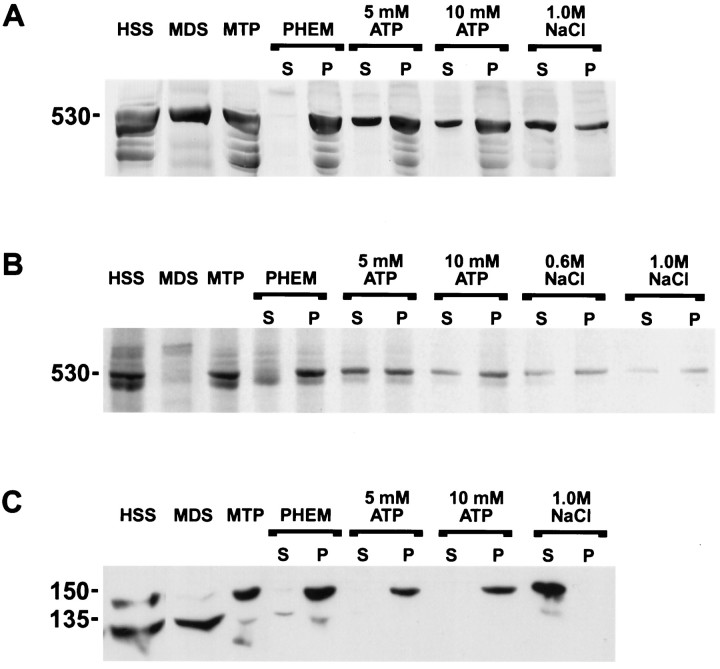
MT binding of dynein and dynactin. Rat brains or radiolabeled optic nerves were removed and homogenized in PHEM buffer supplemented with phosphatase inhibitors, kinase inhibitors, and apyrase; dynactin and dynein were purified via MT affinity, followed by MgATP and NaCl release, as described in Materials and Methods. Aliquots of the supernatant and pellet fractions were resolved by 4% acrylamide and 8 m urea gel electrophoresis. HSS, A high-speed supernatant was prepared from the indicated rat tissue homogenate; either Taxol was added to the brain HSS to assemble MTs, or Taxol-stabilized MTs were added to SCb optic nerve HSS. The MTs were centrifuged through a sucrose cushion, yielding an MT-depleted supernatant (MDS) and an MT-enriched pellet (MTP). PHEM, The MT pellet was resuspended in PHEM buffer and centrifuged, yielding a supernatant (S) and an MT pellet (P). 5 mm ATP, The MT pellet was resuspended in the presence of 5 mm MgATP and centrifuged, yielding a supernatant (S) and MT pellet (P).10 mm ATP, The MT pellet was resuspended in the presence of 10 mm MgATP and centrifuged, yielding a supernatant (S) and MT pellet (P).1.0 m NaCl, The MT pellet was resuspended in the presence of 1.0 m NaCl and centrifuged, yielding a supernatant (S) and MT pellet (P).A, Analysis of brain dynein MT binding and elution. Portion of a gel analyzing the 530 kDa cytoplasmic dynein heavy chain (530), silver-stained. Most of the dynein from brain binds MTs in an ATP-dependent manner. B, SCb dynein binds MTs in an ATP-sensitive manner. Portion of an autoradiograph of a gel analyzing the 530 kDa cytoplasmic dynein heavy chain (530). Two sequential salt elution steps were used in this experiment, 0.6 and 1.0 m NaCl. Most (>90%) of the SCb dynein seems to bind MTs, and the majority of this (∼60%) binds in an ATP-sensitive manner. SCb dynein binding to MTs is nearly indistinguishable from whole-brain dynein binding to MTs.C, Brain p150Glued dynactin binds MTs. Portion of a Western blot probed with affinity-purified UP235, which recognizes all the dynactin p150Gluedsubunit isoforms, including the p135Gluedisoform. (This is from the same experiment as A). The p150Glued and p135Gluedisoform polypeptides are indicated (150,135). Most of the p150Gluedisoform binds the MTs, whereas the p135Gluedisoform does not bind the MTs. The p150Glueddynactin from brain binds MTs in a salt-dependent, but not ATP-dependent, manner.
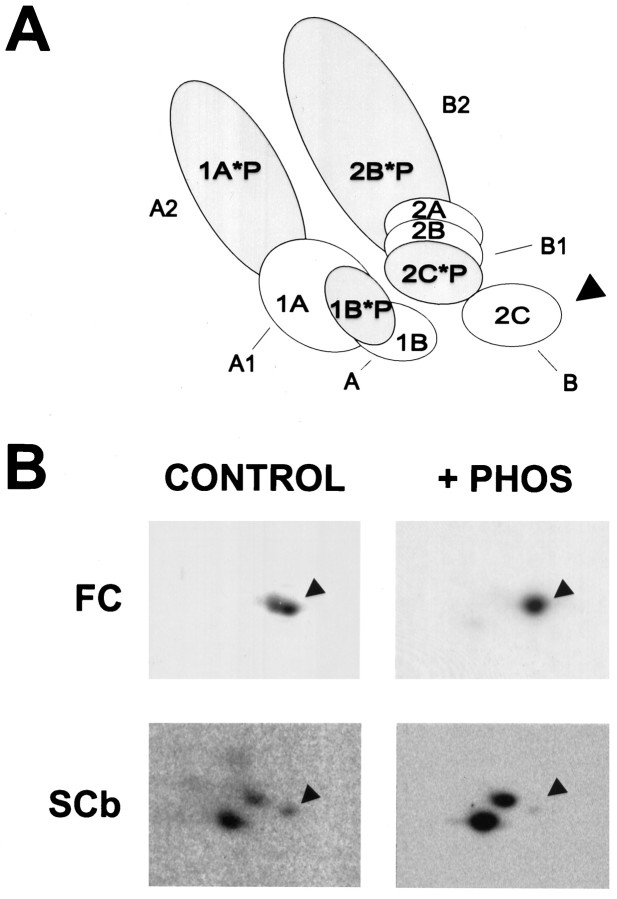
To identify the specific dynein IC74 gene products present in the fast component and SCb pools, we used two-dimensional gel electrophoresis. We have shown recently that the diversity of the dynein IC74 isoforms results from tissue-specific and developmentally regulated phosphorylation and alternative splicing (Dillman and Pfister, 1994;Pfister et al., 1996a,b) of the two IC74 genes (IC74-1 and IC74-2) (Vaughan and Vallee, 1995). Figure 5A is a model summarizing the correlation of the two-dimensional gel pattern of the IC74 isoforms from whole brain with a specific mRNA protein product or phosphoisoform (Pfister et al., 1996a,b). The dynein IC74 isoforms are resolved on two-dimensional gels as two arcs of three spots each: the A arc composed of the A, A1, and A2 spots, all products of gene IC74-1, and the B arc composed of B, B1, and B2 spots, all products of gene IC74-2. The IC74-2C gene product and its phosphoisoform are found in all cells and tissues and are the only IC74 protein isoforms found in glia. This indicates that the IC74-2C gene product is sufficient for general membrane organelle transport in cells. The IC74-1A gene product, however, is found only in neurons, and it, the IC74-2B, and IC74-1B gene products are developmentally regulated, appearing between embryonic days E13-18, just before and during neurite outgrowth in the rat brain.
Fig. 5.
Identification of IC74 gene products associated with fast component and SCb by two-dimensional gel analysis and phosphatase treatment. A, Diagram showing IC74 isoforms from whole rat brain (Pfister et al., 1996a,b). The different gene products (IC74-1A, IC74-1B, IC74-2A, IC74-2B, IC74-2C) are indicatedinside the spots. The *Pdesignation inside the spots indicates phosphoisoforms. The protein spot designations of the A arc (A, A1, A2) and B arc (B, B1, B2) are indicatedoutside the spots. For orientation purposes, the arrowhead points to the B spot (IC74-2C gene product). B, Radiolabeled cytoplasmic dynein immunoprecipitated from fast component (FC; top 2 panels) and slow component b (SCb; bottom 2 panels). Equivalent samples of the immunoprecipitated dynein were treated with buffer alone (CONTROL) or with phosphatase (+ PHOS) and then analyzed by two-dimensional gel electrophoresis and fluorography or storage phosphor autoradiography. For orientation purposes, the arrowhead points to the B spot (IC74-2C gene product). In the control dynein from fast component (top left), both IC74-2C and phospho-IC74-2C are seen, and phospho-IC74-2C is removed by phosphatase treatment, leaving IC74-2C (top right). Although barely detectable in these reproductions, there are faint spots in the A arc that did not reproduce well. In the dynein from SCb (bottom panels), all of the gene products and phosphoisoforms are present, although there is less IC74-2C (B spot, indicated by thearrow) than in whole brain (Pfister et al., 1996a,b), and the phosphoisoforms are removed by treatment with phosphatase.
The two-dimensional gel pattern of dynein IC74 subunits associated with fast component shows two major isoforms, the B and B1 spots (Fig.5B). To determine whether the B1 and B spots are related by phosphorylation, we treated dynein from fast component with phosphatase and then analyzed it by two-dimensional gel electrophoresis and fluorography. Phosphatase treatment resulted in the removal of the B1 spot and an increase in intensity of the remaining B spot, demonstrating that these fast component protein isoforms are related by phosphorylation. Therefore, the major IC74 isoforms associated with the membranous organelles of the fast anterograde component are the IC74-2C gene product and its phosphoisoform (phospho-IC74-2C). A small amount of IC74-1 gene product (the A arc of spots), possibly IC74-1A and its phosphoisoform, was also seen but did not reproduce well.
All the brain dynein IC74 isoforms were found when dynein from SCb was analyzed; however, there was less IC74-2C gene product associated with SCb dynein, as compared with whole-brain dynein (Fig. 5; see alsoPfister et al., 1996a,b). As we showed previously for brain dynein, treatment of SCb dynein with phosphatase resulted in the removal of the A2 and B2 spots (the phospho-IC74-1A and phospho-IC74-2B isoforms;Pfister et al., 1996a,b) and an increase in intensity of the remaining spots (the IC74-1A, IC74-1B, and IC74-2B gene products). Therefore, the apparently constitutive IC74-2C gene product also is found on the membranous organelles of fast axonal transport, whereas the developmentally regulated IC74 isoforms are associated with the SCb transport complex.
Functional properties of SCb dynactin and dynein
Karki and Holzbaur (1995) used affinity chromatography to determine that dynein IC74 interacts directly with the p150Glued subunit of dynactin. To test whether SCb dynein is also capable of direct binding to dynactin, we incubated high-speed supernatants, made from rat optic nerves radiolabeled for 4 d, with p150Glued cross-linked to beads. Figure 6 shows that the p150Gluedbeads bound dynein from SCb supernatants. Thus, SCb dynein binds to the p150Glued subunit of dynactin.
Fig. 6.
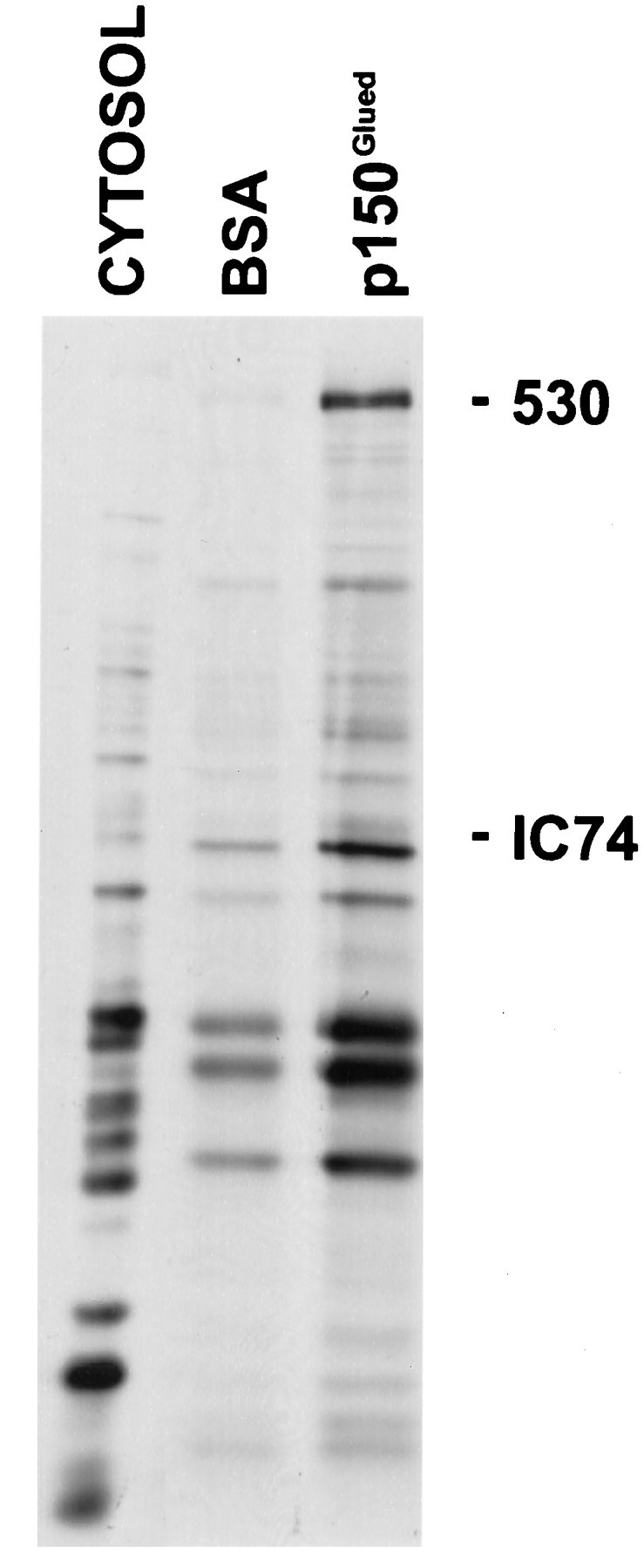
SCb dynein binds p150Gluedin vitro. Radiolabeled optic nerves were homogenized in PHEM, as described. A high-speed supernatant was prepared and incubated with BSA conjugated to agarose beads. The BSA-treated supernatant was then incubated with BSA-beads or p150Gluedbeads. The bound proteins were eluted with sample buffer and analyzed by SDS-PAGE and fluorography. CYTOSOL, SCb protein supernatant added to the beads; BSA, SCb proteins that bound to BSA-beads; p150Glued, SCb proteins that bound to p150Glued beads. Note the enrichment of the dynein heavy chain and IC74 (the LICs are obscured by contaminating bands). The 530 kDa dynein heavy chain (530) and the 74 kDa intermediate chain (IC74) are indicated.
We also compared the ability of dynein and dynactin from brain and rat optic nerve SCb to bind MTs. As others have shown previously (Paschal et al., 1987; Schroer and Sheetz, 1991), dynein from whole brain binds to MTs (Fig. 7A). This dynein is extracted from the MTs with MgATP or 1.0 m NaCl. Dynein from SCb also binds MTs (Fig. 7B) and, like whole-brain dynein, is extracted from the MTs with MgATP or NaCl. Thus, SCb dynein binds MTs in an ATP-sensitive manner. When dynactin from whole brain was analyzed, nearly all of the p150Glued pelleted with the MTs, whereas almost none of the p135Glued pelleted with the MTs (Fig.7C). The MT-associated p150Glued is not extracted from the MTs with MgATP, but it is extracted from the MTs with 1.0 m NaCl. Our finding that p150Glued, but not p135Glued, binds MTs is consistent with the report of Tokito and associates (1996). They found that p135Glued is a neuron-specific alternative splice variant of the p150Glued gene that lacks the p150Glued MT binding site but retains the dynein binding site.
We next examined the MT binding of dynactin from SCb and compared it with dynactin from whole brain. However, when SDS-PAGE was used to analyze the MT supernatant and pellet fractions, several heavily radiolabeled SCb protein bands migrated very near to the p150Glued and p135Gluedbands on the gels and interfered with the analysis (data not shown). Therefore, to analyze SCb dynactin binding to MTs by SDS-PAGE, we immunoprecipitated dynactin from the supernatant fractions. Dynein was also immunoprecipitated from the same fractions as a control. The control analyses of brain and SCb dynein immunoprecipitated from high-speed supernatants (HSS) and MT-depleted supernatants (MDS) shown in Figure 8 agree with the results shown in Figure 7. The greater amount of dynein heavy chain in the HSS lanes, as compared with that in the MDS lanes, demonstrates that the dynein from whole brain and SCb binds to MTs. As was also seen in Figure 7, a greater percentage of the SCb dynein binds MTs than does whole-brain dynein. As expected, when no ATP elution step is used, most of the SCb dynein elutes from the MTs with NaCl. We then examined dynactin binding to MTs. SCb p135Glued, like brain p135Glued, does not bind MTs. However, in contrast to dynein, SCb p150Glued behaves differently from whole-brain p150Glued. When the brain and SCb dynactin panels are compared, it can be seen that, whereas almost all of the brain p150Glued is depleted from the MDS, most of the SCb p150Gluedremains in the MDS. Furthermore, when the proteins released from the MTs by NaCl are analyzed, almost no SCb p150Glued is found in the NaCl supernatant. Therefore, almost none of the SCb p150Gluedbound to MTs. Thus, it seems that there are two functionally distinct pools of dynactin with the p150Glued isoform that can be distinguished on the basis of their binding to MTs. This suggests that the ability of dynactin to bind to MTs is regulated.
To obtain further evidence for the regulation of the MT binding of p150Glued, we determined whether a factor in brain could alter the MT binding properties of SCb p150Glued in vitro. Radiolabeled SCb rat optic nerve was cohomogenized with unlabeled rat brain, and then the MT binding assay was performed (Fig. 8). Fourfold more radiolabeled p150Glued was found in the SCb optic nerve MDS than in the brain plus SCb optic nerve MDS, indicating that a factor in brain increased SCb p150Glued binding to MTs. To further analyze the SCb p150Glued bound to MTs, we compared the amounts of radiolabeled p150Glued that eluted from MTs in the presence of 1 m NaCl (NaCl lane). As expected, there was fourfold more radiolabeled p150Glued in the brain plus SCb optic nerve NaCl fraction than in the SCb optic nerve alone fraction. This demonstrates that the failure of SCb dynactin to bind MTs is not an artifact of the in vitro assay. Rather, it indicates that the MT binding of dynactin is stimulated by a factor found in brain that is lacking in axons; presumably this factor is located either in cell bodies or synapses. This suggests that the binding of dynactin to MTs is modulated for different tasks in different neuronal locations.
It has been observed previously that a small and variable amount of dynactin would elute from MTs with ATP, suggesting that it was bound through dynein (Gill et al., 1991; Schroer and Sheetz, 1991;Waterman-Storer and Holzbaur, 1996). Unlike these previous studies, we routinely included inhibitors of phosphatases and kinases in our homogenization buffers for the MT binding experiments. If phosphorylation is involved in the interaction of dynein and dynactin, adding these inhibitors may have effects on the observed properties of the proteins. A similar effect of phosphatase inhibitors on MT–dynein–dynactin interactions was also noted recently by Niclas et al. (1996). Although it is known that the dynein subunits (Dillman and Pfister, 1994) and dynactin p150Glued (P. Farshori and E. L. F. Holzbaur, unpublished observation) are phosphorylated, the issue of whether phosphorylation directly affects the interaction between dynein and dynactin will require further investigation. The regulation of dynactin binding to MTs also explains the observation that dynactin and tubulin are transported at different rates. If SCb dynactin bound constitutively to the axonal MTs, then it would be expected that either the axonal MTs would be transported at the rate of SCb or dynactin would be transported at the rate of SCa.
DISCUSSION
To gain insight into the possible roles of dynactin and cytoplasmic dynein in axonal transport, we have compared their axonal transport profiles and examined their functional properties. We found that, similar to dynein, the bulk of the axonally transported dynactin complex is associated with SCb of slow axonal transport, whereas only a small pool is associated with the membranous organelles of fast axonal transport. Comparison of the proteins in fast component and SCb revealed different distributions of dynein IC74 isoforms, dynein LIC isoforms, and dynactin p150Glued isoforms in these functionally distinct axonal compartments. Analysis of the functional properties of dynein revealed that, like dynein from brain, dynein from SCb binds MTs in an ATP-sensitive manner and that SCb dynein is capable of direct binding to dynactin. Furthermore, the binding of dynactin to MTs is regulated, and dynactin from SCb does not bind MTs.
Lasek and associates proposed that the MTs and other cytoskeletal elements in slow transport are moved as polymer by sliding (Lasek, 1986; McQuarrie et al., 1986; Brady, 1991; Vallee and Bloom, 1991). Supporting this hypothesis, Baas and Ahmad (1993) used newly cultured rat sympathetic ganglia neurons treated with low concentrations of vinblastine to demonstrate MT transport from the cell body into the axon, and Terasaki et al. (1995) found that short MT segments injected into squid axoplasm would move at rates comparable to slow axonal transport. In addition, Reinsch et al. (1991) used neural tube cultures from Xenopus embryos injected with photo-activatable tubulin to demonstrate that MT polymer does indeed move along axons. Although MT polymer movement has not been demonstrated with photo activation or photo bleaching in other cultured cell systems, it has been observed that, unlike the Xenopus neurons, the other cultured neurons grow so slowly that diffusion alone is sufficient to supply tubulin subunits to the growing axon (Sabry et al., 1995). Furthermore, movement of MTs down the axon does not exclude the possibility that there is exchange of tubulin subunits during axonal transport (Vallee and Bloom, 1991; Black, 1994). Yu and associates (1996) recently have used cultured sympathetic neurons to demonstrate that both MT transport and assembly occur during axonal growth. It is known that both the polarity of dynein force generation and the orientation of the MTs (plus end toward the synapse), are consistent with the hypothesis that dynein slides MTs toward the synapse (Heidemann et al., 1981; Paschal and Vallee, 1987; Brady, 1991).
We considered two possible models to account for our observation that dynein and dynactin are associated with SCb. In one model, the dynein and dynactin associated with SCb are inactive pools transported to the synapse, where they are activated in some manner to function in the retrograde axonal transport of membranous organelles. The alternative model hypothesizes that cytoplasmic dynein, cross-linked via dynactin to the SCb transport complex, has a transient interaction with MTs as a motor protein, thereby moving the MTs along the axon at the slower rate of SCa. To distinguish between these two possible models, we analyzed the properties of SCb dynein and dynactin. SCb dynein binds dynactin, which is consistent with the idea that SCb dynein interacts with dynactin during slow axonal transport. Most importantly, dynein from SCb binds MTs in an ATP-sensitive manner in vitro, as does dynein from whole brain. This observation is inconsistent with the idea that SCb dynein is an inactive transport pool and suggests that SCb dynein indeed has a functional role in slow axonal transport.
Tokito et al. (1996) found two distinct pools of dynactin in brain. Individual dynactin complexes contain either the p150Glued isoform or the p135Glued isoform, which binds dynein but does not bind MTs. We find that the MT binding of dynactin is regulated such that SCb dynactin with the p150Glued isoform does not bind MTs. We also find that the pool of dynactin with the p135Glued isoform is present in SCb. This suggests that the role of axonal SCb dynactin is to bind dynein, not MTs. These data thus support the model in which anterogradely moving dynein linked to the SCb transport complex by dynactin is involved in slow transport of MTs rather than being an inactive transport pool.
Comparison of the SCb and fast component pools of dynein revealed different isoforms of the dynein IC74 and LIC subunits associated with these two functionally distinct axonal compartments. We considered the hypothesis that the additional SCb dynein IC74 and LIC isoforms might function to maintain dynein in an inactive state during transport in SCb. However, that possibility is inconsistent with the observed ATP-sensitive MT binding of SCb dynein. By analogy with flagellar dynein IC subunits, it is thought that IC74 is involved in binding dynein to cargo (King et al., 1995). This raises the possibility that the different isoforms of the IC74s and LICs of dynein perform specific functions. The IC74-2C gene product seems to be involved in binding to membranous organelles, whereas the other IC74 gene products may be involved in axon-specific functions, such as binding to the SCb transport complex and axonal transport. It is interesting that different isoforms of both the dynein IC74 and the dynactin p150Glued subunits are found in fast component as compared with SCb, because these dynein and dynactin subunits interact in vitro (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995).
The data presented here therefore lead us to support the model in which dynactin is tightly associated with the SCb transport complex and serves as a “platform” for dynein binding. Active dynein, which is cross-linked via dynactin to the SCb transport complex, interacts transiently with MTs and moves them at the slower rate of SCa. One important aspect of this model still needs to be addressed experimentally—the association of dynactin with microfilaments. There are several mechanisms by which dynactin could bind to the microfilaments of the SCb transport complex. Dynactin contains a filament composed of actin-related protein 1 (Arp1) (Schafer et al., 1994). Arp1 has been shown to copolymerize with conventional actinin vitro (Melki et al., 1993). Thus, dynactin could interact with the microfilaments in SCb directly through an Arp1/actin filament. Alternatively, another SCb protein, such as spectrin (Levine and Willard, 1981) or a myosin, could cross-link the dynactin Arp1 filament to the axonal microfilaments (Schroer, 1994; Mullins et al., 1996).
It has been reported that the bulk of the proteins transported in SCb are concentrated in the cortex of the subaxolemmal region of the axon, whereas those of SCa are distributed more uniformly in the axon (Heriot et al., 1985). This suggests that SCb dynein and dynactin are also concentrated near the membrane. Thus the model for dynein and dynactin anchored in the cortical actin microfilaments generating MT movement in the axon is likely comparable to the mechanism of cortical-based MT movements observed in various other cell systems, including nuclear migration (Xiang et al., 1994) as well as spindle orientation in fungi (Eshel et al., 1993; Muhua et al., 1994) and Caenorhabditis elegans (Waddle et al., 1994).
One prediction of a model with SCb dynein as the motor for SCa is that the rate of MT movement in SCa will be a function of the amount of interaction between SCb dynein and MTs. Either longer times of interaction or more frequent interactions will result in an apparent increase in the transport velocity of MTs from SCa rates closer to the rate of SCb. If the extent of dynein interaction with MTs is regulated, that could explain the variation in axonal transport constituents and rates that has been observed in different nerves and species (McQuarrie et al., 1986). Our results may also provide an explanation for part of the Drosophila Glued phenotype (Swaroop et al., 1987). Detailed histological studies of photoreceptor cells and laminal neurons from flies possessing the Gl1 Gluedmutation reveal that, in addition to abnormal fiber projection, there is grossly altered morphology of individual axons ranging from short, wide paddle shapes to irregular swellings and spikes (Garen and Kankel, 1983). It is not unreasonable to speculate that such morphological defects result from the aberrant transport of cytoskeletal elements down the axon in slow axonal transport. This hypothesis is consistent with the proposal that disruption of slow axonal transport could lead to symptoms of motor neuron disease (Collard et al., 1995; Cleveland, 1996).
The experiments described in this report demonstrate that cytoplasmic dynein and dynactin are associated with SCb of axonal transport and that there are biochemically and functionally distinct pools of each in axons and brain. Axonal transport has been studied extensively since it was first described by Weiss and Hiscoe (1948). Although much work over the past decade has greatly advanced our understanding of the mechanisms of fast axonal transport, the mechanisms involved in slow axonal transport have remained primarily unknown. This study points to already characterized motor proteins and motor protein-associated complexes as potential players in the mechanisms of slow axonal transport.
Footnotes
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke and National Institutes of Health Biotechnology Training Program at the University of Virginia and by the University of Virginia Cancer Center. We thank Chris Echeverri and Dr. Richard Vallee for the generous gift of p50 antibody. We also thank Dr. John Lye for helpful discussions.
Correspondence should be addressed to Dr. K. Kevin Pfister, Cell Biology Department, Box 439, School of Medicine, University of Virginia Health Sciences Center, Charlottesville, VA 22908.
REFERENCES
- 1.Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. J Cell Biol. 1993;120:1427–1437. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black MM. Microtubule transport and assembly cooperate to generate the microtubule array of growing axons. van Pelt J, Corner MA, Uylings HBM, Lopes da Silva FH. Progress in brain research, Vol 102 1994. 61 77 Elsevier; Amsterdam: Science. [DOI] [PubMed] [Google Scholar]
- 3.Black MM, Lasek RJ. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 1979;171:401–413. doi: 10.1016/0006-8993(79)91045-x. [DOI] [PubMed] [Google Scholar]
- 4.Black MM, Lasek RJ. Slow components of axonal transport: two cytoskeletal networks. J Cell Biol. 1980;86:616–623. doi: 10.1083/jcb.86.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady ST. Molecular motors in the nervous system. Neuron. 1991;7:521–533. doi: 10.1016/0896-6273(91)90365-7. [DOI] [PubMed] [Google Scholar]
- 6.Brady ST, Lasek RJ. Axonal transport: a cell-biological method for studying proteins that associate with the cytoskeleton. Methods Cell Biol. 1982;25:365–398. doi: 10.1016/s0091-679x(08)61434-x. [DOI] [PubMed] [Google Scholar]
- 7.Cleveland DW. Neuronal growth and death: order and disorder in the axoplasm. Cell. 1996;84:663–666. doi: 10.1016/s0092-8674(00)81044-2. [DOI] [PubMed] [Google Scholar]
- 8.Collard J-F, Cote F, Jullien J-P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 9.Dillman JF, III, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillman JF, III, Dabney LP, Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proc Natl Acad Sci USA. 1996;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50 kDa subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elluru RG, Bloom GS, Brady ST. Fast axonal transport of kinesin in the rat visual system: functionality of kinesin heavy chain isoforms. Mol Biol Cell. 1995;6:21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshel D, Urrenstarazu LA, Vissers S, Jauniaux J-C, van Vliet-Reedijk JC, Planta RJ, Gibbons IR. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garen SH, Kankel DR. Analysis of visual system development in Drosophila melanogaster: mutations at the Glued locus. Dev Biol. 1983;99:88–102. doi: 10.1016/0012-1606(83)90256-7. [DOI] [PubMed] [Google Scholar]
- 15.Garner JA, Lasek RJ. Cohesive axonal transport of the slow component b complex of polypeptides. J Neurosci. 1982;2:1824–1835. doi: 10.1523/JNEUROSCI.02-12-01824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons IR. Dynein ATPases as microtubule motors. J Biol Chem. 1988;263:15837–15840. [PubMed] [Google Scholar]
- 17.Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grafstein B, Forman DS. Intracellular transport in neurons. Physiol Rev. 1980;60:1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- 19.Heidemann SR, Landers JJ, Hamborg MA. Polarity of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heriot K, Gambetti P, Lasek RJ. Proteins transported in slow components a and b of axonal transport are distributed differently in the transverse plane of the axon. J Cell Biol. 1985;100:1167–1172. doi: 10.1083/jcb.100.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman PN, Lasek RJ. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975;66:351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 23.King SM, Patel-King RS, Wilkerson CG, Witman GB. The 78,000-Mr intermediate chain of Chlamydomonas outer arm dynein is a microtubule-binding protein. J Cell Biol. 1995;131:399–409. doi: 10.1083/jcb.131.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lasek RJ. Polymer sliding in axons. J Cell Sci Suppl. 1986;5:161–179. doi: 10.1242/jcs.1986.supplement_5.10. [DOI] [PubMed] [Google Scholar]
- 26.Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–643. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lye RJ, Porter ME, Scholey JM, McIntosh JR. Identification of a microtubule-based cytoplasmic motor in the nematode C. elegans. Cell. 1987;51:309–318. doi: 10.1016/0092-8674(87)90157-7. [DOI] [PubMed] [Google Scholar]
- 28.Mans RJ, Novelli GD. Measurement of the incorporation of radioactive amino acids into protein by a filter-paper disk method. Arch Biochem Biophys. 1961;94:48–53. [Google Scholar]
- 29.McGrail MJ, Gepner J, Silvanovich A, Ludmann S, Serr M, Hays TS. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQuarrie IG, Brady ST, Lasek RJ. Diversity in the axonal transport of structural proteins: major differences between optic and spinal axons in the rat. J Neurosci. 1986;6:1593–1605. doi: 10.1523/JNEUROSCI.06-06-01593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melki R, Vainberg IR, Chow RL, Cowan NJ. Chaperonin-mediated folding of vertebrate actin-related protein and gamma-tubulin. J Cell Biol. 1993;122:1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhua L, Karpova TS, Cooper JA. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994;78:669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 33.Mullins RD, Kelleher JF, Pollard TD. Actin’ like actin. Trends Cell Biol. 1996;6:208–212. doi: 10.1016/0962-8924(96)20017-0. [DOI] [PubMed] [Google Scholar]
- 34.Niclas J, Allen VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paschal BM, Vallee RB. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- 36.Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paschal BM, Holzbaur ELF, Pfister KK, Clark S, Meyer DI, Vallee RB. Characterization of a 50 kDa polypeptide in cytoplasmic dynein preparations reveals a complex with p150Glued and a novel actin. J Biol Chem. 1993;268:15318–15323. [PubMed] [Google Scholar]
- 38.Pfister KK, Wagner MC, Stenoien DL, Brady ST, Bloom GS. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989;108:1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfister KK, Salata MW, Dillman JF, III, Vaughan KT, Vallee RB, Torre E, Lye RJ. Differential expression and phosphorylation of the 74 kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996a;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- 40.Pfister KK, Salata MW, Dillman JF, III, Torre E, Lye RJ. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol Biol Cell. 1996b;7:331–343. doi: 10.1091/mbc.7.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plamann M, Minke PF, Tinsley JH, Bruno KS. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994;127:139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinsch SS, Mitchison TJ, Kirschner M. Microtubule polymer assembly and transport during axonal elongation. J Cell Biol. 1991;115:365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabry J, O’Connor TP, Kirschner M. Axonal transport of tubulin in T1 pioneer neurons in situ. Neuron. 1995;14:1247–1256. doi: 10.1016/0896-6273(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 44.Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroer TA. New insights into the interaction of cytoplasmic dynein with the actin related protein, ARP1. J Cell Biol. 1994;127:1–4. doi: 10.1083/jcb.127.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroer TA, Sheetz MP. Functions of microtubule-based motors. Annu Rev Physiol. 1991;53:629–652. doi: 10.1146/annurev.ph.53.030191.003213. [DOI] [PubMed] [Google Scholar]
- 47.Swaroop A, Swaroop M, Garen A. Sequence analysis of the complete cDNA and encoded polypeptide for the Glued gene of Drosophila melanogaster. Proc Natl Acad Sci USA. 1987;84:6501–6505. doi: 10.1073/pnas.84.18.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terasaki M, Schmidek A, Galbraith JA, Gallant PE, Reese TS. Transport of cytoskeletal elements in the squid giant axon. Proc Natl Acad Sci USA. 1995;92:11500–11503. doi: 10.1073/pnas.92.25.11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokito MK, Howland DS, Lee VM, Holzbaur ELF. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol Biol Cell. 1996;7:1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tytell M, Black MM, Garner JA, Lasek RJ. Axonal transport: each major rate component reflects the movement of distinct macromolecular complexes. Science. 1981;214:179–181. doi: 10.1126/science.6169148. [DOI] [PubMed] [Google Scholar]
- 51.Vallee RB, Bloom GS. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- 52.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waddle JA, Cooper JA, Waterston RH. Transient localized accumulation of actin in Caenorhabditis elegans blastomeres with oriented asymmetric divisions. Development. 1994;120:2317–2328. doi: 10.1242/dev.120.8.2317. [DOI] [PubMed] [Google Scholar]
- 54.Waterman-Storer CM, Holzbaur ELF. The product of the Drosophila gene, Glued, is the functional homologue of the p150Glued component of the vertebrate dynactin complex. J Biol Chem. 1996;271:1153–1159. doi: 10.1074/jbc.271.2.1153. [DOI] [PubMed] [Google Scholar]
- 55.Waterman-Storer CM, Karki S, Holzbaur ELF. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss P, Hiscoe H. Experiments on the mechanism of nerve growth. J Exp Zool. 1948;107:315–395. doi: 10.1002/jez.1401070302. [DOI] [PubMed] [Google Scholar]
- 57.Williams RC, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 58.Wray W, Boulidas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 59.Xiang X, Beckwith SM, Morris NR. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci USA. 1994;91:2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu W, Schwei MJ, Baas PW. Microtubule transport and assembly during axonal growth. J Cell Biol. 1996;133:151–157. doi: 10.1083/jcb.133.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]