Abstract
The expression of hepatocyte growth factor/scatter factor (HGF/SF) and its receptor, the c-met proto-oncogene product, was examined by in situ hybridization in the developing and adult murine olfactory system and compared with the expression of a known activator of HGF/SF, tissue-type plasminogen activator (tPA). In the developing olfactory canal, expression of both c-metand tPA was observed in the olfactory neuroepithelium, whereas HGF/SF expression appeared to be confined to the mucosa adjacent to the neuroepithelium. During development of the olfactory bulb, HGF/SF and tPA were expressed within the rostral migratory pathway leading to the olfactory bulb, whereas c-met expression was observed in the mitral cell layer (MCL) of the olfactory bulb and in the anterior olfactory nucleus. In the adult olfactory bulb, expression of HGF/SF was restricted to the periglomerular region of the glomerular layer, whereas c-met was expressed in the MCL and olfactory nerve fiber layers (ONL). tPA expression in the adult olfactory bulb was observed in the ONL, MCL, and granule cell layers. Therefore, tPA expression was relatively coincident with the expression of HGF/SF and/or c-met in the appropriate projection patterns of the developing and adult olfactory system. In addition, antibodies against tPA inhibited the olfactory bulb extract-mediated cleavage of single-chain HGF/SF. These results suggest that tPA may play a regulatory role in the development and maintenance of the olfactory system by activating HGF/SF in the immediate vicinity of its receptor.
Keywords: hepatocyte growth factor, c-met, tissue-type plasminogen activator, olfactory system, olfactory bulb
The development of the mammalian CNS is a complex process involving extensive tissue remodeling, neuronal and non-neuronal cell migration, and neurite outgrowth; processes for which the activity of plasminogen activators (PAs) have been implicated. PAs are believed to aid in these processes by locally degrading the proteins involved in cell–cell and cell–matrix contacts. (Hart and Rehmetulla, 1988; Saksela and Rifkin, 1988; Seeds et al., 1990). Although there is substantial evidence supporting this role, the widespread and differential expression of PAs throughout the CNS suggests a diversity of possible functions. The fact that PAs can activate several latent growth factors raises the possibility that PAs may play a much broader role in regulating CNS processes than previously imagined (Campbell et al., 1992; Brauer and Yee, 1993).
One such growth factor, hepatocyte growth factor/scatter factor (HGF/SF), can elicit a variety of responses including mitogenic and motogenic activities from cells expressing its receptor, thec-met proto-oncogene product (Rubin et al., 1993). Expression of HGF/SF and c-met is essential for development, because deleting either gene in mice produces embryonic lethality (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995). HGF/SF has been implicated in the development and regeneration of several organs of epithelial origin (Santos et al., 1994; Schmidt et al., 1995;Woolf et al., 1995) and in the migration of myogenic precursor cells (Bladt et al., 1995). Although HGF/SF is expressed in brain (Jung et al., 1994), its function in the CNS is currently unknown; however, murine septal neurons in culture respond to HGF/SF by increasing the level of c-fos transcripts (Jung et al., 1994).
HGF/SF is secreted into the extracellular matrix as an inactive single-chain precursor, proHGF/SF. The biologically active form of HGF/SF is produced by proteolytic processing of proHGF/SF (Nakamura et al., 1989; Naka et al., 1992). In vitro, both urokinase-type PA (uPA) and tissue-type PA (tPA), as well as another protease related to blood coagulation factor XII, have been shown to cleave and activate proHGF/SF (Naldini et al., 1992; Mars et al., 1993). In liver, partial hepatectomy results in a rapid increase in the active form of HGF/SF, which is paralleled by an increase in uPA activity, suggesting that uPA serves as an activator of HGF/SF in liver (Mars et al., 1995).
The presence of HGF/SF and/or c-met in the developing olfactory system (Sonnenberg et al., 1993) appears similar to the expression of tPA mRNA in the olfactory canal (OC) (Friedman and Seeds, 1994). The possibility that tPA may play a regulatory role in the development and/or maintenance of some structures of the olfactory system by activating HGF/SF was explored by defining more precisely the elements of the developing and adult murine olfactory system that express HGF/SF, c-met, and tPA mRNA and correlating their spatial and temporal distributions with developmental events in the olfactory system. A brief preliminary report of some of these findings has appeared previously (Thewke and Seeds, 1995).
MATERIALS AND METHODS
Animals. All animal experiments were conducted according to an officially approved institutional protocol. Adult and postnatal C57Bl/6 mice were killed with CO2 gas. The tissues were rapidly excised and immediately snap-frozen in 2-methylbutane at −35°C. Tissues were then kept at −70°C until use. Fetal mice were obtained after controlled mating for 12 hr. The morning after conception was defined as 0.5 d.
Plasmids and probes. The mouse tPA cDNA vector pK2C3z, containing a 515 bp fragment encompassing part of the coding sequence for the mouse tPA kringle 2 and catalytic domains, has been described previously (Friedman and Seeds, 1994). HGF/SF and c-metpartial cDNA clones were obtained by RT-PCR of total mouse liver RNA using the following primers essentially as described by Kawasaki (1990): HGF/SF sense primer 5′-CGAAATCCTCGAGGGGAAGAAGGG-3′ (535–559), HGF/SF antisense primer 5′-CCAACGCTGACAGGGAATTCCATTC-3′ (965–990) sequence, and nucleotide numbers as in Lee et al. (1993);c-met sense primer 5′-GGGACTGCAGCAGCAAAGC-3′ (296–314),c-met antisense primer 5′-GTCTGAGCATCTAGAGTTTCC-3′ (795–815) sequence, and nucleotide numbers as in Chan et al. (1988). RT-PCR products were fractionated on 2% agarose gels, isolated, and subcloned into pGEM3z (Promega, Madison, WI) by standard protocols (Sambrook et al., 1989). The identities of the cloned fragments were confirmed by using a dideoxynucleotide sequencing kit (Amersham, Arlington Heights, IL).
Single-stranded [35S]-UTP-labeled cRNA probes were transcribed from plasmid vectors linearized downstream of the inserted cDNA fragment using either T7 or SP6 RNA polymerase (Promega, Madison, WI). Probes were purified over G-50 Sephadex spin columns (Boehringer-Mannheim, Indianapolis, IN), and their integrity was verified by electrophoresis on 5% sequencing gels. The specificity of the probes was checked by Northern hybridization of blots containing 5 μg of poly(A+) mRNA isolated from adult mouse liver tissue.
Northern hybridization. Total RNA was isolated from tissues using the RNAzol B method, as described by the manufacturer (Tel-Test, Friends-wood, TX). Poly(A+) RNA was isolated from the total RNA using oligo-dT cellulose columns, as described by the manufacturer (Molecular Research Center, Cincinnati, OH). The RNA was fractionated on 1% agarose–2.2 m formaldehyde gels and transferred by capillary action to ζ-probe membrane. The blots were cross-linked by UV irradiation and prehybridized in in situhybridization buffer (Friedman and Seeds, 1994) at 65°C for 15 min. Heat-denatured cRNA probe was then added to a final concentration of 5 × 106 cpm/ml, and hybridization performed for 16 hr at 62°C. The blots were then washed in descending concentrations of SSC (2×, 1×, 0.5×, 0.1×) containing 1 mm DTT. The first three washes were conducted at room temperature, and the final wash was conducted at 65°C. The blots were then exposed for 48 hr to x-ray film with an intensifying screen at −70°C.
In situ hybridization. Frozen tissues were embedded in O.C.T. compound (Miles, Elkhart, IN), and 16 μm cryostat sections were thaw-mounted onto 3-amino-propyltriethoxysilane-coated slides.In situ hybridization was performed as described by Friedman and Seeds (1994) with the following after modifications: the hybridization mixture contained 5 × 106 cpm/ml of cRNA probe, and the slides were hybridized at 62°C for 16 hr. The slides were washed a second time in 0.1× SSC for 15 min at 65°C, and, after dipping in Kodak NTB-2 emulsion, the slides were stored in a dark box at 4°C for 28–35 d.
Zymography. Extracts were prepared from freshly dissected tissue using a Dounce homogenizer and 5 vol of 50 mmTris-HCl, pH 6.8, containing 0.1% SDS, and aliquots were frozen at −70°C until use. The protein concentration of the extracts was determined using a BCA protein assay (Pierce, Rockford, IL) with BSA as the standard. Aliquots of the extract were mixed with nonreducing 4× Laemmli sample buffer (Laemmli, 1970) and heated at 85°C for 10 min before being subjected to electrophoresis on standard 10% polyacrylamide gels containing 1 mg/ml casein and 0.1 U/ml plasminogen (Chromogenix, Molndal, Sweden). Control gels were run in the absence of plasminogen. Bands of caseinolytic activity were visualized by staining with 0.25% Coomassie brilliant blue R250 in 50% methanol/10% acetic acid and briefly destaining in the same solvent. The concentration of PA activity was estimated by scanning the gel on a computing densitometer using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Cleavage of exogenous HGF/SF. Olfactory bulb extracts were prepared by homogenizing freshly dissected olfactory bulb tissue from adult mice in an ice-cold solution of 25 mm sucrose, 5 mm HEPES, pH 6.8, and aliquots were snap-frozen and stored at −70°C until use. Protein concentration of the extract was determined as described above for zymography.
A predominately single-chain preparation containing 0.25 μg of recombinant human proHGF/SF (R & D Systems, Minneapolis, MN) was iodinated using Iodobeads per the manufacturer’s instructions (Pierce, Rockford, IL) and purified by gel filtration over a Sephadex G-25 column. Approximately 4 ng of iodinated proHGF/SF was incubated with 10 μg of extract at 37°C for various periods of time (0.5–3 hr) in siliconized tubes. Before the addition of iodinated proHGF/SF, the extracts were incubated on ice for 30 min with 0.2 μg of antibodies against murine tPA (American Diagnostic, Greenwich, CT) or an equivalent amount of a nonspecific (rabbit anti-goat IgG) IgG fraction as control. The reactions were stopped by adding an equal volume of 2× reducing SDS-PAGE loading buffer (Laemmli, 1970) and heating at 85°C for 10 min. Samples were then analyzed by 10% SDS-PAGE and the bands visualized by autoradiography. For quantification, the dried gels were exposed to a phos-phorimager screen (Molecular Dynamics) for 16 hr.
RESULTS
Although tPA has been shown to be the primary PA expressed in other regions of the brain (Seeds et al., 1992; Sappino et al., 1993), there has been no clear identification of the PA activity expressed in the olfactory bulb. However, both tPA and uPA mRNA were seen (Dent et al., 1993) in the rat olfactory bulb. Because both tPA and uPA are capable of activating HGF/SF, we wished to identify directly the PA activity present in the mouse olfactory bulb. Zymographic analysis revealed primarily tPA (Mr = 65,000) and a small amount of uPA (Mr = 37,000) activity present in olfactory bulb extract (Fig. 1). Quantitative densitometric analysis of the zymographs showed that tPA constituted >94% of the total PA activity present in the extract. All of the caseinolytic activity observed in the gels was plasminogen-dependent, and no other proteolytic activity was detected on the gels. Thus, as in other regions of the mouse brain, tPA represents the majority of PA activity present in murine olfactory bulb.
Fig. 1.
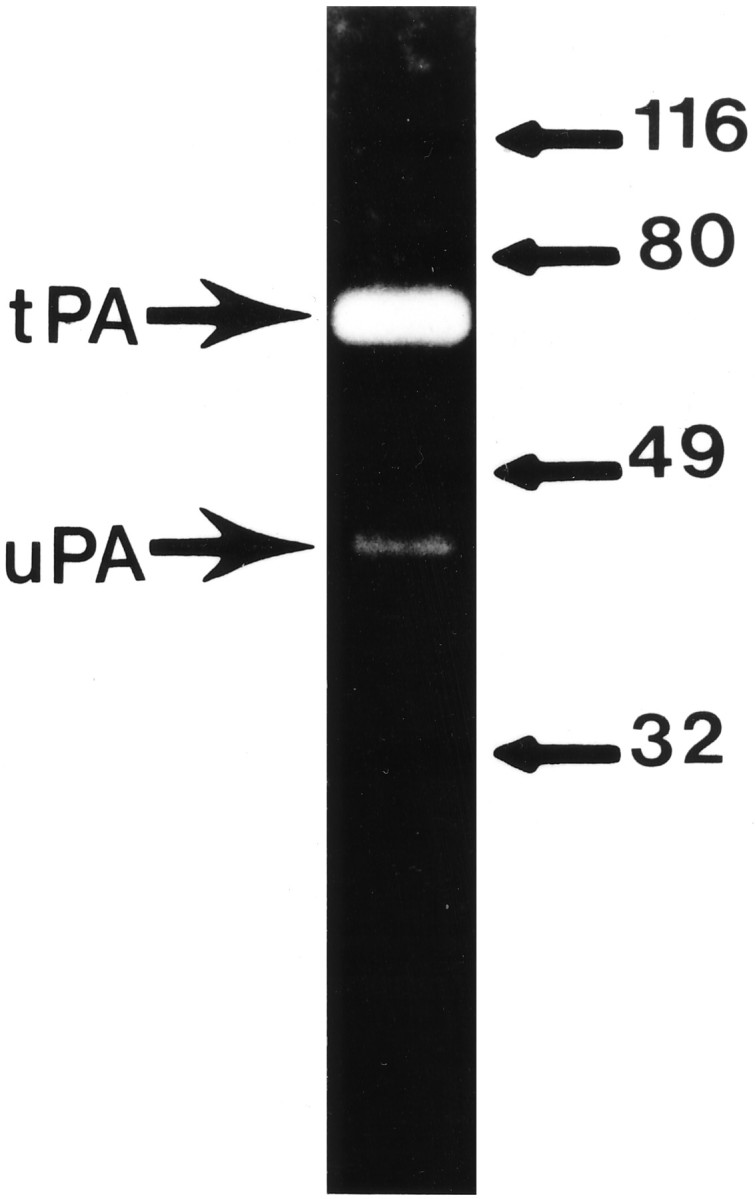
Zymographic analysis of the PA activity present in adult olfactory bulb extract. One microgram of total protein extract obtained from an adult olfactory bulb was subjected to zymographic analysis. The bands of caseinolytic activity corresponding to tPA (Mr = 65,000) and uPA (Mr = 37,000) activity are indicated on theleft.
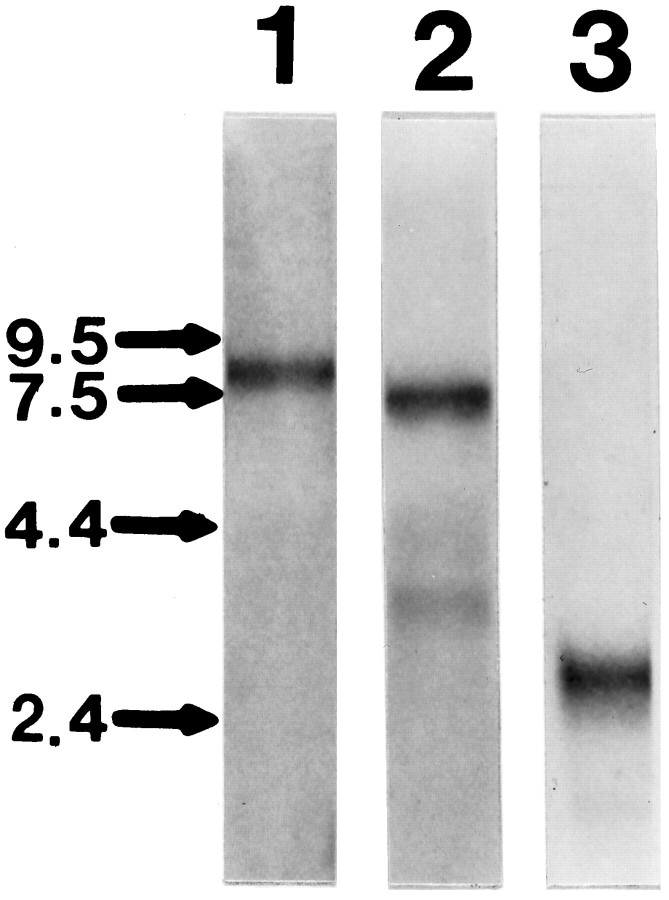
To better characterize their expression in the olfactory system, partial murine HGF/SF and c-met cDNA clones were prepared by RT-PCR of total liver RNA and used to generate probes for Northern blot and in situ hybridization analysis. Northern blot analysis revealed that both HGF/SF and c-met mRNA were expressed in the adult olfactory bulb (Fig. 2). The HGF/SF cRNA probe hybridized to a 6.0 kb transcript and a 3.0 kb transcript, whereas thec-met cRNA probe detected an 8.5 kb transcript. These correspond to the major HGF/SF and c-met transcripts present in other murine tissues (Chan et al., 1988; Lee et al., 1993). In addition, a tPA cRNA probe hybridized to a 2.8 kb transcript corresponding to the major tPA transcript in murine brain (Sappino et al., 1993; Friedman and Seeds, 1995). These results reveal that HGF/SF, c-met, and tPA mRNA all are expressed in the adult murine olfactory bulb and demonstrate the specificity of the probes for their tar- get mRNA.
Fig. 2.
Northern blot analysis of HGF/SF,c-met, and tPA mRNA expression in adult olfactory bulb. Blots containing 5 μg of poly(A+) RNA prepared from the adult olfactory bulbs were hybridized with 35S-labeled cRNA antisense cRNA probes to c-met, HGF/SF, and tPA (lanes 1–3, respectively). The size of RNA standards is as indicated on the left in kb.
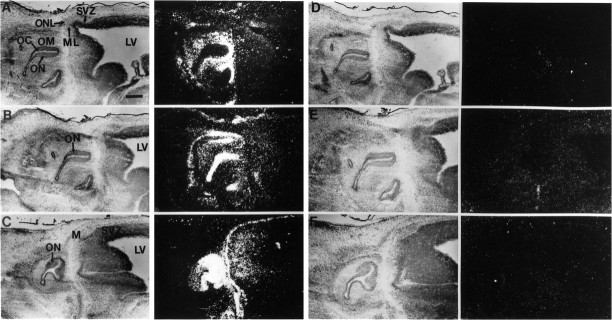
In situ hybridization was performed on mouse embryos of various ages using cRNA probes to HGF/SF, c-met, and tPA mRNA to determine which olfactory structures express each mRNA. In mice, the characteristic organization of the OC and the distribution of cell types in the olfactory epithelium becomes apparent around embryonic day 12 (E12) (Cuschieri and Bannister, 1975; Noda and Harada, 1981). In the developing OC, transcripts of all three mRNAs were detectable at E14, the earliest age examined. At this time,c-met expression was limited to the olfactory neuroepithelium close to the luminal surface, whereas HGF/SF expression was observed in the adjacent olfactory mucosa (OM) (Figs.3, 4). Figure 3 shows tPA mRNA expression throughout the olfactory neuroepithelium, including the portion of the epithelium that expresses c-met mRNA. The hybridization of the antisense cRNA probes was specific as revealed by the absence of hybridization to these structures with control sense cRNA probes (Fig.3). This pattern of expression was observed throughout development of the OC with the highest levels of all three transcripts being observed during the period from E14 to E17. Expression of all three transcripts in the OC was detected at lower levels postnatally (see Fig. 8) (data not shown); however, only tissues up to postnatal day 4 (P4) were examined. These results demonstrate the relatively coordinate expression of tPA, c-met, and HGF/SF mRNA in the developing OC.
Fig. 3.
Expression of HGF/SF, c-met, and tPA in the developing E14 olfactory system. The left panels are bright-field images, and the right panels are corresponding dark-field images. Hybridization of35S-labeled antisense cRNA probes with E14 sagittal sections. A, Expression of HGF/SF is high in the OM of the OC. Lower expression is also observed in the ventricular zone at the anterior end of the LV, which will develop into the olfactory bulb.B, Expression of c-met is observed in the olfactory neuroepithelium lining the OC. C, High-level expression of tPA is observed throughout the olfactory neuroepithelium. Use of 35S-labeled sense HGF/SF, c-met, and tPA (D–F, respectively) cRNA probes detects no specific hybridization. Scale bar, 310 μm.AON, Anterior olfactory nucleus; DON, dorsal anterior olfactory nucleus; EPL, external plexiform layer; GL, also g (Fig. 9), glomerular layer; GCL, granule cell layer;IPL, internal plexiform layer; LON, lateral anterior olfactory nucleus; LV, lateral ventricle; M, meninges; MCL, alsoML (Fig. 3), mitral cell layer; MZ, marginal zone; OC, olfactory canal; OM, olfactory mucosa; ON, olfactory neuroepithelium;ONL, olfactory nerve fiber layer; OV, olfactory ventricle; RMS, rostral migratory stream;SVZ, subventricular zone.
Fig. 4.

Higher-power photomicrograph of the developing OC shown in Figure 3. The left panels are bright-field images, and the right panels are corresponding dark-field images. A, Expression of HGF/SF is detected in the OM underlying the olfactory neuroepithelium. B, Expression of c-met is confined to the portion of the olfactory neuroepithelium lining the OC. C, Expression of tPA is observed throughout the olfactory neuroepithelium. Scale bar, 80 μm. See legend to Figure 3 for abbreviations.
Fig. 8.

Postnatal expression of HGF/SF,c-met, and tPA in the olfactory system. The left panels are bright-field images, and the right panels are corresponding dark-field images. Hybridization of35S-labeled antisense cRNA probes with postnatal sagittal sections. A, Expression of HGF/SF is observed in the RMS from the anterior lateral ventricle into the olfactory bulb at P0.B, Expression of HGF/SF in the RMS at P8.C, Expression of c-met in the P8 olfactory bulb is observed in the MCL and the AON. D, Expression of tPA in the P8 olfactory bulb. Scale bar, 255 μm. See legend to Figure 3 for abbreviations.
At E14, the development of the olfactory bulb is evident by an outpocketing at the anterior end of the lateral ventricle (LV). Several distinct layers are recognizable in the E14 bulb including the subventricular zone (SVZ), the marginal zone (MZ), and the olfactory nerve fiber layer (ONL) (Hinds, 1968). At this time, a low level of HGF/SF expression was observed in the SVZ at the anterior end of the LV (Fig. 3). Expression of HGF/SF in the MZ and ONL was at low or background levels. Neither c-met nor tPA hybridization was evident in any layer of the primordial olfactory bulb (Fig. 3).
By E16, the definitive mitral cell layer (MCL) of the olfactory bulb can be distinguished, and the SVZ has increased in size (Hinds, 1968). At this age, c-met expression was detected in the MCL and in the MZ just superficial to the developing MCL (Figs. 5,6). Expression of c-met was not above background levels in any other area of the E16 bulb. Meanwhile, the expression of HGF/SF had increased throughout the SVZ of both the olfactory and LVs (Fig. 5). Hybridization of HGF/SF outside of the SVZ remained at or below background levels.
Fig. 5.
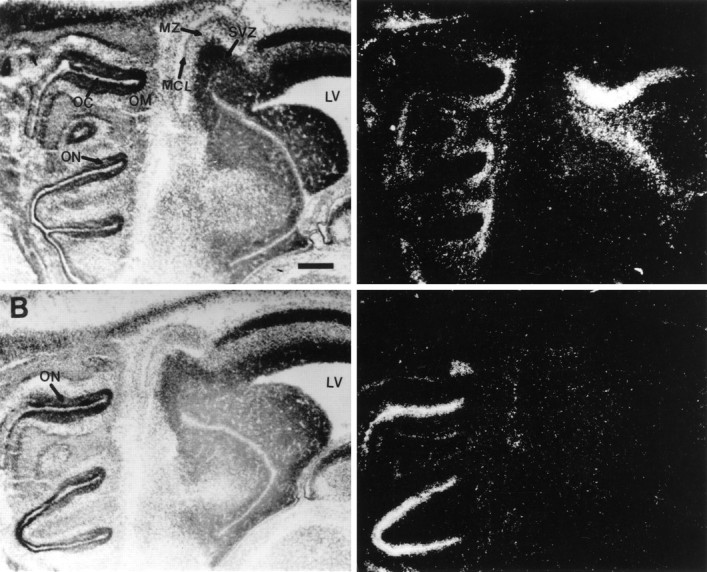
Expression of HGF/SF and c-met in the developing olfactory system at E16. The left panelsare bright-field images, and the right panels are corresponding dark-field images. Hybridization of35S-labeled antisense cRNA probes with E16 sagittal sections. A, Expression of HGF/SF is observed in the OM and in the SVZ of the primordial olfactory bulb. B, Expression of c-met at the luminal surface of the olfactory neuroepithelium and in the MCL of the olfactory bulb. Scale bar, 300 μm. See legend to Figure 3 for abbreviations.
Fig. 6.
Higher-power photomicrograph of HGF/SF andc-met expression in the developing E16 olfactory bulb shown in Figure 5. The left panels are bright-field images, and the right panels are corresponding dark-field images. Hybridization of 35S-labeled antisense cRNA probes with E16 sagittal sections. A, Expression of HGF/SF is high in the SVZ of the primordial bulb. B, Expression of c-met can be seen in the developing MCL. Scale bar, 100 μm. See legend to Figure 3 for abbreviations.
By E18, the characteristic organization of the adult olfactory bulb can be distinguished, including the ONL, MCL, internal plexiform layer (IPL), external plexiform layer (EPL), granule cell layer (GCL), and even a few glomeruli (Hinds, 1968). At this time, expression ofc-met was clearly detected in the MCL (Figs.7); by contrast, the cells in the SVZ and the developing GCL showed little c-met hybridization. Low levels of HGF/SF expression were observed just below the MCL in the developing GCL of the olfactory bulb. Higher levels of HGF/SF expression were observed in the SVZ lining the LVs and olfactory ventricles (OVs) including the region of the SVZ, which extends from the anterior portion of the LV into the olfactory bulb (Fig. 7). This portion of the SVZ serves as a pathway for migrating olfactory interneuron precursors and is often referred to as the rostral migratory stream (RMS) (Lois and Alvarez-Buylla, 1994). Figure 7 demonstrates that tPA was also expressed throughout the SVZ and RMS, coincident with the expression of HGF/SF. Consistent with previous studies (Friedman and Seeds, 1994), a high level of tPA expression was also observed in the meninges (M) surrounding the olfactory bulb and neocortex.
Fig. 7.

Expression of HGF/SF, c-met, and tPA in the developing olfactory system at E18. The left panels are bright-field images, and the right panels are corresponding dark-field images. Hybridization of35S-labeled antisense cRNA probes with E18 sagittal sections. A, HGF/SF expression is observed in the OM of the developing nasal cavity. In the developing bulb, expression of HGF/SF is high in the SVZ of the LVs and OVs including the RMS (indicated by arrows) extending from the LV into the olfactory bulb. B, Expression of c-metoccurs in the ON and MCL. C, Expression of tPA is high in the M and coincident with HGF/SF expression in the SVZ and RMS (indicated by arrows). Scale bar, 280 μm. See legend to Figure 3 for abbreviations.
Expression of HGF/SF in the RMS continued during postnatal development, reaching a peak at ∼P8 before declining in adulthood (Fig.8). Expression of HGF/SF above background levels was not observed outside of the SVZ. During this period, c-metexpression continued in the MCL and could be observed in the anterior olfactory nucleus (AON) (Fig. 8). The expression of tPA mRNA was observed in the AON concurrent with the expression ofc-met mRNA, as well as in the ONL and the dorsal part of the GCL surrounding the SVZ of the P8 bulb.
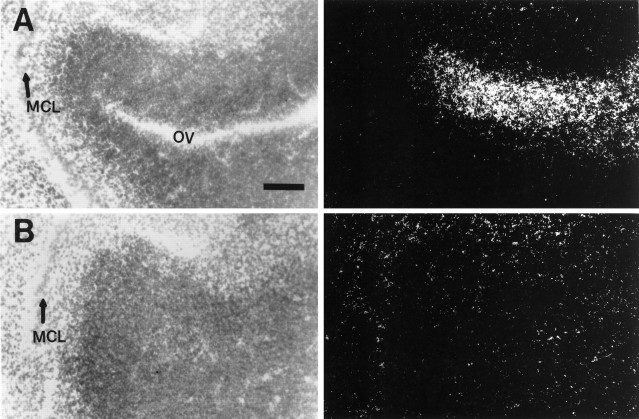
The adult olfactory bulb consists of the six distinct layers: the ONL, glomerular layer (GL), EPL, IPL, MCL, and GCL. In the adult olfactory bulb, expression of HGF/SF was restricted to the cells surrounding the glomeruli. These cells appear to be periglomerular neurons (Fig.9). c-met mRNA was clearly detected in the MCL and ONL (possibly Schwann cells that ensheathe the olfactory nerve fibers) (Fig. 9). Cells in the ONL exhibited a high level expression of tPA mRNA, whereas cells in the MCL and GCL expressed a somewhat lower level of tPA mRNA (Fig. 9). Thus, in the adult olfactory bulb, tPA appears to be expressed in close proximity to HGF/SF-expressing periglomerular neurons onto which c-met-expressing mitral cells synapse. This raises the possibility that tPA may activate HGF/SF produced by periglomerular cells, which would then produce a physiological response from the c-met-expressing mitral cells, the dendrites of which project into the glomeruli.
Fig. 9.

Expression of HGF/SF, c-met, and tPA in the adult olfactory bulb. The left panels are bright-field images, and the right panels are corresponding dark-field images. Hybridization of35S-labeled antisense cRNA probes with adult olfactory bulb sagittal sections. A, Expression of HGF/SF is restricted to the periglomerular cells in the GL. B,c-met expression is confined to the MCL and the ONL.C, Expression of tPA is high in the ONL and lower in the MCLs and GCLs. Scale bar, 255 μm. See legend to Figure 3 for abbreviations.
The results from the in situ hybridization study indicate that the expression of tPA is relatively coincident with the expression of either HGF/SF or its receptor c-met during the development of some structures of the olfactory system, strengthening a possible role for tPA as an activator of HGF/SF in the olfactory system. To determine whether the tPA present in the olfactory bulb could actually cleave proHGF/SF, an iodinated preparation of predominantly single-chain HGF/SF (scHGF/SF) was incubated with olfactory bulb homogenates. When subjected to reducing SDS-PAGE analysis, the iodinated scHGF/SF produced a major band of ∼92 kd as well as minor bands of 62 kd and 32–34 kd (Fig.10A). Previous studies have shown that the 92 kd band represents the scHGF/SF, which is cleaved to form the heavy (62 kd) and light (32–34 kd) chains of the active HGF/SF heterodimer (Gak et al., 1992; Naka et al., 1992). We reasoned that if olfactory bulb extract contained an activator of HGF/SF, the cleavage of scHGF/SF should be accompanied by a decrease in the 92 kd band, and if this activator were tPA, then the decrease should be inhibited by antibodies that neutralize tPA. Figure 10 demonstrates that incubation of iodinated scHGF/SF with adult olfactory bulb homogenate produced a decrease in the amount of the 92 kd band. A similar result was obtained using olfactory bulb homogenates obtained from mice of various postnatal ages and occurred in a concentration- and time-dependent manner (data not shown). We did not observe a concomitant increase in the intensity of the heavy and light chains, probably because of nonspecific proteolysis. Other researchers have noted a similar susceptibility of the heavy and light chains to degradation even when using purified preparations of tPA and uPA (Mars et al., 1993). Using an amount of olfactory bulb homogenate that resulted in an ∼50% decrease in the 92 kd band, preincubation of the homogenate with tPA antibodies greatly inhibited the cleavage of the 92 kd proHGF/SF (Fig.10B). This inhibition was specific, because preincubation of the homogenate with a like amount of a control IgG was without effect. The inclusion of the plasmin inhibitor aprotinin in the assay had no effect on the decrease of the 92 kd band or on the ability of the tPA antibodies to inhibit the decrease (data not shown). This indicates that the decrease in the 92 kd band was not attributable to plasmin in the homogenate and that the observed inhibition by the tPA antibodies was a direct effect on a tPA cleavage and not attributable to inhibition of tPA-mediated conversion of plasminogen to plasmin. Furthermore, preincubation with the uPA inhibitor amiloride was also without effect, indicating that the observed proHGF/SF cleavage was not attributable to the minor amount of uPA activity present in the homogenate (data not shown). These results demonstrate that tPA present in the olfactory bulb extract represents the primary exogenous proHGF/SF cleaving activity. Together with the relatively coincident expression of HGF/SF, c-met, and tPA mRNAs, these results strongly suggest that tPA functions in vivo as an activator of HGF/SF in the murine olfactory system.
Fig. 10.

Cleavage of scHGF/SF by olfactory bulb extract is inhibited by preincubation with antibodies to tPA. Iodinated HGF/SF was subjected to treatment with olfactory bulb homogenate that had been preincubated with either polyclonal antibodies against murine tPA or a like amount of control IgG preparation. A, Autoradiogram showing inhibition of scHGF/SF cleavage by tPA antibodies. Lane 1, HGF/SF incubated in the absence of olfactory bulb homogenate. Lane 2, HGF/SF incubated in the presence of olfactory bulb homogenate. Lane 3, HGF/SF incubated with olfactory bulb homogenate that was preincubated with tPA antibodies.Lane 4, HGF/SF incubated with olfactory bulb homogenate that was preincubated with a control IgG. sc,hc, and lc refer to the single chain, heavy chain, and light chains of HGF/SF, respectively. The molecular weights in kilodaltons of protein standards are as indicated on theright. B, Histogram depicting mean values ± SE (n = 4) for several scHGF/SF cleavage experiments similar to the one shown in A. Iodinated scHGF/SF was incubated with olfactory bulb extract that had been pretreated with either antibodies against tPA (+Ab) or the same amount of a nonspecific IgG preparation (control) for a period of time determined to give an ∼50% decrease in the scHGF/SF band in the absence of antibody treatment (−Ab). The value obtained from an scHGF/SF band from a mock incubation was set at 100% and used to determine the percent scHGF/SF remaining in the sample.
DISCUSSION
In this study, we demonstrate that the expression of tPA mRNA was relatively coincident with c-met mRNA and, in some instances, with HGF/SF mRNA during development of the OC and bulb. In the developing OC, both tPA mRNA and c-met mRNA were expressed in the olfactory neuroepithelium, whereas HGF/SF mRNA was expressed in the adjacent OM. These results extend the earlier preliminary demonstration of HGF/SF and c-met mRNA expression (Sonnenberg et al., 1993) and of tPA mRNA expression (Friedman and Seeds, 1994) in the developing OC to demonstrate the concurrent expression of HGF/SF and c-met mRNA with tPA. We also observed the expression of HGF/SF and c-met mRNA in the developing and adult olfactory bulb and demonstrate the relatively coincident expression of tPA mRNA within this structure. Furthermore, tPA was found to be the predominant PA activity present in olfactory bulb extracts and to be responsible for the majority of exogenous proHGF/SF cleaving activity in these extracts. These results indicate that HGF/SF may play an important role in the adult and developing murine olfactory system were tPA may be localized near c-metreceptors to generate the active, but relatively unstable, HGF/SF in the immediate vicinity of its receptor.
In mice, development of the olfactory epithelium begins at ∼E9 and continues throughout gestation (Hinds, 1968). The expression of HGF/SF,c-met, and tPA mRNA were detectable at the earliest time investigated (E14), and levels of all three mRNAs were high through E18 (Figs. 3, 4, 5, 6, 7). E11–E18 is a period of extensive tissue remodeling and cell migration, as well as the time of greatest olfactory sensory neuron differentiation, axonal outgrowth, and synaptogenesis in the olfactory bulb (Brunjes and Frazier, 1986; Farbman, 1991). tPA may have several functions during this developmental period. The proteolytic activity of tPA may directly influence tissue remodeling and cell migration by degrading extracellular matrix and cell adhesion molecules, as has been proposed previously (Seeds et al., 1980, 1990;Krystosek and Seeds, 1981; Moonen et al., 1982; Pittman 1985). In addition, tPA may also participate in a signaling cascade regulating the activity of HGF/SF and possibly the proliferation, differentiation, and migration of cells in the developing OC. The expression of tPA in other neuronal tissues that do not express HGF/SF or c-metsuggests tPA may have other functions in these structures, perhaps activating other latent growth factors, cytokines, chemokines, and other neuropeptides.
The fact that HGF/SF is secreted into the extracellular matrix as an inactive prohormone implies that cleavage to the active form may be a highly regulated step. If tPA serves as an activator of HGF/SF in vivo, control of HGF/SF activity could be achieved by maintaining a strict balance between tPA and the various matrix molecules that can influence its activity. Regulators of tPA activity that are known to be expressed in the developing murine olfactory system include thrombospondin, an enhancer of tPA activity (O’Shea and Dixit, 1988), and protease nexin-1, an inhibitor of tPA (Mansuy et al., 1993).
The recent observation that c-met expression is essential for myoblast migration and that myoblasts appear to migrate up a concentration gradient of HGF/SF (Bladt et al., 1995) suggests that HGF/SF may play a similar role in the development of the olfactory system. During development of the olfactory system, HGF/SF may act as a chemoattractant for migrating cells and neurites expressingc-met. The growth cones of some neurons are known to express a high-affinity receptor for tPA (Verrall and Seeds, 1989; Seeds et al., 1990, 1992). The binding of tPA to a receptor on the surface of migrating cells and/or growth cones expressing c-met would provide localized activation of HGF/SF in the immediate vicinity ofc-met when the cell/growth cone enters a region expressing proHGF/SF. The observed susceptibility of the heterodimeric form of HGF/SF to nonspecific degradation in vitro may indicate a rapid turnover of the active form in vivo and the importance of such a localized activation mechanism. One area where such a mechanism may function is the developing embryonic OC. Here, high levels of HGF/SF, c-met, and tPA mRNA are expressed at E14 through E19, which is a period of intense cell migration and axonal growth from the neuroepithelium to the developing olfactory bulb (Farbman, 1991). HGF/SF produced in the mucosa may act as a chemoattractant for axonal growth cones of olfactory receptors and/or the migrating epithelial cells that precede the axons out of the epithelium. Once in the mucosa, migrating cells/growth cones may use additional guidance cues for continued migration to the bulb. Although not investigated in mature animals, all three mRNAs were expressed in OC P4, the oldest age examined. If this pattern of expression continues in adult animals, it may reflect a continued role for HGF/SF andc-met in axonal guidance of newly generated olfactory sensory neurons that occurs throughout adulthood in mice (Graziadei and Monti-Graziadei, 1978).
Based on its expression patterns in other regions of the brain, HGF/SF has been proposed to act as a target derived neurotrophic factor (Jung et al., 1994), and HGF/SF has been shown to promote the survival of motor and hippocampal neurons in culture (Honda et al., 1995; Wong et al., 1995). Within the olfactory bulb, the expression patterns of HGF/SF and c-met suggest a neurotrophic role in the formation and/or maintenance of the olfactory glomeruli. HGF/SF expressed by the periglomerular cells may function in the formation and maintenance of synaptic connections among periglomerular dendrites, sensory neuron axons, and mitral cell dendrites, all of which form synapses within the glomeruli (Pinching and Powell, 1971; White, 1973;Hinds and Hinds, 1976). The observed upregulation of c-metmRNA in the MCL (Fig. 5) at an age (E15) when the first synaptic connections in the olfactory bulb are observed supports this suggestion (Hinds and Hinds, 1976; Brunjes and Frazier, 1986). The continued expression of c-met mRNA in the MCL and ONL, and of HGF/SF mRNA by periglomerular cells in the adult bulb, suggests that HGF/SF may continue to function in the maintenance of glomerular synapses and/or the formation of new synaptic connections resulting from the turnover of olfactory sensory neurons. Expression of tPA and c-met in the AON of postnatal mice is also observed (Fig. 8). Because centrifugal fibers from portions of the AON project to the periglomerular region of the olfactory bulb (Luskin and Price, 1983), periglomerular-derived HGF/SF may function as a target-derived neurotrophic factor for some neurons of the AON as well. In all cases, tPA was expressed by cells also expressing c-met, suggesting that tPA localized on the surface of these cells may activate HGF/SF.
An intriguing result was the observation of a low level of tPA expression coincident with HGF/SF expression in the SVZ and RMS (Fig.7). This expression coincides with the peak time (E17–P7) of olfactory interneuron proliferation in the SVZ and the migration of these cells within the RMS (Hinds, 1968; Farbman, 1991). The SVZ, a discrete region at the anterior end of the LV, continues to produce neuronal precursors well into the adult life of the mouse. These precursors migrate via the RMS to the olfactory bulb, where they differentiate into granule and periglomerular neurons (Corotto et al., 1993; Luskin, 1993; Lois and Alvarez-Buylla, 1994; Lois et al., 1996). These cells migrate strictly within the confines of the RMS toward the bulb without the aid of radial glial or axon fibers. The known motogenic and mitogenic activity of HGF/SF on other cell types and the observed coexpression of HGF/SF and tPA in the RMS during the period of peak olfactory interneuron production suggest that HGF/SF may be involved in the proliferation, migration, and/or differentiation of these precursors.
In conclusion, in this report, we identify the components of the developing and adult olfactory system that express HGF/SF and its receptor c-met mRNA and demonstrate that the expression of tPA mRNA was relatively coincident within these structures. Furthermore, tPA was found to be the primary PA activity in the olfactory bulb and to constitute the bulk of exogenous proHGF/SF-cleaving activity in bulb extracts. These results support the proposed role for tPA in regulating olfactory processes via activation of HGF/SF in the vicinity of its receptor and provide the basis for additional research aimed at elucidating the roles of tPA and HGF/SF in the nervous system.
Footnotes
This work was supported by National Institutes of Health Grants NS09818 (N.W.S.) and T32-NS07083 (D.P.T.) and by National Science Foundation Grant IBN-9630458 (N.W.S.). N.W.S. is a Jacob Javits Investigator of the National Institute of Neurological Disorders and Stroke. We thank Dr. Steven Hayden for his helpful comments and suggestions. We also thank Susan Haffke for her help with tissue sectioning as well as Carey Miller and Darcy Williams for their valuable technical assistance.
Correspondence should be addressed to Dr. Nicholas W. Seeds, Neuroscience Program and Department of Biochemistry/Biophysics and Genetics, University of Colorado Health Sciences Center, 4200 East 9th Avenue, B-138, Denver, CO 80262.
REFERENCES
- 1.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 2.Brauer PR, Yee JA. Cranial neural crest cells synthesize and secrete a latent form of transforming growth factor β that can be activated by neural crest cell proteolysis. Dev Biol. 1993;155:281–285. doi: 10.1006/dbio.1993.1026. [DOI] [PubMed] [Google Scholar]
- 3.Brunjes PC, Frazier LL. Maturation and plasticity in the olfactory system of vertebrates. Brain Res Rev. 1986;11:1–45. doi: 10.1016/s0006-8993(86)80188-3. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PG, Novak JF, Yanosick TB, McMaster JS. Involvement of the plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology. 1992;130:1401–1412. doi: 10.1210/endo.130.3.1371448. [DOI] [PubMed] [Google Scholar]
- 5.Chan AM-L, King HWS, Deakin EA, Tempest PR, Hilkens J, Kroezen V, Edwards DR, Wills AJ, Brookes P, Cooper CS. Characterization of the mouse met proto-oncogene. Oncogene. 1988;2:593–599. [PubMed] [Google Scholar]
- 6.Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persist in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri A, Bannister LH. The development of the olfactory mucosa in the mouse: light microscopy. J Anat. 1975;119:277–286. [PMC free article] [PubMed] [Google Scholar]
- 8.Dent MAR, Sumi Y, Morris RJ, Seeley PJ. Urokinase-type plasminogen activator expression by neurons and oligodendrocytes during process outgrowth in developing rat brain. Eur J Neurosci. 1993;5:633–647. doi: 10.1111/j.1460-9568.1993.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 9.Farbman AI. Developmental neurobiology of the olfactory system. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and taste in health and disease. Raven; New York: 1991. pp. 19–33. [Google Scholar]
- 10.Friedman GC, Seeds NW. Tissue plasminogen activator expression in the embryonic nervous system. Dev Brain Res. 1994;81:41–49. doi: 10.1016/0165-3806(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 11.Friedman GC, Seeds NW. Tissue plasminogen activator mRNA expression in granule neurons coincides with their migration in the developing cerebellum. J Comp Neurol. 1995;360:658–670. doi: 10.1002/cne.903600410. [DOI] [PubMed] [Google Scholar]
- 12.Gak E, Taylor WG, Chan AM-L, Rubin JS. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992;311:17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- 13.Graziadei PPC, Monti-Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1978;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 14.Hart D, Rehmetulla A. Plasminogen activators and their inhibitors: regulators of extracellular proteolysis and cell function. Comp Biochem Physiol. 1988;90B:691–708. doi: 10.1016/0305-0491(88)90323-9. [DOI] [PubMed] [Google Scholar]
- 15.Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. II. Cell proliferation and migration. J Comp Neurol. 1968;134:305–322. doi: 10.1002/cne.901340305. [DOI] [PubMed] [Google Scholar]
- 16.Hinds JW, Hinds PL. Synapse formation in the mouse olfactory bulb. I. Quantitative studies. J Comp Neurol. 1976;169:15–40. doi: 10.1002/cne.901690103. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Kagoshima M, Wanaka A, Tohyama M, Matsumoto K, Nakamura T. Localization and functional coupling of HGF and c-Met/HGF receptor in rat brain: implication as neurotrophic factor. Mol Brain Res. 1995;32:197–210. doi: 10.1016/0169-328x(95)00075-4. [DOI] [PubMed] [Google Scholar]
- 18.Jung W, Castren E, Odenthal M, VandeWoude GF, Ishii T, Dienes HP, Lindholm D, Schirmacher P. Expression and functional interaction of hepatocyte growth factor and its receptor c-met in mammalian brain. J Cell Biol. 1994;126:485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki ES. Amplification of RNA. In: Innis MA, Gelfand DH, Seninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic; San Diego: 1990. pp. 21–27. [Google Scholar]
- 20.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;220:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lee C-C, Kozak CA, Yamada KM. Structure, genetic mapping and expression of the mouse hgf/scatter factor gene. Cell Adhes Commun. 1993;1:101–111. doi: 10.3109/15419069309095686. [DOI] [PubMed] [Google Scholar]
- 23.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 24.Lois C, Garcia-Verdugo J-M, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 25.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 26.Luskin MB, Price JL. The topographical organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 27.Mansuy IM, van der Putten H, Schmid P, Meins M, Botter F, Monard D. Variable and multiple expression of protease nexin-1 during mouse organogenesis and nervous system development. Development. 1993;119:1119–1134. doi: 10.1242/dev.119.4.1119. [DOI] [PubMed] [Google Scholar]
- 28.Mars Wm Wm, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143:949–958. [PMC free article] [PubMed] [Google Scholar]
- 29.Mars WM, Liu M-L, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- 30.Moonen G, Grau-Wagemans MP, Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982;298:753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- 31.Naka D, Ishii T, Yoshiyama Y, Miyazawa K, Hara H, Hishida T, Kitamura N. Activation of hepatocyte growth factor by proteolytic conversion of a single chain form to a heterodimer. J Biol Chem. 1992;267:20114–20119. [PubMed] [Google Scholar]
- 32.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 33.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Berchmeier W, Daikuhata Y, Tsubouch H, Blas F, Comoglio PM. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda M, Harada Y. Development of olfactory epithelium in the mouse: scanning electron microscopy. Biomed Res [Suppl] 1981;2:449–454. [Google Scholar]
- 35.O’Shea KS, Dixit VM. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988;107:2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinching AJ, Powell TPS. The neuropil of the periglomerular region of the olfactory bulb. J Cell Sci. 1971;9:379–409. doi: 10.1242/jcs.9.2.379. [DOI] [PubMed] [Google Scholar]
- 37.Pittman RN. Release of plasminogen activator and a calcium-dependent metalloprotease from cultures sympathetic and sensory neurons. Dev Biol. 1985;110:91–101. doi: 10.1016/0012-1606(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 38.Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Bio- chim Biophys Acta. 1993;1155:357–371. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 39.Saksela O, Rifkin D. Cell associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, Ed 2. New York: Cold Spring Harbor Laboratory.
- 41.Santos OF, Barros EJG, Yang X-M, Matsumoto K, Nakamura T, Park M, Nigam SK. Involvement of hepatocyte growth factor in kidney development. Dev Biol. 1994;163:525–529. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- 42.Sappino A-P, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli J-D. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 44.Seeds NW, Haffke S, Krystosek A. Cell migration and recognition in cerebellar reaggregate cultures. In: Giacobini E, editor. Tissue culture in neurobiology. Raven; New York: 1980. pp. 145–154. [Google Scholar]
- 45.Seeds NW, Haffke S, Christensen K, Schoonmaker J. Cerebellar granule cell migration involves proteolysis. In: Lauder JM, editor. Molecular aspects of development and aging of the nervous system. Plenum; New York: 1990. pp. 169–178. [DOI] [PubMed] [Google Scholar]
- 46.Seeds NW, Haffke S, Hawkins R, Krystosek A, McGuire P, Verrall S. Neuronal growth cones: battering rams or lasers? In: Kater SB, editor. The nerve growth cone. Raven; New York: 1992. pp. 219–228. [Google Scholar]
- 47.Sonnenberg E, Weidner KM, Birchmeier C. Expression of the c-met receptor and its ligand during mouse embryogenesis. In: Goldberg ID, Rosen EM, editors. Hepatocyte growth factor-scatter factor (hgf-sf) and the c-met receptor. Birkhäuser; Basel: 1993. pp. 381–395. [Google Scholar]
- 48.Thewke DP, Seeds NW. Coexpression of hepatocyte growth factor, its receptor (c-met), and tissue-type plasminogen activator in murine brain. Soc Neurosci Abstr. 1995;21:2011. doi: 10.1523/JNEUROSCI.16-21-06933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uehara Y, Minoa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 50.Verrall S, Seeds NW. Characterization of 125I-tissue plasminogen activator binding to cerebellar granule neurons. J Cell Biol. 1989;109:265–271. doi: 10.1083/jcb.109.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White EL. Synaptic organization of the mammalian olfactory glomerulus: new findings including an intraspecific variation. Brain Res. 1973;60:299–313. doi: 10.1016/0006-8993(73)90792-0. [DOI] [PubMed] [Google Scholar]
- 52.Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong V, Song Y, Arriaga R, Lindsay RM. CNTF Potentiates the effects of bdnf, gdnf, or hgf in cultured motor neurons. Soc Neurosci Abstr. 1995;21:1535. [Google Scholar]