Abstract
Caenorhabditis elegans UNC-18 protein, homologous to yeast Sec1p, is important in neurotransmitter release, because theunc-18 mutation leads to severe paralysis and presynaptic acetylcholine (ACh) accumulation. To examine the functional conservation in mammals, we tried to isolate unc-18isoforms from mouse and human brain cDNA libraries and obtained two classes of isoforms—neural genes and ubiquitous genes. Neural genes were identical to Munc-18 (also known as n-Sec1 or rbSec1), identified in rat and bovine brains as a syntaxin-binding protein. According to “Munc-18” terminology, we call the neural genes Munc-18-1 and the ubiquitous genes Munc-18-3. These mammalian isoforms exhibit 58% (Munc-18-1) and 42–43% (Munc-18-3) amino acid sequence identity with UNC-18. Next, we constructed transgenic unc-18mutants to test biological activity of mouse Munc-18-1 and Munc-18-3 under the control of C. elegans unc-18promoter. Munc-18-1 compensates for severe locomotion disability and cholinergic defects, e.g., abnormal sensitivities to cholinesterase inhibitors and cholinergic receptor agonists inunc-18 mutants, but Munc-18-3 fails. These data suggest that Munc-18-1 and C. elegans unc-18 may play positive roles in ACh release and that the molecular mechanism of neuronal regulated secretion has been partially conserved from nematodes to mammals.
Keywords: neurotransmitter release, unc-18, Caenorhabditis elegans, ACh, transgenic study
Neurotransmitters stored in the synaptic vesicles are released into the synaptic gap by Ca2+-dependent exocytosis. According to the SNAP receptor (SNARE) hypothesis (Rothman, 1994), the interaction of vesicular and target membrane proteins (referred to as v-SNARE and t-SNARE, respectively) leads to vesicle docking. Several neuronal components have been isolated from synaptosomes of vertebrates, and the molecular and biochemical properties of these components have been elucidated (Südhof and Jahn, 1991; Bennett and Scheller, 1994; Südhof, 1995). It has been suggested that formation of the SNARE complex, consisting of synaptobrevin/VAMP (v-SNARE), syntaxin, and SNAP-25 (t-SNAREs), is followed by association with solubleN-ethylmaleimide-sensitive factor (NSF) and soluble NSF attachment proteins (SNAPs) (Söllner et al., 1993; Rothman, 1994;Söllner and Rothman, 1994). It has been demonstrated recently that there is a common mechanism between regulated secretion and constitutive secretion, because SNAREs belong to large gene families from yeasts to mammals (Bennett and Scheller, 1993; Südhof, 1995).
The unc ( uncoordinated)-18 mutations of Caenorhabditis elegans cause several phenotypes implicating impairment of presynaptic functions, such as acetylcholine (ACh) accumulation and resistance to acetylcholinesterase inhibitors, as well as a paralytic phenotype (Hosono et al., 1987, 1989). The UNC-18 products are homologous to yeast Sec1 protein and are expressed predominantly in the nervous system (Hosono et al., 1992; Gengyo-Ando et al., 1993). Homologous proteins of UNC-18 “Munc-18” (also known as n-Sec1/rbSec1; Hata et al., 1993; Garcia et al., 1994; Pevsner et al., 1994a) identified in bovine and rat brain specifically interact with syntaxin in vitro, and extragenic suppressors ofsec1 encode syntaxin-related proteins (Aalto et al., 1993), suggesting that UNC-18-related proteins may participate in the regulation or formation of presynaptic SNARE complex in neuronal exocytosis.
In the present study, we cloned a brain-specific isoform (Munc-18/n-Sec1/rbSec1; Hata et al., 1993; Garcia et al., 1994; Pevsner et al., 1994a) and a ubiquitous isoform (known as Munc-18c; Tellam et al., 1995) of unc-18 from both the mouse brain cDNA library and the human fetal brain cDNA library. Recently, the neuronal isoform was designated Munc-18-1 and another ubiquitous isoform was designated Munc-18-2, respectively (Hata and Südhof, 1995). Following their terminology, we termed a distinct ubiquitous isoform identified in this study “Munc-18-3.” Predicted proteins share 42–58% identity toC. elegans UNC-18. There is no substituted amino acid of Munc-18-1 between mouse and human, but Munc-18-3 of the two species diverge. Here we demonstrate that neuronal defects in unc-18mutants are partially suppressed by the mouse Munc-18-1 transgene but not by the mouse Munc-18-3 transgene. These results suggest that Munc-18-1 is a functional unc-18 homolog and that the molecular mechanism of the neurosecretory pathway viaunc-18/Munc-18-1 is partially conserved between nematodes and mammals.
MATERIALS AND METHODS
Molecular biology. Molecular biological procedures were performed as described previously, with minor modifications (Maniatis et al., 1989).
Cloning of C. briggsae unc-18. Southern blot analysis of aC. briggsae genomic DNA digested with EcoRI was performed using a C. briggsae cDNA fragment (+29 to +588) as a probe, which was amplified with RT-PCR; a 4 kb band was detected. Genomic DNA fragments of the proper size fraction were cloned into theEcoRI-digested λZAP vector. The resulting phage clones were screened with the same probes under standard conditions. One of four positive clones was analyzed further. Because these clones did not contain the last exon, a cDNA clone of the 3′ end of the message was isolated using the 3′-RACE method.
cDNA isolation. Two degenerate oligonucleotides were designed to be located in highly conserved regions among theunc-18 of C. elegans and C. briggsae,and the sec1 family of S. cerevisiae. The forward oligo AG(A/G)(A/T)(G/C)CCA(A/G)CT(G/C)ATCATCATCGA(C/T)AG(A/G)GG(A/C)TA(C/T)GA, corresponding to amino acid residues 225–236, and the reverse oligo AG(A/G)TC(A/G)TA(A/G)CACAT(G/A)GC(T/C)TG, corresponding to residues 248–254 of UNC-18, were used in RT-PCR with poly(A+) RNA isolated from mouse adult brain. The fragments of the exact predicted size (89 bp) were subcloned and sequenced. Two clones (amp11 and amp15) were found to be ∼70% homologous tounc-18 at the DNA level and were used as DNA probes to isolate cDNA clones. A mouse adult brain cDNA library in the λgt10 vector was constructed and screened using a 32P-labeled insert DNA of amp11. Two of three positive clones (2A-11 and 2B-11) covering the entire open reading frame of Munc-18-1 were subcloned into the pBluescript plasmid vector and sequenced. For cloning of the Munc-18-3 cDNA, a mouse fetal brain cDNA library in λZAPII (Stratagene, La Jolla, CA) was screened with an amp15 cDNA probe, yielding five independent clones of Munc-18-3. The longest cDNA clone (7E-1) was subcloned and sequenced. A sequence corresponding to the 5′ end of the Munc-18-3 cDNA was amplified by the 5′-RACE method using 5′-AmpliFINDER (Toyobo, Tokyo, Japan).
Human Munc-18-1 and Munc-18-3 cDNA clones were isolated from a human fetal brain cDNA library cloned in λZAPII (Stratagene) with the murine Munc-18-1 and Munc-18-3 cDNA probes. Detailed cloning steps of the human homologs will be described elsewhere.
RNA isolation and Northern blot analysis. Total cellular RNA was isolated from adult mouse tissues, and poly(A+) RNA was purified with oligo-dT cellulose columns (Life Technologies, Gaithersburg, MD) and separated on a 1% 0.01 mNaH2PO4 agarose gel. The glyoxylated RNA was transferred to Hybond-N+ (Amersham, Buckinghamshire, UK) for hybridization.
Nematodes. Conditions for growth and maintenance of C. elegans have been described by Brenner (1974).unc-47(e307) was obtained from theCaenorhabditis Genetics Center (St. Paul, MN).
Transgenic animals. For transgenic study, constructs carrying test cDNA (unc-18, mouse Munc-18-1, and Munc-18-3) under the control of unc-18 genomic flanking regions were generated. A 1 kb KpnI–XbaI genomic fragment containing the 3′ poly(A) signal of unc-18 from PE10 (Hosono et al., 1992) was inserted into pUC19. The resulting plasmids were digested with EcoRI and KpnI. Each 1.8 kb test cDNA fragment encoding the entire protein sequence was then amplified with PCR primers designed with EcoRI and KpnI and inserted. The 3 kb EcoRI fragment, derived from theunc-18 upstream promoter region, was also amplified from PE10 using the primers designed with EcoRI and inserted into each construct. All PCR reactions were performed with pfu DNA polymerase (Stratagene).
The resulting DNA constructs, punc-18, pMunc-18-1, and pMunc-18-3 plasmids (20 μg/ml) were injected intounc-18(e81) by standard germline transformation techniques (Fire, 1986; Mello et al., 1991). pRF4 plasmid (50 μg/ml) containing the dominant roller markerrol-6(su1006) was co-injected to identify transgenic animals. The chromosomal integrations of extrachromosomal arrays were performed by gamma ray irradiation as described previously (Way et al., 1991). Two independent integrated lines (kIn2-1 and kIn2--2; kIn4-1 and kIn4-2) were identified from the progenies of 15 kEx2 and 20 kEx4 animals that had been treated with 3780 rad from a137Cs source.
Antibody staining. Immunohistochemistry was carried out using a whole-mount procedure (McIntire et al., 1992) with minor modifications. Animals were fixed in 4% paraformaldehyde, 1% glutaraldehyde at 4°C for 16–24 hr, and then treated with 2-mercaptoethanol and collagenase. To examine reactions to anti-GABA antibodies as precisely as possible, four animal types [wild-type;unc-18(e81); unc-18(e81) carrying mouse Munc-18-1; andunc-47(e307)] were adhered to each poly-l-lysine-coated slide and incubated with polyclonal anti-GABA antibody (SF04B; SFRI Laboratoire, Berganton, France) diluted 1:40,000 at 4°C for 12–16 hr. After being washed with PBS, the slides were incubated with biotinylated anti-rabbit antibody (1:600) and then incubated with ABC complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA). Antigen was detected by the DAB reaction.
RESULTS
Molecular identification of the unc-18 family
As a first step in isolating a mammalian unc-18homolog, we tried to identify an evolutionally conserved region of theunc-18 gene from the nematode C. briggsae, which was expected to be functionally important. To determine the C. briggsae unc-18 sequence, we isolated genomic clones containing the C. briggsae unc-18-coding region. The predicted amino acid sequence of C. briggsae UNC-18 is highly conserved to that of C. elegans UNC-18 (572/590 identical; Fig.1), and the intron–exon pattern of the C. briggsae unc-18 gene is also similar to that of C. elegans unc-18 gene (data not shown).
Fig. 1.
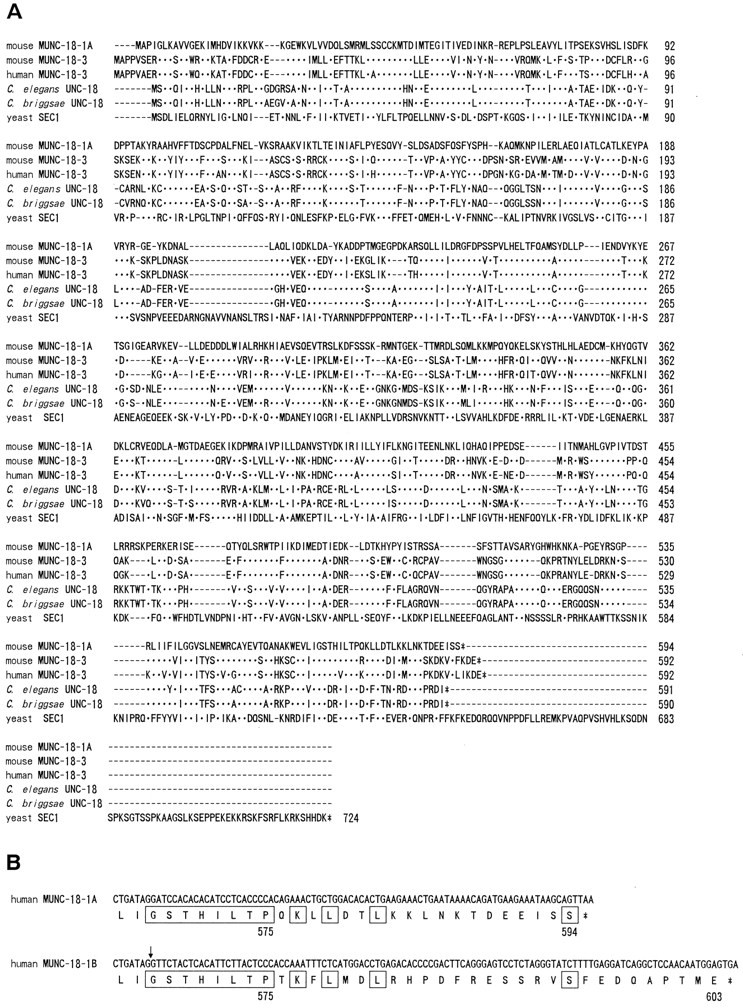
The unc-18 families.A, Alignment of predicted amino acid sequences from theunc-18-related genes. An optimal alignment was produced with the Pile-Up program. Identical residues to the mouse Munc-18-1 are indicated by dots, and gaps caused by the alignment are indicated by dashes. The sequence of human Munc-18-1 is not shown in this alignment because it is identical to mouse Munc-18-1.B, Alternative C-terminal sequences of human Munc-18-1.Boxed sequences indicate amino acids identical in Munc-18-1A and Munc-18-1B. Munc-18-1B cDNA contains 126 bp of an additive sequence at position +1702 (indicated by arrow) of the cDNA sequence of Munc-18-1A and encodes a protein of 603 amino acids.
Degenerate oligonucleotide primers based on the conserved amino acid regions among the UNC-18 of C. elegans and C. briggsae, and the yeast Sec1-related proteins Sec1p (Aalto et al., 1991), Sly1p (Dascher et al., 1991), and Slp1p (Wada et al., 1990), which play essential roles in the yeast secretory pathway, were designed to isolate the mouse homolog of the nematode unc-18gene. DNA fragments of the appropriate size were amplified from mouse adult brain poly(A+) RNA using RT-PCR with these primers. Sequence analyses of cloned PCR products revealed that two cDNA species homologous to unc-18 sequence were amplified. We isolated multiple independent cDNA clones from two mouse brain cDNA libraries to characterize the mouse genes. The complete cDNA structure was determined by sequencing these cDNA clones and overlapping the RACE–PCR-derived 5′ end cDNA clones. A human fetal brain cDNA library was screened with the murine probes to isolate the human Munc-18 cDNA clones. Sequence analysis of these mammalian brain cDNA clones revealed two distinct cDNA classes, referred to here as Munc-18-1 and Munc-18-3. The mouse Munc-18-1 was identical to the mammalian neural homolog identified previously in bovine and rat brain (also known as n-Sec1 or rbSec1; Hata et al., 1993; Garcia et al., 1994; Pevsner et al., 1994a), and the mouse Munc-18-3 was identical to one of the ubiquitous isoforms (Munc-18c; Tellam et al., 1995). The mouse Munc-18-1 protein sequence is fully matched with the human sequence, but the mouse Munc-18-3 protein sequence is only 91% identical to the human sequence (Fig.1A). Both Munc-18-1 and Munc-18-3 proteins are also distantly related to the yeast proteins Sec1p, Sly1p, and Slp1p. Additionally, we obtained an alternative isoform of human Munc-18-1 (Munc-18-1B; Fig. 1B) that diverges after amino acid 575. The same isoform is also identified in rat (Garcia et al., 1995). The hydropathy plots of both Munc-18-3 amino acid sequences do not show any striking hydrophobic region that could serve as a signal peptide or a transmembrane segment, indicating that the Munc-18-3 protein is a typical intracellular cytosolic protein.
Distinct expression pattern of Munc-18-1 and Munc-18-3 mRNA in mouse tissues
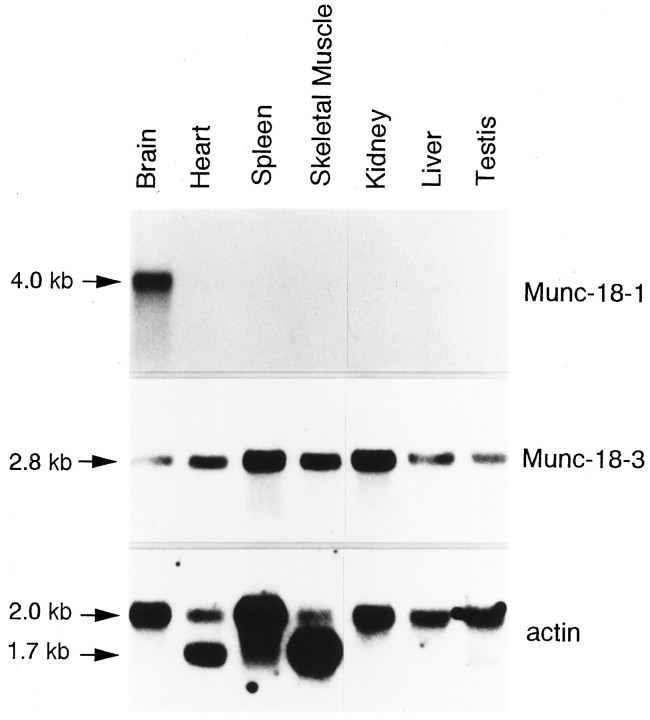
The tissue distribution of Munc-18-1 and Munc-18-3 transcripts was investigated by Northern blot analysis. Poly(A+) RNA purified from various tissues was electrophoresed, blotted, and hybridized to 1.2 kb BamHI–BamHI coding fragments from a murine Munc-18-1 cDNA clone (2B-11) or a 0.7 kbEcoRI–PstI-coding fragment from a murine Munc-18-3 cDNA clone (5A-1).
As expected, Munc-18-1 is expressed only in the brain as an ∼4 kb transcript, whereas Munc-18-3 is expressed constitutively as a message of ∼2.8 kb in all mouse tissues analyzed (Fig. 2).
Fig. 2.
Northern blot analysis of mouse Munc-18 genes. Northern blots of poly(A+) RNA (2 μg) from seven different mouse tissues were hybridized with 32P-labeled probes derived from Munc-18-1, Munc-18-3, and actin. Whereas transcripts for Munc-18-1 are expressed exclusively in the brain, transcripts for Munc-18-3 are expressed in all tissues analyzed.
Munc-18-1 rescues the cholinergic defects inunc-18 mutants
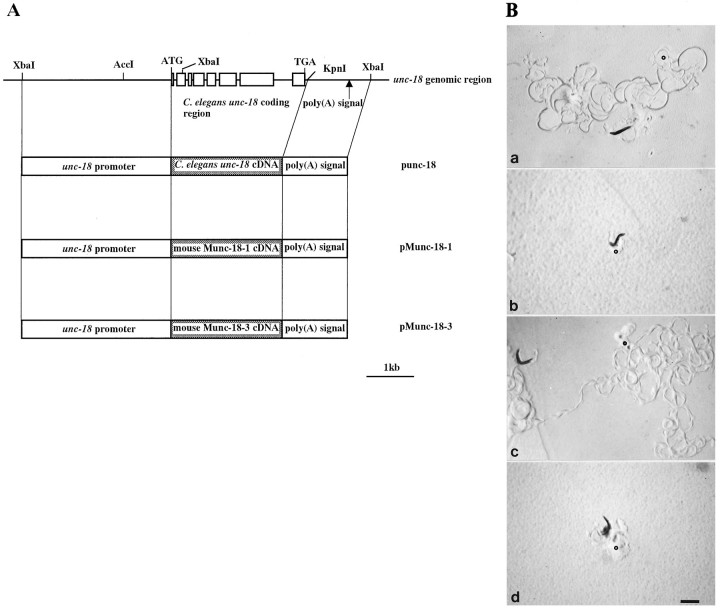
The fact that Munc-18-1 is a neural gene and has higher homology to unc-18 than does Munc-18-3 suggests that the Munc-18-1 protein might function in nervous system in a manner similar to UNC-18. Mutants defective in the unc-18 gene exhibit several characteristic phenotypes implicated in the impairment of presynaptic function, including severe locomotion disability, resistance to cholinesterase inhibitors, and hypersensitivity to cholinergic receptor agonists. To examine whether mouse Munc-18 genes can substitute forunc-18, we designed expression vectors containing the mouse genes under the control of promoter and poly(A) signal derived from theunc-18 genomic sequence (pMunc-18-1 and pMunc-18-3; Fig.3A).
Fig. 3.
For a positive control, an expression vector containing theunc-18 cDNA was also constructed (punc-18; Fig.3A). unc-18(e81) strain was used as the recipient of the transgene, which has no detectable UNC-18 products. Obvious restoration of the coordinated phenotype was observed in animals (kEx2 and kEx3) transgenic for punc-18 as well as wild-type animals (kEx9) transgenic only for rol-6, indicating that the expression system was sufficient to examine the biological activities of mouse Munc-18 genes. Animals (kEx4 and kEx5) transgenic for the pMunc-18-1 construct showed a prominent restoration of locomotion compared to animals (kEx1) transgenic only forrol-6, whereas pMunc-18-3 induced no detectable rescue in two independent transformed lines (kEx6 and kEx7) (Table1, Fig. 3B). We detected no mutation in the Munc-18-3 transgene and observed no obvious dominant effect in the wild-type worms transgenic for pMunc-18-3 (data not shown).
Table 1.
Transgenic C. elegans strains and their properties
| Plasmid | Array | Host genotype | Motility | Sensitivity for cholinergic reagents | |
|---|---|---|---|---|---|
| Trichlorfon (mm) | Levamisole (mm) | ||||
| – | – | unc-18(+) | wild-type | 0.01 | >0.30 |
| – | – | unc-18(e81) | unc | 0.20 | 0.01 |
| pRF4 | kEx9 | unc-18(+) | wild-type | 0.01 | >0.30 |
| pRF4 | kEx1 | unc-18(e81) | unc | 0.20 | 0.01 |
| punc-18; pRF4 | kEx2 | unc-18(e81) | wild-type | 0.02 | >0.30 |
| punc-18; pRF4 | kEx3 | unc-18(e81) | wild-type | 0.02 | >0.30 |
| punc-18; pRF4 | kIn2-1 | unc-18(e81) | wild-type | 0.01 | >0.30 |
| punc-18; pRF4 | kIn2-2 | unc-18(e81) | wild-type | 0.01 | >0.30 |
| pMunc-18-1; pRF4 | kEx4 | unc-18(e81) | wild-type1_a | 0.01 | >0.30 |
| pMunc-18-1; pRF4 | kEx5 | unc-18(e81) | wild-type1_a | 0.01 | >0.30 |
| pMunc-18-1; pRF4 | kIn4-1 | unc-18(e81) | wild-type1_a | 0.01 | >0.30 |
| pMunc-18-1; pRF4 | kIn4-2 | unc-18(e81) | wild-type1_a | 0.01 | >0.30 |
| pMunc-18-3; pRF4 | kEx6 | unc-18(e81) | unc | 0.20 | 0.01 |
| pMunc-18-3; pRF4 | kEx7 | unc-18(e81) | unc | 0.20 | 0.01 |
punc-18, pMunc-18-1, and pMunc-18-3 plasmids were injected intounc-18(e81) with the plasmid pRF4, which confers a dominant Roller phenotype. Extrachromosomal arrays that behave as unstable free duplications are named kEx, and stable arrays integrated into a chromosome are named kIn. The motility and the sensitivity for cholinergic reagents of unc-18mutants are affected by these transgenes.
Wild-type means slightly slower motility than the motility of wild-type animals carrying rol-6. Three larvae were put onto NGM containing 10 different concentrations of reagents (0, 0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.15, 0.2, and 0.3 mm). The highest concentrations are shown in which animals were able to produce F2 progeny within 10 d in triplicate experiments.
To investigate cholinergic functions in transformed C. elegans, we examined the sensitivity of these animals to a cholinesterase inhibitor and to cholinergic receptor agonists. The resistance of unc-18 animals to trichlorfon was fully reversed by the presence of the punc-18 transgene or by the presence of the pMunc-18-1 transgene (Table 1). In addition, pMunc-18-1 rescued the hypersensitivity to the ACh receptor agonists levamisole (Table 1) and carbachol (data not shown). As a control, we demonstrated that the presence of the rol-6 transgene by itself had no effect on the drug responses of transgenic animals. These results suggest that Munc-18-1 can correct presynaptic defects in cholinergic neurons ofunc-18 mutants. In the case of pMunc-18-3 animals, however, there was no obvious change in sensitivity to cholinergic reagents (Table 1). To facilitate a comparison of the sensitivities of punc-18 and pMunc-18-1 animals to cholinergic reagents, we constructed chromosomal integrated lines of these plasmids in which non-Rol animals were not segregated. Figure 4shows the growth rates of these animals on medium containing trichlorfon (Fig. 4A) or levamisole (Fig.4B). The growth rates of wild-type andunc-18(e81) animals carrying onlyrol-6 are also shown for comparison.
Fig. 4.
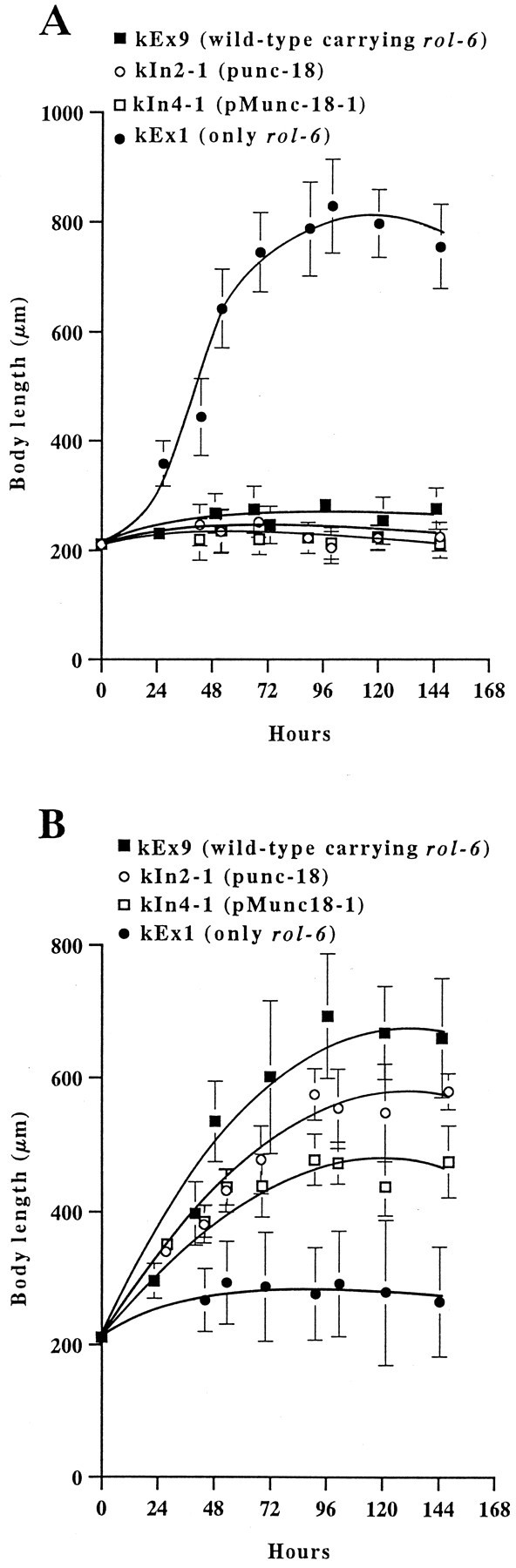
Sensitivities for cholinergic drugs in the transgenic nematodes. Animals were grown on NGM containing 40 μm trichlorfon (A) or 20 μmlevamisole (B) at 20°C. Twenty to forty animal lengths were measured for each time point, and the mean values were plotted. Bars indicate SEM. The cholinergic defects in unc-18mutants are partially suppressed by the Munc-18-1 transgene. Animals transgenic for punc-18 (kIn2-1) or pMunc-18-1 (kIn4-1) are more sensitive to levamisole than wild-type animals transgenic forrol-6. These effects may be attributable to copy number of transgenes.
As expected, we found no detectable difference in sensitivity to trichlorfon between punc-18 and pMunc-18-1 animals. Moreover, pMunc-18-1 animals partially but significantly rescued the hypersensitive phenotype of levamisole.
Abnormal GABA immunoreactivities in unc-18 mutants
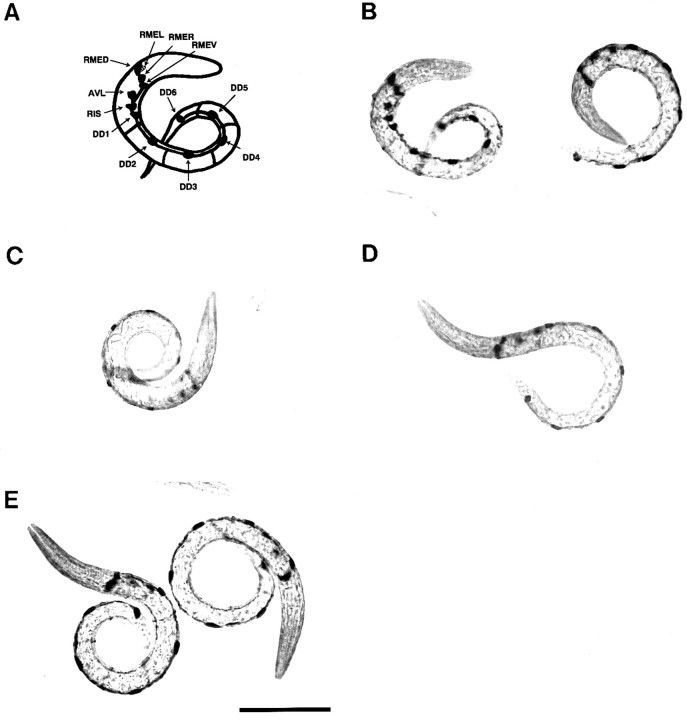
There are seven classes of motor neurons (DA, VA, DB, VB, DD, VD, and AS) innervating body wall muscles in the ventral nerve cord ofC. elegans. Electrophysiological studies have shown that the DA, DB, and AS homologs of Ascaris are excitatory and that the DD and VD homologs are inhibitory (Walrond et al., 1985). The DD and VD motor neurons have been shown to contain the neurotransmitter GABA. A previous report demonstrated that UNC-18 protein is expressed in all motor neurons of the ventral nerve cord (Gengyo-Ando et al., 1993), suggesting that unc-18 gene function may be not restricted in cholinergic processes. To investigate this possibility, immunocytochemical studies using specific anti-GABA antiserum were performed. The first-stage larvae of wild-type,unc-18(e81), and animals transgenic for Munc-18-1 were stained with anti-GABA polyclonal antibody by indirect immunohistochemistry. We also stainedunc-47(e307) animals as a positive control (Fig. 5E), because immunocytochemical studies have shown that GABAergic neurons in this mutant contain abnormally high levels of GABA (McIntire et al., 1993). To compare their immunoreactivities precisely, each animal was confronted with the antibodies on the same slide. Each nematode was stained with an anti-GABA antibody, and six immunoreactive neurons of the ventral nerve cord (DD1–DD6) in each L1 larva were examined (Fig. 5).
Fig. 5.
The higher GABA immunoreactivity inunc-18 mutants. Whole mounts ofunc-18(e81) (B), wild-type N2 (C), and animals transgenic for Munc-18-1(kIn4-1) (D) andunc-47(e307) (E) were stained with an anti-GABA antiserum followed by a secondary biotin-conjugated goat anti-rabbit antiserum and ABC complex. The 12 immunoreactive neurons of the unc-18 animal at theleft of B are schematically represented in A. The unc-18 mutants show abnormally high levels of GABA reactivity, similar to the unc-47mutants. Scale bar, 50 μm.
The GABAergic neurons in unc-18 (Fig. 5B) andunc-47 mutants (Fig. 5E) reacted similarly to anti-GABA antibody, and both reactions were more intense than those exhibited by wild-type N2 (Fig. 5C). These results suggest that mutations in the unc-18 gene cause some defect within GABAergic function. GABA immunoreactivity in transgenic nematodes for Munc-18-1 kIn4-1 (Fig. 5D) and kIn4-2 (data not shown) was slightly weaker than in recipient unc-18(e81).
DISCUSSION
To further our understanding of the precise role of theunc-18 family in vesicle traffic, we isolated and characterized the unc-18-related genes Munc-18-1 and Munc-18-3. Although the neuronal unc-18 isoform Munc-18-1 (n-Sec1/rbSec1) shows the highest homology to C. elegans unc-18 among mammalian homologs and exhibits specific binding to syntaxin (Hata et al., 1993; Garcia et al., 1994; Pevsner et al., 1994a), there is no biological evidence indicating that Munc-18-1 plays a role in neurotransmitter release. In the present report, we demonstrate that the Munc-18-1 gene partially rescues cholinergic defects of unc-18 mutants. Transgenic studies also reveal that there are functional differences between the the neuronal isoform Munc-18-1 and the ubiquitous isoform Munc-18-3, because Munc-18-3 shows no detectable suppressor activity of unc-18 mutation.
It is interesting to clarify the relationship between theunc-18 family and the syntaxin family. In mammalian cells, six syntaxin isoforms with broad tissue distribution are characterized (Bennett et al., 1993b). On the other hand, three classes of Munc-18 isoforms, Munc-18-1, Munc-18-3, and another ubiquitous isoform (Munc-18-2/muSec1/Munc-18b; Hata and Südhof, 1995; Katagiri et al., 1995; Tellam et al., 1995) have been identified. Munc-18-1 can bind to syntaxin isoforms syntaxin 1A, -2, and -3, but not to syntaxin 4 in vitro (Pevsner et al., 1994a; Hata and Südhof, 1995). No significant difference in the binding specificity for syntaxins was observed between Munc-18-2 and Munc-18-1 in yeast two-hybrid assays (Hata and Südhof, 1995). Identification of the syntaxin isoforms that can interact with Munc-18-3 remains to be elucidated.
It has been shown that neurotransmitters common to vertebrates, such as ACh, GABA, serotonin, dopamine, and some neuropeptides, are involved in the functioning of the C. elegans nervous system. Immunocytochemical studies revealed that GABAergic neurons inunc-18 mutants contained abnormally high levels of GABA as well as those in unc-47 mutants (Fig. 5). Therefore, theunc-18 gene also appears to have some function in GABAergic neurons of C. elegans. A murine Munc-18-1 transgene could slightly reduce the abnormal GABA accumulation observed in the DDn motor neurons of unc-18 mutants. Further quantitative analyses of GABA accumulation and investigations of other GABA-related phenotype will facilitate an understanding of the roles of theunc-18 gene in GABAergic neurotransmission.
We found that human Munc-18-1 has an alternative form [referred to as Munc-18-1B (Fig. 1B), the same as in rat rbSec1B;Garcia et al., 1995) that differs from the previously identified Munc-18-1 isoform (referred to as Munc-18-1A) at the C termini. We have not yet identified the alternative form of UNC-18 that is produced by alternative splicing of unc-18 gene in both C. elegans and C. briggsae.
In mutants of the cha-1 and the closely linkedunc-17 genes, which encode choline acetyltransferase and vesicular ACh transporter, respectively, homozygous null mutations lead to lethality in C. elegans, because cholinergic functions are fully blocked (Rand and Russell, 1984; Rand, 1989; Alfonso et al., 1993). A Drosophila homolog of UNC-18, named “rop,” is an important protein participating in both regulated and constitutive secretion, and the null mutants of the rop gene are not viable (Salzberg et al., 1993; Harrison et al., 1994). On the other hand, homozygous mutants of unc-18(e81), which are probably null (because e81 mutants have neitherunc-18 transcripts nor UNC-18 products at a detectable level), show ACh accumulation and severe paralysis; however, they are viable (Hosono et al., 1987, 1989). Thus, these observations raise the possibility that C. elegans has other unc-18isoforms, like mammals, or other redundant mechanisms.
Mutations in the C. elegans unc-18 gene lead to severe synaptic defects, suggesting that unc-18 may have positive functions in neuronal secretion. Binding of Munc-18-1(Munc-18/n-Sec1/rbSec1) to syntaxin, however, inhibits interaction of syntaxin with SNAREs (Pevsner et al., 1994b), indicating that one of the functions of Munc-18-1 might be negative regulation of the formation of functional synaptic complex. In the present study, we demonstrate that Munc-18-1 has the ability to partially substitute forunc-18 function. A simple model in which Munc-18-1 works solely as a negative regulator is unlikely. Further characterization of the unc-18 family will be required to understand their precise roles in neurotransmitter release. Transgenic studies, as described here, would provide further our understanding of the structural and functional requirements of the unc-18 family, by using deletion mutants or reciprocal fusions between differentunc-18 isoforms.
GenBank accession numbers
The accession numbers for the sequences reported in this paper are D63504 (the C. briggsae genomic sequence), D63505 (theC. briggsae cDNA sequence), D45903 (the mouse Munc-18-1 cDNA sequence), D63851 (human Munc-18-1 cDNA sequence), D30798 (the mouse Munc-18-3 cDNA sequence), and D63506 (the human Munc-18-3 cDNA sequence).
Footnotes
This study was supported by grants from the Science and Technology Agency of Japan, the Ministry of Education, Science, Sports, and Culture of Japan, and the Defense Agency of Japan. K.G.-A. acknowledges support by the Special Postdoctoral Researchers Program from the Science and Technology Agency of Japan. We thank Dr. R. Hosono (Kanazawa University) for kindly providing genomicunc-18 clone PE10 and unc-18 cDNA clone ASC, and for commenting on this manuscript. We also thank Dr. M. W. Schein (Rockville, MD) for his editing of this manuscript and Professor M. Noda (Kyoto University) for encouragement. Nematode strainunc-47 (e307) was provided by theCaenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Correspondence should be addressed to Hitoshi Kitayama, Department of Molecular Oncology, Graduate School of Medicine, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto 606, Japan.
REFERENCES
- 1.Aalto MK, Ruohonen L, Hosono K, Keränen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1991;7:643–650. doi: 10.1002/yea.320070613. [DOI] [PubMed] [Google Scholar]
- 2.Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett MK, Scheller RH. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 7.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986;5:2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia EP, Gatti E, Butler M, Burton J, De Camilli P. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc Natl Acad Sci USA. 1994;91:2003–2007. doi: 10.1073/pnas.91.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia EP, McPherson PS, Chilcote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gengyo-Ando K, Kamiya Y, Yamakawa A, Kodaira K, Nishiwaki K, Miwa J, Hosono R. C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron. 1993;11:703–711. doi: 10.1016/0896-6273(93)90080-b. [DOI] [PubMed] [Google Scholar]
- 13.Harrison SD, Broadie K, van de Goor J, Rubin GM. Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 14.Hata Y, Südhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. J Biol Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- 15.Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 16.Hosono R, Sassa T, Kuno S. Mutations affecting acetylcholine levels in the nematode Caenorhabditis elegans. J Neurochem. 1987;49:1820–1823. doi: 10.1111/j.1471-4159.1987.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 17.Hosono R, Sassa T, Kuno S. Spontaneous mutations of trichlorfon resistance in the nematode Caenorhabditis elegans. Zool Sci. 1989;6:697–708. [Google Scholar]
- 18.Hosono R, Hekimi S, Kamiya Y, Sassa T, Murakami S, Nishiwaki K, Miwa J, Taketo A, Kodaira K-I. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J Neurochem. 1992;58:1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 19.Katagiri H, Terasaki J, Murata T, Ishihara H, Ogihara T, Inukai K, Fukushima Y, Anai M, Kikuchi M, Miyazaki J, Yazaki Y, Oka Y. A novel isoform of syntaxin-binding protein homologous to yeast Sec1 expressed ubiquitously in mammalian cells. J Biol Chem. 1995;270:4963–4966. doi: 10.1074/jbc.270.10.4963. [DOI] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch EF, Sambrook J (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- 21.McIntire SL, Garriga G, White J, Jacobson D, Horvitz HR. Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron. 1992;8:307–322. doi: 10.1016/0896-6273(92)90297-q. [DOI] [PubMed] [Google Scholar]
- 22.McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- 23.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pevsner J, Hsu SC, Scheller RH. n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA. 1994a;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pevsner J, Hsu SC, Braun JEA, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994b;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 26.Rand JB. Genetic analysis of the cha-1.unc-17 complex in Caenorhabditis elegans. Genetics. 1989;122:73–80. doi: 10.1093/genetics/122.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rand JB, Russell RL. Choline acetyltransferase deficient mutants of the nematode Caenorhabditis elegans. Genetics. 1984;106:227–248. doi: 10.1093/genetics/106.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 29.Salzberg A, Cohen N, Halachmi N, Kimchie Z, Lev Z. The Drosophila Ras2 and Rop gene pair: a dual homology with a yeast Ras-like gene and a suppressor of its loss-of-function phenotype. Development. 1993;117:1309–1319. doi: 10.1242/dev.117.4.1309. [DOI] [PubMed] [Google Scholar]
- 30.Söllner T, Rothman JE. Neurotransmission: harnessing fusion machinery at the synapse. Trends Neurosci. 1994;17:344–348. doi: 10.1016/0166-2236(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 31.Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 32.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 33.Südhof TC, Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- 34.Tellam JT, McIntosh S, James DE. Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J Biol Chem. 1995;270:5857–5863. doi: 10.1074/jbc.270.11.5857. [DOI] [PubMed] [Google Scholar]
- 35.Wada Y, Kitamoto K, Kanbe T, Tanaka K, Anraku Y. The SLP1 gene of Saccharomyces cerevisiae is essential for vacuolar morphogenesis and function. Mol Cell Biol. 1990;10:2214–2223. doi: 10.1128/mcb.10.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walrond JP, Kass IS, Stretton AOW, Donmoyer JE. Identification of excitatory and inhibitory motoneurons in the nematode Ascaris by electrophysiological techniques. J Neurosci. 1985;5:1–8. doi: 10.1523/JNEUROSCI.05-01-00001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Way JC, Wang L, Run JQ, Wang A. The mec-3 gene contains cis-acting elements mediating positive and negative regulation in cells produced by asymmetric cell division in Caenorhabditis elegans. Genes Dev. 1991;5:2199–2211. doi: 10.1101/gad.5.12a.2199. [DOI] [PubMed] [Google Scholar]