Abstract
The contribution of pharmacologically distinct Ca2+ channels to prepulse-induced facilitation was studied in mouse cerebellar granule cells. Ca2+ channel facilitation was measured as the percentage increase in the whole-cell current recorded during a test pulse before and after it was paired with a positive prepulse. The amount of facilitation was small in recordings made during the first few days in tissue culture but increased substantially after 1 week. L-type channels accounted for the largest proportion of facilitation in 1-week-old cells (60–70%), whereas N-type channels contributed very little (∼3%). The toxins ω-agatoxin IVa or ω-conotoxin MVIIC (after block of N-, L-, and P-type channels) each blocked a small percentage of facilitation (∼12 and 14%, respectively). Perfusion of cells with GTP-γ-S enhanced the facilitation of N-type channels, whereas it inhibited facilitation of L-type channels. During development in vitro, the contribution of L-type channels to the whole-cell current decreased. Single-channel recordings showed the presence of 10 and 15 pS L-type Ca2+ channels in 1-d-old cells. After 1 week in culture, a ∼25 pS L-type channel dominated recordings from cell-attached patches. Positive prepulses increased the activity of the 25 pS channel but not of the smaller conductance channels. The expression of Ca2+channel facilitation during development may contribute to changes in excitability that allow frequency-dependent Ca2+influx during the period of active synaptogenesis.
Keywords: calcium channel, calcium, cerebellar granule cell, development, facilitation, L-type channel, N-type channel, P-type channel, Q-type channel, potentiation
Voltage-gated Ca2+ channels control many important neuronal functions, including membrane excitability, neurotransmitter release, and axon outgrowth. In the CNS, five types of high-threshold Ca2+ channels have been identified to date, on the basis of their sensitivity to various channel blockers. Dihydropyridine antagonists inhibit L-type channels (for review, see Catterall et al., 1988; Tsien et al., 1988; Glossmann and Striessnig, 1990). N-type channels were first characterized in peripheral neurons, where they were shown to be blocked selectively by ω-conotoxin GVIA (ω-CTX-GVIA) (McCleskey et al., 1987; Aosaki and Kasai, 1989; Plummer et al., 1989). Central neurons also express distinct P- and Q-type Ca2+ channels, which are blocked by the toxins ω-agatoxin IVA (ω-aga-IVA) and ω-conotoxin MVIIC (ω-CTX-MVIIC), respectively (Hillyard et al., 1992; Mintz et al., 1992a,b). In addition, a class of R-type channels is insensitive to block by all of the Ca2+ channel blockers (Regan et al., 1991).
In a number of different cell types, a strong depolarization increases the Ca2+ channel current evoked by a subsequent test pulse to a moderate potential. This process, which has been termed prepulse-induced facilitation or potentiation, has been described in cardiac muscle (Lee, 1987; Pietrobon and Hess, 1990; Zygmunt and Maylie, 1991), adrenal chromaffin cells (Artalejo et al., 1991a,b;Fenwick et al., 1982; Hoshi et al., 1984), and peripheral neurons (Kasai and Aosaki, 1989; Pollo et al., 1989; Ikeda, 1991; Kasai, 1991). Several distinct mechanisms have been shown to underlie prepulse-induced Ca2+ channel facilitation in different types of cells. Cardiac and neuronal L-type Ca2+ channels undergo a type of facilitation in which channels enter a long open state at positive potentials (Pietrobon and Hess, 1990; Forti and Pietrobon, 1993). Facilitation of L-type channels also seems to require channel phosphorylation (Yue et al., 1990; Artelejo et al., 1992; Scultoreanu et al., 1993a,b). On the other hand, prepulse-induced facilitation of N-type channels in peripheral neurons involves a different mechanism in which strong depolarization relieves the block of the channel by an activated GTP-binding protein (Elmslie et al., 1990; Ikeda, 1991; Kasai, 1991).
There is little information about prepulse-induced facilitation of Ca2+ channels in central neurons. It is not known, for example, whether the mechanisms that produce facilitation of N- and L-type channels in peripheral neurons can account for the facilitation of these channel types in central neurons. There is also little information on whether facilitation is a property of other types of Ca2+ channels in central neurons. Dissociated cultures of cerebellar cells are enriched in granule cells and have been useful for studying ion channel diversity in central neurons. The aim of this study is to investigate which types of Ca2+ channels contribute to facilitation of the whole-cell Ca2+ current in granule cells and to determine whether the mechanisms are similar to those in peripheral neurons.
Parts of this paper have been published previously (Parri and Lansman, 1994).
MATERIALS AND METHODS
Tissue culture. Cultures of dissociated mouse cerebellar cells were prepared as described previously (Slesinger and Lansman, 1991a). Cerebellar cells were plated at a density of 0.05–0.5 × 106 cells/ml on glass coverslips (Deutsche Spiegelglas) precoated with 25 mg/ml poly-l-lysine (Sigma, St. Louis, MO). Cultures were kept in a humidified atmosphere of 5% CO2/95% air at 37°C in a medium containing Minimum Essential Medium (MEM) with Earle’s basal salts and 2 mm glutamine (UCSF Cell Culture Facility). MEM was supplemented with 10% fetal bovine serum, 25 mm KCl, and 0.12% glucose.
Solutions. For whole-cell recordings, the intracellular solution contained (in mm): 100 aspartic acid, 10 HEPES, 20 TEA-Cl, 10 EGTA, 5 glucose, and 1 MgCl2, adjusted to pH 7.4 by adding CsOH. The intracellular solution also contained 4 mm MgATP and 0.5 GTP mm to minimize rundown of the Ca2+ current. In some experiments, GTP was replaced by either GTP-γ-S or GDP-β-S (Sigma). The osmolarity was adjusted to 320 mOsm by adding TEA-Cl. The external solution contained 120 TEA-Cl, 10 HEPES, 10 glucose, 5 BaCl2, and 1 μm TTX. The pH was adjusted to 7.4 by adding TEA-OH. Currents through single channels were recorded with an electrode-filling solution containing (in mm): 100 BaCl2 [(Aldrich Chemicals, Milwaukee, WI) > 99.9% purity], 10 HEPES, and 10 glucose; the pH was adjusted to 7.4 by adding TEA-hydroxide. The bathing solution contained (in mm): 150 KOH, 5 MgCl2, 60 glucose, 1 EGTA, and 10 HEPES. The pH was adjusted to 7.4 by adding methanesulfonic acid. An isotonic K+ bathing solution was used for the single-channel recordings to zero the cell resting potential so that the patch potential would be the same as the voltage command applied to the patch-clamp amplifier. The voltage error introduced by this procedure was generally <5 mV. An error as large as 10–15 mV was measured in a small number of experiments as a shift in the single-channel i–V relation after patch excision. The contribution of this source of error was minimized by rejecting experiments in which there was a change in the single-channel current after excising the patch at the end of an experiment.
The dihydropyridine antagonists nimodipine and nifedipine (RBI, Natick, MA) were prepared as 0.1 m stock solutions in 100% ethanol. The dihydropyridine agonist (−)Bay K 8644 (RBI) was prepared as a 0.01 m stock solution in 100% ethanol. ω-CTX-GVIA (Sigma) was prepared as a 50 × 10−6 M stock solution in distilled H2O. ω-aga-IVA (Peptides International, Louisville, KY) and ω-CTX-MVIIC (RBI) were prepared as a 1 × 10−4 M stock solution in distilled water. Stock solutions of the toxins were stored at −20°C. Fresh solutions were prepared on each day of the experiments. Drugs were applied by perfusing the cell locally with a second pipette having a tip diameter of ∼50 μm. The perfusion pipette was lowered into the bath and positioned close to the cell under study after a stable recording had been established.
Electrophysiological methods. Individual coverslips were placed in a recording chamber mounted on a Nikon phase-contrast microscope. Whole- cell or single-channel currents were recorded following the method described by Hamill et al. (1981). Patch electrodes were made from Boralex hematocrit glass (Rochester Scientific) and had resistances of 3–5 MΩ with Cs-Asp in the electrode and 5 BaCl2/TEA in the bath (whole-cell recordings) or 5–7 MΩ with 100 Ba2+ in the electrode and K+-methanesulfonate in the bath (single-channel recordings). Current signals were recorded with a List EPC-7 amplifier with a 0.5 GΩ feedback resistor in the headstage. Voltage command pulses were generated, and current responses were simultaneously digitized and stored on a laboratory computer (LSI 11/73, Indec Systems, Sunnyvale, CA). Currents were filtered with an eight-pole, low-pass Bessel filter at 1 kHz (−3dB) and sampled at 5 kHz. The junction potential between the electrode-filling solution and the bath was zeroed before making a seal. After establishment of a whole-cell recording, the cell membrane capacitance was compensated for by an analog circuit that injected a current proportional to the integral of the test voltage step after filtering and scaling. The membrane capacitance was obtained from the compensation circuit, which was determined to be within ∼20% of the actual value, as estimated by measurements on a test circuit. An analog circuit was used to compensate for ∼50–70% of the series resistance. The small size of the currents in these experiments indicated that there was a maximum voltage error of ∼2 mV produced by current flow through the series resistance. Test pulses were delivered at 0.05–0.2 Hz. All current traces shown were corrected for linear leak and capacity current by subtracting, after appropriate scaling, the averaged current response to four voltage steps of one fourth the amplitude of the test pulse. All recordings were made at room temperature (21–24°C).
Analysis of data. Facilitation was expressed either as the percentage increase of the current during a test pulse before and after pairing it with a positive prepulse (percentage facilitation) or the percentage change in the amplitude of the facilitation current (difference between test pulse current before and after pairing with a prepulse) before and after exposure to various channel blockers (percentage contribution to facilitation current). In most experiments, the test pulse was separated from the prepulse by a 5 msec interpulse interval. This was sufficient time to allow open channels to close (Slesinger and Lansman, 1991a). The strong prepulse also produced a slow tail current, which appeared after repolarization (τ = 8–10 msec at −80 mV). This slow tail current reflected the closing of L-type channels that had entered a long open-state gating mode during the prepulse (Pietrobon and Hess, 1990; Forti and Pietrobon, 1993;Slesinger and Lansman, 1991c, 1996). To avoid contamination of the current during the test voltage step, the amplitude of the current was measured 20–30 msec after the onset of the test step. Granule cells in culture elaborate neurites, which may have contributed to spatial nonuniformity of the membrane potential. In experiments on cells during the first week in culture, we chose cells for recordings that did not have elongated neurites. In some experiments, however, we observed a tail current with τ >40 msec that may have been associated with spatial nonuniformity of the membrane potential. Recordings in which these very slow tail currents appeared were excluded from the kinetic analysis.
RESULTS
Prepulse-induced facilitation in cultured cerebellar granule cells
Ca2+ channel currents were isolated with intracellular Cs+ to block current through K+ channels and with 5 mmBa2+ as the charge carrier. Figure1A shows an example of the effect of previous depolarization on the amplitude of the Ca2+ channel current recorded during a test pulse to 0 mV. The recording was made from a cerebellar granule cell that had been in culture 1 week. In the absence of a prepulse, the voltage step to 0 mV activated an inward current that showed both a fast and a slow phase of activation (smaller current record). When the test pulse was preceded by a prepulse to +90 mV, the Ca2+channel current reached a larger peak amplitude and then decayed during the voltage step.
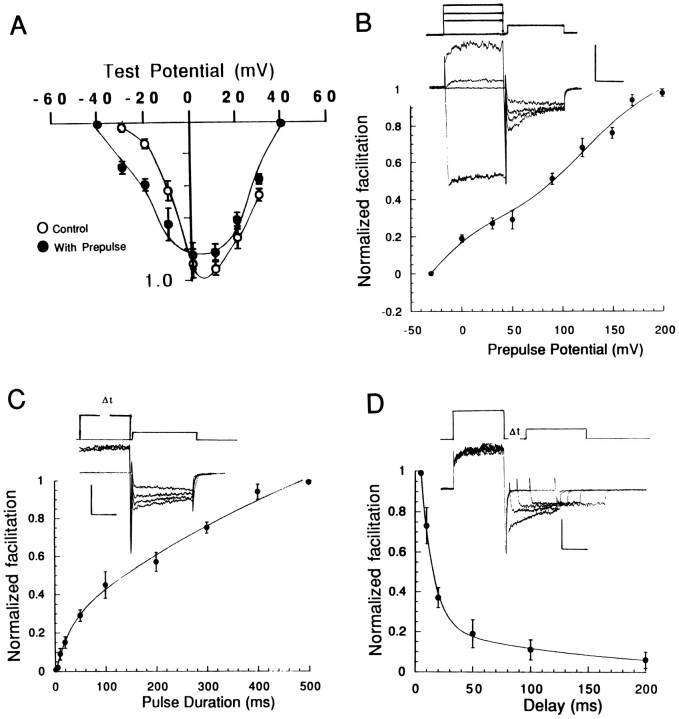
Fig. 1.

Prepulse-induced Ca2+channel facilitation in cerebellar granule cells. A, Ca2+ currents evoked by voltage steps to 0 mV from a holding potential of −80 mV. The voltage step was applied either alone (smaller current) or after a 200 msec prepulse to +90 mV. The bathing solution contained 5 mmBa2+ as the charge carrier. B, Changes in prepulse-induced facilitation during development of granule cells in tissue culture. The amount of facilitation was measured as ratio of the peak current elicited by a test pulse to −20 mV measured with and without a prepulse to +90 mV × 100. Error bars = SEM (n = 7–11).
A number of electrophysiological studies of cerebellar granule cells have documented changes in membrane excitability during development (Hockberger et al., 1987; Haws et al., 1993; Rossi and Slater, 1993). In recordings from mouse cerebellar granule cells, we found that Ca2+ channel facilitation was critically dependent on the length of time the cells had been in tissue culture. Figure 1B shows the change in prepulse-induced facilitation during development in culture. In recordings from cells during the first few days in culture, the prepulse increased the current during the test pulse by ∼35%. After ∼1 week in culture, however, the prepulse roughly doubled the Ca2+ current. The increase in facilitation was not maintained, and facilitation decreased substantially in 2-week-old cells. These results suggest that thein vitro development of granule cells is associated with changes in Ca2+ channel facilitation. In the first series of experiments, we analyzed the pharmacological components of prepulse-induced facilitation to determine which types of Ca2+ channels contributed to the increase in current (see below).
Pharmacological components of prepulse-induced facilitation
Previous studies of Ca2+ currents in cerebellar granule cells showed that a large component is carried by dihydropyridine-sensitive L-type Ca2+ channels, whereas a smaller component is carried by ω-CTX-sensitive, N-type channels (Huston et al., 1990; De Waard et al., 1991; Marchetti et al., 1991; Slesinger and Lansman, 1991a; Haws et al., 1993). The recent availability of the neuronal Ca2+ channel toxins ω-aga-IVA and ω-CTX-MVIIC have made it possible to distinguish components of the dihydropyridine- and ω-CTX-GVIA-insensitive current in granule cells (Zhang et al., 1993; Chavis et al., 1995; Randall and Tsien, 1995). We used these toxins to identify the contribution of pharmacologically distinct channel types to facilitation.
Figure 2A shows an experiment in which a cell was exposed to a saturating concentration of the dihydropyridine antagonist nimodipine (10 μm). Under control conditions (top), the prepulse to +90 mV roughly doubled the peak amplitude of the Ca2+ channel current. Subsequent exposure of the cell to nimodipine (bottom) reduced the control current evoked in the absence of the prepulse by roughly one quarter. In a number of experiments, nimodipine blocked 29 ± 3% (±SD, n = 21) of the current during the test pulse. Note that nimodipine also reduced the outward current elicited by the prepulse. Block of the outward current by nimodipine suggests that it is carried by Cs+ through L-type channels (Lee and Tsien, 1984).
Fig. 2.
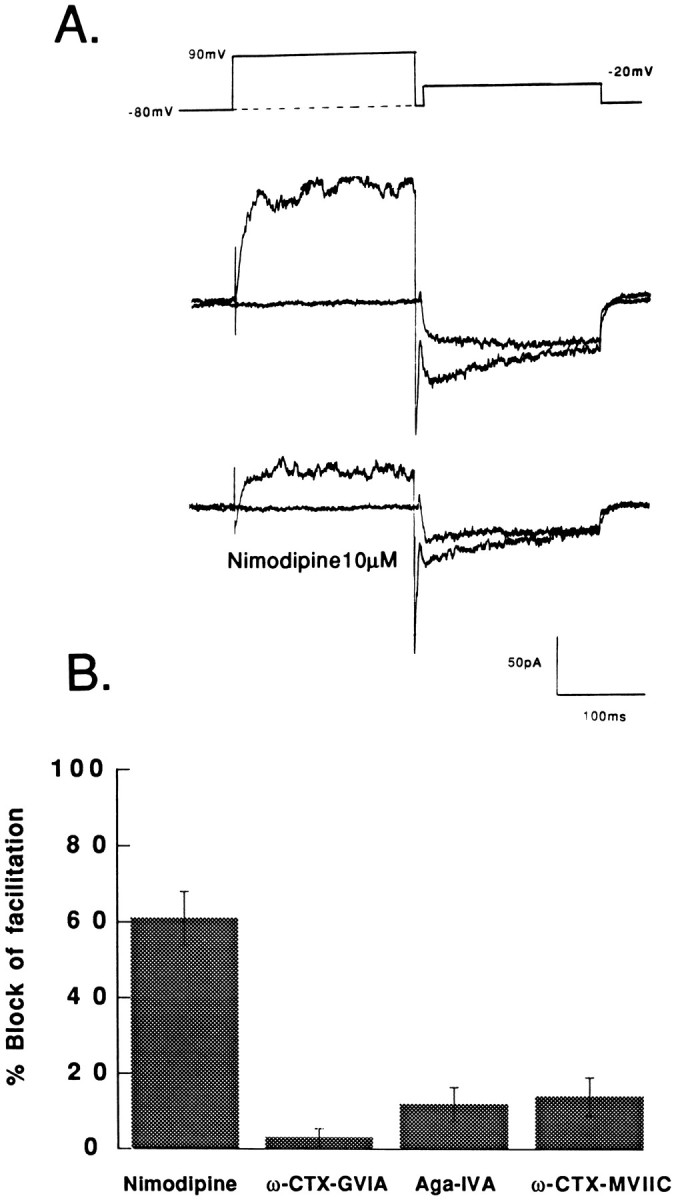
Pharmacological components of facilitation.A, Whole-cell Ca2+ channel currents carried by Ba2+ recorded from a granule cell before (top) and after (bottom) exposure to 10 μm nimodipine. Ca2+channel currents were measured during a test pulse to −20 mV from a holding potential of −80 mV. The voltage step was applied either alone (smaller current) or 5 msec after a prepulse to +90 mV. Note that a large fraction of the outward current activated during the prepulse to +90 mV was blocked by nimodipine, suggesting that the outward current flows through Ca2+ channels. B, The effects of Ca2+ channel blockers on prepulse-induced facilitation. Nimodipine (10 μm) blocked 61 ± 7% (n = 9); ω-CTX-GVIA (3 μm) blocked 3 ± 2.5% (n = 6); ω-Aga-IVA (200 nm) blocked 12 ± 4.5% (n = 6); ω-CTX-MVIIC (3 μm applied after ω-CTX and ω-aga) blocked 14 ± 5% (n = 5) of the facilitation current.
Nimodipine produced a marked inhibition of the facilitation current (current evoked in excess of the control current when the test pulse was paired with a prepulse). Figure 2B shows the percentage reduction of the facilitation current measured in a number of cells that were exposed to nimodipine. As shown in Figure2B, nimodipine inhibited ∼60–70% of the facilitation current. Nimodipine inhibited a much larger fraction of the facilitation current than it inhibited during the test pulse alone (25–45%) (Slesinger and Lansman, 1991a; Haws et al., 1993; Chavis et al., 1995). The effects of nimodipine show that L-type channels contribute a major component to the prepulse-induced facilitation in granule cells. A smaller component, however, is carried by dihydropyridine-insensitive Ca2+ channels.
The dihydropyridine-insensitive facilitation was examined further with neuronal Ca2+ channels toxins. Figure2B shows that the N-type channel blocker ω-CTX-GVIA (3 μm) blocked very little of the facilitation current (∼3%). Moreover, higher doses of ω-CTX-GVIA did not produce any additional inhibition of facilitation (data not shown). This stands in contrast to the effects of ω-CTX-GVIA on the current evoked by the test pulse alone, where it reduced the current by 17 ± 3% (±SD, n = 10) in 1-week-old cells. Although N-type channels contribute to the whole-cell currents, they contribute a negligible component to facilitation of the Ca2+ current in granule cells measured with standard recording solutions.
The spider toxin ω-aga-IVA blocks a class of P-type channels in Purkinje cells and other central neurons (Mintz et al., 1992a,b). Analysis of the actions of ω-aga-IVA, however, is complicated by its slow blocking kinetics at low concentrations and the ability of strong depolarizations to relieve channel block (Mintz et al., 1992a,b). We chose to examine the sensitivity of prepulse-induced facilitation to 200 nm ω-aga-IVA; at this concentration, virtually all of the P-type channels are expected to be blocked, and steady-state block is reached within minutes. Because voltage-dependent relief from ω-aga-IVA block proceeds with a rate that is slower than the onset of facilitation, substantial unblocking would not be expected with the single short prepulses that were used to study facilitation in these experiments.
Recordings from granule cells were made before and after exposure to 200 nm ω-aga-IVA. We found that ω-aga-IVA reduced the current during the test pulse by 18 ± 3% (n = 15). Figure 2B shows that ω-aga-IVA reduced prepulse-induced facilitation by ∼12%, although it blocked the current during the test pulse by 18 ± 3% (n = 11) (also see Chavis et al., 1995). The effects of ω-aga-IVA on Ca2+ currents in granule cells, however, are complex and may involve additional blockade of N- and L-type channels (Pearson et al., 1995) or a class of neuronal Q-type channel (Randall and Tsien, 1995). We found no evidence to indicate that ω-aga-IVA blocked N- or L-type channels. To assess the contribution of Q-type channel inhibition to the actions of ω-aga-IVA, we examined the effects of ω-CTX-MVIIC, which is thought to inhibit N-, P- and Q-type channels (Hillyard et al., 1992). In these experiments, granule cells were exposed continuously to ω-CTX-GVIA (3 μm) and ω-aga-IVA (200 nm). Exposure of granule cells to 3 μm ω-CTX-GVIA would be expected to block all of the N-type channels, whereas 200 nmω-aga-IVA would block 100% of the P-type channels and ∼80% of the Q-type channels (Randall and Tsien, 1995). Subsequent addition of ω-CTX-MVIIC, therefore, would be expected to inhibit very little of the facilitation current if most of the Q-type channels had already been blocked by ω-aga-IVA. We found that 3 μmω-CTX-MVIIC reduced the current during the test pulse by 20 ± 3% (±SD, n = 5) in cells that were exposed continuously to 3 μm ω-CTX-GVIA and 200 nm ω-aga-IVA. Figure 2B shows that ω-CTX-MVIIC reduced prepulse-induced facilitation by 14%. Thus, there is an ω-CTX-MVIIC-sensitive component of facilitation that is not carried by N- or P-type channels. We attribute this to the Q-type channels that were unblocked in the presence of 200 nm ω-aga-IVA. If block of Q-type channels accounted for most of the aga-IVA-sensitive current, then the contribution of P-type channels to facilitation is likely to be negligible. Because the absolute size of the ω-aga-IVA-sensitive component of facilitation was small (∼10 pA in most cells), however, we did not study it further.
Figure 3 shows the analysis of the voltage dependence and kinetics of facilitation in granule cells perfused with a standard intracellular solution. The bathing solution contained 3 μm ω-CTX-GVIA to inhibit the N-type channels. Figure 3A shows the I–V relation of the whole-cell Ca2+ channel current measured in response to a test pulse that was delivered either with (filled circles) or without (open circles) a prepulse to +90 mV. When the test pulse was preceded by a prepulse (open symbols), facilitation was observed at negative test pulse potentials. The apparent reduction of the current measured during positive test pulses may reflect a shift in theI–V relation to more negative potentials. Figure3B shows the effect of the prepulse potential on the facilitation of the current during a test pulse to −20 mV. At this test potential, the prepulse produced the largest increase in the test pulse current. The activation of facilitation was biphasic withV1/2 = ∼0 and +60 mV. Moreover, facilitation increased at very positive potentials without apparent saturation.
Fig. 3.
Voltage dependence and kinetics of facilitation measured with a standard intracellular solution. The bathing solution contained 3 μm ω-CTX-GVIA. A, Effect of a prepulse to +90 mV on the I–Vrelation of the whole-cell Ca2+ current.Open circles, Peak current measured during a test pulse to the potential indicated on the voltage axis. Filled circles, Peak current during a test pulse to the potential indicated on the voltage axis when preceded by a prepulse to +90 mV. The currents are normalized to their maximum amplitude. B, Voltage-dependent activation of facilitation. The graph plots the amount of facilitation (normalized to its maximum value) as a function of the prepulse potentials; the curve was fit by eye. Inset, The current responses to test pulses to −20 mV when preceded by prepulses to 0, +50, and +90 mV (from bottom to top).C, The rate of onset of facilitation measured with a two-pulse voltage-clamp protocol. The amount of facilitation during a test pulse to −20 mV was measured after a prepulse to +90 mV that lasted from 10 to 500 msec. The onset of facilitation was fit with a double exponential with time constants τfast = 12 msec and τslow = 300 msec. Inset, The current responses to test pulses to −20 mV when preceded by a prepulse to +90 mV that lasted 100, 200, and 500 msec. D, The time course of recovery from facilitation measured with a two-pulse voltage-clamp protocol. Facilitation declined along a double-exponential time course with τfast = 7 msec and τslow = 93 msec. Inset, The current responses to voltage steps to −20 mV measured 5, 20, 50, 100, and 200 msec after repolarizing the membrane potential to the holding potential of −80 mV after the prepulse to +90 mV. Data points are the mean ± SEM (n = 5). Current calibration, 100 pA; time calibration, 100 msec.
The kinetics of facilitation was studied with a two-pulse voltage clamp protocol. Figure 3C shows the time course of the development of facilitation measured in response to prepulses of increasing duration. Facilitation developed along a double-exponential time course (τfast = ∼12 msec and τslow = ∼300 msec). Figure 3Dshows the time course of the recovery of facilitation measured after repolarizing the potential to −80 mV for varying times before applying the test pulse. Facilitation also decayed along a double-exponential time course (τfast = 7 msec and τslow = 93 msec).
The complex kinetics of facilitation may reflect the individual contribution of L- and Q-type channels. The contribution to facilitation of these channels can be demonstrated in the presence of ω-CTX-MVIIC, which blocks a large fraction of the non-L, non-N-current in hippocampal neurons (Hillyard et al., 1992). Figure4 shows the contribution of the ω-CTX-MVIIC-sensitive channels to facilitation of the Ca2+ current. Figure 4A shows the current during a test pulse before and after the test pulse was paired with a prepulse. After facilitation of the Ca2+ channel current was measured in the absence of any blockers (control), the cell was exposed to ω-CTX-MVIIC. After adding ω-CTX-MVIIC, the facilitation current evoked in excess of the control current was reduced by ∼30%. Subsequent addition of ω-CTX-MVIIC and nimodipine inhibited virtually all of the facilitation current. Figure 4B shows the effects of ω-CTX-MVIIC and nimodipine on the amplitude of the facilitation current evoked with prepulses to different potentials. Addition of ω-CTX-MVIIC reduced the amplitude of the facilitation current over the entire range of prepulse potentials. Subsequent addition of nimodipine reduced the facilitation current further, although the block by nimodipine was not complete at the most positive prepulse potentials. Thus, the dihydropyridine-insensitive component of facilitation can be accounted for fully by the presence of ω-CTX-MVIIC-sensitive Ca2+ channels.
Fig. 4.
Effects of ω-CTX-MVIIC (3 μm) and nimodipine (10 μm) on facilitation. A, Currents recorded during a test pulse to −20 before and after (+ prepulse) pairing the test pulse with a 200 msec prepulse to +50 mV. After the prepulse, the membrane was repolarized to −80 mV for 5 msec before the test pulse was delivered. This pulse protocol was applied to the cell in the absence of blockers (top) and after sequential exposure to ω-CTX-MVIIC (MVIIC;middle) and ω-CTX-MVIIC and nimodipine (MVIIC + Nimodipine; bottom). B, The amplitude of the facilitation current (current with prepulse–current without pulse) plotted as a function of the prepulse potential. The prepulse dependence of the facilitation current was measured before applying blockers (open circles), after exposure to 3 μm ω-CTX-MVIIC (filled circles), and subsequently after exposure to 3 μm ω-CTX-MVIIC and 10 μm nimodipine (open squares).
Enhancement of N-type channel facilitation by intracellular GTP-γ-S
In peripheral neurons, prepulse-induced facilitation involves a voltage-dependent relief of the block of N-type Ca2+ channels by activated G-proteins (Pollo et al., 1989; Elmslie et al., 1990; Ikeda, 1991; Kasai, 1991). Perfusion of granule cells with GTP-γ-S, however, inhibits L-type as well as N-type channels (Haws et al., 1993). Consequently, we asked whether the facilitation of the whole-cell Ca2+ channel current involved a voltage-dependent relief from inhibition by a G-protein and whether it involved both N- and L-type channels. In these experiments, 0.5 mm GTP-γ-S was introduced into the cell through the recording electrode to produce maximal activation of endogenous G-proteins.
Figure 5A shows a recording from a granule cell that was perfused with GTP-γ-S. The Ca2+current evoked by a step depolarization to 0 mV from a holding potential of −80 mV showed a slow phase of activation characteristic of Ca2+ currents recorded with electrode solutions containing GTP-γ-S (Dolphin and Scott, 1987; Kasai and Aosaki, 1989; Haws et al., 1993). When the test pulse to 0 mV was preceded by a prepulse to +90 mV, the Ca2+current during the test pulse activated more quickly and reached a larger peak amplitude. Prepulse-induced facilitation was measured as the percentage increase in the current that was measured 30 msec after the onset of the test pulse. The results from a number of experiments with GTP-γ-S in the recording electrode are shown in Figure5B.
Fig. 5.
Effect of intracellular GTP-γ-S on facilitation.A, Records of the whole-cell Ca2+channel currents carried by Ba2+ in a cell perfused with GTP-γ-S (0.5 mm). With intracellular GTP-γ-S, the Ca2+ current evoked by a test pulse to 0 mV activates slowly and shows little inactivation during the pulse. The prepulse to +90 mV increased the current during the subsequent test pulse to 0 mV. B, Pharmacological sensitivity of prepulse-induced facilitation in cells perfused with GTP-γ-S. Test pulse = −20 mV for GTP and GDP-β-S; 0 mV for GTP-γ-S, GTP-γ-S + ω-CTX, and GTP-γ-S + nimodipine.
The facilitation observed when cells are perfused with a standard intracellular solution may be the result of a tonic G-protein-mediated inhibition of Ca2+ channels. To test this prediction, cells were perfused with GDP-β-S to prevent formation of activated G-protein. As shown in Figure 5B, there seemed to be somewhat more facilitation in cells perfused with GDP-β-S than in those with GTP, but the difference was not statistically significant (70 ± 20% vs 50 ± 30%, n = 11 and 7, respectively). Perfusion of cells with GDP-β-S had no effect on the Ca2+ current density (20 ± 3% with GDP-β-S vs 22 ± 3% control, n = 16 and 11, respectively), suggesting that it did not alter the relative proportions of the various channel types.
Figure 5B shows that the Ca2+ channel facilitation measured with intracellular GTP-γ-S was blocked completely when the external solution contained 3–6 μm ω-CTX GVIA. Nimodipine, by contrast, produced a small, statistically insignificant increase in the percentage facilitation. This small increase in facilitation, however, could be attributed to the small reduction of the control current by nimodipine (data not shown). Evidently, the mechanism of facilitation changes when cells are perfused with GTP-γ-S. In cells perfused with standard solutions, the facilitation current is carried predominantly by L-type channels, with a smaller component carried by ω-CTX-MVIIC-sensitive channels. Intracellular perfusion of cells with GTP-γ-S, however, enhanced the facilitation of N-type channels while apparently suppressing the facilitation of L- as well as non-L, N-type channels.
We next asked whether facilitation observed in the presence of GTP-γ-S could be distinguished from the facilitation measured with standard intracellular solutions by its voltage dependence and kinetics. Figure 6A shows that facilitation of N-type channels in cells perfused with GTP-γ-S could be observed over a much wider range of test pulse potentials than that measured with standard intracellular solutions. A prepulse to +90 mV increased the current evoked by test pulses from −40 to up to +30 mV. In addition, Figure 6B shows that facilitation increased with the potential of the prepulse along a simple sigmoidal activation curve. Figure 6C shows the time course of the development of facilitation measured with a two-pulse voltage protocol. Facilitation developed rapidly, reached a maximum by 20 msec, and then inactivated (Fig. 6C). The recovery from facilitation was also studied with a two-pulse protocol. Figure 6D shows that facilitation declined at the holding potential along a double-exponential time course (τfast = 15 msec and τslow = 135 msec at −80 mV).
Fig. 6.
Voltage dependence and kinetics of N-type channel facilitation. A, Effect of a prepulse to +90 mV on theI–V relation of the whole-cell Ca2+current. Open circles, Peak current measured during a test pulse to the potential indicated on the voltage axis. Filled circles, Peak current during a test pulse to the potential indicated on the voltage axis when preceded by a prepulse to +90 mV. The currents are normalized to their maximum amplitude. The prepulse increases the peak current over a range of test pulse potentials.B, Voltage-dependent activation of facilitation. Thegraph plots the amount of facilitation (normalized to its maximum value) as a function of the prepulse potential. The relation was fit to a Boltzmann relation with V1/2 = 35.5 mV and steepness k = 24.9 mV. Inset, Amplitude of the current during a test pulse to 0 mV when preceded by a prepulse to either 0 or +90 mV (from bottom totop). C, Time course of the onset of facilitation measured with a two-pulse voltage-clamp protocol. Inset, Amplitude of the current during a test pulse to 0 mV when preceded by prepulses to +90 mV that lasted 1, 5, or 10 msec. Current scale, 100 pA; time scale, 40 msec. D, Time course of recovery from facilitation measured with a two-pulse voltage-clamp protocol. Facilitation declined along a double-exponential time course with τfast = 15 msec and τslow = 135 msec. Inset, The currents evoked by voltage steps to 0 mV measured 5, 10, 20, 50, and 100 msec after repolarizing the membrane to −80 mV after a 200 msec prepulse to +90 mV. Data points are mean ± SEM (n = 5). Current scale, 100 pA; time scale, 100 msec.
Effect of dihydropyridine agonist
Dihydropyridine agonists contribute to the facilitation of cardiac L-type channels by promoting a long-lived open state (Hess et al., 1984; Lacerda and Brown, 1989). Strong positive prepulses also promote a long-lived open state that underlies the facilitation of L-type channels in cardiac muscle (Pietrobon and Hess, 1990), chromaffin cells (Artalejo et al., 1991b), and central neurons (cerebellar granule cells: Slesinger and Lansman, 1991b; Forti and Pietrobon, 1993;Slesinger and Lansman, 1996; hippocampal neurons: Kavalali and Plummer, 1994). We examined the effect of the agonist on facilitation to determine whether it recruits the same population of L-type channels as the prepulse.
Figure 7 shows an experiment in which facilitation was measured before and after exposure of cells to the dihydropyridine agonist Bay K 8644. Figure 7A shows the current evoked by the test pulse to −20 mV alone (smaller current in each set) or after a prepulse to +90 mV. The voltage step protocol was applied to the cell before (top) and after (bottom) exposure to Bay K 8644. Before adding the agonist, the prepulse increased the current during the test pulse by roughly three times. After the cell was exposed to the agonist, the current evoked by the test pulse alone was increased two- to threefold. When the prepulse to +90 mV was applied before the test pulse, however, there was a further doubling of the current. Figure 7B shows the combined results from a number of cells. The small reduction in the percentage facilitation caused by exposure to the agonist can be explained in terms of the increased amplitude of the control current measured in the absence of a prepulse. That the amount of facilitation was not greatly altered by exposure to the agonist suggests that the prepulse and the agonist facilitate the current by different mechanisms.
Fig. 7.
Facilitation of the Ca2+channel current persists after exposure to dihydropyridine agonist. The bathing solution contained 3 μm ω-CTX-GVIA.A, Top shows the whole-cell currents carried by Ba2+ evoked by a test pulse to −20 mV in the presence and absence (large and small currents, respectively) of a prepulse to +90 mV. Bottom shows the currents recorded in response to the same pulse protocol after the cells were exposed for several minutes to 1 μm Bay K 8644 (BayK). B, The effects of Bay K 8644 (Bay K) on prepulse-induced facilitation. In the absence of the dihydropyridine agonist, the prepulse to +90 mV increased the Ca2+ channel current by 202 ± 40% (SEM, n = 4). In the presence of agonist, the prepulse increased the current by 129 ± 11% (SEM,n = 4).
Effects of phosphorylation
To test whether facilitation required phosphorylation of the channel, granule cells were exposed to the nonselective protein kinase inhibitor H7. Cells were treated with H7 (200 μm) by adding it to the bathing solution at the start of an experiment. Facilitation was measured during a test pulse to −20 mV before and after pairing it with a prepulse to +90 mV. The percentage facilitation was 89 ± 27% (SD, n = 8) in cells exposed to H7 and 81 ± 28% (SD, n = 10) in untreated cells from the same isolation. The absence of an effect of kinase inhibition on facilitation contrasts with the finding that kinase inhibition blocks facilitation of L-type channels in chromaffin cells (Artalejo et al., 1992) and those expressed from the neuronal α1C (Bourinet et al., 1994).
Changes in L-type channels during development
We were concerned in subsequent experiments with obtaining information on the mechanism that contributes to the change in facilitation during development. Experiments focused on L-type channels, because they contribute the dominant component of facilitation in 1-week-old cells. The increase in facilitation during the first week in culture could be attributable to an increase in the number of L-type channels; however, Figure 8 shows that the contribution of L-type channels to the whole-cell current decreased with time. Figure 8A shows that the peak Ca2+ current density increased during the first week in culture but remained relatively constant throughout the second week. Figure 8B, however, shows that the proportion of the whole-cell current that was blocked by a saturating dose of nimodipine decreased with the amount of time in culture. Consequently, the increase in facilitation during development in culture is not attributable simply to an increase in the expression of L-type Ca2+ channels in 1-week-old cells.
Fig. 8.

Changes in the whole-cell Ca2+ channel current during development in tissue culture. A, Ca2+ current density measured after different times in culture. The Ca2+ current density was 21 ± 2.4 pA/pF at 1–4 DIV (n = 12), 34 ± 3.8 pA/pF at 6–8 DIV (n = 7), and 39 ± 5.08 pA/pF at 14–15 DIV (n = 12). B, Contribution of L-type channels to the whole-cell Ca2+ current at different times in culture. Nimodipine (10 μm) blocked 35.4 ± 3% of the current at 1–4 DIV (n = 7), 27.3 ± 5.2% of the current at 6–8 DIV (n = 7), and 14.1 ± 4.7% of the current at 14–15 DIV (n = 8).
We also examined whether the increase in facilitation after 1 week could be explained by a change in channel sensitivity to phosphorylation by cyclic AMP-dependent protein kinase. Inclusion of the cyclic AMP-dependent kinase activator SpcAMPs (200 μm) in the recording electrode increased the Ca2+ current density in cells that had been in culture 1–4 d from 9.8 ± 3 pA/pF to 17.2 ± 1.5 pA/pF (±SEM, n = 4). Although the current density was increased, there was no change in the percentage facilitation. In cells that had been in culture 6–8 d, inclusion of SpcAMPs (200 μm) reduced the Ca2+current density from 31.8 (n = 2) to 20.5 ± 4 pA/pF (n = 6, SEM). Phosphorylation via kinase A, therefore, cannot explain the increase in facilitation during the first week in culture.
An alternative mechanism is that the appearance of facilitation involves a change in channel properties. To test this possibility, we recorded single-channel activity from cell-attached patches. Single L-type Ca2+ channels were identified by including the dihydropyridine agonist Bay K 8644 in the bathing solution to prolong the duration of the single-channel openings. Figure9 shows examples of recordings from cell-attached patches on cells that had been in culture either 1 (left column) or 6 (right column) d. Single-channel activity was evoked by a test pulse to −30 mV from a holding potential of −60 mV. In recordings from cells during the first day in culture, the activity of two small-conductance L-type channels with conductances of ∼10 and 15.5 pS dominated the channel activity recorded from cell-attached patches (Fig. 9Ai,iii). Figure 9Aiishows that there was very little change in the single-channel activity when the test pulse to −30 mV was preceded by a prepulse to +90 mV. On the other hand, recordings from cells during the sixth day in culture showed the activity of an L-type channel with a conductance of ∼24 pS (Fig. 9Bi,iii). As shown in Figure 9Bi,ii, channel activity was low during the test pulse to −30 mV, but application of a prepulse to +90 mV before the test pulse dramatically increased channel opening probability. This finding is consistent with those of others in which strong depolarization was found to prolong opening of a 25–27 pS L-type channel (cardiac muscle: Pietrobon and Hess, 1990; granule cells: Slesinger and Lansman, 1991b; Forti and Pietrobon, 1993; hippocampal neurons: Kavalali and Plummer, 1994).
Fig. 9.
Heterogeneity of single L-type Ca2+ channels in cerebellar granule cells developing in culture. A, Single Ca2+channel activity recorded from a cell-attached patch on a cerebellar granule cell after 1 d in culture. The patch electrode contained 90 mm BaCl2. The bath contained 1 μm Bay K 8644 to prolong the opening of L-type Ca2+ channels. i, Single-channel currents evoked by a test pulse to −30 mV.ii, Currents evoked by the test pulse to −30 mV when the test pulse was preceded by a voltage step to +90 mV. The prepulse produced only a small increase in single-channel activity.iii, The I–V relations of the small conductance Ca2+ channels in cells that had been in culture 1 d. B, Single-channel activity recorded from a cell-attached patch on a cerebellar granule cell that had been in culture 6 d. i, Single-channel currents evoked by a test pulse to −30 mV. ii, Currents evoked by the test pulse to −30 mV when the test pulse was preceded by a voltage step to +90 mV. There is a large increase in channel open probability when the test pulse is preceded by the positive prepulse. iii, TheI–V relation of the large conductance (∼25 pS) L-type Ca2+ channel. The 25 pS L-type channel was observed in 0/6 patches on cells at 1–4 DIV and 4/7 patches on cells at 6–9 DIV. The small conductance L-type channels were observed in 6/6 of the patches on cells at 1–4 DIV and 3/7 patches on cells at 6–9 DIV.
DISCUSSION
Prepulse-induced Ca2+ channel facilitation can be detected in cultured cerebellar granule cells, although its magnitude varies with the amount of time the cells are maintained in tissue culture. Facilitation is carried largely by L-type Ca2+ channels in recordings made with standard internal solutions. When cells were perfused with GTP-γ-S, however, facilitation of L-type channels was inhibited, whereas the facilitation of N-type channels was enhanced. The facilitation of N-type channels with intracellular GTP-γ-S was characterized by a slow rate of current activation during the test pulse, with a shift of activation to more negative membrane potentials (Aosaki and Kasai, 1989; Ikeda, 1991). The voltage dependence and kinetics of N-type channel facilitation in granule cells were generally similar to those described in peripheral neurons (Elmslie et al., 1990; Ikeda, 1991).
Although L-type Ca2+ channels contributed the largest component to the facilitation measured with standard recording solutions, a proportion was resistant to inhibition by dihydropyridines. Experiments with ω-CTX-MVIIC showed that the dihydropyridine-resistant facilitation could be accounted for by the contribution of Q-type channels. The participation of P-type channels was more difficult to assess, because block of facilitation by ω-aga-IVA may involve the block of Q-type channels. Because the ω-CTX-MVIIC-sensitive component (during continuous exposure to ω-aga-IVA) was as large as the ω-aga-IVA-sensitive component, the contribution of P-type channels is likely to be small. The effects of ω-aga-IVA, however, cannot be attributed to block of N- and L-type channels, as suggested by Pearson et al. (1995), because we found that ω-aga-IVA blocked much less facilitation than nimodipine and more than ω-CTX-GVIA.
Molecular basis of facilitation in granule cells
Mammalian brain expresses five classes of Ca2+ channel α1 subunits (Snutch et al., 1990; Soong et al., 1993). The class C and D α1 subunits encode L-type Ca2+ channels (Hui et al., 1991; Snutch et al., 1991; Williams et al., 1992) that are expressed throughout the granular layer of the cerebellum (Hell et al., 1993). Expression of the cloned cardiac, smooth muscle, and neuronal α1Csubunits produces Ca2+ currents that are facilitated by positive prepulses (Sculptoreanu et al., 1993b; Bourinet et al., 1994; Kleppisch et al., 1994). Facilitation of the L-type channels in granule cells is generally similar to the cloned α1C in the time course of facilitation and the persistence of facilitation in the presence of dihydropyridine agonist. Facilitation of both the cardiac and neuronal α1C, however, is blocked completely by inhibitors of cyclic AMP-dependent protein kinase (Sculptoreanu et al., 1993b; Bourinet et al., 1994). In contrast to the behavior of the cardiac and neuronal α1C, however, the facilitation of L-type channels in granule cells was not blocked by the kinase inhibitor H7, which was found to block facilitation of the neuronal α1C. Because the granular layer of the cerebellum shows high levels of immunoreactivity toward an antibody to class D L-type channels (Hell et al., 1993), it is possible that class D channels contribute to the whole-cell Ca2+currents. Consequently, the absence of an effect of phosphorylation on facilitation may reflect a fundamental difference between class C and D L-type channels. Alternatively, the difference between L-type channels in granule cells and the neuronal α1C may reflect the contribution of different splice variants or the incorporation of ancillary subunits, such as the β subunit, which was found to be required for facilitation of the neuronal α1C (Bourinet et al., 1994). Single-channel recordings showed that there is considerable diversity of L-type channels in granule cells (also see Slesinger and Lansman, 1991b; Forti and Pietrobon, 1993; Slesinger and Lansman, 1996). To determine whether this diversity represents the contribution of different gene products or different transcripts of a single gene will require additional experiments.
The finding that N-type channels contributed little to facilitation in the absence of G-protein activation is consistent with the results ofBourinet et al. (1994), who showed that the cloned α1B produces nonfacilitating currents. Class A Ca2+ channels are found throughout the brain, including the Purkinje and granule cells of the cerebellum (Stea et al., 1994). Class A channels are blocked strongly by ω-CTX-MVIIC but only weakly by ω-aga-IVA (Sather et al., 1993; Stea et al., 1994). Thus, the dihydropyridine-insensitive facilitation described in this paper has pharmacological properties that are consistent with class A Ca2+ channels (Sather et al., 1993). Although the currents produced by the expression of α1Asubunits do not show facilitation (Bourinet et al., 1994), both the α2δ and β subunits produce marked changes in its gating and permeability characteristics (De Waard and Campbell, 1995).
Physiological significance
During the development of the cerebellum, postmitotic granule cells migrate from the external to the internal granular layer in close contact with glial fibers. The granule cell parallel fibers begin to form synapses on Purkinje cells during the first postnatal week, with active synaptogenesis reaching a peak during the second and third postnatal weeks. A number of studies have pointed to changes that occur in the excitability of granule cells corresponding to the periods of migration and synaptogenesis (Komuro and Rakic, 1992; Rossi and Slater, 1993). Studies of cultured granule cells have also revealed developmentally regulated changes in the pharmacological sensitivity of Ca2+-dependent glutamate release (Gallo et al., 1985) and facilitation of K+-stimulated intracellular Ca2+ signals (Connor et al., 1987). The finding of a developmental change in the facilitation of intracellular Ca2+ responses is consistent with the changes in facilitation of the whole-cell Ca2+ current reported here. Additional experiments, however, are necessary to determine whether such changes occur during normal development in vivo, and if so, whether they participate in the processes of neuronal migration and synaptogenesis. L-type Ca2+ channels play a role in depolarization-dependent neuron survival (Collins and Lile, 1989), regulation of spontaneous neurotransmitter release at developing neuromuscular synapses (Fu and Huang, 1994), and depolarization-dependent changes in gene expression (Murphy et al., 1991; Rosen et al., 1994). It is interesting to speculate that L-type Ca2+ channels play a similar role in spontaneous transmitter release and gene expression during the period of synaptogenesis of granule cells by coupling neuronal activity to events involved in the establishment of synaptic contacts.
Footnotes
Correspondence should be addressed to Dr. Jeffry B. Lansman, Department of Cellular and Molecular Pharmacology, School of Medicine, University of California, San Francisco, CA 94143-0450.
Dr. Parri’s present address: Department of Physiology, University of Wales, Cardiff, UK.
REFERENCES
- 1.Aosaki T, Kasai H. Characterization of two kinds of high voltage-activated calcium channel currents in chick sensory neurons: differential sensitivity to dihydropyridines and ω-conotoxin GVIA. Pflügers Arch. 1989;414:150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- 2.Artalejo CR, Dahmer MK, Perlman RL, Fox AP. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol (Lond) 1991a;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artalejo CR, Rossi S, Perlman RL, Fox AP. Three types of bovine chromaffin cell Ca2+ channels: facilitation increases the opening probability of a 27 pS channel. J Physiol (Lond) 1991b;444:213–240. doi: 10.1113/jphysiol.1991.sp018874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artalejo CR, Rossie S, Perlman RL, Fox AP. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992;358:63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- 5.Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal α1C L-type calcium channel. EMBO J. 1994;13:5032–5039. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall WA, Seager MJ, Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channel. J Biol Chem. 1988;263:3535–3538. [PubMed] [Google Scholar]
- 7.Chavis P, Fagni L, Bockaert J, Lansman JB. Modulation of calcium channels by metabotropic glutamate receptors in cerebellar granule cells. Neuropharmacology. 1995;349:929–937. doi: 10.1016/0028-3908(95)00082-h. [DOI] [PubMed] [Google Scholar]
- 8.Collins F, Lile JD. The role of dihydropyridine-sensitive voltage-gated calcium channels in potassium-mediated neuronal survival. Brain Res. 1989;502:99–108. doi: 10.1016/0006-8993(89)90465-4. [DOI] [PubMed] [Google Scholar]
- 9.Connor JA, Tseng H-Y, Hockberger PE. Depolarization and transmitter-induced changes in intracellular Ca2+of rat cerebellar granule cells in explant cultures. J Neurosci. 1987;7:1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Waard M, Feltz A, Bossu JL. Properties of a high-threshold voltage-activated calcium current in rat cerebellar granule cells. Eur J Neurosci. 1991;3:771–777. doi: 10.1111/j.1460-9568.1991.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 11.De Waard M, Campbell KP (1995) Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol (Lond) 485.3:619–634. [DOI] [PMC free article] [PubMed]
- 12.Dolphin AC, Scott RH. Calcium currents and their inhibition by (−) baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol (Lond) 1987;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmslie KS, Zhou W, Jones SW. LHRH and GTP-γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 14.Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol (Lond) 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forti L, Pietrobon D. Functional diversity of L-type calcium channels in rat cerebellar neurons. Neuron. 1993;10:437–450. doi: 10.1016/0896-6273(93)90332-l. [DOI] [PubMed] [Google Scholar]
- 16.Fu W-M, Huang FL. L-type Ca2+channel is involved in the regulation of spontaneous transmitter release at developing neuromuscular synapses. Neuroscience. 1994;58:131–140. doi: 10.1016/0306-4522(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 17.Gallo V, Ciotti MY, Coletti A, Alolsi F, Levy G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci USA. 1985;79:7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glossmann H, Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- 19.Haws CM, Slesinger PA, Lansman JB. Dihydropyridine- and ω-conotoxin-sensitive Ca2+ currents in cerebellar neurons: persistent block of L-type channels by a pertussis toxin-sensitive G-protein. J Neurosci. 1993;13:1148–1156. doi: 10.1523/JNEUROSCI.13-03-01148.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess P, Lansman JB, Tsien RW. Different modes of calcium channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 22.Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, McIntosh JM, Cruz LJ, Imperial JS, Olivera BM. A new conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- 23.Hockberger PE, Tseng H-Y, Connor JA. Immunocytochemical and electrophysiological differentiation of rat cerebellar granule cells in explant culture. J Neurosci. 1987;7:1370–1383. doi: 10.1523/JNEUROSCI.07-05-01370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshi T, Rothlein J, Smith SJ. Facilitation of Ca2+ channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1984;81:5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui A, Ellinor PT, Krizanova O, Wang JJ, Diebold RJ, Schwarz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the α1 subunit of the voltage-dependent calcium channel. Neuron. 1991;7:35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- 26.Huston E, Scott RH, Dolphin AC. A comparison of the effect of calcium channel ligands and GABABagonists and antagonists on transmitter release and somatic calcium channel currents in cultured neurons. Neuroscience. 1990;38:721–729. doi: 10.1016/0306-4522(90)90065-c. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda S. Double-pulse calcium current facilitation in adult rat sympathetic neurones. J Physiol (Lond) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavalali ET, Plummer MR (1994) Selective potentiation of a novel calcium channel in rat hippocampal neurones. J Physiol (Lond) 480.3:475–484. [DOI] [PMC free article] [PubMed]
- 29.Kasai H. Tonic inhibition and rebound facilitation of a neuronal calcium channel by a GTP-binding protein. Proc Natl Acad Sci USA. 1991;88:8855–8859. doi: 10.1073/pnas.88.19.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasai H, Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflügers Arch. 1989;414:145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- 31.Kleppisch T, Pedersen K, Strübing C, Bosse-Doenecke E, Flockerzi V, Hofmann F, Hescheler J. Double-pulse facilitation of smooth muscle α1-subunit Ca2+ channels expressed in CHO cells. EMBO J. 1994;13:2502–2507. doi: 10.1002/j.1460-2075.1994.tb06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–810. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- 33.Lacerda AE, Brown AM. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989;93:1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K. Potentiation of calcium channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci USA. 1987;84:3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Tsien RW. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol (Lond) 1984;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchetti C, Carignani C, Robello M. Voltage-dependent calcium currents in dissociated granule cells from rat cerebellum. Neuroscience. 1991;43:121–133. doi: 10.1016/0306-4522(91)90422-k. [DOI] [PubMed] [Google Scholar]
- 37.McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, Tsien RW, Yoshikami D. ω-Conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not in muscle. Proc Natl Acad Sci USA. 1987;84:4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintz IM, Venema VJ, Swiderek K, Lee T, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin ω-Aga-IVA. Nature. 1992a;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- 39.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992b;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 40.Murphy TH, Worley PF, Baraban JM (1991) L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron 7:625–635. [DOI] [PubMed]
- 41.Parri HR, Lansman JB. Multiple components of pre-pulse induced facilitation of calcium currents in cerebellar granule cells. Soc Neurosci Abstr. 1994;20:899. [Google Scholar]
- 42.Pearson HA, Sutton KG, Scott RH, Dolphin AC (1995) Characterization of calcium channel currents in cultured rat cerebellar granule neurones. J Physiol (Lond) 482.3:493–509. [DOI] [PMC free article] [PubMed]
- 43.Pietrobon D, Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990;346:651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- 44.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 45.Pollo A, Taglialatela M, Carbone E. Voltage dependent inhibition and facilitation of Ca channel activation by GTP-γ-S and Ca-agonists in adult rat sensory neurons. Neurosci Lett. 1989;123:203–207. doi: 10.1016/0304-3940(91)90931-i. [DOI] [PubMed] [Google Scholar]
- 46.Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan L, Sah DH, Bean BP. Ca2+channels in rat central and peripheral neurons: high threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- 48.Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 49.Rossi DJ, Slater NT. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology. 1993;32:1239–1248. doi: 10.1016/0028-3908(93)90018-x. [DOI] [PubMed] [Google Scholar]
- 50.Sather WA, Tanabe T, Zhang J-F, Mori Y, Adams ME, Tsien RW. Distinctive biophysical and pharmacological properties of class A (B1) calcium channel α subunits. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 51.Scultoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L type Ca channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993a;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 52.Scultoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993b;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slesinger PA, Lansman JB. Inactivation of calcium currents in granule cells cultured from mouse cerebellum. J Physiol (Lond) 1991a;435:101–125. doi: 10.1113/jphysiol.1991.sp018500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slesinger PA, Lansman JB. Inactivating and non-inactivating dihydropyridine-sensitive Ca channels in mouse cerebellar granule cells. J Physiol (Lond) 1991b;439:301–323. doi: 10.1113/jphysiol.1991.sp018668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slesinger PA, Lansman JB. Reopening of Ca channels in mouse cerebellar neurons at resting membrane potentials during recovery from inactivation. Neuron. 1991c;7:755–762. doi: 10.1016/0896-6273(91)90278-8. [DOI] [PubMed] [Google Scholar]
- 56.Slesinger PA, Lansman JB (1996) Reopening of single L-type Ca2+ channels in mouse cerebellar granule cells. Voltage- and ion dependence. J Physiol (Lond) 491.2:335–345. [DOI] [PMC free article] [PubMed]
- 57.Snutch TP, Leonard JP, Gilbert MM, Lester HA, Davidson N. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci USA. 1990;87:3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snutch TP, Tomlinson WJ, Leonard JP, Gilbert MM. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 59.Soong TW, Stea W, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low-voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 60.Stea A, Tomlinson J, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain alpha1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal Ca channels and their modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 62.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 63.Yue DT, Herzig S, Marban E. β-Adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their counterparts in mammalian central neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 65.Zygmunt AC, Maylie J. Stimulation-dependent facilitation of the high-threshold calcium current in guinea-pig ventricular myocytes. J Physiol (Lond) 1991;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]